Abstract
Background
Cyclin-Dependent Kinase (CDK) 4/6 inhibitors have shown significant clinical activity in cancer patients. However, some concerns regarding rare adverse events (AEs) have occurred including interstitial lung disease (ILD)/pneumonitis, for which data are deficient. The aim of this study was to evaluate the overall incidence and risk of ILD/pneumonitis related to CDK4/6 inhibitors in randomized controlled trials (RCTs).
Methods
Electronic databases and ClinicalTrials.gov were searched from inception to October 1, 2021 for RCTs reporting the occurrence of LD/pneumonitis in cancer patients treated with CDK4/6 inhibitors. Peto odds ratios (Peto ORs) and 95% confidence intervals (CIs) were used to pool the study.
Results
12 RCTs with a total of 16,060 patients were eligible. The overall incidence of all-grade ILD/pneumonitis was 1.6% (131/8407) in the treatment group compared with 0.7% (50/7349) in the control group. CDK4/6 inhibitors significantly increased the risk of all-grade ILD/pneumonitis with a pooled Peto OR of 2.12 (95% CI [1.57, 2.86], P < 0.00001) with no heterogeneity (I2 = 0%, χ2 P = 0.98). A higher incidence of grade 3 or higher ILD/pneumonitis was also observed in the treatment group (0.2%, 16/7087) compared with the control group (0.05%, 3/6617) with a Peto OR of 3.22 (95% CI [1.28, 8.09], P = 0.01) with no heterogeneity (I2 = 0%, χ2 P = 0.62). Two grade 5 pneumonitis were reported in the included studies. Subgroup analyses did not show any significant difference.
Conclusions
The risk of all-grade and grade 3 or higher ILD/pneumonitis was higher in patients treated with CDK4/6 inhibitors compared to controls. The awareness for these rare AEs in the application of CDK4/6 inhibitors should be enhanced. Further studies are required to validate the mechanisms and the risk factors of ILD/pneumonitis with CDK4/6 inhibitors.
Keywords: Cyclin-dependent kinase (CDK) 4/6 inhibitors, ILD, Pneumonitis, Meta-analysis, Randomized controlled trials
Highlights
-
•
CDK4/6 inhibitors significantly increased the risk of all-grade ILD/pneumonitis.
-
•
CDK4/6 inhibitors significantly increased the risk of grade ≥3 ILD/pneumonitis.
-
•
Palbociclib and abemaciclib showed the most significant risk of ILD/pneumonitis.
-
•
CDK4/6 inhibitor-related ILD/pneumonitis may lead to fatal outcomes.
1. Introduction
In terms of new cases of cancer reported by the International Agency for Research on Cancer (IARC), breast cancer (BC) has become the most commonly diagnosed cancer in the world, with over 2.3 million new cases occurring in 2020, overtaking lung cancer [1]. Hormone receptor (HR) positive/human epidermal growth factor 2 (HER2) negative (HR+/HER2-) is the most common subtype of BC [2]. Generally, expression of HR is associated with a higher survival rate as a result of targeted endocrine therapy (ET) [3]; nevertheless, the cancer cells can produce resistance to ET, which poses an increasing therapeutic challenge for physicians [4]. The advent of CDK4/6 inhibitors have brought new hope for the treatment of HR + patients. CDK4/6 inhibitors as palbociclib, abemaciclib, ribociclib have been approved by the U.S. Food and Drug Administration (FDA) for patients with HR+/HER2-advanced or metastatic BC, and abemaciclib was also approved for early BC patients with HR+/HER2- [[5], [6], [7], [8]]. Additionally, trilaciclib was the only CDK4/6 inhibitor approved for decrease the incidence of myelosuppression for lung cancer in 2021 [9].
The infrequent adverse events (AEs) were discovered with the continuously increasing use of CDK4/6 inhibitors, such as respiratory disorders. There were a few severe and fatal cases of Interstitial Lung Disease (ILD)/pneumonitis caused by CDK4/6 inhibitors that have been reported, which was warned by FDA in the drug labels [[5], [6], [7], [8], [9]]. The supporting data were generally from clinical trials with a low incidence of 1.0% in patients treated with palbociclib [[10], [11], [12]], [[10], [11], [12]] 3.3% in patients treated with abemaciclib [[13], [14], [15]], 1.1% in patients treated with ribociclib [[16], [17], [18]], and 0.4% in patients treated with trilaciclib [[19], [20], [21]]. However, isolated randomized controlled trials (RCTs) might not be sufficient to assess the overall incidence of CDK4/6 inhibitor-related ILD/pneumonitis. Meanwhile, the risk of ILD/pneumonitis related to CDK4/6 inhibitors remained undetermined.
2. Methods
2.1. Search strategy
The selection and systematic review of trials were performed following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement [22]. PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), Embase, China Biology Medicine disc (CBM disc), China National Knowledge Infrastructure (CNKI), Wanfang, and ClinicalTrials.gov were searched from inception to October 1, 2021. We retrieved both Medical Subject Heading (MeSH) terms and free text words to identify relevant studies. The search terms included the keywords “CDK 4/6 inhibitor”, “Cyclin-Dependent Kinase 4/6 inhibitor”, “palbociclib”, “abemaciclib”, “ribociclib”, “trilaciclib” and “randomized controlled trial”. To identify potential unpublished data, the oncological meeting proceedings from the European Society for Medical Oncology (ESMO), the American Society of Clinical Oncology (ASCO), and the Chinese Society of Clinical Oncology (CSCO) were also searched up to October 1, 2021. The latest FDA drug labels of approved agents (palbociclib, abemaciclib, ribociclib and trilaciclib) were reviewed to identify additional relevant information. The reference list of trials and relative reviews were also searched for additional studies.
2.2. Study selection
Interstitial lung disease and pneumonitis were considered as ILD/pneumonitis events. Our selection criteria included: (a) Phase II or III RCTs with participants assigned to CDK4/6 inhibitors containing group (single agent or in combination) or non-CDK4/6 inhibitors containing controls; (b) Studies with available data reporting ILD/pneumonitis events; (c) Articles published in English or Chinese language. Case reports, reviews, case-control studies, cohort studies, phase I studies, single-arm phase II studies, RCTs without available outcomes, and RCTs with CDK4/6 inhibitors in all arms were excluded.
2.3. Data extraction and quality assessment
Two reviewers independently selected the potential trials, extracted data, and assessed the risk of bias of included trials. Another author was consulted if there was any discrepancy. The baseline demographic data, type of CDK4/6 inhibitor, type of control group, number of patients enrolled, number of patients for safety analysis, and number of patients with ILD/pneumonitis for all-grade and grade 3 or higher in the treatment and control groups were collected from the eligible studies. Included studies were assessed using the Cochrane collaboration's tool for assessing risk of bias.
2.4. Outcomes
The primary outcomes were the incidence and risk of all-grade ILD/pneumonitis in patients treated with CDK4/6 inhibitors compared with control. All available events of ILD/pneumonitis were extracted from the latest publications first, then from ClinicalTrials.gov. The data in publication with the longest follow-up duration was used in case of various follow-up durations reported. We contacted the authors of eight relevant studies asking for further data on ILD/pneumonitis (if interested outcomes were not available from the articles, supporting information appendix or ClinicalTrials.gov). The secondary outcomes were the incidence and risk of grade 3 or higher ILD/pneumonitis. Subgroup analyses were carried out based on the study phase, type of cancer, type of CDK4/6 inhibitor, CDK4/6 inhibitor assignation (CDK4/6 inhibitor monotherapy versus combination of CDK4/6 inhibitors and other chemotherapy), type of controls and type of CDK4/6 inhibitor setting.
2.5. Statistical analysis
The results of the meta-analysis were performed using RevMan version 5.4. Due to the rare incidence of ILD/pneumonitis events, we used the Peto odds ratios (Peto ORs) with 95% confidence intervals (CIs) to pool the data. Higgins inconsistency index (I2) test and Chi-squared (χ2) test with P value were used to evaluate heterogeneity. Significant heterogeneity between studies was defined as χ2 P ≤ 0.1 or I2 > 50%. We did a sensitivity analyses by recalculating the pooled Peto OR estimates after removing one study at a time. Publication bias was assessed by the funnel plots.
3. Results
3.1. Eligible studies and characteristics
Our initial search yielded 2399 reports. After removing obvious duplicates and screening titles and abstracts, we retrieved 33 studies for full-text screening. Finally, seven publications [15,17,18,[23], [24], [25], [26]] from electronic database and five RCTs from ClinicalTrials.gov [[27], [28], [29], [30], [31]] comprising 16,060 patients were included in the quantitative analysis (Fig. 1).
Fig. 1.
Flow diagram of eligible studies.
As was shown in Table 1, this meta-analysis included 9 phase III studies and 3 phase II studies. 9 studies were performed in patients with BC, 2 in non-small cell lung cancer (NSCLC), and 1 in head and neck cancer. Most of the studies included patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. Among the 12 studies, palbociclib was used in 3 trials, abemaciclib in 7 trials, and ribociclib in 2 trials. Risk of bias for each study was illustrated in supplementary appendix Figure S1. All studies reported adequate randomization, and 7 were double-blinded.
Table 1.
Characteristics of eligible studies.
| NCT number Author (Pabulication year) Acronym |
Phase | Cancer type | ECOG | CDK4/6 inhibitor | Patients enrolled | Patient groups |
Age |
ILD/pneumonitis events |
Events reported | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment, n |
Median/Mean, years |
No. of patients for safety analysis |
All-grade |
≥3 grade or SAEs |
||||||||||
| Control, n | Treatment | Treatment | Control | Treatment | Control | Treatment | Control | |||||||
|
NCT 02513394 Mayer (2021) PALLAS |
III | BC | 0–1 | Palbociclib | 5760 | Palbociclib 125 mg qd + ET (n = 2883) versus ET (n = 2877) | 52 (45–61) | 2840 | 2930 | 13 | 5 | 1 | 0 | Publication |
|
NCT 03155997 Johnston (2020) MONARCHE |
III | BC | 0–1 | Abemaciclib | 5637 | Abemaciclib 150 mg bid + ET (n = 2808) versus ET (n = 2829) | 51 (23–89) | 2791 | 2800 | 76 | 33 | 10 | 1 | Publication |
|
NCT 02675231 Tolaney (2020) MONARCHER |
II | BC | 0–1 | Abemaciclib | 237 | Abemaciclib 150 mg bid + Trastuzumab + Fulvestrant (n = 79) Abemaciclib 150 mg bid + Trastuzumab (n = 79) versus Standard chemotherapy + Trastuzumab (n = 79) |
55 (47–62) 54 (47–62) |
78 77 |
72 / |
3 3 |
1 | 1 1 |
1 | Publication |
|
NCT 02763566 Zhang (2020) MONARCH plus |
III | BC | 0–1 | Abemaciclib | 463 | Abemaciclib 150 mg bid + NSAI (n = 207) versus Placebo + NSAI (n = 99) Abemaciclib 150 mg bid + Fulvestrant (n = 104) versus Placebo + Fulvestrant (n = 53) |
54 (32–83) 60 (36–80) |
207 104 |
99 53 |
13 1 |
3 1 |
1 0 |
1 0 |
Publication |
|
NCT 02422615 Slamon (2019) MONALEESA-3 |
III | BC | 0–1 | Ribociclib | 726 | Ribociclib 600 mg qd + NSAI (n = 484) versus Placebo + NSAI (n = 242) | 63 (31–89) | 327 | 161 | 6 | 2 | 1 | 0 | Publication |
|
NCT 02278120 Im (2019) MONALEESA-7 |
III | BC | 0–1 | Ribociclib | 672 | Ribociclib 600 mg qd + NSAI/Tamoxifen + Goserelin (n = 335) versus Placebo + NSAI/Tamoxifen + Goserelin (n = 337) | 43 (25–58) | 335 | 337 | 1 | 0 | 0 | 0 | Publication |
|
NCT 02246621 Goetz (2017) MONARCH-3 |
III | BC | 0–1 | Abemaciclib | 493 | Abemaciclib 150 mg bid + NSAI (n = 328) versus Placebo + NSAI (n = 165) | 63 (38–87) | 328 | 165 | 1 | 0 | 1 | 0 | Publication |
|
NCT 01740427 PALOMA-2 |
III | BC | 0–2 | Palbociclib | 666 | Palbociclib 125 mg bid + Letrozole (n = 444) versus Placebo + Letrozole (n = 222) | 61.7 | 444 | 222 | 2 | 0 | 2 | 0 | ClinicalTrials.gov |
|
NCT 02107703 MONARCH-2 |
III | BC | 0–1 | Abemaciclib | 669 | Abemaciclib 150 mg bid + Fulvestrant (n = 446) versus Placebo + Fulvestrant (n = 223) | 59.9 | 441 | 223 | 4 | 0 | 4 | 0 | ClinicalTrials.gov |
| NCT 02499120 | II | Head and neck | NA | Palbociclib | 125 | Palbociclib 125 mg qd + Cetuximab (n = 65) versus Placebo + Cetuximab (n = 60) | 59.5 | 64 | 60 | 1 | 1 | 1 | 1 | ClinicalTrials.gov |
| NCT 02450539 | II | NSCLC | 0–1 | Abemaciclib | 159 | Abemaciclib 200 mg bid (n = 106) versus Docetaxel (n = 53) | 63.5 | 106 | 52 | 3 | 1 | 3 | 1 | ClinicalTrials.gov |
|
NCT 02152631 JUNIPER |
III | NSCLC | 0–1 | Abemaciclib | 453 | Abemaciclib 200 mg bid (n = 270) versus Erlotinib (n = 183) | 62.5 | 265 | 175 | 4 | 3 | 4 | 3 | ClinicalTrials.gov |
Abbreviation: ECOG, Eastern Cooperative Oncology Group; SAEs, severe adverse events; T, treatment group; C, control group; BC, breast cancer; NSCLC, non-small cell lung cancer; qd, once daily; bid, twice daily; ET, endocrine therapy; NSAI, nonsteroidal aromatase inhibitor; NA, not available.
3.2. Incidence and risk of ILD/Pneumonitis
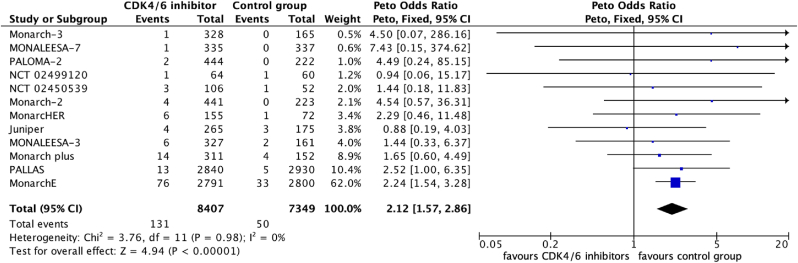
According to the 12 RCTs, the incidence of all-grade ILD/pneumonitis in patients treated with CDK4/6 inhibitors was 1.6% (131/8407) compared to 0.7% (50/7349) in the control group. CDK4/6 inhibitors significantly increased the risk of all-grade ILD/pneumonitis compared to the control group (Peto OR 2.12, 95% CI [1.57, 2.86], P < 0.00001, Fig. 2) with no significant heterogeneity across studies (I2 = 0%, χ2 P = 0.98). In addition, CDK4/6 inhibitors significantly increased both the risk of all-grade ILD and all-grade pneumonitis compared to the control group. (Peto OR 2.23, 95% CI [1.55, 3.21], P < 0.0001, Figure S2 in supplementary appendix; Peto OR 1.86, 95% CI [1.07, 3.25], P = 0.03, Figure S3 in supplementary appendix, respectively). In terms of the risk of grade 3 or higher ILD/pneumonitis, a significant association with CDK4/6 inhibitors was observed (Peto OR 3.22, 95% CI [1.28, 8.09], P = 0.01, Fig. 3), with event rates of 0.2% (16/7087) in the treatment group and 0.05% (3/6617) in the control group with no significant heterogeneity across studies (I2 = 0%, χ2 P = 0.62).
Fig. 2.
Pooled Peto odds ratio for all-grade ILD/pneumonitis in the treatment group compared to the control group.
Fig. 3.
Pooled Peto odds ratio for grade 3 or higher ILD/pneumonitis in the treatment group compared to the control group.
3.3. Subgroups analyses
We listed the incidence and risk of all-grade CDK4/6 inhibitor-related ILD/pneumonitis in the subgroups in Table 2. Higher Peto ORs of all-grade ILD/pneumonitis associated with CDK4/6 inhibitors were observed among trials of BC (Peto OR 2.24, 95% CI [1.63, 3.06]), combination therapy (Peto OR 2.22, 95% CI [1.63, 3.02]), phase III trials (Peto OR 2.16, 95% CI [1.58, 2.94]), endocrine therapy as control group (Peto OR 2.24, 95% CI [1.63, 3.07]), and CDK4/6 inhibitors as adjuvant therapy (Peto OR 2.28, 95% CI [1.61, 3.24]) compared to the trials of other cancer type, monotherapy, phase II trials, other control groups and other settings for CDK4/6 inhibitors. But none of the differences between these subgroups were statistically significant.
Table 2.
Subgroup analyses for the incidence and risk of all-grade ILD/pneumonitis related to CDK4/6 inhibitors.
| Group/Subgroup | No. of studies | ILD/pneumonitis |
|||||
|---|---|---|---|---|---|---|---|
| No. of patients |
Peto Odds Ratio |
95% CI | I [2] (%), |
P value | |||
| Treatment | Control | χ [2] p value | |||||
| Overall | 12 | 131/8407 (1.6%) | 50/7349 (0.7%) | 2.12 | 1.57, 2.86 | 0%, 0.98 | <0.00001 |
| Phase | 0.70 | ||||||
| II | 3 | 10/325 (3.1%) | 3/184 (1.6%) | 1.70 | 0.53, 5.44 | 0%, 0.85 | 0.37 |
| III | 9 | 121/8082 (1.5%) | 47/7165 (0.7%) | 2.16 | 1.58, 2.94 | 0%, 0.92 | < 0.00001 |
| Cancer type | 0.19 | ||||||
| BC | 9 | 123/7972 (1.5%) | 45/7062 (0.6%) | 2.24 | 1.65, 3.06 | 0%, 0.98 | < 0.00001 |
| Other | 3 | 8/435 (1.8%) | 5/287 (1.7%) | 1.02 | 0.33, 3.16 | 0%, 0.93 | 0.97 |
| CDK4/6 inhibitor assignation | 0.24 | ||||||
| monotherapy | 2 | 7/371 (1.9%) | 4/227 (1.8%) | 1.04 | 0.30, 3.58 | 0%, 0.71 | 0.95 |
| In combination withother chemotherapy | 10 | 124/8036 (1.5%) | 46/7122 (0.6%) | 2.22 | 1.63, 3.02 | 0%, 0.99 | < 0.00001 |
| CDK4/6 inhibitor | 0.92 | ||||||
| Palbociclib | 3 | 16/3348 (0.5%) | 6/3212 (0.2%) | 2.41 | 1.04, 5.59 | 0%, 0.73 | 0.04 |
| Abemaciclib | 7 | 108/4397 (2.5%) | 42/3639 (1.2%) | 2.10 | 1.51, 2.92 | 0%, 0.88 | < 0.00001 |
| Ribociclib | 2 | 7/662 (1.1%) | 2/498 (0.4%) | 1.77 | 0.44, 7.10 | 0%, 0.44 | 0.42 |
| Control group setting | 0.42 | ||||||
| Other chemotherapy | 2 | 9/261 (3.4%) | 2/124 (1.6%) | 1.93 | 0.54, 6.94 | 0%, 0.73 | 0.32 |
| Endocrine therapy | 8 | 117/7817 (1.5%) | 44/6990 (0.6%) | 2.24 | 1.63, 3.07 | 0%, 0.97 | < 0.00001 |
| Anti-EGFR agents | 2 | 5/329 (1.5%) | 4/235 (1.7%) | 0.89 | 0.23, 3.39 | 0%, 0.97 | 0.87 |
| CDK4/6 inhibitor setting | 0.44 | ||||||
| Adjuvant setting | 2 | 89/5631 (1.6%) | 38/5730 (0.7%) | 2.28 | 1.61, 3.24 | 0%, 0.82 | < 0.00001 |
| Other setting | 10 | 42/2776 (1.5%) | 12/1619 (0.7%) | 1.76 | 0.99, 3.10 | 0%, 0.96 | 0.05 |
Abbreviation: BC, breast cancer. Significant differences (P value < 0.05) between treatment group and control group are shown in bold.
Stratified by types of CDK4/6 inhibitors, the incidence of all-grade ILD/pneumonitis in patients treated with palbociclib, abemacicilb and ribociclib was 0.5% (16/3348), 2.5% (108/4397), and 1.1% (7/662), respectively. Compared with the control group, palbociclib and abemaciclib significantly increased the risk of CDK4/6 inhibitor-related ILD/pneumonitis (Peto OR 2.41, 95% CI [1.04, 5.59], P = 0.04; Peto OR 2.10, 95% CI [1.51, 2.92], P < 0.00001; respectively),while a similar risk was found between ribociclib and the control group (Peto OR 1.77, 95% CI [0.44, 7.10], P = 0.42). However, the test for subgroup differences suggested that there was no statistically significant subgroup effect (P = 0.92).
3.4. Sensitivity analyses and publication bias
Sensitivity analyses were performed to assess the stability of the meta-analysis. The exclusion of studies one by one did not result in significant alterations in outcomes (Table S1 in the supplementary appendix). There was no detectable asymmetry in the funnel plot suggesting a low risk of publication bias in our meta-analysis (Figure S4 in the supplementary appendix).
4. Discussion
To the best of our knowledge, this is the latest and most comprehensive meta-analysis to provide an evaluation of the incidence and risk of all-grade and grade 3 or higher ILD/pneumonitis in cancer patients treated with CDK4/6 inhibitors.
The efficacy of CDK4/6 inhibitors has been verified in several RCTs [[10], [11], [12], [13], [14], [15], [16], [17], [18]] primarily in patients with metastatic HR+/HER2- BC. According to the FDA drug labels, palbociclib, abemaciclib, and ribociclib can be used in combination with aromatase inhibitor or fulvestrant for HR+/HER2-advanced or metastatic BC patients, while only abemaciclib is available as monotherapy [[5], [6], [7]]. In addition, abemaciclib was approved by the FDA in 2021 with ET for adjuvant treatment of HR+/HER2-early BC patients with high risk of recurrence and a Ki-67 score ≥20% [8]. Trilaciclib can be used to decrease the incidence of myelosuppression for small cell lung cancer [9]. Furthermore, CDK4/6 inhibitors have shown consistent activity in preclinical models such as colon cancer [[32], [33], [34]] glioblastoma [35,36], prostate carcinoma, sarcomas [[37], [38], [39], [40], [41]], pancreatic ductal adenocarcinoma, melanoma, NSCLC, head and neck cancer and esophageal adenocarcinoma [42]. Considering the potential clinical use of CDK4/6 inhibitors, a thorough understanding of all associated toxicities is necessary to ensure that patients can achieve maximal clinical benefits with minimum risks. In general, CDK4/6 inhibitors are well-tolerated agents with predictable AEs [43]. Hematological toxicities such as neutropenia were especially associated with palbociclib and ribociclib, whereas abemaciclib showed a high rate of gastrointestinal toxicities such as diarrhea and ribociclib was associated with a high rate of liver injury and QTc prolongation [43,44]. Besides, increased thromboembolic events associated all CDK4/6 inhibitors have attracted clinical attention [45]. The major side effects associated with CDK4/6 inhibitors are similar, including neutropenia and gastrointestinal side effects [43,46,47]. With the continuously increasing use of CDK4/6 inhibitors, several cases of ILD/pneumonitis related to these agents have been reported, indicating a potential association between CDK4/6 inhibitors and ILD/pneumonitis [[48], [49], [50], [51], [52], [53]].
Our meta-analysis demonstrated a significantly increased risk of all-grade ILD/pneumonitis related to CDK4/6 inhibitors compared to the control group. According to a pharmacovigilance assessment study, CDK4/6 inhibitors were detected with signals of ILD as a class compared to all other drugs in the FDA Adverse Event Reporting System (FAERS) database, corresponding to reporting odds ratio (ROR) 1.50 (95% CI [1.28, 1.74]) [44]. The significantly increased risk of grade 3 or higher ILD/pneumonitis in the treatment group compared to the control group was shown in our study, which indicated that ILD/pneumonitis related to CDK4/6 inhibitors might result in serious outcomes in clinical practice. Of 117 patients who reported ILD/pneumonitis, 14 (12.0%) were grade 3 and 4, and 2 (1.7%) resulted in death with abemaciclib [12,24]. However, death was recorded in 29% (46/161) of CDK4/6 inhibitors related- ILD/pneumonitis cases in the real-world study [44]. Several reasons might explain the discrepency. First, AEs reported in ClinicalTrials.gov were not classified according to the Common Terminology Criteria for Adverse Events (CTCAE) [54], resulting in missing reports of deaths. Second, insufficient follow-up duration might lead to an underestimation of mortality.
In the subgroup analyses, the higher Peto ORs of all-grade ILD/pneumonitis were found in phase III trials, BC patients, patients treated with abemaciclib and palbociclib, patients treated with a CDK4/6 inhibitor in combination with other chemotherapy, endocrine therapy as control group, and CDK4/6 inhibitors as adjuvant treatment but none of these differences was statistically significant. Phase II trials, patients with other cancers, patients treated with ribociclib, and CDK4/6 inhibitors monotherapy might not have enough power to detect or rule out the differences due to the small sample size of these subgroups. Moreover, these subgroup analyses were performed for exploratory purposes only, and no definitive conclusions should be drawn. Studies based on individual patient data and large clinical trials are needed to determine the risk factors for the development of CDK4/6 inhibitor-related ILD/pneumonitis.
Among different CDK4/6 inhibitors, abemaciclib and palbociclib were noted to be statistically significantly associated with ILD/pneumonitis with Peto OR 2.10 (P < 0.00001) and 2.41 (P = 0.04), respectively. This finding was consistent with previous pharmacovigilance assessment study [55], in which the significant pharmacovigilance signals were found with abemaciclib and palbociclib (ROR 4.8, 95% CI [3.8, 6.1]; ROR 1.3, 95%CI [1.1, 1.4], respectively). It is speculated that abemaciclib may cause more AEs through the following points. First, compared with palbociclib and ribociclib, abemaciclib possesses unique pharmacological properties [44]. Second, abemaciclib exhibits a wider selectivity towards other CDKs and kinases, i.e., it has a low specificity for CDK4/6 [43,[55], [56], [57]]. Third, abemaciclib acts through other mechanisms of action in addition to inducing G1 cell cycle arrest, meaning that it may act independently of the CDK4/6-cyclin D-RB pathway [56,[58], [59], [60]]. Therefore, the potential toxicity of abemaciclib is presumed to be greater by the differences between abemaciclib and the other two CDK4/6 inhibitors.
The mechanisms of CDK4/6 inhibitor-related ILD/pneumonitis are still unclear. An in vivo study suggested that palbociclib augmented inflammatory cell recruitment (including macrophages and T cells) in the bronchoalveolar lavage fluid, which could be a consequence of the palbociclib-induced cell cycle arrest with relevant cellular senescence [44,61]. It was further proposed that in the presence of cell cycle inhibitor like palbociclib, the inflammatory cell milieu was altered, which could leading to cell senescence [51,62]. This speculation might explain the reason why CDK4/6 inhibitors caused lung injury.
In addition, previous real-world study based on the FAERS database showed that a median latency of 63 days (range 21–136) for CDK4/6 inhibitor-associated ILD [44]. This finding was in line with the results from an adverse event-time analysis of abemaciclib and palbociclib using data from Japanese Adverse Drug Event Report (JADER) database, suggesting that both abemaciclib and palbociclib were associated with the onset of ILD after 1–2 months from the start of treatment [55]. A retrospective study of drug-induced ILD demonstrated that, there was a certain similar trend in the timing of the occurrence of ILD induced by most anticancer drugs, most of which occurred within 3 months [63].
Our study has several limitations. First, five studies included were open-label, introducing high chances of performance bias and detection bias. Second, the severity of ILD/pneumonitis events was defined differently from electronic databases and ClinicalTrials.gov, with seven studies classified by the CTCAE [54], whereas the others classified by all AEs and severe adverse events (SAEs). Third, data were extracted from clinical trial results, and individual information on patient with ILD/pneumonitis was not available. Therefore, the potential risk factors for developing ILD/pneumonitis were not included in our study. Fourth, studies included were not specifically designed to assess the AEs, which might lead to an underestimation of ILD/pneumonitis related to CDK4/6 inhibitors. Finally, trials included were performed from multicenter, where the diagnostic criteria of ILD/pneumonitis and the ability to detect these events might be different. However, the strength of our study was that we set strict selection criteria with the most comprehensive studies included from both electronic databases and ClinicalTrials.gov, and the heterogeneity among included studies was low.
5. Conclusion
Our study demonstrated that patients treated with CDK 4/6 inhibitors had a higher risk of all-grade and grade 3 or higher ILD/pneumonitis compared to the control group. CDK4/6 inhibitor-related ILD/pneumonitis events are rare but may be life-threatening, which should attract clinical attention. Future studies are required to confirm the mechanism and explore the possible risk factors.
Author contributions
The data acquisition, statistical analysis, article drafting and revising were performed by Zhuo Ma and Yi Zhang. Xin Feng and Zhuoling An conceived and supervised the study. The final manuscript was read, checked and approved by all authors.
Funding source
None.
Ethical approval
None.
Declaration of competing interest
All authors have no significant disclosures.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.02.011.
Contributor Information
Xin Feng, Email: fengxin1115@126.com.
Zhuoling An, Email: anzhuoling@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.S H., F J., RL S., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.H N., Am N., K M., et al. National Cancer Institute; Bethesda, MD: 1975-2016. SEER cancer statistics review.https://seer.cancer.gov/csr/1975_2016/ based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 3.Kz T., Tw H., B S., S S., S A., O T.H. Venous thromboembolism risk in patients with hormone receptor-positive HER2-negative metastatic breast cancer treated with combined CDK 4/6 inhibitors plus endocrine therapy versus endocrine therapy alone: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res Treat. 2020;183(2):479–487. doi: 10.1007/s10549-020-05783-3. [DOI] [PubMed] [Google Scholar]
- 4.Ag W., W E.P. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 5.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103Orig1s012lbl.pdf.
- 6.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/209935s013lbl.pdf.
- 7.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208716s006s007s008lbl.pdf.
- 8.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208716s006s007s008lbl.pdf.
- 9.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214200s000lbl.pdf.
- 10.Rs F., Jp C., I L, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 11.Rs F., M M, Hs R., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 12.Nc T., J R, F A, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 13.Dickler M.N.T.S., Rugo H.S., Cortés J., Diéras V., Patt D., Wildiers H., Hudis C.A., O'Shaughnessy J., Zamora E., Yardley D.A., Frenzel M., Koustenis A., Baselga J. MONARCH 1, A phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory hr+/HER2- metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218–5224. doi: 10.1158/1078-0432.CCR-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jr S.G., M T, P N, et al. Monarch 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 15.Mp G., M T, M C, et al. Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 16.Gn H., Sm S., Ha B., et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 17.Dj S., P N, S C, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2019;382(6):514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 18.Sa I., Ys L., A B, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 19.https://www.clinicaltrials.gov/ct2/show/NCT02499770?term=02499770&draw=2&rank=1.
- 20.https://www.clinicaltrials.gov/ct2/show/NCT02514447?term=02514447&draw=1&rank=1.
- 21.https://www.clinicaltrials.gov/ct2/show/NCT03041311?term=03041311&draw=2&rank=1.
- 22.Boap M.J., D M, Pm B., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EL M., Ac D., M M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212–222. doi: 10.1016/S1470-2045(20)30642-2. [DOI] [PubMed] [Google Scholar]
- 24.Srd J., N H, R H, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020;38(34) doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sm T., Am W., S Z, et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21(6):763–775. doi: 10.1016/S1470-2045(20)30112-1. [DOI] [PubMed] [Google Scholar]
- 26.Qy Z., T S, Ym Y., et al. MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2- advanced breast cancer: the multinational randomized phase III study. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920963925. 1758835920963925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.https://www.clinicaltrials.gov/ct2/show/NCT01740427?term=01740427&draw=2&rank=1.
- 28.https://www.clinicaltrials.gov/ct2/show/results/NCT02107703?term=02107703&draw=2&rank=1.
- 29.https://www.clinicaltrials.gov/ct2/show/NCT02450539?term=02450539&draw=2&rank=1.
- 30.https://www.clinicaltrials.gov/ct2/show/NCT02499120?term=02499120&draw=2&rank=1.
- 31.https://www.clinicaltrials.gov/ct2/show/NCT02152631?term=02152631&draw=2&rank=1.
- 32.S F., Ds I., Cg R., et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol. 2018;8:608. doi: 10.3389/fonc.2018.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.F D., C M., C S., et al. Durable response to palbociclib and letrozole in ovarian cancer with CDKN2A loss. Cancer Biol Ther. 2020;21(3):197–202. doi: 10.1080/15384047.2019.1685291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.C A., F D., Am N., et al. Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut. 2018;67(12):2142–2155. doi: 10.1136/gutjnl-2017-315144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M K., Da S., E O, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70(8):3228–3238. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wr W., If D, Sn Q., et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci U S A. 2010;107(25):11501–11506. doi: 10.1073/pnas.1001613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.B J., T Bs, B S., et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42(8):715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.P M., S M.-G., Mp G., M J., C A. Efficacy of CDK4 inhibition against sarcomas depends on their levels of CDK4 and p16ink4 mRNA. Oncotarget. 2015;6(38):40557–40574. doi: 10.18632/oncotarget.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.M V., Mh H.-R., Ew S., et al. Targeting cyclin-dependent kinases in synovial sarcoma: palbociclib as a potential treatment for synovial sarcoma patients. Ann Surg Oncol. 2016;23(9):2745–2752. doi: 10.1245/s10434-016-5341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R S., JL B., Ap M., et al. Pharmacologic inhibition of cyclin-dependent kinase 4/6 activity arrests proliferation in myoblasts and rhabdomyosarcoma-derived cells. Mol Cancer Therapeut. 2006;5(5):1299–1308. doi: 10.1158/1535-7163. [DOI] [PubMed] [Google Scholar]
- 41.Yx Z., E S, Jt C., et al. Antiproliferative effects of CDK4/6 inhibition in CDK4-amplified human liposarcoma in vitro and in vivo. Mol Cancer Therapeut. 2014;13(9):2184–2193. doi: 10.1158/1535-7163.MCT-14-0387. [DOI] [PubMed] [Google Scholar]
- 42.Je K., Ah Z., An O., et al. CDK4/6 dual inhibitor abemaciclib demonstrates compelling preclinical activity against esophageal adenocarcinoma: a novel therapeutic option for a deadly disease. Oncotarget. 2017;8(59):100421–100432. doi: 10.18632/oncotarget.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.T M., S M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918793326. 1758835918793326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.R E., F M., A A., P E., Dp F. Cyclin-dependent kinase 4/6 inhibitors and interstitial lung disease in the FDA adverse event reporting system: a pharmacovigilance assessment. Breast Cancer Res Treat. 2021;186(1):219–227. doi: 10.1007/s10549-020-06001-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raschi E., Fusaroli M., Ardizzoni A., Poluzzi E., Dp F. Thromboembolic events with cyclin-dependent kinase 4/6 inhibitors in the FDA adverse event reporting system. Cancers (Basel) 2021 Apr 7;13(8):1758. doi: 10.3390/cancers13081758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lm S., ML Z., M B., B A. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: practical considerations and recommendations. Oncologist. 2017;22(9):1039–1048. doi: 10.1634/theoncologist.2017-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R R., A J., P A., et al. CDK4/6 inhibitors in breast cancer treatment: potential interactions with drug, gene, and pathophysiological conditions. Int J Mol Sci. 2020;21(17):6350. doi: 10.3390/ijms21176350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ka J., Gt B., N D, J A Can CDK4/6 inhibitors cause fatal lung injury? Expert Rev Anticancer Ther. 2019;19(11):917–919. doi: 10.1080/14737140.2019.1674651. [DOI] [PubMed] [Google Scholar]
- 49.K R., Na I., S B, S M., Sm G., C C.E. Palbociclib enhances pulmonary fibrosis in patients undergoing thoracic radiation therapy: a case series and review of the literature. Int J Radiat Oncol Biol Phys. 2018;102 [Google Scholar]
- 50.O L., E P., Y S., et al. Fatal palbociclib -related interstitial pneumonitis. Arch Clin Med Case Rep. 2019;3:162–166. [Google Scholar]
- 51.M N., J A., Ag A., Ao J., A S CDK 4/6 inhibitor induced lung injury: a case report and review of literature. Ecanc Med Sci. 2021;15:1245. doi: 10.3332/ecancer.2021.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.A G., Aa B., Ma E., A-T T. Ribociclib-induced pneumonitis: a case report. Breast Care (Basel) 2021;16(3):307–311. doi: 10.1159/000507647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.G J., M C, Kw Y., J W, Y Y, J M A single institution experience with palbociclib toxicity requiring dose modifications. Breast Cancer Res Treat. 2018;168(2):381–387. doi: 10.1007/s10549-017-4606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
- 55.N H., N T., Y K., G M., Z Y., I K. Cancer Rep; Hoboken): 2021. Evaluation of potential complication of interstitial lung disease with abemaciclib and palbociclib treatments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qy C., Zh K., NL B., et al. A unique CDK4/6 inhibitor: current and future therapeutic strategies of abemaciclib. Pharmacol Res. 2020;156:104686. doi: 10.1016/j.phrs.2020.104686. [DOI] [PubMed] [Google Scholar]
- 57.SL S., DL T., B K.L. HR+, HER2- advanced breast cancer and CDK4/6 inhibitors: mode of action, clinical activity, and safety profiles. Curr Cancer Drug Targets. 2017;17(7):637–649. doi: 10.2174/1568009617666170330120452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lm G., S C, X L, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest N Drugs. 2014;32(5):825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.G V., Mr V., A F, P V Abemaciclib: safety and effectiveness of a unique cyclin-dependent kinase inhibitor. Expet Opin Drug Saf. 2020;19(8):945–954. doi: 10.1080/14740338.2020.1781814. [DOI] [PubMed] [Google Scholar]
- 60.M M., B M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 61.B A., E B., B V., et al. CDK4/6 inhibition enhances pulmonary inflammatory infiltration in bleomycin-induced lung fibrosis. Respir Res. 2020;21(1):167. doi: 10.1186/s12931-020-01433-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.S R.R., S D.R. Safety and tolerability of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in Oncology. Drug Saf. 2019;42(2):181–198. doi: 10.1007/s40264-018-0772-x. [DOI] [PubMed] [Google Scholar]
- 63.M O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. 2012;13(1):39. doi: 10.1186/1465-9921-13-39. 2012 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.