Abstract
There is an urgent need for interventions that improve healing time, prevent amputations and recurrent ulceration in patients with diabetes‐related foot wounds. In this randomised, open‐label trial, participants were randomised to receive an application of non‐cultured autologous skin cells (“spray‐on” skin; ReCell) or standard care interventions for large (>6 cm2), adequately vascularised wounds. The primary outcome was complete healing at 6 months, determined by assessors blinded to the intervention. Forty‐nine eligible foot wounds in 45 participants were randomised. An evaluable primary outcome was available for all wounds. The median (interquartile range) wound area at baseline was 11.4 (8.8‐17.6) cm2. A total of 32 (65.3%) index wounds were completely healed at 6 months, including 16 of 24 (66.7%) in the spray‐on skin group and 16 of 25 (64.0%) in the standard care group (unadjusted OR [95% CI]: 1.13 (0.35‐3.65), P = .845). Lower body mass index (P = .002) and non‐plantar wounds (P = .009) were the only patient‐ or wound‐related factors associated with complete healing at 6 months. Spray‐on skin resulted in high rates of complete healing at 6 months in patients with large diabetes‐related foot wounds, but was not significantly better than standard care (Australian New Zealand Clinical Trials Registry: ACTRN12618000511235).
Keywords: amputation, diabetic foot, skin, ulcer, wound healing
1. INTRODUCTION
Globally, one person loses a limb every 20 seconds, usually as a direct consequence of a diabetes‐related foot ulcer (DFU). 1 Impaired healing 2 and episodes of infection of DFU 3 , 4 may result in hospitalisation, prolonged antibiotic therapy and major limb amputations (MLA) with substantial costs to the health system. 5 Ulcer recurrence is estimated to be 40% and 65% at 1 and 3 years, respectively. 2 Five‐year mortality rates are high among those who had any form of lower extremity amputations, exceeding those of many cancers. 6 These poor outcomes have persisted despite increasing awareness of the medical, economic and social burden of diabetes‐related foot complications. 7
Wound healing time is a key cost driver and influences the overall cost‐benefit analysis for any DFU intervention. 8 It is estimated that the mean healing time for a diabetes‐related foot wound managed without amputation is 6 months, rising to 12 months if an amputation is required. 9 , 10 Outpatient management accounts for the majority of the total costs and suggests that reductions in the time to healing are likely to have major benefits, particularly for direct costs related to home nursing visits, dressings and outpatient appointments. 5 Therefore, there is an urgent need to test interventions to improve healing time, reduce recurrent ulceration and the incidence of MLA in patients with diabetes‐related foot wounds, while optimising function and quality of life.
We hypothesised that the use of non‐cultured autologous skin grafting (“spray‐on skin”; ReCell, Avita Medical) in DFU would increase the proportion of wounds healed at 6 months, improve healing trajectory and reduce overall cost of treatment. Autologous 'spray‐on' skin aids epithelial regeneration and has been used successfully in the treatment of scars, burns and other ulcers, 11 particularly for areas where traditional split skin grafting is not feasible. The first randomised trial of ReCell demonstrating comparative efficacy for venous leg ulceration has only recently been published. 12 Promising results for ReCell have been reported in non‐comparative studies and small case series of DFU. 13 , 14 The aim of this trial was to assess the potential benefit of spray‐on skin as a superior and cost‐effective management strategy for diabetes‐related foot wounds.
2. PARTICIPANTS AND METHODS
2.1. Trial registration and ethics
The trial was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12618000511235; April 9, 2018) and a full description of the methods and analytical plan are published as a protocol elsewhere. 15 Written informed consent was obtained from all participants prior to randomisation. The trial was approved by the South Metropolitan Health Service Health Research Ethics Committee (HREC; RGS000000722).
2.2. Design and eligibility
This was a 6‐month, open‐label randomised controlled trial of spray‐on skin compared with standard care for patients presenting to Fiona Stanley (FSH) or Royal Perth Hospitals (RPH) inpatient or outpatient multidisciplinary foot ulcer units between July 12, 2018 and November 6, 2019 with a diabetes‐related foot wound. Eligible participants (i) were aged ≥18 years, (ii) had either type 1 or 2 diabetes mellitus according to international consensus guidelines, (iii) had wound area > 6 cm2 (measured using Silhouette [Aranz Medical]), (iv) had wound characteristics (location, contour, shape and wound base) considered suitable for administration of spray‐on skin, (v) did not have any further debridement or amputation planned, (vi) had an adequately vascularised wound defined by the presence of at least one palpable pulse in the affected foot, or at least single vessel run‐off identified by arterial Doppler ultrasonography, MRI, CT or conventional angiography (including if present following a revascularisation procedure), (vii) were able to be followed up by community nursing services (Silver Chain Group) and viii) were competent and willing to provide written informed consent.
The primary wound requiring treatment was considered the index wound for enrolment and the location was categorised as either being fore‐, mid‐ or hindfoot. All clinical procedures and follow‐up were at FSH. If a participant had two wounds eligible for enrolment on different feet, both were randomised separately. This could apply either to concurrent or sequential ulcers.
2.3. Clinical procedures
All participants identified for inclusion into the trial received the same pre‐intervention wound management before randomisation. This run‐in phase lasted 2 weeks (±6 days) from the last significant surgical debridement or minor amputation and allowed surgical wound sites to demonstrate wound healing potential. During this phase, for wounds >1 cm deep, negative pressure wound therapy dressings were applied with a Prontosan (B. Braun) soak at each change. For wounds ≤1 cm deep, IntraSite Conformable dressings (Smith & Nephew) with a Prontosan (B. Braun) soak were used.
On the day of randomisation, the index wound was prepared using low‐frequency ultrasonic debridement (LFUD) to remove any residual biofilm or devitalised tissue. Full details related to the harvest and application of the spray‐on skin (ReCell [Avita Medical]) are provided elsewhere. 15 Briefly, a small split‐thickness skin graft was collected from the upper thigh. The process of disaggregating the cells is performed with a ReCell [Avita Medical] kit and relies on the enzyme trypsin to allow the epidermis to be separated from the dermis. The cells at the epidermal–dermal junction can then be scraped off using a scalpel and are collected and filtered before being dropped or sprayed onto the wound site. The area of the harvest site was recorded and a small amount of the autologous cell suspension was reapplied to the harvest site to promote healing. The harvest site was dressed with Surfasoft (Tauren) and Mepilex Border (Mölnlycke). The index wound was dressed with a Surfasoft (Tauren) primary dressing for a minimum of 5 days without any disturbance. Secondary absorbent dressings were changed as required without disturbing the Surfasoft layer.
Participants randomised into the control arm continued with standard wound care procedures as per normal day‐to‐day proceedings of the outpatient clinic following LFUD. All participants received standard care related to ongoing diabetes and infection management. A pressure offloading plan was provided to all participants and classified as either (i) total contact casting, (ii) removable below knee device (ProCare Xceltrax CAM Walker), (iii) below ankle, stiff sole shoe (Darco APB) or (iv) wheelchair.
2.4. Follow‐up visits
In addition to baseline, visits at 4, 10, 18, 26, 39 and 52 weeks from randomisation were scheduled. At each visit, a detailed assessment of the index wound was performed which included wound depth, dimensions, volume and area (as measured by Silhouette [Aranz Medical]), probe to bone test and formal photography. Both feet were assessed for new wounds. Blood tests including lipids, C‐reactive protein, full blood examination and renal and liver function tests were performed as clinically indicated. An HbA1c was measured at baseline, 26 and 52 weeks. To ascertain changes in health‐related quality of life, an EQ‐5D‐5L questionnaire 16 was performed at baseline, 26 and 52 week visits. 17 The primary outcome was assessed at the 26‐week visit.
2.5. Outcomes
The primary outcome was a dichotomous outcome of complete healing of the index wound at 26 weeks, defined by full epithelialisation, after debridement of callus, lasting for at least 2 weeks. Primary outcome arbitration at the interim analysis and at the final analysis was performed using clinical images assessed by two independent senior clinicians. The primary outcome assessors were not investigators on the trial, used only baseline and 26‐week images to make their decision and were blinded to the treatment allocation. Any discordant outcome assessments were resolved by consensus. An MLA of the index limb occurring prior to the primary outcome was considered to be not healed.
Pre‐specified secondary outcomes included: (i) index ulcer healing at 12 months, (ii) time to full epithelialisation of the index ulcer, (iii) trajectory of wound healing of the index ulcer (defined as volume and measured using Silhouette), (iv) major adverse events, (v) any minor or major limb amputation, (vi) all‐cause mortality, (vii) re‐ulceration of the index wound, (viii) the development of any new ulcers, (ix) costs for ambulatory care nursing staff, (x) readmission to hospital and (xi) health‐related quality of life (as measured by EQ‐5D‐5L). 16
The definitions for secondary outcomes were consistent with international guidelines. 18 Re‐ulceration was defined as healing of index ulcer followed by subsequent ulceration at the same location. A minor amputation was an amputation below the ankle including toe, metatarsal‐phalangeal and mid‐foot amputations. A major amputation was defined as amputation above the ankle including below knee and above knee amputations.
2.6. Outpatient nursing costs
Detailed data regarding ambulatory wound care costs were obtained from the local community nursing care service provider and included nursing time and wound care consumables costs for each individual patient at each visit, but not costs related to nurse travel, outpatient parenteral antibiotic therapy if required or general administration.
2.7. Safety and monitoring
A Data Safety and Monitoring Board (DSMB) was established, which included an independent researcher and two independent clinicians, including one with experience in clinical trials and the other with managing diabetic foot infections. Severe adverse events (SAE) and adverse events (AE) were pre‐specified and reported to the DSMB in accordance with the Australian National Health and Medical Research Council position statement of monitoring and reporting of clinical trials. An SAE form was defined as any of the following: (i) death from any cause, (ii) major limb amputation of the ipsilateral leg up to 12 months from enrolment or (iii) major infection of the harvest site as defined as the requirement for admission to hospital, surgical debridement or intravenous antibiotics. An AE was defined as: (i) readmission for any reason related to infection or deterioration of the index ulcer, (ii) minor amputation unrelated to the index ulcer, but on the same foot as the index ulcer (after enrolment), (iii) minor infection of the harvest site as defined by erythema and the requirement for oral antibiotic therapy for this or (iv) delayed healing of the harvest site as defined by persistent need for a dressing on the harvest site at or beyond the 4 week visit.
2.8. Randomisation
Participants were randomised in a 1:1 ratio to intervention (ReCell) or control (standard care) was performed by using REDCap [version 9.2.5 Vanderbilt University]. All investigators were blinded to the randomisation algorithm, which included variable block sizes of 2, 4 or 8. Randomisation was also stratified according to recruitment site and whether the ulcer was fore‐, mid‐ or hindfoot.
2.9. Sample size calculations
Local and international data informed sample size calculations indicating that 45% of participants would achieve complete healing at 6 months. 9 , 10 , 19 It was estimated that 136 (with continuity correction) participants were required to have 80% chance of detecting, at the 5% level of significance, an increase in the primary outcome measure from 45% in the standard care group to 70% in the spray‐on skin group. 11 To account for drop‐outs, recruitment of 150 participants was planned.
2.10. Analytical plan
All analyses were conducted according to the intention‐to‐treat (ITT) principle with the statistical plan provided in the published protocol. 15 Data were analysed using IBM SPSS Statistics 25 and graphs presented using GraphPad Prism (version 8.0.1 for Windows, GraphPad Software, San Diego, CA,).
2.11. Statistical methods
Baseline comparability of the two treatment groups was assessed to confirm the success of the randomisation procedure. Data were summarised as percentages, mean ± SD and median [interquartile range (IQR)]. Two‐sample comparisons of two independent samples were carried out with Student's t‐test for normally distributed variables and by Mann‐Whitney U‐test for non‐normally distributed data. Comparisons of proportions used Fishers' exact test. For the primary efficacy endpoint, the proportion of participants with complete healing of the index DFU at 6 months in the two treatment groups, ReCell versus usual care was compared using unadjusted logistic regression. Multiple logistic regression (using backward conditional, stepwise modelling with P for entry = .049 and for removal = .050) was used to determine clinically plausible prognostic factors with P < .20 in bivariable analyses that were independently associated with the primary endpoint. The efficacy of treatment on this outcome was then assessed after adjustment for these factors using multiple logistic regression. Time to complete wound healing at 6 months, in those achieving the primary outcome, was compared by treatment group with the Student's t‐test.
Paired t‐tests were used to compare the EQ‐5D‐5L VAS score at randomisation and 6 months, separately for each treatment group. One‐way ANCOVA was conducted to compare the efficacy of treatment on the change in EQ‐5D‐5L VAS score before and after adjusting for VAS score at randomisation. For each EQ‐5D‐5L dimension, McNemar tests compared the percentage of participants who reported any problem at randomisation versus 6 months separately for each treatment group. Multiple logistic regression was used to assess whether the percentage of participants who reported any problem changed over time (randomisation versus 6 months) by treatment group. All P values were two‐sided and P < .05 was considered to indicate statistical significance.
Pre‐specified sub‐group analyses were performed using the dichotomous primary study endpoint described above. Sub‐groups included wound site (categorical variable; fore‐, mid‐ or hind foot, plantar/dorsal), WiFI Clinical Stage 20 at baseline, pre‐existent moderate to severe renal disease (creatinine clearance ≤30 or > 30 mL/min), age (age ≤60 or >60 years), long‐term glycaemic control at presentation (HbA1c ≤9% or >9%), primary pre‐randomisation surgical procedure performed (minor amputation versus LFUS or surgical debridement).
2.12. Data management
Study data were collected and managed using REDCap [9.2.5 Vanderbilt University] hosted at the University of Western Australia. 21
3. RESULTS
A total of 254 patients were screened and 49 eligible diabetes‐related foot wounds (in 45 participants) were randomised (Figure 1). Two participants were recruited with co‐existing bilateral hindfoot wounds, each of which were eligible for randomisation. Two participants subsequently developed an eligible wound on the contralateral foot. Two hundred patients did not meet inclusion criteria and five patients declined to participate. Of the 200 ineligible patients, 100 (50.0%), 53 (26.5%) and 20 (10.0%) had wounds that were <6 cm2, were out of the hospital catchment area or with wounds that were deemed inappropriate by the clinicians, respectively. Trial recruitment was terminated early on advice from the DSMB. The initial catalyst for requesting a DSMB review was concern of an excess of SAE and AE in the intervention group, slow recruitment rates and lack of an ongoing funding stream. Following review, the DSMB requested an interim analysis of primary and key secondary outcomes for the 42 wounds with primary outcome data available, together with an updated sample size calculation. Based on a futility assessment, an updated sample size calculation of 890 in each group and slower than anticipated recruitment, the DSMB recommended trial cessation on March 3, 2020 because it was no longer feasible to complete. All participants recruited to this point were followed up as planned.
FIGURE 1.
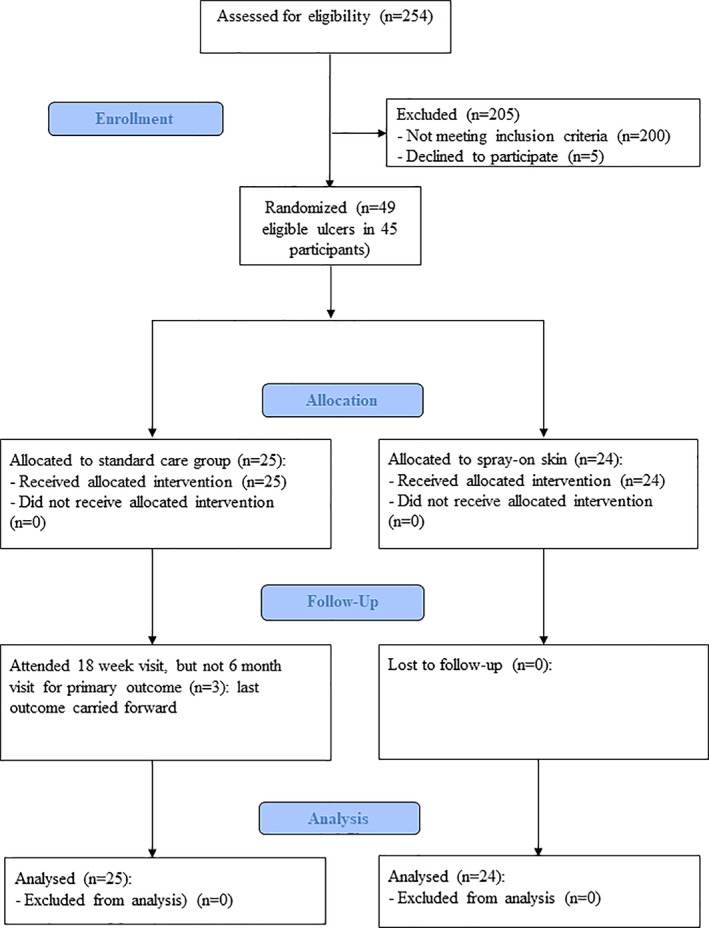
Consort diagram for spray‐on skin trial for diabetic foot ulcers
Of the 49 eligible foot wounds, 24 (49.0%) were allocated to spray‐on skin, and 25 to standard care. All participants received the allocated intervention. Three participants did not attend their 6‐month visit. All three were in the usual care group and had attended the 18‐week visit. In accordance with the protocol, these participants had this time point as the last observation carried forward (LOCF) with blinded outcome assessments performed, as for the other participants. At the 39 and 52 week time points, there were outcome data available for 48 and 47 participants, respectively (including those participants who died or had an MLA).
The baseline characteristics of the participants enrolled according to intervention are shown (Table 1). The median (IQR) wound area at baseline was 11.4 (8.8‐17.6) cm2. There were no significant differences in any of the socio‐demographic, clinical or EQ‐5D‐5L variables. Most wounds were post‐surgical following minor amputation (32 [65.3%]) with surgical debridement or LFUS in 9 (18.4%) and 8 (16.3%) wounds, respectively.
TABLE 1.
Baseline socio‐demographic and clinical characteristics by treatment group at randomisation
| Standard care | Spray‐on skin | P value | |
|---|---|---|---|
| N | 25 | 24 | |
| Age at randomisation (years) | 58.1 ± 12.5 | 61.5 ± 14.3 | .375 |
| Male (%) | 84.0 | 79.2 | .725 |
| Aboriginal (%) | 4.0 | 4.2 | >.999 |
| Smoking status (%) | >.999 | ||
| Never | 44.0 | 45.8 | |
| Ex‐ | 48.0 | 45.8 | |
| Current | 8.0 | 8.3 | |
| Ulcer side (% right) | 36.0 | 58.3 | .156 |
| Type 2 diabetes (%) | 84.0 | 83.3 | >.999 |
| Diabetes duration (years) | 17.0 [15.5‐29.0] | 25.5 [11.5‐30.8] | .787 |
| HbA1c (%) | 8.4 [7.5‐9.4] | 7.6 [6.6‐9.2] | .129 |
| BMI (kg/m2) | 32.7 ± 7.6 | 30.9 ± 6.2 | .363 |
| Palpable DP pulse (%) | 64.0 | 54.2 | .567 |
| Palpable PT pulse (%) | 40.0 | 58.3 | .258 |
| Loss of sensation (%) | 92.0 | 100 | .490 |
| Hypertension (%) | 80.0 | 75.0 | .742 |
| Ischaemic heart disease (%) | 40.0 | 37.5 | >.999 |
| Intermittent claudication (%) | 16.0 | 25.0 | .496 |
| Prior revascularisation (%) | 16.0 | 29.2 | .321 |
| Prior diabetic foot infection (%) | 52.0 | 79.2 | .072 |
| Prior foot deformity (%) | 25.0 | 16.7 | .724 |
| Prior healed diabetes‐related foot ulcer (index limb, %) | 48.0 | 62.5 | .393 |
| Prior minor lower extremity amputation (%) | 32.0 | 58.3 | .088 |
| Prior major lower extremity amputation (%) | 4.0 | 0 | >.999 |
| Neuropathy (%) | 100 | 95.8 | .490 |
| Retinopathy (%) | 44.0 | 33.3 | .561 |
| Nephropathy (%) | 28.0 | 29.2 | >.999 |
| CKD stage (%): | .159 | ||
| No CKD or Stage 1 | 68.0 | 41.7 | |
| Stage 2 or Stage 3A | 8.0 | 12.5 | |
| Stage 3B or worse | 24.0 | 45.8 | |
| Immunosuppressed (%) | 4.0 | 0 | >.999 |
| Cirrhosis/hepatic failure (%) | 0 | 4.2 | |
| Index wound area (cm2) | 15.3 [9‐23.8] | 11 [8.4‐15.3] | .27 |
| Location of index wound (%): | |||
| Fore | 72.0 | 79.2 | .742 |
| Mid | 16.0 | 4.2 | .349 |
| Hind | 12.0 | 16.7 | .702 |
| Index wound on dorsal surface (%) | 44.0 | 33.3 | .561 |
| Index wound on plantar surface (%) | 36.0 | 29.2 | .762 |
| Surgical procedure prior to randomisation (%) | .43 | ||
| Minor amputation | 60.0 | 70.8 | |
| Surgical or low‐frequency ultrasonic debridement | 40 | 29.2 | |
| WIfI amputation risk (20): | .566 | ||
| Very low (%) | 56 | 58.3 | |
| Low (%) | 36 | 41.7 | |
| Moderate (%) | 4 | 0 | |
| High (%) | 4 | 0 | |
| Index wound pedis depth (%) | .394 | ||
| Superficial | 44.0 | 58.3 | |
| Involving subcutaneous structures | 52.0 | 33.3 | |
| Involving bone/joint | 4.0 | 8.3 | |
| EQ‐5D‐5L: | |||
| Any mobility problems (%) | 76.0 | 75.0 | >.999 |
| Any problems with self‐care (%) | 24.0 | 25.0 | >.999 |
| Any problems with doing usual activities (%) | 64.0 | 70.8 | .762 |
| Any pain or discomfort (%) | 56.0 | 62.5 | .773 |
| Any anxiety or depression (%) | 28.0 | 37.5 | .551 |
| VAS score | 75 [50‐88] | 78 [52‐89] | .936 |
| Pressure offloading (%): | .297 | ||
| Below knee removable device (%) | 32.0 | 16.7 | |
| Stiff‐soled ankle height shoe (%) | 68.0 | 79.2 | |
| Wheelchair (%) | 0 | 4.2 | |
Note: Baseline data are summarised as means ± SD, median [interquartile range] or percentages. WIfI, Wound, Ischemia, and foot Infection. 20
An ITT analysis using logistic regression showed no significant difference for the primary outcome measure. A total of 32 (65.3%) index DFUs were completely healed at 6 months, including 16 of 24 (66.7%) in the spray‐on skin group and 16 of 25 (64.0%) in the standard care group (unadjusted OR [95% CI]: 1.13 (0.35‐3.65), P = .845). In bivariable analysis (Table S1), a lower BMI was significantly associated with complete healing of the index DFU at 6 months (P = .002), while an index DFU located on the plantar surface was significantly less likely to be completely healed at 6 months (P = .009). After adjusting for these factors in the most parsimonious logistic regression model, treatment with spray‐on skin was not independently associated with a higher proportion of healed wounds (adjusted OR (95% CI): 0.65 (0.14‐2.93), P = .571).
Unadjusted ORs (95% CIs) for the primary outcome according to pre‐specified subgroups are shown (Figure S1). Some subgroups had too few outcomes for valid ORs to be derived. The confidence intervals for the ORs for all subgroups crossed unity and therefore were not statistically significant. There were no significant interactions between treatment and subgroups (P > .14).
The mean ± SD time for complete DFU healing was 17.0 ± 8.6 weeks in the spray‐on skin group versus 18.0 ± 9.6 weeks in the standard care group (P = .775). Wound healing trajectories are shown according to wound area and baseline size (Figure 2; Panels A and B, respectively).
FIGURE 2.
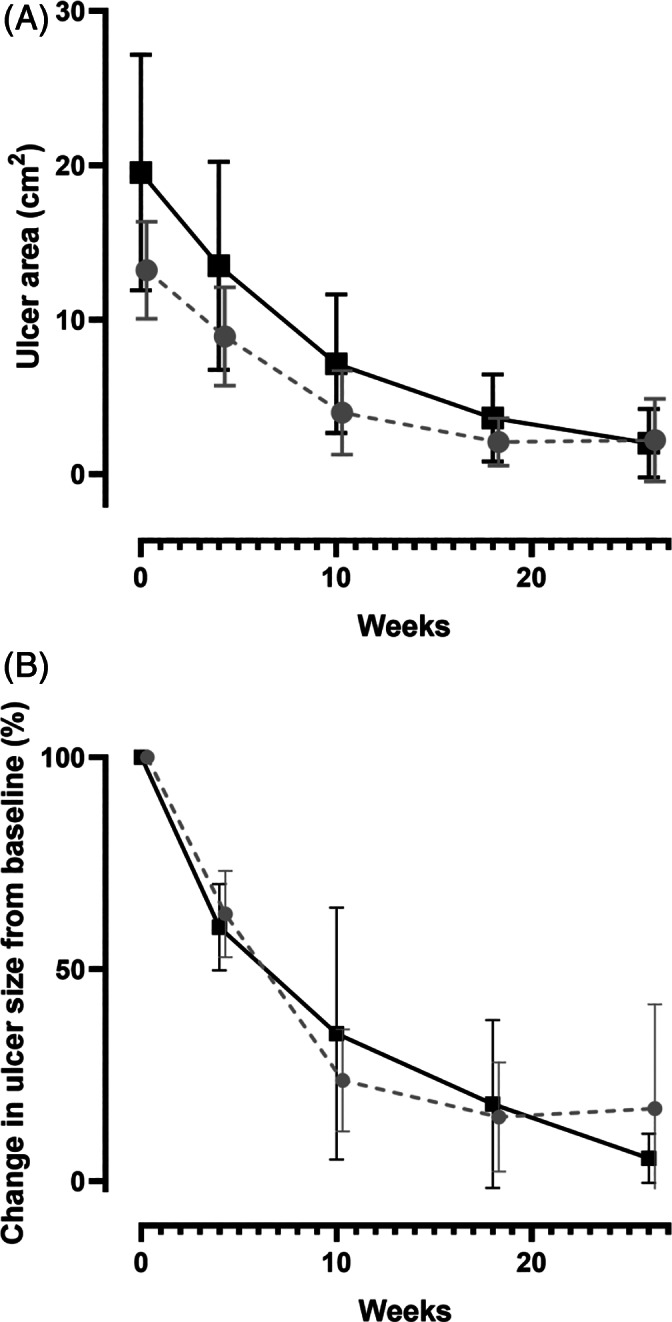
Mean (with 95% confidence intervals) healing trajectories for ulcer healing with spray‐on skin (grey) or standard of care (black). Panel A depicts changes in area (cm2) while panel B shows changes relative to the baseline ulcer size (%)
Forty‐two wounds were assessed at 12 months (20 in the spray‐on skin arm). Of these, 33 were completely healed (17 in the ReCell arm). Of the seven DFUs not assessed at 12 months, three patients had died before the assessment, all with previously healed DFU, one had a healed DFU at week 18, one had a MLA and their DFU was consequently considered unhealed, and the remaining two had their last observation carried forward as not having a healed DFU. Therefore, complete healing at 12 months was deemed to have occurred in 18 DFUs in each treatment arm (P > .999). An ITT analysis using logistic regression showed no significant difference for the pre‐specified secondary outcome measure, DFU completely healed at 12 months, for those treated with spray‐on skin compared with standard care (OR 1.17 [0.33‐4.16], P = .812).
The mean change (95% CI) in the EQ‐5D‐5L visual analogue score (VAS; 0‐100) for the spray‐on skin group between randomisation and 6 months was −8.8 (−17.7 to 0.2) (paired t‐test, P = .054), compared with 0.5 (−6.5 to 7.5) (P = .880) for the standard care group. Using one‐way ANCOVA, the difference (95% CI) in the mean change between treatment groups was −9.3 (−20.3 to 1.7), P = .096. Adjustment for the VAS score at randomisation decreased the difference modestly to −8.9 (−19.1 to 1.2; P = .084).
There were no statistically significant differences for changes in the EQ‐5D‐5L domains of mobility, self‐care, usual activities or anxiety/depression with spray‐on skin. The proportion with any pain/discomfort in the spray‐on skin group decreased significantly from 62.5% to 29.2% (McNemar's test, P = .021), while it decreased non‐significantly from 56.0% to 52.0% in the usual care group, P > .999. The interaction between treatment and time was not statistically significant (P = .140).
The frequency of adverse events according to treatment allocation is shown in Table 2. The three deaths occurred between 6 and 12 months in the spray‐on skin group were reported to the DSMB and designated as unrelated to the intervention. There were two MLA of the ipsilateral limb performed; one prior to the 6 month primary outcome (considered to be not healed) and the other between the 6‐month and 9‐month scheduled visits (considered not healed at the 52‐week time point). The median [IQR] harvest site area was 3.8 [3.2‐4.8] cm2. There were no harvest site SAEs. One participant had delayed healing of the harvest site, without infection.
TABLE 2.
Serious adverse events and adverse events according to treatment allocation
| Serious adverse events (SAE) | Standard care | Spray‐on skin |
|---|---|---|
| Death | 0 | 3 a |
| Major limb amputation | 1 | 1 |
| Major donor site complication | 0 | 0 |
| Total SAE | 1 | 4 |
| Adverse event (AE) | ||
| Readmission related to index wound deterioration or infection | 2 | 7 b |
| Minor amputation on ipsilateral foot as index wound | 1 | 3 |
| Minor donor site infection | 0 | 0 |
| Delayed donor site healing | 0 | 1 |
| Total (SAE and AE) | 3 | 11 |
| Number of participants contributing to total AEs | 3 | 9 |
All deaths during enrolment to this project occurred after the 6‐month primary outcome assessment. These were reported to the DSMB and considered unrelated to the intervention.
Two of these recorded adverse events were related to one participant with two enrolled wounds (one on either foot). This participant was readmitted to hospital with a Staphylococcus aureus bacteraemia within 7 days of intervention. Based on assessment from an infectious diseases physician, it was determined that the source of this was a new foot ulcer unrelated to the trial wounds. Due to the significance of the admission, it was determined that an AE should be recorded.
The overall community nursing costs associated with dressings and nursing time to perform and document the dressing procedures were captured by Silver Chain Group. At 6 months the median [IQR] cost in the spray‐on skin group was $1421 ($1002‐$2223) compared with $2256 ($1563‐$3837) in the standard care group (Mann‐Whitney U‐test P = .034). This did not include the costs of the ReCell device.
4. DISCUSSION
Based on these data, there is no evidence that spray‐on skin offers any benefit over standard care for large, well‐vascularised diabetes‐related foot wounds in terms of 6‐ or 12‐month cure rates, wound healing trajectory, or quality of life measurements. The skin harvest procedure was safe and well‐tolerated, with only one minor complication related to delayed healing of the harvest site.
This trial had a number of strengths that reduced the possibility of bias. There was near complete follow‐up, which enabled an evaluable primary outcome to be determined for all patients. For the few participants where the 6‐month visit was not available, an approach using the LOCF was pre‐specified in the protocol. Primary outcome assessments were performed by experienced clinicians who were blinded to the intervention.
The main limitation of the present trial, and the only deviation from the original protocol, was the unplanned interim analysis, which resulted in early termination of the trial. This was requested by the DSMB due to an unexpected high number of severe adverse events in the intervention group, but also in the context of acknowledged slow recruitment rates. At the request of the DSMB, an interim analysis was undertaken, which did not show any likely benefit for the intervention for the primary or secondary outcomes. Furthermore, a revised sample size of 890 participants was beyond the scope of what was feasible given the recruitment rates to that point, and the funding available. On the basis of these assessments, the DSMB recommended that the trial was ceased.
The healing trajectory and overall cure rates are very similar to a recently published single‐arm pilot feasibility study of spray‐on skin for diabetes‐related foot wounds in a different setting. Here, 13 of 16 participants (with a mean wound area of 15.5 cm2) received up to two applications of ReCell. As with the present study, there was a period of run‐in prior to the delivery of spray‐on skin and participants were followed for 26 weeks, using a comparable schedule and assessment methods. Of those with an evaluable outcome, 7 (54%) had complete healing and the mean change in wound area from baseline was 85%. 14 By comparison, the present trial had an overall healing rate of 66.7% and mean change in wound area from baseline of 83%.
The proportion of participants in the control group that achieved complete healing (64%) was considerably higher than originally expected. The expected rate of 45% in the control group specified in the sample size estimates was based on data from observational studies. 9 , 10 , 19 One of these, prospective, observational study described an overall healing rate of 45% at 12 months, but included poorly vascularised neuroischaemic ulcers. 10
A number of factors are likely to have contributed to the unexpectedly high rates of cure in the control group. First, a high proportion of wounds were recruited following debridement or minor amputations, so the wound was effectively a post‐surgical wound bed. Second, all wounds were well vascularised with at least single vessel run‐off to the foot, confirmed by either clinical assessment or radiological imaging. Finally, a “Hawthorne Effect” may have accounted for a better outcome in the control group. This well‐documented phenomenon describes the non‐specific effects of participation in clinical trials where patient or provider behaviour such as the extra frequency or completeness of assessments result is better than expected outcomes. 22 However, for the present trial, the follow‐up schedule was the same, so it might be expected that this effect should apply equally to both the control and intervention arms.
The slow recruitment rate was an expected challenge, which is difficult to overcome. Studies have demonstrated that only a third of well‐funded trials manage to maintain planned recruitment schedules. 23 This seems to be particularly relevant for wound healing trials for both diabetes‐related foot wounds and venous leg ulcers. The recent single‐arm pilot feasibility study of ReCell recruited only 16 participants from three centres over ~30 months, while a recent randomised trial of ReCell for venous leg ulcers required 30 months to report week 14 healing rates for 52 patients from seven participating sites. 12 Another example is a recent single‐centre trial of vitamin C supplementation for diabetes‐related foot ulcers, which had a target of 200 participants (ACTRN12617001142325) with an 8‐week primary outcome, but recruitment was ceased early after only 16 patients had achieved the primary outcome. 24 Taken together, the challenges observed with this study and other published trials highlight the difficulty of performing large‐scale wound healing trials with small effect sizes and provide further justification for the DSMB recommendation to cease the trial early.
There was a significant difference in cost between treatment arms in favour of spray‐on skin. However, the cost analyses did not factor in the cost of the ReCell kit, which would likely offset any observed cost‐benefit.
For the present study, the commonest exclusions related to the wound size and accessibility. In the case of the spray‐on skin intervention for the present trial, the goal was to maximise generalisability to as many participants with a DFU at “moderate” risk of delayed healing. Because the additional costs were likely to be sensitive to the cost of ReCell, smaller wounds with a high likelihood of healing were not a suitable application for this intervention. Such wounds would include a transphalangeal amputation site in a participant with good blood supply. Likewise, limiting the intervention to a salvage therapy for wounds with an extremely high chance of clinical failure would also compromise the chances of demonstrating a meaningful treatment response. In relation to accessibility, Western Australia covers a large area, and the catchment for both participating hospitals covers patients from distances greater than 500 km. Patients more than 60 minutes travel away struggled to access ambulatory care services and were frequently unable to attend regular visits. Conversely, patients receiving spray‐on skin did not require any wound debridement by podiatrists as is commonly carried out in general care, nor did they require any specialised dressings to their wound, suggesting a possible role for spray‐on skin for rural patients with limited access to podiatric and nursing services.
Significant heterogeneity in lower limb vascular supply, ulcer location and size, long‐term diabetes control, infection extent, antibiotic efficacy and adherence to offloading amplify the challenges with conducting trials of healing in patients with diabetes‐related foot wounds. 2
The detrimental effects of increased BMI and a plantar ulcer location on healing in this trial were important findings that highlight the importance of optimised offloading in trials of diabetes‐related foot wounds. Further research in this area is desirable.
5. CONCLUSION
Interventions that improve healing time, reduce recurrent ulceration and the incidence of MLA in patients with diabetes‐related foot wounds remain a priority. At the present time, there is no evidence to support the routine use of spray‐on skin to achieve these goals for this subset of patients. Further work is required in the clinical characterisation of the wounds, their healing trajectory and the role of wound healing interventions. Future trials in this field will need to recruit from many centres, and have a robust easily assessable primary outcome, preferably measured by the patient without the need for regular travel to the trial centre.
CONFLICT OF INTEREST
ReCell was developed in WA by Professor Fiona Wood (FW), who is an investigator on the study. To avoid perceived conflict of interest, FW assigned all Intellectual Property for ReCell to a charitable foundation (Fiona Wood Foundation). All ReCell kits used by WA Health are provided on a not‐for‐profit basis. In the case of the present study, the ReCell kits were donated by the Fiona Wood Foundation. The manufacturers (Avita Medical) had no role in the study design. None of the other investigators report a real or perceived conflict of interest for this study.
Supporting information
Figure S1: Forest plot demonstrating odds ratios (95% confidence interval) for 6‐month cure rates according to pre‐specified subgroups. Odds ratios >1 favour “spray‐on” skin
Table S1: Baseline socio‐demographic and clinical characteristics by primary outcome (completely healed index diabetic foot wound by 6 months with last outcome carried forward)
ACKNOWLEDGEMENTS
The authors would like to acknowledge the commitment from the Fiona Stanley Hospital Silver Chain liaison nurse Angela Schmeiss and Silver Chain area coordinator Ann Tuvik in facilitating the community nursing for all trial participants. The authors would also like to acknowledge the work done by the Fiona Stanley Hospital Medical Illustrations team and in particular Michelle Bavcevic (Coordinator Medical Illustrations) for their help in amalgamating the wound photographs. The Western Australia Department of Health was the main funder of this trial, through the 2017 Research Translation Project Grants (Round 11).
Manning L, Ferreira IB, Gittings P, et al. Wound healing with “spray‐on” autologous skin grafting (ReCell) compared with standard care in patients with large diabetes‐related foot wounds: an open‐label randomised controlled trial. Int Wound J. 2022;19(3):470–481. 10.1111/iwj.13646
Ivana Bastos Ferreira and Paul Gittings contributed equally to this work.
Emma Jane Hamilton and Jens Carsten Ritter contributed equally to this work.
Erica Ryan and Mendel Baba contributed equally to this work.
Funding information The Western Australia Department of Health
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions
REFERENCES
- 1. Schaper NC, van Netten JJ, Apelqvist J, et al. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(Suppl 1):e3266. [DOI] [PubMed] [Google Scholar]
- 2. Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care. 2018;41(4):645‐652. [DOI] [PubMed] [Google Scholar]
- 3. Alavi A, Sibbald RG, Mayer D, et al. Diabetic foot ulcers: part I. pathophysiology and prevention. J Am Acad Dermatol. 2014;70(1):1e‐18e. quiz 9‐20. [DOI] [PubMed] [Google Scholar]
- 4. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132‐e173. [DOI] [PubMed] [Google Scholar]
- 5. Kerr M, Barron E, Chadwick P, et al. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet Med. 2019;36(8):995‐1002. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lazzarini PA, Hurn SE, Kuys SS, et al. Direct inpatient burden caused by foot‐related conditions: a multisite point‐prevalence study. BMJ Open. 2016;6(6):e010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc. 2010;100(5):335‐341. [DOI] [PubMed] [Google Scholar]
- 9. Jeffcoate WJ, Chipchase SY, Ince P, Game FL. Assessing the outcome of the management of diabetic foot ulcers using ulcer‐related and person‐related measures. Diabetes Care. 2006;29(8):1784‐1787. [DOI] [PubMed] [Google Scholar]
- 10. Ndosi M, Wright‐Hughes A, Brown S, et al. Prognosis of the infected diabetic foot ulcer: a 12‐month prospective observational study. Diabet Med. 2018;35(1):78‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Angelis B, Migner A, Lucarini L, Agovino A, Cervelli V. The use of a non cultured autologous cell suspension to repair chronic ulcers. Int Wound J. 2015;12(1):32‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayes PD, Harding KG, Johnson SM, et al. A pilot multi‐Centre prospective randomised controlled trial of RECELL for the treatment of venous leg ulcers. Int Wound J. 2020;17(3):742‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chant H, Woodrow T, Manley J. Autologous skin cells: a new technique for skin regeneration in diabetic and vascular ulcers. J Wound Care. 2013;22(10 Suppl):S10‐S15. [DOI] [PubMed] [Google Scholar]
- 14. Rashid ST, Cavale N, Bowling FL. A pilot feasibility study of non‐cultured autologous skin cell suspension for healing diabetic foot ulcers. Wound Repair Regen. 2020;28(6):719‐727. [DOI] [PubMed] [Google Scholar]
- 15. Manning L, Hamilton EJ, Raby E, et al. Spray on skin for diabetic foot ulcers: an open label randomised controlled trial. J Foot Ankle Res. 2019;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20(10):1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sothornwit J, Srisawasdi G, Suwannakin A, Sriwijitkamol A. Decreased health‐related quality of life in patients with diabetic foot problems. Diabetes Metab Syndr Obes. 2018;11:35‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Netten JJ, Bus SA, Apelqvist J, et al. Definitions and criteria for diabetic foot disease. Diabetes Metab Res Rev. 2020;36(1):e3268. [DOI] [PubMed] [Google Scholar]
- 19. Smith‐Strom H, Iversen MM, Igland J, et al. Severity and duration of diabetic foot ulcer (DFU) before seeking care as predictors of healing time: a retrospective cohort study. PLoS One. 2017;12(5):e0177176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mills JL Sr, Conte MS, Armstrong DG, et al. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(1):220‐34 e1‐2. [DOI] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reynolds T. Clinical trials: can technology solve the problem of low recruitment? BMJ. 2011;342:d3662. [DOI] [PubMed] [Google Scholar]
- 24. Gunton JE, Girgis CM, Lau T, Vicaretti M, Begg L, Flood V. Vitamin C Improves Healing of Foot Ulcers; A Randomised, Double‐Blind. Placebo‐Controll Trial Br J Nutr. 2020;1‐21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Forest plot demonstrating odds ratios (95% confidence interval) for 6‐month cure rates according to pre‐specified subgroups. Odds ratios >1 favour “spray‐on” skin
Table S1: Baseline socio‐demographic and clinical characteristics by primary outcome (completely healed index diabetic foot wound by 6 months with last outcome carried forward)
Data Availability Statement
Data available on request due to privacy/ethical restrictions


