Summary
Intravital studies of cellular morphology in structures such as the hypothalamus are challenging because of their location at the bottom of the brain. Here, we describe an intravital imaging protocol based on gradient refractive index (GRIN) lenses in conjunction with confocal microscopy to inspect fluorescent cells at high resolution in deep-brain areas. The approach relies on implanted guide-tubes for the interchangeable use of GRIN lenses, thereby allowing imaging at different magnifications and increasing the effective field of view.
For complete details on the use and execution of this profile, please refer to Butiaeva et al. (2021).
Subject areas: Metabolism, Microscopy, Model Organisms, Neuroscience
Graphical abstract

Highlights
-
•
A protocol for deep-brain intravital imaging at subcellular resolution in mouse
-
•
A technique to longitudinally track fluorescently labelled cells and blood vessels
-
•
A microendoscope and guide cannula-based imaging approach to expand field of view
-
•
In vivo inspection of deeply seated brain structures at different magnifications
Intravital studies of cellular morphology in structures such as the hypothalamus are challenging because of their location at the bottom of the brain. Here, we describe an intravital imaging protocol based on gradient refractive index (GRIN) lenses in conjunction with confocal microscopy to inspect fluorescent cells at high resolution in deep-brain areas. The approach relies on implanted guide-tubes for the interchangeable use of GRIN lenses, thereby allowing imaging at different magnifications and increasing the effective field of view.
Before you begin
We describe a gradient-refractive index (GRIN) lens based deep-brain imaging method that allows simultaneous, longitudinal in vivo tracking of multiple fluorescently tagged neural cells and their fine processes. Central to this approach is the use of GRIN lens accommodating guide tubes. These guide tubes are permanently implanted dorsal to the area of interest (Figure 1A) and serve as shafts to accommodate GRIN lenses that differ in field of view (FOV) and magnification. GRIN lenses act as optical relays as their radial refractive index profile varies parabolically along the radius of the lens (Flores et al., 1989), and are thin enough to be embedded into the brain of a living mouse (Messerschmidt and Matz, 2014). The guide tube-based micro-endoscopy approach described in this protocol provides an intravital modality that enables imaging of target area at different magnifications due to the interchangeable use of GRIN lenses and also is cost-effective as individual GRIN lenses can be used across multiple animals.
Figure 1.
Guide tube fabrication, implantation, and GRIN lens imaging set up
(A) schematic illustrating implanted guide-tube GRIN lens assembly for imaging of a deeply seated brain structure (MBH).
(B) the stereotaxic set up used for implantation surgery.
(C) illustration of guide tube fabrication.
(D–F) schematic depiction of the 2 GRINTECH lens types (D and E) used for imaging and upright/inverted imaging modality (F).
(G) the GRIN lens holder assembly that attaches to the microscope objective.
(H) holding frame with mouse positioned underneath GRIN lens-holder assembly that is attached to the microscope objective.
(I) illustration of guide tube bottom image shape when tube and GRIN lens are aligned properly.
We will first describe the fabrication process for a guide tube to fit GRIN lenses of various thicknesses. This way the brain region at the tip of the guide tube can be imaged at different magnifications through lens exchange. Furthermore, for GRIN lenses that are considerably thinner than the tube, imaging content can be increased by systematic lens repositioning within the tube implant between scans.
We will then detail the tube implantation at the mediobasal hypothalamus (MBH), where we imaged mice that expressed tdTomato in leptin receptor (LepR) positive cells (LepR-tdTom mice) (Madisen et al., 2010; DeFalco et al., 2001).
The final section of the protocol describes the intravital image acquisition process using high and low NA GRIN microendoscopes, respectively. To minimize optical distortion, a custom-built lens holder was employed, which is attached to the microscope objective. For studies involving the vasculature, imaging followed acute intravenous injections of a fluorescent dye to highlight blood vessels.
All animal procedures were carried out in accordance with the recommendations of the Canadian Council on Animal Care (CCAC) and have been approved by the McGill University Animal Care Committee.
Note: The guide tube fabrication, implantation, and imaging procedures described here are based on the work of Barretto and Schnitzer (2012a).
Preparation for guide tube fabrication
Timing: ∼15 min
-
1.
Decide on the GRIN lens design for the experiment. Choose a capillary glass tubing that exceeds the diameter of the GRIN lens to be used by a minimum of 10%.
Note: If the goal is to maximize the field of view, a wider glass tubing should be chosen to allow for the displacement of the lens inside the tube.
Note: To enable imaging using the two GRIN lenses described here (1.4 mm and 0.7 mm diameter), a glass tubing with the inner diameter of 1.5 mm was chosen. The cannula is wide enough to accommodate either lens which in turn are stably attached underneath the microscope objective via a custom GRIN lens holder (see below).
-
2.
Sterilize all surgical tools and disinfect the surfaces (surgical table, stereomicroscope, stereotaxic devices) with 70% alcohol.
Preparation for guide tube implantation
Timing: ∼30 min
-
3.
Acquire or generate mice that express a fluorescent reporter in the cells of interest.
-
4.
Prepare anesthesia cocktail [ketamine (100 mg/kg) / xylazine (10 mg/kg) / acepromazine (3 mg/kg)] and 0.9% saline solution. This anesthesia cocktail will also be used for intravital imaging.
-
5.Prepare stereotaxic frame for surgery (Figure 1B)
-
a.Carefully mount the guide tube (at its mounting section; see Figure 1C) onto the cannula holder of one arm of the stereotaxic frame.
-
b.Attach a blunt-tip-needle (17–21 gauge if implanting a 1.5 mm guide tube) to a disposable, conventional 1-mL syringe, and mount the syringe onto the holder of the second arm of the stereotaxic frame.
-
a.
Note: Make sure the blunt-tip-needle is tightly attached to the syringe to ensure effective aspiration.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| FITC dextran | MilliporeSigma | FD2000S |
| Experimental models: Organisms/Strains | ||
| Mouse: Ai9 (RCL-tdTomato); 8–12 week old female and male | Madisen et al., 2010; The Jackson Laboratory | 007909 |
| Mouse: Lepr-Cre, 8–12 week old female and male | DeFalco et al. (2001); The Jackson Laboratory | 008320 |
| Software and algorithms | ||
| ZEN 2012 SP2 Black | Zeiss | https://www.zeiss.com |
| Other | ||
| #0 thickness coverglass | Electron Microscopy Sciences | Cat# 72198-10 |
| Absorption triangles | VWR | Cat# MSPP-1810503 |
| BD conventional 1-mL syringe | BD | Cat# 309659 |
| Blunt-tip needle, 17 gauge | Hamilton | Cat# 91017 |
| Cannula holder | Kopf Instruments | Model 1771 |
| Capillary glass tubing | Vitrocom | Cat# CV1518 |
| Confocal microscope | Zeiss | LSM 710 NLO |
| Dental cement liquid | Lang Dental | Cat# 1320CLR |
| Dental cement powder | Lang Dental | Cat# 1304CLR |
| Diamond Scriber | Electron Microscopy Sciences | Cat# 62107-BT |
| Dissecting microscope | Leica | Model EZ4 |
| GRIN lenses | GRINTECH, Germany | Cat# GT-MO-080-018-810 Cat# NEM-050-50-10-860-DL |
| GRIN lens holder with plastic discs | Custom-fabricated | The holder is shown on Figure 1G. It consists of the holder cuff that will be fixed around the microscope objective using the tightening screw and two screws for aligning the lens vertically. Plastic discs have a bore that fits the GRIN lens of choice. |
| Instant adhesive | Henkel | https://www.henkel-adhesives.com/ca/en/product/instant-adhesives/loctite_454.html |
| Ketamine | Vetoquinol | 02374994 |
| Micro Dissecting Forceps | Roboz | Cat# RS-5137 |
| Microdrill | Roboz | Model RS-6300 |
| Microdrill burr | Fine Science Tools | Item No. 19008-07 |
| Optical adhesive | Norland Products | Cat# NOA 81 |
| Reusable adhesive | Scotch | https://www.staples.com/3m-Scotch-Adhesive-Putty-Removable-White-2-oz-860/product_602158 |
| Sanding paper | Norton | https://www.homedepot.ca/product/orton-sandwet-9-inch-x11-inch-sanding-sheets-ultra-fine-600-grit-5-pack-/1000100829 |
| Stereotaxic frame for surgery | Kopf Instruments | Model 962LS |
| Stereotaxic frame for imaging | Kopf Instruments | Model 1900 B |
| Syringe holder | Kopf Instruments | Model 1772 |
| UV light source for adhesive curing | Intertek | Model UV36W |
| White craft glue | Dollarama, Canada | https://www.dollarama.com/en-CA/p-white-glue-3pk/0502341 |
| Xylazine | Bayer | 02169592 |
Step-by-step method details
Guide tube fabrication (day 1)
Timing: ∼1 h
This step will produce the GRIN lens guide tube to be used for implantation. Guide tubes should be fabricated no later than one day before the implantation surgery, to allow glue to set in. The compound guide tube consists of two sections: an upper mounting and a lower implant section which are glued together tip-to-tip (Figure 1C). The compound guide tube will be mounted into the stereotaxic arm holder via the upper section which will be detached upon tube implantation (Figures 1B and 1C).
Note: It is critical to choose the appropriate length for the guide tube. When implanted it should not protrude more than 1–2 mm from the skull surface to minimize risks of damage while the animal is freely moving in the cage between imaging sessions which could be days to months apart. However, some protrusion is required to leave enough room for proper cementation to skull. The mounting section of the guide tube should be long enough (e.g., 15 mm) for successful attachment to the cannula holder of the stereotaxic frame.
-
1.Cut two pieces of thin-walled capillary glass tubing (1.5 mm inner diameter, 0.3 mm wall thickness) which will serve as the implantation and the mounting sections of the guide tube. For MBH imaging in the lateral hypothalamus region, we chose 6.5 mm for the implantation and 15 mm for the mounting section, respectively.
-
a.Align the glass capillary with a ruler under a stereomicroscope and mark cut positions lightly using a diamond scriber.
-
b.Set aside the ruler and scratch all around the perimeter of the tubing at the cut positions using a diamond scriber.
-
c.Carefully bend the tubing by hand until it breaks at the marked position.
-
a.
CRITICAL: The directionality of the 2 tube pieces is important as they will be glued back together at the point of breakage at the very end of the fabrication process. Make a note which side corresponds to the “broken” side.
-
2.
Polish the bottom tip of the section that will be inserted into the brain with sanding paper. Polish until surface is planar and perpendicular to the tube walls to enable proper sealing. Clean with sterile water and/or a compressed air duster to ensure absence of dust at the luminal side of the capillary walls.
-
3.Create a cover glass disk for sealing the lower tip of the implantation section.
-
a.Use diamond cutter to cut out a small disk (1.8–2.0 mm in diameter, if glass tubing inner diameter is 1.5 mm) from a #0-thickness (80–130 μm) cover glass under the stereomicroscope.
-
b.Carefully trim extra glass from disk using forceps.
-
c.Inspect disk surface under the stereomicroscope for the absence of any scratches or dust. If dust cannot be completely removed, discard the disk.
-
a.
-
4.Seal the polished tip of the guide tube (step 2) with the disk.
-
a.Apply a small amount of UV light-activatable glue onto the rim of the polished tip using a fine applicator (e.g., a needle tip).
-
b.Immediately thereafter place cover glass disk on the capillary tip.
-
i.Hold the disk with forceps, positioning it right above the capillary tip, and then loosen the forceps so that the disk lands right on the glue coated tip.
-
ii.Make sure the glue is not spreading along the inner walls of the guide tube or further onto the cover glass. Otherwise remove and discard cover glass and repeat step 4.
-
i.
-
c.Once the cover glass is positioned properly, proceed with glue curing by UV light. Glue curing time is ∼15–20 s.
-
a.
-
5.
Carefully remove any protruding portions of the cover glass using sanding paper. A diamond cutter could be used in this step if the disk is too large, however, extra care should be applied.
-
6.Glue the implantation and the mounting sections of the guide tube together tip-to-tip.
-
a.Align both “broken” parts under the stereomicroscope.
-
b.Apply a thin layer of white craft glue to the tube outer surfaces where the two pieces will be joined. The glue should not seep into the tubing when joining the parts.
-
c.Ensure that the glue completely dries (i.e., transforms into a clear film).
-
d.Rinse the guide tube with 70% ethanol.
-
a.
CRITICAL: We recommend waiting at least 12 h before proceeding with the next step.
-
7.Prepare a pedestal for the guide tube
-
a.Cut a circular piece from standard printing paper twice the diameter of the guide tube and render it into a collar through two perpendicular incisions at the center as illustrated in Figure 1C (red dashed lines). The extent of the incisions should roughly match the outer diameter of the guide tube.
-
b.Slip the collar (which serves as the pedestal) over the guide tube with the four cut corners facing in the upward direction as illustrated in Figure 1C (in red). The pedestal should be movable along the vertical axis of the guide tube yet sit tight enough to remain where positioned.
-
a.
Store fabricated guide tubes in sterile 6-well plates until further use.
Guide tube implantation (day 2)
Timing: ∼1 h
This step will result in guide tube implantation. For intravital hypothalamic imaging, the following coordinates were used: anteroposterior −1.8 mm, lateral +0.95 mm, dorsoventral −5.0 mm below the skull. We found that survival rate critically depends on pre-operative age, weight, and general health of the mouse. 10–15 weeks old mice that weighed no less than 23–25 g seem optimal for this procedure and recover well from surgery. After implantation, house mice individually for the duration of study to minimize risk of guide tube displacement or damage. See “before you begin” before proceeding.
-
8.
Anesthetize mouse by intraperitoneal injections of the ketamine/xylazine cocktail and apply ophthalmic ointment to eyes. Shave most dorsal portion of mouse head and disinfect the exposed area with betadine. Place anesthetized mouse in surgical stereotaxic frame. Keep animal on a heating blanket during the surgery.
-
9.
Expose the cranium around the area by skin incisions at midline ∼1 cm in length using a scalpel, secure the skin with surgical clamps, and remove the periosteum with a cotton swab damped in 0.9% saline solution.
-
10.
Conduct circular craniotomy above the area of interest using a microdrill with the drill hole diameter exceeding the guide tube diameter by 20%–25%. Remove dura using forceps. Stop bleeding with triangular-shaped cotton absorption swab if needed.
-
11.Stereotaxically position the blunt-tip syringe needle over the area of interest and lower the tip into the brain parenchyma down to 0.5 mm above the area of interest (e.g., −4.5 mm below skull for the MBH).
-
a.When desired depth is reached, pull the syringe plunger until the piston’s distal edge meets the 0.1 mL mark and slowly (∼0.5 mm/s) lift the stereotaxic arm holding the needle.
-
b.Once the needle exits the brain, the parenchymal tissue column (∼1.4 mm in diameter and 4.5 mm in height) will be automatically aspirated into the syringe barrel due to negative pressure. If aspiration is insufficient (assessed upon inspection of the aspirated tissue column, now inside the barrel), repeat aspiration until all of the desired tissue is removed.
-
c.If more than one aspiration cycle is required, rinse drill hole with 0.9% saline in between cycles and remove excess fluids/blood with the triangular absorption swab.
-
a.
-
12.
Apply a small amount of instant adhesive at the bottom side of the pedestal. Be careful to not apply the glue to the guide tube itself.
-
13.
Stereotaxically lower the guide tube into the aspirated path. Once the guide tube reaches the desired coordinates, depress pedestal/collar onto the skull. Allow for the glue to dry.
-
14.
Apply a small amount of instant glue between each of the four corners of the pedestal and the guide tube wall and gently press corners against the wall. Let dry.
-
15.
Apply a thin layer of dental cement on top of the pedestal. Let it harden completely.
-
16.
Using a stereomicroscope, carefully peel off the clear glue film where the tube sections connect using a sharp needle tip. Gently lift and remove the mounting section.
-
17.
Apply one more layer of dental cement to fully secure the guide tube implant. Be careful to not let any dental cement enter the inside of the tube.
-
18.
Seal the top of the implant with reusable adhesive or tape to prevent debris from entering into tube.
-
19.
Clip extra skin around the guide tube if necessary. Administer carprofen and place mouse in a cage kept on a heating blanket while recovering from anesthesia. Inject 1 mL of warm (37C) sterile saline subcutaneously to prevent dehydration. Troubleshooting 1.
Note: Allow 3–4 weeks for animal to recover from surgery before proceeding to the next step.
Intravital imaging (day 3, ∼3–4 weeks after surgery)
Timing: Up to 1.5 h
This step describes the intravital imaging procedure for the visualization of fluorescently-labeled cells that reside below the tip of the implanted guide tube. Guide tube-based imaging enables interchangeable use of multiple GRIN lenses within a single imaging session.
Note: In our particular case, two types of GRIN micro-objectives (GRIN-TECH, Germany) were used with the following specifications: A) 0.7 mm diameter; object side: 800 μm working distance (WD), 0.50 numerical aperture (NA); image side: 100 μm WD, 0.19 NA; geometrical length: 15.86 mm (Figure 1D); B) 1.4 mm diameter; object side: 200 μm WD, 0.8 NA; image side: 200 μm WD, 0.18 NA; geometrical length: 7.39 mm; additionally features a plano-convex lens at the object side (Figure 1E).
Note: Type A) was used for low resolution and type B) for high resolution, cellular imaging. The GRIN lenses we chose were between ∼7–15 mm long. Endoscope length can be varied at increments of 0.5 pitch. Use of shorter endoscopes is desirable to minimize aberration, but may impede handling and compatibility with the lens holder. The lenses were used in two modalities – upright and inverted (Figure 1F) - which enabled imaging at low (inverted) and high (upright) magnifications to capture population overviews or cellular details, respectively.
Note: A custom-fabricated GRIN-lens holder (Figures 1G and 1H) was employed to ensure stable alignment of the GRIN lens with the 20× microscope objective (Plan-Apochromat 20×/0.8 M27, WD=0.55 mm, Zeiss) (Figure 1H).
Note: A typical imaging session may last up to 1.5 h. Isoflurane anesthesia can be possibly used as alternatively to ketamine-xylazine, however anesthesia depth may not be sufficient to prevent breathing-induced movements which may create imaging artifacts. Note, that we used the same high NA 20× microscope objective regardless of GRIN lens type or position (upright versus inverted). Thus objective NA and the endoscope image-side NA where either matching (high NA endoscope in inverted position) or the image-side NA was lower, which is in line with previous recommendations in the context of GRIN lens-based deep brain imaging (Barretto and Schnitzer, 2012b). However, while we were able to obtain high resolution images in the (sub)micron range despite mismatching NAs, matching NAs may generally help with resolving fine structures such as synaptic spines (Meng et al., 2019).
-
20.
Anesthetize mouse by intraperitoneal injection of a ketamine-xylazine cocktail. Apply ophthalmic ointment to eyes to prevent drying during the imaging session. Place and secure anesthetized mouse in holding frame (Kopf) to be positioned underneath objective. Keep animal on a heating blanket during the imaging session.
-
21.
Position the mouse head under the dissecting microscope and carefully remove the protective seal/adhesive that covers the top of the guide tube with forceps.
Note: Focus on the bottom of the guide tube and adjust the head position of the mouse so that when looking down through both eyepieces simultaneously, the bottom of the guide tube forms an oval as illustrated in Figure 1I.
Note: A more circular shape indicates misalignment between objective and tube and thus the mouse head position should be corrected to allow for a perfectly vertical in-line alignment of the guide tube and the objective-attached GRIN lens. Position correction will be attained by careful adjustments to the head holder of the stereotaxic frame which is freely adjustable in all 3 dimensions.
-
22.
Inspect the tube inner wall and the cover glass for dust particles using the stereomicroscope. If necessary, inject H2O using 1 mL insulin syringe and dry with absorbing tissue (Kimwipes).
CRITICAL: Extra caution should be taken when rinsing the guide tube to prevent damage to the cover glass at the tube bottom).
Optional: For vasculature labeling, inject e.g., fluorescein-isothiocyanate dextran (20 mg/ml saline) intravenously into mouse tail vein immediately prior to the imaging session. Troubleshooting 2.
-
23.
Attach GRIN lens holder to microscope. Slip holder cuff without the disk onto the 20× objective and tighten holder screw (Figures 1G and 1H).
-
24.For low magnification, wide FOV, insert 1.4 mm GRIN lens in inverted direction through the central bore of the custom-fabricated plastic disc (Figure 1G).
-
a.Insert disc into holder, secure with clips (see Figure 1G).Note: The upper tip of the GRIN lens should be positioned so that the distance to the objective equals the sum of the objective’s and GRIN lens’ focal plane distances. Depending on the design of the custom holder, this can be done manually or using the holder’s z-axis positioning.
-
b.Magnification can be simply changed by reversing the orientation of the GRIN lens in disc. To provide a different set of magnifications, the 0.7 mm GRIN lens can be employed. Troubleshooting 3 and 4.Note: Parameters for one/two-photon imaging depend on the experimental design. For example, we used the following excitation/emission filters for confocal mode imaging: ∼480/520 nm for fluorescein-conjugated dextran and ∼555/580 nm for tdTomato protein. Image resolution was typically 512 × 512 pixels acquired at scan speeds of 0.5–1 pixels/μs with a pin hole size of 2–14 airy units using the microscope’s image acquisition software (ZEN). Lowest possible laser power should be used to minimize imaging-induced tissue damage.
-
a.
-
25.Position the stereotaxic imaging frame which holds the mouse underneath the GRIN lens so that it is aligned with the guide tube (Figure 1H).
-
a.Gently lower the objective-GRIN lens assembly into the guide tube.
-
b.When the lens tip approaches the bottom of the guide tube, a fluorescence signal from labeled cells/vasculature should become detectable.
-
c.Lower the objective-GRIN assembly further until the desired imaging area is reached. Troubleshooting 5.
-
a.
CRITICAL: We recommend that the lens holder allows for some movement of the GRIN lens along the vertical axis. This is to prevent damage to the cover glass at the guide tube bottom in case the lens is accidentally lowered too far into the guide tube.
-
26.
To maximize the effective field of view, the GRIN lens X-Y position within the guide tube can be altered by manually changing the X-Y position of the microscope stage (in cases where the guide tube is substantially wider than GRIN lens).
Note: Visualization of the vasculature by i.v. fluorescent dextran may help with tracking individual cells over time.
-
27.
Once imaging is completed, lift the objective-GRIN lens assembly, replace the lens with a different lens if necessary or end the imaging session by resealing the guide tube with protective adhesive. Return animal to its home cage placed on a heating blanket until full recovery from anesthesia. Animals can be re-imaged at intervals ranging from days to at least a few months.
Expected outcomes
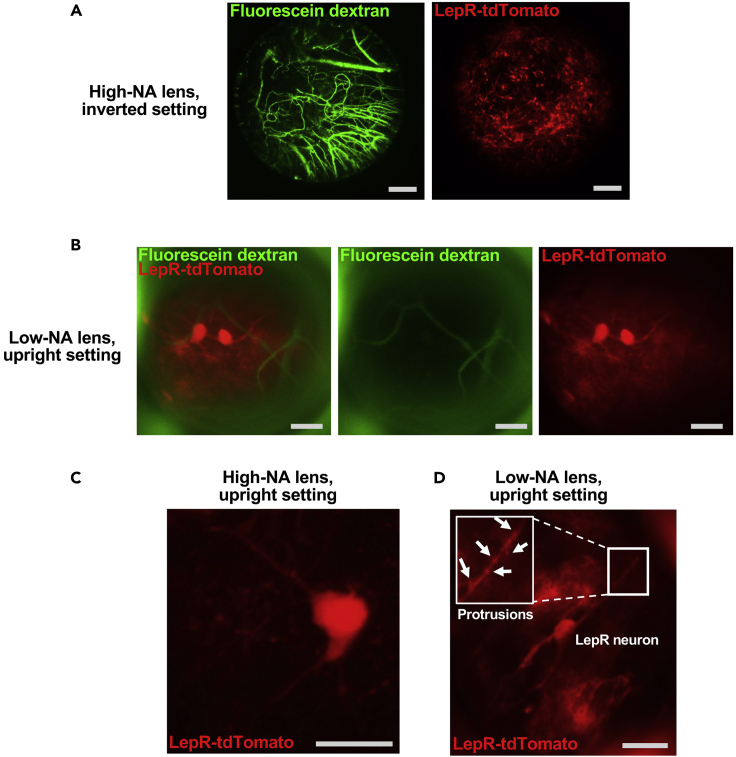
Employing various GRIN lenses in conjunction with a guide tube of 1.5 mm inner diameter allows for imaging of dozens of cells near the tip of the guide tube (Figure 2A) with the possibility to focus on individual cells for imaging at submicron resolution through lens exchange or change of lens position (upright versus inverted, Figures 2B–2D). High NA GRIN lens imaging can reveal fine cellular processes (Figures 2C and 2D) and protrusions reminiscent of dendritic spines can be visualized (Figure 2D). Visualization of blood vessels upon, e.g., i.v. FITC-dextran delivery (Figure 2A and 2B) can facilitate tracking of individual cells across imaging sessions.
Figure 2.
Guide-tube based GRIN lens imaging of Tomato-expressing LepR cells and fluorescein-labeled vasculature in the MBH
(A) low resolution, large FOV images using the high-NA lens in inverted mode after i.v. fluorescent dextran injection.
(B) same as in (A), except the low-NA lens was used in upright position.
(C) high-resolution image of a LepR neuron using the high-NA lens in upright position.
(D) Low-NA lens image (upright position) revealing fine subcellular structures such as neuronal process protrusions. Scale bars, 200 μm (A), 30 μm (B and D), and 15 μm (C).
Limitations
This protocol was optimized to image cells and blood vessels of the mediobasal hypothalamus in anesthetized animals. Considering previous accounts where thinner GRIN lenses with smaller FOV were employed to image deep-brain structures in head-fixed awake animals using guide cannulas (Bocarsly et al., 2015), our large diameter guide tube approach might be similarly amenable to awake animals using implanted head-bars. However, one has to consider the possibility of stress-dependent artifacts in the awake state, which may especially hamper the study of stress-related neural circuits dynamics. To minimize the risk, animals may be habituated to head fixation by applying gradual exposure (Schwarz et al., 2010).
Imaging of certain deep-seated brain regions may not be amenable to the guide-tube approach described here. For instance, imaging of cells in the hypothalamic ArcN requires guide tube insertion along the midline which would likely entail damage to the third ventricle (3V) with the consequence of a greatly reduced survival rate and physiological artifacts. Guide tube implantation at an angle may prevent damage to the ventricle and may thus represent a viable strategy to enable imaging of areas close or below the ventricular system. Intravital imaging per se will not be impacted as long as the tilted guide tube implant is aligned along the vertical axis of the microscope objective.
Due to the highly invasive nature of the procedure, we do not recommend using mice that are younger than 10 weeks and/or weigh less than 23 g. Younger mice may however be amenable to smaller diameter guide tube/GRIN lens designs.
Although our imaging approach significantly extends the effective field of view compared to other methods previously employed (Messerschmidt and Matz, 2014; Bocarsly et al., 2015), data collection is limited to the brain parenchyma below the guide tube bottom. More sophisticated GRIN lens designs employing prisms attached to the lens tip in conjunction with square guide tube profiles may enable imaging along the guide tube walls thereby profoundly increasing imaging information content per individual mouse.
Troubleshooting
Problem 1
Recovery from the surgery is poor (step 19).
Potential solution
The procedure is invasive, thus surgery success may depend on a variety of factors, including animal’s age, weight, the guide tube design, implantation coordinates, and implant positioning (degree of tilt) within the brain. Changes to any of these parameters may significantly affect recovery.
In addition, it is critical that tissue has been sufficiently aspirated (step 11). Otherwise, the guide tube could create intracranial pressure/lead to tissue damage that may impact recovery.
Due to the relatively long duration of surgery, it is important to provide adequate thermal support via external heating devices (warm pads) to prevent excessive heat loss/drop in core body temperature until full recovery from anesthesia.
Problem 2
Low to undetectable fluorescence signal from vasculature or cells (step 22, optional).
Potential solution
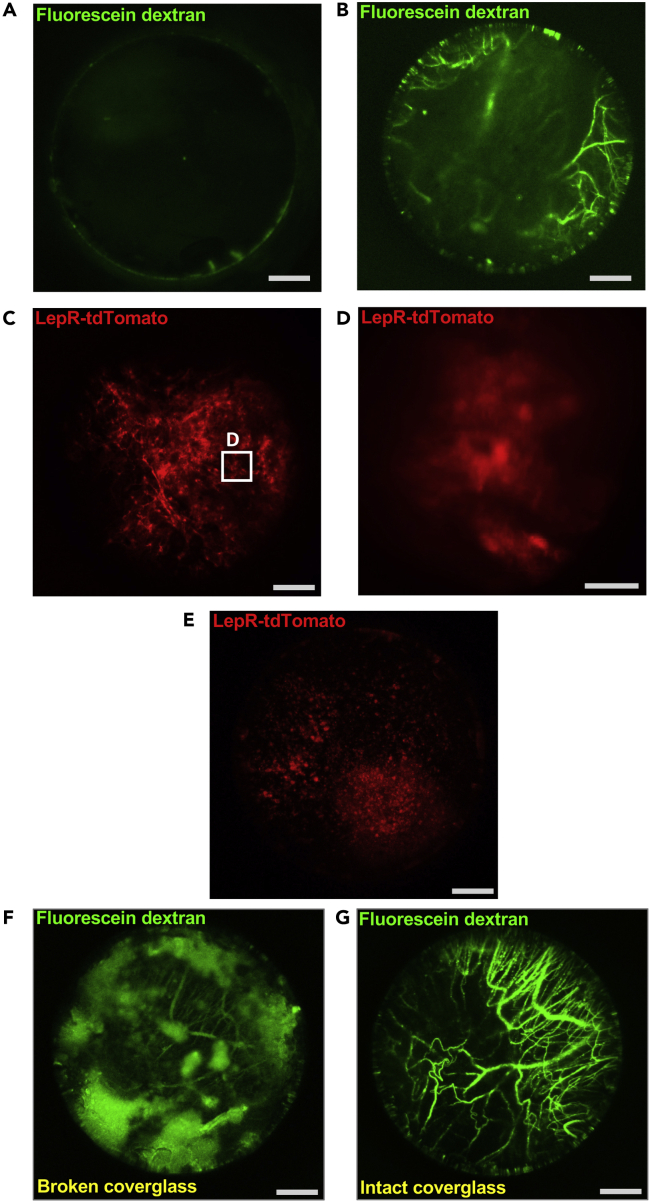
Low or absent fluorescence signal from the vasculature (Figure 3A) may suggest that no/not enough labeled dextran reached the brain circulation. One may retry the i.v. dextran injection. If vasculature signal is clear, but cellular signal is absent, the mouse is likely not expressing fluorescent protein in the cells of interest. Absence of cellular fluorescence could also result from mistargeting of the guide tube. Subsequent immunohistochemical analysis of the brain should confirm if fluorescent protein is misexpressed, absent, or if the guide tube has been mistargeted.
Figure 3.
Guide tube-based GRIN lens imaging troubleshooting
(A) lack of fluorescent signal from vasculature upon i.v. fluorescein dextran injection.
(B–E) (partially) blurry images due to air-filled space below guide tube implant bottom. Boxed area in (C) is shown in (D).
(F and G) cloudy fluorescent signal (F) due to broken cover glass; control image shown in (G). Images taken with the high-NA lens in upright (D) or inverted position (A–C and E–G). Scale bars, 200 μm (A–C and E–G) and 30 μm (D).
Problem 3
Fluorescence signal from cells or vasculature is present but blurry (step 24).
Potential solution
If cellular signal appears blurry or otherwise indistinctive, ensure that the animal has been given enough time for recovery from surgery (∼2–3 weeks) as surgery related inflammation at the guide tube tip may possibly contribute to the aberrant signal.
Check z-axis positioning of the GRIN lens. Distance to the microscope objective may be suboptimal or the lens is not appropriately aligned with the objective and/or the guide tube, which both can account for blurry images.
Alternatively, cells of interest are located too far away from the bottom of the guide tube. GRIN lenses with a longer WD may provide a solution in such cases.
Fluorescence that appears blurry could also suggest presence of an air-filled space between the guide tube bottom and the brain parenchyma (Figures 3B–3E). This may be due to aspiration of extra tissue under the guide tube tip. It is also possible that the guide tube displaced upwards before fixation to skull. Extra attention should be given to the guide tube implantation step to avoid such a problem.
Problem 4
Fluorescence signal is uneven, shows patchy cloudiness (step 24).
Potential solution
If image shows uneven, cloudy fluorescence (Figures 3F and 3G), the guide tube cover glass may be damaged/broken. In such case the mouse is no longer useful for intravital imaging as the tissue below the guide tube might have been displaced too far away from the GRIN lens focal plane. As preventative measure, extra attention should be given to cover glass trimming/ guide tube cleaning and/or the insertion/lowering of the GRIN lens into the guide tube.
Also, dirt/dust on the GRIN lens surfaces or at the guide tube bottom can account for patchy image quality.
Problem 5
When lowering the lens into the guide tube, the lens displaces upwards in the holding disk insert of the lens holder despite the fact that the lens tip has not yet reached the desired depth level within the guide tube (step 25).
Potential solution
The flexible nature of the GRIN lens holder allows for some Z-axis displacement when positioning the lens inside the guide tube, thus the GRIN-lens will displace in case of resistance. If the GRIN lens displaces before reaching the guide tube bottom, the tube is likely tilted/misaligned with the lens. Lift the lens out of the guide and re-adjust the head of the mouse for proper alignment between tube and lens.
Resource availability
Lead contact
Requests for further information on methods, resources, and reagents should be directed to and will be fulfilled by the lead contact, Dr. Maia Kokoeva (maia.kokoeva@mcgill.ca).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We thank Walter Kucharski for manufacturing the customized GRIN lens holders, Ian Bates (Zeiss) for assistance with confocal microscopy, and Xiaohong Liu for technical assistance. This work was supported by grants to M.V.K. from the Canadian Institutes of Health Research (PJT-148790) and Canadian Foundation for Innovation.
Author contributions
M.V.K. and L.I.B. designed and developed the methods and wrote the manuscript. L.I.B. carried out the experimental procedures.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate or analyze datasets or code.
References
- Barretto R.P., Schnitzer M.J. In vivo microendoscopy of the hippocampus. Cold Spring Harbor Protoc. 2012;2012:1092–1099. doi: 10.1101/pdb.prot071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto R.P., Schnitzer M.J. In vivo optical microendoscopy for imaging cells lying deep within live tissue. Cold Spring Harbor Protoc. 2012;2012:1029–1034. doi: 10.1101/pdb.top071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocarsly M.E., Jiang W.C., Wang C., Dudman J.T., Ji N., Aponte Y. Minimally invasive microendoscopy system for in vivo functional imaging of deep nuclei in the mouse brain. Biomed. Opt. Express. 2015;6:4546–4556. doi: 10.1364/BOE.6.004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butiaeva L.I., Slutzki T., Swick H.E., Bourguignon C., Robins S.C., Liu X., Storch K.F., Kokoeva M.V. Leptin receptor-expressing pericytes mediate access of hypothalamic feeding centers to circulating leptin. Cell Metab. 2021;33:1433–1448.e5. doi: 10.1016/j.cmet.2021.05.017. [DOI] [PubMed] [Google Scholar]
- DeFalco J., Tomishima M., Liu H., Zhao C., Cai X., Marth J.D., Enquist L., Friedman J.M. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Flores J.R., Gómez-Reino C., Acosta E., Linares J. Geometrical optics of gradient index lenses. Opt. Eng. 1989;28:281173. [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G., Liang Y., Sarsfield S., Jiang W.C., Lu R., Dudman J.T., Aponte Y., Ji N. High-throughput synapse-resolving two-photon fluorescence microendoscopy for deep-brain volumetric imaging in vivo. Elife. 2019;8:e40805. doi: 10.7554/eLife.40805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmidt B., Matz G. Enabling endomicroscopy using GRIN-microoptics: current applications, challenges and development trends. Optik Photonik. 2014;9:57–61. [Google Scholar]
- Schwarz C., Hentschke H., Butovas S., Haiss F., Stüttgen M.C., Gerdjikov T.V., Bergner C.G., Waiblinger C. The head-fixed behaving rat—procedures and pitfalls. Somatosens. Mot. Res. 2010;27:131–148. doi: 10.3109/08990220.2010.513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate or analyze datasets or code.