Abstract
Deep surgical site infection (DSSI) is a serious complication affecting the surgical outcome of displaced intra‐articular calcaneal fracture, and a risk prediction model based on the identifiable risk factors will provide great clinical value in prevention and prompt interventions. This study retrospectively identified patients operated for calcaneal fracture between January 2014 and December 2019, with a follow‐up ≥1 year. The data were extracted from electronic medical records, with regard to demographics, comorbidities, injury, surgery and laboratory biomarkers at admission. Univariate and multivariate logistics regression analyses were used to identify the independent factors for DSSI, thereby the risk prediction model was developed. Among 900 patients included, 2.7% developed a DSSI. The multivariate analyses identified five factors independently associated with DSSI, including current smoking (OR, 2.8; 95% confidence interval [CI], 1.3‐6.4; P = .021), BMI ≥ 26.4 kg/m2 (OR, 3.1; 95% CI, 1.6‐8.4; P = .003), ASA ≥II (OR, 1.3; 95% CI, 1.0‐5.1; P = .043), incision level of II (OR, 3.8; 95% CI, 1.3‐12.6; P = .018) and NLR ≥6.4 (OR, 3.2; 95% CI, 1.3‐7.5; P = .008). A score of 14 as the optimal cut‐off value was corresponding to sensitivity of 0.542 and specificity of 0.872 (area, 0.766; P < .001); ≥14 was associated with 8.1‐times increased risk of DSSI; a score of 7 was corresponding sensitivity of 100% and 10 corresponding to sensitivity of 0.875. The risk prediction model exhibited excellent performance in distinguishing the risk of DSSI and could be considered in practice for improvement of wound management, but its validity requires to be verified by better‐design studies.
Keywords: calcaneal fracture, deep surgical site infection, multivariate analysis, receiver operating characteristic (ROC), risk prediction model
1. INTRODUCTION
Displaced intra‐articular calcaneal fracture (DIACF) is prevalent in department of orthopaedic trauma, involving approximately 2% of overall fractures and approximately 30% of foot and ankle fractures. 1 , 2 By far, open reduction and internal fixation (ORIF) remains the standard modality of operative treatment, but is not without drawbacks. The first concern was the high rate of wound‐related complications, especially the deep surgical site infection (DSSI), which affected 2.9% to 15.4% of surgically treated patients. 3 , 4 , 5 DSSI was associated with substantial health care burden from repeated wound debridement, revision or implant removal, and social consequences due to the occasional amputation or sequela of limb dysfunction. 6 , 7
In practice, the importance of preventing DSSI cannot be overemphasised. This is especially true for high‐risk patients, who, in other word, may gain most benefits from targeted preventive interventions. More importantly, identification of the high‐risk patients via the identified factors and accordingly targeting them with interventions is the most cost‐effective method. In fact, in clinical investigations, it is the consistent aim to keep identifying risk factors (especially the modifiable or controllable factors) for the development of a DSSI. 4 , 8 , 9 , 10 , 11 However, due to heterogeneity in institutional policy (operating room availability, operation schedule reasonability, prophylactic antibiotic use, wound care), surgeon preference (wound drainage or type of prophylactic antibiotics), patients' conditions (comorbidities, obesity, smoking, non‐compliance) and surgery‐related factors (inadequate surgical skills, surgical approach), the results available from these studies might not be so generalizable. In addition, the relatively small sample size and no inclusion of so many variables for adjustment made them difficult to obtain conclusive results, and possibly there remain some factors that have never been investigated or noticed.
In this study, we used the large sample of calcaneal fracture surgically treated by ORIF in a tertiary referral institution to address our aims: (1) to investigate the incidence rates of DSSI; (2) to identify the factors independently associated with DSSI; and (3) on basis of these factors, if any, to form a risk model for predicting the DSSI and evaluate its predictive power.
2. METHODS
2.1. Inclusion and exclusion criteria
This study retrospectively identified patients aged 18 years or older who underwent surgical treatment of acute closed calcaneal fractures by ORIF in our institution between January 2014 and December 2019, with postoperative follow‐up period of a minimum of 12 months. Patients with complete follow‐up data were deemed to be eligible for inclusion. The exclusion criteria were age less than 18 years, open calcaneal fracture, bilateral calcaneal fracture, pathological fractures, old fractures (> 21 days from initial injury), multiple fractures, polytrauma, treatments rather than ORIF (conservative, closed reduction, percutaneous fixation, external fixation et al), presence of infections or signs before index fracture operation, wound issues other than DSSIs (superficial infection, wound edge necrosis, erythema), missing data for any variable of interest or incomplete follow‐up data. This study was approved by the local ethics committee, which waived the need for informed consent.
2.2. Perioperative management
For ORIF procedure via extended lateral approach or sinus tarsi approach, 1 to 2 g of cefazolin on the basis of weight was administered to all patients within 30 minutes before skin incision, and in case of procedure predicted to last over 3 hours, another dose was given. Within 24 hours after wound closure, prophylactic use of 1 to 2 g of cefazolin was routinely given, and for patients with high‐risk infection, the period could be appropriately extended. Pneumatic tourniquets, bone‐grafting and postoperative drainage use were left to the discretion of the treating surgeon. Postoperatively, all patients were instructed to follow the same protocol on wound care and physical exercises.
2.3. Data collection
Two investigators (K.L and T.M) independently extracted the data from the inpatient electronic medical records and documented them using the EpiData software (version 3.1, The EpiData Association, Odense, Denmark), and any discrepancies were resolved by consensus. Then, these data were exported into the Excel worksheet (Office version 2016) for the purpose of statistical description and analysis.
The collected data were demographics (age, sex, body weight, height, occupation, education level), lifestyles (current smoking status, alcohol consumption), comorbidities or conditions (hypertension, diabetes mellitus, chronic heart disease), injury‐related (injury mechanism and fracture type based on Sander' classification), surgery‐related (time from injury to surgery, surgical approach, surgical duration, blood loss, allogeneic blood transfusion, anaesthesia type, American Society of Anesthesiologists [ASA] grade, bone‐grafting, postoperative drainage use), and laboratory indexes measured at admission (count of white blood cell, neutrophil, lymphocyte, red blood cell and platelet, level of plasma albumin, total protein, haemoglobin and fasting blood glucose). We also calculated the values of ratio of neutrophil to lymphocyte and platelet to lymphocyte, and investigated whether they are in relation to the development of DSSI, both of which have demonstrated to be associated with multiple adverse outcomes (venous thromboembolism, infection, mortality) across a wide range of specialties (trauma, cancer, cardia‐cerebrovascular disease). 8 , 10 , 12 , 13 , 14
Occupation was categorised as retirement, office work, manual work and others (students, unemployment). Based on attainment of years, education level was categorised as illiteracy, <6, 6 to 12 and >12 years. Body mass index was calculated by dividing the square of height in metre by the weight in kilogram, and was divided into non‐obesity (<28 kg/m2) and obesity (≥28 kg/m2) based on the criteria fit for Chinese people, and further was dichotomised according to the cut‐off value determined by algorithm. Current smoking or alcohol consumption was defined as patients' self‐reported smoking activity or drinking of wine, beer or any other alcoholic beverage alcohol within 12 months of the index operation. Considering the clinical relevance for explorable analysis, the laboratory indexes were divided into two or three categories, as appropriate. Injury mechanism was dichotomised as high‐impact trauma (e.g. traffic accidents, fall from height ≥1 m, and other violent injuries) and low‐to medium‐impact trauma (fall from height <1 m or standing height).
2.4. Definition and confirmation of DSSI
Definition of DSSI is based on the criteria issued by the US Centers for Disease Control and Prevention, referring to an infection directly related to the wound and involving the tissues surpassing the deep fascia. DSSI was identified and confirmed by checking the descriptions in the electronic medical records on the basis of at least one of the following incision‐related signs: pus discharging; dehiscence or separation; various examinations or medication prescriptions and dispensations providing evidence for SSI; and debridement or/and removal of implant performed. Of note, for superficial SSI or other minor wound issues such as wound edge necrosis or erythema that resolved by wound care or oral antibiotics alone, we did not include them in the analysis for the purpose of ruling out potential confounding effects.
For those who were readmitted within the 1 year of index operation, we used their national identification card number rather than the inpatient record number to confirm the potential DSSI cases, because only the former was unique for one person.
2.5. Statistical analysis
The continuous variables were presented as mean ± standard deviation (SD), using Kolmogorov‐Smirnov test and Levene's test to evaluate the normality status and homogeneity of variances, respectively. Student t‐test or Mann‐Whitney U‐test was used to detect the difference between groups, as appropriate. Categorical data were presented as a number with a percentage and compared by Chi‐square test or Fisher exact test, as appropriate.
Receiver operating characteristic (ROC) curve was constructed to determine the optimal cut‐off value for age, BMI, NLR and PLR, when sensitivity +1‐specificity (namely, Youden index) was maximised. Based on the cut‐off values (BMI, 26.4 kg/m2; NLR, 6.4; PLR, 150; age, 45 years), they were dichotomised and compared between groups in univariate analyses. Variables tested with P < .10 were further entered into the multivariate logistics regression model to determine their independent effects on development of DSSI, using stepwise backward method. The goodness‐of‐fit of the final model was evaluated using Hosmer‐Lemeshow (H‐L) test, with P value >.05 considered as the acceptable result. Variables with statistical level of P < .10 were retained in the final model. Odds ratio (OR) and its 95% confidential interval indicated the magnitude of association effect.
For each independent variable, a scoring point (integer value, derived from the rounded‐up ORs) was assigned; therefore, the potential assigned point was zero, or otherwise the rounded‐up OR value. For any patient, the total score was calculated by totalling the scores from all independent variables existing for him/her. Again, ROC curve was made for determining the rate of DSSI for every possible value for the total score (independent factor), while the optimal cut‐off value was calculated with the rate of DSSI as the dependent factor. The validity of the cut‐off value was evaluated by means of comparing the area under the ROC curve (AUC), as previously described in detail. 15
The statistical significance was set as P < .05 and all the analyses were performed using SPSS24.0 (IBM Corporation, New York, USA).
3. RESULTS
Within the study period, 1407 calcaneal fractures were retrieved and 507 patients were excluded based on our rigorous criteria, leaving 900 for data analysis (details presented in Figure 1). Among them, male patients predominated overwhelmingly (92.8%, 835/900). The age averaged 41.8 ± 11.8 years at injury, with ≤45 years in 62.5% of patients. These ORIF procedures were performed by 32 surgeons. Approximately 40% of patients were operated within 7 days, and 93.8% within 14 days after injury. The predominant surgical approach was extended lateral approach in 71.0% of patients.
FIGURE 1.
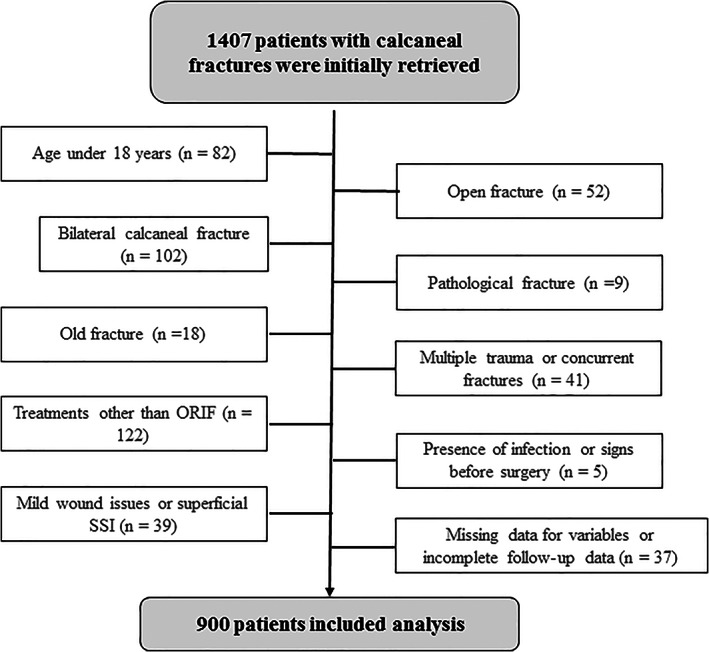
The flow chart showing the screening of patients meeting the criteria for inclusion for data analysis
DSSI was developed in 24 patients, representing an accumulated incidence of 2.7%. The median interval was 27 days (range 6‐206 days) between operation DSSI and 70.8% (17/24) occurred within 3 months.
Compared with those without developing a DSSI, patients with a DSSI had a significantly prolonged hospital stay (24.1 ± 24.6 vs 16.1 ± 8.5, P = .001). Twenty patients received reoperation that was debridement alone in eight patients, implant removal in five patients, flap repair procedure in two patients and others in five patients. No amputation was performed. The average number of surgical procedures needed for control of infection was 1.8, 2 or more in three‐fifths (12/20) of the patients (Table 1).
TABLE 1.
Comparisons between SSI and non‐SSI group in terms of demographics, patient, injury surgery variables and the biomarkers measured at admission
| Variable | Non‐SSI group (n = 876) | SSI group (n = 24) | P value |
|---|---|---|---|
| Sex (male) | 812 (92.7) | 23 (95.8) | .558 |
| Age (years) | 41.9 ± 11.2 | 41.5 ± 8.7 | .863 |
| ≤45 | 518 (59.1) | 15 (62.5) | .740 |
| BMI (kg/m2) | 25.1 ± 3.5 | 26.8 ± 3.6 | .023 |
| ≥26.4 (vs <26.4) | 273 (31.2) | 15 (62.5) | .001 |
| ≥28.0 (vs <28.0) | 150 (17.1) | 8 (33.3) | .039 |
| Occupation | .857 | ||
| Office work | 109 (12.4) | 2 (7.7) | |
| Labouring | 448 (51.3) | 15 (62.5) | |
| Retirement | 67 (7.6) | 2 (7.7) | |
| Manual work | 150 (17.2) | 3 (12.5) | |
| Others | 102 (11.7) | 4 (15.4) | |
| Attainment of education (years) | .570 | ||
| Illiteracy | 57 (6.5) | 2 (8.3) | |
| <6 | 227 (25.9) | 9 (37.5) | |
| 6‐11.9 | 482 (55.0) | 11 (45.8) | |
| ≥12 | 110 (12.6) | 2 (8.3) | |
| Current smoking | 135 (15.4) | 8 (33.3) | .018 |
| Current drinking | 278 (31.7) | 9 (37.5) | .550 |
| Hypertension | 76 (8.7) | 3 (12.5) | .514 |
| Diabetes mellitus | 42 (4.8) | 2 (8.3) | .754 |
| Heart or cardiovascular disease | 23 (2.4) | 1 (4.2) | 1.000 |
| History of any surgery | 32 (3.7) | 2 (8.3) | .235 |
| Injury mechanism (high‐impact trauma) | 625 (71.3) | 21 (87.5) | .107 |
| Fracture types (Sanders grades) | .953 | ||
| II | 445 (50.8) | 14 (56.5) | |
| III | 288 (32.9) | 7 (30.4) | |
| IV | 143 (16.3) | 3 (13.0) | |
| Time from injury to operation (day) | 8.0 ± 4.6 | 8.0 ± 4.9 | .990 |
| <7 | 352 (40.2) | 10 (41.7) | .413 |
| 7‐10 | 337 (38.5) | 6 (25.0) | |
| 11‐14 | 128 (14.6) | 5 (20.8) | |
| >14 | 59 (6.7) | 3 (12.5) | |
| Length of hospital stay | 16.1 ± 8.5 | 24.1 ± 24.6 | .001 |
| Operative time | 126.7 ± 70.8 | 134.2 ± 79.2 | .610 |
| Intraoperative blood loss | 167.9 ± 186.9 | 172.6 ± 113.9 | .905 |
| Incision level | .023 | ||
| I | 845 (96.5) | 21 (87.5) | |
| II | 31 (3.5) | 3 (12.5) | |
| ASA grade | .089 | ||
| I | 153 (17.5) | 1 (4.2) | |
| II or greater | 723 (82.5) | 23 (95.8) | |
| Anaesthesia mode | .848 | ||
| Regional (epidural/spinal) | 679 (77.5) | 19 (79.2) | |
| General | 197 (22.5) | 5 (20.8) | |
| Surgical approach | .371 | ||
| Tarsal sinus approach | 256 (29.2) | 5 (20.8) | |
| Extended lateral approach | 620 (70.8) | 19 (79.2) | |
| Bone‐grafting | .454 | ||
| Yes | 72 (8.2) | 3 (12.5) | |
| No | 804 (91.8) | 21 (87.5) | |
| Drainage use | 353 (40.3) | 7 (29.2) | .272 |
| Allogeneic blood transfusion | 69 (7.9) | 4 (16.7) | .120 |
| TP (g/L) | 65.0 ± 6.1 | 67.8 ± 5.7 | .024 |
| <60 g/L | 165 (18.8) | 1 (4.2) | .068 |
| Albumin (g/L) | 41.2 ± 5.1 | 44.4 ± 2.9 | .002 |
| <35 g/L | 95 (10.8) | 0 | .171 |
| HCRP (mg/L) | 30 ± 33.6 | 26.2 ± 44.8 | .603 |
| >8 mg/L | 604 (68.9) | 13 (54.2) | .124 |
| LDH (U/L) | 235.7 ± 117.7 | 223.2 ± 53.3 | .605 |
| >250 U/L | 292 (33.3) | 10 (41.7) | .394 |
| Sodium concentration (mmol/L) | 139.0 ± 3.2 | 139.9 ± 2.5 | .167 |
| <135 mmol/L | 202 (23.1) | 1 (4.2) | .029 |
| FBG (mmol/L) | 5.8 ± 1.5 | 5.6 ± 1.2 | .663 |
| ≥6.1 mmol/L | 192 (21.9) | 6 (25.0) | .719 |
| WBC (×109/L) | 8.9 ± 2.6 | 10.1 ± 3.3 | .036 |
| >10 × 109/L | 331 (37.8) | 14 (58.3) | .041 |
| Neutrophil (109/L) | 6.5 ± 2.4 | 7.8 ± 3.3 | .011 |
| >6.3 × 109/L | 413 (47.1) | 15 (62.5) | .137 |
| Lymphocyte (×109/L) | 1.6 ± 0.6 | 1.5 ± 0.5 | .444 |
| <1.1 × 109/L | 162 (18.5) | 6 (25.0) | .420 |
| platelet (×109/L) | 228.3 ± 79.7 | 229.1 ± 62.3 | .960 |
| >300 × 109/L | 124 (14.2) | 5 (20.8) | .357 |
| NLR | 4.8 ± 3.1 | 6.0 ± 3.8 | .044 |
| <6.4 | 696 (79.5) | 14 (58.3) | .012 |
| ≥6.4 | 180 (20.5) | 10 (41.7) | |
| PLR | 160.5 ± 83.9 | 162.1 ± 48.2 | .927 |
| <150 | 518 (59.1) | 10 (41.7) | .086 |
| ≥150 | 358 (40.9) | 14 (58.3) | |
| RBC (×1012/L) | 4.2 ± 0.6 | 4.5 ± 0.5 | .089 |
| <Lower limit | 168 (19.2) | 2 (8.3) | .181 |
| Haemoglobin (g/L) | 134.5 ± 17.3 | 138.5 ± 14.4 | .256 |
| <Lower limit | 117 (13.4) | 2 (8.3) | .474 |
| HCT (percentage) | 39.7 ± 5.3 | 40.9 ± 4.1 | .280 |
| <Lower limit | 120 (13.7) | 4 (2.7) | .677 |
Note: Data presentation, mean ± SD (standard deviation) or number (percentage); BMI, body mass index; ASA, American Society of Anesthesiologists; TP, total protein; HCRP, hyper‐sensitive C‐reaction protein; HDH, lactate dehydrogenase; WBC, white blood cell; FBG, fasting blood glucose; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; RBC, red blood cell, reference range: female, 3.5 to 5.0 × 1012/L; male, 4.0 to 5.5 × 1012/L. Haemoglobin, reference range: Female, 110 to 150 g/L; male, 120 to 160 g/L. HCT, haematocrit, reference range: female, 35% to 45%; male, 40% to 50%.
The optimal cut‐off values were determined for NLR (6.4), PLR (150), BMI (26.4 kg/m2) and age (45 years). (Figure 2) Compared with the non‐DSSI group, patients with a DSSI had a significantly higher BMI (26.8 ± 3.6 vs 25.1 ± 3.5, P = .023) and a higher proportion of BMI ≥26.4 kg/m2 (P = .001) and ≥ 28.0 (P = .039), were more likely current smokers (33.3% vs 15.4%), and had an incision of greater level (II, 12.5% vs 3.5%), TP level (67.8 ± 5.7 vs 65.0 ± 6.1, P = .024), albumin level (44.4 ± 2.9 vs 41.2 ± 5.1, P = .002), WBC count (10.1 ± 3.3 vs 8.9 ± 2.610*109/L, P = .036) and proportion of WBC > 10*109/L (58.3% vs 37.8%, P = .041), neutrophil count (10.1 ± 3.3 vs 8.9 ± 2.6* 10*109/L, P = .011), NLR value (6.0 ± 3.8 vs 4.8 ± 3.1, P = .044) and higher proportion of NLR > 6.4 (41.7% vs 20.5%, P = .012). For other variables, no significant differences were found.
FIGURE 2.
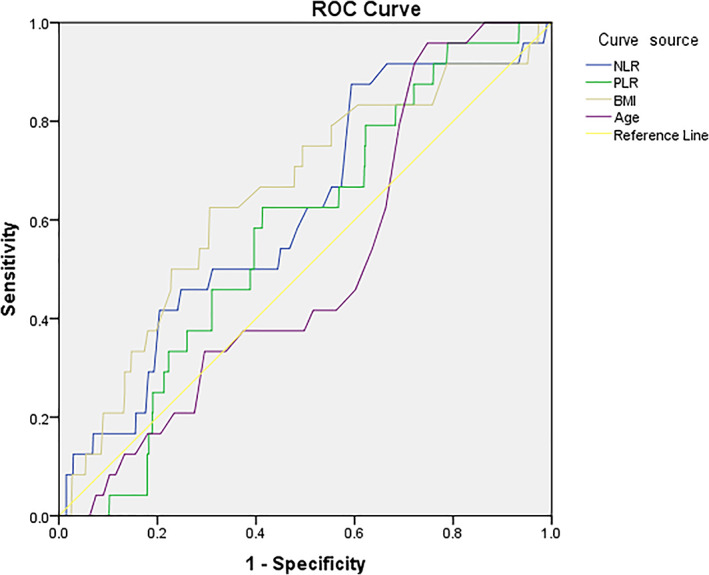
ROC curve for determination of the optimal cut‐off values for NLR (6.4), PLR (150), BMI (26.4 kg/m2) and age (45 years). Horizontal axis represents the 1‐specificity and vertical axis indicates the sensitivity of each variable in predicting development of DSSI. The lines in different colours represent the different variables. The area under the curve (AUC) represents the ability to discriminate the DSSI cases
Entered in the multivariate logistics regression model were the above significant variables and additionally those with P < .10 who were ASA grade, TP and PLR in dichotomised category, and RBC in continuous variable. The final results showed that current smoking (OR, 2.8; P = .021), BMI ≥26.4 kg/m2 (OR, 3.1; P = .003), ASA ≥II (OR, 1.3; P = .043), incision level of II (OR, 3.8; P = .018) and NLR ≥6.4 (OR, 3.2; P = .008) were independently associated with the development of DSSI. For each variable, the corresponding score was assigned (Table 2). Hosmer‐Lemeshow test demonstrated an acceptable goodness‐of‐fit of the model (X 2 = 4.118, P = .306).
TABLE 2.
Assigned score for each variable based on their independent association of magnitude with DSSI
| Variable | Association of magnitude (OR and 95% CI) | P | Assigned score | |
|---|---|---|---|---|
| OR | 95% CI | |||
| Current smoking | 2.8 | 1.3‐6.4 | .021 | 3 |
| BMI ≥26.4 kg/m2 | 3.1 | 1.6‐8.4 | .003 | 3 |
| ASA (≥II vs I) | 1.3 | 1.0‐5.1 | .043 | 1 |
| Incision level (II vs I) | 3.8 | 1.3‐12.6 | .018 | 4 |
| NLR (≥6.4 vs <6.4) | 3.2 | 1.3‐7.5 | .008 | 3 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidential interval; DSSI, deep surgical site infection; NLR, neutrophil/lymphocyte ratio; OR, odd ratio.
Based on our predefined algorithm, the average score for one patient was 10 (median, 11; range 4‐21). The ROC results showed the AUC was 0.766 (95% CI, 0.670‐0.863; P < .001) and a score of 14 was the optimal cut‐off value, corresponding to sensitivity of 0.542 and specificity of 0.872. One hundred and twenty‐five patients had a score of ≥14 among whom 13 (10.4%) developed a DSSI; while 775 patients had a score of <14, and 11 (0.13%) developed a DSSI. This suggested a score of ≥14 was associated with 8.1‐times increased risk of DSSI (Chi‐square test, 95% CI, 3.5‐18.4; P < .001). We also investigated another 2 seconds optimal cut‐off values for sensitivity of most clinical relevance, which had a score of 7 (sensitivity, 100%) and a score of 10 (sensitivity, 0.875) (Figure 3).
FIGURE 3.
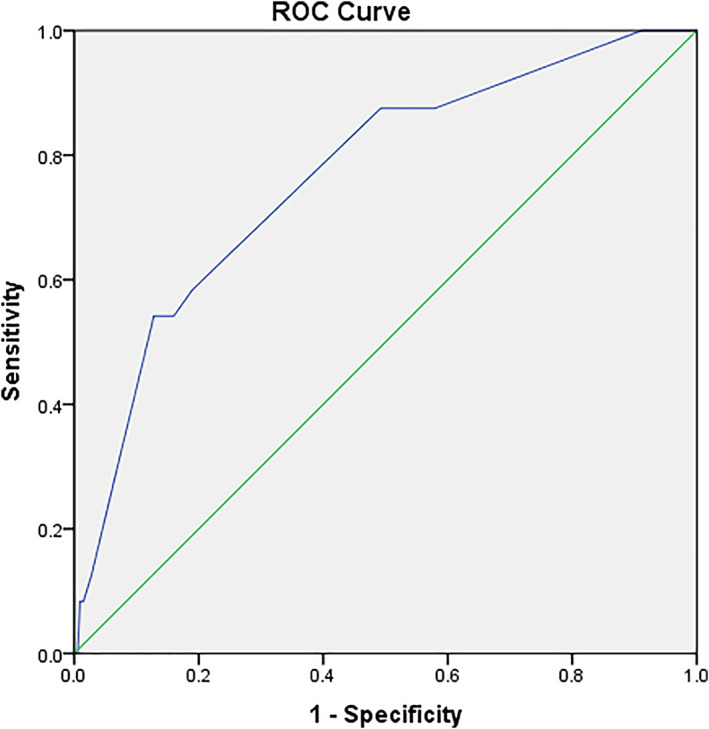
ROC curve for the summed score to determinate its optimal cut‐off for distinguishing the DSSIs from non‐DSSIs. The AUC was 0.766 (95% CI, 0.670‐0.863; P < .001) and a score of 14 was the optimal cut‐off value, corresponding to sensitivity of 0.542 and specificity of 0.872. A score of 7 as cut‐off value is corresponding to the sensitivity of 100%, and of 10 responding to sensitivity of 0.875. Horizontal axis represents the 1‐specificity and vertical axis indicates the sensitivity of each variable in predicting the development of DSSI
4. DISCUSSION
DSSI in the field of orthopaedic trauma surgery has been consistently a focus in both clinical practice and scientific research. In this study, we identified several important factors, thereby forming a valuable risk prediction model. The model showed that a score of ≥10 should alert the risk of DSSI (sensitivity, 0.875), a score of 14 or more is strongly predictive of development of DSSI (OR, 8.1), and a score of <7 almost could rule out the possibility of DSSI.
Compared with the high variable rates of SSI (5.0%‐25.0%) 11 , 16 , 17 or DSSI (2.9%‐15.4%) 3 , 4 , 5 in the literature, we reported a relatively lower incidence rate of 2.7%. This was mainly decided by the predefined rigorous inclusion and exclusion criteria and the narrower definition of DSSI. Some injuries or medical conditions at high risk of DSSI had been excluded, including open fracture, multiple trauma or bilateral calcaneal fractures. 6 , 11 The rapid improvement in operative techniques, implant material property, standardisation of antibiotics prophylaxis and wound care in orthopaedic trauma during the past 30 years has contributed to decreasing the SSIs overall. 18 Another reason might be that we only inquired index hospitalisation or re‐hospitalisation medical records for confirmation of DSSIs, leaving those with DSSI treated in other institutions excluded acquiescently.
Identification of risk factors and accordingly targeting prophylactic measures do help with effective perioperative management of surgical wound. In the present study, we used a thoroughly selected population with a huge amount of sample to address the risk factors of DSSI following calcaneal fracture. It is of note, four out of five factors identified had been repeatedly investigated and their role was well‐established across studies with different levels of evidence. 4 , 8 , 9 , 10 , 19 The only difference among them might be the determination of cut‐off values of BMI, for which we used both the traditional value (28.0 g/m2, for definition of obesity for Chinese people) and the current value of 26.4 kg/m2 to make a dichotomization. As a result, the latter demonstrated to be more sensitive in prediction of DSSI (P < .001). We inferred that the younger age (mean, 41.8 years) and slimmer figure (BMI, mean, 25.1 kg/m2) in this selected group contributed to this discrepancy.
As a novel indicator, NLR was firstly identified as an independent factor for predicting the development of DSSI in this study, which could be explained by it being an indicator of magnitude of body's systemic inflammatory response 20 and further the cumulative effects of persistent acute inflammatory response after surgery. 13 Our finding complemented the recent reports regarding the prediction role in adverse results following orthopaedic surgeries. For example, NLR demonstrated to be capable of predicting the mortality and cardiovascular complications after hip fracture, 13 the risk of venous thromboembolic events (VTE) after total knee arthroplasty, 12 the degree of inflammatory response/infection and onset of myocardial injury in orthogeriatric patients. 21 In addition, in non‐orthopaedics specialties, NLR has also demonstrated its relation with adverse outcomes, onset of acute or chronic complications, or poor conditions. 14 , 22 , 23 In this study, patients with NLR ≥6.4 are highly vulnerable to the development of a DSSI (OR, 3.2), to whom specific attention should be paid to control those combined modifiable factors (smoking, dose of prophylactic antibiotics).
By far as we know, this is the first report of development of a risk model for predicting DSSI specifically following calcaneal fracture. Its feasibility in clinical application and potential advantages were predictable. First, this model was formed based on four well‐established risk factors in the literature and one novel biomarker. Particularly, the latter was derived from the blood routine examination results (neutrophil and lymphocyte count), both of which are readily available and hence without adding any extra cost for patients. Second, this model uses the score of 10 (with a sensitive of 0.875) to stratify patients into high‐risk and low‐risk categories, which was conducive to targeted prevention of DSSIs. It is of particular note that a score of ≥14 was identified to be associated with 8.1‐times increased risk of DSSI, and patients in this category should be paid more attention to and if conditional, specifically protocolised surveillance could be developed. Third, all the DSSI cases occurred in patients with a score of ≥7, and hence a score of <7 contributes to almost completely rule out the possibility of DSSI, possibly avoiding the generalised coverage of infection control resources.
Several limitations to this study should be mentioned. First, the retrospective nature of this study limited the accuracy and precision in data collection, although the double‐entry might partly offset this bias. For some variables (smoking) volume and frequency might be particularly important parameters in infection complications, but detailed data were not available. Second, the volume of surgeon might affect the results, because surgical treatment of calcaneal fracture needs a learning curve, especially for tarsal sinus. 19 As plenty of surgeons perform operations, the sample size of operations for each surgeon in 1 year is too small to obtain effective statistical analyses. Third, as with every multivariate analysis, there remain the unknown or unmeasured confounders to bias the results, despite numerous variables included in this study. Fourth, the single‐centre setting may limit the generalizability of our findings, because patients admitted or transferred commonly have severe injury or poorer comorbid conditions. Fifth, as we discussed previously, we could not identify those who had developed a DSSI but sought treatment in other institutions, resulting in underestimation of such key complication.
In summary, the incidence rate of DSSI following ORIF of calcaneal fractures was 2.7% and five independent factors were identified, most of which were well‐established in the literature. The risk prediction model exhibited its excellent capability in distinguishing those with high‐ and low‐risk of DSSI, with different but appropriate cut‐off values. Our findings should be looked upon in the context of specific or general limitations, and the validity of the risk prediction model should be verified by prospective and multicentre studies of a larger sample size.
CONFLICT OF INTEREST
The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article. ICMJE forms for all authors are available online.
AUTHOR CONTRIBUTIONS
Kaosheng Lu designed the study; Kaosheng Lu, Tiaoxiao Ma followed up the patients and collected the data. Chunyan Yang and Haibo Liu analysed and interpreted the data; Qu Q seared the relevant literature; Kaosheng Lu wrote the manuscript and all the authors approved the final version of the manuscript.
ETHICAL APPROVAL
Permission was obtained from the Institutional Review Board.
ACKNOWLEDGEMENTS
We are grateful to Zhang and Liu K of the Department of Orthopaedics, and to Zhang X of the Department of Statistics and Applications for their kind assistance. All the authors declare that they have no conflict of interest.
Lu K, Ma T, Yang C, Qu Q, Liu H. Risk prediction model for deep surgical site infection (DSSI) following open reduction and internal fixation of displaced intra‐articular calcaneal fracture. Int Wound J. 2022;19(3):656-665. 10.1111/iwj.13663
DATA AVAILABILITY STATEMENT
Peer review of empirical data will be conducted to confirm that the data reproduce the analytic results reported in the paper.
REFERENCES
- 1. Epstein N, Chandran S, Chou L. Current concepts review: intra‐articular fractures of the calcaneus. Foot Ankle Int. 2012;33:79‐86. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y. Clinical Epidemiology of Orthopaedic Trauma. 2nd ed. Stuttgart: Thieme; 2016. [Google Scholar]
- 3. Backes M, Spijkerman IJ, de Muinck‐Keizer RO, Goslings JC, Schepers T. Determination of pathogens in postoperative wound infection after surgically reduced calcaneal fractures and implications for prophylaxis and treatment. J Foot Ankle Surg: Off Publ Am Coll Foot Ankle Surg. 2018;57:100‐103. [DOI] [PubMed] [Google Scholar]
- 4. Wang H, Pei H, Chen M, Wang H. Incidence and predictors of surgical site infection after ORIF in calcaneus fractures, a retrospective cohort study. J Orthop Surg Res. 2018;13:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Backes M, Spierings K, Dingemans S, Goslings J, Buckley R, Schepers T. Evaluation and quantification of geographical differences in wound complication rates following the extended lateral approach in displaced intra‐articular calcaneal fractures—a systematic review of the literature. Injury. 2017;48:2329‐2335. [DOI] [PubMed] [Google Scholar]
- 6. Dickens JF, Kilcoyne KG, Kluk MW, Gordon WT, Shawen SB, Potter BK. Risk factors for infection and amputation following open, combat‐related calcaneal fractures. J Bone Joint Surg Am. 2013;95:e24. [DOI] [PubMed] [Google Scholar]
- 7. Backes M, Schep NW, Luitse JS, Goslings JC, Schepers T. The effect of postoperative wound infections on functional outcome following intra‐articular calcaneal fractures. Arch Orthop Trauma Surg. 2015;135:1045‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ding L, He Z, Xiao H, Chai L, Xue F. Risk factors for postoperative wound complications of calcaneal fractures following plate fixation. Foot Ankle Int. 2013;34:1238‐1244. [DOI] [PubMed] [Google Scholar]
- 9. Soni A, Vollans S, Malhotra K, Mann C. Association between smoking and wound infection rates following calcaneal fracture fixation. Foot Ankle Spec. 2014;7:266‐270. [DOI] [PubMed] [Google Scholar]
- 10. Court‐Brown C, Schmied M, Schmidt M, Schutte B. Factors affecting infection after calcaneal fracture fixation. Injury. 2009;40:1313‐1315. [DOI] [PubMed] [Google Scholar]
- 11. Ho CJ, Huang HT, Chen CH, Chen JC, Cheng YM, Huang PJ. Open reduction and internal fixation of acute intra‐articular displaced calcaneal fractures: a retrospective analysis of surgical timing and infection rates. Injury. 2013;44:1007‐1010. [DOI] [PubMed] [Google Scholar]
- 12. Barker T, Rogers VE, Henriksen VT, et al. Is there a link between the neutrophil‐to‐lymphocyte ratio and venous thromboembolic events after knee arthroplasty? A pilot study. J Orthop Traumatol: Off J Ital Soc Orthop Traumatol. 2016;17:163‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forget P, Moreau N, Engel H, et al. The neutrophil‐to‐lymphocyte ratio (NLR) after surgery for hip fracture (HF). Arch Gerontol Geriatr. 2015;60:366‐371. [DOI] [PubMed] [Google Scholar]
- 14. Forrest EH, Storey N, Sinha R, et al. Baseline neutrophil‐to‐lymphocyte ratio predicts response to corticosteroids and is associated with infection and renal dysfunction in alcoholic hepatitis. Aliment Pharmacol Ther. 2019;50:442‐453. [DOI] [PubMed] [Google Scholar]
- 15. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. 2010;5:1315‐1316. [DOI] [PubMed] [Google Scholar]
- 16. Strømsøe K, Mørk E, Hem ES. Open reduction and internal fixation in 46 displaced intraarticular calcaneal fractures. Injury. 1998;29:313‐316. [PubMed] [Google Scholar]
- 17. Zwipp H, Tscherne H, Thermann H, Weber T. Osteosynthesis of displaced intraarticular fractures of the calcaneus. Results in 123 cases. Clin Orthop Relat Res. 1993;(290):76‐86. [PubMed] [Google Scholar]
- 18. Spierings KE, Min M, Nooijen LE, Swords MP, Schepers T. Managing the open calcaneal fracture: a systematic review. Foot Ankle Surg: Off J Eur Soc Foot Ankle Surg. 2019;25:707‐713. [DOI] [PubMed] [Google Scholar]
- 19. Spierings KE, Sanders FRK, Nosewicz TL, Schepers T. Risk factors for surgical site infections with the sinus tarsi approach in displaced intra‐articular calcaneal fractures; a prospective cohort study with a minimum of one year follow‐up. Injury. 2020;51:1676‐1680. [DOI] [PubMed] [Google Scholar]
- 20. Ahsen A, Ulu MS, Yuksel S, et al. As a new inflammatory marker for familial Mediterranean fever: neutrophil‐to‐lymphocyte ratio. Inflammation. 2013;36:1357‐1362. [DOI] [PubMed] [Google Scholar]
- 21. Fisher A, Srikusalanukul W, Fisher L, Smith P. The neutrophil to lymphocyte ratio on admission and short‐term outcomes in Orthogeriatric patients. Int J Med Sci. 2016;13:588‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manohar V, Prasad SB, Raj S, Sreekrishnan TP, Gireesh Kumar KP. The Eminence of neutrophil‐lymphocyte count ratio in predicting bacteremia for community‐acquired infections at an emergency medicine Department in a Tertiary Care Setting. J Emerg Trauma Shock. 2018;11:271‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou J, Xu E, Shao K, et al. Circulating platelet‐neutrophil aggregates as risk factor for deep venous thrombosis. Clin Chem Lab Med. 2019;57:707‐715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Peer review of empirical data will be conducted to confirm that the data reproduce the analytic results reported in the paper.


