Abstract
The use of agronomic alternatives such as plant hormone sprays has been considered a tool to mitigate drought stress. This research aimed to evaluate the use of foliar brassinosteroid analogue DI-31 (BRs) sprays on plant growth, leaf exchange and chlorophyll a fluorescence parameters, and biochemical variables in lulo (Solanum quitoense L. cv. septentrionale) seedlings grown under drought stress conditions. Seedlings were grown in plastic pots (3 L) using a mix between peat and sand (1:1 v/v) as substrate. Lulo plants were subjected to drought stress by suppressing 100% of the water needs at 30–37 and 73–80 days after transplanting (DAT). Foliar BRs analogue (DI-31) sprays were carried out at four different rates (0, 1, 2, 4, or 8 mL of analogue per liter) at different times (30, 33, 44, 60, 73, and 76 DAT). Drought stress caused a reduction in the Fv/Fm ratio, leaf gas exchange properties, total biomass, and relative water content. Foliar DI-31 sprays enhanced leaf photosynthesis in well-watered (WW) (∼10.7 μmol m−2 s−1) or water-stressed plants (WS) (∼6.1 μmol m−2 s−1) when lulo plants were treated at a dose of 4 and 8 mL·L−1 compared to their respective controls (0 mL·L−1 for WW: 8.83 μmol m−2 s−1 and WS: 2.01 μmol m−2 s−1). Also, DI-31 sprays enhanced the photochemical efficiency of PSII, and plant growth. They also increased the concentration of photosynthetic pigments (TChl and Cx + c) and reduced lipid peroxidation of membranes (MDA) under drought conditions. The results allow us to suggest that the use of DI-31 at a dose of 4 or 8 mL·L−1 can be a tool for managing water stress conditions caused by low water availability in the soil in lulo-producing areas to face situations of moderate water deficit at different times of the year.
Keywords: Drought stress, Andean fruit species, Leaf photosynthesis, Foliar spray, Malondialdehyde, Plant hormones
Drought stress; Andean fruit species; Leaf photosynthesis; Foliar spray; Malondialdehyde; Plant hormones.
1. Introduction
Lulo or naranjilla (Solanum quitoense Lam.) is an important tropical fruit tree belonging to the Solanaceae family and its origin is in Ecuador and Colombia (Ávila et al., 2019). Its fruits are characterized by their refreshing and intense aroma, with potential interest for international markets, such as the United States and Europe (Forero et al., 2016; Ávila et al., 2019). Lulo is grown at altitudes between 1000 and 1990 m above sea level in Colombia, Ecuador, Panama and Costa Rica (Fló rez – Velasco et al., 2015; Ramírez et al., 2018). In Colombia, lulo crops had a cultivated area of 8,821 ha with an average yield of 10.1 t·ha−1 in 2018 (Agronet, 2021).
Climate change poses increasingly severe risks to crop production as it affects weather patterns, resulting in extreme heat events, frequent frosts, waterlogging, and drought (Dahal et al., 2019; Menezes– Silva et al., 2019). Drought stress is one of the principal limitations to agricultural productivity worldwide (Nawaz et al., 2015). World regions most affected by drought include South and Central Asia, South America, Central Europe, and the Southeastern United States (Goñi et al., 2018). Drought events can also be associated with climate variability phenomena such as El Niño-Southern Oscillation (ENSO), generating more frequent and intense drought periods (Zambrano Mera et al., 2018; Oñ ate – Valdivieso et al., 2020). In Colombia, ENSO phenomena cause reductions in rainfall and increases in average temperatures (Sá nchez – Reinoso et al., 2018). As reported by the Organization for Economic Cooperation and Development (OECD) (2014), decreases in the average annual precipitation rate of 4 mm year−1 are projected, which may generate extreme drought events in Colombia for the year 2100. A transition can be expected from a semi-humid to a semi-arid climate in the main lulo-producing areas in Colombia (Andean region) (OECD, 2014; Agronet, 2021).
Drought can influence plant metabolism, growth, and productivity (Abid et al., 2018; Kumar et al., 2018; Liang et al., 2020). Net photosynthesis (PN) is the physiological process most affected by this type of stress (Li et al., 2018; Talaat, 2020). Photosynthetic deterioration during water scarcity is mainly caused by stomatal (stomatal closure due to CO2 reduction) or non-stomatal (decrease in chlorophyll content, inhibition of Rubisco, and photochemical efficiency of PSII) limitations that alter carbohydrate metabolism and dry matter partitioning (Zahoor et al., 2017; Li et al., 2018; Zhao et al., 2020). Liu et al. (2015) observed a decrease in growth, PN, stomatal conductance (gs), and chlorophyll content of tomato (Solanum lycopersicum L.) plants under conditions of drought stress. The above has allowed concluding that the estimation of gas exchange parameters such as PN, gs, and transpiration (E) is a useful technique to evaluate plant responses to drought and its mitigation strategies (Hu et al., 2013; Gill et al., 2017).
Leaf photosynthetic pigments such as carotenoids (Cx + c) and total chlorophyll (TChl) have become important elements in monitoring drought stress and quantifying agronomic management techniques in plants (Chen et al., 2016; Shah et al., 2017). Batra et al. (2014) recorded a gradual decrease in the TChl content and an increase in Cx + c values of three genotypes of mung bean (Vigna radiata L.) exposed to drought. However, the application of agronomic management strategies such as mineral fertilizers, compost, and plant growth-promoting rhizobacteria (PGPR) showed an increase in TChl of 82% in wheat plants under conditions of drought stress (Kanwal et al., 2017).
Chlorophyll fluorescence parameters are also a rapid, complementary, and non-destructive technique to estimate the effects of stress (including drought) on the functioning of the photosynthetic system (Liu et al., 2019). Chlorophyll fluorescence parameters such as quantum efficiency of PSII (Fv/Fm), photochemical quenching (qP), and non-photochemical quenching (NPQ) have been used as indicators of tolerance and acclimatization to drought stress conditions in cotton (Gossypium hirsutum L.) plants (Singh et al., 2014) and Moso Bamboo (Phyllostachys edulis) (Wu et al., 2018). Raja et al. (2020) recorded drops in Fv/Fm and qP values, and higher NPQ in tomato plants under a 10-day drought period. In contrast, Pourghasemian et al. (2020) recorded an increase in Fv/Fm after the use of biostimulants (salicylic acid and beeswax waste and licorice extracts) as effective agronomic practices in sesame (Sesamum indicum L.) plants under water deficit conditions.
Drought stress also causes the buildup of reactive oxygen species (ROS). High levels of these molecules are harmful since they damage membranes, proteins, chlorophyll, and nucleic acids in plants (Yuan et al., 2015; Talaat, 2020). Malondialdehyde (MDA) is generated from the lipid peroxidation of plant cell membranes and has been used as a biochemical indicator of membrane damage under drought conditions (Cheng et al., 2018; Liang et al., 2019). MDA has also been used as a biochemical marker to evaluate the effect of agronomic strategies for optimizing crop resilience to water deficit conditions (Gill et al., 2017; Gou et al., 2017). These same authors recorded a lower MDA content after the use of agronomic strategies such as the application of nitrogen fertilizers or plant hormones, mitigating stress caused by water shortage in barley (Hordeum vulgare L.) and maize (Zea mays L.) plants, respectively.
Exogenous applications of plant hormones or biostimulants are also an agronomic strategy used as an adaptation method to improve crop productivity and water use efficiency under drought conditions (Hussain et al., 2018; Pourghasemian et al., 2020). Foliar applications of brassinosteroids (BRs) have been considered an alternative to lessen the damage caused by drought conditions in plants (Lima and Lobato, 2017; Khamsuk et al., 2018). Exogenous BRs mitigate the negative effects of this type of stress on growth, gas exchange parameters (increasing PN, gs, and water use efficiency (WUE)), photosynthetic pigment contents, or chlorophyll fluorescence measurements such as Fv/Fm in cultivated plants (Khamsuk et al., 2018; Lima and Lobato, 2017; Talaat, 2020). Foliar applications of BRs help plants to cope with drought stress by improving water relations and plant growth, leaf gas exchange parameters (PN, gs, and E), photosynthetic pigment contents (TChl and Cx + x), and chlorophyll fluorescence parameters such as Fv/Fm, qP and NPQ in chili pepper [Capsicum annuum L. var. frutescens (L.) Kuntze] (Khamsuk et al., 2018) and cowpea [Vigna unguiculata (L.) Walp.] plants (Lima and Lobato, 2017). Yuan et al. (2010) also found that this plant hormone can reduce oxidative damage reflected in a lower MDA accumulation in tomato plants. One of the most used BRs is 24-Epibrassinolide (EBL) since it improves the growth, yield, and photosynthetic parameters of plants under drought conditions (Tanveer et al., 2019; Khan et al., 2021). However, the application of this plant hormone results in increased costs to producers (Serna et al., 2015). Therefore, the use of brassinosteroid analogues (DI-31) results in a cost-efficient tool to alleviate the negative effects of abiotic stress (Pérez-Borroto et al., 2021). BRs analogues exhibit greater biological activity and longer average life under field conditions (Sasse, 2003). These characteristics, along with a growth-promoting effect in different plant species and lower cost, make these compounds a good option to replace some of the most commonly used hormones such as EBL (Pérez-Borroto et al., 2021). DI-31 has been reported to improve crop yield and increase the photosynthetic rate of plant species grown under field conditions (Serna et al., 2012a, 2012b, 2015).
Different studies on plant ecophysiology have been mainly focused on the effect of the water stress (drought or waterlogging) or light intensity on leaf gas exchange and chlorophyll fluorescence parameters, morphoanatomical changes, and plant growth (Medina et al., 2006; Cardona et al., 2016; Sá nchez-Reinoso et al., 2019). The literature reports the use of foliar nitrogen sprays to enhance lulo tolerance to water stress (waterlogging) (Fló rez – Velasco et al., 2015). Research on the use of agronomic management strategies to mitigate drought stress conditions is scarce in lulo. Additionally, no studies have reported the use of a brassinosteroid analogue on the growth and physiological behavior of an Andean fruit species such as lulo under drought stress. This study hypothesized that the exogenous application of BRs analogue DI-31 would be an agronomical tool to alleviate the negative impacts of drought by enhancing physiological and biochemical traits in lulo plants. Therefore, the aim of this research was to determine if DI-31 can lessen the negative effects caused by water deficit on plant growth, leaf gas exchange parameters, photosynthetic pigments, chlorophyll fluorescence, and lipid peroxidation of membranes and the possible contribution of these plant hormones in increasing the tolerance to this abiotic condition in lulo plants.
2. Materials and methods
2.1. Plant material and growing conditions
This experiment was performed in the greenhouses of the Universidad Nacional de Colombia, Bogotá campus (4°35′56´´N, 74°4′51´´W; 2556 masl) between August and December 2015 (16 weeks). Two-month-old lulo (Solanum quitoense cv. Septentrionale) seedlings purchased at a local nursery were transplanted into 3 L plastic pots (one plant per pot) containing a mixture of quartz sand and nutrient-free peat (Base 1 substrate, Klasman-Deilmann GmbH, Geeste, Germany) as low moisture retention substrate (1:1 v/v). The conditions of the greenhouse during the experiment were as follows: 22 ± 4 °C average temperature, relative humidity between 60 and 90%, and a day length of 12 h with a maximum photosynthetically active radiation (PAR) of 1500 mol m−2 s−1 recorded at noon. The seedlings underwent an acclimatization period of 30 days after transplantation.
During the experiment, plants were watered with a nutrient solution based on a complete liquid fertilizer (Nutriponic®; Walco SA, Colombia) at a dose of 5 mL·L−1 H2O. The nutrient solution concentration used was the following: 2.08 mM Ca (NO3)2·4H2O, 1.99 mM MgSO4·7 H2O, 2.00 mM NH4H2PO4, 10.09 mM KNO3, 46.26 nM H3BO3, 0.45 nM Na2MoO4·2H2O, 0.32 nM CuSO4·5H2O, 9.19 nM MnCl2·4H2O, 0.76 nM ZnSO4·7H2O, and 19.75 nM FeSO4·H2O. The irrigation volume of the nutrient solution was adjusted throughout the experiment depending on the pot capacity and plant growth to meet its water requirements. The following irrigation volume was used: 450 mL plant−1 week−1 from 1 to 30 days after transplanting (DAT), 600 mL plant−1 week−1 from 31 to 57 DAT, and 750 mL plant−1 week−1 from 58 to 80 DAT (end of the experiment). Plant irrigation requirements were estimated considering the plant's evapotranspiration demand (ETo) (quantified daily) using the gravimetric technique (Hainaut et al., 2016). Finally, the experiment lasted 80 days.
2.2. Drought stress treatments and foliar brassinosteroid analogue (DI-31) doses
After the acclimatization period (30 DAT), the lulo seedlings were divided into two groups of 25 plants each. The first group corresponded to seedlings under well-irrigated conditions, and the second consisted of water-stressed seedlings. Well-irrigated plants were watered up to 100% of their daily ETo needs for the duration of the experiment. On the other hand, water-stress treatment consisted of suppressing 100% of the daily ETo needs. Water-stressed plants were exposed to two water deficit periods (without water supply) for 7 consecutive days. Both water stress periods were carried out on the following dates: from 30 to 37 DAT (Period 1) and from 73 to 80 DAT (Period 2). Between stress periods, plants under water stress were subjected to a recovery time of 35 days, supplying 100% of their daily water needs.
Seedlings from both irrigation regimes (well-irrigated and water-stressed) were also subdivided into five other groups to establish foliar treatments with five different doses of a brassinosteroid analogue (DI-31). The doses were as follows: 0, 1, 2, 4, or 8 mL·L−1 of DI-31 [(25 R) – 3β. 5α – dihydroxy-spirostan-6-one] (Biomex DI-31®, Minerales exclusivos SA, Bogotá, Colombia). The concentrations of this analogue were selected based on the available literature on the use of brassinosteroid and analogues in plants under water stress conditions (Farooq et al., 2009; Yuan et al., 2010; Wang et al., 2015; Pérez-Borroto et al., 2021).
Foliar applications of DI-31 were performed at the beginning and during each period of water stress as well as in the recovery period. A total of six foliar sprays of the different DI-31 doses were carried out at 30, 33, 44, 60, 73, and 76 DAT. The application times were selected according to Fló rez – Velasco et al. (2015); these authors showed that multiple application sprays are appropriate to help lulo plants cope with abiotic stresses. Seedlings of the dose 0 mL·L−1 of DI-31 of both irrigation regimes (well-irrigated and water-stressed) were only sprayed with distilled water. Ten treatment groups were obtained at the end of the experiment: i) well-irrigated plants with foliar application of 1, 2, 4, or 8 mL·L−1 of DI-31; ii) well-irrigated plants without DI-31 application; iii) plants with water stress and foliar application of 1, 2, 4 or 8 mL·L−1 of DI-31, and iv) plants with water stress without DI-31 application. Finally, foliar applications of DI-31 were carried out between 07:00 and 09:00 h, using a Style 1.5 compression sprinkler (Matabi, Spain). The application volume of the sprinkler was 15 ml H2O per plant and the upper and lower surfaces of leaves were wetted until dripping. All foliar applications carried a surfactant adjuvant (INEX-A, Cosmoagro, Colombia) at a dose of 0.1% (v/v). A completely randomized factorial design (irrigation level vs. DI-31 doses) with five replicates was used to arrange the treatments, and each replicate consisted of a plant.
2.3. Leaf gas exchange parameters
One fully expanded leaf per plant from the upper part of the canopy was selected to carry out leaf gas exchange readings. The leaves were adapted to the surrounding environmental conditions without touching their surface to prevent stomatal closure before measurements. Stomatal conductance (gs) readings were taken over two days before the end of the experiment (79 and 80 DAT) using a portable porometer (SC-1, Decagon Devices Inc., Pullman, WA, US). Net photosynthesis (PN) was estimated using a portable meter (LiCOR 6200, Lincoln, Nebraska, USA). One set of 2-d measurements (79 and 80 DAT) was carried out to determine PN and gs between 1100 and 1200 h following the methodology described by Lombardini et al. (2009). The conditions within the LiCOR chamber during PN readings were the following: leaf temperature of 25 ± 5 °C, PAR ≥800 μmol m−2∙s−1, and leaf-to-air vapor pressure difference of 1.8 ± 0.5 kPa. Intrinsic water use efficiency (WUEi) was determined as the PN to gs ratio (Flexas et al., 2001). Finally, the gravimetric technique described by Sá nchez – Reinoso et al. (2014) was used to obtain the total plant transpiration (E). Pots were covered with plastic wrap to prevent water loss by evaporation and weighted daily before and after irrigation during the second water stress period (73–80 DAT). The transpiration rate was expressed in mg H2Og−1FW∙h−1.
2.4. Chlorophyll fluorescence parameters
Chlorophyll a fluorescence parameters were determined on the same leaves used to estimate gas exchange parameters (PN and gs) utilizing a modulated fluorometer (MINI-PAM, Walz, Effeltrich, Germany). The leaves were previously adapted to the dark using clips for 20 min. After dark adaptation, we calculated the maximum quantum efficiency of PSII (Fv/Fm), photochemical quenching (qP), and non-photochemical quenching (NPQ) using an actinic light pulse of up to 2,600 μmol m−2∙s−1 on the adaxial leaf surface to determine fluorescence parameters. These readings were taken at 80 DAT.
2.5. Screening of water stress tolerance in terms of Fv/Fm ratio
The decrease in the maximum quantum efficiency of PSII (DQE) under water stress was calculated using the Fv/Fm ratio readings obtained at the end of the experiment (80 DAT). compared to foliar doses of DI-31. DQE was estimated using Eq. (1) described by Chá ves – G ó mez et al., 2020.
| (1) |
where WI represents well-irrigated plants without foliar applications of DI-31, and WS represents plants subjected to water stress and with foliar applications of DI-31 at the evaluated doses (0, 1, 2, 4, and 8 mL·L−1 of DI-31).
Subsequently, foliar treatments with different DI-31 doses were classified into the following four categories: high tolerance: DQE ≤25; moderate tolerance: between 26 and 41; low tolerance: between 42 and 55; susceptible ≥56.
2.6. Leaf photosynthetic pigments
The equations by Wellburn (1994) were used to determine the contents of total chlorophyll (TChl) and carotenoids (Cx + c) in leaves at 80 DAT. Leaf tissue samples of approximately 30 mg were collected from the same leaves used to estimate PN and gs. Samples were homogenized in 3 mL of 80% acetone (v/v) and then centrifuged (Model 420101, Becton Dickinson Primary Care Diagnostics, MD, USA) at 5000 rpm for 10 min to eliminate particles. Acetone was added to dilute the supernatant to a final volume of 6 mL (Sims and Gamon, 2002). A spectrophotometer (Spectronic BioMate 3 UV-vis Thermo, Madison, WI) was used to determine the content of TChl at 663 and 646 nm, and Cx + c at 470 nm.
2.7. Production of malondialdehyde (MDA)
Malondialdehyde (MDA) production (lipid peroxidation of membranes) was estimated using the thiobarbituric acid (TBA) method by Hodges et al. (1999). Approximately 300 mg of plant material from the same leaves used to measure gas exchange parameters was homogenized and stored in liquid nitrogen. Subsequently, the samples were centrifuged (Model 420101, Becton Dickinson Primary Care Diagnostics, MD, USA) at 5000 rpm for 10 min, and a spectrophotometer (Spectronic BioMate3UV-Vis, Thermo, Madison, WI) was used to estimate absorbances at 440, 532, and 600 nm. The extinction coefficient (157 M mL−1) was considered to determine the MDA concentration, and MDA readings were also taken at 80 DAT.
2.8. Leaf relative water content
Fully expanded leaves were collected from the middle portion of the canopy to determine the relative water content (RWC) also at 80 DAT. After determining the fresh weights (FW), the leaves were put in distilled water at 4 °C for 24 h in a dark room to determine the turgid weight (TW). The dry weight (DW) was calculated by heating turgid leaves in an oven at 70 °C for 48 h. The RWC was estimated according to Smart and Bingham (1974), using Eq. (2):
| (2) |
2.9. Plant growth parameters
Plant height was recorded at the end of the experiment (80 DAT) using a ruler. Lulo plants were then harvested, and their biomass was separated into each of the organs (leaves, stems, and roots). Photographs of all the leaves in the plant canopy (D3300, Nikon, Thailand) were taken to determine the leaf area (LA) of each plant. All photographs were saved as TIFF (Tagged Image File Format) images and then processed using Java software (Image J; National Institute of Mental Health, Bethesda, MD) to calculate LA. Plant organs were dried in an oven at 70 °C for 72 h until constant weight to estimate the dry weights of leaves (LDW), stems, roots, and total (TDW); additionally, the dry matter (DM) partitioning was determined. Finally, the following indices were obtained: specific leaf area (SLA = LA/LWD; c m2·g−1) (Gunn et al., 1999), leaf mass per area (LMA = LDW/LA; mg·cm2) (Cheng et al., 2014) and equivalent water thickness (EWT = (leaf fresh weight (LFW) – LDW)/LA; mg·cm2) (Yilmaz et al., 2008).
2.10. Relative tolerance index (RTI)
The effect of foliar applications of DI-31 on the tolerance of lulo plants to water stress conditions was determined using the relative tolerance index (RTI). RTI was estimated using the values of net photosynthesis (PN) of the different treatments with DI-31 under drought stress conditions in relation to the treatment under well-irrigated conditions and without DI-31 applications. The RTI was calculated at 80 DAT, using Eq. (3) from Chá vez – Arias et al. (2020) with some modifications:
| (3) |
2.11. Experimental design and statistical analysis of data
For data analysis, a factorial arrangement was used where the plant water status (well-irrigated plants vs. water-stressed plants) was the first factor and the DI-31 doses (0, 1, 2, 4, or 8 mL·L−1) corresponded to the second factor. Five plants were used per treatment. We performed an analysis of variance (ANOVA) and a post hoc Tukey test for comparison of means when significant differences (P ≤ 0.05) were observed. A correlation analysis between RTI and TDW or MDA was conducted to find the best treatments under water deficit conditions (water-stressed plants). The arcsine function was used to transform percentage values. Data were processed using the software Statistix v 9.0 (Analytical Software, Tallahassee, FL.). All figures and the three-dimensional graph were prepared using SigmaPlot (version 12.0; Systat Software, San José, CA, USA), and the correlation analysis was conducted by the same software.
3. Results
3.1. Leaf growth parameters and relative water content (RWC)
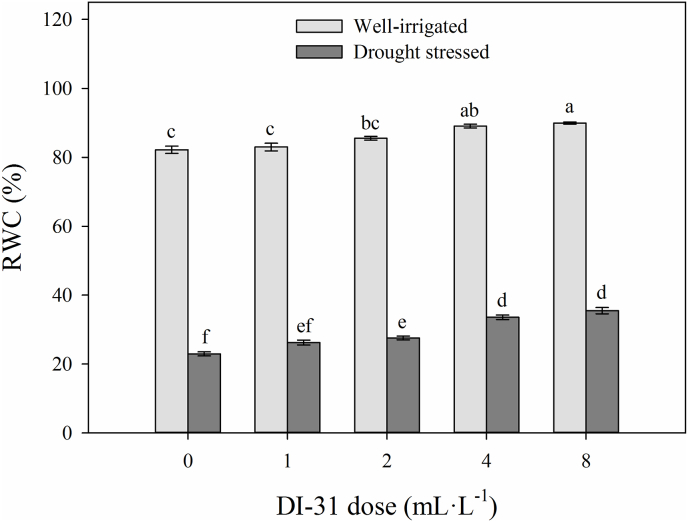
Significant differences were observed in the interaction between irrigation regimes and doses of brassinosteroid analogue on the relative water content (RWC) (p = 0.02) and leaf growth parameters such as leaf area (LA) (p = 0.000), leaf dry weight (LDW) (p = 0.014), specific leaf area (SLA) (p = 0.033), leaf mass per area (LMA) (p = 0.019) and equivalent water thickness (EWT) (p = 0.000) at 80 DAT. The RWC increased significantly with foliar application of DI-31 in plants under both irrigation regimes. In the group of well-irrigated plants, foliar applications with 4 and 8 mL·L−1 of DI-31 increased RWC by 9% (mean values of 89.5%) compared to plants without DI-31 application (82.2%). A much higher increment in RWC was observed in drought-stressed plants. RWC increased significantly by 20.2, 46.3, and 54.7% after exogenous application of 2, 4, and 8 mL·L−1 of DI-31, respectively, compared to the treatment with 0 mL·L−1 of DI-31 in the same group of plants (22.9%) (Figure 1).
Figure 1.
Relative water content (RWC) in well-irrigated (light grey bars) and drought-stressed (dark gray bars) lulo (Solanum quitoense Lam.) plants sprayed with 1, 2, 4 and 8 mL·L−1 of brassinosteroid analogue (DI-31) at 80 days after transplanting (DAT). Each column represents the mean of five data ± standard error (n = 5). Bars followed by different letters indicate statistically significant differences according to the Tukey test (p ≤ 0.05).
LA, LDW, SLA, and EWT were also highly affected by the drought stress condition, registering the lowest values in plants without foliar application of DI-31 (Table 1). A positive response was observed in plants under both irrigation regimes when sprayed with DI-31. Foliar applications with 8 mL·L−1 of DI-31 caused the greatest increases of 30.1% in LA, 25.8% in LDW, 19.8% in SLA, and 18.8% in EWT compared to the other treatments in drought-stressed plants. In contrast, LMA showed an inverse behavior since the highest values of this variable were observed in drought-stressed plants without exogenous application of DI-31 (12.1 mg cm2). A much lower LMA value was recorded in all the doses of DI-31 used (1, 2, 4, and 8 mL·L−1) registering mean values of 11.6 mg cm2 in lulo plants subjected to water deficit stress. The highest values of LA, LDW, and SLA in well-irrigated plants were recorded in foliar treatments with 4 and 8 mL·L−1 of DI-31. All DI-31 doses did not show any effect on the variables LMA and EWT in this group of plants (Table 1).
Table 1.
Leaf area (LA), leaf dry weight (LDW), specific leaf area (SLA), leaf mass per area LMA) and equivalent water thickness (EWT) of lulo (Solanum quitoense Lam.) plants exposed to drought and sprayed with 1, 2, 4 and 8 mL·L−1 of brassinosteroid analogue (DI-31) at 80 days after transplanting (DAT).
| Irrigation treatment | DI-31 dose (mL·L−1) | LA |
LDW |
SLA |
LMA |
EWT |
|---|---|---|---|---|---|---|
| (cm2) | (g dry matter) | (cm2·g−1) | (mg·cm−2) | (mg·cm−2) | ||
| Well-irrigated | 0 | 1353.7 ca | 11.1 b | 123.4 c | 8.2 c | 42.7 a |
| 1 | 1389.6 bc | 11.4 b | 123.9 c | 8.1 c | 43.6 a | |
| 2 | 1464.4 b | 11.6 b | 126.9 bc | 7.9 c | 43.9 a | |
| 4 | 1612.7 a | 12.2 ab | 132.7 ab | 7.8 c | 44.3 a | |
| 8 | 1679.3 a | 13.3 a | 134.1 a | 7.6 c | 44.6 a | |
| Drought-stressed | 0 | 524.5 e | 6.3 d | 72.3 e | 12.8 a | 31.1 d |
| 1 | 554.3 e | 7.3 cd | 76.3 e | 12.1 b | 34.4 c | |
| 2 | 663.2 d | 7.7 c | 85.7 d | 11.7 b | 35.3 bc | |
| 4 | 672.3 d | 7.8 c | 86.6 d | 11.5 b | 36.3 bc | |
| 8 | 682.3 d | 7.9 c | 86.9 d | 11.4 b | 36.6 b | |
| Significance (p value) | 0.0000 | 0.0143 | 0.0193 | 0.0333 | 0.0005 | |
| CV (%)b | 3.45 | 5.33 | 3.41 | 3.18 | 2.42 | |
Values (n = 5) within the same column and followed by different letters are significantly different according to the Tukey test (p ≤ 0.05).
CV: Coefficient of variation.
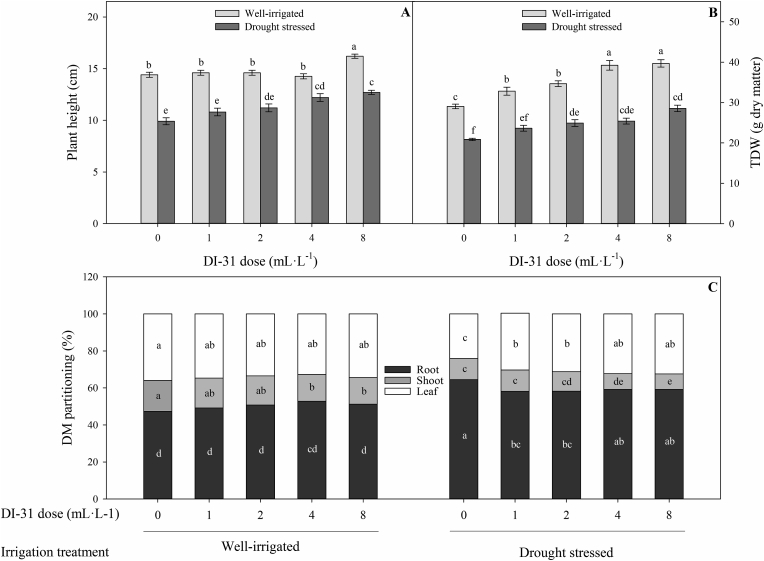
Plant growth parameters such as plant height (p = 0.02), total dry weight (TDW) (p = 0.011), and dry matter (DM) partitioning were also affected by the interaction between irrigation regimes and doses of DI-31 at 80 DAT (Figure 2). In treatments with well-irrigated plants, the application of 8 mL·L−1 of DI-31 significantly increased the plant height (16.2 cm) compared to the other treatments (0, 1, 2, and 4 mL·L−1 of DI-31), registering mean values of 14.4 cm (Figure 2A). A progressive increase in TDW was also observed with the increment in the foliar dose of DI-31. The highest values of TDW were recorded in the treatments with 4 and 8 mL·L−1 of DI-31 (∼39.4 g DM) compared to plants without DI-31 application (29.1 g DM) (Figure 2B). The foliar application of DI-31 also generated a positive effect on plant height and TDW in plants subjected to drought stress. These growth parameters were favored mainly by the foliar application of 4 (12.2 cm for plant height and 25.4 g DM for TDW) and 8 mL·L−1 (12.7 cm for plant height and 28.5 g DM for TDW) of DI-31 in plants under drought conditions compared to plants without exogenous application of DI-31 (9.9 cm for plant height and 20.9 g DM for TDW) (Figure 2A and B).
Figure 2.
Plant height (A), total dry weight (TDW) (B) and dry matter (DM) partitioning (C) in well-irrigated (light grey bars) and drought-stressed (dark gray bars) lulo (Solanum quitoense Lam.) plants sprayed with 1, 2, 4 and 8 mL·L−1 of brassinosteroid analogue (DI-31) at 80 days after transplanting (DAT). Each bar chart summarizes the mean of five data ± standard error (n = 5). Bars followed by different letters indicate statistically significant differences according to the Tukey test (p ≤ 0.05).
Foliar applications of DI-31 did not alter the DM partitioning in leaves and roots of well-irrigated plants. However, the application of 4 and 8 mL·L−1 of DI-31 generated a lower percentage of DM accumulation in the stem (∼14.4%) compared to the other treatments under well-irrigated conditions (∼16.2%). Under conditions of drought stress, the foliar application of this analogue showed two different effects on the DM partitioning of lulo plants: the first effect was an increase in the percentage of leaf DM with the application of mainly 4 and 8 mL·L−1 of DI-31, registering mean values of 32.3% compared to untreated plants (24.1%). The second effect was a decrease in the percentage of stem DM with the exogenous application of 4 and 8 mL·L−1 of DI-31 since this group of plants showed mean values of 8.4% compared to plants without foliar application of DI-31 (11.4%). A reduction in the percentage of root DM was also recorded after the exogenous application of 1, 2, 4, or 8 mL·L−1 of DI-31, registering mean values of 58.6% compared to treatment plants with 0 mL·L−1 of DI-31 (64.4%) (Figure 2C).
3.2. Leaf gas exchange parameters
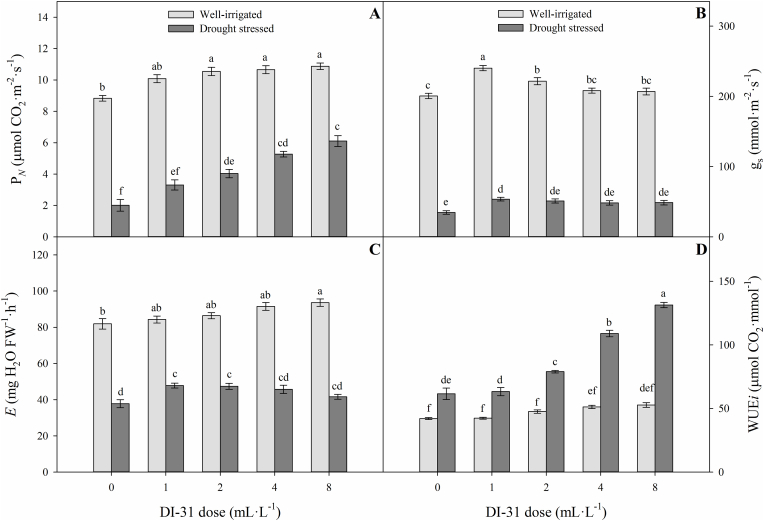
Significant differences were also observed on leaf gas exchange parameters (PN, gs, E, and WUEi) of lulo plants for the interaction between irrigation regimes and foliar doses of BRs analogue (DI-31) at 80 DAT (Figure 3). In drought-stressed plants, foliar application of DI-31 at the doses evaluated (1, 2, 4, and 8 mL·L−1 of DI-31) showed two responses on gas exchange parameters. In the first case, PN and WUEi showed increased values with the increment in the foliar dose of DI-31, obtaining the highest values with the treatment of 8 mL·L−1 of DI-31 (6.1 μmol CO2 m−2·s−1 for PN and 131.3 μmol CO2·mmol H2O−1 for WUEi) compared to untreated plants (2.01 μmol CO2 m−2·s−1 for PN and 61.5 μmol CO2·mmol H2O−1 for WUEi) (Figure 3A and D). In the second case, plants with foliar application of 1 mL·L−1 of DI-31 enhanced acclimatization expressed as the highest gs values (53.6 mmol CO2 m−2·s−1) and E (47.8 mg H2O FW−1·h−1) compared to untreated lulo plants (34.6 mmol CO2 m−2·s−1 for gs and 37.8 mg H2O FW−1·h−1 for E) (Figure 3B and C).
Figure 3.
Net photosynthetic rate (PN) (A), stomatal conductance (gs) (B), transpiration rate (E) (C) and intrinsic water-use efficiency (WUEi) (D) in well-irrigated (light grey bars) and drought-stressed (dark gray bars) lulo (Solanum quitoense Lam.) plants sprayed with 1, 2, 4 and 8 mL·L−1 of brassinosteroid analogue (DI-31) at 80 days after transplanting (DAT). Each bar chart summarizes the mean of five data ± standard error (n = 5). Bars followed by different letters indicate statistically significant differences according to the Tukey test (p ≤ 0.05).
Foliar applications of DI-31 positively affected the gas exchange parameters (PN, gs, E, and WUEi) of well-irrigated plants. Increased values of PN were observed with exogenous applications of 2, 4, and 8 mL·L−1 of DI-31, obtaining mean values of 10.7 μmol CO2 m−2·s−1 compared to untreated plants (8.8 μmol CO2 m−2·s−1) (Figure 3A). Higher values of gs were also recorded with the foliar application of 1 and 2 mL·L−1 of DI-31 (∼230.9 mmol CO2 m−2·s−1) compared to plants without application of plant hormones (200.5 mmol CO2 m−2·s−1) (Figure 3B). On the other hand, E values of lulo plants under well-irrigated conditions significantly increased by 14.3% compared to the treatment with 0 mL·L−1 of DI-31 (81.9 mg H2O FW−1·h−1) after foliar spray with 8 mL·L−1 of DI-31 (Figure 3C). WUEi values did not significantly differ between treatments with various concentrations of DI-31 in well-irrigated plants (Figure 3D).
3.3. Chlorophyll fluorescence parameters
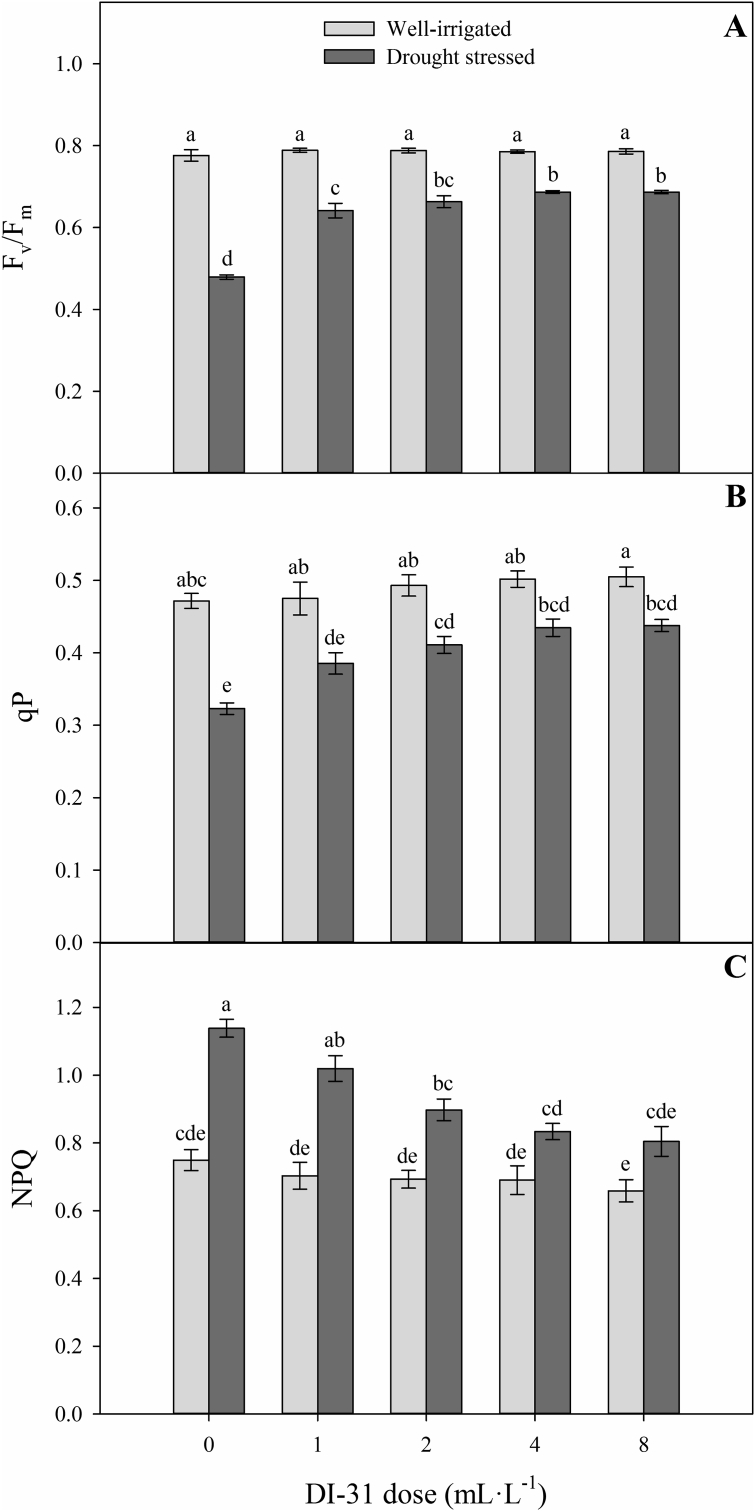
Figure 4 shows the chlorophyll fluorescence parameters evaluated in lulo plants at 80 DAT. Significant differences were also found on the Fv/Fm ratio (p = 0.000), qP (p = 0.025) and NPQ (p = 0.002) for the interaction between irrigation regimes and foliar doses of DI-31. Plants of the treatments under well-irrigated conditions did not show differences in chlorophyll fluorescence parameters between foliar doses of DI-31 (0, 1, 2, 4, and 8 mL·L−1 of DI-31) (Figure 4). Increased Fv/Fm ratio and qP values were observed with higher doses of DI-31 under conditions of water deficit. The highest values of these variables were registered in plants with foliar sprays of 4 and 8 mL·L−1 of DI-31 (0.69 for Fv/Fm and 0.44 for qP) compared to plants of the 0 mL·L−1 treatment (0.48 for Fv/Fm and 0.32 for qP) (Figure 4A and B). In contrast, the values of NPQ showed a drop with increasing foliar doses of DI-31. The lowest values of this variable were obtained in the treatments of 4 and 8 mL·L−1 of this plant hormone (∼0.81) compared to plants without foliar application of DI-31 (1.14) (Figure 4C).
Figure 4.
Maximum quantum efficiency of PSII (Fv/Fm) (A), photochemical quenching (qP) (B) and non-photochemical quenching (NPQ) (C) in well-irrigated (light grey bars) and drought-stressed (dark gray bars) lulo (Solanum quitoense Lam.) plants sprayed with 1, 2, 4 and 8 mL·L−1 of brassinosteroid analogue (DI-31) at 80 days after transplanting (DAT). Each bar chart summarizes the mean of five data ± standard error (n = 5). Bars followed by different letters indicate statistically significant differences according to the Tukey test (p ≤ 0.05).
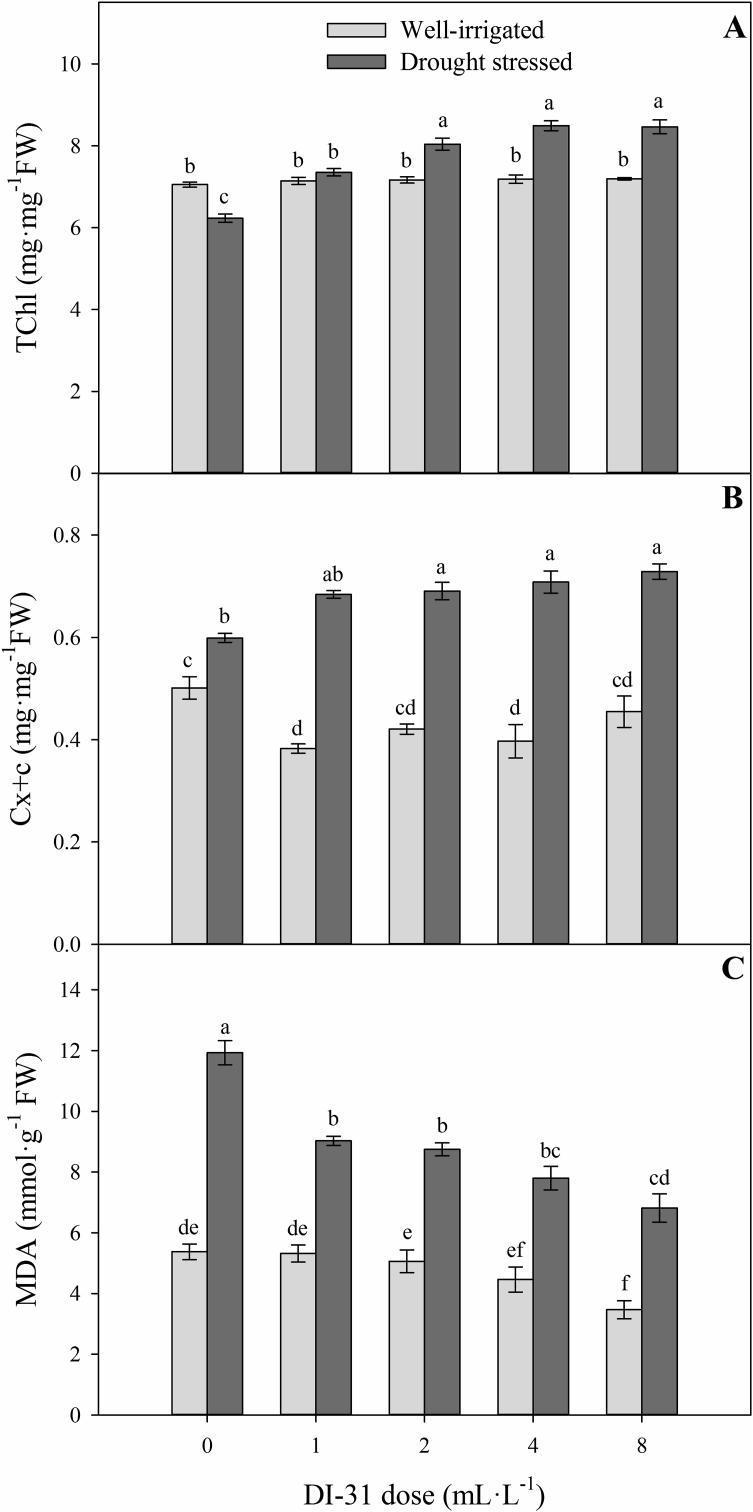
3.4. Photosynthetic pigment content and malondialdehyde (MDA) production
The contents of total chlorophyll (TChl) and carotenoids (Cx + c) were also affected by the interaction of irrigation regimes and foliar doses of BRs analogue (p = 0.000 and p = 0.000, respectively) at 80 DAT (Figure 5). Foliar application of DI-31 at the doses evaluated did not show differences in TChl in well-irrigated lulo plants. However, Cx + c values decreased with the application of treatments with 1 and 4 mL·L−1 of DI-31 (0.38 mg mg−1 FW and 0.4 mg mg−1 FW, respectively) compared to the treatment of 0 mL·L−1 of DI-31 (0.5 mg mg−1 FW) in the same group of plants (Figure 5A and B). When plants were under drought stress conditions, the foliar application of 2, 4, and 8 mL·L−1 of DI-31 showed a positive effect on photosynthetic pigments (∼8.3 mg mg−1 FW for TChl and ∼0.71 mg mg−1 FW for Cx + c) compared to plants without foliar treatment with DI-31 (6.2 mg mg−1 FW for TChl and of 0.6 mg mg−1 FW for Cx + x) (Figure 5A and B).
Figure 5.
Total chlorophyll content (TChl) (A), carotenoids (Cx + c) (B) and malondialdehyde production (MDA) (C) in well-irrigated (light grey bars) and drought-stressed (dark gray bars) lulo (Solanum quitoense Lam.) plants sprayed with 1, 2, 4 and 8 mL·L−1 of brassinosteroid analogue (DI-31) at 80 days after transplanting (DAT). Each bar chart summarizes the mean of five data ± standard error (n = 5). Bars followed by different letters indicate statistically significant differences according to the Tukey test (p ≤ 0.05).
Statistical differences (p = 0.0001) were also found for MDA production in the interaction between irrigation regimes and foliar doses of DI-31 at 80 DAT (Figure 5C). In general, a progressive decrease in MDA content was recorded with increasing doses of DI-31 in both irrigation groups. In well-irrigated lulo plants, MDA content was lower in the treatment with the exogenous application of 8 mL·L−1 of DI-31 (3.5 mmol g−1 FW) compared to plants without any DI-31 application (5.4 mmol g−1 FW). Exposure to drought conditions generated the highest values of MDA production (11.9 mmol g−1 FW) in lulo plants without foliar treatment. However, the application of the doses evaluated registered a drop in the values of this variable, obtaining lower lipid peroxidation (MDA) with the foliar application of 8 mL·L−1 of DI-31 (6.8 mmol g−1 FW).
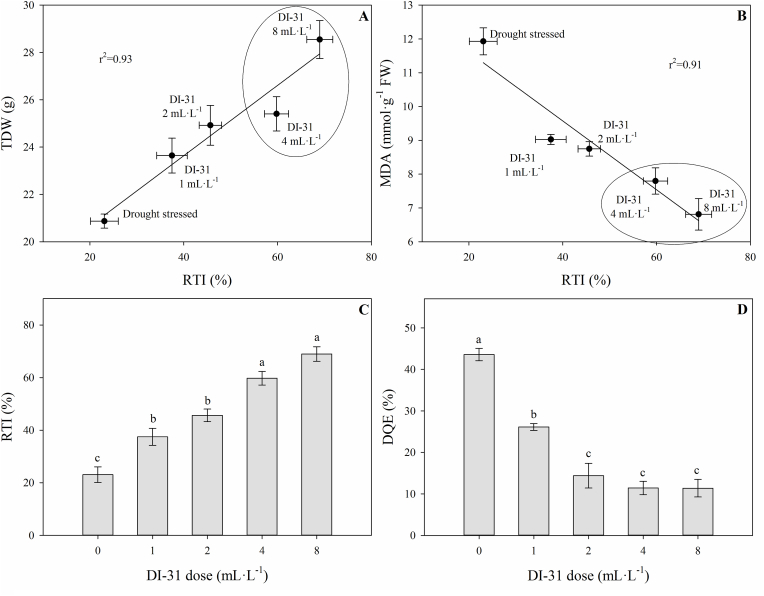
3.5. Detection of tolerance to water stress promoted by foliar application of brassinosteroid analogue DI-31
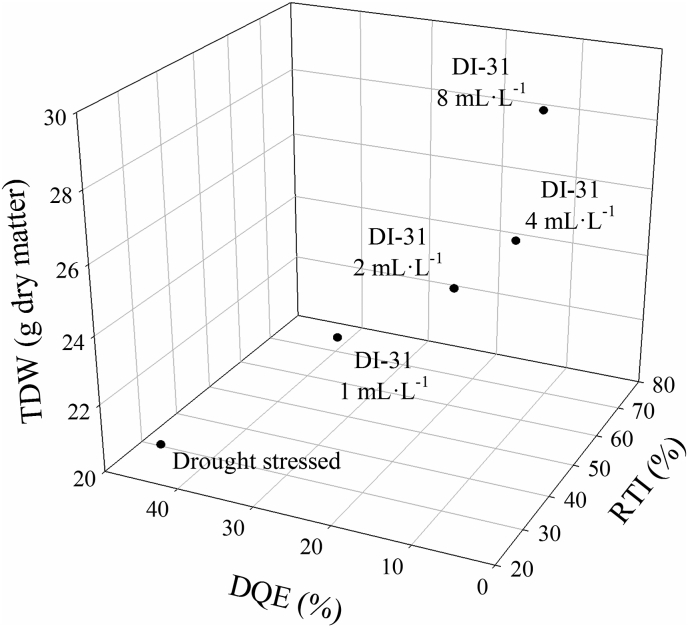
The relative tolerance index (RTI), correlations between RTI and TDW or MDA, and the decrease in the maximum quantum efficiency of PSII (DQE) were determined at 80 DAT to identify the foliar doses of DI-31 that could be used as a possible strategy for the management of drought stress in lulo plants (Figure 6). The RTI and its correlations with TDW (r2 = 0.93) and MDA production (r2 = 0.91) showed that the treatments with foliar applications of 4 and 8 mL·L−1 of DI-31 exhibited a higher accumulation of dry matter in the entire plant and lower lipid peroxidation of membranes in drought-stressed plants (Figure 6A and B). This was reflected in a higher RTI for the dose of 8 mL·L−1 of DI-31 (69%) followed by the dose of 4 mL·L−1 of DI-31 (59.8%) in lulo plants under water deficit conditions (Figure 6C). DQE confirmed all the previous observations; this index showed that drought stress had a lesser effect on the quantum efficiency of PSII in lulo plants treated with 2 (14.4%), 4 (11.4%), and 8 (11.3%) mL·L−1 of DI-31 with values lower than 25%. As a result, these plants were classified with a good level of tolerance to conditions of water stress (Figure 6D and Table 2). Finally, the three-dimensional graph (TDW, RTI, and DQE) also confirmed the results of the correlation analysis where lulo plants treated with exogenous applications of 4 and 8 mL·L−1 of DI-31 showed enhanced physiological behavior (lower affectation of PN, Fv/Fm ratio, and photosynthetic pigments and a lower MDA production) under conditions of drought stress (Figure 7).
Figure 6.
Correlation between the total dry weight (TDW) (A) or malondialdehyde production (MDA) (B) and the relative tolerance index (RTI) (C), and the decrease in the maximum quantum efficiency of PSII (DQE) (D) in lulo (Solanum quitoense Lam.) plants exposed to drought and sprayed with 1, 2, 4 and 8 mL·L−1 of brassinosteroid analogue (DI-31) at 80 days after transplanting (DAT). Bars and points represent the mean of five data ± standard error (n = 5). Bars followed by different letters indicate statistically significant differences according to the Tukey test (p ≤ 0.05).
Table 2.
Classification of treatments with foliar applications of brassinosteroid analogue (DI-31) based on the decrease in the maximum quantum efficiency of photosystem II (Fv/Fm ratio) (DQE) in lulo (Solanum quitoense Lam.) plants under drought stress at 80 days after transplanting (DAT).
| DQE (%)a | Classification | Treatments studied |
|---|---|---|
| DQE ≤25 | Good tolerance | DI-31 2 mL·L−1, DI-31 4 mL·L−1, DI-31 8 mL·L−1 |
| 26 ≥ DQE ≤41 | Moderate tolerance | DI-31 1 mL·L−1 |
| 42 ≥ DQE ≤55 | Low tolerance | Drought-stressed plants |
| DQE ≥56 | Very low tolerance | - |
DQE - decrease in the maximum quantum efficiency of photosystem II (Fv/Fm ratio) in lulo plants exposed to drought and sprayed with DI-31 compared to untreated well-irrigated plants.
Figure 7.
Three-dimensional plot (total dry weight (TDW), decrease in the maximum efficiency of PSII (DQE) and relative tolerance index (RTI)) for lulo (Solanum quitoense Lam.) plants exposed to drought and sprayed with 1, 2, 4 and 8 mL·L−1 of brassinosteroid analogue (DI-31) at 80 days after transplanting (DAT). Data represents the mean of five data ± standard error (n = 5).
4. Discussion
The results of this research indicated that water deficit stress (irrigation suspension) caused significant damage to lulo plants. Drought conditions caused a negative impact on plant water status (lower RWC), plant growth, and photosynthetic function (lower PN, gs, Fv/Fm, and TChl) and promoted a higher lipid peroxidation (higher MDA content). Leaf RWC is considered one of the most appropriate indicators to estimate the water status of plants exposed to water stress conditions (Jungklang et al., 2017). In this study, RWC was reduced because of water stress in lulo plants treated only with distilled water, confirming that this group of plants was subjected to stressful conditions. Similar results were observed by Jangid and Dwivedi (2017) in tomato (Solanum lycopersicum L.) plants and Plazas et al. (2019) in eggplant (Solanum melongena L.) plants registering a reduction in RWC of 27.6 and 35%, respectively, under conditions of water deficit stress. In this study, a significant increase in RWC was observed at higher doses of DI-31 (4 or 8 mL·L−1) under drought stress. Pérez-Borroto et al. (2021) also reported that foliar applications of DI-31 increased RWC by 16% in Arabidopsis thaliana L. plants under drought. A higher RWC in lulo plants under conditions of water stress may be associated with the fact that DI-31 is involved in the plant's osmotic adjustment process, promoting soluble carbohydrate accumulation (starch and sucrose), and the development of primary and lateral roots in plants under drought stress conditions (Khamsuk et al., 2018; Pérez-Borroto et al., 2021).
Low water availability reduced growth parameters such as LA, LDW, TDW, plant height, and DM partitioning of lulo plants (Table 1 and Figure 2). The reduction in growth caused by drought has also been described in other Solanaceae species such as potato (Solanum tuberosum L.) (Banik et al., 2016; Hill et al., 2021), tomato (Shi et al., 2016), and Russian box thorn (Lycium ruthenicum Murr.) (Guo et al., 2016). However, the exogenous application of DI-31 resulted, in general, in increased shoot growth in lulo plants exposed to water deficit. These responses may be related to the beneficial effects of analogue BRs (DI-31) on gas exchange parameters including a higher PN (Figure 3). The increase in PN may be mediated by the analogue's effect to promote a higher photosynthetic area and growth rate, and adequate stomatal regulation. This BRs analogue also plays an important role in the process of cell division in an environment of abiotic stress (Lima and Lobato, 2017; Tanveer et al., 2019; Pérez-Borroto et al., 2021). Serna et al. (2012a) also described positive results of DI-31 applications on growth parameters such as fresh weight and yield of endive (Cichorium endivia L.) plants. Finally, lower dry matter partitioning to roots was observed under water stress conditions despite the application of the analogue. This allows us to infer that root growth may have been inhibited by the abiotic stress rather than by the use of the analogue. This statement is based on the DM partitioning in well-irrigated plants. Hola (2019) reports that the lack of response may be due to the fact that the exogenous application of BRs or the analogue is limited locally to the site of application and is not transported to other organs.
Drought stress reduced leaf gas exchange proprieties (PN, gs, and E) in lulo plants. Liang et al. (2020) observed that drought stress also reduced PN, gs, and E in tomato plants. However, foliar DI-31 sprays improved leaf PN of lulo plants under stress conditions. An increase in the values of PN was also recorded in lettuce (Lactuca sativa L.) plants after the exogenous application of DI-31 (Serna et al., 2012b). It has been reported that BRs analogues such as DI-31 promote photosynthetic capacity through the upregulation of Rubisco (the enzyme in charge of intercellular CO2 assimilation) and chlorophyllase, and the transcription of encoded genes involved in photosynthesis under stress conditions (Anwar et al., 2018; Hussain et al., 2020; Pérez-Borroto et al., 2021). On the other hand, foliar sprays of analogue BRs (DI-31) at low concentrations can induce stomatal opening under stress conditions, improving leaf gas exchange properties and plant water status (Serna et al., 2012c; Chá vez – Arias et al., 2020). In this study, the lower foliar concentrations of DI-31 (1 or 2 mL·L-1) caused higher gs values related to the increase in E (Figure 3B and C). Likewise, foliar applications also increased the WUEi values in lulo plants under stress. Pereira et al. (2019) obtained an increment in WUEi of more than 250% caused by the application of 50 and 100 nM of BRs in soybean (Glycine max L.) plants subjected to water deficit stress compared to untreated plants. BRs can enhance water relations through their participation in the modification and/or manipulation of the structure and stability of the plasma membrane under stress conditions (Hayat et al., 2010; Ramakrishna and Rao, 2015).
Drought stress altered leaf chlorophyll a parameters (a high NPQ, and low Fv/Fm and qP). The determination of chlorophyll a fluorescence parameters is a rapid and non-destructive tool used to study the tolerance or level of acclimatization of plants to environmental stress (Thussagunpani et al., 2015; Chá vez – Arias et al., 2019). Raja et al. (2020) also recorded lower Fv/Fm and qP, and higher NPQ values of tomato plants exposed to water stress. In contrast, the foliar DI-31 application increased the values of Fv/Fm and qP and caused a decrease in NPQ in this experiment. BRs have been reported to alleviate the photoinhibition promoted by drought related to greater light use efficiency and dissipation of excitation energy in PSII antennae (Lima and Lobato, 2017; Hu et al., 2019). Foliar BRs sprays increased qP as a result of higher light absorption and flow of electrons accepted by plastoquinone, causing a high quantum efficiency of PSII and electron transport rate (Thussagunpanit et al., 2015; Lima and Lobato, 2017). The exogenous application of DI-31 promoted the reduction of NPQ in plants under drought stress. Pereira et al. (2019) report that reductions in NPQ values in plants treated with BRs indicate a decrease in the loss of photons mainly in the form of heat, by optimizing light use in photochemical processes.
The foliar application of DI-31 in lulo plants under drought stress generated increases in photosynthetic pigments (TChl and Cx + x). Treatments with DI-31 or BRs also caused higher photosynthetic pigments in Arabidopsis thaliana L. (Pérez-Borroto et al., 2021), soybean (Pereira et al., 2019), and sheepgrass (Leymus chinensis (Trin.) (Lv et al., 2020) plants exposed to water deficit. BRs analogues (DI-31) can inhibit the synthesis or activity of enzymes involved in the process of chlorophyll decomposition (Chl b reductase), promote a higher content of ascorbates, phenolic compounds, and tannic acid, and enhance the activity of antioxidant enzymes (Hussain et al., 2020). BRs also participate in the regulation of chlorophyll biosynthesis and decrease lipid peroxidation (Thussagunpanit et al., 2015; Sadura and Janeczko, 2018).
MDA production is the product of lipid peroxidation of membranes and has been considered a direct indicator of membrane damage under drought conditions (Yuan et al., 2010). Tani et al. (2018) recorded a significantly higher MDA content of eggplant (Solanum melongena L.) plants exposed to water stress. In this study, an increased MDA content was also found in lulo plants subjected to drought stress without the application of DI-31. Exogenous sprays of analogue BRs cause lower membrane lipid peroxidation (lower MDA) since this molecule favors the antioxidant system and improves the structure and stability of membranes (Yuan et al., 2010; Barros Junior et al., 2020; Hussain et al., 2020). Chá vez – Arias et al., 2020 also observed a decrease in the accumulation of MDA after the foliar application of DI-31 in cape gooseberry (Physalis peruviana L.) plants under water stress conditions.
BRs and analogues such as DI-31have been reported to improve plant responses to drought through the expression of genes involved in the tolerance mechanism to this abiotic stress (Serna et al., 2012a; Ahanger et al., 2018; Pérez-Borroto et al., 2021). Resilience to drought conditions in lulo plants after exogenous application of DI-31 may be related to the improvement of the activity of the major ROS-scavenging molecules (enzymatic and non-enzymatic) (Serna et al., 2012b; Ahanger et al., 2018; Pérez-Borroto et al., 2021). Additionally, BRs analogues may induce the expression of genes of proline synthesis that promote a higher proline (Yadav et al., 2012; Fu et al., 2019), and promote the elimination of ROS, the stability of plant cell membrane, and plant water status (Yadav et al., 2012; Vardhini and Anjum, 2015; Pérez-Borroto et al., 2021).
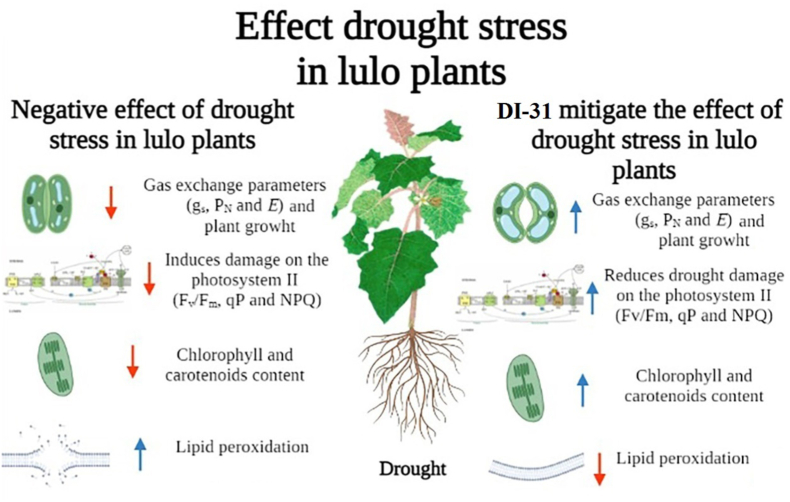
In Colombia, studies on lulo have been recently focused on the evaluation of plant responses to environmental stresses (waterlogging and light) using biochemical, morphoanatomical, or physiological parameters (Medina et al., 2006; Cardona et al., 2016; Sá nchez-Reinoso et al., 2019). However, the use of techniques to manage abiotic stress in lulo has been studied for waterlogging conditions in the country (Fló rez – Velasco et al., 2015). For this reason, foliar BRs analogue (DI-31) sprays constitute a suitable crop management technique since this molecule can stimulate the production of growth-promoting hormones (auxins and gibberellins). The application of this analogue can also induce differentially expressed genes related to drought tolerance, enhancing physiological and biochemical responses under drought conditions (Jiroutova et al., 2018; Huang et al., 2020; Díaz et al., 2021). Foliar DI-31 applications increased the tolerance (low DQE and high RTI) of lulo plants and enhanced the physiological and biochemical responses of plants subjected to drought stress conditions. The principal responses of lulo plants under drought were a decrease in leaf gas exchange parameters (PN, gs, and transpiration), RWC, total chlorophyll, and carotenoid content. Additionally, plants showed increased PSII damage (lower values of chlorophyll fluorescence parameters such as Fv/Fm ratio and qP, and higher NPQ values), and membrane lipid peroxidation. In contrast, these negative effects were ameliorated when lulo plants were foliar treated with BR (Figure 8).
Figure 8.
Concept model of the impact of the drought stress and foliar brassinosteroid analogue (DI-31) sprays in lulo plants. Red arrows and blue arrows indicate the negative or positive effect of interaction between drought stress and foliar DI-31 applications on the physiological and biochemical responses, respectively. gs: stomatal conductance; PN: net photosynthesis rate; E: transpiration; Fv/Fm: maximum quantum efficiency of PSII; qP: photochemical quenching; NPQ: non-photochemical quenching.
5. Conclusion
In summary, this study revealed that this species shows a high susceptibility to drought mainly related to lower values of photosynthetic rate and water status. However, foliar BRs analogue (DI-31) applications, mainly at concentrations of 4 or 8 mL·L−1, helped plants cope with the adverse effects by improving physiological (leaf photosynthesis, photochemical efficiency of PSII, and plant growth) and biochemical [total chlorophyll and carotenoids concentration, and lipid peroxidation (malondialdehyde)] variables. Foliar DI-31 sprays can be a tool for managing water stress conditions because this plant hormone can generate a beneficial effect, helping lulo acclimation under low rainfall periods in producing areas. The results of this research allow us to go deeper into the agronomic management techniques to face water stress conditions generated by climate change and variability scenarios in Andean fruit crops.
Declarations
Author contribution statement
Cristian Camilo Castañeda-Murillo; Javier Gustavo Rojas-Ortiz; Alefsi David SánchezReinoso: Performed the experiments.
Cristhian Camilo Chávez-Arias: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hermann Restrepo-Díaz: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors do not have permission to share data.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
This article is a part of the Irrigation and Fertilizer Management Special issue.
References
- Abid M., Ali S., Qi L.K., Zahoor R., Tian Z., Jiang D., Snider J.L., Dai T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.) Sci. Rep. 2018;8:4615. doi: 10.1038/s41598-018-21441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agronet . 2021. Área, Producción y Rendimiento Nacional por Cultivo.https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1 Uchuva. [Google Scholar]
- Ahanger M.A., Ashraf M., Bajguz A., Ahmad P. Brassinosteroids regulate growth in plants under stressful environments and crosstalk with other potential phytohormones. J. Plant Growth Regul. 2018;37:1007–1024. [Google Scholar]
- Anwar A., Liu Y., Dong R., Bai L., Yu X., Li Y. The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol. Res. 2018;51:46. doi: 10.1186/s40659-018-0195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila A.C., Ochoa J., Proaño K., Martínez M.C. Jasmonic acid and nitric oxide protects naranjilla (Solanum quitoense) against infection by Fusarium oxysporum f. sp. quitoense by eliciting plant defense responses. Physiol. Mol. Plant Pathol. 2019;106:129–136. [Google Scholar]
- Banik P., Zeng W., Tai H., Bizimungu B., Tanino K. Effects of drought acclimation on drought stress resistance in potato (Solanum tuberosum L.) genotypes. Environ. Exp. Bot. 2016;126:76–89. [Google Scholar]
- Barros Junior U.O., Lima M.D., Alsahli A.A., Lobato A.K. Unraveling the roles of brassinosteroids in alleviating drought stress in young Eucalyptus urophylla plants: implications on redox homeostasis and photosynthetic apparatus. Physiol. Plantarum. 2020;2020:1–14. doi: 10.1111/ppl.13291. [DOI] [PubMed] [Google Scholar]
- Batra N.G., Sharma V., Kumari N. Drought–induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J. Plant Interact. 2014;9:712–721. [Google Scholar]
- Cardona W., Bautista-Montealegre L., Flórez-Velasco N., Fischer G. Desarrollo de la biomasa y raíz en plantas de lulo (Solanum quitoense var. septentrionale) en respuesta al sombrío y anegamiento. Rev. Colomb. Ciencias Hortícolas. 2016;10:53–65. [Google Scholar]
- Cháves–Gómez J.L., Becerra–Mutis L.M., Chávez–Arias C.C., Restrepo–Díaz H., Gómez–Caro S. Screening of different Physalis genotypes as potential rootstocks or parents against vascular wilt using physiological markers. Front. Plant Sci. 2020;11:806. doi: 10.3389/fpls.2020.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez–Arias C.C., Gómez–Caro S., Restrepo–Díaz H. Physiological, biochemical and chlorophyll fluorescence parameters of Physalis peruviana L. seedlings exposed to different short–term waterlogging periods and Fusarium wilt infection. Agronomy. 2019;9:213. [Google Scholar]
- Chávez–Arias C.C., Gómez–Caro S., Restrepo–Díaz H. Mitigation of the impact of vascular wilt and soil hypoxia on cape gooseberry plants by foliar application of synthetic elicitors. Hortscience. 2020;55:121–132. doi: 10.3390/plants9020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Wang S., Cao B., Cao D., Leng G., Li H., Yin L.N., Shan L., Deng X.P. Genotypic variation in growth and physiological response to drought stress and Re–Watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016;6:1241. doi: 10.3389/fpls.2015.01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T., Rivard B., Sánchez–Azofeifa A.G., Féret J., Jacquemoud S., Ustin S.L. Deriving leaf mass per area LMA from foliar reflectance across a variety of plant species using continuous wavelet analysis. ISPRS J. Photogrammetry Remote Sens. 2014;87:28–38. [Google Scholar]
- Cheng L., Han M., Yang L., Yang L., Sun Z., Zhang T. Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis georgi under drought stress. Ind. Crop. Prod. 2018;122:473–482. [Google Scholar]
- Dahal K., Li X.–Q., Tai H., Creelman A., Bizimungu B. Improving potato stress tolerance and tuber yield under a climate change scenario – a current overview. Front. Plant Sci. 2019;10:563. doi: 10.3389/fpls.2019.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz K., Espinoza L., Carvajal R., Silva-Moreno E., Olea A.F., Rubio J. Exogenous application of brassinosteroid 24-norcholane 22 (S)-23-dihydroxy type analogs to enhance water deficit stress tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2021;22(3):1158. doi: 10.3390/ijms22031158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M., Wahid A., Basra S.M.A. Improving water relations and gas exchange with brassinosteroids in rice under drought stress. J. Agron. Crop Sci. 2009;195(4):262–269. [Google Scholar]
- Flexas J., Gulías J., Jonasson S., Medrano H., Mus M. Seasonal patterns and control of gas exchange in local populations of the Mediterranean evergreen shrub Pistacia lentiscus L. Acta Oecol. 2001;22:33–43. [Google Scholar]
- Flórez–Velasco N., Balaguera–López H.E., Restrepo–Díaz H. Effects of foliar urea application on lulo (Solanum quitoense cv. septentrionale) plants grown under different waterlogging and nitrogen conditions. Sci. Hortic. 2015;186:154–162. [Google Scholar]
- Forero D.P., Masatani C., Fujimoto Y., Coy–Barrera E., Peterson D.G., Osorio C. Spermidine derivatives in lulo (Solanum quitoense Lam.) fruit: sensory (taste) versus biofunctional (ACE–inhibition) properties. J. Agric. Food Chem. 2016;64:5375–5383. doi: 10.1021/acs.jafc.6b01631. [DOI] [PubMed] [Google Scholar]
- Fu J., Sun P., Luo Y., Zhou H., Gao J., Zhao D., Pubu Z., Liu J., Hu T. Brassinosteroids enhance cold tolerance in Elymus nutans via mediating redox homeostasis and proline biosynthesis. Environ. Exp. Bot. 2019;167:103831. [Google Scholar]
- Gill M.B., Cai K.F., Zhang G.P., Zeng F.R. Brassinolide alleviates the drought–induced adverse effects in barley by modulation of enzymatic antioxidants and ultrastructure. Plant Growth Regul. 2017;82(3):447–455. [Google Scholar]
- Goñi O., Quille P., O’Connell S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018;126:63–73. doi: 10.1016/j.plaphy.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Gou W., Zheng P., Tian L., Gao M., Zhang L., Akram N.A., Ashraf M. Exogenous application of urea and a urease inhibitor improves drought stress tolerance in maize (Zea mays L.) J. Plant Res. 2017;130:599–609. doi: 10.1007/s10265-017-0933-5. [DOI] [PubMed] [Google Scholar]
- Gunn S., Farrar J.F., Collis B.E., Nason M. Specific leaf area in barley: individual leaves versus whole plants. New Phytol. 1999;143:45–51. [Google Scholar]
- Guo Y.Y., Yu H.Y., Kong D.S., Yan F., Zhang Y.J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica. 2016;54:524–531. [Google Scholar]
- Hainaut P., Remacle T., Decamps C., Lambert R., Sadok W. Higher forage yields under temperate drought explained by lower transpiration rates under increasing evaporative demand. Eur. J. Agron. 2016;72:91–98. [Google Scholar]
- Hayat S., Hasan S.A., Hayat Q., Ahmad A. Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma. 2010;239:3–14. doi: 10.1007/s00709-009-0075-2. [DOI] [PubMed] [Google Scholar]
- Hill D., Nelson D., Hammond J., Bell L. Morphophysiology of Potato (Solanum tuberosum) in response to drought stress: paving the way forward. Front. Plant Sci. 2021;11:597554. doi: 10.3389/fpls.2020.597554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges D.M., DeLong J.M., Forney C.F., Prange R.K. Improving the thiobarbituric acid–reactive–substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- Hola D. In: Brassinosteroids: Plant Growth and Development. Hayat S., Yusuf M., Bhardwaj R., Bajguz A., editors. Springer Singapore; Singapore): 2019. Role of brassinosteroids in the plant response to drought: do we know anything for certain; pp. 101–168. [Google Scholar]
- Hu L., Wang Z., Huang B. Effects of cytokinin and potassium on stomatal and photosynthetic recovery of Kentucky bluegrass from drought stress. Crop Sci. 2013;53:221–231. [Google Scholar]
- Hu W.H., Yan X.H., He Y., Xi R. 24–epibrassinolide alleviate drought–induced photoinhibition in Capsicum annuum via up–regulation of AOX pathway. Sci. Hortic. 2019;243:484–489. [Google Scholar]
- Huang L., Zhang L., Zeng R., Wang X., Zhang H., Wang L., Liu S., Wang X., Chen T. Brassinosteroid priming improves peanut drought tolerance via eliminating inhibition on genes in photosynthesis and hormone signaling. Genes. 2020;11(8):919. doi: 10.3390/genes11080919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Farooq S., Hasan W., Ul–Allah S., Tanveer M., Farooq M., Nawaz A. Drought stress in sunflower: physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018;201:152–166. [Google Scholar]
- Hussain M.A., Fahad S., Sharif R., Jan M.F., Mujtaba M., Ali Q., Ahmad H., Amin N., Ajayo B.S., Sun C., Gu L., Ahmad I., Jiang Z., Hou J. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul. 2020:1–16. [Google Scholar]
- Jangid K.K., Dwivedi P. Physiological and biochemical changes by nitric oxide and brassinosteroid in tomato (Lycopersicon esculentum Mill.) under drought stress. Acta Physiol. Plant. 2017;39:73. [Google Scholar]
- Jiroutova P., Oklestkova J., Strnad M. Crosstalk between brassinosteroids and ethylene during plant growth and under abiotic stress conditions. Int. J. Mol. Sci. 2018;19(10):3283. doi: 10.3390/ijms19103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungklang J., Saengnil K., Uthaibutra J. Effects of water–deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017;24:1505–1512. doi: 10.1016/j.sjbs.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal S., Ilyas N., Batool N., Arshad M. Amelioration of drought stress in wheat by combined application of PGPR, compost, and mineral fertilizer. J. Plant Nutr. 2017;40:1250–1260. [Google Scholar]
- Khamsuk O., Sonjaroon W., Suwanwong S., Jutamanee K., Suksamrarn A. Effects of 24–epibrassinolide and the synthetic brassinosteroid mimic on chili pepper under drought. Acta Physiol. Plant. 2018;40:1–12. [Google Scholar]
- Khan I., Awan S.A., Ikram R., Rizwan M., Akhtar N., Yasmin H., et al. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plantarum. 2021;172(2):696–706. doi: 10.1111/ppl.13237. [DOI] [PubMed] [Google Scholar]
- Kumar S., Sachdeva S., Bhat K.V., Vats S. In: Biochemical and Molecular Basis in Biotic and Abiotic Stress Tolerance in Plants. Vats S., editor. Springer Nature Singapore Pte. Ltd.; Singapore: 2018. Plants responses to drought stress: physiological. [Google Scholar]
- Li L., Gu W., Li J., Li C., Xie T., Qu D., Meng Y., Li C., Wei S. Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol. Biochem. 2018;129:35–55. doi: 10.1016/j.plaphy.2018.05.017. [DOI] [PubMed] [Google Scholar]
- Liang G., Bu J., Zhang S., Jing G., Zhang G., Liu X. Effects of drought stress on the photosynthetic physiological parameters of Populus× euramericana “Neva”. J. Res. 2019;30:409–416. [Google Scholar]
- Liang G., Liu J., Zhang J., Guo J. Effects of drought stress on photosynthetic and physiological parameters of tomato. J. Am. Soc. Hortic. Sci. 2020;145:12–17. [Google Scholar]
- Lima J.V., Lobato A.K.S. Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol. Mol. Biol. Plants. 2017;23:59–72. doi: 10.1007/s12298-016-0410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.L., Wang W.X., Wang L.Y., Sun Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 2015;77:317–326. [Google Scholar]
- Liu B., Liang J., Tang G., Wang X., Liu F., Zhao D. Drought stress affects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Sci. Hortic. 2019;250:230–235. [Google Scholar]
- Lombardini L., Restrepo-Diaz H., Volder A. Photosynthetic light response and epidermal characteristics of sun and shade pecan leaves. J. Am. Soc. Hortic. Sci. 2009;134(3):372–378. [Google Scholar]
- Lv J., Zong X.F., Ahmad A.S., Wu X., Wu C., Li Y.P., Wang S.G. Alteration in morpho–physiological attributes of Leymus chinensis (Trin.) Tzvelev by exogenous application of brassinolide under varying levels of drought stress. Chil. J. Agric. Res. 2020;80:61–71. [Google Scholar]
- Medina C.I., Martínez E., Lobo M., López J.C., Riaño N.M. Comportamiento bioquímico y del intercambio gaseoso del Lulo (Solanum quitoense Lam.) a plena exposición solar en el bosque húmedo montano bajo del oriente antiqueño Colombiano. Rev.Fac.Nal.Agr.Medellín. 2006;59(1):3123–3146. [Google Scholar]
- Menezes–Silva P.E., Loram–Lourenco L., Alves R.D.F.B., Sousa L.F., Almeida S.E.D., Farnese F.S. Different ways to die in a changing world: consequences of climate change for tree species performance and survival through an ecophysiological perspective. Ecol. Evol. 2019;9:11979–11999. doi: 10.1002/ece3.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz F., Ahmad R., Ashraf M.Y., Waraich E.A., Khan S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015;113:191–200. doi: 10.1016/j.ecoenv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- OECD (Organization for Economic Cooperation and Development) 2014. Survey of Policies – Water and Climate Change Adaptation Colombia.https://www.oecd.org/env/resources/OECD%20(2014)%20Survey%20of%20Water%20and%20Climate%20Change%20Adaptation%20––%20Colombia.pdf [Google Scholar]
- Oñate–Valdivieso F., Uchuari V., Oñate–Paladines A. Large–scale climate variability patterns and drought: a case of study in south–America. Water Resour. Manag. 2020;34:2061–2079. [Google Scholar]
- Pereira Y.C., Rodrigues W.S., Lima E.J.A., Santos L.R., Silva M.H.L., Lobato A.K.S. Brassinosteroids increase electron transport and photosynthesis in soybean plants under water deficit. Photosynthetica. 2019;57:181–191. [Google Scholar]
- Pérez-Borroto L.S., Toum L., Castagnaro A.P., González-Olmedo J.L., Coll-Manchado F., Pardo E.M., Coll-García Y. Brassinosteroid and brassinosteroid-mimic differentially modulate Arabidopsis thaliana fitness under drought. Plant Growth Regul. 2021;95:33–47. [Google Scholar]
- Plazas M., Nguyen H.T., González–Orenga S., Fita A., Vicente O., Prohens J., Boscaiu M. Comparative analysis of the responses to water stress in eggplant (Solanum melongena) cultivars. Plant Physiol. Biochem. 2019;143:72–82. doi: 10.1016/j.plaphy.2019.08.031. [DOI] [PubMed] [Google Scholar]
- Pourghasemian N., Moradi R., Naghizadeh M., Landberg T. Mitigating drought stress in sesame by foliar application of salicylic acid, beeswax waste and licorice extract. Agric. Water Manag. 2020;231:105997. [Google Scholar]
- Raja V., Qadir S.U., Alyemeni M.N., Ahmad P. Impact of drought and heat stress individually and in combination on physio–biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech. 2020;10:1–18. doi: 10.1007/s13205-020-02206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna B., Rao S.S. Foliar application of brassinosteroids alleviates adverse effects of zinc toxicity in radish (Raphanus sativus L.) plants. Protoplasma. 2015;252:665–677. doi: 10.1007/s00709-014-0714-0. [DOI] [PubMed] [Google Scholar]
- Ramírez F., Kallarackal J., Davenport T.L. Lulo (Solanum quitoense Lam.) reproductive physiology: a review. Sci. Hortic. 2018;238:163–176. [Google Scholar]
- Sadura I., Janeczko A. Physiological and molecular mechanisms of brassinosteroid–induced tolerance to high and low temperature in plants. Biol. Plant. (Prague) 2018;62:601–616. [Google Scholar]
- Sánchez–Reinoso A.D., Garces–Varon G., Restrepo–Diaz H. Biochemical and physiological characterization of three rice cultivars under different daytime temperature conditions. Chil. J. Agric. Res. 2014;74:373–379. [Google Scholar]
- Sánchez–Reinoso A.D., Ligarreto–Moreno G.A., Restrepo–Díaz H. Physiological and biochemical expressions of a determinated growth common bean genotype (Phaseolus vulgaris L.) to water deficit stress periods. J. Anim. Plant Sci. 2018;28:119–127. [Google Scholar]
- Sánchez-Reinoso A.D., Jiménez-Pulido Y., Martínez-Pérez J.P., Pinilla C.S., Fischer G. Chlorophyll fluorescence and other physiological parameters as indicators of waterlogging and shadow stress in lulo (Solanum quitoense var. septentrionale) seedlings. Rev. Colomb. Ciencias Hortícolas. 2019;13(3):325–335. [Google Scholar]
- Sasse J.M. Physiological actions of brassinosteroids: an update. J. Plant Growth Regul. 2003;22(4):276–288. doi: 10.1007/s00344-003-0062-3. [DOI] [PubMed] [Google Scholar]
- Serna M., Hernández F., Coll F., Coll Y., Amorós A. Effects of brassinosteroid analogues on total phenols, antioxidant activity, sugars, organic acids and yield of field grown endive (Cichorium endivia L.) J. Sci. Food Agric. 2012;93(7):1765–1771. doi: 10.1002/jsfa.5968. [DOI] [PubMed] [Google Scholar]
- Serna M., Hernández F., Coll F., Amorós A. Brassinosteroid analogues effect on yield and quality parameters of field-grown lettuce (Lactuca sativa L.) Sci. Hortic. 2012;143:29–37. [Google Scholar]
- Serna M., Hernández F., Coll F., Coll Y., Amorós A. Brassinosteroid analogues effects on the yield and quality parameters of greenhouse-grown pepper (Capsicum annuum L.) Plant Growth Regul. 2012;68(3):333–342. [Google Scholar]
- Serna M., Coll Y., Zapata P.J., Botella M.Á., Pretel M.T., Amorós A. A brassinosteroid analogue prevented the effect of salt stress on ethylene synthesis and polyamines in lettuce plants. Sci. Hortic. 2015;185:105–112. [Google Scholar]
- Shah S.H., Houborg R., McCabe M.F. Response of chlorophyll, carotenoid and SPAD–502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.) Agronomy. 2017;7:61. [Google Scholar]
- Shi Y., Zhang Y., Han W., Feng R., Hu Y., Guo J., Gong H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016;7:196. doi: 10.3389/fpls.2016.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims D.A., Gamon J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002;81:337–354. [Google Scholar]
- Singh R., Naskar J., Pathre U.V., Shirke P.A. Reflectance and cyclic electron flow as an indicator of drought stress in cotton (Gossypium hirsutum) Photochem. Photobiol. 2014;90(3):544–551. doi: 10.1111/php.12213. [DOI] [PubMed] [Google Scholar]
- Smart R.E., Bingham G.E. Rapid estimates of relative water content. Plant Physiol. 1974;53:258–260. doi: 10.1104/pp.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat N.B. 24–Epibrassinolide and Spermine combined treatment sustains maize (Zea mays L.) drought tolerance by improving photosynthetic efficiency and altering phytohormones profile. J. Soil Sci. Plant Nutr. 2020;20:516–529. [Google Scholar]
- Tani E., Kizis D., Markellou E., Papadakis I., Tsamadia D., Leventis G., Makrogianni D., Karapanos I. Cultivar-dependent responses of eggplant (Solanum melongena L.) to simultaneous Verticillium dahliae infection and drought. Front. Plant Sci. 2018;9:1181. doi: 10.3389/fpls.2018.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanveer M., Shahzad B., Sharma A., Khan E.A. 24–Epibrassinolide application in plants: an implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019;135:295–303. doi: 10.1016/j.plaphy.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Thussagunpanit J., Jutamanee K., Sonjaroon W., Kaveeta L., Chai–Arree W., Pankean P., Suksamrarn A. Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica. 2015;53:312–320. [Google Scholar]
- Vardhini B.V., Anjum N.A. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015;2:67. [Google Scholar]
- Wang Z., Zheng P., Meng J., Xi Z. Effect of exogenous 24-epibrassinolide on chlorophyll fluorescence, leaf surface morphology and cellular ultrastructure of grape seedlings (Vitis vinifera L.) under water stress. Acta Physiol. Plant. 2015;37(1):1729. [Google Scholar]
- Wellburn A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994;144:307–313. [Google Scholar]
- Wu Z.Z., Ying Y.Q., Zhang Y.B., Bi Y.F., Wang A.K., Du X.H. Alleviation of drought stress in Phyllostachys edulis by N and P application. Sci. Rep. 2018;8:228. doi: 10.1038/s41598-017-18609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Hayat S., Wani A.S., Irfan M., Ahmad A. Comparison of the influence of 28–homobrassinolide and 24–epibrassinolide on nitrate reductase activity, proline content, and antioxidative enzymes of tomato. Int. J. Veg. Sci. 2012;18:161–170. [Google Scholar]
- Yilmaz M.T., Hunt E.R., Jr., Jackson T.J. Remote sensing of vegetation water content from equivalent water thickness using satellite imagery. Remote Sens. Environ. 2008;112:2514–2522. [Google Scholar]
- Yuan G.F., Jia C.G., Li Z., Sun B., Zhang L.P., Liu N., Wang Q.M. Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci. Hortic. 2010;126:103–108. [Google Scholar]
- Yuan X., Yang Z., Li Y., Liu Q., Han W. Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica. 2015;54:28–39. [Google Scholar]
- Zahoor R., Dong H., Abid M., Zhao W., Wang Y., Zhou Z. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017;137:73–83. [Google Scholar]
- Zambrano Mera Y.E., Rivadeneira Vera J.F., Pérez–Martín M.Á. Linking El Niño Southern Oscillation for early drought detection in tropical climates: the Ecuadorian coast. Sci. Total Environ. 2018;643:193–207. doi: 10.1016/j.scitotenv.2018.06.160. [DOI] [PubMed] [Google Scholar]
- Zhao W., Liu L., Shen Q., Yang J., Han X., Tian F., Wu J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water. 2020;12:2127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.