Abstract
Common bean (Phaseolus vulgaris L.), soybean (Glycine max L.) and mung bean (Vigna radiata L. Wilczek) seedlings were treated with methyl jasmonate (MeJA); then, dose-response and time-course experiments were carried out. Isoflavonoid composition was evaluated by high performance liquid chromatography. As a result of MeJA induction, all leguminous plants increase the amount of isoflavonoids, at levels that depend on the concentration of the elicitor and the time after induction. However, the application of MeJA in concentrations higher than 2.22 mM showed deleterious effects on seedlings and strong decreases in the concentration of isoflavonoids. In addition, a series of compounds structurally related to MeJA, such as jasmonic acid, cis-jasmone, coronatine, and indanoyl derivatives, were evaluated as elicitors. The results show that coronatine and the indanoyl-amino acids conjugates displayed a significant elicitor effect of isoflavonoids in common bean (cvs. Cargamanto Mocho and Corpoica LAS 106) and soybean (cv. Soyica P-34) seedlings, even higher than that found with the recognized elicitors, benzo (1,2,3) thiadiazole-7-carbothioic acid S-methyl ester (acibenzolar S-methyl) and benzo-(1,2,3) thiadiazole-7-carbothioic acid (acibenzolar acid). Leguminous plants can be treated with jasmonates and indanoyl derivatives to increase levels of bioactive isoflavonoids and consequently improve biological and functional properties and resistance against pests.
Keywords: Phytoalexins, Elicitors, Phaseollin, Coronatine, Methyl jasmonate
Highlights
-
•
The accumulation of isoflavonoids in edible legume seedlings treated with jasmonates and structurally related compounds was analyzed.
-
•
Time-course and dose-response experiments were performed using methyl jasmonate as elicitor.
-
•
The application of jasmonates and structurally related compounds increased the concentration of bioactive isoflavonoids.
-
•
The amount of isoflavonoids depended on the cultivar, the concentration and structure of the elicitor, and the post-induction time.
Phytoalexins, Elicitors, Phaseollin, Coronatine, Methyl jasmonate.
1. Introduction
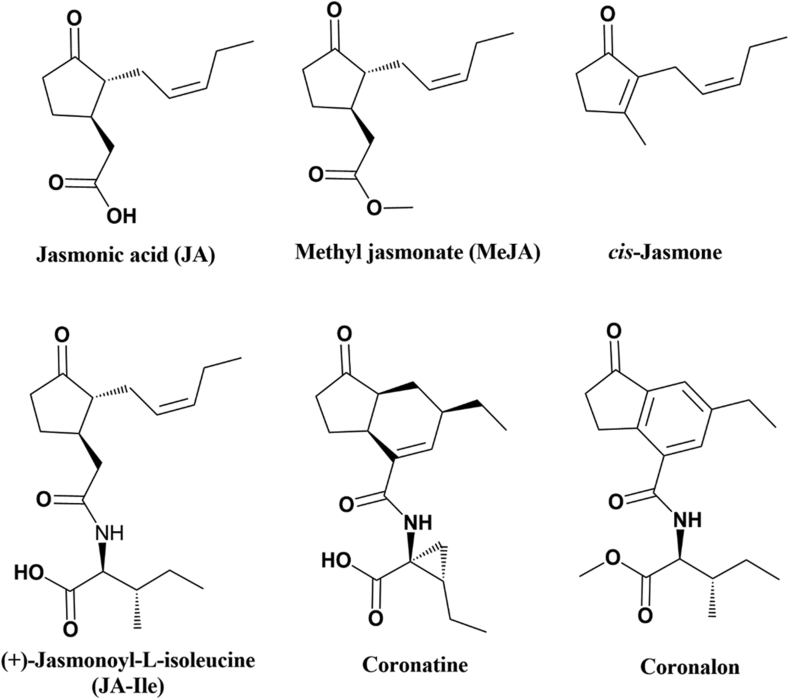
Jasmonates, like jasmonic acid (JA, Figure 1) and methyl jasmonate (MeJA), trigger secondary metabolic pathways in plants, leading to an arsenal of antimicrobial bioactive metabolites (such as isoflavonoid and volatile compounds) of a wide variety of structures and with different precursor biochemical pathways (Henkes et al., 2008). In recent decades, compounds that mimic jasmonates and activate JA-specific intracellular signaling pathways have been studied. For example, Coronatine, a non-host substance that mimics the plant hormone (+)-7-iso-jasmonoyl-L-isoleucine (an active form of JA), induces a wide range of biological activities (Geng et al., 2014). Since coronatine is able to mimic (+)-7-iso-jasmonoyl-L-isoleucine, it has been used as a tool to design structural analogs at the synthetic level, which could be applied in agriculture as stimulators of defense mechanisms in plants (Xie et al., 2008). However, access to useful amounts of coronatine, either by bacterial fermentation or chemical synthesis, has been a challenge, and structure-activity studies have been limited (Littleson et al., 2016; Mithöfer et al., 2004).
Figure 1.
Structure of some jasmonate compounds and structurally related compounds.
To overcome these difficulties, Krumm et al. (1995) designed and developed structurally simpler and more readily available indanoyl-amino acid conjugates, as functional analogs of coronatine. These compounds would have greater facilities for the preparation by chemical synthesis and, consequently, better prospects for large-scale application in the field, as protective agents for agricultural crops (Mithöfer et al., 2004; Schüler et al., 2001, 2004; Botero et al., 2021; Lauchli et al., 2002).
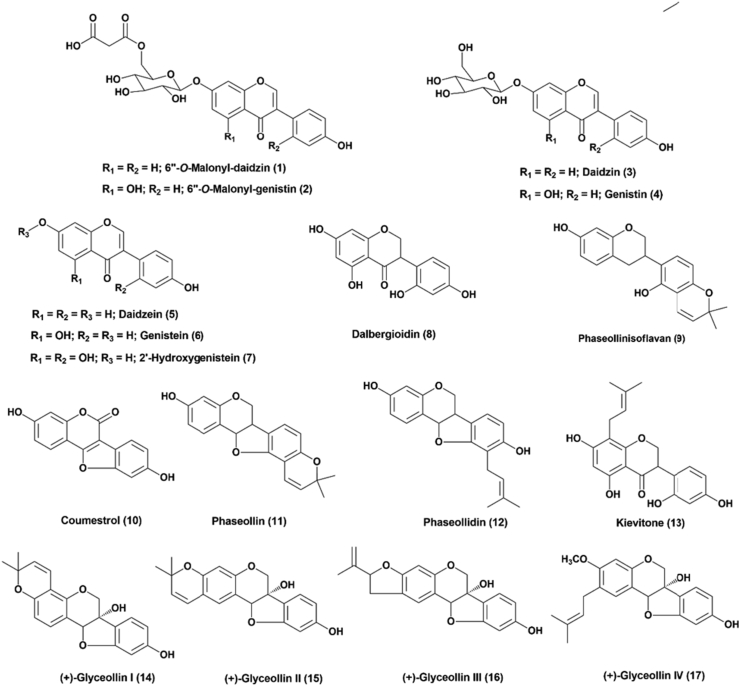
On the other hand, as a result of microbial infections and elicitations, some leguminous plants produce a series of preformed and induced antimicrobial isoflavonoids called phytoanticipins and phytoalexins, respectively (Pedras and Yaya, 2015). The production of isoflavones (genistein, 2′-hydroxygenistein, daidzein, kievitone and dalbergioidin, Figure 2), coumestans (coumestrol), isoflavans (phaseollinisoflavan) and pterocarpans (phaseollin and phaseollidin) in common bean (Phaseolus vulgaris L.) and mung bean (Vigna radiata L. Wilczek), has been associated with chemical defense mechanisms against infections and responses to stress (Durango et al., 2013). The speed and level at which phaseollin is produced have been linked to common bean resistance to anthracnose, a fungal disease (Durango et al., 2002). Similarly, soybean (Glycine max L.) produces a variety of isoflavones such as aglycones (daidzein and genistein), 7-O-glucosyl-conjugates (daidzin and genistin) and 6″-O-malonyl conjugates (6″-O-malonyldaidzin and 6″-O-malonylgenistin), coumestrol and pterocarpans (Glyceollin I, II, III and IV) in response to pathogen attack (Lygin et al., 2013; Nwachukwu et al., 2013). Furthermore, isoflavonoids also have beneficial effects on human health and are used as raw materials in the chemical and pharmaceutical industries (Vitale et al., 2013; Yu et al., 2016). Unfortunately, there are few studies of the effect of methyl jasmonate and structurally related compounds on the accumulation of isoflavonoids in leguminous plants.
Figure 2.
Structure of defense-related isoflavonoids in soybean, common bean, and mung bean.
In the present work, common bean (P. vulgaris L.), soybean (G. max L.), and mung bean (V. radiata L. Wilczek) seedlings were treated with MeJA, and the concentration of defense-related isoflavonoids was analyzed. Dose-effect and time-course experiments were carried out. In addition, a series of jasmonate compounds (MeJA, JA, cis-jasmone, and coronatine) and structurally related indanoyl-amino acids conjugates were evaluated as elicitors in common bean and soybean.
2. Materials and methods
2.1. Chemicals
Genistein, genistin, daidzin, daidzein, jasmonic acid (JA), coronatine, benzo(1,2,3) thiadiazole-7-carbothioic acid S-methyl ester (acibenzolar S-methyl), and benzo(1,2,3) thiadiazole-7-carbothioic acid (acibenzolar acid) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Coumestrol, 2′-hydroxygenistein, dalbergioidin, phaseollin, phaseollidin, kievitone, and phaseollinisoflavone were obtained and identified according to Durango et al. (2002). Methyl jasmonate (MeJA), cis-jasmone and 2-carboxy cinnamic acid were from Alfa-Aesar Co. (Ward Hill, MA, USA). Methanol, ethyl acetate, NaOH, silica gel and all other chemicals were purchased from Merck KGaA (Darmstadt, Germany). 1-oxoindanoyl-amino acid conjugates were prepared from 2-carboxy cinnamic acid according to Krumm et al. (1995) and Botero et al. (2021).
2.2. Plant material
Soybean (G. max L.) seeds, cultivars Soyica P34 and SK-7 were obtained from Monsanto Colombia and Semillas Kamerún S.A.S. (Cartago, Valle del Cauca, Colombia), respectively. Seeds of common bean (P. vulgaris L.), cultivars ICA (Instituto Colombiano Agropecuario)-Cerinza, ICA-Quimbaya, Uribe Rosado, Calima, ICA-Bachué, and Andino were purchased from Semicol S.A.S. (Bogotá D.C., Colombia). Cultivars Cargamanto Mocho and CORPOICA LAS 106 were from Semillas & Semillas (Medellín, Antioquia, Colombia) and Corpoica (La Selva, Rionegro, Antioquia, Colombia), respectively. Seeds of mung bean (V. radiata L. Wilczek) were obtained from Semicol S.A.S (Santa Fe de Bogotá, Bogotá D.C., Colombia). ICA-Quimbaya is resistant to anthracnose (Colletotrichum lindemuthianum Sacc. & Magn.) and tolerant to rust (Uromyces appendiculatus Pers., Unger) (Rios-B. et al., 1989). ICA-Cerinza is tolerant to the most common diseases in Colombia (rust, anthracnose, leaf spot, and root rot) (Diaz and Santana, 2004). ICA-Bachué is resistant to anthracnose and rust (Ligarreto et al., 1994). CORPOICA LAS 106 is resistant to anthracnose (Peláez and Rios, 2001). All seeds were subjected to surface sterilization with NaClO (2.0%), and then, washed with tap water. Seeds were sown in pre-sterilized moist vermiculite. Germination was conducted under dark conditions and 22 ± 3 °C. Six- and five-day-old seedlings of common bean and soybean, respectively, were harvested and washed with tap water. Mung seedlings were four days old. Then, teguments present on the seedlings were carefully removed.
2.3. Treatments
2.3.1. Dose-response experiments
In sterile plastic trays, the uprooted seedlings (7 g for soybean and 10 g for common bean cv. ICA-Cerinza and mung beans) were placed vertically and the roots were submerged in MeJA solutions at various concentrations (0.04, 0.16, 0.44, 2.22, and 4.40 mM). MeJA was initially dissolved in ethanol (0.2%), in order to increase its aqueous solubility. Solutions were then prepared by adding tap water to achieve the specific concentration. After 4 h of induction, the MeJA solutions were discarded. Cellucotton soaked with distilled water was used to carefully cover the roots. Then, the seedlings were incubated for 96 h at 22 ± 3 °C in the dark, in trays sealed with plastic film. Seedlings treated with ethanol (0.2%) instead of the elicitor and stored under the same conditions were used as a control experiment. The experiments were carried out at least twice.
2.3.2. Time-course experiments
In sterile plastic trays under aseptic conditions, the uprooted seedlings (7 g for soybean and 10 g for common and mung beans) were placed vertically; the roots were immersed for 4 h in MeJA solutions (0.16 mM for soybean, 0.66 mM for mung bean and for cultivars ICA-Bachué, Andino, Cargamanto Mocho and ICA-Quimbaya of common bean, and 0.44 mM for the cultivars Uribe Rosado and ICA-Cerinza). The elicitor solutions were then discarded and cellucotton soaked with distilled water was used to cover the roots. The plant material inside the trays covered with plastic film was stored in the dark at 22 ± 3 °C for different times (24, 48, 72, 96 and 120 h). Every 48 h, the seedlings were sprayed with water and the trays were covered again. Similarly, seedlings treated with ethanol (0.2%) instead of MeJA solution and stored for 120 h were used as control experiments. Assays were performed at least twice.
2.3.3. Elicitor effect of structurally related compounds to MeJA
In sterile plastic trays, soybean (G. max L.) (7 g) and common bean (P. vulgaris L. cvs. Cargamanto Mocho and CORPOICA LAS 106) (10 g) seedlings were placed vertically; the roots were immersed for 4 h in solutions (0.16 mM for soybean and 0.44 mM for common bean) of MeJA, JA, cis-jasmone, coronatine, and 1-oxoindanoyl 4-carboxylic acid-amino acid conjugates (L-leucine methyl ester, D-leucine methyl ester, and glycine methyl ester). Treatments with acibenzolar-S-methyl and acibenzolar acid were used as positive controls. All solutions were obtained dissolving the potential elicitor compounds in 0.2% ethanol. Then, solutions containing the elicitor were discarded, and the roots were carefully covered with cellucotton moistened with distilled water. The trays with the plant material were closed using stretch film and the seedlings were incubated for 96 h at 22 ± 3 °C in the dark. Experiments were performed at least twice.
2.4. Sample preparation
In a mortar, plant material was ground with 20 mL ethanol (95%). The resulting solution was centrifuged (3400 rpm, 6 min) and subsequently filtered through filter paper (Whatman No.1). Ethanol was eliminated from the filtrate under vacuum at 40 °C (Rotavapor Buchi R-210). The remaining aqueous phase was submitted to liquid-liquid extraction with ethyl acetate (EtOAc, 3 × 20 mL). After the combination of the organic phases, the solvent was removed (to dryness) by evaporation in vacuo. Methanol was added to the extracts (HPLC-grade MeOH, 5.0 mL), and the solutions were filtered using a syringe sterile filter (0.45-mm pore size). A 0.5 mL aliquot of the resulting solution was stored in an amber glass vial at -20 °C until HPLC analysis.
2.5. Detection and quantification of isoflavonoids
The analysis of the defense-related isoflavonoids was performed on a Shimadzu chromatograph (LC-20AT) with a diode array detector (SPD-20AT, Prominence), using a Phenomenex Security Guard cartridge C18 (4.0 × 3.0 mm) followed by a Phenomenex Luna 5μ C18 (2) reverse-phase column (150 mm × 4.6 mm i.d., 5 μm) (Torrance, USA). The isoflavonoids were eluted at a flow rate of 0.7 mL/min using as mobile phase methanol and 0.05% acetic acid in water, as follows: from 10 to 70 % methanol in 40 min, then from 70 to 90% methanol in 20 min. 20 μL of the sample was injected. Detection was at the wavelengths of maximum absorption of each isoflavonoid (248, 254, 270, 286, and 310 nm) (Durango et al., 2013).
For the quantification of defense-related isoflavonoids, standard calibration curves were constructed by plotting peak areas versus compound concentration. Five working solutions were prepared for genistein, genistin, daidzin, daidzein, dalbergioidin, 2′-hydroxygenistein, coumestrol, and phaseollin in ethanol at 1, 10, 25, 50, and 100 mg/L. Due to genistin and daidzin show molar extinction coefficients like those of the malonyl-conjugates (Durango et al., 2018), the standard curves of genistin and daidzin were used for quantifying the malonyl conjugates and adjusted for molecular weight differences. The concentration of glyceollins was calculated from the total area of peaks for the four isomers, by interpolation in the calibration curve for phaseollin (a structurally related pterocarpan). Relatively high linearity was presented for all calibration curves (correlation coefficient R2 > 0.96). The regression equations were: genistein, Y =16164X – 46085 (R2 of 0.9703); dalbergioidin, Y = 5.0x107X – 39883 (R2 of 0.9970); daidzein, Y = 159171X + 146477 (R2 of 0.9881); genistin, 16164X – 46085 (R2 of 0.9703); daidzin, Y = 159171X + 146477 (R2 of 0.9881); coumestrol, Y = 5.0x106X + 76532 (R2 of 0.9651); 2′-hydroxygenistein, Y = 8.0x107X – 17193 (R2 = 0.9985), and phaseollin, Y = 106380X + 414987 (R2 of 0.9684). Data for each peak were recorded using the wavelength that provides a maximum response (Durango et al. 2013, 2018). The results were expressed as μg isoflavonoid/g fresh material and presented as mean values ±standard deviation.
2.6. Confirmation of identity of isoflavonoids
Defense-related isoflavonoids were previously identified by different methods: genistein, 2′-hydroxygenistein, dalbergioidin, coumestrol, phaseollin, phaseollidin, and phaseollinisoflavan were purified and confirmed by mass and NMR spectra (Durango et al., 2002, 2013). In addition, the identification of daidzein, genistein, genistin, and daidzin was verified by co-elution of HPLC-DAD peaks with authentic samples (Durango et al., 2018) on a Shimadzu chromatograph (LC-20AT) equipped with a diode array detector (SPD-20AT, Prominence), and the same chromatographic conditions as described above (Section 2.5). Compounds 6″-O-malonyl-genistin, 6″-O-malonyl-daidzin, and glyceollins were also previously confirmed by liquid chromatography with mass spectrometry (LC-MS) according to Durango et al. (2018). The reverse-phase column, mobile phase, and flow rate were the same as described above (Section 2.5).
2.7. Statistical analysis
A one-way ANOVA was used to analyze the results and the mean values were compared using the Fisher's least significant differences (LSD) at the 0.05 probability level.
3. Results and discussion
3.1. Chromatographic profiles
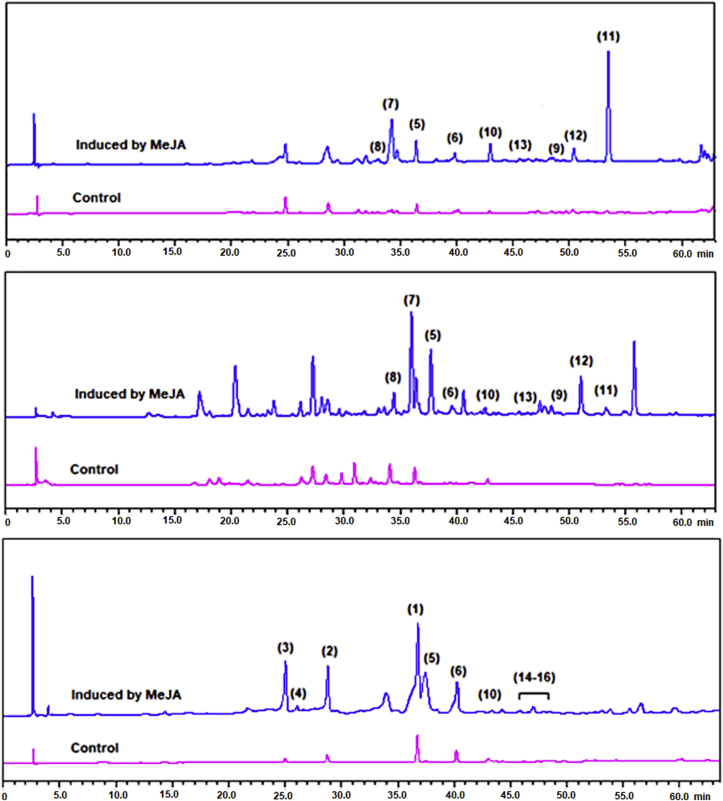
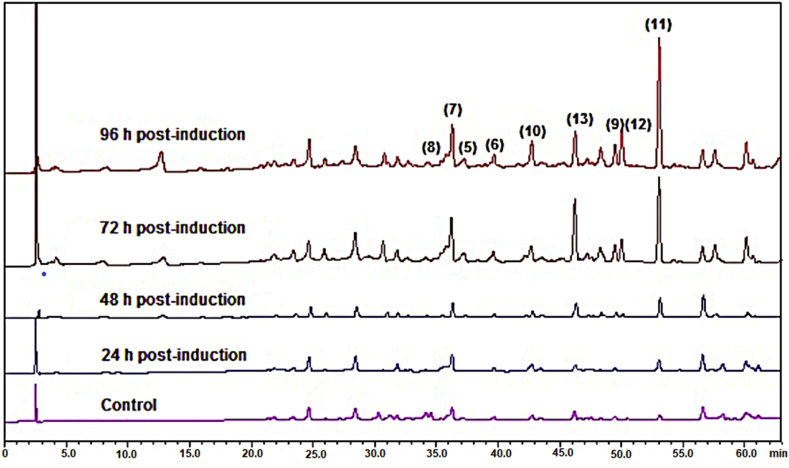
Characteristic chromatographic profiles of soybean, common bean, and mung bean seedlings treated with water (control) and MeJA are shown in Figure 3. All components of the samples were clearly separated. For soybean seedlings, the peaks at retention times (Rt) 23.5–24.5 min and 28.5–36.0 min correspond to the glucosyl- (daidzin and genistin) and the malonyl-glycosyl (6-O″-malonyl-daidzin and 6-O″-malonyl-genistin) derivatives, respectively. The aglycones daidzein and genistein were found in that order at Rt 36.5 and 40.0 min. The lower retention times for the glucosyl- and malonyl-glucosyl conjugated isoflavonoids are consistent with their higher polarities compared to the aglycones. The coumestrol peak appeared at Rt 43.0 min. Glyceollins were detected at 45.2, 45.9, and 46.3–46.6 min, indicating the lowest polarity among soy defense-related isoflavonoids. Daidzein derivatives were found at significantly higher levels than genistein derivatives. The major metabolite detected in soybean seedlings was daidzin. Coumestrol and glyceollins were absent in water-treated seedlings.
Figure 3.
Characteristic chromatographic profiles of seedlings of common bean (P. vulgaris L. cv. ICA-Cerinza) (a), mung bean (V. radiata) (b), and soybean (G. max L. cv. SK-7) (c) elicited by methyl jasmonate (MeJA). 1, 6″-O-malonyl-daidzin; 2, 6″-O-malonyl-genistin; 3, daidzin; 4, genistin; 5, daidzein; 6, genistein; 7, 2′-hydroxygenistein; 8, dalbergioidin; 9, phaseollinisoflavan; 10, coumestrol; 11, phaseollin; 12, phaseollidin; 13, kievitone; 14–16, glyceollins. λ analysis: 248 (for soybean) and 278 (for common and mung beans) nm.
Daidzein, genistein, and coumestrol were also found in common bean and mung bean seedlings. Furthermore, the 2′-hydroxygenistein and dalbergioidin compounds were detected at 35.6 and 34.2 min, respectively. The peaks of the prenylated compounds (kievitone, phaseollin, phaseollidin, and phaseollinisoflavan) appear between 49.0 and 53.0 min. The main compound found in common and mung beans was genistein. The peaks for kievitone, phaseollin, phaseollidin, and phaseollinisoflavan showed a low intensity in seedlings treated with water compared to those treated with MeJA.
3.2. Elicitation with MeJA
3.2.1. Dose-response experiments
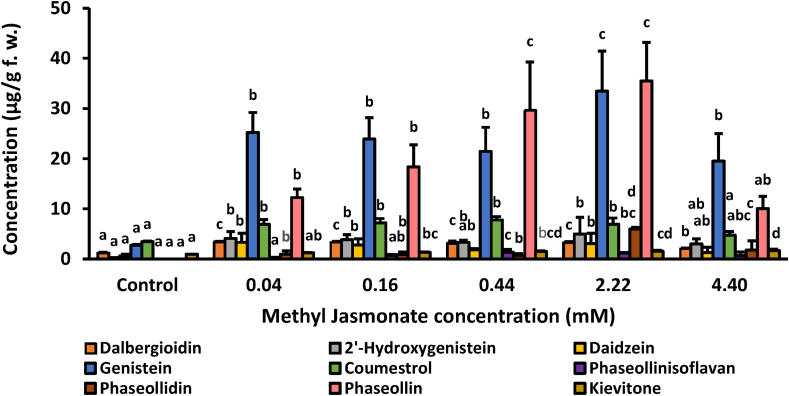
The exogenous application of MeJA and its derivatives has been shown to be effective in inducing the biosynthesis of secondary metabolites and activating the induced defense system in plants (Singh and Bwivedi, 2018; Wasternack, 2007; Wasternack and Feussner, 2018; Farhangi-Abriz and Ghassemi-Golezani, 2019). However, treatments with high concentrations of MeJA can generate signs of phytotoxicity (Jiang et al., 2017). Therefore, a dose-response experiment was initially carried out to choose an optimal concentration. For the dose-response experiments, the hypocotyls-roots of common bean cv. Ica Cerinza were treated with MeJA solutions at different concentrations (0.04, 0.16, 0.44, 2.22, and 4.40 mM), and the content of isoflavonoid was evaluated at 96 h. The results of dose-response experiments in common bean hypocotyls-roots are summarized in Figure 4. After MeJA treatment, the concentration of defense-related isoflavonoids increased significantly relative to control. Interestingly, at the lowest concentrations of the elicitor, 0.04 and 0.16 mM, the major phytoalexin was genistein followed by phaseollin. However, MeJA at 0.44 and 2.22 mM, elicited the highest amounts of phaseollin. In general, the concentration of isoflavonoids in common bean increased progressively in a dose-dependent manner until reaching its maximum levels at 2.22 mM. When 4.40 mM MeJA was used, isoflavonoid concentrations declined rapidly. Under these conditions (4.40 mM MeJA and 96 h post-induction), common bean hypocotyls-roots began to show necrosis symptoms (they turned brownish). Therefore, MeJA at 2.22 mM and below, is safe and can be used as an elicitor in common bean. An earlier report of common bean tissue elicitation showed similar symptoms of necrosis following treatment with salicylic acid at 3.62 mM and above (Durango et al., 2014). Saini et al. (2013) reported significant increases in the concentration of isoflavones in soybeans by treatment with MeJA between 0.1 and 0.5 mM. Similarly, Li et al. (2017) reported that treatment of mung bean sprouts with MeJA at different levels (1.0, 10.0, 100.0 μmol/L, 1.0 and 10.0 mmol/L) significantly increased total phenolic amounts, and antioxidant activity.
Figure 4.
Accumulation of defense-related isoflavonoids on hypocotyls-roots of common bean (P. vulgaris L. cv. ICA-Cerinza) treated with methyl jasmonate (MeJA) at different concentrations and after 96 h post-induction. For each compound, bars with different letters (a, b, c, d, and e) indicate statistically significant differences (p = 0.05; Fisher's LSD test).
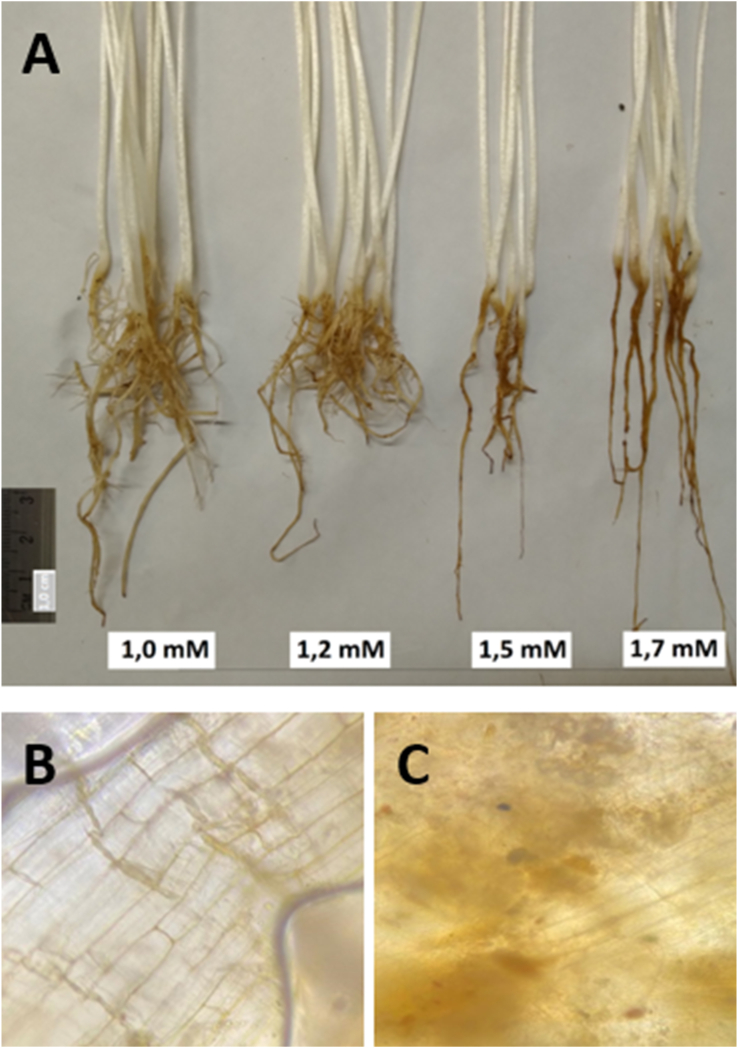
On the other hand, mung bean and soybean seedlings exhibited higher sensitivity to MeJA treatment, showing wilting symptoms at concentrations as low as 2.22 and 0.44 mM, respectively (Data not shown). For mung bean, elicitation with 1.0 mM MeJA and higher exhibited changes in root coloration (turned brown) and a reduction in the number of lateral roots and root hairs (Figure 5). Exogenous application of MeJA or JA in high concentrations is known to inhibit root growth in some plant species (Henkes et al., 2008; Wasternack, 2007). Consequently, for the subsequent assays, MeJA was used at 0.44, 0.66, and 0.16 mM for common bean, mung bean, and soybean, respectively.
Figure 5.
Effect of the application of methyl jasmonate (MeJA) at different concentrations on root elongation and the number of secondary roots in mung beans (A). Deteriorative effect on the integrity of the epidermal tissues in common bean (P. vulgaris L.) roots untreated (B) and treated with 4.44 mM of MeJA (C).
3.2.2. Time-course experiments
In order to investigate the effect of MeJA application in the course of time, hypocotyls-roots of common bean (P. vulgaris L. cvs. ICA-Cerinza, ICA-Quimbaya, Cargamanto Mocho, Uribe Rosado, ICA-Bachué, Andino, Calima, and CORPOICA LAS 106), mung bean (V. radiata L. Wilczek) and soybean (G. max L. cv. SK-7) were immersed for 4 h in MeJA solutions at 0.16 mM for soybean, 0.66 mM for mung bean and the common bean cultivars ICA-Bachué, Andino, Cargamanto Mocho and ICA-Quimbaya, and 0.44 mM for the cultivars Uribe Rosado and ICA-Cerinza. Plant materials were then stored in the dark at different times (24, 48, 72, 96, and 120 h). Defense-related isoflavonoid concentrations over time for hypocotyls-roots of common bean cvs. ICA-Cerinza, Uribe Rosado, and Andino are summarized in Figure 6. For all cultivars, isoflavonoid content increased significantly after MeJA application. In general, the accumulation of isoflavonoid compounds increased progressively with time after induction. In Uribe Rosado and Andino, two cultivars susceptible to anthracnose, the major compound found was genistein. The highest level of genistein was detected during the first 72 h after induction; then, the amount of genistein decreased rapidly. The other compounds presented levels lower than 22 μg/g during the analysis time, for the Uribe Rosado and Andino cultivars. After 96 h post-induction, the main isoflavonoid compounds for Uribe Rosado, Andino, and Cargamanto Mocho were genistein (36.5 μg/g), coumestrol (21.1 μg/g), and kievitone (44.4 μg/g), respectively. For the ICA-Cerinza, ICA-Bachué, and ICA-Quimbaya cultivars, resistant and tolerant to anthracnose, the main defense-related isoflavonoids after 96 h were phaseollin, phaseollidin, and kievitone. In particular, after 96 h post-induction, phaseollin reached levels of 102.0, 20.1, 29.4 μg/g for ICA-Quimbaya, ICA- Bachué, and ICA-Cerinza, respectively. In general, the highest amounts of genistein, daidzein, 2′-hydroxygenistein, and dalbergioidin were detected during the first 72 h after induction. Then, the concentration of these compounds decreased, coinciding with an increase in the concentration of phaseollin, phaseollidin, phaseollinisoflavan, kievitone, and coumestrol (Figure 7). These results are consistent with previous reports about elicitation in common bean tissues with other elicitors (Durango et al., 2013; Botero et al., 2021). As can be seen for Uribe Rosado and ICA-Cerinza, after 120 h post-induction, the amount of isoflavonoid compounds is similar to that found in the controls (water-treated hypocotyls-roots). Reductions in isoflavonoid levels after post-induction times greater than 120 h have been reported for other common bean cultivars treated with salicylic acid in cotyledons and hypocotyls-root (Durango et al., 2013, 2014).
Figure 6.
Accumulation of defense-related isoflavonoids on hypocotyls-roots of common bean (P. vulgaris L.) cvs. ICA-Cerinza (top), Uribe Rosado (middle), and Andino (bottom) treated, respectively, with 0.44, 0.44, and 0.66 mM MeJA at different post-induction times. For each compound, bars with different letters (a, b, c, d, and e) indicate statistically significant differences (p = 0.05; Fisher's LSD test).
Figure 7.
Chromatographic profiles in the course of time for hypocotyls-roots of common bean (cv. Andino) treated with 0.66 mM methyl jasmonate (MeJA). Compounds: 5, daidzein; 6, genistein; 7, 2′-hydroxygenistein; 8, dalbergioidin; 9, phaseollinisoflavan; 10, coumestrol; 11, phaseollin; 12, phaseollidin; 13, kievitone.
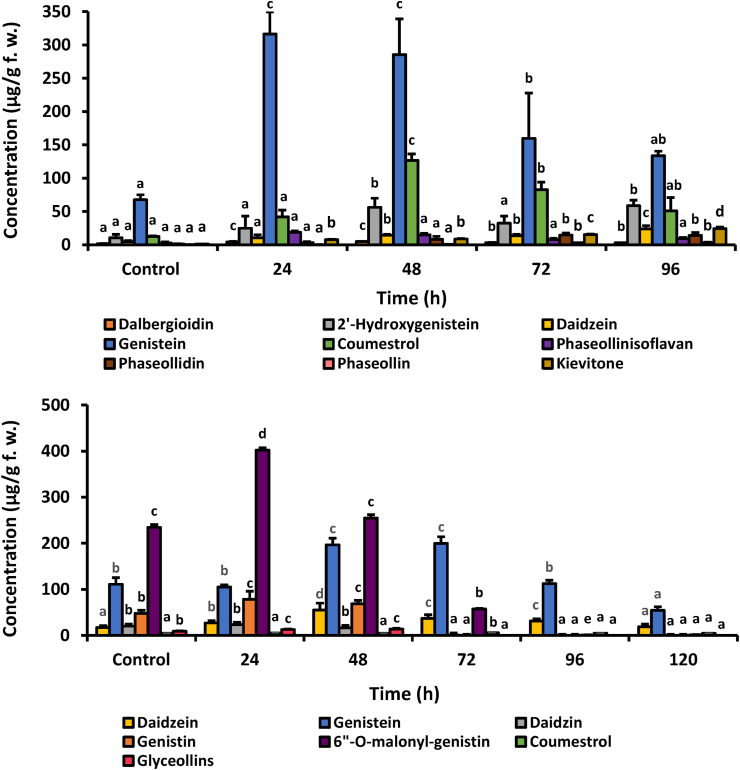
The results of mung bean (V. radiata L. Wilczek) showed that the concentration of isoflavonoids is higher than that of common bean. The main isoflavonoid compounds were genistein, followed by coumestrol and 2′-hydroxygenistein, throughout the analysis time (Figure 8). The amount of genistein ranged from 67.6 (control; water-treated seedlings) to 316.6 μg/g (24 h post-induction). Coumestrol and 2′-hydroxygenistein reached the highest level at 48 h, with 126.8 and 56.3 μg/g, respectively. Then, the concentration of coumestrol and 2′-hydroxygenistein decreased, coinciding with a slight increase in the concentration of phaseollidin and kievitone. Li et al. (2017) reported that MeJA application also significantly increased the concentration of genistein and daidzein in mung beans.
Figure 8.
Accumulation of defense-related isoflavonoids on hypocotyls-roots of mung bean (V. radiata L. Wilczek) (top) and soybean (G. max L. cv. SK-7) (bottom) treated with methyl jasmonate (MeJA) at 0.66 and 0.16 mM respectively, and different post-induction times. For each compound, bars with different letters (a, b, c, d, and e) indicate statistically significant differences (p = 0.05; Fisher's LSD test).
Treatment of soybean seedlings with 0.16 mM MeJA significantly increased the amount of all isoflavonoid compounds except coumestrol compared with the control. The main metabolite was 6″-O-malonylgenistin, followed by genistein and genistin. The concentration of 6″-O-malonylgenistin reached the maximum (402.4 μg/g) at 24 h. Coumestrol and glyceollins showed amounts less than 14 μg/g during the analysis. Jeong et al. (2018) reported that MeJA treatment increased the production of three glycosidic isoflavones (daidzin, malonyldaidzin, and malonylgenistin) by activating structural genes involved in isoflavonoid biosynthesis.
3.2.3. Elicitor effect of structurally related compounds to MeJA
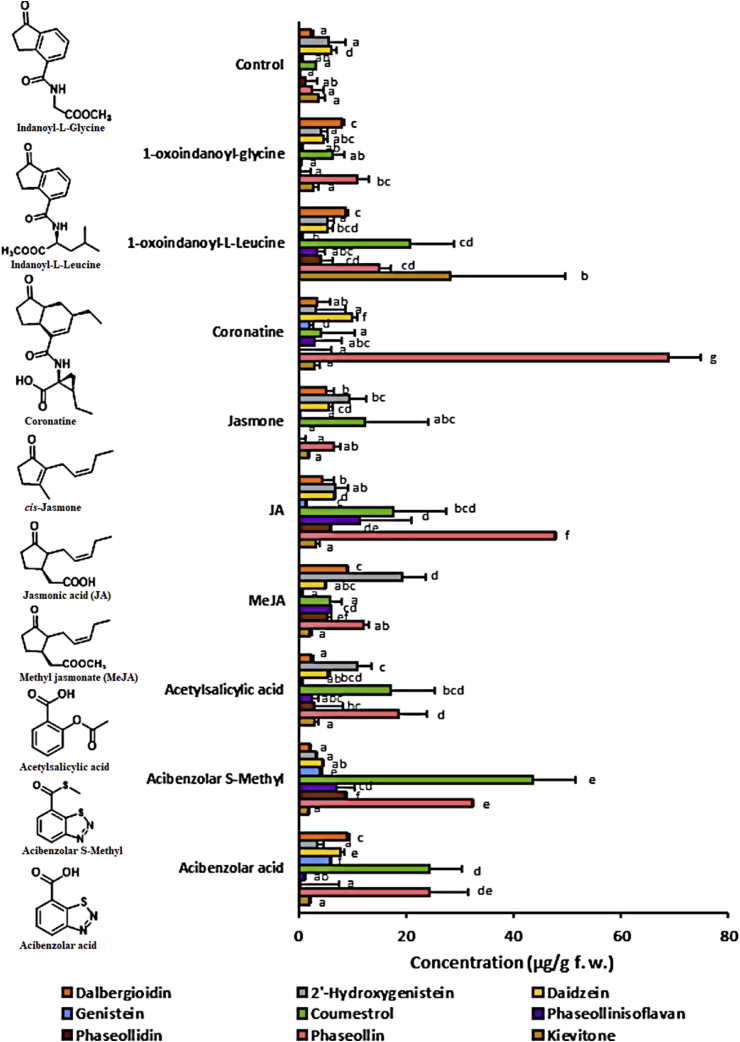
Currently, there is a growing interest in the control of important plant diseases through the use of elicitors. Acibenzolar acid, acibenzolar S-methyl, acetylsalicylic acid, MeJA, JA, cis-jasmone, coronatine, 1-oxoindanoyl-L-leucine methyl ester, and 1-oxoindanoyl-glycine methyl ester (Figure 9) were evaluated for their inducing effect of isoflavonoids on common bean hypocotyls-roots (P. vulgaris L. cvs. CORPOICA LAS 106 and Cargamanto Mocho). As can be seen in Figure 9, jasmonates have a strong elicitor effect, being even higher than that shown by the recognized elicitors, acibenzolar-S-methyl and acibenzolar acid. Significant differences were found in the concentration of all defense-related isoflavonoids with respect to the control (tissues treated with 0.2% ethanol). The greatest accumulation of dalbergioidin was found to be elicited by the two indanoyl derivatives, the MeJA, and the acibenzolar acid. Similarly, MeJA and 1-oxoindanoyl-L-leucine methyl ester treatments exhibited the highest amounts of 2′-hydroxygenistein and phaseollidin, and kievitone, respectively. The highest levels of daidzein and genistein were shown in coronatine-treated hypocotyls-roots. In addition, coronatine caused the highest accumulation of phaseollin (17 times higher than control). Coronatine-treated soybean seedlings also showed significant increases in the amounts of daidzin (47.7 μg/g; 5 times higher than control) and 6″-O-malonyldaidzin (26.3 μg/g; twice the concentration found in the control) (Data not shown). Coumestrol reached the highest level in common bean tissues treated with acibenzolar S-methyl, followed by acibenzolar acid and 1-oxoindanoyl-L-leucine methyl ester. In general, coumestrol and phaseollin showed similar accumulations in common bean tissues elicited by benzoyl-containing compounds such as salicylic acid, acibenzolar-S-methyl, acibenzolar acid, and 1-oxoindanoyl-L-leucine methyl ester. These results agree with previous reports on elicitation with salicylic acid and derivatives in other common bean cultivars (Durango et al., 2013, 2014). Surprisingly, phaseollin showed substantially greater accumulation than coumestrol in common bean tissues treated with compounds containing the oxocyclopentyl-system, such as coronatine and JA. In common bean, the elicitation of isoflavonoid-phytoalexins seems to depend largely on the structural characteristics of the indanoyl derivatives; even small differences in structure are sufficient to differentially induce phytoalexins. Thus, cis-jasmone was a poor elicitor of defense-related isoflavonoids in common bean compared to JA and MeJA, despite small structural differences. For indanoyl-derivatives, Krumm et al. (1995) and Lauchli et al. (2002) have reported that even the loss of a –CH2- group from the amino acid, leads to a complete loss of biological activity. As can be seen (Figure 9, Table 1), the accumulation of defense-related isoflavonoids was higher in hypocotyl-roots tissues treated with 1-oxoindanoyl-L-leucine methyl ester compared to 1-oxoindanoyl-glycine methyl ester. Botero et al. (2021) previously reported the antifungal and elicitor activities in common bean tissues of the derivative 1-oxoindanoyl-L-leucine methyl ester.
Figure 9.
Accumulation of defense-related isoflavonoids on hypocotyls-roots of common bean (P. vulgaris L. cv. Corpoica LAS 106) treated with jasmonate compounds at 0.44 mM, and 96 h post-induction. For each compound, bars with different letters (a, b, c, d, and e) indicate statistically significant differences (p = 0.05; Fisher's LSD test).
Table 1.
Accumulation of isoflavonoid-phytoalexins in common bean (P. vulgaris L.) tissues of cultivars CORPOICA LAS 106 (anthracnose-resistant) and Cargamanto Mocho (anthracnose-susceptible) treated with indanoyl derivatives.
| DALB | 2′-OHG | DAID | GEN | COU | PHAIS | PHAD | PHAS | KIEV | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Hypocotyls-Roots CORPOICA LAS 106 | Indanoyl-L-Leucine | 8.74 ± 0.46b | 5.36 ± 1.30ab | 5.49 ± 0.84a | 0.67 ± 0.24a | 20.73 ± 8.32b | 3.46 ± 1.44b | 4.19 ± 0.38ab | 15.08 ± 2.11c | 28.28 ± 21.42a |
| Indanoyl-glycine | 8.04 ± 0.36a | 4.12 ± 1.20a | 4.64 ± 0.84a | 0.65 ± 0.26a | 6.43 ± 2.02a | 0.24 ± 0.11a | 0.11 ± 0.06a | 10.99 ± 2.10b | 2.64 ± 1.12a | |
| Indanoyl-D-Leucine | 8.12 ± 0.88ab | 3.88 ± 0.45a | 3.94 ± 1.12a | 0.38 ± 0.12a | 8.55 ± 1.53a | 6.15 ± 0.88c | 0.21 ± 0.12a | 15.44 ± 1.77c | 4.68 ± 0.95a | |
| Control | 8.27 ± 0.63ab | 6.70 ± 2.52b | 4.65 ± 0.58a | 0.40 ± 0.08a | 4.65 ± 1.33a | 5.05 ± 1.10bc | 6.64 ± 5.51b | 4.34 ± 1.02a | 2.61 ± 0.69a | |
| Hypocotyls-Roots Cargamanto Mocho | Indanoyl-L-Leucine | 7.78 ± 1.86b | 11.62 ± 4.46b | 8.53 ± 3.35a | 4.24 ± 2.20b | 20.63 ± 7.91b | 9.46 ± 6.80b | 3.24 ± 2.03b | 12.38 ± 1.06b | 3.49 ± 1.34a |
| Indanoyl-glycine | 4.04 ± 0.45a | 14.44 ± 1.23b | 8.68 ± 4.24a | 4.05 ± 0.75b | 19.18 ± 2.23b | 1.93 ± 0.51ab | 2.28 ± 0.26ab | 11.98 ± 1.05b | 6.69 ± 2.08b | |
| Indanoyl-D-Leucine | 4.28 ± 0.95a | 11.32 ± 0.28b | 6.10 ± 1.93a | 2.68 ± 1.13b | 27.06 ± 0.62b | 7.05 ± 2.33ab | 3.94 ± 2.69b | 18.05 ± 1.35c | 2.53 ± 0.32a | |
| Control | 3.50 ± 1.50a | 3.16 ± 1.03a | 3.97 ± 0.05a | 0.20 ± 0.14a | 6.19 ± 2.35a | 0.21 ± 0.11a | 0.32 ± 0.14a | 6.64 ± 2.54a | 3.30 ± 0.93a | |
| CotyledonsCargamanto Mocho | Indanoyl-L-Leucine | 10.73 ± 3.43a | 28.53 ± 13.12c | 5.30 ± 0.13bc | 0.37 ± 0.21ab | 18.19 ± 11.73b | 12.60 ± 6.36b | 14.56 ± 6.93a | 7.39 ± 1.96b | 10.93 ± 5.33b |
| Indanoyl-glycine | 4.37 ± 1.66a | 5.31 ± 1.72a | 4.54 ± 0.04b | 0.10 ± 0.04a | 15.05 ± 10.56b | 11.27 ± 1.63b | 11.87 ± 5.53a | 4.32 ± 0.07a | 3.31 ± 0.00a | |
| Indanoyl-D-Leucine | 8.39 ± 2.14a | 9.78 ± 3.40ab | 6.67 ± 0.81c | 0.82 ± 0.13ab | 33.91 ± 9.77c | 16.42 ± 6.85b | 14.20 ± 5.89a | 14.32 ± 1.24c | 4.72 ± 0.00a | |
| Control | 8.63 ± 3.91a | 23.00 ± 20.34bc | 2.67 ± 1.89a | 0.92 ± 0.86b | 2.74 ± 2.60a | 0.51 ± 0.68a | 8.30 ± 6.97a | 3.37 ± 2.20a | 2.08 ± 1.59a |
DALB: Dalbergioidin; 2′-OHG: 2′-Hydroxygenistein; DAID: Daidzein; GEN: Genistein; COU: Coumestrol; PHAIS: Phaseollinisoflavan; PHAD: Phaseollidin; PHAS: Phaseollin; KIEV: Kievitone. For each isoflavonoid, bars with different letters are significantly differents (p = 0.05). 96 h post-induction.
Studies using different configurations for the chiral amino acid indicate that 1-oxoindanoyl-D-leucine methyl ester elicited significantly different amounts of some phytoalexins in hypocotyls-roots and cotyledons of common bean. In particular, coumestrol (8.55 ± 1.53 μg/g), phaseollidin (0.21 ± 0.12 μg/g), and kievitone (4.68 ± 0.95 μg/g) reached significantly lower amounts using 1-oxoindanoyl-D-leucine methyl ester compared to its L-substituted analog (coumestrol, 20.73 ± 8.32 μg/g; phaseollidin, 4.19 ± 0.38 μg/g; and kievitone, 28.28 ± 21.42 μg/g), in hypocotyl-roots of cultivar CORPOICA LAS 106 (anthracnosis-resistant cultivar).
In Cargamanto Mocho, hypocotyls-roots treated with 1-oxoindanoyl-D-leucine methyl ester accumulated phaseollin (18.05 ± 1.35 μg/g) and dalbergioidin (4.28 ± 0.95 μg/g) in levels significantly higher and lower, respectively, than the L-substituted analog (phaseollin, 12.30 ± 1.06 μg/g; dalbergioidin, 7.78 ± 1.86 μg/g). On the other hand, the amounts of phaseollin and coumestrol in the cotyledons treated with the compound 1-oxoindanoyl-D-leucine methyl ester were significantly higher than in the tissues treated with 1-oxoindanoyl-L-leucine methyl ester. It can be suggested that the interaction of the indanoyl elicitor and the receptor that triggers the biological activity is very specific, discriminating small differences in the carbon chain of the amino acid, or even between the L and D configurations (Krumm et al., 1995).
4. Conclusions
The exogenous application of jasmonate-type compounds, such as methyl jasmonate (MeJA) and jasmonic acid, along with coronatine and structurally related compounds (1-oxo-indanoyl amino acids conjugates) increases the amount of bioactive isoflavonoid compounds on soybean, common bean, and mung bean. In general, defense-related isoflavonoid contents on elicited tissues of leguminous plants depended on the species and cultivar, the concentration and structure of elicitor, and the time after induction. Our results show that jasmonate compounds, coronatine and the 1-oxo indanoyl amino acids conjugates can be used to enhance the biological and functional properties of extracts from common bean, soybean, and mung bean, and the resistance against pests.
Declarations
Author contribution statement
Karen Gómez, Franklin Quenguang, Diego Aristizábal: Performed the experiments.
Gustavo Escobar: Contributed reagents, materials, analysis tools or data.
Winston Quiñones: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Olimpo García-Beltrán: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Diego Durango: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by MINCIENCIAS/Departamento Administrativo de Ciencia, Tecnología e Innovación (111874558342; FP44842-057-2017) and Universidad Nacional de Colombia, Sede Medellín (50136; Quipu: 201010026836).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Botero L., Vizcaíno S., Quiñones W., Echeverri F., Gil J., Durango D. Increased accumulation of isoflavonoids in common bean (Phaseolus vulgaris L.) tissues treated with 1-oxo-indane-4-carboxylic acid derivatives. Biotechnol. Rep. (Amst). 2021;29 doi: 10.1016/j.btre.2021.e00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz C.A., Santana G.E. Corpoica-Agrosavia. Santa Rosa de Osos; Antioquia (Colombia): 2004. Revisión de las fichas técnicas de las variedades comerciales de fríjol (Phaseolus vulgaris) en Colombia. 184 pp. [Google Scholar]
- Durango D., Murillo J., Echeverri F., Escobar G., Quiñones W. Isoflavonoid composition and biological activity of extracts from soybean seedlings treated by different elicitors. An Acad. Bras. Cienc. 2018;90(2 suppl 1):1955–1971. doi: 10.1590/0001-3765201820170785. [DOI] [PubMed] [Google Scholar]
- Durango D., Pulgarin N., Echeverri F., Escobar G., Quiñones W. Effect of salicylic acid and structurally related compounds in the accumulation of phytoalexins in cotyledons of common bean (Phaseolus vulgaris L.) cultivars. Molecules. 2013;18(9):10609–10628. doi: 10.3390/molecules180910609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durango D., Pulgarin N., Gil J., Escobar G., Echeverri F., Quiñones W. Differential accumulation of defense-related isoflavonoids in hypocotyls/roots of common bean (Phaseolus vulgaris L.) cultivars treated with salicylic acid and structurally related compounds. Bol. Latinoam. Caribe Plantas Med. Aromat. 2014;13(4):381–405. [Google Scholar]
- Durango D., Quiñones W., Torres F., Rosero Y., Gil J., Echeverri F. Phytoalexin accumulation in Colombian bean varieties and aminosugars as elicitors. Molecules. 2002;7(11):817–832. [Google Scholar]
- Farhangi-Abriz S., Ghassemi-Golezani K. Jasmonates: mechanisms and functions in abiotic stress tolerance of plants. Biocatal. Agric. Biotechnol. 2019;20 article101210. [Google Scholar]
- Geng X., Jin L., Shimada M., Kim M.G., Mackey D. The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta. 2014;240(6):1149–1165. doi: 10.1007/s00425-014-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkes G.J., Thorpe M.R., Minchin P.E.H., Schurr U., Rose U.S.R. Jasmonic acid treatment to part of the root system is consistent with simulated leaf herbivory, diverting recently assimilated carbon towards untreated roots within an hour. Plant Cell Environ. 2008;31(9):1229–1236. doi: 10.1111/j.1365-3040.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- Jeong Y.J., An C.H., Park S.-C., Pyun J.W., Lee J., Kim S.W., Kim H.-S., Kim H., Jeong J.C., Kim C.Y. Methyl jasmonate increases isoflavone production in soy-bean cell cultures by activating structural genes involved in isoflavonoid biosynthesis. J. Agric. Food Chem. 2018;66:4099–4105. doi: 10.1021/acs.jafc.8b00350. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Ye J., Li S., Niinemets U. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: a high-resolution analysis of dose dependence. J. Exp. Bot. 2017;68(16):4679–4694. doi: 10.1093/jxb/erx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumm T., Katja B., Boland W. Induction of volatile biosynthesis in the Lima bean (Phaseolus lunatus) by leucine- and isoleucine conjugates of 1-oxo- and 1-hydroxyindan-4-carboxylic acid: evidence for amino acid conjugates of jasmonic acid as intermediates in the octadecanoid signalling pathway. FEBS Lett. 1995;377(3):523–529. doi: 10.1016/0014-5793(95)01398-9. [DOI] [PubMed] [Google Scholar]
- Lauchli R., Schüler G., Boland W. Selective induction of secondary metabolism in Phaseolus lunatus by 6-substituted indanoyl isoleucine conjugates. Phytochemistry. 2002;61(7):807–817. doi: 10.1016/s0031-9422(02)00397-7. [DOI] [PubMed] [Google Scholar]
- Li L., Dong Y., Ren H., Xue Y., Meng H., Li M. Increased antioxidant activity and polyphenol metabolites in methyl jasmonate treated mung bean (Vigna radiata) sprouts. Food Sci. Technol. 2017;37(3):411–417. [Google Scholar]
- Ligarreto G.A., Vargas A., Chaparro J. ICA. Bogotá-Colombia; 1994. ICA Bachué: Variedad de fríjol arbustivo para clima frio. 4 pp. [Google Scholar]
- Littleson M.M., Russell C.J., Frye E.C., Ling K.B., Jamieson C., Watson A.J.B. Synthetic approaches to coronafacic acid, coronamic acid, and coronatine. Synthesis. 2016;48(20):3429–3448. [Google Scholar]
- Lygin A.V., Zernova O.V., Hill C.B., Kholina N.A., Widholm J.M., Hartman G.L., Lozovaya V.V. Glyceollin is an important component of soybean plant defense against Phytophthora sojae and Macrophomina phaseolina. Phytopathology. 2013;103(10):984–994. doi: 10.1094/PHYTO-12-12-0328-R. [DOI] [PubMed] [Google Scholar]
- Mithöfer A., Maitrejean M., Boland W. Structural and biological diversity of cyclic octadecanoids, jasmonates, and mimetics. J. Plant Growth Regul. 2004;23(3):170–178. [Google Scholar]
- Nwachukwu I.D., Luciano F.B., Udenigwe C.C. The inducible soybean glyceollin phytoalexins with multifunctional health-promoting properties. Food Res. Int. 2013;54(1):1208–1216. [Google Scholar]
- Pedras M.S.C., Yaya E.E. Plant chemical defenses: are all constitutive antimicrobial metabolites phytoanticipins? Nat. Prod. Commun. 2015;10(1):209–218. [PubMed] [Google Scholar]
- Pelaez L.G., Rios M.J. Fríjol Corpoica 106: nueva variedad de fríjol voluble resistente a la antracnosis para zona de clima frio moderado. Corpoica. Rionegro-Antioquia-Colombia. 2001:8. [Google Scholar]
- Rios-B M.J., Roman-V A., Florez-O G., Posada-S H. ICA-CIAT-CENICAFE-Secretaría de Agricultura de Antioquia. Rionegro-Antioquia; Colombia: 1989. ICA Quimbaya: variedad de fríjol arbustivo rojo para clima medio. 6 pp. [Google Scholar]
- Saini R.K., Akithadevi M.K., Giridhar P., Ravishankar G.A. Augmentation of major isoflavones in Glycine max L. through the elicitor-mediated approach. Acta Bot. Croat. 2013;72(2):311–322. [Google Scholar]
- Schüler G., Göris H., Boland W. 6-Substituted indanoyl isoleucine conjugates mimic the biological activity of coronatine. Eur. J. Org. Chem. 2001;9:1663–1668. [Google Scholar]
- Schüler G., Mithöfer A., Baldwin I.T., Berger S., Ebel J., Santos J.G., Herrmann G., Hölscher D., Kramell R., Kutchan T.M., Maucher H., Schneider B., Stenzel I., Wasternack C., Boland W. Coronalon: a powerful tool in plant stress physiology. FEBS Lett. 2004;563(1-3):17–22. doi: 10.1016/S0014-5793(04)00239-X. [DOI] [PubMed] [Google Scholar]
- Singh A., Bwivedi P. Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: a review. J. Pharmacogn. Phytochem. 2018;7(1):750–757. [Google Scholar]
- Vitale D.C., Piazza C., Melilli B., Drago F., Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013;38(1):15–25. doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007;100(4):681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C., Feussner I. The oxylipin pathways: biochemistry and function. Annu. Rev. Plant Biol. 2018;69(1):363–386. doi: 10.1146/annurev-arplant-042817-040440. [DOI] [PubMed] [Google Scholar]
- Xie Z., Duan L., Tian X., Wang B., Eneji A.E., Li Z. Coronatine alleviates salinity stress in cotton by improving the antioxidative defense system and radical-scavenging activity. J. Plant Physiol. 2008;165(4):375–384. doi: 10.1016/j.jplph.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Yu J., Bi X., Yu B., Chen D. Isoflavones: anti-inflammatory benefit and possible caveats. Nutrients. 2016;8(6):361. doi: 10.3390/nu8060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.