Abstract
Epithelioid leiomyoma of the uterus is rare, and its prognostic factors have not been well established. Moreover, radiologic findings of this disease have not been previously documented. This is a case of a 49-year-old woman with epithelioid leiomyoma of the uterus. Magnetic resonance imaging (MRI) revealed a heterogeneous high-intensity mass with multiple ordinary uterine leiomyomas. The mass showed a slightly diffusion-restricted site. Since benign tumors could not be confidently diagnosed using these MRI findings, total abdominal hysterectomy with bilateral salpingectomy was performed, and a pathological diagnosis of epithelioid leiomyoma of the uterus was established. Microscopically, this lesion showed edematous changes and cyst formation, causing a heterogeneous appearance on T2-weighted images. In addition, the diffusion-restricted site is considered to be consistent with areas of solid and dense proliferation of tumor cells. The patient survived and was well 10 months after the surgery. It is important to recognize this benign variant of leiomyoma with an unusual appearance, to provide appropriate therapeutic management.
Keywords: Epithelioid leiomyoma, MRI, Uterine tumor
Highlights
-
•
Epithelioid leiomyoma of the uterus is rare.
-
•
Radiologic findings of this disease have not been documented previously.
-
•
It is important to recognize this benign variant of leiomyoma with an unusual appearance.
1. Introduction
Leiomyomas are the most common benign smooth muscle tumors of the uterus. They have several histological variants, such as atypical, cellular, myxoid, and epithelioid, accounting for approximately 10% of all leiomyoma cases [1]. Epithelioid leiomyoma of the uterus is a rare variant of leiomyoma composed of round or polygonal clear cells rather than typical spindle-shaped cells. The prognostic factors of epithelioid leiomyoma of the uterus have not been well established. However, since variant leiomyomas may have a greater risk of recurrence than ordinary leiomyomas, recognition of these rare and malignancy-mimicking leiomyomas is crucial to prevent inappropriate treatment. Epithelioid leiomyoma of the uterus has been documented in several reports; however, no imaging findings are available. The magnetic resonance imaging (MRI) findings of this rare tumor are presented herein.
2. Case Presentation
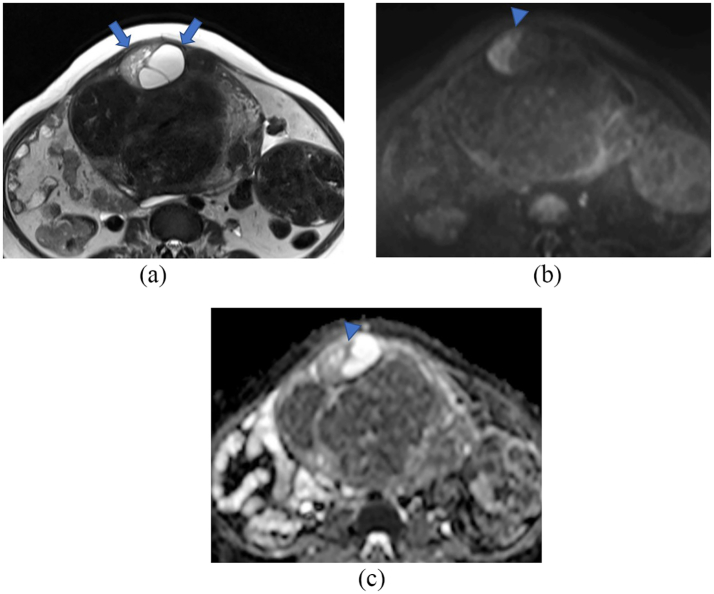
A 49-year-old menopausal woman, gravida 0, was referred to hospital for abdominal discomfort and an increased uterine mass lesion. She had no symptoms of hypermenorrhea or dysmenorrhea and no notable medical history except for Graves' disease. Her uterus was enlarged to that of 9–10 months of gestation. On speculum examination, the cervix appeared normal. Transvaginal ultrasonography revealed a mass occupying the pelvis. MRI of the lower abdomen was performed using a 3-T Magnetom Skyra (Siemens Healthcare, Erlangen, Germany). Multiple well-defined uterine masses were observed: one of them exhibited isointensity to the myometrium on T1-weighted images and heterogeneous high intensity on T2-weighted images (Fig. 1A). On diffusion-weighted images (DWI) with a b-value of 800 s/mm2, the solid portion of the mass showed high intensity, but low intensity on the apparent diffusion coefficient (ADC) map, and the lowest ADC value of the lesion was slightly low (1.3 × 10−3 mm2/s) (Fig. 1B, C).
Fig. 1.
Magnetic resonance images.
(A): T2-weighted axial section, (B, C): Diffusion-weighted images. Within the myometrium of the anterior wall of the uterus, a heterogeneous high-intensity mass (arrows) coexisting with multiple ordinary leiomyomas is observed. In the solid portion of the mass, reduced diffusion is observed (B, C; arrowhead).
Although the MRI findings did not positively suggest malignant tumors, the possibility of smooth muscle tumors of uncertain malignant potential (STUMP) or malignancy could not be ruled out; therefore, a total abdominal hysterectomy with bilateral salpingectomy was performed. Pathological examination revealed multiple uterine masses. The cut sections of those masses showed gray-white areas. On the ventral side, the tumor was found to be 5 cm in diameter (Fig. 2). Hematoxylin and eosin staining revealed that the tumor cells had oval nuclei, eosinophilic cytoplasm, dense proliferation, and showed epithelioid arrangement (Fig. 3). Edematous change, cyst formation, and abundant muscular arteries were observed inside the tumor. The boundary between the tumor and surrounding tissue was clear, and no infiltration of the tumor was observed. The mitotic count was 2/10 high-power field (HPF). Immunohistochemical examination revealed positivity for alpha-smooth muscle actin (α-SMA). In addition, desmin and caldesmon were focally positive, suggesting differentiation into smooth muscles. The Ki-67 proliferation index was approximately 5% positive at the hot spot, with an average of 3% positive staining. AE1/AE3, Melan A, HMB45, S100P, cyclin D1, and CD10 were negative.
Fig. 2.
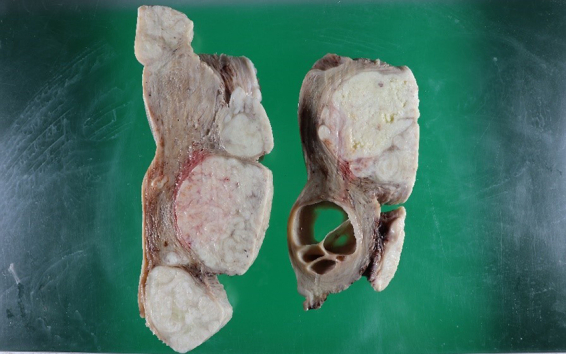
Image of gross examination of a uterus with multiple giant tumors.
Cyst formation is observed inside the tumor attached to the anterior wall of the uterus.
Fig. 3.
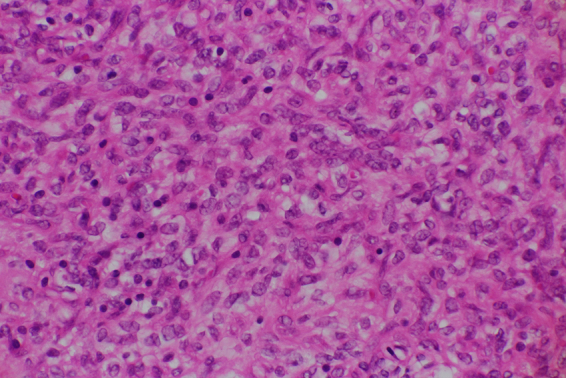
Histologic features of the intramural uterine mass.
High-powered view (400 ×) of hematoxylin and eosin-stained specimens showing cells with oval nuclei and epithelioid arrangement.
3. Discussion
The malignant counterpart of uterine leiomyoma is leiomyosarcoma, which is also the most common uterine nonepithelial malignant tumor. Leiomyosarcoma accounts for approximately 1.3% of uterine malignancies. Although most leiomyosarcomas arise de novo, the malignancy may develop in pre-existing leiomyomas as well [1]. Leiomyomas may have unusual growth patterns and many histopathological variants, such as atypical, cellular, mitotically active, myxoid, and epithelioid [1]. Epithelioid leiomyoma is a rare atypical smooth muscle tumor of the uterus and has not been documented extensively. Irrespective of the different morphological variations, management is the same for all leiomyomas, except for certain variants. However, awareness of tumor variants and unconventional growth patterns is critical for appropriate classification and patient management. Pathological findings have been reported in a few cases of uterine epithelioid leiomyoma [2]. However, MRI findings of this rare tumor have not been reported.
DWI of MRI shows tissue characteristics based on the diffusion motion of water molecules. In general, high-cellularity tumors demonstrate restricted diffusion, whereas normal tissues do not. Thus, DWIs can delineate malignant lesions as a hyperintense area with excellent tissue contrast, and cases with hypointense signals on DWIs may be regarded as benign lesions in smooth muscle tumors of the uterus [3,4]. In this case, MRI revealed a solid lesion with a cystic component. Since the solid part was consistent with high intensity on DWI and the ADC value was slightly low, malignant tumors, including STUMP, could not be ruled out. In contrast, previous studies reported that there were overlaps in ADC values between a uterine leiomyoma, such as cellular leiomyoma and bizarre leiomyoma, and uterine sarcoma, suggesting that DWIs alone are insufficient to establish a definite diagnosis [[4], [5], [6], [7], [8]]. Recently, several investigators have reported the high diagnostic capabilities of positron emission tomography (PET) using 2-[18F] fluoro-2-deoxy-d-glucose (FDG) for malignancies of various organs [9,10]. In cases where ADC values for the solid component of the tumor are not definitive for differentiating benign from malignant or borderline components, FDG-PET/computed tomography (CT) or FDG-PET/MRI could be useful. Unfortunately, however, FDG-PET cannot be used to differentiate leiomyosarcoma from leiomyoma. Patients with any future fertility desires may have the option of undergoing myomectomy and careful follow-up after surgery if the imaging tests results do not positively suggest malignancy [11,12].
In this case, the tumor was 5 cm in diameter. It was encapsulated with clear cells, and extensive hyalinization was observed. No necrosis was observed, and the mitotic count was 2/10 HPF [13]. Therefore, a benign lesion was considered. Ki-67, which is a typical immunohistochemical marker for cell proliferation, of the current lesion was approximately 3% positive. Higher expression of Ki-67 correlates with poor clinical outcomes in several cancers. In a group of 68 cases, Mills et al. reported an average proliferation index Ki-67 of 2% in the case of atypical leiomyomas, 3% in the case of leiomyomas, and 25% in leiomyosarcomas [14], which is consistent with these results.
In summary, the case presented herein is an epithelioid leiomyoma of the uterus with multiple ordinary leiomyomas. This rare tumor may show a complex cystic mass on CT and magnetic resonance images and cannot be adequately distinguished from other uterine tumors. However, DWI might be useful while considering the possibility of a variant type of uterine leiomyoma. Nevertheless, further study on the correlation between identification of malignant uterine masses using DWI MRI is warranted.
Acknowledgments
Contributors
Ayaka Kita contributed to patient management, data collection, and manuscript writing.
Tetsuo Maeda contributed to data collection and manuscript editing.
Kazuhiro Kitajima contributed to data collection and manuscript editing.
Homare Murakoshi contributed to patient management and manuscript editing.
Takahiro Watanabe contributed to data collection and manuscript editing.
Mieko Inagaki contributed to patient management and manuscript editing.
Shigeki Yoshida contributed to manuscript editing and supervision.
All authors approved the final submitted article.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Provenance and peer review
This article was not commissioned and was peer reviewed.
Acknowledgments
Conflict of interest statement
The authors declare that they have no conflict of interest regarding the publication of this case report.
References
- 1.Kefeli M., Baris S., Aydin O., Yıldız L., Yamak S., Kandemir B. An unusual case of an osteosarcoma arising in a leiomyoma of the uterus. Ann. Saudi. Med. 2012;32:544–546. doi: 10.5144/0256-4947.2012.23.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulita F., Iliopoulos F., Plachouri K.M., Kehagias I. Uterine leiomyoblastoma. BMJ. Case. Rep. 2021;14 doi: 10.1136/bcr-2020-241533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namimoto T., Yamashita Y., Awai K., Nakaura T., Yanaga Y., Hirai T., Saito T., Katabuchi H. Combined use of T2-weighted and diffusion-weighted 3-T MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur. Radiol. 2009;19:2756–2764. doi: 10.1007/s00330-009-1471-x. [DOI] [PubMed] [Google Scholar]
- 4.Sato K., Yuasa N., Fujita M., Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am. J. Obstet. Gynecol. 2014;210 doi: 10.1016/j.ajog.2013.12.028. 368.e1–368.e8. [DOI] [PubMed] [Google Scholar]
- 5.Tamai K., Koyama T., Saga T., Morisawa N., Fujimoto K., Mikami Y., Togashi K. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur. Radiol. 2008;18:723–730. doi: 10.1007/s00330-007-0787-7. [DOI] [PubMed] [Google Scholar]
- 6.Thomassin-Naggara I., Dechoux S., Bonneau C., Morel A., Rouzier R., Carette M.F., Daraï E., Bazot M. How to differentiate benign from malignant myometrial tumours using MR imaging. Eur. Radiol. 2013;23:2306–2314. doi: 10.1007/s00330-013-2819-9. [DOI] [PubMed] [Google Scholar]
- 7.Lin G., Yang L.Y., Huang Y.T., Ng K.K., Ng S.H., Ueng S.H., Chao A., Yen T.C., Chang T.C., Lai C.H. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma / smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J. Magn. Reson. Imaging. 2016;43:333–342. doi: 10.1002/jmri.24998. [DOI] [PubMed] [Google Scholar]
- 8.Barral M., Placé V., Dautry R., Bendavid S., Cornelis F., Foucher R., Guerrache Y., Soyer P. Magnetic resonance imaging features of uterine sarcoma and mimickers. Abdom. Radiol. (NY). 2017;42:1762–1772. doi: 10.1007/s00261-017-1076-9. [DOI] [PubMed] [Google Scholar]
- 9.Kitajima K., Ebina Y., Sugimura K. Present and future role of FDG-PET/CT imaging in the management of gynecologic malignancies. Jpn. J. Radiol. 2014;32:313–323. doi: 10.1007/s11604-014-0317-x. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen N.C., Beriwal S., Moon C.H., D’Ardenne N., Mountz J.M., Furlan A., Muthukrishnan A., Rangaswamy B. Diagnostic value of FDG PET/MRI in females with pelvic malignancy-a systematic review of the literature. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.519440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitajima K., Murakami K., Sugimura K. Spectrum of FDG PET/CT findings of uterine tumors, AJR. Am. J. Roentgenol. 2010;195:737–743. doi: 10.2214/AJR.09.4074. [DOI] [PubMed] [Google Scholar]
- 12.Ly A., Mills A.M., McKenney J.K., Balzer B.L., Kempson R.L., Hendrickson M.R., Longacre T.A. Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. Am. J. Surg. Pathol. 2013;37:643–649. doi: 10.1097/PAS.0b013e3182893f36. [DOI] [PubMed] [Google Scholar]
- 13.Hendrickson M., Tavassali F., Kempson R., Mccluggage W., Haller U., Kubik-Huch R. IARC Press; 2003. Mesenchymal Tumours and Related Lesions. World Health Organization Classification of Tumors. Pathology and Genetics of Tumours of Breast and Female Genital Organs; pp. 233–244. [Google Scholar]
- 14.Mills A.M., Ly A., Balzer B.L., Hendrickson M.R., Kempson R.L., McKenney J.K., Longacre T.A. Cell cycle regulatory markers in uterine atypical leiomyoma and leiomyosarcoma: Immunohistochemical study of 68 cases with clinical follow-up. Am. J. Surg. Pathol. 2013;37:634–642. doi: 10.1097/PAS.0b013e318287779c. [DOI] [PubMed] [Google Scholar]