Abstract
As the use of closed incision negative pressure therapy (ciNPT) becomes more widespread, dressing designs have evolved to address implementation challenges and meet surgeon demand. While traditional application of ciNPT was limited to the immediate suture line, a novel dressing that covers the incision and additional surrounding tissues has become available. To expand upon previous ciNPT recommendations and provide guidance on this new dressing, an expert panel of plastic surgeons convened to review the current literature, identify challenges to the implementation and sustainability of ciNPT, and use a modified Delphi technique to form a consensus on the appropriate use of ciNPT with full‐coverage dressings. After three rounds of collecting expert opinion via the Delphi method, consensus was reached if 80% of the panel agreed upon a statement. This manuscript establishes 10 consensus statements regarding when ciNPT with full‐coverage foam dressings should be considered or recommended in the presence of patient or incision risk factors, effective therapeutic settings and duration, precautions for use, and tools and techniques to support application. The panel also discussed areas of interest for future study of ciNPT with full‐coverage dressings. High‐quality, controlled studies are needed to expand the understanding of the benefits of ciNPT over the incision and surrounding tissues.
Keywords: Delphi technique, negative pressure wound therapy, plastic surgery, surgical wound, wound healing
1. INTRODUCTION
In 2012, the estimated global surgical volume was 312.9 million operations, representing a 33.6% increase from 8 years prior. 1 Among general surgical procedures, the reported incidence of 30‐day surgical site complications (SSC) ranges from 5.8% to 43.5%. 2 These complication rates vary depending on incisional, institutional, and patient risk factors, and are associated with prolonged hospitalizations and higher mortality. The current standard of postoperative care for closed surgical incisions is application of occlusive dressings, although other options include antimicrobial dressings and closed incision negative pressure (ciNPT). The use of ciNPT with foam dressings as an effective method for managing and protecting the incision site has been thoroughly documented in multiple surgical specialties. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 In 2017, an international panel of multidisciplinary experts identified key SSC risk factors and recommendations for the appropriate application of ciNPT. 24 Since then, multiple meta‐analyses have reported an association between ciNPT and reduced SSCs when applied after arthroplasty, 25 abdominal, 13 , 23 , 26 vascular, 4 , 19 , 22 and caesarean surgery, 27 among other surgery types. 14 , 17 , 28 , 29 , 30
Historically, the application of ciNPT with reticulated open‐cell polyurethane foam dressings has been limited to the closed incision and 1 to 2 cm of immediately surrounding tissue. However, reports have emerged showing the utility of applying negative pressure beyond the narrow incision area, especially when the surrounding tissue has been undermined. 31 , 32 These efforts required the clinician to cut the foam dressing to shape and use a contact layer (such as petrolatum gauze or polyurethane foil) to protect the intact skin. Recently, a novel ciNPT foam dressing design with a contoured shape and built‐in skin interface layer has been made commercially available, allowing for convenient application. This ciNPT dressing covers a larger area of peri‐incisional soft tissue and addresses difficulties in dressing placement over complex surgical sites with non‐linear incisions, non‐planar surface areas, or areas of high tension. By applying ciNPT to a wider area, this new, full‐coverage dressing may be able to provide greater mechanical support and bolster traumatised tissue after surgery.
Due to the novelty of this ciNPT dressing, there is currently no published literature to guide its effective use. To assess the advantages of the unique design and form consensus guidelines, an expert panel of plastic surgeons convened to review the current literature, identify conditions in which ciNPT with full‐coverage dressings is most appropriate, and address challenges to the implementation and sustainability of ciNPT. In the absence of high‐quality studies, the group agreed that an established expert consensus would be a valuable resource to clinicians.
2. METHODS
The process for creating a consensus document consisted of a literature review, an in‐person meeting, and consensus‐building via a modified Delphi technique. 33
2.1. Panellist selection and meeting
A panel of experts was assembled based on the following criteria: (1) they had documented experience using ciNPT with both the conventional and novel dressings in their practise, (2) they previously presented or published on the subject of negative pressure therapy, (3) they must be able to present cases demonstrating their use of ciNPT with the novel dressings at the panel meeting, and (4) they must be able to understand and participate in the consensus formation process. The selection criteria were formulated by industry (3M Company) personnel. Before the in‐person meeting, the panel members were provided with the results of a literature search of PubMed and OVID databases including the terms: “negative pressure wound therapy,” “vacuum assisted closure,” “subatmospheric pressure therapy,” or “topical negative pressure,” along with “breast,” “mastectomy,” “mastopexy,” and “mammaplasty.”
The meeting occurred between 3 December 2019 and 4 December 2019, in Dallas, Texas. The schedule included a presentation of the technology and mechanism of action supporting the use of ciNPT, and a review of the current literature of ciNPT in plastic surgery. The panellists presented their individual cases and experiences using ciNPT with the full‐coverage dressings, followed by a discussion of technical pearls, key takeaways, and impediments to implementation. Future studies that would provide evidence and clarify the benefits of ciNPT were identified. Lastly, the process for reaching consensus agreement was outlined.
2.2. Formation of consensus statements
The consensus methodology was based upon the modified Delphi technique, 33 which involved three rounds of input to gather feedback and identify topics with potential for agreement. Subjects for discussion included infection risk factors that were identified in Willy et al, 24 therapy settings, and techniques for ciNPT application with the new full‐coverage foam dressing (3M Prevena Restor Bella Form Dressing; 3M Company, San Antonio, Texas). The process began with an open‐ended feedback session at the in‐person meeting, which was consolidated into potential consensus statements that were sent to the panellists for a second round of open‐ended input to further refine wording and substance. In the third round, the statements were submitted to the panellists in survey format, allowing the experts to register anonymous agreement or disagreement with each statement. Consensus was defined as ≥80% agreement among panel members. Statements that did not reach the consensus threshold were excluded, but this does not necessarily suggest that they are inappropriate or ill‐advised. For select consensus statements, the panellists were given the opportunity to specify whether ciNPT with the new dressing “should be considered” or “is recommended” for incision management.
3. RESULTS
3.1. Consensus statement 1
ciNPT with full‐coverage dressings is recommended for use in patients with ≥2 patient risk factors, and in patients with the following specific risk factors: (1) diabetes (Haemoglobin A1c ≥ 6.5%), (2) obesity (BMI > 30 kg/m2), (3) hypoalbuminemia (serum albumin level < 3 g/dL), (4) chronic renal insufficiency, (5) chronic obstructive pulmonary disease, (6) current tobacco use, (7) tobacco cessation within the past 3 months, (8) corticosteroid use, (9) recent or current chemotherapy (Table 1).
TABLE 1.
Patient risk factors considerations and recommendations for ciNPT with full‐coverage dressings survey results
| Consensus statements | “Should be considered” | “Is recommended” | ||||
|---|---|---|---|---|---|---|
| Yes | No | Consensus | Yes | No | Consensus | |
| ciNPT … for incision management in diabetic (Haemoglobin A1c ≥6.5%) patients | 11 (100%) | 0 (0%) | Yes | 10 (90.9%) | 1 (9.1%) | Yes |
| ciNPT … for incision management in patients of advanced age (>65 years of age) | 7 (63.6%) | 4 (36.4%) | No | 6 (54.6%) | 5 (45.4%) | No |
| ciNPT … for incision management in patients with an ASA score ≥3 and <6 | 9 (81.8%) | 2 (18.2%) | Yes | 7 (63.6%) | 4 (36.4%) | No |
| ciNPT … for incision management in obese (BMI >30) patients | 10 (90.9%) | 1 (9.1%) | Yes | 9 (81.8%) | 2 (18.2%) | Yes |
| ciNPT … for incision management in patients that are active tobacco users | 10 (90.9%) | 1 (9.1%) | Yes | 11 (100%) | 0 (0%) | Yes |
| ciNPT … for incision management in patients that have ceased tobacco use within the past 3 months | 10 (90.9%) | 1 (9.1%) | Yes | 9 (81.8%) | 2 (18.2%) | Yes |
| ciNPT … for incision management in patients that have ceased tobacco use more than 3 months ago | 8 (72.7%) | 3 (27.3%) | No | 5 (45.4%) | 6 (54.6%) | No |
| ciNPT … for incision management in patients with hypoalbuminemia (serum albumin level <3 g/dL) | 10 (90.9%) | 1 (9.1%) | Yes | 9 (81.8%) | 2 (18.2%) | Yes |
| ciNPT … for incision management in patients using corticosteroids | 10 (90.9%) | 1 (9.1%) | Yes | 10 (90.9%) | 1 (9.1%) | Yes |
| ciNPT … for incision management in patients with active alcoholism | 11 (100%) | 0 (0%) | Yes | 6 (54.6%) | 5 (45.4%) | No |
| ciNPT … for incision management in patients with chronic renal insufficiency | 11 (100%) | 0 (0%) | Yes | 9 (81.8%) | 2 (18.2%) | Yes |
| ciNPT … for incision management in patients with chronic obstructive pulmonary disease | 11 (100%) | 0 (0%) | Yes | 9 (81.8%) | 2 (18.2%) | Yes |
| ciNPT … for incision management in patients who have undergone or are currently undergoing chemotherapy | 11 (100%) | 0 (0%) | Yes | 10 (90.9%) | 1 (9.1%) | Yes |
| ciNPT … for incision management in patients with abnormal liver function tests | 8 (72.7%) | 3 (27.3%) | No | 7 (63.6%) | 4 (36.4%) | No |
| ciNPT … for management of incisions with ≥1 patient risk factors | 8 (72.7%) | 3 (27.3%) | No | 8 (72.7%) | 3 (27.3%) | No |
| ciNPT … for management of incisions with ≥2 patient risk factors | 10 (90.9%) | 1 (9.1%) | Yes | 9 (81.8%) | 2 (18.2%) | Yes |
| ciNPT … for management of incisions with ≥3 patient risk factors | 10 (90.9%) | 1 (9.1%) | Yes | 11 (100%) | 0 (0%) | Yes |
Abbreviations: ASA, American Society of Anaesthesiologists; ciNPT, closed incision negative pressure therapy with full‐coverage dressings.
As acknowledged in the 2017 guidelines, the complication rate is impacted by key patient risk factors, and additional supportive care in the immediate postoperative period may be necessary. 24 The panel recommended the use of ciNPT with full‐coverage dressings in patients with two or more of these risk factors. Additionally, the panel evaluated specific risk factors that can individually impact incision healing. Higher body mass index, greater breast volume, and smoking have been correlated with higher rates of nipple‐areolar complex (NAC) ischaemia and other complications after skin‐ and nipple‐sparing mastectomy with immediate breast reconstruction. 34 , 35 , 36 , 37 Recent or concurrent chemotherapy, commonly present in mastectomies and breast reconstructions, can impair cellular replication, inflammation, and other mechanisms of tissue repair. 38
3.2. Consensus statement 2
ciNPT with full‐coverage dressings should be considered for use in patients with the following patient risk factors: (1) active alcoholism and (2) an ASA score ≥3 and <6 (Table 1).
Active alcoholism is a risk factor for surgical site infection and delayed closure, possibly necessitating additional support to protect the incision from exposure to infectious materials and reduce lateral tension. 39 The ASA score was created by the American Society of Anesthesiologists to classify the physical status of patients prior to surgery. 40 ASA scores of 3 to 5 indicate patients that have severe systemic disease, with or without a constant threat to life, or are moribund and not expected to survive without the operation. As with the previously identified risk factors, these patient characteristics can increase the risk of SSC, and clinicians should take into consideration additional information to decide whether ciNPT could benefit incision healing.
3.3. Consensus statement 3
ciNPT with full‐coverage dressings is recommended for management of incisions with ≥2 incision risk factors, and on incisions with the following specific risk factors: (1) are high tension, (2) result from repeated incisions or revision surgeries, (3) have extensive undermining, (4) involve traumatised soft tissue, (5) have oedema, (6) have undergone pre‐surgical radiation therapy, (7) result from post‐bariatric abdominoplasty, (8) have soilage risk, or (9) have compromised perfusion (Table 2).
TABLE 2.
Incision risk factors considerations and recommendations for ciNPT with full‐coverage dressings survey results
| Consensus statements | “Should be considered” | “Is recommended” | ||||
|---|---|---|---|---|---|---|
| Yes | No | Consensus | Yes | No | Consensus | |
| ciNPT … for incision management of surgically closed wounds | 10 (90.9%) | 1 (9.1%) | Yes | 6 (54.6%) | 5 (45.4%) | No |
| ciNPT … for management of nipple‐areolar complex incisions | 11 (100%) | 0 (0%) | Yes | 6 (54.6%) | 5 (45.4%) | No |
| ciNPT … for management of high‐tension incisions | 10 (90.9%) | 1 (9.1%) | Yes | 11 (100%) | 0 (0%) | Yes |
| ciNPT … for management of repeated incisions and revision surgeries | 11 (100%) | 0 (0%) | Yes | 10 (90.9%) | 1 (9.1%) | Yes |
| ciNPT … for management of incisions with extensive undermining | 10 (90.9%) | 1 (9.1%) | Yes | 10 (90.9%) | 1 (9.1%) | Yes |
| ciNPT … for management of incisions with traumatised soft tissue | 10 (90.9%) | 1 (9.1%) | Yes | 9 (81.8%) | 2 (18.2%) | Yes |
| ciNPT … for management of incisions with oedema | 10 (90.9%) | 1 (9.1%) | Yes | 11 (100%) | 0 (0%) | Yes |
| ciNPT … for management of incisions resulting from an emergency procedure | 10 (90.9%) | 1 (9.1%) | Yes | 7 (63.6%) | 4 (36.4%) | No |
| ciNPT … for management of incisions resulting from procedures with a prolonged operation time | 10 (90.9%) | 1 (9.1%) | Yes | 6 (54.6%) | 5 (45.4%) | No |
| ciNPT … for management of incisions undergoing pre‐surgical radiation | 10 (90.9%) | 1 (9.1%) | Yes | 10 (90.9%) | 1 (9.1%) | Yes |
| ciNPT … for management of incisions resulting from postbariatric abdominoplasty | 10 (90.9%) | 1 (9.1%) | Yes | 11 (100%) | 0 (0%) | Yes |
| ciNPT … for management of incisions resulting from breast reconstruction | 10 (90.9%) | 1 (9.1%) | Yes | 8 (72.7%) | 3 (27.3%) | No |
| ciNPT … for management of incisions resulting from large soft tissue defects | 11 (100%) | 0 (0%) | Yes | 7 (63.6%) | 4 (36.4%) | No |
| ciNPT … for management of incisions with soilage risk | 10 (90.9%) | 1 (9.1%) | Yes | 9 (81.8%) | 2 (18.2%) | Yes |
| ciNPT … for management of incisions with compromised perfusion | 10 (90.9%) | 1 (9.1%) | Yes | 11 (100%) | 0 (0%) | Yes |
| ciNPT … for management of incisions with ≥1 incision risk factors | 8 (72.7%) | 3 (27.3%) | No | 8 (72.7%) | 3 (27.3%) | No |
| ciNPT … for management of incisions with ≥2 incision risk factors | 10 (90.9%) | 1 (9.1%) | Yes | 10 (90.9%) | 1 (9.1%) | Yes |
| ciNPT … for management of incisions with ≥3 incision risk factors | 10 (90.9%) | 1 (9.1%) | Yes | 11 (100%) | 0 (0%) | Yes |
Abbreviation: ciNPT, closed incision negative pressure therapy with full‐coverage dressings.
Patient and incisional risk factors can lead to compromised perfusion, resulting in soft tissue necrosis, dehiscence, or flap failure. Although ciNPT is not indicated to manage compromised perfusion, positive outcomes have been observed following the use of ciNPT over incisions exhibiting signs of diminished blood flow. 41 In a prospective RCT of 17 patients with abdominoplasty incisions managed with conventional wound dressing or ciNPT, ICG angiography revealed an earlier and stronger enhancement of perfusion parameters with ciNPT, which coincided with higher oxygen saturation on postoperative day 3. 42 Likewise, Atkins et al reported a 100% increase in peristernal perfusion in patients undergoing mammary artery harvesting followed by ciNPT, compared with a 25.7% reduction in the control group. 43
The application of negative pressure has also been associated with reduced oedema in multiple clinical settings, an observation that was affirmed by the panel. 32 , 44 , 45 , 46 Although the mechanism of oedema reduction has been debated, preclinical studies suggest that restoring impaired lymphatic clearance may reduce fluid retention. 47 , 48 In a study of 24 patients undergoing inguinal lymphadenectomy for cancer, Tauber et al reported that patients managed with 7 days of ciNPT revealed significantly lower rates of lymphoceles, lymphorrhea, and lymphedema compared with control‐treated patients. 49 Further high‐quality human studies are required to validate the hypothesised mechanism in the clinical setting.
Breast surgeries, especially in the case of post‐mastectomy reconstruction, often exhibit two or more of these incision risk factors. Mastectomy may be followed by radiation, which can pose a risk to wound healing. 50 Additionally, repeated incisions may be necessary for delayed or staged reconstruction. For elective breast procedures, breast weight and natural skin folds can place tension across the incision. Undermining of the surrounding tissue may also be employed to reduce tension and shape the breast. Application of ciNPT with full‐coverage dressings can provide external structural support of the breasts, as seen in Figure 1.
FIGURE 1.
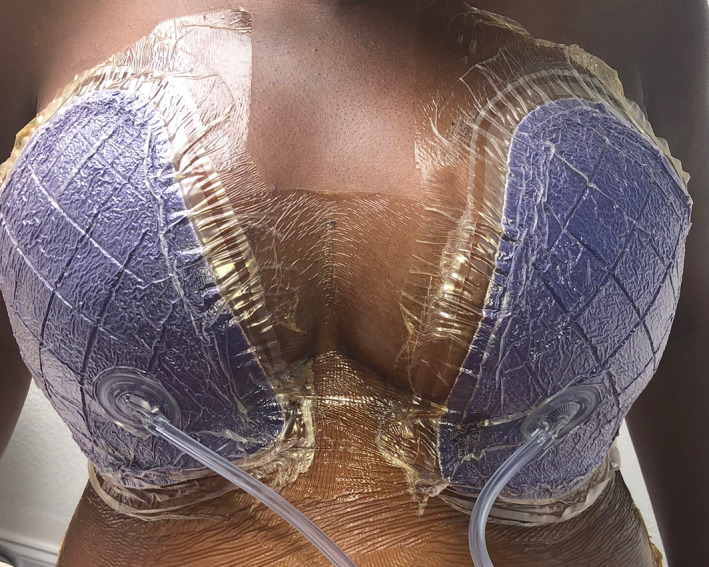
Front view of patient sitting upright with ciNPT and full‐coverage foam dressings on postoperative Day 7 after breast reduction surgery. ciNPT, closed incision negative pressure therapy
The use of ciNPT full‐coverage dressings is not limited to any area of the body, and surgeons may find it practical to apply ciNPT over any surgical site that could benefit from increased coverage, such as areas with multiple incisions or incision junctions (eg, abdominoplasties, Figure 2), incisions with high lateral tension (eg, medial sternal incisions, Figure 3), or peri‐incisional tissue at risk of exposure to contamination (eg, groin incisions).
FIGURE 2.
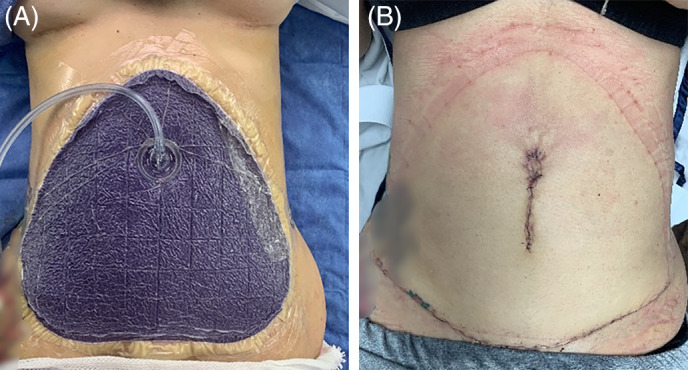
Application of ciNPT full‐coverage dressing over incisions resulting from abdominoplasty. A, A seal is created over the incisions and surrounding area. B, Incision appearance after 7 days of ciNPT. ciNPT, closed incision negative pressure therapy
FIGURE 3.
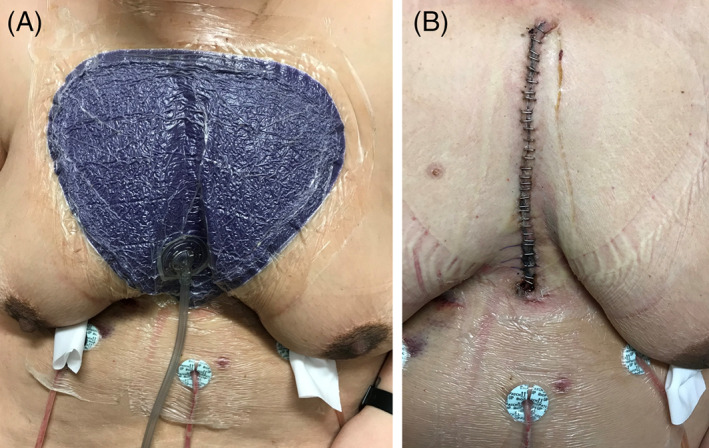
Application of ciNPT full‐coverage dressing over a sternal incision resulting from cardiothoracic surgery. A, Dressing placement in the operating room. B, Incision appearance after 7 days of ciNPT. ciNPT, closed incision negative pressure therapy
3.4. Consensus statement 4
ciNPT with full‐coverage dressings should be considered for the management of surgically closed wounds in general, and specifically those with the following incision risk factors: (1) involve the NAC, (2) result from an emergency procedure, (3) result from procedures with a prolonged operation time, (4) result from breast reconstruction, or (5) involve closure of large soft tissue defects (Table 2).
These incision types may be inherently vulnerable to postoperative complications, yet only stand to benefit from ciNPT if other risk factors are present. For example, breast reconstruction may be relatively low‐risk (eg, following prophylactic mastectomy), yet should be paid special consideration for other contributing SSC risk factors.
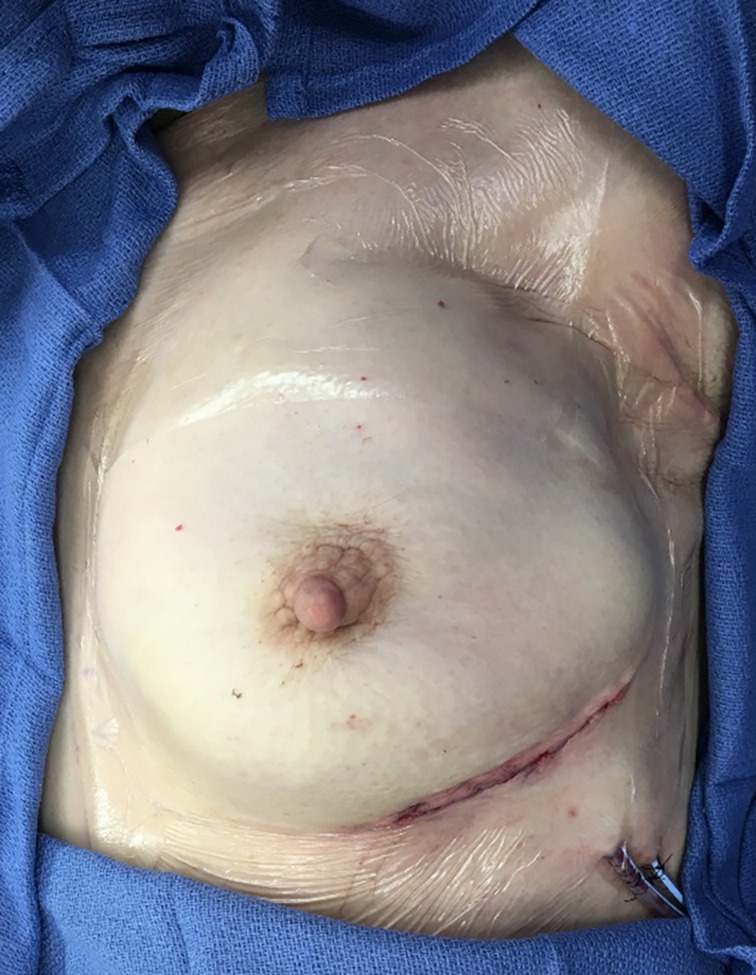
Nipple‐sparing mastectomies are often preferred when possible to optimise aesthetic outcome and patient satisfaction. Although periareolar incisions have a well‐documented risk of nipple necrosis, this incision is generally elected because it facilitates dissection of the inner quadrants and provides a more easily hidden scar in the absence of complications. 35 Full‐coverage ciNPT dressings are available in variable sizes to allow the selection of dressing large enough to be able to cover incisions along the outside and centre of the breast, including the NAC (Figure 4). ciNPT has been safely applied in nipple‐sparing mastectomies with no report of adverse effects. 51 , 52 , 53
FIGURE 4.
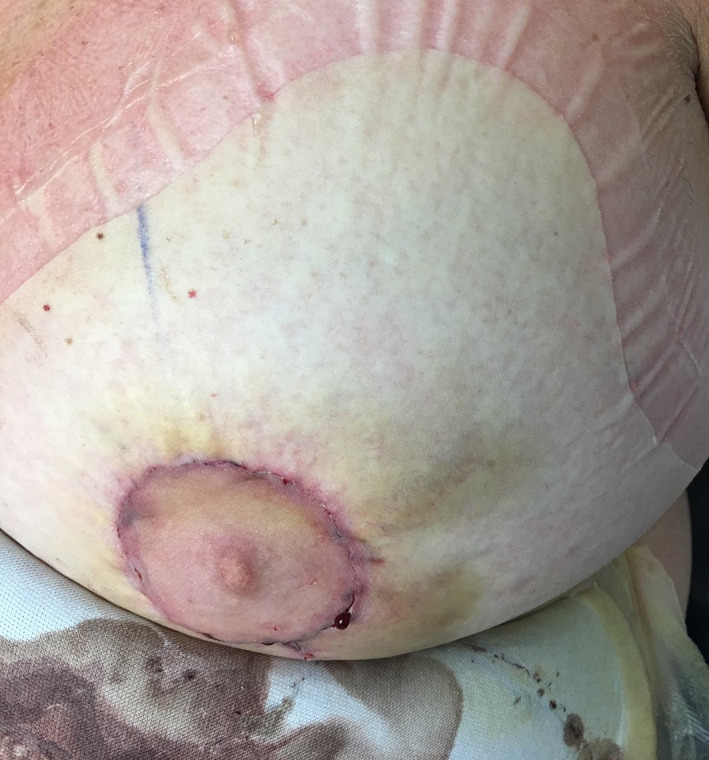
Nipple appearance upon removal of ciNPT full‐coverage dressings on postoperative Day 7 shows signs of healthy perfusion and some epithelialization of the incision involving the nipple‐areolar complex. ciNPT, closed incision negative pressure therapy
3.5. Consensus statement 5
An appropriate pressure setting for ciNPT with full‐coverage dressings is −125 mmHg (Table 3).
TABLE 3.
Pressure settings for ciNPT with full‐coverage dressings survey results
| Consensus statements | Yes | No | Consensus |
|---|---|---|---|
| An appropriate ciNPT pressure setting is −75 mmHg | 2 (18.2%) | 9 (81.8%) | No |
| An appropriate ciNPT pressure setting is −100 mmHg | 5 (45.4%) | 6 (54.6%) | No |
| An appropriate ciNPT pressure setting is −125 mmHg | 11 (100%) | 0 (0%) | Yes |
| An appropriate ciNPT pressure setting is −150 mmHg | 1 (9.1%) | 10 (90.9%) | No |
| An appropriate ciNPT pressure setting is −175 mmHg | 1 (9.1%) | 10 (90.9%) | No |
Abbreviation: ciNPT, closed incision negative pressure therapy with full‐coverage dressings.
Commercial ciNPT units available for single‐use, outpatient setting (3M Prevena 125 and Prevena Plus 125 Therapy Units; 3M Company) are designed to provide −125 mmHg for up to 7 days. Standard and full‐coverage ciNPT dressings are also compatible with units capable of applying pressures ranging from −50 to −200 mmHg. According to the manufacturer's instructions, ciNPT should be applied at −125 mmHg, the setting that was also recommended by the panel. This pressure setting is based upon earlier preclinical and human studies conducted on the effect of negative pressure delivered by conventional negative pressure wound therapy systems. In the earliest publication on negative pressure wound therapy, Morykwas et al studied the effect of negative pressure with foam dressings on tissue perfusion in a swine model, observing a 4‐fold increase in blood flow at −125 mmHg. 54 Timmers et al investigated the impact of negative pressure ranging from −25 to −500 mmHg applied with foam dressings on cutaneous blood flow in healthy forearm intact skin. 55 Non‐invasive laser Doppler probes detected significant increases in cutaneous blood flow with polyurethane foam dressings applying up to −300 mmHg, but this effect was limited by each increase of −100 mmHg correlating with higher pain scores. ciNPT at −125 mmHg is the most commonly studied pressure setting, and it has been shown to be effective and safe in multiple wound types.
3.6. Consensus statement 6
An appropriate number of therapy days for ciNPT with full‐coverage dressings, with dressing changes at least once per week, are 7 to 14 days (Table 4).
TABLE 4.
Therapy duration for ciNPT with full‐coverage dressings survey results
| Consensus statements | Yes | No | Consensus |
|---|---|---|---|
| An appropriate number of therapy days for ciNPT is 2‐7 days | 8 (72.7%) | 3 (27.3%) | No |
| An appropriate number of therapy days for ciNPT is 7‐10 days | 11 (100%) | 0 (0%) | Yes |
| An appropriate number of therapy days for ciNPT is 10‐14 days | 10 (90.9%) | 1 (9.1%) | Yes |
| An appropriate number of therapy days for ciNPT is 14‐21 days | 4 (36.4%) | 7 (63.6%) | No |
| An appropriate number of therapy days for ciNPT is >21 days | 2 (18.2%) | 9 (81.8%) | No |
| There is no appropriate maximum duration of ciNPT | 6 (54.6%) | 5 (45.4%) | No |
Abbreviation: ciNPT, closed incision negative pressure therapy with full‐coverage dressings.
In most cases, signs of healing can be observed after a single application of ciNPT. The panel members unanimously agreed that 7 to 10 days of ciNPT was an appropriate duration of therapy with dressing changes occurring at least every 7 days. Ten to 14 days may also be appropriate to obtain benefit from ciNPT. However, in the case that additional support of the surgical site is needed, ciNPT may be reapplied as necessary.
3.7. Consensus statement 7
ciNPT can be applied concurrently or in the absence of surgical drains (Table 5).
TABLE 5.
Techniques for ciNPT with full‐coverage dressings survey results
| Consensus statements | Yes | No | Consensus |
|---|---|---|---|
| ciNPT can be applied concurrently with the placement of a surgical drain | 11 (100%) | 0 (0%) | Yes |
| ciNPT can be applied in the absence of a surgical drain | 11 (100%) | 0 (0%) | Yes |
| ciNPT can be applied over incisions closed with sutures | 11 (100%) | 0 (0%) | Yes |
| ciNPT can be applied over incisions closed with staples | 11 (100%) | 0 (0%) | Yes |
| ciNPT can be applied over incisions closed with surgical adhesives | 7 (63.6%) | 4 (36.4%) | No |
| Protecting the surrounding skin with barrier film (eg, skin protectant) should be considered with ciNPT | 11 (100%) | 0 (0%) | Yes |
| Protecting the surrounding skin with barrier film (eg, skin protectant) is recommended with ciNPT | 8 (72.7%) | 3 (27.3%) | No |
Abbreviation: ciNPT, closed incision negative pressure therapy with full‐coverage dressings.
The intraoperative placement of surgical drains may be necessary to prevent fluid accumulation at the surgical site, preventing the formation of hematomas, lymphoceles, or seromas. Although necessary, a surgical drain can independently increase the risk of infection in breast surgeries. 56 The panel confirmed that ciNPT can be safely and effectively used concomitant to drain placement (Figure 5), or in the absence of drains.
FIGURE 5.
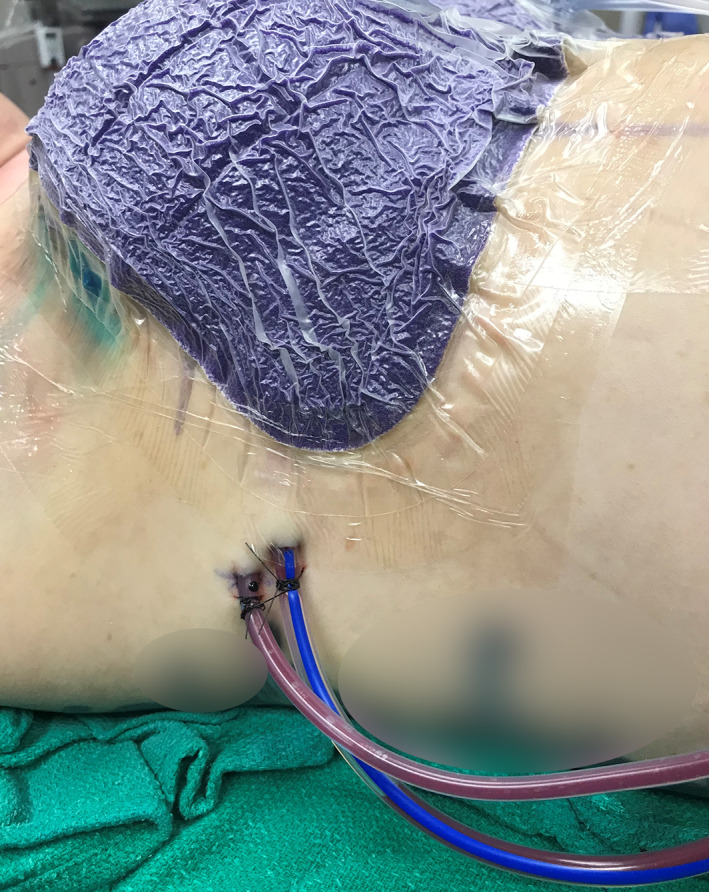
Lateral view of patient with drains and ciNPT with full‐coverage dressings. ciNPT, closed incision negative pressure therapy
Studies have reported reduced drain output or shorter time‐to‐drain removal when ciNPT was utilised compared with control therapies for incision management of abdominoplasties, 57 sternal incisions, 58 hernioplasties, 59 trauma incisions, 60 and breast reconstructions. 51 High‐drain output associated with breast procedures has been linked to the increased likelihood of seroma formation, indicating that reducing drainage may be a key target for reducing SSCs. 61 , 62 , 63 Furthermore, several studies have shown that incisions managed with ciNPT, in comparison to standard care, exhibited a lower incidence or volume of hematoma after breast reconstruction, 51 arthroplasty, 64 , 65 hemiarthroplasty, 66 and trauma surgery. 60 These findings do not suggest that ciNPT can be used as a replacement for drains. Physicians should make independent assessments of the need for drain placement based on exudate volume.
3.8. Consensus statement 8
ciNPT can be applied over incisions closed with sutures or staples.
ciNPT should only be applied over clean, completely closed incisions without obstructions over the incision edges. The panel unanimously agreed that primary closure with sutures and staples are compatible with ciNPT.
3.9. Consensus statement 9
Protecting the surrounding skin with a barrier film (eg, skin protectant) should be considered when using ciNPT with full‐coverage dressings.
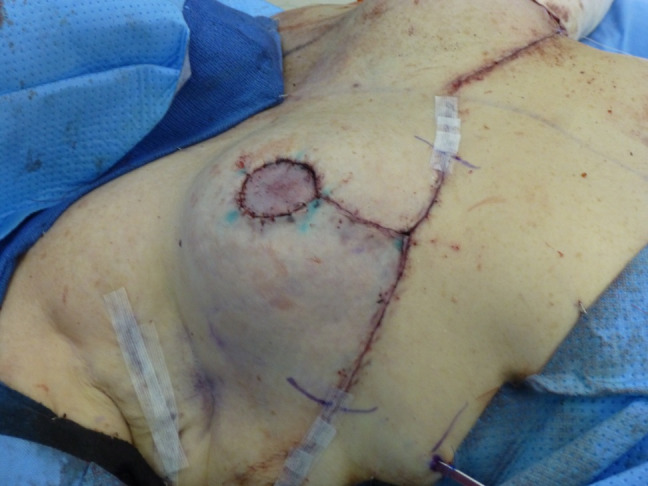
The adhesive drape that is used to secure ciNPT dressings may apply tension to the skin, especially on patients with heavy breasts or fragile skin. In such cases, the panel advised consideration of a skin barrier film, which should be applied around surgical site edges near dressing borders, to avoid tears or other irritations. ciNPT dressing kits include transparent tape that can be used to windowpane the edges of the breast (Figure 6).
FIGURE 6.
Use of transparent tape to windowpane around the breast with an inframammary incision before application of ciNPT. ciNPT, closed incision negative pressure therapy
When incisions are too long to cover completely with the ciNPT dressing, these areas should be protected by occlusive film or tape before dressing placement (Figure 7). This protects the incision from coming into direct contact with the adhesive drape, while also aiding the creation of a seal.
FIGURE 7.
Use of protective strips to cover incision edges and maintain a seal
3.10. Consensus statement 10
ciNPT with full‐coverage dressings should not be used for incision management in patients who are allergic or hypersensitive to acrylic adhesives or silver.
The interface layer of ciNPT dressing may contain silver to reduce bacterial colonisation into the dressing. Acrylic adhesives are present in the clear drape that is used to create the vacuum seal. The panel unanimously agreed that if patients have a known allergy or hypersensitivity to these substances, or begin to exhibit symptoms of an allergic reaction, use of ciNPT dressings with acrylic adhesives or silver should be avoided.
3.11. Challenges with implementation
As research demonstrating the utility of ciNPT has continued to expand, meta‐analyses have identified significant benefits of ciNPT in abdominal, thoracic, groin, and lower extremity incisions. 19 , 67 , 68 Despite this, one primary concern identified by the panel was the perception that ciNPT induces compression. Once negative pressure has been applied, the foam dressings exhibit a collapsed appearance, which may suggest to some clinicians that the device inhibits blood flow to the surgical site. However, both preclinical and clinical studies have shown increased blood flow in peri‐incisional tissues during the application of negative pressure. 42 , 43 The addition of high‐quality clinical studies examining tissue perfusion during ciNPT would reassure clinicians concerned about blood flow and elucidate the underlying mechanisms of therapy.
The panel also discussed the premature removal of dressings to check incision appearance before 5 to 7 days of therapy. Any action that breaks the vacuum seal and disrupts the sterile environment creates an opportunity for contamination, possibly undermining the benefit of ciNPT. This can be especially common with nipple‐sparing mastectomies and incisions involving the NAC, as visual assessment of the NAC are often used as an indicator of incision health. The growing number of studies demonstrating the safe and effective use of ciNPT may reassure cautious clinicians that NAC perfusion is not compromised under ciNPT and can benefit from application. 51 , 52 If the preoperative concern for compromised blood flow is high, extra vigilance can be undertaken to evaluate and address adequate blood flow intraoperatively. 69
4. DISCUSSION
The recommendations presented in these guidelines augment previous consensus on the use of ciNPT with reticulated open‐cell polyurethane foam dressings to inform the appropriate use of ciNPT with novel, full‐coverage dressings. These new dressings apply the benefits of negative pressure to both the incision and the surrounding tissue, supporting surgical sites that are at an elevated risk of complication. The panel identified multiple patient and incision risk factors that warrant consideration or recommendation of ciNPT. Notably, the panel recommended ciNPT with full‐coverage dressings when two or greater risk factors for SSC are present. In addition to these recommendations, conditions for when ciNPT should not be applied were also discussed: ciNPT cannot be used as treatment for open or dehisced incisions, nor should it be used as a replacement for incision and drainage of cutaneous abscesses.
The panellists highlighted potential clinical studies and endpoints that would advance the understanding of the effects and underlying mechanisms of ciNPT. There was significant interest in further studying tissue perfusion under ciNPT full‐coverage dressings, especially after nipple‐sparing mastectomy, collecting blood flow measurements before and after ciNPT. Further evidence is also needed to clarify how ciNPT affects the mechanisms of fluid retention and clearance.
For highly visible incisions, or those resulting from cosmetic procedures, scar appearance is also an important outcome. Improved scar appearance has been observed in incisions managed with ciNPT versus control dressings for abdominal flap donor sites, 70 abdominoplasty, 71 and breast surgery in high‐risk patients. 52 A recent systematic review identified a moderate level of evidence supporting the use of ciNPT to improve scarring characteristics. 72 The panel indicated a need for evidence that ciNPT benefits scar aesthetics in additional incision types and normal or low‐risk patient populations.
The limitations of these recommendations include the inherent bias in panel member selection, the relatively small number of panel members, and potential peer‐to‐peer influence on opinion. To address these limitations, there was a strong effort to create and adhere to a consensus‐building protocol, based on the Delphi method, to allow for the free expression of opinions, facilitate exchange of ideas, and gather anonymous, controlled input for reaching final consensus. Requiring ≥80% agreement among voting panel members for consensus enabled convergence on areas of shared confidence, avoiding areas of uncertain or experimental use of ciNPT.
These consensus guidelines present a practical framework for the application of ciNPT with novel, full‐coverage dressings, particularly in the context of plastic and breast surgeries. The novel dressing design allows for the evolution of ciNPT to deliver negative pressure not only to the incision, but to the surrounding soft tissues, thus broadening the area of effect. These new recommendations take this feature into consideration and build upon the ciNPT guidelines published in 2017. 24 Future studies of ciNPT should focus on overcoming barriers to implementation and identifying benefits of use.
CONFLICT OF INTEREST
Drs. Silverman and Kuang are employees of 3M Company (St. Paul, MN). Drs. Apostolides, Chatterjee, Dardano, Fearmonti, Gabriel, Grant, Johnson, Koneru, Moreira, and Sigalove have consulting agreements with 3M Company. Funding for the advisory panel meeting was provided by 3M.
ACKNOWLEDGEMENTS
The authors thank Mikaela Sifuentes, PhD, (3M Company) for assistance with manuscript preparation and Ricardo Martinez (3M Company) for providing project guidance.
Silverman RP, Apostolides J, Chatterjee A, et al. The use of closed incision negative pressure therapy for incision and surrounding soft tissue management: Expert panel consensus recommendations. Int Wound J. 2022;19(3):643-655. 10.1111/iwj.13662
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Weiser TG, Haynes AB, Molina G, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385(suppl 2):S11. [DOI] [PubMed] [Google Scholar]
- 2. Tevis SE, Kennedy GD. Postoperative complications and implications on patient‐centered outcomes. J Surg Res. 2013;181(1):106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ailaney N, Johns WL, Golladay GJ, Strong B, Kalore NV. Closed Incision negative pressure wound therapy for elective hip and knee arthroplasty: a systematic review and meta‐analysis of randomized controlled trials. J Arthroplasty. 2021;36(7):2402–2411. [DOI] [PubMed] [Google Scholar]
- 4. Antoniou GA, Onwuka CC, Antoniou SA, Russell D. Meta‐analysis and trial sequential analysis of prophylactic negative pressure therapy for groin wounds in vascular surgery. J Vasc Surg. 2019;70(5):1700–1710. [DOI] [PubMed] [Google Scholar]
- 5. Boland PA, Kelly ME, Donlon NE, et al. Prophylactic negative pressure wound therapy for closed laparotomy wounds: a systematic review and meta‐analysis of randomised controlled trials. Ir J Med Sci. 2021;190(1):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cagney D, Simmons L, O'Leary DP, et al. The efficacy of prophylactic negative pressure wound therapy for closed incisions in breast surgery: a systematic review and meta‐analysis. World J Surg. 2020;44:1526‐1537. [DOI] [PubMed] [Google Scholar]
- 7. De Vries FE, Wallert ED, Solomkin JS, et al. A systematic review and meta‐analysis including GRADE qualification of the risk of surgical site infections after prophylactic negative pressure wound therapy compared with conventional dressings in clean and contaminated surgery. Medicine. 2016;95(36):e4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Vries FEE, Wallert ED, Solomkin JS, et al. Prophylactic negative pressure wound therapy to reduce the risk of surgical site infections. A systematic review and meta‐analysis. Hernia. 2016;20(suppl 2):S233. [Google Scholar]
- 9. Gombert A, Dillavou E, D'Agostino R Jr, Griffin L, Robertson JM, Eells M. A systematic review and meta‐analysis of randomized controlled trials for the reduction of surgical site infection in closed incision management versus standard of care dressings over closed vascular groin incisions. Vascular. 2020;28(3):274‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gwilym BL, Dovell G, Dattani N, et al. Systematic review and meta‐analysis of wound adjuncts for the prevention of groin wound surgical site infection in arterial surgery. Eur J Vasc Endovasc Surg. 2021;61:636‐646. [DOI] [PubMed] [Google Scholar]
- 11. Kuper TM, Murphy PB, Kaur B, Ott MC. Prophylactic negative pressure wound therapy for closed laparotomy incisions: a meta‐analysis of randomized controlled trials. Ann Surg. 2020;271(1):67–74. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Wu B, Liu Y. The effect of negative pressure therapy on closed wound after the orthopedic surgery of lower limb: a meta‐analysis. Surg Innov. 2020;27(2):165–172. [DOI] [PubMed] [Google Scholar]
- 13. Sahebally SM, McKevitt K, Stephens I, et al. Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta‐analysis. JAMA Surg. 2018;153(11):e183467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandy‐Hodgetts K, Watts R. Effectiveness of negative pressure wound therapy/closed incision management in the prevention of post‐surgical wound complications: a systematic review and meta‐analysis. JBI Database System Rev Implement Rep. 2015;13(1):253‐303. [DOI] [PubMed] [Google Scholar]
- 15. Sexton F, Healy D, Keelan S, Alazzawi M, Naughton P. A systematic review and meta‐analysis comparing the effectiveness of negative‐pressure wound therapy to standard therapy in the prevention of complications after vascular surgery. Int J Surg. 2020;76:94–100. [DOI] [PubMed] [Google Scholar]
- 16. Shiroky J, Lillie E, Muaddi H, Sevigny M, Choi WJ, Karanicolas PJ. The impact of negative pressure wound therapy for closed surgical incisions on surgical site infection: a systematic review and meta‐analysis. Surgery. 2020;167:1001‐1009. [DOI] [PubMed] [Google Scholar]
- 17. Singh DP, Gabriel A, Silverman RP, Griffin LP, D'Agostino McGowan L, D'Agostino RB Jr. Meta‐analysis comparing outcomes of two different negative pressure therapy systems in closed incision management. Plast Reconstr Surg Glob Open. 2019;7(6):e2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strugala V, Martin R. Meta‐analysis of comparative trials evaluating a prophylactic single‐use negative pressure wound therapy system for the prevention of surgical site complications. Surg Infect (Larchmt). 2017;18(7):810‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svensson‐Björk R, Zarrouk M, Asciutto G, Hasselmann J, Acosta S. Meta‐analysis of negative pressure wound therapy of closed groin incisions in arterial surgery. Br J Surg. 2019;106(4):310–318. [DOI] [PubMed] [Google Scholar]
- 20. Swanson EW, Cheng HT, Susarla SM, Lough DM, Kumar AR. Does negative pressure wound therapy applied to closed incisions following ventral hernia repair prevent wound complications and hernia recurrence? A systematic review and meta‐analysis. Plast Surg. 2016;24(2):113‐118. [PMC free article] [PubMed] [Google Scholar]
- 21. Wang C, Zhang Y, Qu H. Negative pressure wound therapy for closed incisions in orthopedic trauma surgery: a meta‐analysis. J Orthop Surg Res. 2019;14(1):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wee IJ, Syn N, Choong AM. Closed incision negative pressure wound therapy in vascular surgery: a systematic review and meta‐analysis. Eur J Vasc Endovasc Surg. 2019;58(3):446‐454. [DOI] [PubMed] [Google Scholar]
- 23. Wells CI, Ratnayake CB, Perrin J, Pandanaboyana S. Prophylactic negative pressure wound therapy in closed abdominal incisions: a meta‐analysis of randomised controlled trials. World J Surg. 2019;43(11):2779‐2788. [DOI] [PubMed] [Google Scholar]
- 24. Willy C, Agarwal A, Andersen CA, et al. Closed incision negative pressure therapy: international multidisciplinary consensus recommendations. Int Wound J. 2017;14(2):385‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang L, Xu X, Cao JG, Liu J. Negative pressure wound therapy in total hip and knee arthroplasty: a meta‐analysis. J Comp Eff Res. 2019;8:791‐797. [DOI] [PubMed] [Google Scholar]
- 26. Tran BN, Johnson AR, Shen C, Lee BT, Lee ES. Closed‐incision negative‐pressure therapy efficacy in abdominal wall reconstruction in high‐risk patients: a meta‐analysis. J Surg Res. 2019;241:63‐71. [DOI] [PubMed] [Google Scholar]
- 27. Yu L, Kronen RJ, Simon LE, Stoll CRT, Colditz GA, Tuuli MG. Prophylactic negative‐pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2018;218(2):200‐210.e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hyldig N, Birke‐Sorensen H, Kruse M, et al. Meta‐analysis of negative‐pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li HZ, Xu XH, Wang DW, Lin YM, Lin N, Lu HD. Negative pressure wound therapy for surgical site infections: a systematic review and meta‐analysis of randomized controlled trials. Clin Microbiol Infect. 2019;25:1328‐1338. [DOI] [PubMed] [Google Scholar]
- 30. Li L, Liu W, Tao H, et al. Efficacy and safety of negative pressure versus natural drainage after thyroid surgery: a systematic review and meta‐analysis. Medicine. 2018;97(31):e11576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dragu A, Schnurer S, Unglaub F, et al. Wide topical negative pressure wound dressing treatment for patients undergoing abdominal dermolipectomy following massive weight loss. Obes Surg. 2011;21(11):1781‐1786. [DOI] [PubMed] [Google Scholar]
- 32. Shim HS, Choi JS, Kim SW. A role for postoperative negative pressure wound therapy in multitissue hand injuries. Biomed Res Int. 2018;2018:3629643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Custer RL, Scarcella JA, Stewart BR. The modified Delphi technique ‐ a rotational modification. J Career Tech Educ. 1999;15(2):50–58. [Google Scholar]
- 34. Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple‐sparing mastectomy: predictors of complications, reconstruction outcomes, and 5‐year trends. Plast Reconstr Surg. 2014;133(3):496‐506. [DOI] [PubMed] [Google Scholar]
- 35. De Vita R, Zoccali G, Buccheri EM, Costantini M, Botti C, Pozzi M. Outcome evaluation after 2023 nipple‐sparing mastectomies: our experience. Plast Reconstruct Surg. 2017;139(2):335e‐347e. [DOI] [PubMed] [Google Scholar]
- 36. Dent BL, Small K, Swistel A, Talmor M. Nipple‐areolar complex ischemia after nipple‐sparing mastectomy with immediate implant‐based reconstruction: risk factors and the success of conservative treatment. Aesthet Surg J. 2014;34(4):560‐570. [DOI] [PubMed] [Google Scholar]
- 37. Davies K, Allan L, Roblin P, Ross D, Farhadi J. Factors affecting post‐operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast. 2011;20(1):21‐25. [DOI] [PubMed] [Google Scholar]
- 38. Budrukkar A, Pandit P, Wadasadawala T, et al. Impact of adjuvant systemic chemotherapy on wound healing and cosmetic outcome in 224 women treated with accelerated partial breast irradiation using interstitial brachytherapy. Brachytherapy. 2017;16(5):935‐942. [DOI] [PubMed] [Google Scholar]
- 39. Trevejo‐Nunez G, Kolls JK, de Wit M. Alcohol use as a risk factor in infections and healing: a clinician's perspective. Alcohol Res. 2015;37(2):177‐184. [PMC free article] [PubMed] [Google Scholar]
- 40. Anesthesiologists ASo . ASA Physical Status Classification System. www asahq org. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. 2019.
- 41. Gabriel A, Pfaffenberger M, Eldenburg E. Successful salvage of a lower extremity local flap using multiple negative pressure modalities. Plast Reconstr Surg Glob Open. 2020;8(6):e2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Renno I, Boos AM, Horch RE, Ludolph I. Changes of perfusion patterns of surgical wounds under application of closed incision negative pressure wound therapy in postbariatric patients. Clin Hemorheol Microcirc. 2019;72(2):139‐150. [DOI] [PubMed] [Google Scholar]
- 43. Atkins BZ, Tetterton JK, Petersen RP, Hurley K, Wolfe WG. Laser Doppler flowmetry assessment of peristernal perfusion after cardiac surgery: beneficial effect of negative pressure therapy. Int Wound J. 2011;8(1):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Young SR, Hampton S, Martin R. Non‐invasive assessment of negative pressure wound therapy using high frequency diagnostic ultrasound: oedema reduction and new tissue accumulation. Int Wound J. 2013;10(4):383‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kamolz LP, Andel H, Haslik W, Winter W, Meissl G, Frey M. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns. 2004;30(3):253‐258. [DOI] [PubMed] [Google Scholar]
- 46. Kement M, Baskiran A. Efficacy of negative pressure wound therapy in the management of acute burns. Ulus Travma Acil Cerrahi Derg. 2018;24(5):412‐416. [DOI] [PubMed] [Google Scholar]
- 47. Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen. 2011;19(5):588‐596. [DOI] [PubMed] [Google Scholar]
- 48. Yuan Y, Niu Y, Xiao W, Qi B, Hu X, Yu A. The effect and mechanism of negative pressure wound therapy on lymphatic leakage in rabbits. J Surg Res. 2019;235:329‐339. [DOI] [PubMed] [Google Scholar]
- 49. Tauber R, Schmid S, Horn T, et al. Inguinal lymph node dissection: epidermal vacuum therapy for prevention of wound complications. J Plast, Reconstruct Aesthetic Surg. 2013;66(3):390‐396. [DOI] [PubMed] [Google Scholar]
- 50. Karalashvili L, Mardaleishvili K, Uhryn M, Chakhunashvili D, Kakabadze Z. Current condition and challenges in treatment of non‐healing wound after radiation therapy (Review). Georgian Med News. 2018;7(280):23‐28. [PubMed] [Google Scholar]
- 51. Gabriel A, Sigalove S, Sigalove N, et al. The impact of closed incision negative pressure therapy on postoperative breast reconstruction outcomes. Plast Reconstruct Surg ‐ Global Open. 2018;6(8):e1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferrando PM, Ala A, Bussone R, Beramasco L, Actis Perinetti F, Malan F. Closed incision negative pressure therapy in oncological breast surgery: comparison with standard care dressings. Plast Reconstruct Surg ‐ Global Open. 2018;6(6):e1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gabriel A, Sigalove SR, Maxwell GP. Initial experience using closed incision negative pressure therapy after immediate postmastectomy breast reconstruction. Plast Reconstr Surg Glob Open. 2016;4(7):e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553‐562. [DOI] [PubMed] [Google Scholar]
- 55. Timmers MS, Le Cessie S, Banwell P, Jukema GN. The effects of varying degrees of pressure delivered by negative‐pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55(6):665‐671. [DOI] [PubMed] [Google Scholar]
- 56. Chim JH, Borsting EA, Thaller SR. Urban myths in plastic surgery: postoperative management of surgical drains. Wounds. 2016;28(2):35‐39. [PubMed] [Google Scholar]
- 57. Abesamis GM, Chopra S, Vickery K, Deva AK. A comparative trial of incisional negative‐pressure wound therapy in abdominoplasty. Plast Reconstr Surg Glob Open. 2019;7(5):e2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chowdhry SA, Wilhelmi BJ. comparing negative pressure wound therapy with instillation and conventional dressings for sternal wound reconstructions. Plast Reconstr Surg Glob Open. 2019;7(1):e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Olona C, Duque E, Caro A, et al. Negative‐pressure therapy in the postoperative treatment of incisional hernioplasty wounds: a pilot study. Adv Skin Wound Care. 2014;27(2):77‐80. [DOI] [PubMed] [Google Scholar]
- 60. Stannard JP, Robinson JT, Anderson ER, McGwin G, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. J Trauma. 2006;60(6):1301‐1306. [DOI] [PubMed] [Google Scholar]
- 61. Barwell J, Campbell L, Watkins RM, Teasdale C. How long should suction drains stay in after breast surgery with axillary dissection? Ann R Coll Surg Engl. 1997;79(6):435‐437. [PMC free article] [PubMed] [Google Scholar]
- 62. Yii M, Murphy C, Orr N. Early removal of drains and discharge of breast cancer surgery patients: a controlled prospective clinical trial. Ann R Coll Surg Engl. 1995;77(5):377‐379. [PMC free article] [PubMed] [Google Scholar]
- 63. Kopelman D, Klemm O, Bahous H, Klein R, Krausz M, Hashmonai M. Postoperative suction drainage of the axilla: for how long? Prospective randomised trial. Eur J Surg. 1999;165(2):117‐120. [DOI] [PubMed] [Google Scholar]
- 64. Pachowsky M, Gusinde J, Klein A, et al. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop. 2012;36(4):719‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Redfern RE, Cameron‐Ruetz C, O'Drobinak S, Chen J, Beer KJ. Closed incision negative pressure therapy effects on postoperative infection and surgical site complication after total hip and knee arthroplasty. J Arthroplasty. 2017;32(11):3333‐3339. [DOI] [PubMed] [Google Scholar]
- 66. Pauser J, Nordmeyer M, Biber R, et al. Incisional negative pressure wound therapy after hemiarthroplasty for femoral neck fractures ‐ reduction of wound complications. Int Wound J. 2016;13(5):663‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Semsarzadeh NN, Tadisina KK, Maddox J, Chopra K, Singh DP. closed incision negative‐pressure therapy is associated with decreased surgical‐site infections: a meta‐analysis. Plast Reconstr Surg. 2015;136(3):592‐602. [DOI] [PubMed] [Google Scholar]
- 68. Singh DP, Gabriel A, Parvizi J, Gardner MJ, D'Agostino R Jr. Meta‐analysis of comparative trials evaluating a single‐use closed‐incision negative‐pressure therapy system. Plast Reconstr Surg. 2019;143(suppl 1):41S‐46S. [DOI] [PubMed] [Google Scholar]
- 69. Rancati A, Irigo M, Angrigiani C. Management of the ischemic nipple‐areola complex after breast reduction. Clin Plast Surg. 2016;43(2):403‐414. [DOI] [PubMed] [Google Scholar]
- 70. Fang CL, Changchien CH, Chen MS, Hsu CH, Tsai CB. Closed incision negative pressure therapy following abdominoplasty after breast reconstruction with deep inferior epigastric perforator flaps. Int Wound J. 2020;17(2):326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Abatangelo S, Saporiti E, Giatsidis G. closed incision negative‐pressure therapy (ciNPT) reduces minor local complications in post‐bariatric abdominoplasty body contouring: a retrospective case‐control series. Obes Surg. 2018;28(7):2096‐2104. [DOI] [PubMed] [Google Scholar]
- 72. Zwanenburg PR, Timmermans FW, Timmer AS, et al. A systematic review evaluating the influence of incisional negative pressure wound therapy on scarring. Wound Repair Regen. 2021;29(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


