Abstract
Background:
Moxibustion is increasingly used for treatment of irritable bowel syndrome (IBS). This study investigated the long-term effects of moxibustion for IBS with diarrhea (IBS-D).
Methods:
Patients with IBS-D were assigned to receive moxibustion or sham moxibustion (52 each, 3× per week, 6 weeks) and were followed up to 24 weeks. The acupoints were bilateral ST25 and ST36, body surface temperatures at acupoints were 43°C ± 1°C and 37°C ± 1°C for the moxibustion and sham groups, respectively. Primary outcome was changes in IBS Adequate Relief (IBS-AR) from baseline to 6 weeks. Secondary outcomes included the following: IBS symptom severity scale (IBS-SSS), Bristol stool form scale (BSS), IBS quality of life (IBS-QOL), and Hospital Anxiety and Depression Scale (HADS).
Results:
Based on an intention-to-treat analysis, the rate of IBS-AR in the moxibustion group was significantly higher than the sham group at 6 weeks (76.9% versus 42.3%; p < 0.001); the mean decrease of total IBS-BSS score in the moxibustion group was lower than that of the sham group (−116.9 versus −61.5; p < 0.001), both of which maintained throughout the follow-up period. Five specific domains of the IBS-SSS were lower in the moxibustion group than the sham, throughout (p < 0.001). At week 6, the rate of reduction >50 points in IBS-SSS of the treatment group was significantly higher than that of the sham (p < 0.001), which persisted throughout the follow-up period. Similar long-lasting improvements were observed in BSS, stool frequency, and stool urgency (p < 0.001). Improvements of IBS-QOL and HADS were comparable between the groups.
Conclusions:
Moxibustion treatment benefits the long-term relief of symptoms in IBS-D patients.
Trial registration:
Clinical trials.gov (NCT02421627). Registered on 20 April 2015.
Keywords: acupuncture, alternative therapy, irritable bowel syndrome, randomized controlled trial
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disease. Its main clinical manifestations include abdominal pain, bloating, and changes in bowel habits (diarrhea, constipation or alternative). The pathogenesis of the disease is still unclear. Without specific biomarker for the disease, IBS is diagnosed mainly based on clinical symptoms. The prevalence of IBS is 10% to 25% in the world and 5% to 10% in China, with the majority being diarrhea-predominant IBS (IBS-D). 1 IBS not only manifests as intestinal symptoms but also is accompanied by stress and anxiety, severely affecting the quality of life (QOL) of patients. Frequent hospital visits of these patients greatly increase the medical and social burden. 2
Since specific therapeutic interventions are still lacking, the treatment of IBS presently focuses on the relief of symptoms. However, the efficacy of evidence-based pharmacological drugs is not high while the side effects can be intolerable. 3 A more effective therapy is needed to deal with this distressful disease. Moxibustion is a commonly used treatment in Traditional Chinese Medicine (TCM). It involves burning processed mugwort (moxa) on acupuncture points of the body, in combination with acupuncture or alone. In many countries, it is used to treat a variety of gastrointestinal diseases.4 –7 According to TCM theory, acupoints Tianshu (ST25) and Zusanli (ST36) can regulate gastrointestinal function and are often used in the treatment of IBS-D. 8 Acupuncture stimulation on ST36 can regulate intestinal motility; improve the rectal sensory threshold, defecation urge, and pain threshold; and effectively relieve abdominal pain caused by visceral hypersensitivity. 9 ST25 and the gastrointestinal tract are innervated by the thoracic 10 spinal cord segment. Acupuncture on this point can inhibit diarrhea and abdominal pain caused by excessive gastrointestinal motility. 10 However, clinical trials of moxibustion for IBS-D are limited. Previous studies have shown that patients with IBS-D treated with moxibustion experienced greater improvement of symptoms than did patients given sham treatment 5 or electroacupuncture. 6 Recent meta-analyses 7 reported that trials of moxibustion treatment for IBS-D are limited by lack of adequate sample size, randomization, allocation concealment, and blinding of participants. Furthermore, most are single-center studies without long-term follow-up, leading to the relatively higher risk of bias in the test results.
To overcome the major weaknesses of previous studies discussed above, we conducted this multicenter randomized clinical trial to evaluate the long-term efficacy and safety of moxibustion at ST25 and ST36 for the treatment of IBS-D relative to a sham control, with parallel grouping and 24-week follow-up. The specific aim was to provide more convincing evidence on the efficacy of moxibustion at ST25 and ST36 in relieving symptoms in patients with IBS-D, especially in the long-term follow-up.
Methods
Study design
This was a multicenter, parallel group randomized and sham-controlled clinical trial with a 24-week follow-up. The clinical trial protocol (No. 2015-006) was reviewed and approved by the Institutional Review Board of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of TCM and registered in Clinicaltrials.gov (No. NCT02421627). The study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with ICH-Good Clinical Practice guidelines. All participants gave their informed consent prior to their inclusion in the study.
Subjects
IBS-D patients were recruited from May 2015 to October 2017 at three centers including the Bowel Disease Specialist Outpatient Clinic, Shanghai Research Institute of Acupuncture and Meridian, the Department of Acupuncture and Moxibustion, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of TCM, and the Department of Acupuncture and Moxibustion, Shanghai TCM-Integrated Hospital, and Shanghai University of TCM.
All the enrollees met the Rome III diagnostic criteria of IBS-D, were aged between 18 and 65 years, and signed informed consent. Patients with any of the following conditions were excluded: other subtypes of IBS, organic gastrointestinal disease, medications for IBS or other medications such as antibiotics, probiotics, prebiotics or traditional Chinese medicine used for the previous month, severe diseases of the heart, brain, liver, kidney, or hematopoietic system, confirmed diagnosis or family history of mental illness, pregnant or plan to become pregnant, breastfeeding, or history of abdominal surgery or moxibustion treatment.
All eligible subjects completed baseline assessments, including demographics, moxibustion expectation, and IBS-D-related features such as severity and QOL (Supplementary Figure 1). 11
Randomization and masking
Subject allocation was by a random computer-generated sequence, which was stored in sequentially coded, opaque envelopes and delivered to qualified subjects. In this way, subjects were equally and randomly assigned to receive moxibustion or sham moxibustion. All patients were blinded to their group assignment. All treatments were conducted in a single isolated room to avoid communication between patients. The outcome evaluators and statisticians were blinded to the grouping.
Procedures
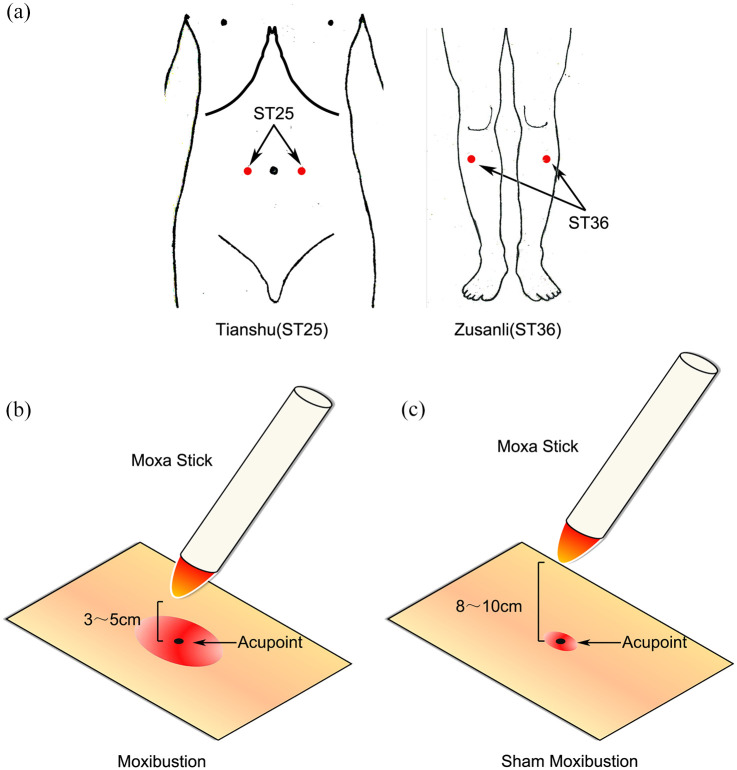
For both groups, the acupuncture points were bilateral ST25 and ST36, defined according to the World Health Organization standard (Figure 1). A pure moxa stick (diameter 2.8 cm, Hanyi, Nanyang Hanyi Moxa, Henan, P.R. China) was ignited and placed on a moxa stand with the burning end of the moxa stick perpendicular to the acupoint, 3–5 and 8–10 cm away from the acupoint for the moxibustion and sham groups, respectively. The temperatures on the skin of acupoints were maintained at 43°C ± 1°C and 37°C ± 1°C for the moxibustion and sham groups, respectively. The ash of the burning end of the moxa stick was scraped off every 5 min. Each treatment lasted for 30 min. Each patient underwent the assigned treatment, 3×/week, once every other day, for 6 weeks (18×). Follow-up was at 12, 18, and 24 weeks from baseline.
Figure 1.
Acupoints and method of moxibustion. (a) Locations of Zusanli (ST36) and Tianshu (ST25), (b) verum moxibustion, and (c) sham moxibustion had different distance between the ignited moxa stick and acupuncture points.
All acupuncturists had received special training for this study and had at least 5 years of clinical experience.
Outcome
The primary outcome was the rate of adequate relief of IBS symptoms (IBS-AR) during the 6-week treatment. IBS-AR, including whether abdominal pain and abdominal discomfort are adequately relieved, was assessed weekly. Patients who reported adequate relief for more than 3 weeks within the 6-week treatment period were characterized as a responder, otherwise as a nonresponder. 12 At the end of treatment, the difference in the proportion of respondents between the two groups was compared.
The secondary outcome measures comprised the following assays: IBS-AR at 12, 18, and 24 weeks, IBS severity scoring system (IBS-SSS), IBS-SSS responders, Bristol stool form scale (BSS), mean frequency of diarrhea/week, bowel urgency, IBS-QOL, Hospital Anxiety and Depression Scale (HADS), adverse events, and patients’ blinded guess of treatment. These secondary outcome measures are detailed below.
The IBS-SSS score reflected the severity of symptoms based on degree and days of abdominal pain, severity of abdominal distension, satisfaction with bowel habits, and interference in general life. The full score for each aspect was 100 points and a higher score indicated greater severity. 13 A positive IBS-SSS response was considered a 50-point reduction in total IBS-SSS scores. 13
The BSS assessed stool from constipation to diarrhea, from 1 to 7 points. 14 The mean frequency of diarrhea/week was determined from the patient’s diarrhea diary. 15 The bowel urgency score was based on patient report, ranging from 0 (mild) to 100 (strong). 15
Health-related QOL was judged according to the IBS-QOL, 16 evaluated in eight domains, with higher scores indicating better health-related QOL: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual, and relationships. The HADS consisted of subscales for anxiety and depression. 17 The safety assessment evaluated occurrence and outcomes of adverse events during treatments.
At the end of treatment, the subjects were asked to give their blinded guess about specific treatment they received.
Statistical analysis
The rates of IBS-AR at 6 weeks were expected to be 80% (moxibustion) and 49% (sham).18,19 The estimation of sample size was based on the sample rates (α = 0.05, 1 − β = 0.9). The sample size required for each group was 45. Considering a 15% dropout, each group required 52 cases, and the study altogether, >104 cases.
The primary outcome (IBS-AR at week 6) and rate of IBS-AR secondary outcome were analyzed using the intention-to-treat principle and per-protocol analysis (patients who completed the trial, did not violate the protocol, and completed 80–100% of treatments). Demographic and baseline characteristics, and other secondary outcomes, were analyzed using the intention-to-treat principle. Blind assessment was performed using per-protocol analysis. Missing data for continuous variables were filled in using the last observation carried forward. The missing dichotomous outcome data (IBS-AR) were analyzed after filling by nonresponders. Safety assessments included patients who received at least one treatment.
The categorical data are shown as percentage, analyzed by chi-square, Fisher’s exact, or Cochran–Mantel–Haenszel tests. Quantitative variables are shown as mean ± standard deviation, mean (95% confidence interval), or median (interquartile range), analyzed by independent sample t test or Wilcoxon rank-sum test. The primary and secondary outcomes (inter-group differences for IBS-AR and IBS-SSS response rates) were analyzed by chi-square or Fisher’s exact tests. For other secondary outcomes, inter-group comparisons of the IBS-QOL and HADS changes from baseline used an independent samples t test, intra-group comparisons were performed using a paired t test. IBS-SSS, BSS, mean frequency of diarrhea/week, and bowel urgency were analyzed by a linear mixed effect model and independent samples t test. For exploratory outcomes, subgroup analyses of the primary outcome were performed using the Cochran–Mantel–Haenszel test, with gender, IBS-SSS severity, and moxibustion expectation as stratified factors. All were two-sided tests with a type I error probability of 0.05. SPSS Version 21.0 (IBM, Armonk, NY, USA) was used for the analyses.
Results
Demographic baseline characteristics
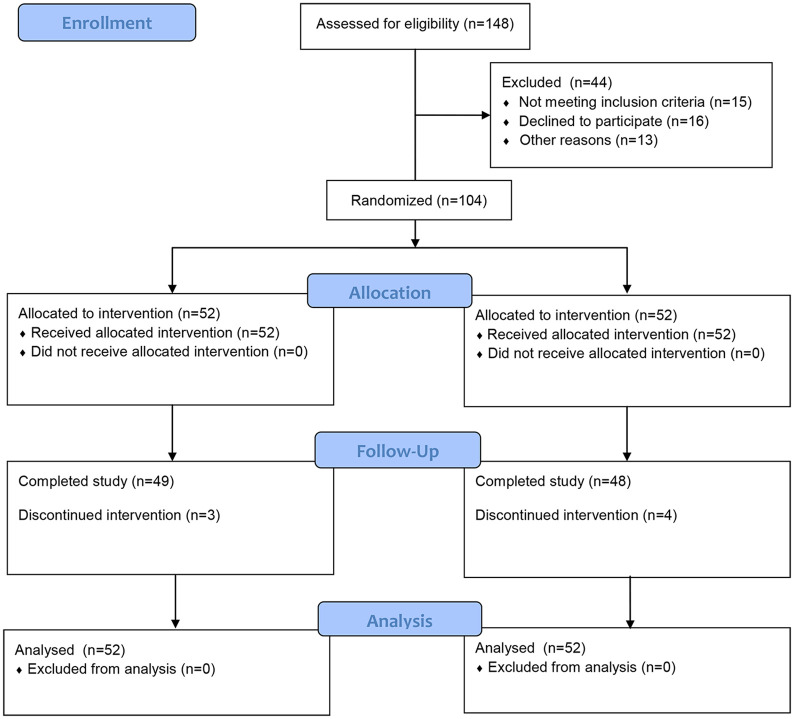
A total of 148 patients with IBS-D were recruited, and 104 satisfied the study criteria, with 52 each in the moxibustion and sham groups (Table 1). The demographic and clinical characteristics of the groups were similar.
Table 1.
Baseline demographics and clinical characteristics of the moxibustion and sham groups. a
| Moxibustion | Sham | p | |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 23 (46.9) | 28 (53.9) | 0.327 |
| Female | 29 (55.8) | 24 (46.1) | |
| Age, y, mean (SD) | 47.6 ± 11.9 | 45.2 ± 14.7 | 0.357 |
| Height, cm, mean (SD) | 166.2 ± 8.0 | 167.3 ± 9.2 | 0.534 |
| Weight, kg, mean (SD) | 60.7 ± 12.9 | 63.0 ± 12.1 | 0.357 |
| Education, years, mean (SD) | 14.2 ± 2.6 | 15.6 ± 15.4 | 0.525 b |
| Duration of IBS, years, mean (SD) | 6.8 ± 7.9 | 8.1 ± 7.1 | 0.353 |
| Pt’s expectation, n (%) c | |||
| Slight | 7 (13.5) | 5 (9.6) | 0.673 d |
| Moderate | 12 (23.1) | 13 (25.0) | |
| Extreme | 33 (63.4) | 34 (65.4) | |
| IBS-SSS, mean (SD) | |||
| Total score | 253.6 ± 72.2 | 252.9 ± 77.5 | 0.958 |
| Abdominal pain, severity | 37.3 ± 30.4 | 37.7 ± 32.4 | 0.950 |
| Abdominal pain duration, days | 35.4 ± 28.5 | 38.3 ± 32.3 | 0.630 |
| Abdominal distension severity | 37.3 ± 30.4 | 37.7 ± 32.4 | 0.950 |
| Satisfaction with bowel habits | 69.6 ± 22.8 | 68.1 ± 26.6 | 0.752 |
| Interference in general life | 67.3 ± 23.4 | 65.2 ± 23.0 | 0.643 |
| IBS-SSS, n (%) | |||
| Mild | 6 (11.5) | 10 (19.2) | 0.352d |
| Moderate | 33 (63.5) | 31 (59.6) | |
| Severe | 13 (25) | 11 (21.2) | |
| BSS, mean (SD) | |||
| Total score | 5. 92 ± 0.86 | 6.10 ± 0.80 | 0.290 |
| Frequency of diarrhea/week | 3.25 ± 1.20 | 3.61 ± 1.36 | 0.159 |
| Bowel urgency score | 88.5 ± 9.4 | 88.7 ± 8.9 | 0.915 |
| IBS-QOL, mean (SD) | |||
| Total score | 58.5 ± 18.9 | 60.1 ± 17.9 | 0.659 |
| Dysphoria | 53.6 ± 23.1 | 55.3 ± 22.8 | 0.670 |
| Interference with activity | 51.0 ± 22.4 | 50.6 ± 24.1 | 0.940 |
| Body image | 73.1 ± 20.0 | 76.2 ± 18.7 | 0.412 |
| Health worry | 56.1 ± 21.7 | 55.9 ± 22.5 | 0.971 |
| Food avoidance | 40.9 ± 30.0 | 46.2 ± 27.3 | 0.350 |
| Social reaction | 66.6 ± 20.6 | 68.6 ± 24.7 | 0.648 |
| Sexual activity | 71.6 ± 28.9 | 73.6 ± 28.7 | 0.734 |
| Relationships | 70.0 ± 23.8 | 71.0 ± 22.6 | 0.833 |
| HADS, mean (SD) | |||
| Anxiety | 7.6 ± 4.1 | 8.4 ± 4.3 | 0.346 |
| Depression | 6.1 ± 4.3 | 6.1 ± 4.4 | 0.964 |
BSS, Bristol stool form scale; HADS, Hospital Anxiety and Depression Scale; IBS, irritable bowel syndrome; QOL, quality of life; SSS, symptom severity scale.
n = 52 in each group.
Satterthwaite test.
Patients’ expectation of the efficacy of moxibustion treatment.
Row mean scores differ test.
Among the 104 patients, 97 (93.3%) completed the trial (Figure 2). Three dropouts were in the moxibustion group (one each due to going abroad, work conflict, or unknown reason). Four patients dropped out of the sham group (two due to work conflicts and one each due to travel or unknown reason).
Figure 2.
CONSORT flowchart of patients throughout the study.
Primary and secondary outcomes
At the end of treatment (6 weeks), both the intention-to-treat and per-protocol analyses showed that the percentage of patients in the moxibustion group who achieved IBS-AR (76.9%, 81.6%, respectively) was significantly higher than that of the sham group (42.3%, 45.8%, respectively, p < 0.001, both, Table 2). At each timepoint of the follow-up, the rate of IBS-AR of the moxibustion group was higher than that of the sham (p < 0.001).
Table 2.
Proportion of IBS-AR in the two groups, n(%). a
| Week | Moxibustion | Sham | Difference (95% CI) | |
|---|---|---|---|---|
| Intention-to-treat | 6 | 40 (76.9) | 22 (42.3) | 34.6 (17.0 to 52.3) |
| 12 | 48 (92.3) | 15 (28.8) | 63.5 (49.2 to 77.7) | |
| 18 | 48 (92.3) | 11 (21.1) | 71.2 (57.9 to 84.4) | |
| 24 | 48 (92.3) | 9 (17.3) | 75.0 (62.4 to 87.6) | |
| Per-protocol | 6 | 40 (81.6) | 22 (45.8) | 35.8 (18.0 to 53.6) |
| 12 | 48 (98.0) | 15 (31.3) | 66.7 (53.0 to 80.4) | |
| 18 | 48 (98.0) | 11 (22.9) | 75.1 (62.5 to 87.6) | |
| 24 | 48 (98.0) | 9 (18.8) | 79.2 (67.5 to 90.9) |
AR, Adequate Relief; CI, confidence interval; IBS, irritable bowel syndrome.
n = 52 in each group, p < 0.001 in each category.
At the end of treatment, the IBS-SSS response rate of the moxibustion group (84.6%) was significantly higher than that of the sham group (50.0%, p < 0.001), and this trend was maintained throughout the follow-up (p < 0.001, Table 3).
Table 3.
IBS-SSS response rate of the moxibustion and sham groups, n (%). a
| Moxibustion | Sham | Difference (95% CI) | |
|---|---|---|---|
| Week 6 | 44 (84.6) | 26 (50.0) | 34.6 (17.9 to 51.4) |
| Week 12 | 44 (84.6) | 33 (63.5) | 21.1 (4.8 to 37.5) |
| Week 18 | 46 (88.5) | 36 (69.2) | 19.3 (4.0 to 34.5) |
| Week 24 | 48 (92.3) | 32 (61.5) | 30.8 (15.7 to 45.9) |
CI, confidence interval; IBS, irritable bowel syndrome; SSS, symptom severity scale.
n = 52 in each group, p < 0.001 in each category.
IBS-SSS and domains
At the end of treatment (6 weeks), from 6 to 24 weeks, and over the entire study period (24 weeks), the reduction of total IBS-SSS score in the moxibustion group was significantly greater than that of the sham group (p < 0.001, each, Supplementary Table 1).
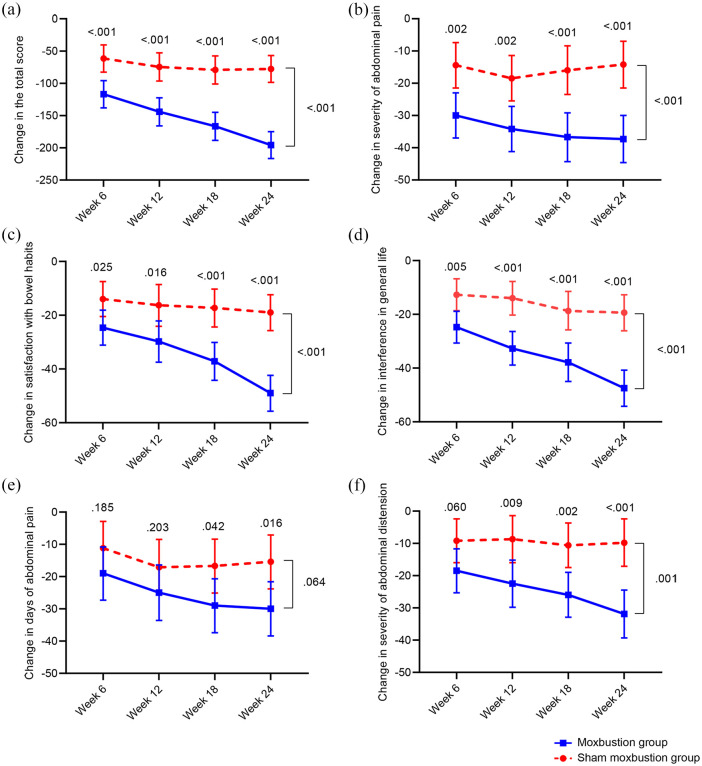
Consistent with the IBS-SSS total score, the reductions of the following specific IBS-SSS domains at the end of treatment were significantly higher in the moxibustion group than the sham group: abdominal pain severity, dissatisfaction of bowel habits, and interference of life in general (p = 0.002, 0.025, 0.005, respectively, Figure 3, Supplementary Table 1). The differences between the groups were significant during the follow-up. The moxibustion group experienced greater improvements in days of abdominal pain at 18 and 24 weeks (p = 0.042, 0.016, respectively) and abdominal distension severity at 12, 18, and 24 weeks (p = 0.009, 0.002, <0.001, respectively), compared with the sham group, as well as abdominal distention throughout the study period (p = 0.001). However, for the entire 24 weeks, the days of abdominal pain in the two groups did not differ significantly (p = 0.064).
Figure 3.
Inter-group differences in change of IBS-SSS score and five domain items from baseline at weeks 6, 12, 18, and 24. Variables are present as mean (95% confidence interval). (a) Moxibustion treatment significantly improved the overall IBS-SSS score, (b) severity of abdominal pain, (c) satisfaction with bowel habits, and (d) interference in general life after 6-week treatment. It also significantly reduced the (e) days of abdominal pain and (f) severity of abdominal distension at the 18-week follow-up.
Changes in BSS, mean frequency of diarrhea per week, and bowel urgency
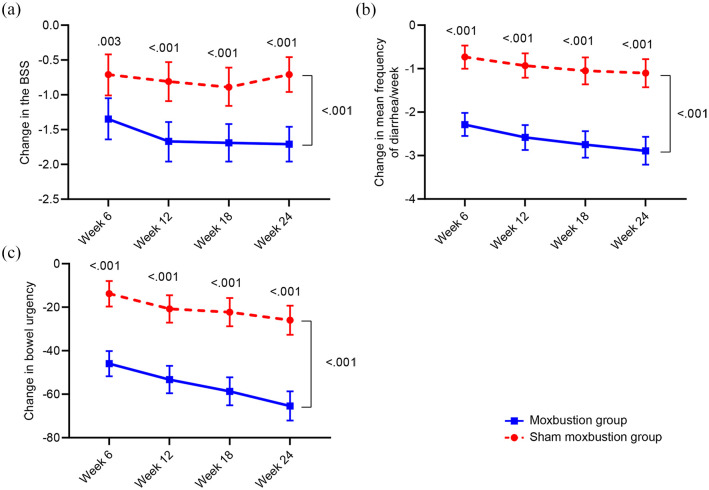
At the end of treatment (6 weeks), the moxibustion group had experienced significantly greater decreases in BSS score, frequency of diarrhea per week, and bowel urgency than the sham group (p = 0.003, <0.001, <0.001, respectively, Figure 4, Supplementary Table 2). The decreases remained significantly greater in the moxibustion group throughout the follow-up period.
Figure 4.
(a) Inter-group differences in changes of BSS score, (b) mean frequency of diarrhea/week, and (c) bowel urgency score from baseline at weeks 6, 12, 18, and 24. Variables are present as mean (95% confidence interval).
Health-related QOL
At 6 weeks, the health-related QOL had significantly improved from the baseline in both the moxibustion (p < 0.001) and sham (p = 0.004) groups, with no significant difference between the two groups in degree of improvement (p = 0.613, Supplementary Table 3). Regarding specific domains of the IBS-QOL, significant improvements in the domains of anxiety, behavioral disorders, and health concerns (p < 0.001, 0.001, 0.005, respectively) were observed in the moxibustion group. In the sham group, significant improvements were observed in the following four domains: anxiety, behavioral disorders, health concerns, and interpersonal relationships (p = 0.002, 0.001, 0.026, 0.024, Supplementary Table 4).
HADS
After 6 weeks of treatment, both the anxiety and depression scores of the moxibustion group had significant improvement (p < 0.001, both, Supplementary Tables 5 and 6). In the sham group, both the anxiety and depression scores improved, but only the change in anxiety score reached statistical significance (p = 0.003, 0.156, respectively). The moxibustion and sham groups were comparable in degree of improvement in both anxiety (p = 0.573) and depression (p = 0.054).
Subgroup analysis
At the end of treatment, the men (but not women) of the moxibustion group had a higher rate of remission compared with the sham group (Supplementary Table 7). Of patients with slight or moderate (but not severe) IBS-SSS score, the moxibustion group experienced a higher rate of remission compared with the sham group. For patients at all levels of expectation of improvement with treatment, the moxibustion group had a significantly higher rate of remission than the sham group.
Safety outcome
No serious adverse events occurred in this study. One person in each group suffered mild burn and recovered after symptomatic treatment.
Patients’ blinded guess regarding treatment type
At the end of treatment, the subjects were asked to guess as to the specific treatment they received. In the moxibustion and sham groups, 89.80% (44/49) and 83.33% (40/48), respectively, considered that they had received actual moxibustion treatment (p = 0.35).
Discussion
This is the first parallel group randomized and sham-controlled clinical trial of moxibustion at ST25 and ST36 for the treatment of IBS-D, with a 24-week follow-up. In 6 weeks, 18 moxibustion treatments were administered to each subject, and the long-term effects were assessed after 18 weeks. We found that patients who received moxibustion had a higher adequate response rate, significantly lower IBS-SSS scores and frequency of diarrhea, and better improvement in stool characteristics and bowel urgency than the sham treatment. The 6.7% dropout rate and low adverse event indicate that moxibustion has good compliance and is safe for relief of IBS-D symptoms.
At the end of treatment, 76.9% of patients in the moxibustion group showed adequate response, which was maintained at 92.3% during the follow-up. In contrast, the adequate response of the sham group decreased from 42.3% to 17.3%. The trend in IBS-SSS scores was consistent with the IBS-AR rates. This shows that moxibustion can significantly improve IBS symptoms and was associated with significant posttreatment effects. This may be related to the effect of moxibustion in promoting self-healing, which can continue to restore the body to normal condition even after stopping moxibustion. Similar posteffects have been observed in a recent large-scale acupuncture study. 20 In the present study, the placebo effect of the sham moxibustion diminished over time, consistent with the 44% and 42% placebo/sham acupuncture effects demonstrated in previous studies.19,21 Begtrupet al. 22 found that after 6 months of oral administration of probiotic capsules, patients with IBS had an adequate response rate of 52%, which was similar to the placebo (41%), and dropped to 38% after an additional 6-month follow-up, with side effects of treatment-related rash. Chey et al. 23 studied the mixed μ- and κ-opioid receptor agonist and δ-opioid receptor antagonist eluxadoline for treatment of IBS-D. After 1 month of treatment, 22.8% to 24.6% of patients showed adequate response, but about one in four of them failed to respond during the subsequent 3- to 6- month follow-up. Side effects including abdominal pain, nausea, and constipation occurred in 6% to 8% of patients. In contrast, moxibustion treatment in the present study had better rate of adequate response and less side effects. In addition, the exploratory subgroup analysis showed that moxibustion was associated with a higher adequate response rate in men (cf. women), and patients with moderate or severe IBS-D (cf. mild), while patients’ expectations for moxibustion did not affect the results.
The improvement in total IBS-SSS score from baseline after moxibustion was also significantly better than that of the sham group, which reached maximum at the last follow-up at 24 weeks. The studies of Kim et al. 24 and Ma et al. 25 showed that 4 weeks of moxibustion treatment led to a significantly lower symptom severity score and was more advantageous than either sham moxibustion or pinaverium bromide at the 8-week follow-up. 25
Abdominal pain is an important factor that troubles IBS patients and negatively influences daily QOL and emotional psychology. 26 In this study, patients who received moxibustion had significant improvement in IBS-SSS scores for abdominal pain severity, satisfaction with bowel habits, and interference in general life. Among them, the severity of abdominal pain showed the greatest decrease from baseline at the end of treatment (6 weeks). The effects of moxibustion on improving severity of abdominal distension and days of abdominal pain took longer to appear (at 12 and 18 weeks, respectively). This suggests that moxibustion is best for the relief of severe abdominal pain, and we speculate that this effect leads to improvement in satisfaction of bowel habits and interference in general life.
Moxibustion also showed consistent advantages in improving BSS, mean frequency of diarrhea per week, and bowel urgency. These effects affect satisfaction with bowel habits and interference in general life, and these improvements were maintained to the end of the study period. Zhao et al. 6 obtained similar findings. In the present study, the improvement of abdominal pain after moxibustion occurred much earlier than that of abdominal distension, suggesting that moxibustion may be more suitable to patients with abdominal pain as main complaint.
In the present study, both groups showed improvements in IBS-QOL, as reflected by reduced dysphoria, interference with activities, and health worries, and better scores for anxiety and depression. Thus, the prospect for moxibustion treatment may have induced psychological comfort to the patients. As abnormal emotions are shown to worsen IBS symptoms and affect IBS-QOL, 27 we speculate that the reductions in dysphoria and interference with activities and health worries were due to a relief of anxiety and depression. Furthermore, a warm sensation during sham or verum moxibustion can soothe patients and generate a calming and stabilizing effect on emotions. 28
In this study, the weak inter-group differences in IBS-QOL and emotions may be due to the effect of warm sensation from both sham and verum moxibustion in a disease that is prone to placebo effect.19,29 Although lifting the height of the burning moxa stick from the acupoint greatly reduced the heat, there was still a slightly higher temperature and a small amount of infrared radiation at the skin of acupoint. The warm sensation during the treatment may be an important factor in giving the subjects a psychological placebo effect. In addition, the acupoints ST25 and ST36 used that are mainly for regulating gastrointestinal symptoms instead of emotional improvement may also contribute to the lack of significant differences between the two groups in emotion-related outcomes. This interesting finding provides additional evidence that our blinding protocol was successful.
Heat stimulation is critical in the effect of moxibustion. Reports have shown that an increase in body surface temperature to above 43°C ± 1°C during moxibustion can activate the transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor in the acupoints related to visceral pain. These stimulation signals are transmitted to the spinal cord through afferent nerve, which inhibits hyperexcitability of the spinal dorsal horn cells, increases pain threshold, and regulates the release of related brain-gut peptides, thereby alleviating the symptoms of IBS. 30 As the temperature of sham moxibustion in the present study was controlled at 37°C ± 1°C, the heat stimulation is likely subthreshold for activating TRPV1 and other afferent input and does not induce significant clinical effects.
There are several shortcomings in this study. First, there was no waiting group, which makes it impossible to rule out the influence of the natural course of the disease. Second, no comparison of efficacy was made with standard therapies. Third, changes in the severity of diarrhea during menstrual cycle in women are not tracked, as diarrhea may worsen during menstruation. It is necessary to improve these shortcomings in future research to further verify the results of this study.
In summary, this study demonstrates that moxibustion treatment at ST25 and ST36 is associated with a sustained beneficial clinical effect in the treatment of patients with IBS-D for at least 24 weeks. The benefits include improvements in disease severity, stool characteristics, diarrhea frequency, and bowel urgency. Although QOL and anxiety improved in both groups, verum moxibustion is more effective for the relief of depression. This study suggests that moxibustion is a safe, effective, and highly acceptable therapy for improving and maintaining IBS-D symptom relief over a long time period.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848221075131 for Long-term effect of moxibustion on irritable bowel syndrome with diarrhea: a randomized clinical trial by Chunhui Bao, Luyi Wu, Yin Shi, Zheng Shi, Xiaoming Jin, Jiacheng Shen, Jing Li, Zhihai Hu, Jianhua Chen, Xiaoqing Zeng, Wei Zhang, Zhe Ma, Zhijun Weng, Jinmei Li, Huirong Liu and Huangan Wu in Therapeutic Advances in Gastroenterology
Acknowledgments
All the authors thank the subjects who participated in the study, and Chunye Wang from Yueyang Hospital of Integrated Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine for the recruitment of subjects.
Footnotes
Author contributions: Chunhui Bao: Conceptualization; Data curation; Funding acquisition; Investigation; Writing – original draft.
Luyi Wu: Data curation; Investigation; Methodology; Project administration; Writing – original .
Yin Shi: Data curation; Methodology; Resources; Writing – review & editing.
Zheng Shi: Data curation; Investigation; Methodology; Writing – review & editing.
Xiaoming Jin: Methodology; Supervision; Writing – review & editing.
Jiacheng Shen: Formal analysis; Software; Writing – review & editing.
Jing Li: Data curation; Resources; Writing – review & editing.
Zhihai Hu: Data curation; Resources; Writing – review & editing.
Jianhua Chen: Methodology; Validation; Writing – review & editing.
Xiaoqing Zeng: Data curation; Methodology; Writing – review & editing.
Wei Zhang: Formal analysis; Methodology.
Zhe Ma: Data curation; Writing – review & editing.
Zhijun Weng: Software; Visualization.
Jinmei Liu: Data curation; Investigation; Writing – review & editing.
Huirong Liu: Conceptualization; Funding acquisition; Project administration; Supervision; Writing – review & editing.
Huangan Wu: Conceptualization; Funding acquisition; Project administration; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the National Key Basic Research Program of China (973 Program, No. 2015CB554500 and 2009CB522900), the Science Foundation of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine (No. 2021yyjm04), the National Natural Science Foundation of China (No. 81503656), the Shanghai Leading Talent Program (No. 2016-037), and the Chinese Medicine Inheritance and Innovation ‘100 Million’ Talent Project (Qi Huang Scholar).
ORCID iD: Chunhui Bao  https://orcid.org/0000-0002-9763-0046
https://orcid.org/0000-0002-9763-0046
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chunhui Bao, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China; Key Laboratory of Acupuncture and Immunological Effects, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Luyi Wu, Key Laboratory of Acupuncture and Immunological Effects, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Yin Shi, Department of Outpatient, Shanghai Research Institute of Acupuncture and Meridian, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Zheng Shi, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China; Key Laboratory of Acupuncture and Immunological Effects, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Xiaoming Jin, Department of Anatomy and Cell Biology, Stark Neurosciences Research Institute, Indiana University School of Medicine, Indianapolis, IN, USA.
Jiacheng Shen, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Jing Li, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Zhihai Hu, Department of Acupuncture and Moxibustion, Shanghai Traditional Chinese Medicine-Integrated Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Jianhua Chen, Shanghai Clinical Research Center for Mental Health, Shanghai Key Laboratory of Psychotic Disorders, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Xiaoqing Zeng, Department of Gastroenterology, Zhongshan Hospital, Fudan University, Shanghai, China.
Wei Zhang, Department of Biostatistics, School of Public Health, Fudan University, Shanghai, China.
Zhe Ma, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Zhijun Weng, Key Laboratory of Acupuncture and Immunological Effects, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Jinmei Li, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Huirong Liu, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, No. 11o, Ganhe Road, Hongkou District, Shanghai 200437, China; Key Laboratory of Acupuncture and Immunological Effects, Shanghai University of Traditional Chinese Medicine, No. 650, Wanping South Road, Xuhui District, Shanghai 200030, China.
Huangan Wu, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, No. 11o, Ganhe Road, Hongkou District, Shanghai 200437, China; Key Laboratory of Acupuncture and Immunological Effects, Shanghai University of Traditional Chinese Medicine, No. 650, Wanping South Road, Xuhui District, Shanghai 200030, China.
References
- 1. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014; 6: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cashman MD, Martin DK, Dhillon S, et al. Irritable bowel syndrome: a clinical review. Curr Rheumatol Rev 2016; 12: 13–26. [DOI] [PubMed] [Google Scholar]
- 3. Li CY, Li SC. Treatment of irritable bowel syndrome in China: a review. World J Gastroenterol 2015; 21: 2315–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schneider A, Streitberger K, Joos S. Acupuncture treatment in gastrointestinal diseases: a systematic review. World J Gastroenterol 2007; 13: 3417–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu Y, Wu Z, Ma X, et al. Brain regions involved in moxibustion-induced analgesia in irritable bowel syndrome with diarrhea: a functional magnetic resonance imaging study. BMC Complement Altern Med 2014; 14: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao JM, Lu JH, Yin XJ, et al. Comparison of electroacupuncture and moxibustion on brain-gut function in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. Chin J Integr Med 2015; 21: 855–865. [DOI] [PubMed] [Google Scholar]
- 7. Tang BZ, Zhang JL, Yang ZG, et al. Moxibustion for diarrhea-predominant irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2016; 2016: 5105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu LP, Ma YH, Ye SS, et al. Acupuncture for diarrhoea-predominant irritable bowel syndrome: a network meta-analysis. Evid Based Complement Alternat Med 2018; 2018: 2890465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xing JH, Larive B, Mekhail N, et al. Transcutaneous electrical acustimulation can reduce visceral perception in patients with the irritable bowel syndrome: a pilot study. Altern Ther Health Med 2004; 10: 38–42. [PubMed] [Google Scholar]
- 10. Zhao C, Qi L, Wu LY, et al. Suspended moxibustion at Tianshu (ST25) inhibits prokineticin 1 and prokineticin receptor 1 expression in the spinal cord of rats with chronic visceral hypersensitivity. Neural Regen Res 2012; 7: 1145–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bao C, Zhang J, Liu J, et al. Moxibustion treatment for diarrhea-predominant irritable bowel syndrome: study protocol for a randomized controlled trial. BMC Complement Altern Med 2016; 16: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mangel AW, Hahn BA, Heath AT, et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res 1998; 26: 76–81. [DOI] [PubMed] [Google Scholar]
- 13. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997; 11: 395–402. [DOI] [PubMed] [Google Scholar]
- 14. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32: 920–924. [DOI] [PubMed] [Google Scholar]
- 15. Chen CQ, Tao CH, Liu ZC, et al. A randomized clinical trial of berberine hydrochloride in patients with diarrhea-predominant irritable bowel syndrome. Phytother Res 2015; 29: 1822–1827. [DOI] [PubMed] [Google Scholar]
- 16. Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci 1998; 43: 400–411. [DOI] [PubMed] [Google Scholar]
- 17. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 18. Qi L, Li N, Liu HR, et al. Clinical and experimental studies on moxibustion for treatment of irritable bowel syndrome. China J Tradit Chin Med Pharm 2010; 12: 2224–2227. [Google Scholar]
- 19. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008; 336: 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu ZS, Liu Y, Xu HF, et al. Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence a randomized clinical trial. JAMA 2017; 317: 2493–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowe C, Aiken A, Day AG, et al. Sham acupuncture is as efficacious as true acupuncture for the treatment of IBS: a randomized placebo controlled trial. Neurogastroenterol Motil 2017; 29: 13040. [DOI] [PubMed] [Google Scholar]
- 22. Begtrup LM, de Muckadell OB, Kjeldsen J, et al. Long–term treatment with probiotics in primary care patients with irritable bowel syndrome – a randomised, double-blind, placebo controlled trial. Scand J Gastroenterol 2013; 48: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 23. Chey WD, Dove LS, Andrae DA, et al. Early response predicts a sustained response to eluxadoline in patients with irritable bowel syndrome with diarrhoea in two Phase 3 studies. Aliment Pharmacol Ther 2017; 45: 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim T, Chung J, Bae S, et al. Efficacy and safety of different doses of moxibustion for irritable bowel syndrome: a randomised controlled pilot trial. Eur J Integr Med 2018; 20: 79–83. [Google Scholar]
- 25. Ma YX, Liu X, Liu CZ, et al. Randomized clinical trial: the clinical effects of herb-partitioned moxibustion in patients with diarrhoea-predominant irritable bowel syndrome. Evid Based Complement Alternat Med 2013; 2013: 605460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akbar A, Yiangou Y, Facer P, et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008; 57: 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larsen AR, Engsbro AL, Bytzer P. Screening instruments for anxiety and depression in patients with irritable bowel syndrome are ambiguous. Dan Med J 2014; 61: A4785. [PubMed] [Google Scholar]
- 28. Nakamura M, Yoda T, Crawshaw LI, et al. Regional differences in temperature sensation and thermal comfort in humans. J Appl Physiol (1985) 2008; 105: 1897–1906. [DOI] [PubMed] [Google Scholar]
- 29. Patel SM, Stason WB, Legedza A, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil 2005; 17: 332–340. [DOI] [PubMed] [Google Scholar]
- 30. Huang R, Zhao J, Wu L, et al. Mechanisms underlying the analgesic effect of moxibustion on visceral pain in irritable bowel syndrome: a review. Evid Based Complement Alternat Med 2014; 2014: 895914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848221075131 for Long-term effect of moxibustion on irritable bowel syndrome with diarrhea: a randomized clinical trial by Chunhui Bao, Luyi Wu, Yin Shi, Zheng Shi, Xiaoming Jin, Jiacheng Shen, Jing Li, Zhihai Hu, Jianhua Chen, Xiaoqing Zeng, Wei Zhang, Zhe Ma, Zhijun Weng, Jinmei Li, Huirong Liu and Huangan Wu in Therapeutic Advances in Gastroenterology