Abstract
Background:
Despite the significant difference between men and women in incidence of anterior cruciate ligament (ACL) injuries, there is a paucity of consistent information on the influence of patient sex on outcomes after ACL reconstruction. A previous meta-analysis has demonstrated that female patients have worse outcomes with regard to laxity, revision rate, Lysholm score, and Tegner activity score and are less likely to return to sports (RTS).
Purpose:
To conduct a systematic review and meta-analysis to evaluate and compare sex-specific outcomes after ACL reconstruction.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A systematic review was performed using PubMed, PubMed Central, Embase, OVID, and Cochrane databases per PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The following search terms were used: “anterior cruciate ligament reconstruction” OR “ACL reconstruction” OR “anterior cruciate ligament” OR “ACL” AND “gender” OR “sex” OR “male” OR “female” AND “outcome” AND “2015-Present” to gather all relevant articles between 2015 and 2020. A risk-of-bias assessment and quality assessment was conducted on included studies.
Results:
Of 9594 studies initially identified, 20 studies with 35,935 male and 21,455 female patients were included for analysis. The 7 studies reporting International Knee Documentation Committee (IKDC) scores showed that male patients had statistically significantly higher postoperative scores (mean difference, 3.02 [95% CI, 1.19-4.84]; P< .01; I 2 = 66%), and 7 studies that reported the rate of ACL revision showed there was no significant difference between male and female patients (odds ratio, 0.85 [95% CI, 0.45-1.60]; P = .61; I 2 = 94%). The 7 studies that reported rates of rerupture showed that males were significantly more likely than females to have a graft rerupture (odds ratio, 1.35 [95% CI, 1.22-1.50]; P < .01; I 2 = 0%). Male patients reported a higher RTS rate than did their female counterparts (59.82% compared with 42.89%); however, no formal statistical analysis could be done because of the variability in reporting techniques.
Conclusion:
Male and female patients with ACL injuries demonstrated similar outcomes regarding their rates of revision; however, male patients were found to have statistically significantly higher postoperative IKDC scores but at the same time higher rerupture rates. Our findings suggest that sex-based differences in outcomes after ACL reconstruction vary based on which metric is used. These results must be considered when counseling patients with ACL injuries.
Keywords: ACL, knee, outcomes, sex, surgical repair
Anterior cruciate ligament (ACL) tears are a common knee-related injury, with approximately 120,000 ACL reconstructions (ACLRs) performed in the United States each year. 21,60 The incidence of ACLR has increased from 32.9 per 100,000 person-years to 43.5 per 100,000 person-years over a recent 12-year span. 9 Many different risk factors, such as sex, age, and sport played, have been studied to determine their role in the incidence of ACL ruptures, but few studies have looked at the effect that these factors play on the outcomes of ACLR. Specifically, the incidence of ACL ruptures has been shown to be higher in patients who are female 12,67 ; younger 62 ; and play sports with frequent cutting or landing maneuvers, such as basketball, ice hockey, field hockey, football, and volleyball. 1
In general, the literature demonstrates that ACLR results in optimal outcomes for patients, regardless of age or concomitant injuries. 47,49,58 Many factors are considered when looking at the effectiveness of an ACLR, including, but not limited to, reoperative rate, rerupture rate, functional tests, objective tests, return-to-sports (RTS) rate, and the visual analog scale (VAS) pain scale. Many previous studies have evaluated the extent to which various risk factors (eg, age, athletic ability, graft, surgical technique, and rehabilitation program) play a role in the success of ACLR. ¶ However, little work has been done to examine the effect that patient sex has on outcomes after ACLR. A systematic review published in 2014, evaluating sex-based differences in ACLR outcomes, found that there were no significant differences between male and female patients in the 13 papers included in the review. 51 In 2016, Tan et al 68 performed another systematic review and meta-analysis to evaluate outcomes of ACLR based on patient sex. The authors found that, postoperatively, female patients had inferior outcomes in instrumented laxity, revision rate, Lysholm score, Tegner activity score, and RTS. All other outcomes analyzed, including anterior drawer test, Lachman test, pivot-shift test, and single-leg hop tests, were comparable.
Despite these 2 systematic reviews, there is still a very limited understanding of the effect of patient sex on outcomes after ACLR. In this study, we aimed to update the study published by Tan et al 68 to include information regarding sex-specific ACLR outcomes between 2015 and 2020.
Methods
Search Strategy and Study Selection
This study was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. 65 Two authors (A.C.M., D.J.F.) performed a manual study selection using the PubMed, PubMed Central, Cochrane, OVID, and Embase databases with discrepancies being resolved via discussion with a third author (M.L.V.). The following search terms were used: “anterior cruciate ligament reconstruction” OR “ACL reconstruction” OR “anterior cruciate ligament” OR “ACL” AND “gender” OR “sex” OR “male” OR “female” AND “outcome” AND “2015-Present.” These databases were searched between January 2015 and March 2020. Titles and abstracts were screened to assess for removal per our eligibility criteria, and then the full text was reviewed for the remaining studies to further assess for eligibility using the criteria stated below. All included studies included distinct cohorts from separate research groups.
Eligibility Criteria
Studies that met the following criteria were included: published in the English language, used human participants, and evaluated for sex-specific outcomes of ACLR. Our initial search terms were applied to the aforementioned databases. All studies that resulted from the search were initially included. The abstracts of all studies were then screened, and studies were excluded if any of the following were present: non—English language publication, cadaveric study, nonhuman participants, and no evaluation for any of the sex-specific ACLR outcomes of interest. The methods of the remaining studies were reviewed, and if the study included injuries of concomitant cartilage procedure, multiligamentous injury, or a quadriceps/patellar tendon rupture, it was also excluded. Finally, only studies that separated their outcomes based on sex in an extractable manner were included in our final meta-analysis. For example, studies were excluded because they only reported which sex had better results, without any data, or the study was the only one that reported a specific outcome (eg, osteoarthritis rate), in which case we would not be able to perform statistical analysis.
Outcomes
Patient characteristics that were collected from the eligible studies included patient sex, age at time of injury, age at time of surgery, follow-up time, body mass index, treatment technique, ACL graft type used, and sport played. Outcomes for which we were screening in our papers to possibly conduct a meta-analysis included functional outcomes, retear rate, reoperative rate, contralateral injury rate, postoperative range of motion (flexion and extension), postoperative stability (Lachman test and pivot-shift test), rate of RTS, time required to RTS, VAS for pain, KT-1000 arthrometer side-to-side difference in laxity, Tegner score, International Knee Documentation Committee (IKDC) score, osteoarthritis rates, limb symmetry index score, and maximal voluntary isometric contraction torque. We were able to identify at least 1 of these values separated by sex in 61 studies. If these outcomes were reported in >1 study in an extractable manner, then they were included in our analysis. Only outcomes of IKDC scores, revision rates, rerupture rates, RTS rates, and contralateral ACL injury rates were reported in >1 study to allow for analysis.
Quality Assessments
We used an assessment of bias table to address the levels of bias within each study included in our study. Studies were ranked as having a low risk, high risk, or unclear risk of bias in 7 different categories. These categories included 2 assessments for selection bias, 1 assessment for performance bias, 1 assessment for detection bias, 2 assessments for attrition bias, and 1 assessment for any other type of bias.
We also used a modified version of the Coleman Methodology Score to assess the quality of the studies included. Each study is given a score based on 10 evaluation standards. A score ≥85 is considered excellent, 70 to 84 is considered good, 50 to 69 is considered moderate, and <50 is considered poor.
Statistical Analysis
Studies that reported postoperative IKDC scores, rerupture rates, and revision rates were included in the meta-analysis. For studies that reported a range instead of an SD for IKDC scores, the SD was estimated by using range divided by 4. Random-effects meta-analysis was utilized. 22 The inverse variance method was used for continuous outcomes, and the Mantel-Haenszel method was used for binary outcomes. The mean difference, along with 95% CI, was calculated for IKDC scores. For the rerupture and revision rates, odds ratios (ORs) and 95% CIs were calculated. Heterogeneity was examined using the I 2 statistic. A high I 2 (>50%) indicated that the studies were inconsistent in what they found. Low I 2 indicated that the studies were consistent with each other. (Version 3.6.3; R Core Team) was used for all statistical analysis. P < .05 was considered significant.
Results
A total of 9594 studies were identified in our initial search; of these, 9129 studies were removed per our study selection and eligibility criteria, leaving 465 studies for full-text review. After the full text was reviewed and assessed, 61 of the studies met all inclusion criteria; however, only 20 of these studies reported outcomes that were also reported in other studies included so that analysis could be performed (ie, 7 studies reported IKDC scores so that analysis could be performed, but only 1 study reported postoperative range of motion, which is why postoperative range of motion is not included in our analysis). Other outcomes for which only 1 study reported sex-based differences were postoperative tibial slope, flexion and extension angles, maximum torque, KT-1000 arthrometer laxity, Tegner score, Lachman score, anterior drawer test, and limb symmetry index.
Of the 61 eligible studies, 20 (33%) reported data in their results in an extractable manner, separated by sex, and thus were included in the meta-analysis. # A total of 57,390 patients were included in these 20 studies (35,935 male, 21,455 female). Figure 1 denotes the study selection process.
Figure 1.
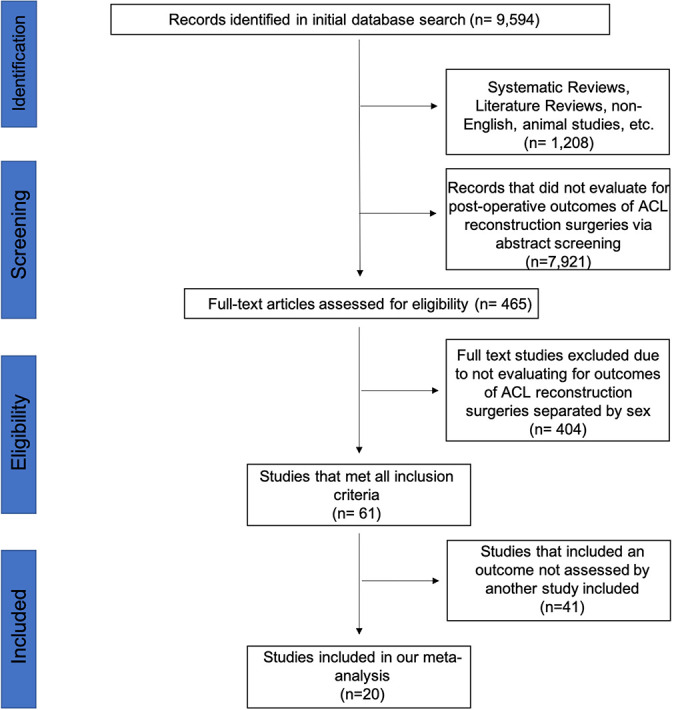
Flow diagram of study selection following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). ACLR, anterior cruciate ligament reconstruction.
Of the 20 studies included in the meta-analysis, 7 included IKDC scores, 14,29 –31,45,60,71 6 included revision rates, 6,16,40,55,63,75 6 included rerupture rate, 15,26,52,62,66,70 and 1 paper included both revision and rerupture rates. 43
IKDC Scores
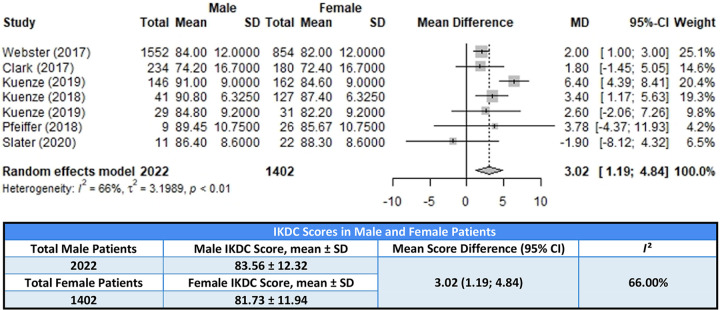
Postoperative IKDC scores were included in 7 studies that were used for statistical analysis. 14,30 –32,46,61,72 Within these studies, scores from 2022 male and 1402 female patients were included. Male patients reported a statistically significant higher postoperative IKDC score with a mean difference of 3.02 (95% CI, 1.19-4.84; P < .01; I 2 = 66%). A forest plot showing the results and weight for the IKDC scores is shown in Figure 2, and the characteristics of the studies are listed in Table 1.
Figure 2.
Forest plot of studies used in meta-analysis of International Knee Documentation Committee (IKDC) scores. MD, mean difference. First Kuenze 2019 is reference 31, second Kuenze 2019 is reference 30.
TABLE 1.
Characteristics of Studies Used in IKDC Score Meta-analysis a
| Study | LOE | Graft Used | Surgical Technique |
|---|---|---|---|
| Webster (2017) 72 | 3 | Hamstring, patellar tendon, allograft | Single bundle |
| Clark (2017) 14 | 2 | Hamstring | NR |
| Kuenze (2019) 30 | 4 | Patellar tendon, hamstring | NR |
| Kuenze (2018) 32 | 4 | Patellar tendon, hamstring, allograft | NR |
| Kuenze (2019) 31 | 4 | Patellar tendon, hamstring, allograft | NR |
| Pfeiffer (2018) 46 | 4 | Patellar tendon autograft | NR |
| Slater (2020) 61 | 4 | Patellar tendon, hamstring, allograft | NR |
a IKDC, International Knee Documentation Committee; LOE, level of evidence; NR, not reported.
Revision Rates
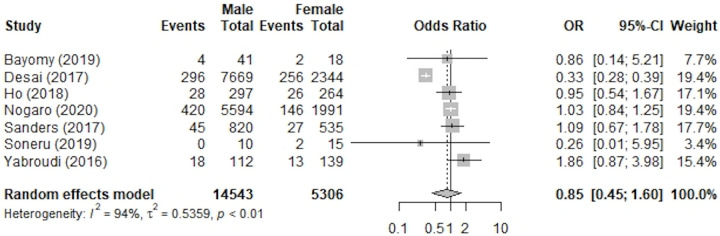
The revision rates of ACLR in both male and female patients were included in 7 studies that were evaluated quantitatively. 6,16,23,40,55,63,75 Overall, data from 19,849 patients (14,543 male and 5,306 female) were included. The overall population had a total of 1283 revisions, which was 6.46% of the study population. There was a total of 811 revisions for male patients from this pool for an incidence rate of revision of 5.58%. For female patients, there was a total of 472 revisions from this pool for an incidence of revision of 8.90%. While male patients did have a lower incidence of revisions from this pool, the rate of revision in male patients as compared with their female counterparts was not statistically significant (OR, 0.85 [95% CI, 0.45-1.60]; P = .61; I 2 = 94%). A forest plot of the results and weight for revision rates is shown in Figure 3, and the characteristics of the studies are listed in Table 2.
Figure 3.
Forest plot of studies included in meta-analysis of revision rates. ACLR, anterior cruciate ligament reconstruction; OR, odds ratio.
Table 2.
Studies Used in Rate of Revision Meta-analysis
| Study | Level of Study | Reported Graft Used | Surgical Technique |
|---|---|---|---|
| Bayomy (2019) 6 | III-Case-Control | Autologous Hamstring Tendon | Transphyseal ACR |
| Desai (2017) 16 | III | Hamstring | Single bundle |
| Ho (2018) 23 | III | Not Specified | Not Specified |
| Nogaro (2020) 40 | III | Not Specified | Not Specified |
| Sanders (2017) 55 | II | Patellar, Hamstring, Allograft | Not Specified |
| Soneru (2019) 63 | II | Achilles tendon bone graft, patellar | Not Specified |
| Yabroudi (2016) 75 | III-Case-Control | Autograft, Allograft, Mixed | Single Bundle, Double Bundle |
Rerupture Rate
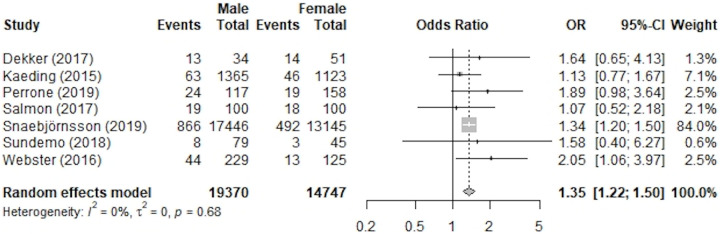
The rerupture rates after ACLR were included in 7 studies that were evaluated quantitatively. 15,26,44,52,62,66,70 A total of 34,117 patients were involved in our analysis (19,370 male and 14,747 female), and a total of 1642 reruptures occurred for an overall incidence of 4.81%. Of the male patients involved in this quantitative analysis, 1037 experienced an ACL rerupture, with an incidence of 5.35%. Comparatively, 605 female patients experienced an ACL rerupture (incidence rate, 4.10%). Based on these results, male patients were significantly more likely to have a rerupture than were female patients, with an OR of 1.35 (95% CI, 1.22-1.50; P < .01; I 2 = 0%). A forest plot showing each study’s results and weight for the rerupture rates is shown in Figure 4, and the studies’ characteristics are listed in Table 3.
Figure 4.
Forest plot of studies included in meta-analysis of rerupture rates. ACLR, anterior cruciate ligament reconstruction; OR, odds ratio.
TABLE 3.
Studies Used in Rate of Rerupture Meta-analysis
| Study | Level of Evidence | Reported Graft Used | Surgical Technique |
|---|---|---|---|
| Dekker (2017) 15 | IV | Patellar, Hamstring, Hamstring autograft and allograft combined | Adult-Type Reconstruction, Vertical Transphyseal Reconstruction, Physeal Preserving |
| Kaeding (2015) 26 | III | Patellar, Hamstring, Allograft | Not Listed |
| Perrone (2019) 44 | IV | Hamstring | Arthroscopic |
| Salmon (2017) 52 | III | Hamstring | Arthroscopic |
| Snaebjörnsson (2019) 62 | II | Hamstring, Patellar | Not Listed |
| Sundemo (2018) 66 | III | Hamstring, Patellar | Transtibial technique |
| Webster (2016) 70 | III | Hamstring | Arthroscopic |
RTS Rate
Reporting of RTS varied greatly depending on whether the rates were separated by patient sex and/or age. In general, average time to RTS varied from as short as 8.3 months to as long as 11.1 months. 11,15,27,41,42 The average RTS rates for both male and female patients combined also varied greatly, with rates reported as low as 63.7% to as high as 96%. 11,15,17,27,41,74
The RTS rates by sex were reported by 8 of the included studies, 10,17,27,29,50,56,71,74 with a total of 3632 patients (2541 male and 1091 female). No formal data analysis could be performed due to the difference in reporting of RTS among studies. The papers included did not consistently report their results based on age, which appears to have a significant effect on RTS rates. 71 However, in the studies that reported rates of RTS, male patients had a higher RTS rate (59.82%) compared with their female counterparts (42.89%).
Contralateral Knee Injuries
The number of contralateral knee injuries were recorded in only 5 studies (n = 17,078 patients) of the 61 included in our quantitative analysis. 40,44,52,66,70 There were 12,276 male patients, of whom 411 had a contralateral ACL injury (incidence, 3.35%). There was a total of 4802 female patients, 197 of whom sustained a contralateral ACL injury (incidence, 4.10%). We were unable to perform a statistical analysis for these results given that 16,125 patients came from a single study. 39
Risk-of-Bias and Quality Assessments
There was a high risk of bias for the majority of papers included in our meta-analysis, as the majority of the included studies were cohort studies (Table 4). The quality of the studies as assessed via the modified Coleman Methodology Score indicated that all of the included studies fell in the moderate and poor scoring categories (Table 5).
TABLE 4.
Risk-of-Bias Assessment a
| Lead Author (Year) | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Attrition Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Bayomy (2019) 6 | • | • | • | • | • | • | • |
| Clark (2017) 14 | • | • | • | • | • | • | • |
| Dekker (2017) 15 | • | • | • | • | • | • | • |
| Desai (2017) 16 | • | • | • | • | • | • | • |
| Kaeding (2015) 26 | • | • | • | • | • | • | • |
| Kuenze (2019) 30 | • | • | • | • | • | • | • |
| Kuenze (2019) 31 | • | • | • | • | • | • | • |
| Kuenze (2018) 32 | • | • | • | • | • | • | • |
| Nogaro (2020) 40 | • | • | • | • | • | • | • |
| Perrone (2019) 44 | • | • | • | • | • | • | • |
| Pfeiffer (2018) 46 | • | • | • | • | • | • | • |
| Salmon (2017) 52 | • | • | • | • | • | • | • |
| Sanders (2017) 55 | • | • | • | • | • | • | • |
| Slater (2020) 61 | • | • | • | • | • | • | • |
| Snaebjörnsson (2019) 62 | • | • | • | • | • | • | • |
| Soneru (2019) 63 | • | • | • | • | • | • | • |
| Sundemo (2018) 66 | • | • | • | • | • | • | • |
| Webster (2016) 70 | • | • | • | • | • | • | • |
| Webster (2017) 72 | • | • | • | • | • | • | • |
| Yabroudi (2016) 75 | • | • | • | • | • | • | • |
a Red indicates a high risk of bias, green represents a low risk of bias, and yellow represents an unclear risk of bias in each category.
TABLE 5.
Modified Coleman Methodology Scores a
| Lead Author (Year) | Study Size | Mean Follow-up | No. of Different Versions (of Implant) Used | Type of Study | Description of Indications/ Diagnosis |
Description of Surgical Technique | Survivorship Analysis | Outcome Criteria | Outcome Assessment | Subject Selection Process | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayomy (2019) 6 | 7 | 4 | 10 | 0 | 5 | 5 | 0 | 7 | 5 | 5 | 48 |
| Clark (2017) 14 | 10 | 0 | 10 | 0 | 5 | 3 | 0 | 7 | 8 | 10 | 53 |
| Dekker (2017) 15 | 10 | 4 | 0 | 0 | 5 | 3 | 0 | 7 | 3 | 5 | 37 |
| Desai (2017) 16 | 10 | 7 | 0 | 0 | 5 | 3 | 0 | 5 | 4 | 0 | 34 |
| Kaeding (2015) 26 | 10 | 4 | 0 | 0 | 5 | 0 | 0 | 5 | 4 | 5 | 33 |
| Kuenze (2019) 30 | 10 | 4 | 0 | 0 | 5 | 0 | 0 | 7 | 5 | 5 | 36 |
| Kuenze (2019) 31 | 7 | 0 | 0 | 0 | 5 | 0 | 0 | 7 | 12 | 5 | 36 |
| Kuenze (2018) 32 | 10 | 4 | 0 | 0 | 5 | 0 | 0 | 5 | 8 | 5 | 37 |
| Nogaro (2020) 40 | 10 | 10 | 0 | 0 | 5 | 0 | 0 | 5 | 4 | 5 | 39 |
| Perrone (2019) 44 | 10 | 7 | 10 | 0 | 5 | 5 | 0 | 5 | 5 | 5 | 52 |
| Pfeiffer (2018) 46 | 4 | 0 | 0 | 0 | 5 | 0 | 0 | 2 | 5 | 5 | 21 |
| Salmon (2017) 52 | 10 | 10 | 10 | 0 | 5 | 5 | 0 | 7 | 11 | 5 | 63 |
| Sanders (2017) 55 | 10 | 10 | 0 | 0 | 5 | 0 | 0 | 5 | 5 | 5 | 40 |
| Slater (2020) 61 | 7 | 0 | 0 | 0 | 5 | 0 | 0 | 2 | 5 | 5 | 24 |
| Snaebjörnsson (2019) 62 | 10 | 4 | 0 | 10 | 5 | 0 | 0 | 7 | 9 | 5 | 50 |
| Soneru (2019) 63 | 4 | 4 | 0 | 0 | 5 | 0 | 0 | 5 | 8 | 5 | 31 |
| Sundemo (2018) 66 | 10 | 10 | 0 | 0 | 5 | 5 | 0 | 7 | 9 | 5 | 51 |
| Webster (2016) 70 | 10 | 7 | 10 | 0 | 5 | 5 | 0 | 7 | 5 | 5 | 54 |
| Webster (2017) 72 | 10 | 7 | 10 | 0 | 5 | 0 | 0 | 5 | 5 | 5 | 47 |
| Yabroudi (2016) 75 | 10 | 4 | 0 | 0 | 5 | 0 | 0 | 5 | 5 | 5 | 34 |
a A score ≥85 points is considered excellent, 70-84 is considered good, 50-69 is considered moderate, and anything <50 is considered poor.
Discussion
To our knowledge, this is the most recent and in-depth study on the effect of patient sex on outcomes after ACLR. We found that male patients reported higher levels of IKDC scores after ACLR but at the same time had a higher rate of rerupture. We found no difference in the rate of revision between male and female patients. We also found that male patients had a higher rate of RTS, but this was influenced by age, making interpretation of the data difficult.
In this study, the differences based on sex were dependent upon the metric used, with no clear-cut, overall difference noted. This theme was found to be relatively consistent with previous papers that evaluated outcomes of ACLR. Tan et al 68 found that, in the majority of the measures included, men and women had statistically similar results. This included measures such as the anterior drawer test, Lachman test, pivot-shift test, single-leg hop test, quadriceps/hamstring testing, flexion/extension loss, and IKDC knee examination scores. However, they found that female patients had inferior outcomes in instrumented laxity (standardized mean difference [SMD], 0.24 [95% CI, 0.11 to 0.37]), revision rate (relative risk, 1.15 [95%CI, -0.49 to -0.24]), Lysholm score (SMD, -0.33 [95% CI, -0.55 to -0.11]), Tegner Activity score (SMD, -0.37 [95% CI, -0.49 to -0.24]), and incidence of not returning to sport (relative risk, 1.12 [95% CI, 1.04 to 1.21]). The systematic review by Ryan et al 51 looking at ACLR outcomes by sex found that both male and female patients had significantly similar graft rupture risk, contralateral ACL rupture risk, knee laxity, instrumented laxity, and patient-reported outcomes.
Of the 7 studies included in our analysis of IKDC scores, 3 reported no statistical difference, 1 reported that male patients had higher scores, 1 reported that female patients had higher scores, and 2 did not complete a statistical analysis on their results. 14,30 –32,46,61,72 A report published by the AOSSM Outcomes Task Force recommended that a minimal clinically significant difference is between 3.19 and 16.7. 25 Therefore, while our finding of male patients reporting higher IKDC scores than female patients is statistically significant, it would not be clinically significant based on the AOSSM criterion. The IKDC is still regarded as a trustworthy measure of quality of life postoperatively, and these results may still be a useful tool for clinicians when predicting outcomes and counseling patients after ACLR.
Of the 7 studies included in our analysis of IKDC scores, 3 reported no statistical difference, 1 reported that male patients had higher scores, 1 reported that female patients had higher scores, and 2 did not complete a statistical analysis on their results. 14,30 –32,46,61,72 A report published by the AOSSM Outcomes Task Force recommended that a minimal clinically significant difference is between 3.19 and 16.7. 25 Therefore, while our finding of male patients reporting higher IKDC scores than female patients is statistically significant, it would not be clinically significant based on the AOSSM criterion. The IKDC is still regarded as a trustworthy measure of quality of life postoperatively, and these results may still be a useful tool for clinicians when predicting outcomes and counseling patients after ACLR.
Another significant finding of our study was that men and women did not differ significantly in the number of revision surgeries they underwent. Of the 7 studies included in our study, 5 found no statistical difference between sexes in regard to revisions, and the other 2 did not complete a statistical analysis on their results. 6,16,40,44,55,63,75 This is similar to the results of a previous systematic review of Danish, Sweden, and Norwegian registries that found no significant difference in revision surgeries between male and female patients. 16,19,34,45,48 Interestingly, a study that looked at the Kaiser Permanente registry, which included 17,682 patients, reported that male patients have a 38% increased risk for revision after ACLR (95% CI, 1.14-1.69). 37 It appears that there is no general consensus in the literature on the effect that sex plays on the rates of revision after ACLR. One potential explanation for this observation is that it appears that the type of sport played postoperatively may affect the incidence of revision. 42,44,56 A study by Snaebjörnsson et al 62 showed that soccer was the most common sport associated with primary ACL injuries and recurrent injuries. It has also been reported that revision rates for soccer players are as high as 28.7%, which is in contrast to our overall revision rate for both sexes of 6.5%. 56 There may be other factors, in addition to type of sport played, such as age of patient, time to RTS, and rehabilitation protocol, that may act as covariables affecting revision rates.
The final finding of our meta-analysis that male patients had a higher rerupture rate than did female patients is similar to that of other studies that have reported that male patients are at a higher risk for graft injury than are female patients. 8 Of the 7 studies included in our statistical analysis, 5 did not report a statistically significant difference in rerupture rates, 1 reported that rerupture rates were higher in male patients, and 1 study did not conduct a statistical analysis. 15,26,44,52,62,66,70 Schilaty et al 57 also showed that risk for second ACL injuries differs by sex and age, reporting that they occur at a higher rate in female patients aged <25 years and a higher rate in male patients aged 26 to 45 years. Other studies that have looked at causes for graft ruptures have found that although female patients do have greater laxity post-ACLR, sex does not play a role in rerupture rates. 44,53,54 As with revision surgery rates, comorbidities in addition to sex likely play a role in the risk for rerupture.
Unfortunately, due to the variable methods of reporting RTS rates by studies, we were unable to perform any formal statistical analyses. Reporting of RTS varied greatly, depending on whether the rates were separated by patient sex and/or age. The rate of RTS assessed by patient sex varied significantly, which may have been due to the large range in number of patients, as well as numbers of men and women, included in each study. Rates of RTS for male and female patients have been reported to be as low as 42.39% and 5.55%, respectively. 10 However, Cheecharern 10 included only 18 female and 92 male participants in their study, which may explain the low percentage. Excluding this study, the results of RTS rates were more consistent, with the lowest rates for male and female patients being 44.6% and 30.4%, respectively. 50,71 Due to our inability to perform a statistical analysis, caution must be used when interpreting these results.
The differences in RTS rates between male and female patients may be affected by many confounding factors. There may be a significant difference in effect of age on RTS rates between male and female patients: in 2017, Webster reported that male patients younger than 35 years of age had a higher RTS rate than did female patients within the same age group. 72 Conversely, men older than 35 years of age had a lower RTS rate than did women within the same age group. Unfortunately, the other studies did not separate RTS rates by both patient sex and age, making it difficult to compare the results, although highlighting an area for future research.
Another significant issue is the rate of contralateral ACL injuries after ACLR. We found that the rates of contralateral ACL injuries were similar between the sexes, with male patients having a rate of 3.35% and female patients having a rate of 4.10%. The reliability of these results is questionable, however, due to the fact that 96% of the male and 91% of the female patients from the statistical analysis came from 1 study. 39 However, other studies have also documented that female patients experience a higher rate of contralateral ACL tears. 39,43
Limitations
There are several limitations to this study. As mentioned previously, there is a fairly large risk of bias in the studies included due to the majority being cohort studies. The assessment of quality using the Coleman score also shows that many studies were not of the highest research quality, again due to the nature of studies included. Randomized controlled trials present the highest quality studies, but they are not logical for the nature of our research. Another limitation is that some studies did not specifically state their inclusion criteria, which makes it difficult to evaluate whether or not they should be included. For example, if a study did not explicitly state whether they included or excluded patients with concomitant ligamentous injuries, that study was included. Finally, many studies reported their data in different ways and included some descriptive data that other studies did not. This makes it difficult to analyze the data on a larger scale. There were many data points that we would have liked to have analyzed but were unable to analyze due to lack of datapoints or the variance in reporting methods. This creates the risk of missing significant data about ACLR outcomes.
Conclusion
This study assessed the more recent literature to determine the effect of sex on outcomes of ACLRs. One of the primary concerns noted from this review was that only about one-third of studies that met initial inclusion criteria presented their data in an extractable format separated by sex. Given the differences in ACL injury incidence and intermediate and long-term outcomes of ACL injuries, it is crucial that future studies include assessment of data based on sex. Similar to the paper published by Tan et al, 68 we were not able to demonstrate a significant difference between sexes for the majority of the outcomes that we studied, potentially related to the paucity of data. Further studies are needed to evaluate the effect of patient sex, age, graft, and sport played on outcomes after ACLR to increase the breadth of knowledge in this area and to better counsel all patients on anticipated results.
Footnotes
Final revision submitted November 12, 2021; accepted November 29, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: M.L.V. has received education payments from DePuy and Titan Surgical and hospitality payments from Stryker and Zimmer Biomet. J.P.S. has received education payments from Arthrex/Titan Surgical, consulting fees from Vericel, and hospitality payments from Zimmer Biomet. M.K.M has received education payments from Arthrex, Alon Medical Technology, and QuestMedical; nonconsulting fees from Arthrex; and hospitality payments from Zimmer Biomet. B.G.V. has received education payments from Titan Surgical, consulting fees from DePuy, and hospitality payments from Smith & Nephew and Stryker. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Agel J, Rockwood T, Klossner D. Collegiate ACL injury rates across 15 sports. Clin J Sport Med. 2016;26(6):518–523. doi:10.1097/JSM.0000000000000290 [DOI] [PubMed] [Google Scholar]
- 2. Araujo FX, Castro MP, Schell MS, et al. Twenty-year follow-up study comparing operative versus nonoperative treatment of anterior cruciate ligament ruptures in high-level athletes: letter to the editor. Am J Sports Med. 2018;46(11):NP55–NP57. doi:10.1177/0363546518788318 [DOI] [PubMed] [Google Scholar]
- 3. Ardern CL, Sonesson S, Forssblad M, Kvist J. Comparison of patient-reported outcomes among those who chose ACL reconstruction or non-surgical treatment. Scand J Med Sci Sports. 2017;27(5):535–544. doi:10.1111/sms.12707 [DOI] [PubMed] [Google Scholar]
- 4. Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34(2):86–92. [PMC free article] [PubMed] [Google Scholar]
- 5. Baldassarri M, Perazzo L, Ghinelli D, Ricciarelli M, Pilla F, Buda R. Return to sport after ACL surgery: a comparison between two different reconstructive techniques. J Knee Surg. 2019;32(6):513–518. doi:10.1055/s-0038-1653948 [DOI] [PubMed] [Google Scholar]
- 6. Bayomy AF, Bompadre V, Schmale GA. The impact of transphyseal anterior cruciate ligament reconstruction on lower extremity growth and alignment. Arthroscopy. 2019;35(3):940–949. [DOI] [PubMed] [Google Scholar]
- 7. Biswal UK, Balaji G, Nema S, Poduval M, Menon J, Patro DK. Correlation of tunnel widening and tunnel positioning with short-term functional outcomes in single-bundle anterior cruciate ligament reconstruction using patellar tendon versus hamstring graft: a prospective study. Eur J Orthop Surg Traumatol. 2016;26(6):647–655. doi:10.1007/s00590-016-1809-4 [DOI] [PubMed] [Google Scholar]
- 8. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985–1992. doi:10.1177/0363546512454414 [DOI] [PubMed] [Google Scholar]
- 9. Buller LT, Best MJ, Baraga MG, Kaplan LD. Trends in anterior cruciate ligament reconstruction in the United States. Orthop J Sports Med. 2015;3(1):232596711456366. doi:10.1177/2325967114563664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheecharern S. Return to sport and knee functional scores after anterior cruciate ligament reconstruction: 2 to 10 years’ follow-up. Asia Pac J Sport Med Arthrosc Rehabil Technol. 2018;12:22–29. doi:10.1016/j.asmart.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chicorelli AM, Micheli LJ, Kelly M, Zurakowski D, MacDougall R. Return to sport after anterior cruciate ligament reconstruction in the skeletally immature athlete. Clin J Sport Med. 2016;26(4):266–271. doi:10.1097/jsm.0000000000000275 [DOI] [PubMed] [Google Scholar]
- 12. Cimino F, Volk BS, Setter D. Anterior cruciate ligament injury: diagnosis, management, and prevention. Am Fam Physician . 2010;82(8):917–922. http://www.ncbi.nlm.nih.gov/pubmed/20949884 [PubMed] [Google Scholar]
- 13. Cinque ME, Chahla J, Moatshe G, et al. Outcomes and complication rates after primary anterior cruciate ligament reconstruction are similar in younger and older patients. Orthop J Sports Med. 2017;5(10):2325967117729659. doi:10.1177/2325967117729659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark RA, Bell SW, Feller JA, Whitehead TS, Webster KE. Standing balance and inter-limb balance asymmetry at one year post primary anterior cruciate ligament reconstruction: sex differences in a cohort study of 414 patients. Gait Posture. 2017;52:318–324. doi:10.1016/j.gaitpost.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 15. Dekker TJ, Godin JA, Dale KM, Garrett WE, Taylor DC, Riboh JC. Return to sport after pediatric anterior cruciate ligament reconstruction and its effect on subsequent anterior cruciate ligament injury. J Bone Joint Surg Am. 2017;99(11):897–904. [DOI] [PubMed] [Google Scholar]
- 16. Desai N, Andernord D, Sundemo D, et al. Revision surgery in anterior cruciate ligament reconstruction: a cohort study of 17,682 patients from the Swedish National Knee Ligament Register. Knee Surg Sports Traumatol Arthrosc. 2017;25(5):1542–1554. doi:10.1007/s00167-016-4399-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards PK, Ebert JR, Joss B, et al. Patient characteristics and predictors of return to sport at 12 months after anterior cruciate ligament reconstruction: the importance of patient age and postoperative rehabilitation. Orthop J Sports Med. 2018;6(9):23259 67118797575. doi:10.1177/2325967118797575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Failla MJ, Logerstedt DS, Grindem H, et al. Does extended preoperative rehabilitation influence outcomes 2 years after ACL reconstruction? A comparative effectiveness study between the MOON and Delaware-Oslo ACL cohorts. Am J Sports Med. 2016;44(10):2608–2614. doi:10.1177/0363546516652594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Faunø P, Rahr-Wagner L, Lind M. Risk for revision after anterior cruciate ligament reconstruction is higher among adolescents. Orthop J Sports Med. 2014;2(10):2325967114552405. doi:10.1177/2325967114552405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geffroy L, Lefevre N, Thevenin-Lemoine C, et al. Return to sport and re-tears after anterior cruciate ligament reconstruction in children and adolescents. Orthop Traumatol Surg Res. 2018;104(8 suppl):S183–S188. doi:10.1016/j.otsr.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 21. Gordon M, Steiner M. Anterior cruciate ligament injuries. In: Garrick JE, ed. Orthopaedic Knowledge Update Sports Medicine. 3rd ed. American Academy of Orthopaedic Surgeons; 2004:169–170. [Google Scholar]
- 22. Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ho B, Edmonds EW, Chambers HG, Bastrom TP, Pennock AT. Risk factors for early ACL reconstruction failure in pediatric and adolescent patients: A review of 561 cases. J Pediatr Orthop 2018;38(7):388–392. [DOI] [PubMed] [Google Scholar]
- 24. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in National Collegiate Athletic Association Division I soccer athletes: a study of the Southeastern Conference. Am J Sports Med. 2016;44(2):433–439. [DOI] [PubMed] [Google Scholar]
- 25. Irrgang J. Summary of Clinical Outcome Measures for Sports-Related Knee Injuries; 2012. Accessed June 2020. https://www.sportsmed.org/AOSSMIMIS/members/downloads/research/ClinicalOutcomeMeasuresKnee.pdf
- 26. Kaeding CC Pedroza AD Reinke EK Huston LJ; MOON Consortium; Spindler KP. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 Primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43(7):1583–1590. doi:10.1177/0363546515578836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. King E, Richter C, Jackson M, et al. Factors influencing return to play and second anterior cruciate ligament injury rates in level 1 athletes after primary anterior cruciate ligament reconstruction: 2-year follow-up on 1432 reconstructions at a single center. Am J Sports Med. 2020;48(4):812–824. doi:10.1177/0363546519900170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kondo E, Yasuda K, Azuma H, Tanabe Y, Yagi T. Prospective clinical comparisons of anatomic double-bundle versus single-bundle anterior cruciate ligament reconstruction procedures in 328 consecutive patients. Am J Sports Med. 2008;36(9):1675-1687. doi:10.1177/0363546508317123 [DOI] [PubMed] [Google Scholar]
- 29. Kostyun RO, Burland JP, Kostyun KJ, Milewski MD, Nissen CW. Male and female adolescent athletes’ readiness to return to sport after anterior cruciate ligament injury and reconstruction. Clin J Sport Med. 2021;31(4):383–387. doi:10.1097/jsm.0000000000000751 [DOI] [PubMed] [Google Scholar]
- 30. Kuenze C, Lisee C, Birchmeier T, et al. Sex differences in quadriceps rate of torque development within 1 year of ACL reconstruction. Phys Ther Sport. 2019;38:36–43. doi:10.1016/j.ptsp.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 31. Kuenze C, Pietrosimone B, Lisee C, et al. Demographic and surgical factors affect quadriceps strength after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(3):921–930. [DOI] [PubMed] [Google Scholar]
- 32. Kuenze CM, Trigsted S, Lisee C, Post E, Bell DR. Sex differences on the landing error scoring system among individuals with anterior cruciate ligament reconstruction. J Athl Train. 2018;53(9):837–843. doi:10.4085/1062-6050-459-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JK, Lee S, Lee MC. Outcomes of anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(9):2323–2329. doi:10.1177/0363546516650666 [DOI] [PubMed] [Google Scholar]
- 34. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40(7):1551–1557. doi:10.1177/0363546512446000 [DOI] [PubMed] [Google Scholar]
- 35. Lind M, Nielsen TG, Soerensen OG, Mygind-Klavsen B, Fauno P. Quadriceps tendon grafts does not cause patients to have inferior subjective outcome after anterior cruciate ligament (ACL) reconstruction than do hamstring grafts: a 2-year prospective randomised controlled trial. Br J Sports Med. 2020;54(3):183–187. doi:10.1136/bjsports-2019-101000 [DOI] [PubMed] [Google Scholar]
- 36. Maletis GB, Chen J, Inacio MCS, Love RM, Funahashi TT. Increased risk of revision after anterior cruciate ligament reconstruction with bone-patellar tendon-bone allografts compared with autografts. Am J Sports Med. 2017;45(6):1333–1340. [DOI] [PubMed] [Google Scholar]
- 37. Maletis GB, Inacio MCS, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641–647. doi:10.1177/0363546514561745 [DOI] [PubMed] [Google Scholar]
- 38. Mohtadi N, Barber R, Chan D, Paolucci EO. Complications and adverse events of a randomized clinical trial comparing 3 graft types for ACL reconstruction. Clin J Sport Med. 2016;26(3):182–189. doi:10.1097/jsm.0000000000000202 [DOI] [PubMed] [Google Scholar]
- 39. Nawasreh Z, Adams G, Pryzbylkowski O, Logerstedt D. Influence of patient demographics and graft types on ACL second injury rates in ipsilateral versus contralateral knees: a systematic review and meta-analysis. Int J Sports Phys Ther. 2018;13(4):561–574. doi:10.26603/ijspt20180561 [PMC free article] [PubMed] [Google Scholar]
- 40. Nogaro MC, Abram SGF, Alvand A, Bottomley N, Jackson WFM, Price A. Paediatric and adolescent anterior cruciate ligament reconstruction surgery. Bone Joint J. 2020;102(2):239–245. [DOI] [PubMed] [Google Scholar]
- 41. Nwachukwu BU, Voleti PB, Berkanish P, et al. Return to play and patient satisfaction after ACL reconstruction: study with minimum 2-year follow-up. J Bone Joint Surg Am. 2017;99(9):720–725. doi:10.2106/jbjs.16.00958 [DOI] [PubMed] [Google Scholar]
- 42. Paterno MV, Huang B, Thomas S, Hewett TE, Schmitt LC. Clinical factors that predict a second ACL injury after ACL reconstruction and return to sport: preliminary development of a clinical decision algorithm. Orthop J Sports Med. 2017;5(12):2325967117745279. doi:10.1177/2325967117745279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paterno MV, Rauh M, Schmitt LC, Ford KR, Hewett TE. Incidence of second anterior cruciate ligament (ACL) injury 2 years after primary ACL reconstruction and return to sport. Orthop J Sports Med. 2013;1(4 suppl):2325967113S00002. doi:10.1177/2325967113S00002 [Google Scholar]
- 44. Perrone GS, Webster KE, Imbriaco C, et al. Risk of secondary ACL injury in adolescents prescribed functional bracing after ACL reconstruction. Orthop J Sports Med. 2019;7(11):2325967119879880. doi:10.1177/2325967119879880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Persson A, Fjeldsgaard K, Gjertsen J-E, et al. Increased risk of revision with hamstring tendon grafts compared with patellar tendon grafts after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(2):285–291. doi:10.1177/0363546513511419 [DOI] [PubMed] [Google Scholar]
- 46. Pfeiffer SJ, Blackburn JT, Luc-Harkey B, et al. Peak knee biomechanics and limb symmetry following unilateral anterior cruciate ligament reconstruction: associations of walking gait and jump-landing outcomes. Clin Biomech (Bristol, Avon). 2018;53:79–85. doi:10.1016/j.clinbiomech.2018.01.020 [DOI] [PubMed] [Google Scholar]
- 47. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505–511. [PubMed] [Google Scholar]
- 48. Rahardja R, Zhu M, Love H, Clatworthy MG, Monk AP, Young SW. Factors associated with revision following anterior cruciate ligament reconstruction: a systematic review of registry data. Knee. 2020;27(2):287–299. doi:10.1016/j.knee.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 49. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents. Am J Sports Med. 2014;42(11):2769–2776. doi:10.1177/0363546513510889 [DOI] [PubMed] [Google Scholar]
- 50. Rodriguez-Roiz JM, Caballero M, Ares O, Sastre S, Lozano L, Popescu D. Return to recreational sports activity after anterior cruciate ligament reconstruction: a one- to six-year follow-up study. Arch Orthop Trauma Surg. 2015;135(8):1117–1122. [DOI] [PubMed] [Google Scholar]
- 51. Ryan J, Magnussen RA, Cox CL, Hurbanek JG, Flanigan DC, Kaeding CC. ACL reconstruction: do outcomes differ by sex? J Bone Joint Surg Am. 2014;96(6):507–512. doi:10.2106/JBJS.M.00299 [DOI] [PubMed] [Google Scholar]
- 52. Salmon L, Heath E, Akrawi H, Roe J, Pinczewski L. 20 Year outcomes of ACL reconstruction with hamstring tendon autograft: the catastrophic effect of age and posterior tibial slope. J Sci Med Sport. 2017;20:79. doi:10.1016/j.jsams.2017.09.354 [DOI] [PubMed] [Google Scholar]
- 53. Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(8):948–957. doi:10.1016/j.arthro.2005.04.110 [DOI] [PubMed] [Google Scholar]
- 54. Salmon LJ, Refshauge KM, Russell VJ, Roe JP, Linklater J, Pinczewski LA. Gender differences in outcome after anterior cruciate ligament reconstruction with hamstring tendon autograft. Am J Sports Med. 2006;34(4):621–629. doi:10.1177/0363546505281806 [DOI] [PubMed] [Google Scholar]
- 55. Sanders TL, Pareek A, Hewett TE, et al. Long-term rate of graft failure after ACL reconstruction: a geographic population cohort analysis. Knee Surg Sport Traumatol Arthrosc. 2017;25(1):222–228. doi:10.1007/s00167-016-4275-y [DOI] [PubMed] [Google Scholar]
- 56. Sandon A, Werner S, Forssblad M. Factors associated with returning to football after anterior cruciate ligament reconstruction. Knee Surg Sport Traumatol Arthrosc. 2015;23(9):2514–2521. doi:10.1007/s00167-014-3023-4 [DOI] [PubMed] [Google Scholar]
- 57. Schilaty ND, Nagelli C, Bates NA, et al. Incidence of second anterior cruciate ligament tears and identification of associated risk factors from 2001 to 2010 using a geographic database. Orthop J Sports Med. 2017;5(8):2325967117724196. doi:10.1177/2325967117724196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Seng K, Appleby D, Lubowitz JH. Operative versus nonoperative treatment of anterior cruciate ligament rupture in patients aged 40 years or older: an expected-value decision analysis. Arthroscopy. 2008;24(8):914–920. doi:10.1016/j.arthro.2008.01.021 [DOI] [PubMed] [Google Scholar]
- 59. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. Am J Sports Med. 1991;19(4):332–336. doi:10.1177/036354659101900402 [DOI] [PubMed] [Google Scholar]
- 60. Sims M, Mulcahey MK. Sex-specific differences in psychological response to injury and return to sport following ACL reconstruction. JBJS Rev. 2018;6(7):e9. [DOI] [PubMed] [Google Scholar]
- 61. Slater LV, Blemker SS, Hertel J, Saliba SA, Weltman AL, Hart JM. Sex affects gait adaptations after exercise in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2020;71:189–195. doi:10.1016/j.clinbiomech.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 62. Snaebjörnsson T, Svantesson E, Sundemo D, et al. Young age and high BMI are predictors of early revision surgery after primary anterior cruciate ligament reconstruction: a cohort study from the Swedish and Norwegian knee ligament registries based on 30,747 patients. Knee Surg Sport Traumatol Arthrosc. 2019;27(11):3583–3591. doi:10.1007/s00167-019-05487-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Soneru A, Sarwark JF. Survivorship of allograft ACL reconstruction in adolescent patients. J Orthop. 2019;16(1):11–13. doi:10.1016/j.jor.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steadman JR, Matheny LM, Hurst JM, Briggs KK. Patient-centered outcomes and revision rate in patients undergoing ACL reconstruction using bone-patellar tendon-bone autograft compared with bone-patellar tendon—bone allograft: a matched case-control study. Arthroscopy. 2015;31(12):2320–2326. doi:10.1016/j.arthro.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 65. Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data. JAMA. 2015;313(16):1657. doi:10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 66. Sundemo D, Sernert N, Kartus J, et al. Increased postoperative manual knee laxity at 2 years results in inferior long-term subjective outcome after anterior cruciate ligament reconstruction. Am J Sports Med. 2018;46(11):2632–2645. doi:10.1177/0363546518786476 [DOI] [PubMed] [Google Scholar]
- 67. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41–50. doi:10.5435/JAAOS-21-01-41 [DOI] [PubMed] [Google Scholar]
- 68. Tan SH, Lau BP, Khin LW, Lingaraj K. The importance of patient sex in the outcomes of anterior cruciate ligament reconstructions: a systematic review and meta-analysis. Am J Sports Med. 2016;44(1):242–254. doi:10.1177/0363546515573008 [DOI] [PubMed] [Google Scholar]
- 69. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men’s professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744–750. doi:10.1136/bjsports-2015-095952 [DOI] [PubMed] [Google Scholar]
- 70. Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(11):2827–2832. [DOI] [PubMed] [Google Scholar]
- 71. Webster KE, Feller JA. Return to level I sports after anterior cruciate ligament reconstruction: evaluation of age, sex, and readiness to return criteria. Orthop J Sports Med. 2018;6(8):2325967118788045. doi:10.1177/2325967118788045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Webster KE, Feller JA. Younger patients and men achieve higher outcome scores than older patients and women after anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2017;475(10):2472–2480. doi:10.1007/s11999-017-5418-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Webster KE, Feller JA, Hartnett N, Leigh WB, Richmond AK. Comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction: a 15-year follow-up of a randomized controlled trial. Am J Sports Med. 2016;44(1):83–90. doi:10.1177/0363546515611886 [DOI] [PubMed] [Google Scholar]
- 74. Webster KE, Feller JA, Whitehead TS, Myer GD, Merory PB. Return to sport in the younger patient with anterior cruciate ligament reconstruction. Orthop J Sports Med. 2017;5(4):23259 67117703399. doi:10.1177/2325967117703399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yabroudi MA, Björnsson H, Lynch AD, et al. Predictors of revision surgery after primary anterior cruciate ligament reconstruction. Orthop J Sports Med. 2016;4(9):2325967116666039. doi:10.1177/2325967116666039 [DOI] [PMC free article] [PubMed] [Google Scholar]