Abstract
The fungus Fusarium sacchari was isolated repeatedly from the blood of an immunosuppressed host. The infection was treated successfully with a small dose of amphotericin B. The strain was resistant to this antifungal in vitro. MICs and minimum fungicidal concentrations of six antifungals for the clinical isolate are provided. To our knowledge, this is the first report involving this fungus in a case of fungemia.
Systemic infections due to Fusarium species are increasingly reported in immunosuppressed patients. The majority of these infections have a very poor outcome, especially if the host defenses do not recover. However, the pattern of infection as well as the prognosis may vary according to the species involved. In this paper, we report the first known case of fungemia due to Fusarium sacchari.
Case report.
A 40-year-old man received a cadaveric renal transplant. During surgery, there was an accidental rupture of the bladder and a drain was inserted into the bladder. The patient was started on corticosteroids and azathioprine. A few days later, acute graft rejection was diagnosed and anti-OKT3 was added to the immunosuppressive therapy. On the 11th postoperative day, the patient developed a low-grade fever and leukocytosis. Three blood samples (10 ml each) were taken from peripheral veins at intervals of 15 min. Each blood culture sample was processed as follows. Each of two vented aerobic bottles containing 45 ml of brain heart infusion broth received 5 ml of blood from one sample. The bottles were incubated at 37°C, and after 6 to 24 h of incubation, blind subcultures were made. For the subculture, we used a biocontainment hood to inoculate plates of blood agar. New subcultures were performed only if an alteration in the broth was observed (hemolysis, turbidity, etc.). Both the bottles and the plates were produced in-house following strict quality control measures regarding sterility. After 5 days of incubation, two bottles were turbid and subcultures were performed. The subcultures were made by taking 0.5 ml of the liquid from the bottle with disposable syringes and inoculating it into four tubes containing one of the following solid media: brain heart infusion agar (Merck KgaA, Darmstadt, Germany), Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich.), mycobiotic agar (Difco), and Niger seed agar. Fusarium colonies grew abundantly in all subcultures. They formed numerous confluent colonies, with more than 10 colonies in each tube. The patient had no central venous catheter, was well, and had no skin lesions or other complaints. On the 24th postoperative day, two blood samples were taken (15 min apart) from the peripheral veins; the four blood culture bottles were positive for the same Fusarium sp. A sample of urine was taken for culture and was negative. The patient's creatinine was checked and found to be 11 mg/dl, allowing us to start with a small dose of amphotericin B (0.5 mg/kg of body weight daily). During his course of treatment, the patient remained afebrile, with no evidence of deep-seated infection. Amphotericin B was discontinued after two other sets of blood cultures were negative. A cumulative dose of 500 mg of amphotericin B was administered. The patient was discharged.
Mycological study and diagnosis.
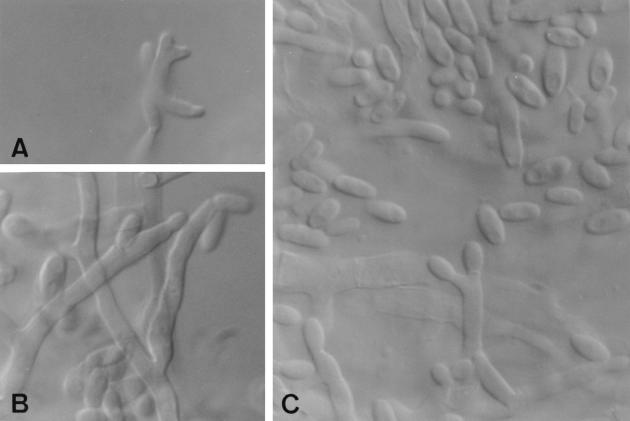
Isolates from different bottles were cultured on potato dextrose agar and oatmeal agar at 25°C in the dark. Colonies on oatmeal agar attained a diameter of 58 to 60 mm after 7 days. They were flat and white, with sparse aerial mycelium. On potato dextrose agar over the same incubation period, the colonies reached 57 to 60 mm in diameter and were densely cottony, white at first but soon becoming pastel red at the center and with whitish aerial mycelium and dark violet substrate mycelium towards the edges. The reverse was brownish red to violet brown. The isolates did not develop macroconidia in any medium, but microconidia were abundant. They were produced in false heads from prostrate conidiophores with mono- and polyphialides (Fig. 1) which measured 10 to 23 μm by 2.8 to 4 μm. Microconidia were oval, ellipsoidal, more or less cylindrical or allantoid, and commonly aseptate, measuring 5 to 11 μm by 2 to 3.5 μm (Fig. 1B and C), or very rarely septate (one to two septa), measuring 11 to 14 μm by 2.8 to 4 μm. A representative isolate of the strain is maintained in the mycology laboratory at the Faculty of Medicine in Reus, University Rovira i Virgili, Tarragona, Spain, as FMR 6487.
FIG. 1.
Fusarium sacchari FMR 6487. (A) Polyphialide; (B and C) microconidia and mono- and polyphialides. Magnification by Nomarski optics, ×1,600.
Because of the abundance of short and lateral monophialides, the isolate was first identified as Fusarium oxysporum, but due to the absence of chlamydospores and the presence of polyphialides, it was definitively identified as F. sacchari, mainly on the basis of the criteria of Gerlach and Nirenberg (3) and Nirenberg and O'Donnell (7). This fungus is characterized by its vinaceous to violet colonies and, microscopically, by producing microconidia in false heads from mono- and polyphialides. However, these features are shared with Fusarium subglutinans, another human pathogenic Fusarium from the section Liseola. For this reason, some authors (2, 6) considered the two species synonymous. However, on the basis of recent molecular data, O'Donnell et al. (8) and Nirenberg and O'Donnell (7) demonstrated that they are different species, although morphologically difficult to distinguish from each other. These authors differentiated the two species by the host plant and by morphology: F. subglutinans has erect conidiophores (conidiophores arising directly from the substrate) and oval to fusiform microconidia which are aseptate or have one to three septa, while the conidiophores of F. sacchari are prostrate (conidiophores arising from hyphae that grow horizontally above the substrate surface). The microconidia of F. sacchari, although similar in shape to those of F. subglutinans, are usually aseptate (3).
Antifungal susceptibility testing.
The strain was tested to determine its susceptibility to six antifungal drugs (amphotericin B, itraconazole, miconazole, fluconazole, ketoconazole, and flucytosine) (Table 1). As all the isolates showed the same growth pattern, very similar colonies, and practically identical microscopic features, only one isolate was tested. Tests were carried out by a previously described microdilution method (10), mainly according to the guidelines for yeast of the National Committee for Clinical Laboratory Standards by using RPMI 1640 medium (buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid), an inoculum of 1.1 × 106 CFU/ml, an incubation temperature of 30°C, a second-day reading (48 h), and an additive drug dilution procedure. Three different criteria were used to define the MIC: (i) the lowest drug concentration that showed prominent (approximately 50%) growth inhibition, (ii) the lowest drug concentration that matched a 75% inhibition standard, and (iii) the lowest concentration at which no growth occurred. To determine the minimum fungicidal concentration (MFC), 10 μl from each of the wells at or above the MIC was plated on Sabouraud glucose agar. The plates were incubated at 25°C for 72 h. The MFC was defined as the lowest drug concentration at which no colonies developed on the agar plate.
TABLE 1.
Antifungal susceptibility of the clinical strain of Fusarium sacchari
| Antifungal | MIC (μg/ml)a
|
MFC | ||
|---|---|---|---|---|
| 50 | 75 | 100 | ||
| Amphotericin B | 2 | 2 | ||
| Fluconazole | 32 | 64 | >64 | |
| Flucytosine | >128 | >128 | >128 | |
| Itraconazole | 1 | >16 | >16 | |
| Ketoconazole | 4 | 8 | >16 | |
| Miconazole | 1 | 4 | >16 | |
Column headings (50, 75, and 100) represent the MICs at which 50%, 75%, or 100% of growth was inhibited.
The results of susceptibility testing showed that the MIC of amphotericin B for the present isolate was similar to those for F. oxysporum (2.13 μg/ml) and Fusarium verticillioides (1.98 μg/ml) and lower than that for Fusarium solani (3.47 μg/ml), the other opportunistic species of Fusarium which we have tested previously (11). Of all Fusarium species, F. solani showed the highest MICs of amphotericin B and is also the most virulent species in experimental studies (5), which agrees with its behavior in vivo. Infections caused by F. solani have the poorest prognosis. On the other hand, F. sacchari showed lower MICs of miconazole and ketoconazole than the other species tested previously. F. solani, F. oxysporum, and F. verticillioides showed MICs of ketoconazole and miconazole of more than 30 and more than 69 μg/ml, respectively. The differences between the two MICs (which inhibited 50 and 75% of fungal growth, respectively) were important in the case of itraconazole (which were of more than 4 dilutions). With fluconazole and ketaconazole, there was a difference of only 1 dilution; with miconazole, there was a difference of 2 dilutions.
This is the first reported case of fungemia due to F. sacchari. This species had previously only caused several cases of keratitis (9, 13). The species that has most frequently been involved in invasive or systemic infections so far is clearly F. solani, followed by F. verticillioides and F. oxysporum (4). Other species that can also cause severe infections, although more rarely, are Fusarium chlamydosporum, Fusarium dimerum, Fusarium napiforme, Fusarium nygamai, Fusarium proliferatum, and now F. sacchari. In the literature, cases in which the fungus was recovered only from blood were associated with a better clinical outcome. Sometimes giving small doses of amphotericin B or simply removing the catheter was enough to resolve the infection (4, 12).
In our case, the patient had no central venous catheter and the portal of entry of the fungus was not determined. In this case, no correlation existed between in vitro results and clinical outcome. The patient did well when receiving low doses of amphotericin B, even though the MIC and MFC of this antifungal in vitro were high. It is worth mentioning, however, that the patient was nonneutropenic, and fusarial infections in nonneutropenic patients have a better prognosis. It is also possible that the pathogenicity of F. sacchari was low in comparison to other fusaria because the patient did well even though immunosuppression was not reduced or removed.
Acknowledgments
We thank Arvind A. Padhye (CDC, Atlanta, Ga.) for reviewing the manuscript.
This work was supported by CICYT (Ministerio de Educación y Ciencia of Spain) grant PM98-0059 and Fundació Ciència i Salut.
REFERENCES
- 1.Boutati E I, Anaissie F J. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years experience at a cancer center and implications for management. Blood. 1997;90:999–1008. [PubMed] [Google Scholar]
- 2.de Hoog G S, Guarro J. Atlas of clinical fungi. Baarn, The Netherlands: Centraalbureau voor Schimmelcultures; 1995. [Google Scholar]
- 3.Gerlach W, Nirenberg H. The genus Fusarium–a pictorial atlas. Berlin-Dahlem, Germany: Biologische Bundesanstalt für Land- und Forstwirtschaft;; 1982. [Google Scholar]
- 4.Guarro J, Gené J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:741–754. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- 5.Mayayo E, Pujol I, Guarro J. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J Med Microbiol. 1999;48:363–366. doi: 10.1099/00222615-48-4-363. [DOI] [PubMed] [Google Scholar]
- 6.Nelson P E, Toussoun T A, Marasas W F O. Fusarium species. An illustrated manual for identification. London, England: Pennsylvania State University Press; 1983. [Google Scholar]
- 7.Nirenberg H, O'Donnell K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia. 1998;90:434–458. [Google Scholar]
- 8.O'Donnell K, Cigelnik E, Nirenberg H. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia. 1998;90:465–493. [Google Scholar]
- 9.Polenghi F, Lasagni A. Observation on a case of mycokeratitis and its treatment with BAY b 5097 (Canesten) Mykosen. 1976;19:223–226. doi: 10.1111/j.1439-0507.1976.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 10.Pujol I, Guarro J, Llop C, Soler L, Fernández-Ballart J. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob Agents Chemother. 1996;40:2106–2110. doi: 10.1128/aac.40.9.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pujol I, Guarro J, Gené J, Sala J. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J Antimicrob Chemother. 1997;39:163–167. doi: 10.1093/jac/39.2.163. [DOI] [PubMed] [Google Scholar]
- 12.Velasco E, Martins C, Nucci M. Successful treatment of catheter-related fusarial infection in immunocompromised children. Eur J Clin Microbiol Infect Dis. 1995;14:697–699. doi: 10.1007/BF01690877. [DOI] [PubMed] [Google Scholar]
- 13.Zapater R C. Opportunistic fungus infection—Fusarium infections—keratomycosis by Fusarium. Jpn J Med Mycol. 1986;27:68–69. [Google Scholar]