Abstract
Negative pressure wound therapy (NPWT) decreases postoperative complications of various surgeries. However, the use of NPWT for oncological surgical wounds remains controversial. To evaluate the association of NPWT with oncologic recurrence in surgical wounds without residual malignancy, we analysed studies that compared NPWT with conventional non‐pressure dressings for cancer surgical wounds without residual tumour by August 12, 2020. We compared tumour recurrence rates and postoperative complications between the two procedures. The six studies included 118 patients who received NPWT, and 149 patients who received conventional non‐pressure wound care. The overall quality of the included studies was high based on the Newcastle–Ottawa scale score of 7.5. Tumour recurrence after NPWT was not significantly different compared with conventional non‐negative pressure wound care (9.3% versus 11.4%, P = 0.40). There was no significant heterogeneity between the studies (I2 = 3%). Although NTWT was associated with a lower complication rate compared with the control group, the result was non‐significant (P = 0.15). Application of NPWT in oncologic resection wounds without residual malignancy revealed no difference in local recurrence and may reduce the risk of postoperative complications compared with conventional non‐negative pressure dressings. NPWT can be considered an alternative method for reconstruction in challenging cases.
Keywords: malignant neoplasms, melanoma, negative‐pressure wound therapy, recurrence, sarcoma
1. INTRODUCTION
Negative pressure wound therapy (NPWT) benefits patients with difficult surgical wounds by reducing the risk of surgical site infections (SSIs) 1 and surgical complications, including dehiscence, seroma/hematoma, skin necrosis/blistering, 2 and postoperative mortality rate as shown in previous meta‐analyses. 3 , 4 Recently, following the advancement of NPWT use in closed incisional wounds, a meta‐analysis also found that NPWT applied to closed surgical incisions reduces the risk of SSIs across all surgical procedures, including general and colorectal surgery, 5 , 6 and also significantly lowers the rate of seroma and overall wound complications when used in orthopaedic procedures 7 and caesarean wounds. 8 From the beginning, the manufacturers of NPWT devices have constrained the use of NPWT to non‐malignant wounds. One reason (also from a legal stand point) was that it was feared that physicians might simply apply NPWT to existing tumours. Therefore, NPWT use in wounds even after cancer removal has been considered as a relative contraindication by some physicians because of concerns that the angiogenic effect may stimulate tumoral recurrence or seeding. 9 , 10 There is no meta‐analysis performed specifically for NPWT use in oncological surgical wounds without residual malignancy. Therefore, we aimed to perform a systematic review and meta‐analysis to evaluate the feasibility and oncological recurrence of NPWT use in cancer surgical wounds without residual malignancy.
2. MATERIALS AND METHODS
2.1. Study selection
The protocol was registered in PROSPERO with the registration number CRD42020207486. We defined our eligibility criteria prior to the literature search (Table S1). We searched for all studies that compared NPWT with conventional non‐negative pressure dressings for oncologic surgical wounds without residual malignancy and compared tumour recurrence and postoperative complications between the two groups.
There were at least five patients in each group. The primary outcome was tumour recurrence. Wound complications, hospitalisation days, and time to wound closure were secondary outcomes. We excluded studies without information on tumour recurrence, studies with inaccurate designs or less than five patients in each group, those without comparators, and studies with patients already with a metastatic disease.
2.2. Literature search
We performed a systematic search and meta‐analysis according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines.
The keywords used in the search, i.e., negative pressure wound therapy, cancer, and recurrence, included their synonyms and controlled vocabulary (MeSH or Emtree terms), when available. Search terms were as follows:
#1 (Negative Pressure Wound Therapy* OR Negative‐Pressure Wound Therapy* OR Topical Negative‐Pressure Therapy* OR Negative‐Pressure Dressing* OR Negative Pressure Dressings OR Vacuum‐Assisted Closure OR Vacuum Assisted Closure OR Negative‐Pressure Wound Therapy);
#2 (cancer* OR Malignancy* OR Malignant Neoplasm OR Malignant Neoplasms OR Neoplasia* OR Neoplasm OR tumour * OR Neoplasms OR melanoma or sarcoma);
#3 (recurrence OR recurrence * OR relapse* OR metastasis OR metastases);
#4: #1 AND #2 AND #3.
The search strategy is detailed in the Table S2. Relevant studies were identified from Medline, Embase, Cochrane Library, and trial registers (www.clinicaltrials.gov), from their start to 12 August, 2020, by two investigators (YJ. Wang, XF. Yao) and one experienced librarian (P‐J Li) from the teaching hospital. We also assessed additional studies by manually searching reference sections of full‐text reviews and contacted experts from the field. Only human studies reported in English were included.
2.3. Data extraction
Two reviewers (YJ. Wang, XF. Yao) independently extracted data regarding authors, year of publication, inclusion and exclusion criteria, population characteristics, malignancy, intervention, recurrence rate or numbers, postoperative complications, and follow‐up periods. The authors of the retrieved studies were contacted if the data of the full‐text papers were not available. All data were extracted and subsequently cross‐checked to rule out any discrepancies. Any disagreement was resolved through negotiation or a consensus meeting with a third investigator.
2.4. Statistical analysis
Statistical analysis was performed using the RevMan 5.1 statistical software (Cochrane Collaboration). We calculated the summary of outcome as a relative risk ratio (RR) with 95% confidence intervals (CIs), using the DerSimonian and Laird random effects model. No‐event RR was used in cases when no events were observed. All statistical tests were two‐sided, and a P‐value < .05 was considered as a statistically significant outcome.
2.5. Risk of bias and quality assessment
The quality of studies was evaluated using the Newcastle–Ottawa scale (NOS), which was developed to assess the quality of non‐randomised cohort studies. Studies with a NOS score of 7 or higher were considered of high quality. 11 Publication bias was assessed by the funnel plot analysis (Figures S1 and S2).
3. RESULTS
3.1. Literature search
Figure 1 illustrates the study selection process in our analysis. The initial search strategy yielded 485 articles, and 42 duplicates among them were removed. Based on the screening criteria for titles and abstracts, 349 articles were excluded. After full‐text reviews, we excluded 90 articles. Finally, six eligible studies were subjected to a qualitative review.
FIGURE 1.
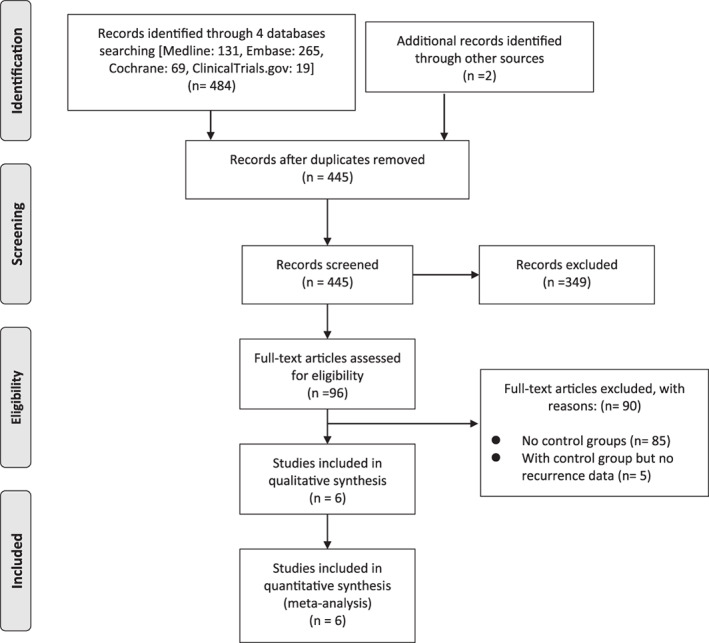
PRISMA flow chart
3.2. Quality assessment of the studies and risk of bias
The mean NOS score was 7.5, indicating a high quality of the included studies. Table 1 presents the quality of six included studies evaluated by NOS. Funnel plot analysis showed no significant publication bias (Table S2).
TABLE 1.
Quality of the six included studies evaluated using Newcastle‐Ottawa Scale (NOS)
| Study | Selection (4) | Comparability (2) | Exposure (3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Case definition adequate (1) | Representativeness of the cases (1) | Selection of controls (1) | Definition of controls (1) | Comparability based on design or analysis (2) | Ascertainment of exposure (1) | Same method of ascertainment for cases and controls (1) | Non‐response rate (1) | Total (9) | |
| Bedi 13 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Campagnari 32 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Denzinger 14 2007 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Narducci 22 2011 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Oh 12 2012 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Seo 33 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
Note: The quality of studies was evaluated using the Newcastle‐Ottawa scale (NOS), which was developed to assess the quality of non‐randomised cohort studies. Studies with an NOS score of 7 or higher were considered of high quality.
The patients in all studies were diagnosed with cancer by pathological assessments. Most of the studies were considered representative, as malignant tumours are mostly treated at medical centres, which is consistent with our included studies. All included studies reported recurrence rates and wound complications and the follow‐up period. Follow‐up data were obtained by an e‐mail contact with an author of one study. 12 Only two studies mentioned lost follow‐up patients, with higher incidence in the control group. Postoperative wound complications were not further classified in one study. 13
3.3. Included study characteristics and details of study interventions
This meta‐analysis included six independently conducted observational studies published between 2007 and 2019, with a total of 267 patients enrolled. All the studies were retrospective cohort analyses. Table 2 presents the characteristics and details of the interventions in each study. All studies included patients who underwent surgery for their malignant tumour, 118 patients were managed with NPWT, and 149 patients were treated with conventional non‐negative pressure wound dressings. Among the NPWT patients, 39 had incisional NPWT, 7 had NPWT on the artificial dermis, and 57 had NPWT directly over the wounds. There was no tumour recurrence in the follow‐up period in two studies. 12 , 14 Tumour recurrence included both local recurrence and distant metastasis.
TABLE 2.
Characteristics of included studies
| Author, y, country | NP/C | Age (y) (mean/range) | Malignancy | Intervention | Recurrence number/type | Wound complication number | Definition of Wound complication | Wound healing time (d) | Hospitalised duration (d) | Follow‐up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NPWT group | Convention group | ||||||||||
|
Bedi 13 2019 USA |
NP: 39 C: 84 |
NP: 54 (28‐81) C: 56.5 (19‐91) |
Soft tissue sarcoma | Preoperative radiation ± chemotherapy → limb‐sparing resection → incisional NPWT (75 mmHg) | Preoperative radiation ± chemotherapy → limb‐sparing resection → wound closure |
NP: 0/39 C: 3/84 (3 local recur) |
NP: 3/39 C: 39/84 |
Not specified | Not mentioned | Not mentioned | 3.9 (1‐15) y |
|
Campagnari 32 2017 Brazil |
NP: 7 C: 6 |
62.6 (39‐86) | 7 carcinoma, 2 melanoma, 4 sarcoma | Tumour excision → artificial dermis → NPWT | Tumour Excision → artificial dermis → Brown's dressing |
NP: 2/7 (1 distant metastasis, 1 local recur) C: 1/6 (1 local recur) |
NP: 3/7 C: 3/6 |
Wound infection graft loss | Not mentioned | Not mentioned | 20 mo (mentioned in one case) |
| Denzinger 14 2007 Germany |
NP: 5 C: 9 |
NP: 64 (42‐80) C: 67 (40‐86) |
Penile cancer | Inguinal lymphadenectomy → NPWT | Inguinal lymphadenectomy → conventional wound care |
NP: 0/5 C: 0/9 |
NP: 0/5 C: 0/9 |
Bleeding wound infection |
NP: 38.9 (36‐43) C: 69.8 (42‐112) |
NP: 13.2 (8‐27) C: 28.4 (16‐39) |
44 (6‐70) mo |
|
Narducci 22 2011 France |
NP: 30 C: 24 |
NP: 68.4 ± 12.6 C: 70.6 ± 12.5 |
Vulvar carcinoma | Vulvectomy → NPWT a | Vulvectomy → conventional wound care |
NP: 8/30 C: 11/24 |
NP: 6/30 C: 7/24 |
Vestibular stenosis flap necrosis |
NP: 44.4 ± 18.4 C: 60.2 ± 28.7 |
NP: 17.8 ± 8.7 b C: 18.4 ± 9.9 |
19.1 ± 11.2 mo |
|
Oh 12 2012 Korea |
NP: 9 C: 13 |
NP: 58.3 ± ;13.3 C: 64.7 ± 10.7 |
Acral lentiginous melanoma | Tumour excision → NPWT | Tumour excision → secondary healing |
NP: 0/9 C: 0/13 |
NP: 0/9 C: 8/13 |
infection seroma necrosis |
NP: 88.7 (53‐113) C: 125.8 (73‐386) |
Not mentioned |
NP: 16 (7‐23) mo C: 33.5 (28‐39) mo |
|
Seo 33 2016 Korea |
NP: 28 C: 13 |
NP: 61.8 ± 15.4 C: 64.9 ± 13.9 |
Acral lentiginous melanoma |
13 tumour excision → NPWT 15 tumour excision+ punch grafting → NPWT |
Tumour excision → secondary healing |
NP: 1/28 (local recurrence) C: 2/13 (local recurrence and distant metastasis) |
NP: 1/28 C: 0/13 |
Infection seroma necrosis |
NP: 86.8 (53‐135) C: 101.1 (63‐173) |
Not mentioned |
NP: 1518 ± 270.6 d C: 1722 ± 185.3 d |
Abbreviations: C, conventional non‐negative pressure wound care; NP, negative pressure wound therapy.
NPWT was also placed in patients who had inguinofemoral lymphadenectomy wounds, but not on the sentinel lymph node dissection wounds.
Length of use of VAC was 11 days.
3.4. Tumour recurrence
The six studies included 118 patients who received NPWT, and 149 patients who received conventional non‐negative pressure wound care (Figure 2). Tumour recurrence rate was not statistically different between the NPWT and conventional wound care groups (9.3% versus 11.4%, P = .25) The relative risk (RR) of “non‐recurrence rate” for NPWT over non‐negative pressure dressings was 1.03 (95% CI, 0.98‐1.10). There was no statistically significant heterogeneity between the studies (P = .40, I2 = 3%).
FIGURE 2.
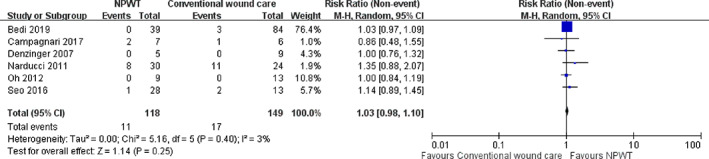
Forest plot shows the relative risk (RR) of “tumour non‐recurrence rate.” A random‐effects model was used to compare the RR between NPWT and conventional non‐negative pressure dressings
3.5. Wound complication, healing time, and hospitalisation duration
Patients treated with NPWT had a lower post‐operative complication rate (10.17%) compared with the conventional non‐negative pressure wound dressings group (38.26%). NPWT was associated with a RR of “non‐complication rate” of 1.28 (95% CI, 0.92‐1.79) (Figure 3). However, the result was not significant (P = .15) with a high heterogeneity test (P < .00001, I2 = 87%). Four studies presented time to complete re‐epithelialization, and NPWT was associated with a significantly shortened healing time in these four studies. However, because only one study presented the standard deviation, we did not perform an analysis for wound healing time. The details of wound healing time and hospitalisation duration are listed in Table 2.
FIGURE 3.
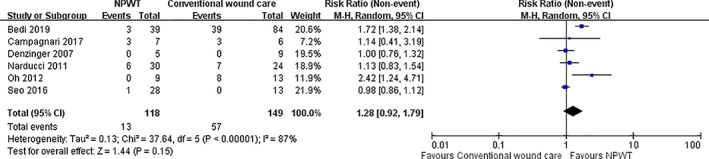
Forest plot shows the relative risk (RR) of “non‐complication rate.” A random‐effects model was used to compare the RR between NPWT and conventional non‐negative pressure dressings
4. DISCUSSION
Most cancer surgical wounds can be managed by flaps, grafts, or free tissue microvascular reconstruction. However, certain types of oncological surgery wounds are prone to develop postoperative complications. For sarcoma, many studies have suggested that preoperative radiation followed by wide local excision is associated with comparable survival and local control rates to amputation. 15 However, many surgeons are reluctant to use preoperative radiation for proximal lower extremity sarcoma because incidence of postoperative wound complications is reported to be as high as 50%. 13 , 16 , 17 Wounds after inguinal lymphadenectomy for melanoma, penile, urethra, and vulva cancers are associated with complications including seroma, SSI, lymphoceles, persistent lymphorrhoea, lymphedema, wound breakdown, significantly longer time of hospitalisation, and reinterventions. 14 , 18 , 19 , 20 Breakdown of wounds with protracted secondary healing following inguinal lymphadenectomy for penile cancer was reported in up to 50% of cases. 14 , 21 In a meta‐analysis, inguinal lymphadenectomy of lower part body melanoma was associated with overall complications of 52%. 20 Vulvectomy not only has a significant psychological impact on patients but is also complicated by complex wound failures in 26% to 85% of cases, resulting in long hospital stays and cicatrisation. 22 , 23 , 24 , 25 Advanced age (mean age: 70 years) and obesity (BMI: 27‐29) also influence this clinical picture. 22 Large defects of the scalp with exposed dura and skull also pose unusual challenges on reconstruction. 26 , 27 , 28 Free vascularised muscle flaps are not always feasible and may fail because of peripheral vascular disease or previous radiation therapy. 26 When a defect is on the sole of the foot, repair is difficult owing to insufficient local skin pool and lack of mobility of the sole skin. In addition, a bulky flap on the sole may interfere with daily activities such as walking and wearing normal shoes.
The implementation and modification of NPWT, including incisional NPWT and NPWT with computer‐controlled wound irrigation, is a major improvement in wound healing. 29 , 30 It serves not only as a delivery device for cold plasma, growth factors, stem cells, and other wound healing factors to the wound bed, but also decreases the harmful inflammation and bacterial load. 29 , 31 NPWT is readily accessible and its use did not compromise oncological control in studies included in our analysis. 13 For proximal lower extremity soft tissue sarcomas with preoperative radiation, NPWT use at the time of resection is associated with a lower risk of wound complications and secondary operative procedures, and the duration of the treatment until complete closure of the wound is significantly shorter. 14 , 18 Likewise, conventional care of vulvectomy wound takes 2 to 3 months until wound recovery and is even longer when patients are of old age, obese, and have multiple cormobidities. 22 , 25 Using NPWT dressing immediately after vulvectomy reduces the total length of cicatrisation by approximately 16 days. 22
Reconstruction options for large scalp and cranial tumours may be limited, especially when free vascularised muscle flaps fail. In such cases, adjunctive use of NPWT helps achieve rapid formation of granulation tissue on the dura to allow subsequent skin grafting. 26 , 32 In the two studies involving acral melanoma surgical wounds, NPWT use was associated with significantly shorter time to complete re‐epithelialization, and favourable cosmetic outcomes. 12 , 33 Furthermore, NPWT devices allow for ambulatory treatment, earlier hospital discharge or even same‐day discharge. A wound study of cost‐utility analysis clearly demonstrated a clear cost benefit to NPWT. 34
The facilitation of angiogenesis surrounding the wound bed is one of the most significant beneficial factors of NPWT. Do the same mechanisms by which NPWT promotes wound healing really facilitate tumorigenesis? Angiogenesis in normal tissues is different from that in cancer in several aspects. In normal tissue, the initiation of angiogenesis (angiogenic switch) is tightly regulated. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 Wound‐site angiogenesis is mechanically initiated through microdeformation, which establishes hypoxia and a subsequent vascular endothelial growth factor (VEGF) gradient that drives directional endothelial tip cell migration, resulting in vessel growth. 43 Tissue hypoxia induces and stabilises hypoxia‐inducible factor‐1α (HIF‐1α). Temporary reduction of blood flow at the wound edge stimulates angiogenesis through the (HIF‐1α)–VEGF pathway, which in turn stimulates VEGF expression. 35 , 36 , 37 , 38 , 44 Thus, NPWT promotes VEGF‐induced angiogenesis and increased capillary quality via promoting structural integrity and functional stabilisation at various stages of wound healing. 45 , 46
In cancer, intratumoral hypoxia stimulates the expression of angiogenic factors and the angiogenic switch is almost always activated. 42 , 47 While physiological angiogenesis leads to the generation of functional vessels that enhance perfusion, 41 tumoral angiogenesis develops abnormal vasculature with variable sizes, shapes, and architecture, as well as with abnormal functionality. 48 These abnormal features are acquired by tumour endothelial cells, 42 which are notably phenotypically different from normal endothelial cells and display distinct gene expression profiles. 49 Compared with normal endothelial cells, tumour endothelial cells are exposed to a highly mechanically heterogeneous extracellular matrix (ECM), and are subjected to intratumoral hypoxia, low pH, mechanical stresses, and soluble mediators released by surrounding cancer and stromal cells. 49 ECM composition and matrix stiffness both direct tumour endothelial cell network formation. 48 The ECM becomes stiffer during solid tumour progression. The overall effect of tumoral angiogenesis is a paradoxical decrease in perfusion that aggravates hypoxia in a feed‐forward loop promoting HIF‐1α stabilisation. 47 In brief, tumour angiogenesis is affected by numerous chemical and mechanical signalling events. 50 , 51 , 52 NPWT‐induced angiogenesis is regulated by normal endothelial cells and controlled by tight environmental regulation. Local hypoxia or elevated VEGF itself cannot trigger tumorigenesis. 53
Our findings concerning NPWT‐related reduction in cancer surgical wound complications must be interpreted with caution because of significant clinical heterogeneity. First, the included studies were on different malignant tumours, including carcinoma, sarcoma, and melanoma. Therefore, treatment plans, outcome, and length of follow‐up for recurrence were different. The majority of melanomas in Caucasians are a superficial‐spreading type located on the trunk and extremities and usually pose no difficulty in reconstruction. The two studies involving melanoma in this meta‐analysis were all acral‐type melanomas in Asians posing challenges for reconstruction. Considering the limited number of included studies, subgroup analysis by cancer type in our study was not feasible. Therefore, the conclusion may not be applicable to all types of cancer. Second, the two main outcomes in our study were tumour recurrence and complication rate. Postoperative complications include SSI, hematoma, seroma, partial graft loss, need for debridement, vulva vestibular stenosis, etc., but one study did not clearly define the post‐operation complication types. 13 On top of the publication bias, the outcome is also related to the techniques used by physicians and the overall condition of the health care system. Thus, more studies from different countries and medical domains are definitely needed to evaluate these findings. Nevertheless, because of the clear definition of participation and intervention comparison, we can still conclude that the results in the present analysis have high clinical significance.
Our study has some limitations. Because application to oncologic resection wounds was more or less unofficial, the number of papers is naturally limited and the data need to be interpreted cautiously. First, the follow‐up period for recurrence was a prerequisite for inclusion into the analysis; there were various lengths of the follow‐up periods given different types of malignant tumours included in this analysis. Some patients with 12‐ to 24‐month follow‐up may still be at risk for local recurrence. Second, recurrences were noted in both groups, as they are not only NPWT‐related. Lastly, there were some differences between the NPWT procedures used in these studies. One study used incisional NPWT, 13 one study used NPWT on the artificial dermis, 32 and the other four studies used NPWT directly on the wounds. 12 , 14 , 22 , 33 Despite these limitations, we conclude that tumour recurrence after NPWT was not significantly different compared with conventional non‐negative pressure wound care. NPWT is associated with a lower risk of wound complications, shortened hospitalisation duration, and wound closure time. This helps tumour and reconstructive surgeons in so far as it gives data to legitimise NPWT on tumour free former cancer wounds, as is frequently necessary bridging the time before wound coverage or final reconstruction until the final histological clearance is guaranteed.
5. CONCLUSION
Our meta‐analysis revealed that there was no significant difference in the recurrence rate between NPWT and conventional non‐negative pressure dressings in oncological surgical wounds without residual malignancy. The use of NPWT was associated with fewer complications. Therefore, NPWT can be considered as an alternative method to promote wound healing for cancer reconstruction in different scenarios.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Appendix S1: Supporting Information
Table S1. Inclusion and exclusion criteria: participants, interventions, comparators, outcomes, time, setting, and study designs (PICOTS‐SD)
Table S2. Search terms and search strategy
Figure S1. Funnel plot of recurrence in the six included studies
Figure S2. Funnel plot of wound complication in the six included studies
ACKNOWLEDGEMENTS
We thank Pei‐Jin Li for her help in the literature scanning.
Wang Y‐J, Yao X‐F, Lin Y‐S, Wang J‐Y, Chang C‐C. Oncologic feasibility for negative pressure wound therapy application in surgical wounds: A meta‐analysis. Int Wound J. 2022;19(3):573-582. 10.1111/iwj.13654
Yen‐Jen Wang and Xiao‐Feng Yao contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Li HZ, Xu XH, Wang DW, Lin YM, Lin N, Lu HD. Negative pressure wound therapy for surgical site infections: a systematic review and meta‐analysis of randomized controlled trials. Clin Microbiol Infect. 2019;25(11):1328‐1338. [DOI] [PubMed] [Google Scholar]
- 2. Ge D. The safety of negative‐pressure wound therapy on surgical wounds: an updated meta‐analysis of 17 randomized controlled trials. Adv Skin Wound Care. 2018;31(9):421‐428. [DOI] [PubMed] [Google Scholar]
- 3. Cirocchi R, Birindelli A, Biffl WL, et al. What is the effectiveness of the negative pressure wound therapy (NPWT) in patients treated with open abdomen technique? A systematic review and meta‐analysis. J Trauma Acute Care Surg. 2016;81(3):575‐584. [DOI] [PubMed] [Google Scholar]
- 4. Atema JJ, Gans SL, Boermeester MA. Systematic review and meta‐analysis of the open abdomen and temporary abdominal closure techniques in non‐trauma patients. World J Surg. 2015;39(4):912‐925. [DOI] [PubMed] [Google Scholar]
- 5. Shiroky J, Lillie E, Muaddi H, Sevigny M, Choi WJ, Karanicolas PJ. The impact of negative pressure wound therapy for closed surgical incisions on surgical site infection: a systematic review and meta‐analysis. Surgery. 2020;167(6):1001‐1009. [DOI] [PubMed] [Google Scholar]
- 6. Sahebally SM, McKevitt K, Stephens I, et al. Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta‐analysis. JAMA Surg. 2018;153(11):e183467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hyldig N, Birke‐Sorensen H, Kruse M, et al. Meta‐analysis of negative‐pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu L, Kronen RJ, Simon LE, Stoll CRT, Colditz GA, Tuuli MG. Prophylactic negative‐pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta‐analysis. Am J Obstetr Gynecol. 2018;218(2):200‐10.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mermerkaya U, Bekmez S, Alkan E, Ayvaz M, Tokgozoglu M. Evaluation of vacuum‐assisted closure in patients with wound complications following tumour surgery. Int Wound J. 2016;13(3):394‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pappalardo V, Frattini F, Ardita V, Rausei S. Negative pressure therapy (NPWT) for Management of Surgical Wounds: effects on wound healing and analysis of devices evolution. Surg Technol Int. 2019;34:56‐67. [PubMed] [Google Scholar]
- 11. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 12. Oh BH, Lee SH, Nam KA, Lee HB, Chung KY. Comparison of negative pressure wound therapy and secondary intention healing after excision of acral lentiginous melanoma on the foot. Br J Dermatol. 2013;168(2):333‐338. [DOI] [PubMed] [Google Scholar]
- 13. Bedi M, King DM, DeVries J, Hackbarth DA, Neilson JC. Does vacuum‐assisted closure reduce the risk of wound complications in patients with lower extremity sarcomas treated with preoperative radiation? Clin Orthop Relat Res. 2019;477(4):768‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Denzinger S, Lübke L, Roessler W, Wieland WF, Kessler S, Burger M. Vacuum‐assisted closure versus conventional wound care in the treatment of wound failures following inguinal lymphadenectomy for penile cancer: a retrospective study. Eur Urol. 2007;51(5):1320‐1325. [DOI] [PubMed] [Google Scholar]
- 15. Capanna R. CORR insights®: does vacuum‐assisted closure reduce the risk of wound complications in patients with lower extremity sarcomas treated with preoperative radiation? Clin Orthop Relat Res. 2019;477(4):775‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ziegele M, King DM, Bedi M. Tumor volume is a better predictor of post‐operative wound complications compared to tumor size in soft tissue sarcomas of the proximal lower extremity. Clin Sarcoma Res. 2016;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cannon CP, Ballo MT, Zagars GK, et al. Complications of combined modality treatment of primary lower extremity soft‐tissue sarcomas. Cancer. 2006;107(10):2455‐2461. [DOI] [PubMed] [Google Scholar]
- 18. Jorgensen MG, Toyserkani NM, Thomsen JB, Sorensen JA. Prophylactic incisional negative pressure wound therapy shows promising results in prevention of wound complications following inguinal lymph node dissection for melanoma: a retrospective case‐control series. J Plast Reconstr Aesthet Surg. 2019;72:1178‐1183. [DOI] [PubMed] [Google Scholar]
- 19. Tauber R, Schmid S, Horn T, et al. Inguinal lymph node dissection: epidermal vacuum therapy for prevention of wound complications. J Plast Reconstr Aesthet Surg. 2013;66(3):390‐396. [DOI] [PubMed] [Google Scholar]
- 20. Söderman M, Thomsen JB, Sørensen JA. Complications following inguinal and ilioinguinal lymphadenectomies: a meta‐analysis. J Plast Surg Hand Surg. 2016;50(6):315‐320. [DOI] [PubMed] [Google Scholar]
- 21. Muñoz Guillermo V, Rosino Sánchez A, Rivero Guerra Á, et al. Video endoscopic inguinal lymphadenectomy in penile cancer: systematic review. Arch Esp Urol. 2019;72(10):992‐999. [PubMed] [Google Scholar]
- 22. Narducci F, Samouelian V, Marchaudon V, et al. Vacuum‐assisted closure therapy in the management of patients undergoing vulvectomy. Eur J Obstetr Gynecol Reprod Biol. 2012;161(2):199‐201. [DOI] [PubMed] [Google Scholar]
- 23. Hu J, Haefner HK. The Management of Vacuum‐assisted Closure Following Vulvectomy with skin grafting. Plast Reconstr Surg Glob Open. 2018;6(4):e1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Llueca A, Herraiz JL, Del Moral R, et al. Use of negative pressure wound therapy after infection and flap dehiscence in radical vulvectomy: a case report. Int J Surg Case Rep. 2017;41:370‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quercia V, Saccone G, Raffone A, et al. Use of negative pressure wound therapy systems after radical Vulvectomy for advanced vulvar cancer. Cancer Invest. 2020;38(8–9):531‐534. [DOI] [PubMed] [Google Scholar]
- 26. Donovan DJ, Person DA. Giant eccrine adenocarcinoma of the scalp with intracranial invasion: resection and reconstruction using a vacuum‐assisted closure device: technical case report. Neurosurgery. 2006;58(4 suppl 2):E371. [DOI] [PubMed] [Google Scholar]
- 27. Cunningham T, Marks M. Vacuum‐assisted closure device and skin substitutes for complex Mohs defects. Dermatol Surg. 2014;40(suppl 9):S120‐S126. [DOI] [PubMed] [Google Scholar]
- 28. Makler V, Litt JS, Litofsky NS. Palliative coverage of cranial defect following failed cranial flap for advanced squamous cell carcinoma: case report. J Palliat Med. 2018;21(1):109‐113. [DOI] [PubMed] [Google Scholar]
- 29. Horch RE, Ludolph I, Müller‐Seubert W, et al. Topical negative‐pressure wound therapy: emerging devices and techniques. Expert Rev Med Devices. 2020;17(2):139‐148. [DOI] [PubMed] [Google Scholar]
- 30. Ludolph I, Fried FW, Kneppe K, Arkudas A, Schmitz M, Horch RE. Negative pressure wound treatment with computer‐controlled irrigation/instillation decreases bacterial load in contaminated wounds and facilitates wound closure. Int Wound J. 2018;15(6):978‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geierlehner A, Horch RE, Müller‐Seubert W, Arkudas A, Ludolph I. Limb salvage procedure in immunocompromised patients with therapy‐resistant leg ulcers‐the value of ultra‐radical debridement and instillation negative‐pressure wound therapy. Int Wound J. 2020;17(5):1496‐1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campagnari M, Jafelicci AS, Carneiro HA, Brechtbühl ER, Bertolli E, Duprat Neto JP. Dermal substitutes use in reconstructive surgery for skin tumors: a single‐center experience. Int J Surg Oncol. 2017;2017:9805980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seo J, Kim J, Nam KA, Zheng Z, Oh BH, Chung KY. Reconstruction of large wounds using a combination of negative pressure wound therapy and punch grafting after excision of acral lentiginous melanoma on the foot. J Dermatol. 2016;43(1):79‐84. [DOI] [PubMed] [Google Scholar]
- 34. Ker H, Al‐Murrani A, Rolfe G, Martin R. WOUND study: a cost‐utility analysis of negative pressure wound therapy after Split‐skin grafting for lower limb skin cancer. J Surg Res. 2019;235:308‐314. [DOI] [PubMed] [Google Scholar]
- 35. Darby IA, Hewitson TD. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016;365(3):553‐562. [DOI] [PubMed] [Google Scholar]
- 36. Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr Diab Rep. 2018;18(1):2. [DOI] [PubMed] [Google Scholar]
- 37. D'Alessandro S, Magnavacca A, Perego F, et al. Effect of hypoxia on gene expression in cell populations involved in wound healing. Biomed Res Int. 2019;2019:2626374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Portou MJ, Yu R, Baker D, Xu S, Abraham D, Tsui J. Hyperglycaemia and Ischaemia impair wound healing via toll‐like receptor 4 pathway activation in vitro and in an experimental murine model. Eur J Vasc Endovasc Surg. 2020;59(1):117‐127. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Yuan F, Liu L, et al. The role of the miR‐21/SPRY2 Axis in modulating Proangiogenic factors, epithelial phenotypes, and wound healing in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2019;60(12):3854‐3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW. Regulation of wound healing and fibrosis by hypoxia and hypoxia‐inducible factor‐1. Mol Cells. 2014;37(9):637‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kir D, Schnettler E, Modi S, Ramakrishnan S. Regulation of angiogenesis by microRNAs in cardiovascular diseases. Angiogenesis. 2018;21(4):699‐710. [DOI] [PubMed] [Google Scholar]
- 42. Dong G, Lin XH, Liu HH, et al. Intermittent hypoxia alleviates increased VEGF and pro‐angiogenic potential in liver cancer cells. Oncol Lett. 2019;18(2):1831‐1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Erba P, Ogawa R, Ackermann M, et al. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg. 2011;253(2):402‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toffoli S, Roegiers A, Feron O, et al. Intermittent hypoxia is an angiogenic inducer for endothelial cells: role of HIF‐1. Angiogenesis. 2009;12(1):47‐67. [DOI] [PubMed] [Google Scholar]
- 45. Ma Z, Shou K, Li Z, Jian C, Qi B, Yu A. Negative pressure wound therapy promotes vessel destabilization and maturation at various stages of wound healing and thus influences wound prognosis. Exp Ther Med. 2016;11(4):1307‐1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hohendorff J, Drozdz A, Borys S, et al. Effects of negative pressure wound therapy on levels of Angiopoetin‐2 and other selected circulating signaling molecules in patients with diabetic foot ulcer. J Diabetes Res. 2019;2019:1756798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schito L. Hypoxia‐dependent angiogenesis and Lymphangiogenesis in cancer. Adv Exp Med Biol. 2019;1136:71‐85. [DOI] [PubMed] [Google Scholar]
- 48. Zanotelli MR, Reinhart‐King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol. 2018;1092:91‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khan KA, Naylor AJ, Khan A, et al. Multimerin‐2 is a ligand for group 14 family C‐type lectins CLEC14A, CD93 and CD248 spanning the endothelial pericyte interface. Oncogene. 2017;36(44):6097‐6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuol N, Stojanovska L, Apostolopoulos V, Nurgali K. Role of the nervous system in tumor angiogenesis. Cancer Microenviron. 2018;11(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leone P, Buonavoglia A, Fasano R, et al. Insights into the regulation of tumor angiogenesis by micro‐RNAs. J Clin Med. 2019;8(12):2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li T, Kang G, Wang T, Huang H. Tumor angiogenesis and anti‐angiogenic gene therapy for cancer. Oncol Lett. 2018;16(1):687‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barrientos S, Brem H, Stojadinovic O, Tomic‐Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22(5):569‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Table S1. Inclusion and exclusion criteria: participants, interventions, comparators, outcomes, time, setting, and study designs (PICOTS‐SD)
Table S2. Search terms and search strategy
Figure S1. Funnel plot of recurrence in the six included studies
Figure S2. Funnel plot of wound complication in the six included studies
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


