Abstract
Negative pressure wound therapy (NPWT) has become the prevailing standard of care for treating complex soft tissue wounds and is now being considered for use in alternative applications including improving skin graft take. While it is generally agreed that negative pressure leads to improved wound healing, universal consensus on its optimal application is not supported in the literature. We describe the design and validation of a bioreactor to determine the prospective benefits of NPWT on skin grafts and engineered skin substitutes (ESS). Clinically relevant pressures were applied, and the native human skin was able to withstand greater negative pressures than the engineered substitutes. Both skin types were cultured under static, flow‐only, and −75 mm Hg conditions for 3 days. While it remained intact, there was damage to the epidermal‐dermal junction in the ESS after application of negative pressure. The normal skin remained viable under all culture conditions. The engineered skin underwent apoptosis in the flow‐only group; however, the application of negative pressure reduced apoptosis. Vascular endothelial growth factor levels were significantly higher in the normal flow‐only group, 152.0 ± 75.1 pg/mg protein, than the other culture conditions, 81.6 ± 35.5 pg/mg for the static and 103.6 ± pg/mg for the negative pressure conditions. The engineered skin had a similar trend but the differences were not significant. This bioreactor design can be used to evaluate the impacts of NPWT on the anatomy and physiology of skin to improve outcomes in wounds after grafting with normal or engineered skin.
Keywords: bioreactor, NPWT, skin graft, skin substitute
1. INTRODUCTION
Negative pressure wound therapy (NPWT) has become the prevailing standard of care for complex acute and chronic wounds; however, clinicians have yet to reach a consensus regarding the best practices and mechanisms of action. 1 While traditionally used when treating open wounds, NPWT is increasingly being used for closed wounds, fluid management, and as an adjunct to improve skin graft adherence of both meshed and sheet grafts. NPWT has been shown to lead to decreased graft failure rates, 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 decreased hospital stay, 3 , 15 reduced reoperation rate, 8 and a decreased number of complications, 12 , 13 , 14 along with increased patient satisfaction. 15 , 16 While frequently implemented for wound care in the paediatric population, additional studies are still warranted to establish standard treatment guidelines. 17 Currently, the NPWT devices are approved for wound closure and removal of exudate; however, use with skin grafts is not contraindicated per the U.S. Food and Drug Administration guidelines. 18
In vitro testing is an important complement to clinical trials and animal studies as it allows control of the environmental conditions, screen several conditions quickly, and study the anatomic and physiologic mechanisms of action of NPWT more easily. Multiple groups have studied the responses of cells and tissues to negative pressure. Many have chosen to apply negative pressure to a chamber in which culture dishes were placed. 19 , 20 , 21 While this method could be used with intact skin, it does not fully recapitulate the clinical use of NPWT. NPWT is traditionally applied via a tube with a suction cup placed on top of open‐cell foam with an impermeable adhesive layer placed on top; the pressure is measured at the pump and not at the wound site. While negative pressures, or suction, may be found directly under the suction cup, it is evident that there are also positive pressures that exert forces onto the edges of the tissue or wound being treated. These pressures also increase proportionally to an increase in pump pressure settings. 22 The magnitude of the pressure quickly dissipates with distance from the suction cup where the negative pressure originates leaving many questions regarding mechanisms of action, and the ability to correlate pressure settings to the physiology of wound healing. Wilkes et al. developed a bioreactor that applied negative pressure to cell‐seeded hydrogels in a modified six‐well dish. 23 This bioreactor incorporated the foam and adhesive with the pressure being applied centrally, similar to clinical applications. It also included medium flow below the hydrogels to provide nutrients for longer culture times. This system, or similar, was utilised to study responses to sub‐atmospheric pressures in osteogenic differentiation of adult stem cells, 24 , 25 morphological changes of fibroblasts, 26 dressing types on dermal fibroblast energetics, 27 and cellular proliferation and viability. 28 While a well‐designed system, it is unclear if it could be used to apply clinically relevant pressures to normal healthy skin (NHS) or engineered skin substitutes (ESS) that could be used for skin grafting.
The objective of this study was to develop a bioreactor that could be used to test responses to clinically relevant NPWT settings and applications by NHS and ESS in vitro. To accomplish this, an upper chamber where clinically relevant negative pressures could be applied and a lower chamber where nutrients would be supplied were both necessary. The upper chamber was designed to be able to test grafts of thicknesses ranging from full‐thickness to the thin cultured endothelial autografts (CEA). This paper presents the design and validation of a system intended to study the effects of NPWT and the mechanisms behind them.
2. MATERIALS AND METHODS
2.1. Bioreactor design for application of NPWT
A custom‐made bioreactor was used to apply NPWT to the NHS and ESS while providing nutrients to the grafts. The bioreactor is shown in Figure 1. It consists of four separate chambers for testing skin samples. The graft material was placed on top of a layer of wicking material (Whatman filter paper) on a perforated stainless‐steel grate. V.A.C. GranuFoam Silver™ Dressing (KCI, San Antonio, Texas) was placed on top of the graft. Negative pressure was applied using a Medela Vario 18 vacuum pump (Medela LLC‐Healthcare, McHenry, Illinois); a small suction cup was affixed on top of the dressing with adhesive V.A.C. drape (KCI). The suction cup size was chosen so that the graft samples would experience similar pressures as would be expected in vivo with a vacuum at the suction cup and compression on the edges. As performed clinically, the pressure was measured at the pump. Culture medium was circulated under the grafts; the medium reservoir included a tube with a filter on it for oxygen exchange. The culture medium composition is described below under ESS fabrication. The culture medium flow rate was controlled by a multi‐channel, analogue peristaltic pump (Cole‐Parmer, Vernon Hills, Illinois). Each sample had a separate flow loop where the medium was re‐circulated. Due to fact that the pump had analogue controls, the exact flow rate had to be empirically determined by measuring the amount of water pumped into a graduated cylinder in 1 minute. The lowest flow rate the pump could be achieved repeatably with the pump head and tubing that was used, at setting number one, was 4.64 ± 1.51 mL/min. This rate was considered appropriate because the rate was comparable to the optimal flow rates determined by Kalyanaraman et al. for ESS. 29 The bioreactor was sized such that it, along with the medium reservoirs, fit in a standard incubator and the wound vacuum and peristaltic pump just outside.
FIGURE 1.
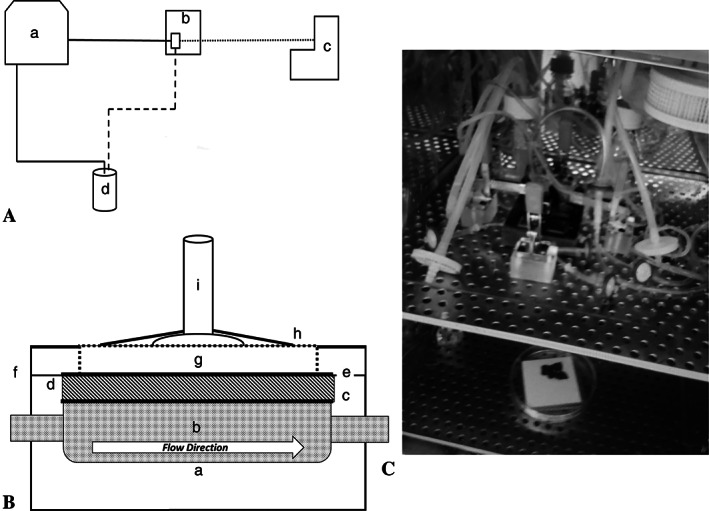
Custom‐made NPWT bioreactor. A, An overview of one flow loop. (a) The peristaltic pump is used to re‐circulate the culture medium through the chamber of the bioreactor (b), pulling the medium from the medium reservoir (d). The wound vac (c) is applied to the top of the sample in the bioreactor. B, Each sample is placed in an individual bioreactor chamber (a) through which the culture medium (b) can flow below the sample. A perforated stainless‐steel grate (c, solid line) allows for medium exchange and a wicking material (d, hashed) is placed on top to help with medium distribution. The skin or ESS (e, solid line) is placed on top of the wicking material (c). A medical sponge (g) is placed on top of the skin or ESS as would be done clinically. A suction port (i) is placed on top of the sponge and sterile adhesive dressing (h) is applied. A vacuum line or a sterile filter is attached to the vacuum cup for the NPWT or flow‐only conditions, respectively. C, The assembled bioreactor in the incubator. There are four separate channels with individual flow loops and culture medium. The peristaltic pump and the wound vac are outside of the incubator. ESS, engineered skin substitutes; NPWT, negative pressure wound therapy
2.2. Skin sources
Commercial allograft skin was procured and used as NHS; it was tested along with ESS that were fabricated in our laboratory. PureSkin™ cryopreserved non‐meshed grafts from two donors were purchased from AlloSource after proposal approval by the manufacturer (RR‐351). These grafts were stored at −80°C before use. The graft material was dissected under aseptic conditions while frozen before experimentation to fit the chambers and be used for paired testing. Graft samples were thawed before use per the manufacturer's recommendations.
The ESS were fabricated using cells isolated from de‐identified discard tissue from elective procedures performed at the University of Cincinnati Medical Center or Shriners Hospital for Children‐Cincinnati. The University of Cincinnati Institutional Review Board determined that this does not constitute human subjects research and is exempt from requirements for informed consent according to 45CFR46.101(b). 4 Human dermal fibroblasts (hDFs) and keratinocytes (hKs) were isolated and cultured as previously described. 30 Briefly, full‐thickness skin samples were incubated overnight at 4°C in Dispase II (Sigma‐Aldrich, St. Louis, Missouri). The epidermis was then mechanically separated from the dermis. To isolate the hDF, the dermis was minced and digested using type II collagenase (Worthington, Lakewood, New Jersey) with periodic agitation. The hDF culture medium consisted of Dulbecco's modified eagle medium (DMEM, Gibco, Grand Island, New York) supplemented with 4% vol/vol fetal bovine serum (FBS, Gibco), 10 ng/mL epidermal growth factor (EGF, Peprotech, Rocky Hill, New Jersey), 0.5 μg/mL hydrocortisone (Sigma‐Aldrich), 5 μg/mL human insulin (Sigma‐Aldrich), 0.1 M l‐ascorbic acid 2‐phosphate sesquimagnesium salt hydrate (AA2P, Sigma), and 1% vol/vol antibiotic‐antimycotic (Gibco). The hKs were isolated from the minced epidermis using 0.025% tyrpisin‐0.01% EDTA (Sigma) solution with gentle agitation. The hKs were cultured in flasks coated with recombinant human type I collagen (Coating Matrix; Invitrogen/Thermo Fisher Scientific) with selective hK culture medium consisting of modified MCDB153, which was prepared in‐house and supplemented with 1 ng/mL EGF, 5 μg/mL human insulin, 0.5 mg/mL hydrocortisone, 1% antibiotic‐antimycotic, and 0.2% bovine pituitary extract (Hammond Cell Tech, Windsor, California). Cells were expanded and utilised between passages 1 and 3. Cells from two separate donors were used.
2.3. ESS fabrication
ESS were fabricated using the process adapted from Boyce. 31 Briefly, collagen‐glycosaminoglycan scaffolds (thickness between 0.25 and 0.35 mm) were inoculated with hDF (at 5 × 105 cells/cm2) and cultured in hDF culture medium. The hDF‐inoculated scaffolds were incubated for 2 days before hKs were inoculated (at 1 × 106 cells/cm2), at which time the medium was changed to DMEM/F12 (Sigma‐Aldrich) supplemented with 1 mM strontium chloride, 0.3% vol/vol FBS, 1xITS Supplement (Sigma‐Aldrich), 10 μg/mL linoleic acid, 5 ng/mL keratinocyte growth factor (Peprotech), 0.1 mM AA2P, 20 pm triiodothyronine, 5 μg/mL hydrocortisone, 1 ng/mL basic FGF, and 1% vol/vol antibiotic‐antimycotic for the duration of the fabrication process. 32 Three days after seeding the hK, the ESS were raised to the liquid‐air interface and cultured for an additional 10 days before bioreactor culture. Three sets of ESS were fabricated with each patient‐matched set of donor cells. The ESS were dissected into samples for testing under the different bioreactor conditions.
2.4. Validation of the NPWT bioreactor
The maximum pressure achievable without damaging the tissue was determined. For the purpose of initial validation, damage is defined as tears that could be assessed macroscopically. After assembly, the pressure was manually increased to failure, ie, when pressures could not be further increased. Once the bioreactor was disassembled, the visible damage to the tissue could be seen. All of the samples were able to maintain some level of clinically relevant pressures. Morykwas et al. 33 reported the use of −125 mm Hg pressures and this pressure has remained the most used setting ever since. 34 Settings as low as −20 mm Hg have been tested 35 , 36 ; −75 mm Hg is the most used for skin graft settings. 37 , 38 , 39 The NHS could withstand −125 mm Hg pressure; however, the ESS was only able to achieve −75 mm Hg; therefore, −75 mm Hg was used for validation testing.
For testing the samples under NPWT, the NHS and ESS were divided into 2.5 cm × 3 cm samples. Three conditions were used for each skin type: (a) −75 mm Hg of negative pressure applied to the skin in the bioreactor with circulating medium (NPWT); (b) 0 mm Hg negative pressure in the bioreactor with circulating medium (flow‐only), and (c) static controls cultured under standard conditions in the same incubator (static). For the flow‐only controls, the sponge dressing was applied and a suction port with a sterile syringe filter was affixed in place via the V.A.C. drape. The culture medium for the static samples was changed daily, and each of the samples in the bioreactor had separate channels for 250 mL of circulating medium. All three groups were incubated under standard conditions (37°C, 5% CO2) for 3 days or 72 hours. At the end of the incubation period, the skin samples were divided, and were placed in lysis buffer for protein assays, fixed in buffered formalin for histological evaluation, or stored at −80°C before analysis.
Two sets of paired samples were run at a time for the validation testing with a total of three runs per sample type. This leads to an n of 12 for each condition. Two of the chambers had NPWT applied and the other two chambers each had a vacuum cup with a filter affixed for oxygen exchange instead of a connection to the NPWT pump. Static controls were cultured at the liquid‐air interface in a large culture dish on the bottom shelf of the same incubator.
2.5. VEGF, DNA, and protein quantification
The skin samples were homogenised in RIPA buffer (MilliporeSigma, St. Louis, Missouri) and stored at −80°C prior to any testing. Vascular endothelial growth factor (VEGF) levels in the tissue homogenates were determined by ELISA (R&D Systems, Minneapolis, Minnesota) per manufacturer protocols. DNA quantification was performed using Hoechst 33258 dye in a 7.4 pH Tris‐EDTA buffer (MilliporeSigma) for 15 minutes at room temperature. Samples were run in duplicate. The fluorescence was determined using a SpectraMax fluorometer (Molecular Devices, San Jose, California) at an excitation of 365 and an emission of 458 nm. Calf thymus DNA standards (MilliporeSigma) were used for the standard curve. The DNA quantities in the tissue were normalised to protein content, which was determined by Pierce™ BCA assay (ThermoFisher, Rockford, Illinois) per manufacturer's guidelines. Duplicates of all samples were run in the assays, and the two data points were averaged together.
2.6. Histological evaluation
The samples were paraffin‐embedded and 5 μm sections were cut. Sections were stained with Masson's trichrome to assess morphology and collagen density. Viability was assessed via the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay (ab66110, Abcam, Cambridge, Massachusetts). Hoechst dye (Millipore) was used to counterstain the nuclei. The images of the Masson's trichrome stained sections were taken with a Nikon DS‐Vi1 camera on a Nikon SMX745T stereomicroscope (Nikon, Melville, New York). The TUNEL stained sections were imaged using the Cytation 5 Cell Imaging Multi‐Mode Reader (BioTek, Winooski, Vermont).
2.7. Statistical analysis
Quantitative data are presented as mean ± SE of the mean. Multivariate analysis of variance (MANOVA) was performed to compare the VEGF and DNA contents of the NHS samples to those of the ESS. The Bartlett test of homogeneity of variances and Mardia's multivariate normality test were used to check the assumptions of the MANOVA, and a post hoc Hotelling's t‐squared test was applied. P value <.05 was considered statistically significant.
3. RESULTS
3.1. Grafts were able to withstand clinically relevant pressures
Clinically relevant values were achieved for both the ESS and NHS, −75 and −125 mm Hg respectively, during preliminary testing. The ESS, though much thinner than the NHS, did not display any visible signs of damage when either −75 or −125 mm Hg was applied, so we elected to use the −75 mm Hg setting for both skin types to make comparisons between the two tissue types.
Masson's trichrome staining was used to visualise the collagen in the ESS as well as the gross histology after culture with negative pressure or control conditions (Figure 2). In both tissues, there is denser collagen in the dermis of the samples that were cultured in the bioreactor regardless of the application of NPWT. The NHS remained intact in all conditions. Under NPWT, the epidermal layer of the EDS remained attached; however, there appeared to be disruption to the epidermal‐dermal junction as the clear distinction between the two layers is lost (Figure 2). Additionally, this damage appears to be more towards the centre of the graft where the suction port was placed. The edges of the ESS that were exposed to NPWT looked similar to those in the flow‐only group.
FIGURE 2.
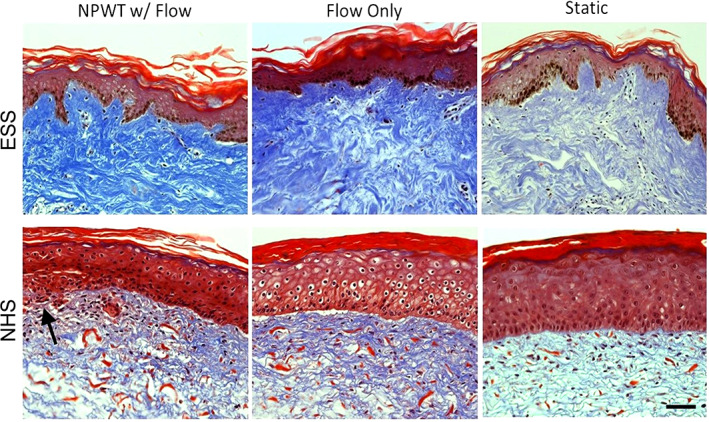
Masson's Trichrome staining. The collagen looks denser in both the flow‐only and negative pressure wound therapy samples compared with static for both skin types. While the epidermis is still intact, the NPWT does appear to cause some damage to the ESS but not to the NHS (indicated by arrow). Scale bar = 50 μm. ESS, engineered skin substitutes; NHS, normal healthy skin
3.2. Changes in DNA and VEGF contents
The DNA contents of the homogenised ESS and NHS were evaluated for all culture conditions. DNA content was used as a surrogate for proliferation. The DNA content was statistically lower in the NHS than in the ESS for each of the three test conditions (Figure 3); however, there were no differences among the DNA contents due to incubation conditions for either the ESS or the NHS.
FIGURE 3.
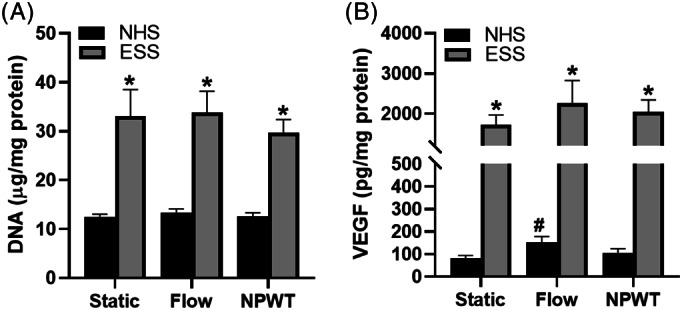
DNA and VEGF contents in grafts. A, The DNA content did not significantly change with any of the culture conditions; however, it was significantly higher in the ESS than in the NHS. B, VEGF content changes showed a similar pattern between the ESS and the NHS due to treatment; however, only the NHS had significantly more VEGF after flow alone than static culture. The VEGF content was significantly higher in the ESS than in the NHS. n = 12; *P < .05 compared with NHS of the same treatment; # P < .05 compared with static control of same skin type. ESS, engineered skin substitutes; NHS, normal healthy skin
VEGF, which promotes angiogenesis, was assayed and normalised to DNA content. The normalised VEGF content was statistically different between the NHS and the ESS for each of the three test conditions (Figure 3). Both skin types yielded the lowest VEGF content in the static condition samples and the highest VEGF content in the flow condition samples; however, this difference was only statistically significant within the ESS flow group. No other significant differences in VEGF were found.
3.3. Cell viability
The viability of the NHS and ESS was determined by TUNEL assay (Figure 4) where fragmented DNA is labelled fluorescent red. Fragmented DNA may represent apoptosis. In all cases, the NHS had very low levels of cell death with slightly more apoptotic cells in the dermis of the skin undergoing NPWT. Overall, the ESS had more apoptotic cells and more cells than the NHS. ESS cultured in static conditions had fewer apoptotic cells than those cultured in the bioreactor, and the apoptotic cells were often in the epidermis. The ESS subjected to the flow conditions had significant numbers of apoptotic cells that stained very brightly. When NPWT was applied along with flow, the number of stained cells was reduced, and apoptotic cells were more prevalent in the dermis.
FIGURE 4.
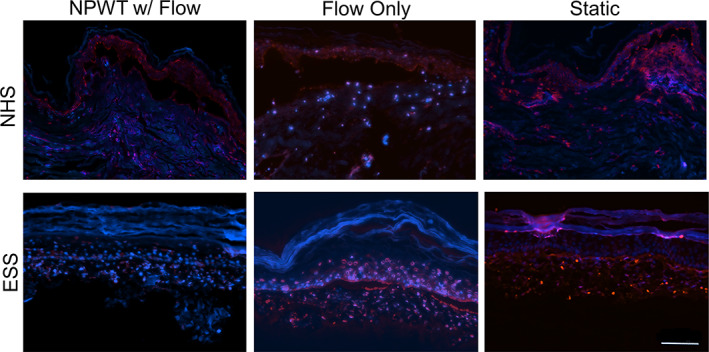
TUNEL staining. Red staining indicates DNA nicks, blue DNA counterstaining was used for comparison. Some red autofluorescence can be seen, especially in the epidermis. The NHS remained highly viable in all culture conditions. The ESS showed significant DNA damage in the Flow‐only culture; however, this was reduced with the application of negative pressure. The static controls had minimal DNA damage. Scale bar = 100 μm. ESS, engineered skin substitutes; NHS, normal healthy skin; TUNEL, transferase dUTP nick end labelling;
4. DISCUSSION
This is the first bioreactor described that can test both NHS and ESS using a system that replicates the clinical application of NPWT in vitro. Another similar bioreactor reported in the literature is designed to test cell‐seeded hydrogels. 23 The decision to perform testing for 3‐day periods was intended to follow clinical conventional protocols in wound care aimed at removing saturated sponges, reducing microbial load, and providing gentle debridement of the wound. 33 Our bioreactor design was able to incubate NHS for 3 days without significant cell death. The ESS that were tested did undergo some apoptosis; however, this was attenuated when negative pressure was applied. This result is interpreted as due to the NPWT improving the mass transfer of nutrients and mitogens into the samples. This follows the hypothesis that negative and positive pressure may lead to cellular strain and stretch and thus stimulate proliferation and differentiation. Investigation of this hypothesis is part of our ongoing studies. The cell death in the ESS may be due to the choice of silver‐coated foam dressing. Results from a previous study suggest that the ionic silver‐coated material may be cytotoxic to cells in ESS. 40 Unfortunately, there was no control for the dressing so this cannot be confirmed at this point and we cannot rule out issues with the mass transfer of nutrients. Longer‐term studies can be conducted prospectively on NHS with the current design while additional work should be continued to improve the viability outcomes of the ESS. Improvements may include alternative media formulations, changes to the flow rates or wicking material to facilitate nutrient exchange and the viability of the ESS. When grafting ESS, the actual situation is likely somewhere between the flow and static conditions. There is not a large volume of medium or blood; however, based on the clinical success of the graft materials, 41 the wound bed likely provides more nutrient supply through imbibition than what the ESS were getting in the flow condition.
Samples incubated in the bioreactor had denser collagen in the dermal layer than those cultured in static conditions. NPWT may have resulted in compression of the dermis, as we saw previously 42 ; however, the flow‐only groups also had denser collagen than the static samples. This suggests that there was some collagen synthesis. There may be compression due to the application of the sponge dressing and suction port; however, it would be expected that the compression would be greater with the application of NPWT. The flow itself may have helped with the circulation of nutrients, which may stimulate collagen synthesis. Further work needs to be done to confirm this. VEGF levels in both NHS and ESS had similar patterns under the different incubation conditions; however, the only significant difference compared with control was the ESS flow group. The differences in VEGF levels between the NHS and ESS are likely because the scaffold used for the ESS is a hydrogel. It is less dense than native tissue and therefore there is less protein content, and it is more hydrated and will contain more of the secreted VEGF than the NHS. There is likely less oxygen supplied to the tissues in the bioreactor than what is available in the static condition; an increase in VEGF levels is likely due to lower oxygen levels and increased hypoxia‐inducible factor in these tissues. 43 , 44
Based on these preliminary results, it is evident that NPWT may lead to favourable physiologic conditions allowing improved viability and engraftment success of the skin graft materials. In the case of ESS or epithelial autografts, a lower pressure should be used as even pump pressures as low as −75 mm Hg caused damage to the dermal‐epidermal junction in the ESS. This may also be a concern for open wounds. These data regarding effects of higher negative and positive pressure on dermal‐epidermal structures would suggest the pump pressure setting should be adjusted over time to the specific wound morphology, and that a single pressure setting utilised may not be optimal for all wound healing applications.
The described bioreactor was designed to be able to accommodate grafts of varying thickness, from full‐thickness to thin CEA. The data presented are from split‐thickness allografts and thin ESS that includes both epithelium and a thin dermis. One preliminary test using full‐thickness skin was completed and was viable for the duration of the study; however, the data were not included as there were no replicates. While we did not test CEAs, we are confident that we could test CEA by placing it on the same wicking material used for the ESS. The negative pressure would likely need to be reduced for CEA such as was reported by Goh et al. 45 While this was designed for skin grafts, it could be used for additional tissues such as the muscle layer of the wound or possibly for wound interface studies. It is limited to a depth of approximately 6 mm; however, the thicker the tissue the more difficult it is to provide nutrients to the top layers. The design could be modified to improve this and to increase the area of the tissue that could be tested; however, to test significantly deeper wounds, a different bioreactor design would be required.
The bioreactor designed for this study served as an affordable, reusable device that can support an in vitro method for comparing ESS and NHS to supplement human and animal studies. While an in vitro model will not include the complete, complex physiology that an in vivo study would provide, an in vitro skin study offers more control over mechanisms of action in the samples than studies involving live animal or human subjects. This model can be modified to further investigate the optimisation of NPWT, and if this optimisation leads to shorter treatment times or fewer dressing changes, it could potentially increase the rate of patient compliance in this therapy option. 46
Any in vitro system such as this has inherent limitations; these can aid in reducing variation to understand some mechanisms of action in wound healing, but also decrease the biologic complexity that exists in vivo. Clinically relevant results were desired, so the pressure supplied was measured at the vacuum pump. However, this does not reflect the pressures that are seen in the tissue, which will vary based on the area of the graft, depth of the wound, potential drop of pressure over the length of the vacuum tube, and distance from the application of negative pressure. Differences in the area and thickness of the samples could also affect the actual pressures in the tissues. Commercial allograft material was chosen to reduce the variation in the thickness and processing of the allografts. A template was used to assist with cutting the allograft and ESS sections to size for the bioreactor; however, variability does occur. Only three of the four possible culture conditions were used, making it more challenging to delineate what results are due to NPWT alone versus what may be due to the combinations of NPWT and flow. An NPWT only sample was not tested due to concerns with cell death due to lack of nutrients without having medium circulating under the sample. A time zero sample may have provided more information on changes in cellularity; however, for validation, we were more concerned with differences between conditions. The results are only based on 3 days of testing; longer time frames are likely to provide more information, especially at the protein level. The material tested was non‐meshed allograft or ESS, not an open wound. There were no variations in the depth or size of the wound modelled in this study. The ESS contained only a small number of cell types in a much less dense material, it lacked an immune system, and a simplified culture medium was used instead of blood or plasma. Any interpretations must take these factors into account; however, this system can be used as a valuable complement to in vivo testing. The bioreactor design provides nutrients to the graft by wicking medium from the reservoir below the tissues. This assumes a fairly vascularised tissue. This design would need to be validated to see if the results replicated those of poorly vascularised tissues such as those in the foot. Shon et al. 47 found that oxygenation in the foot was reduced with the application of NPWT, unlike what was found in more vascularised tissues. Similar to other NPWT bioreactor designs, 19 , 20 , 21 , 23 , 24 , 25 , 26 , 27 , 28 this model does not provide any means of quantifying the exact pressure applied at the wound site. This is something that should be explored and would be best done using finite element modelling.
In conclusion, the bioreactor described here can be used to test NHS and ESS at physiologically relevant negative pressures. The ESS have similar responses to the NHS; however, they may not always have the same responses and use as a surrogate to NHS should be carefully considered. The ESS, having less strength and cell density than native tissue, may provide insights into the use of NPWT on dermal templates and epithelial autografts. Future studies testing NPWT on tissue before and after grafting to wounds on immunocompromised mice may provide benefits as there are significant changes to the ESS after in vivo culture and remodelling. These tissues may benefit from NPWT, but lower pressures may be desired based on the histologic evaluation of the ESS. More work to understand different pressure protocols and results at different time points is needed, but we now have a device that can screen these conditions in vitro and can be used to better understand the mechanisms involved and to screen for the most promising conditions to test in vivo.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the following individuals for their contributions to this study: Ron Hudepohl, Jacob Bertrand, PhD, Melanie Weyant, and the 1819 Innovation Hub for assistance in designing and fabricating the bioreactor; Christopher M. Lloyd, Victoria Dershem, and Jennifer Hahn for assistance with the in vitro studies with ESS; and Marepalli Rao, PhD for the help in identifying the appropriate statistical analysis. Histology services were provided by the University of Cincinnati Histopathology Core Laboratory. This work was supported by startup funding from Shriners Hospitals for Children.
Notorgiacomo G, Klug J, Rapp S, Boyce ST, Schutte SC. A bioreactor for studying negative pressure wound therapy on skin grafts. Int Wound J. 2022;19(3):633-642. 10.1111/iwj.13661
Funding information Shriners Hospitals for Children; University of Cincinnati
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum‐assisted closure (VAC) device: a review. Am J Clin Dermatol. 2005;6(3):185‐194. [DOI] [PubMed] [Google Scholar]
- 2. Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg. 2002;137(8):930‐933. [DOI] [PubMed] [Google Scholar]
- 3. Llanos S, Danilla S, Barraza C, et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double‐masked, controlled trial. Ann Surg. 2006;244(5):700‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petkar KS, Dhanraj P, Kingsly PM, et al. A prospective randomized controlled trial comparing negative pressure dressing and conventional dressing methods on split‐thickness skin grafts in burned patients. Burns. 2011;37(6):925‐929. [DOI] [PubMed] [Google Scholar]
- 5. Jeschke MG, Rose C, Angele P, Fuchtmeier B, Nerlich MN, Bolder U. Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative‐pressure therapy for reconstruction of acute and chronic wounds. Plast Reconstr Surg. 2004;113(2):525‐530. [DOI] [PubMed] [Google Scholar]
- 6. Molnar JA, DeFranzo AJ, Hadaegh A, Morykwas MJ, Shen P, Argenta LC. Acceleration of Integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg. 2004;113(5):1339‐1346. [DOI] [PubMed] [Google Scholar]
- 7. Inatomi Y, Kadota H, Kamizono K, Hanada M, Yoshida S. Securing split‐thickness skin grafts using negative‐pressure wound therapy without suture fixation. J Wound Care. 2019;28(Sup8):S16‐S21. [DOI] [PubMed] [Google Scholar]
- 8. Yin Y, Zhang R, Li S, Guo J, Hou Z, Zhang Y. Negative‐pressure therapy versus conventional therapy on split‐thickness skin graft: a systematic review and meta‐analysis. Int J Surg. 2018;50:43‐48. [DOI] [PubMed] [Google Scholar]
- 9. Shen X, Zhan T, Wei D, Zhang H. Comparison of efficacy and complications between negative pressure wound therapy and conventional mechanical fixation in skin grafts: a retrospective analysis. Wounds. 2019;31(8):213‐218. [PubMed] [Google Scholar]
- 10. Leong S, Lo ZJ. Use of disposable negative pressure wound therapy on split‐thickness skin graft recipient sites for peripheral arterial disease foot wounds: a case report. Int Wound J. 2020;17(3):716‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pereima MJL, Feijo R, Oenning da Gama F, de Oliveira Boccardi R. Treatment of burned children using dermal regeneration template with or without negative pressure. Burns. 2019;45(5):1075‐1080. [DOI] [PubMed] [Google Scholar]
- 12. Mujahid AM, Khalid FA, Ali N, Sajjad Y, Khan H, Tarar MN. Vacuum‐assisted closure in integration of skin graft over scalp wounds: a randomised control trial. J Coll Phys Surg Pak. 2020;30(2):163‐167. [DOI] [PubMed] [Google Scholar]
- 13. Diehm YF, Fischer S, Gazyakan E, et al. Negative pressure wound therapy as an accelerator and stabilizer for incorporation of artificial dermal skin substitutes—a retrospective, non‐blinded, and non‐randomized comparative study. J Plast Reconstr Aesthet Surg. 2021;74:357‐363. [DOI] [PubMed] [Google Scholar]
- 14. Maduba CC, Nnadozie UU, Modekwe VI, Onah II. Split skin graft take in leg ulcers: conventional dressing versus locally adapted negative pressure dressing. J Surg Res. 2020;251:296‐302. [DOI] [PubMed] [Google Scholar]
- 15. Maduba CC, Nnadozie UU, Modekwe VI, Nwankwo EU. Comparing hospital stay and patient satisfaction in a resource poor setting using conventional and locally adapted negative pressure wound dressing methods in management of leg ulcers with split skin grafts: a comparative prospective study. Pan Afr Med J. 2020;36:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kantak NA, Mistry R, Varon DE, Halvorson EG. Negative pressure wound therapy for burns. Clin Plast Surg. 2017;44(3):671‐677. [DOI] [PubMed] [Google Scholar]
- 17. Pedrazzi NE, Naiken S, La Scala G. Negative pressure wound therapy in pediatric burn patients: a systematic review. Adv Wound Care. 2021;10(5):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. U.S. Food and Drug Administration . Non‐powered Suction Apparatus Device Intended for Negative Pressure Wound Therapy (NPWT) ‐ Class II Special Controls Guidance for Industry and FDA Staff. 2018.
- 19. Liu W, Fu X, Yang Z, et al. Moderate intermittent negative pressure increases invasiveness of MDA‐MB‐231 triple negative breast cancer cells. Breast. 2018;38:14‐21. [DOI] [PubMed] [Google Scholar]
- 20. Pandit V, Nesbitt SR, Kim DY, Mixon A, Kotha SP. Combinatorial therapy using negative pressure and varying lithium dosage for accelerated wound healing. J Mech Behav Biomed Mater. 2015;44:173‐178. [DOI] [PubMed] [Google Scholar]
- 21. Dong J, Qing C, Song F, Wang X, Lu S, Tian M. Potential molecular mechanisms of negative pressure in promoting wound healing. Int Wound J. 2020;17(5):1428‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kairinos N, Solomons M, Hudson DA. The paradox of negative pressure wound therapy—in vitro studies. J Plast Reconstr Aesthet Surg. 2010;63(1):174‐179. [DOI] [PubMed] [Google Scholar]
- 23. Wilkes RP, McNulty AK, Feeley TD, Schmidt MA, Kieswetter K. Bioreactor for application of subatmospheric pressure to three‐dimensional cell culture. Tissue Eng. 2007;13(12):3003‐3010. [DOI] [PubMed] [Google Scholar]
- 24. Zhu J, Yu A, Qi B, Li Z, Hu X. Effects of negative pressure wound therapy on mesenchymal stem cells proliferation and osteogenic differentiation in a fibrin matrix. PLoS One. 2014;9(9):e107339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu H, Zheng X, Chen L, et al. Negative pressure wound therapy promotes muscle‐derived stem cell osteogenic differentiation through MAPK pathway. J Cell Mol Med. 2018;22(1):511‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu F, Ogawa R, Nguyen DT, et al. Microdeformation of three‐dimensional cultured fibroblasts induces gene expression and morphological changes. Ann Plast Surg. 2011;66(3):296‐300. [DOI] [PubMed] [Google Scholar]
- 27. McNulty AK, Schmidt M, Feeley T, Villanueva P, Kieswetter K. Effects of negative pressure wound therapy on cellular energetics in fibroblasts grown in a provisional wound (fibrin) matrix. Wound Repair Regen. 2009;17(2):192‐199. [DOI] [PubMed] [Google Scholar]
- 28. McNulty AK, Schmidt M, Feeley T, Kieswetter K. Effects of negative pressure wound therapy on fibroblast viability, chemotactic signaling, and proliferation in a provisional wound (fibrin) matrix. Wound Repair Regen. 2007;15(6):838‐846. [DOI] [PubMed] [Google Scholar]
- 29. Kalyanaraman B, Boyce ST. Wound healing on athymic mice with engineered skin substitutes fabricated with keratinocytes harvested from an automated bioreactor. J Surg Res. 2009;152(2):296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boyce ST. Methods for the serum‐free culture of collagen‐GAG‐based skin substitutes. In: Morgan JR, Yarmusch ML, eds. Methods in Molecular Medicine, Vol 18: Tissue Engineering Methods and Protocols. Totowa, NJ: Humana Press; 1999:365‐389. [DOI] [PubMed] [Google Scholar]
- 31. Boyce ST. Design principles for composition and performance of cultured skin substitutes. Burns. 2001;27(5):523‐533. [DOI] [PubMed] [Google Scholar]
- 32. Boyce ST, Lloyd CM, Kleiner MC, Swope VB, Abdel‐Malek Z, Supp DM. Restoration of cutaneous pigmentation by transplantation to mice of isogeneic human melanocytes in dermal‐epidermal engineered skin substitutes. Pigment Cell Melanoma Res. 2017;30(6):531‐540. [DOI] [PubMed] [Google Scholar]
- 33. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553‐562. [DOI] [PubMed] [Google Scholar]
- 34. Malmsjο M, Borgquist O. NPWT setting and dressing choices made easy. Wounds Int. 2010;1(3):1‐6. [Google Scholar]
- 35. Borgquist O, Gustafsson L, Ingemansson R, Malmsjo M. Micro‐ and macromechanical effects on the wound bed of negative pressure wound therapy using gauze and foam. Ann Plast Surg. 2010;64(6):789‐793. [DOI] [PubMed] [Google Scholar]
- 36. Borgquist O, Ingemansson R, Malmsjo M. The influence of low and high pressure levels during negative‐pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg. 2011;127(2):551‐559. [DOI] [PubMed] [Google Scholar]
- 37. Joo HS, Lee SJ, Lee SY, Sung KY. The efficacy of negative pressure wound therapy for Split‐thickness skin grafts for wounds on the trunk or the neck: a randomized controlled trial. Wounds. 2020;32(12):334‐338. [PubMed] [Google Scholar]
- 38. Evangelista MS, Kim EK, Evans GR, Wirth GA. Management of skin grafts using negative pressure therapy: the effect of varied pressure on skin graft incorporation. Wounds. 2013;25(4):89‐93. [PubMed] [Google Scholar]
- 39. Gupta S. Optimal use of negative pressure wound therapy for skin grafts. Int Wound J. 2012;9(Suppl 1):40‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Supp AP, Neely AN, Supp DM, Warden GD, Boyce ST. Evaluation of cytotoxicity and antimicrobial activity of Acticoat burn dressing for management of microbial contamination in cultured skin substitutes grafted to athymic mice. J Burn Care Rehabil. 2005;26(3):238‐246. [PubMed] [Google Scholar]
- 41. Boyce ST, Simpson PS, Rieman MT, et al. Randomized, paired‐site comparison of autologous engineered skin substitutes and Split‐thickness skin graft for closure of extensive, full‐thickness burns. J Burn Care Res. 2017;38(2):61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rapp SJ, Dershem V, Zhang X, Schutte SC, Chariker ME. Varying negative pressure wound therapy acute effects on human Split‐thickness autografts. J Burn Care Res. 2020;41(1):104‐112. [DOI] [PubMed] [Google Scholar]
- 43. Tufro‐McReddie A, Norwood VF, Aylor KW, Botkin SJ, Carey RM, Gomez RA. Oxygen regulates vascular endothelial growth factor‐mediated vasculogenesis and tubulogenesis. Dev Biol. 1997;183(2):139‐149. [DOI] [PubMed] [Google Scholar]
- 44. Ramakrishnan S, Anand V, Roy S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol. 2014;9(2):142‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goh BKL, Chua AWC, Chew KY, et al. The use of negative‐pressure wound therapy over a cultured epithelial autograft for full‐thickness wounds secondary to purpura fulminans in an infant. Arch Plast Surg. 2021;48(3):338‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Capobianco CM, Zgonis T. An overview of negative pressure wound therapy for the lower extremity. Clin Podiatr Med Surg. 2009;26(4):619‐631. [DOI] [PubMed] [Google Scholar]
- 47. Shon YS, Lee YN, Jeong SH, Dhong ES, Han SK. Influence of negative‐pressure wound therapy on tissue oxygenation of the foot. Arch Plast Surg. 2014;41(6):668‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


