Abstract
Alcohol use disorder (AUD) is a major health problem that causes millions of deaths annually world-wide. AUD is considered to be a chronic pain disorder, that is exacerbated by alcohol withdrawal, contributing to a high (∼80%) relapse rate. Chronic alcohol consumption has a marked impact on the gut microbiome, recognized to have a significant effect on chronic pain. We tested the hypothesis that modulating gut microbiota through feeding rats with probiotics can attenuate alcohol-induced muscle mechanical hyperalgesia. To test this hypothesis, rats were fed alcohol (6.5%, 4 days on 3 days off) for 3 weeks, which induced skeletal muscle mechanical hyperalgesia. Following alcohol feeding, at which time nociceptive thresholds were ∼37% below pre-alcohol levels, rats received probiotics in their drinking water, either Lactobacillus Rhamnosus GG (Culturelle) or De Simone Formulation (a mixture of 8 bacterial species) for 8 days; control rats received plain water to drink. When muscle mechanical nociceptive threshold was evaluated 1 day after beginning probiotic feeding, nociceptive thresholds were significantly higher than rats not receiving probiotics. Mechanical nociceptive thresholds continued to increase during probiotic feeding, with thresholds approaching pre-alcohol levels 5 days after starting probiotics; nociceptive threshold in rats not receiving probiotics remained low. After probiotics were removed from the drinking water, nociceptive thresholds gradually decreased in these two groups, although they remained higher than the group not treated with probiotic (21 days after ending alcohol feeding). These observations suggest that modification of gut microbiota through probiotic feeding has a marked effect on chronic alcohol-induced muscle mechanical hyperalgesia. Our results suggest that administration of probiotics to individuals with AUD may reduce pain associated with alcohol consumption and withdrawal, and may be a novel therapeutic intervention to reduce the high rate of relapse seen in individuals with AUD attempting to abstain from alcohol.
Keywords: Ethanol, myalgia, muscle pain, nociceptors, probiotics, microbiome
Introduction
Chronic alcohol consumption can lead to alcohol use disorder (AUD), which is highly comorbid with chronic pain,1–3 with AUD itself being considered a chronic pain disorder. 4 Approximately 30% of the U.S. population meets AUD criteria on a lifetime basis, 5 and AUD has substantially increased world-wide in the last three decades. 6 In addition to being a risk factor for the development of chronic pain, 2 individuals with AUD undergoing acute alcohol withdrawal exhibit greater pain sensitivity,7–9 which in turn can lead to relapse in recovering from AUD. 2 Unfortunately, there are few effective therapies to treat AUD-mediated pain and individuals undergoing AUD treatment programs have a very high, 70–85%, relapse rate.10,11 Therefore, new therapeutic approaches are needed to treat AUD, in particular AUD-associated pain.
It is well established that chronic alcohol consumption has a marked impact on the gastrointestinal (gut) microbiome, as well as increasing gut permeability.12,13 Given the recent appreciation of the ability of gut microbiota to modulate chronic pain,14–17 and that gut microbial diversity is markedly affected by alcohol consumption,18–20 we hypothesized that feeding probiotics, to ameliorate alcohol-induced dysbiosis, reduces alcohol-induced muscle hyperalgesia.
Methods
Animals
Adult (260–300 g) male Sprague Dawley rats were obtained from Charles River (Hollister, CA), and were housed in the Laboratory Animal Resource Center of the University of California, San Francisco, under a 12 h light/dark cycle (lights on 7 a.m.–7 p.m.) and environmentally controlled conditions; ambient room temperature (21°–23°C), with food and water available ad libitum. Their care and use in experiments conformed to National Institutes of Health guidelines and measures were taken to minimize pain and discomfort. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Chronic alcohol consumption
Rats were fed a Lieber-DeCarli liquid diet (ethanol rat diet #710260, Dyets Inc, Bethlehem, PA), 21 containing 6.5% ethanol; the liquid diet contains all necessary nutrients to replace rat chow. This is the standard ethanol-containing liquid diet for rats from this manufacturer, and this concentration of ethanol in liquid diet has been used by us22–27 and others.28,29 We employed our ‘binge drinking’ model, 26 in which rats were exposed to weekly cycles of 4 days on 6.5% ethanol-containing liquid diet and 3 days of standard laboratory rat chow, for 3 weeks (i.e. ethanol diet on days 0–4, 7–11 and 14–18).
Administration of probiotics
On day 20, two days after the alcohol feeding period ended, rats were divided into 3 groups: the control group received drinking water containing only tap water, another group received Lactobacillus rhamnosus GG in their drinking water (10 billion CFU/150 ml L. rhamnosus, Culturelle®, Amerifit, Inc, Cromwell, CT), and the third group received De Simone Formulation (DSF) in their drinking water (112.5 billion CFU/150 ml mixture of Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus delbrueckii subspecies bulgaricus, Bifidobacterium breve, B. longum, B. infantis, and Streptococcus salivarius subspecies thermophilus, DSF, VSL Pharmaceuticals, Inc, Towson, MD). Drinking water containing probiotics was made fresh each day. Water or probiotic-containing water was provided to rats ad libitum for 8 days (day 20–28).
Mechanical nociceptive threshold in muscle
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a Chatillon digital force transducer (model DFI2, Amtek Inc, Largo, FL). 30 Rats were placed in cylindrical acrylic restrainers designed to minimize restraint stress and allow for hind leg extension from lateral ports. To acclimatize rats to the testing procedure, they were trained in the nociceptive testing protocol daily for 3 days prior to starting experiments. On the day of the experiment, rats were placed in a restrainer for 30 min before experimental manipulations. To measure nociceptive threshold, the hind limb was gently extended, and a 6 mm diameter probe attached to the force transducer was applied to the gastrocnemius muscle to deliver an increasing compression force. The nociceptive threshold was defined as the force, in Newtons, at which the rat withdrew its hind leg. Baseline withdrawal threshold was defined as the mean of 3 readings taken at 5 min intervals. All behavioral testing was conducted by the same individual, between 10 a.m. and 4 p.m., who was blind to treatment condition.
Statistical analyses
Group data are expressed as mean ± SEM of n independent observations. Statistical analyses of experimental data were conducted using repeated‐measures two-way analysis of variance (ANOVA), using Prism 9 (GraphPad Software, San Diego, CA). Where ANOVA showed a significant main difference between treatments groups, Tukey’s multi-comparison post hoc test was used to determine the pair of treatment groups that were different. The accepted level for statistical significance was P < 0.05.
Results
Probiotics markedly attenuated alcohol-induced hyperalgesia
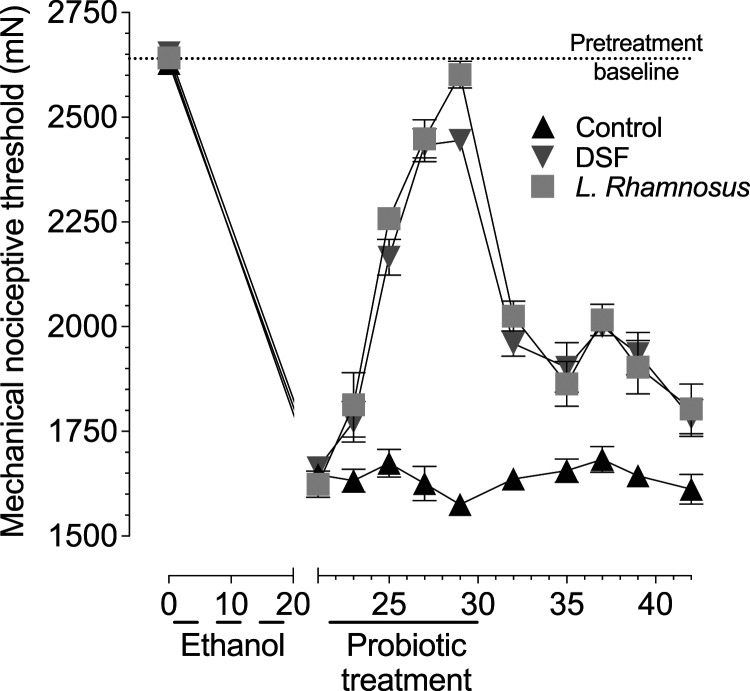
Mechanical nociceptive threshold decreased ∼37% following the 3 weeks of 4 days on/3 days off alcohol feeding protocol, demonstrating robust alcohol-induced hyperalgesia. On day 21 (after alcohol feeding), rats’ drinking water contained either plain tap water (control group), DSF or L. rhamnosus (Figure 1). When mechanical nociceptive threshold was evaluated 1 day later, there was a small, but significant, reversal of hyperalgesia in the probiotic-fed rats compared to control group (Control vs DSF P = 0.028; Control vs L. rhamnosus P = 0.003). During the 8 days of probiotic feeding (day 20–28), nociceptive threshold was markedly increased (days 25, 27, and 29, P < 0.0001 for both DSF and L. rhamnosus compared to control), reaching pre-alcohol baseline nociceptive threshold. Following cessation of probiotic feeding, nociceptive threshold in the probiotic groups decreased over time, although it was still significantly higher than the control group by day 42 (Control vs DSF P = 0.006; Control vs L. rhamnosus P = 0.002).
Figure 1.
Administration of DSF or L. rhamnosus attenuates chronic alcohol-induced muscle mechanical hyperalgesia. Time course of muscle mechanical hyperalgesia induced by chronic alcohol consumption, shows that rats receiving three cycles of ethanol diet (4 days on, 3 days off) produces robust mechanical hyperalgesia by day 21 (nociceptive threshold was ∼37% lower compared to pre-alcohol feeding). After alcohol feeding, rats were divided into three groups, receiving water alone (control), or DSF and L. rhamnosus in their drinking water, beginning on day 22. The two-way repeated measures ANOVA showed a significant group × time interaction (F20,150 = 33.83; P < 0.0001) and a significant main effect of group (F2,15 = 66.16; P < 0.0001), indicating that the groups differed significantly in both time course and magnitude. Tukey’s multiple comparison showed significant differences between control and DSF, and between control and L. rhamnosus, for all time points, beginning on day 23 (1 day after probiotic feeding), and continuing through last time point measured (day 42).
Discussion
In this study, we observed that alcohol-induced mechanical hyperalgesia was markedly attenuated in rats receiving probiotics, L. rhamnosus or DSF, suggesting that modification of gut microbiota has a pronounced effect on chronic alcohol-induced muscle mechanical hyperalgesia. A relationship between gut microbiota and pain has previously been suggested, with preclinical and clinical studies showing that visceral hyperalgesia and abdominal pain are associated with gut dysbiosis,31–34 and that visceral pain can be attenuated by administration of probiotics, including DSF35–37 and L. rhamnosus.37–39 However, there are very few published studies that have examined whether associations between gut dysbiosis and pain syndromes occur for pain outside the abdominal region. It has been reported that pain syndromes, such as back pain, 40 as well as systemic pain conditions, such as chronic widespread pain 41 and fibromyalgia,42,43 and chemotherapy-induced painful peripheral neuropathy 44 are affected by the gut microbiome. However, to the best of our knowledge our current study is the first to suggest that modifying the gut microbiome can have a marked effect at reversing alcohol-induced muscle mechanical hyperalgesia.
While our finding that alcohol-induced hyperalgesia is reversed by consumption of probiotics, the mechanism underlying this effect is yet to be determined. In individuals with AUD, as well as in preclinical models of chronic alcohol feeding, there are significant changes in the gut microbiome.45–48 However, it is not possible at this point to determine which bacterial species may contribute to muscle hyperalgesia given the gut’s vast and diverse microbial composition of 500–1000 species of bacteria, 49 many of which are up- or down-regulated by chronic alcohol consumption.12,45,47,50,51 Furthermore, in addition to bacteria, there are immense populations of fungi, viruses, archaea, and helminths in the gut 52 that may also be affected by alcohol consumption in humans and contribute to changes in nociceptive threshold. However, there is strong evidence from meta-analyses that bacteria alone affect pain, 51 and clinical studies showing that the severity of fibromyalgia pain is correlated with gut Bacteroides spp. levels. 53 Therefore, our observation that DSF and L. rhamnosus reverses alcohol-induced muscle mechanical hyperalgesia may be due to the probiotics rebalancing the gut microbiota in rats chronically consuming ethanol; both DSF and L. rhamnosus have been shown to normalize gut dysbiosis produced by a number of factors, including alcohol consumption.46,50,54–58
In addition to causing gut dysbiosis, chronic alcohol consumption disrupts intestinal epithelial tight junctions and the mucosal barrier, thereby increasing gut permeability.12,13,59,60 Increased permeability allows for translocation of microbes and bacterial products from the intestine to the systemic circulation 13 ; bacteria can directly activate nociceptors via their constitutive elements and products,14,61,62 such as bioactive lipids, and short chain fatty acids, such as butyrate, 49 that increases nociceptor sensitivity, 63 as well as lipopolysaccharide, the ligand for toll-like receptor 4 that markedly increases nociceptor sensitization. 64 Therefore, the reduction of alcohol-induced muscle hyperalgesia produced by DSF and L. rhamnosus may be related to their ability to reverse alcohol-induced increased gut permeability. Administration of both DSF 65 and L. rhamnosus treatment46,66 has been shown to attenuate alcohol-induced increased gut permeability to decrease the translocation of lipopolysaccharide and other bacterial products into the circulation.46,65 Additional studies will be needed to determine which of the 8 bacterial species in DSF are able to reverse alcohol-induced muscle hyperalgesia.
Individuals with AUD undergoing alcohol withdrawal experience pain and hyperalgesia, which is believed to contribute to relapse drinking.8,67,68 In addition, reduction in pain during treatment for AUD is associated with lower risk for relapse. 69 With AUD causing 2.3 million deaths per year, 70 and with a relapse rate of ∼80% one year post-withdrawal,10,11,71 there is an urgent need for new therapies to treat AUD. Our results suggest that consumption of probiotics may reduce pain in individuals with AUD, which could suppress their tendency to relapse. However, our study shows that relatively short-term feeding of probiotics does not result in persistent protection from alcohol-induced hyperalgesia, following cessation of probiotic feeding. Whether a prolonged feeding could result in persistent protection beyond probiotic feeding is an open question. There is evidence that probiotic microbes persist for only a few weeks after consumption.72,73 However, establishment and persistence of probiotics may be dependent on strain and diet. 74 Thus, from a therapeutic standpoint, probiotics may need to be consumed chronically to provide ongoing antihyperalgesia protection. Additional studies will be needed to determine whether specific probiotic species may confer long-lasting antihyperalgesic effects in alcohol and other neuropathies, and to determine the mechanisms of action, as well as clinical studies to evaluate the potential efficacy in patients with alcohol-induced pain.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases grant AR063312.
ORCID iD
Paul G Green https://orcid.org/0000-0001-7648-6826
References
- 1.Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S. Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav 2013; 112: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maleki N, Tahaney K, Thompson BL, Oscar-Berman M. At the intersection of alcohol use disorder and chronic pain. Neuropsychology 2019; 33: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robins MT, Heinricher MM, Ryabinin AE. From pleasure to pain, and back again: the intricate relationship between alcohol and nociception. Alcohol Alcohol 2019; 54: 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 2012; 36: 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tucker JA, Chandler SD, Witkiewitz K. Epidemiology of recovery from alcohol use disorder. Alcohol Res 2020; 40: 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD, DAIIAPC . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown RA, Cutter HSG. Alcohol, customary drinking behavior, and pain. J Abnormal Psychol 1977; 86: 179–188. [DOI] [PubMed] [Google Scholar]
- 8.Jochum T, Boettger MK, Burkhardt C, Juckel G, Bär K-J. Increased pain sensitivity in alcohol withdrawal syndrome. Eur J Pain 2010; 14: 713–718. [DOI] [PubMed] [Google Scholar]
- 9.You DS, Hahn HA, Welsh TH, Meagher MW. Hyperalgesia after a drinking episode in young adult binge drinkers: a cross-sectional study. Alcohol Alcohol 2020; 55: 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batra A, Müller CA, Mann K, Heinz A. Alcohol dependence and harmful use of alcohol: diagnosis and treatment options. Dtsch Ärztebl Int 2016; 113: 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradizza CM, Stasiewicz PR, Paas ND. Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: a review. Clin Psychol Rev 2006; 26: 162–178. [DOI] [PubMed] [Google Scholar]
- 12.Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci 2014; 111: E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maccioni L, Gao B, Leclercq S, Pirlot B, Horsmans Y, De Timary P, Leclercq I, Fouts D, Schnabl B, Stärkel P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 2020; 12: 1782157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defaye M, Gervason S, Altier C, Berthon J-Y, Ardid D, Filaire E, Carvalho FA. Microbiota: a novel regulator of pain. J Neural Transm 2020; 127: 445–465. [DOI] [PubMed] [Google Scholar]
- 15.Guo R, Chen LH, Xing C, Liu T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth 2019; 123: 637–654. [DOI] [PubMed] [Google Scholar]
- 16.Lin B, Wang Y, Zhang P, Yuan Y, Zhang Y, Chen G. Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. The J Headache Pain 2020; 21: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoni M, Miccini F, Battelli N. Gut microbiota, immunity and pain. Immunol Lett 2021; 229: 44–47. [DOI] [PubMed] [Google Scholar]
- 18.Gupta H, Suk KT, Kim DJ. Gut microbiota at the intersection of alcohol, brain, and the liver. J Clin Med 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendes BG, Schnabl B. From intestinal dysbiosis to alcohol-associated liver disease. Clin Mol Hepatol 2020; 26: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G, Liu Q, Guo L, Zeng H, Ding C, Zhang W, Xu D, Wang X, Qiu J, Dong Q, Fan Z, Zhang Q, Pan J. Gut microbiota and relevant metabolites analysis in alcohol dependent mice. Front Microbiol 2018; 9: 1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcoholism 1989; 24: 197–211. [PubMed] [Google Scholar]
- 22.Alvarez P, Ferrari LF, Levine JD. Muscle pain in models of chemotherapy-induced and alcohol-induced peripheral neuropathy. Ann Neurol 2011; 70: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dina OA, Khasar SG, Alessandri-Haber N, Green PG, Messing RO, Levine JD. Alcohol-induced stress in painful alcoholic neuropathy. Eur Jl Neuroscience 2008; 27: 83–92. [DOI] [PubMed] [Google Scholar]
- 24.Dina OA, Gear RW, Messing RO, Levine JD. Severity of alcohol-induced painful peripheral neuropathy in female rats: role of estrogen and protein kinase (A and Cε). Neuroscience 2007; 145: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dina OA, Khasar SG, Alessandri-Haber N, Bogen O, Chen X, Green PG, Reichling DB, Messing RO, Levine JD. Neurotoxic catecholamine metabolite in nociceptors contributes to painful peripheral neuropathy. Eur J Neurosci 2008; 28: 1180–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dina OA, Messing RO, Levine JD. Ethanol withdrawal induces hyperalgesia mediated by PKCε. Eur J Neurosci 2006; 24: 197–204. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari LF, Levine JD. Alcohol consumption enhances antiretroviral painful peripheral neuropathy by mitochondrial mechanisms. Eur J Neurosci 2010; 32: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gameiro GH, Arthuri MT, Tambeli CH, Veiga MCFA. Effects of ethanol on deep pain evoked by formalin injected in TMJ of rat. Life Sci 2003; 73: 3351–3361. [DOI] [PubMed] [Google Scholar]
- 29.Gatch MB, Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res 1999; 23: 328–333. [PubMed] [Google Scholar]
- 30.Green PG, Alvarez P, Gear RW, Mendoza D, Levine JD. Further validation of a model of fibromyalgia syndrome in the rat. J Pain 2011; 12: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J Gastroenterol 2016; 22: 2219–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moloney RD, Johnson AC, O’Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan J F. Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther 2016; 22: 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pusceddu MM, Gareau MG. Visceral pain: gut microbiota, a new hope? J Biomed Sci 2018; 25: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theodorou V, Ait-Belgnaoui A, Agostini S, Eutamene H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes 2014; 5: 430–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai C, Guandalini S, Zhao DH, Jiang M. Antinociceptive effect of VSL#3 on visceral hypersensitivity in a rat model of irritable bowel syndrome: a possible action through nitric oxide pathway and enhance barrier function. Mol Cell Biochem 2012; 362: 43–53. [DOI] [PubMed] [Google Scholar]
- 36.Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One 2013; 8: e63893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutten JMTM, Korterink JJ, Venmans LMAJ, Benninga MA, Tabbers MM. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics 2015; 135: 522–535. [DOI] [PubMed] [Google Scholar]
- 38.Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther 2011; 33: 1302–1310. [DOI] [PubMed] [Google Scholar]
- 39.Kannampalli P, Pochiraju S, Chichlowski M, Berg BM, Rudolph C, Bruckert M, Miranda A, Sengupta JN. ProbioticLactobacillus rhamnosusGG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterology Motil 2014; 26: 1694–1704. [DOI] [PubMed] [Google Scholar]
- 40.Dekker Nitert M, Mousa A, Barrett HL, Naderpoor N, de Courten B. Altered gut microbiota composition is associated with back pain in overweight and obese individuals. Front Endocrinol 2020; 11: 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freidin MB, Stalteri MA, Wells PM, Lachance G, Baleanu AF, Bowyer RCE, Kurilshikov A, Zhernakova A, Steves CJ, Williams FMK. An association between chronic widespread pain and the gut microbiome. Rheumatology 2021; 60: 3727–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clos-Garcia M, Andrés-Marin N, Fernández-Eulate G, Abecia L, Lavín JL, van Liempd S, Cabrera D, Royo F, Valero A, Errazquin N, Vega MCG, Govillard L, Tackett MR, Tejada G, Gónzalez E, Anguita J, Bujanda L, Orcasitas AMC, Aransay AM, Maíz O, López de Munain A, Falcón-Pérez JM. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 2019; 46: 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minerbi A, Gonzalez E, Brereton NJB, Anjarkouchian A, Dewar K, Fitzcharles M-A, Chevalier S, Shir Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019; 160: 2589–2602. [DOI] [PubMed] [Google Scholar]
- 44.Ramakrishna C, Corleto J, Ruegger PM, Logan GD, Peacock BB, Mendonca S, Yamaki S, Adamson T, Ermel R, McKemy D, Borneman J, Cantin EM. Dominant role of the gut microbiota in chemotherapy induced neuropathic pain. Scientific Rep 2019; 9: 20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barr T, Sureshchandra S, Ruegger P, Zhang J, Ma W, Borneman J, Grant K, Messaoudi I. Concurrent gut transcriptome and microbiota profiling following chronic ethanol consumption in nonhuman primates. Gut Microbes 2018; 9: 338–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, Kong M, Barker D, McClain C, Barve S. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 2013; 8: e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang L, Chu H, Gao B, Lang S, Wang Y, Duan Y, Schnabl B. Transcriptomic profiling identifies novel hepatic and intestinal genes following chronic plus binge ethanol feeding in mice. Dig Dis Sci 2020; 65: 3592–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol 2012; 302: G966–G978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo R, Cristiano C, Avagliano C, De Caro C, La Rana G, Raso GM, Canani RB, Meli R, Calignano A. Gut-brain axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem 2018; 25: 3930–3952. [DOI] [PubMed] [Google Scholar]
- 50.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res 2009; 33: 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qamar N, Castano D, Patt C, Chu T, Cottrell J, Chang SL. Meta-analysis of alcohol induced gut dysbiosis and the resulting behavioral impact. Behav Brain Res 2019; 376: 112196. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One 2013; 8: e66019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erdrich S, Hawrelak JA, Myers SP, Harnett JE. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: a systematic review. BMC Musculoskelet Disord 2020; 21: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen L, Li H, Chen Y, Yang Y. Probiotic lactobacillus rhamnosus GG reduces mortality of septic mice by modulating gut microbiota composition and metabolic profiles. Nutrition 2020; 78: 110863. [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Li H, Li J, Chen Y, Yang Y. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int J Mol Med 2019; 43: 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han Y, Wu L, Ling Q, Wu P, Zhang C, Jia L, Weng H, Wang B. Intestinal dysbiosis correlates with sirolimus-induced metabolic disorders in mice. Transplantation 2021; 105: 1017–1029. [DOI] [PubMed] [Google Scholar]
- 57.Jena PK, Sheng L, Li Y, Wan Y-JY. Probiotics VSL#3 are effective in reversing non-alcoholic steatohepatitis in a mouse model. Hepatobiliary Surg Nutr 2020; 9: 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang XL, Gu XY, Zhou XX, Chen XM, Zhang X, Yang YT, Qin Y, Shen L, Yu WF, Su DS. Intestinal dysbacteriosis mediates the reference memory deficit induced by anaesthesia/surgery in aged mice. Brain Behav Immun 2019; 80: 605–615. [DOI] [PubMed] [Google Scholar]
- 59.Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 2008; 42: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skinner C, Thompson AJ, Thursz MR, Marchesi JR, Vergis N. Intestinal permeability and bacterial translocation in patients with liver disease, focusing on alcoholic aetiology: methods of assessment and therapeutic intervention. Ther Advances Gastroenterol 2020; 13: 1756284820942616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Wardenburg JB, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013; 501: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lagomarsino VN, Kostic AD, Chiu IM. Mechanisms of microbial-neuronal interactions in pain and nociception. Neurobiol Pain 2021; 9: 100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsubota M, Matsui K, Nakano M, Kajitani R, Ishii Y, Tomochika K, Nishikawa Y, Fukushi S, Yamagata A, Sekiguchi F, Okada T, Toyooka N, Kawabata A. Essential role of Cav3.2 T-type calcium channels in butyrate-induced colonic pain and nociceptor hypersensitivity in mice. Eur J Pharmacol 2020; 887: 173576. [DOI] [PubMed] [Google Scholar]
- 64.Diogenes A, Ferraz CCR, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 2011; 90: 759–764. [DOI] [PubMed] [Google Scholar]
- 65.Chang B, Sang L, Wang Y, Tong J, Zhang D, Wang B. The protective effect of VSL#3 on intestinal permeability in a rat model of alcoholic intestinal injury. BMC Gastroenterol 2013; 13: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 2009; 43: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Fu C, Liu H, Fu R, Zuo W, Kang S, Chen P, Gregor D, Paulose R, Bekker A, Ye J-H. Electroacupuncture attenuates hyperalgesia in rats withdrawn from chronic alcohol drinking via habenular mu opioid receptors. Alcohol Clin Exp Res 2017; 41: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson I, Thompson T. Association between medically assisted detoxification and neuropathic pain. Nurs Stand 2021. [DOI] [PubMed] [Google Scholar]
- 69.Jakubczyk A, Ilgen MA, Kopera M, Krasowska A, Klimkiewicz A, Bohnert A, Blow FC, Brower KJ, Wojnar M. Reductions in physical pain predict lower risk of relapse following alcohol treatment. Drug Alcohol Dependence 2016; 158: 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, Giovino GA, West R, Hall W, Griffiths P, Ali R, Gowing L, Marsden J, Ferrari AJ, Grebely J, Farrell M, Degenhardt L. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018; 113: 1905–1926. [DOI] [PubMed] [Google Scholar]
- 71.Schellekens AFA, De Jong CAJ, Buitelaar JK, Verkes RJ. Co-morbid anxiety disorders predict early relapse after inpatient alcohol treatment. Eur Psychiatry 2015; 30: 128–136. [DOI] [PubMed] [Google Scholar]
- 72.Khalesi S, Bellissimo N, Vandelanotte C, Williams S, Stanley D, Irwin C. A review of probiotic supplementation in healthy adults: helpful or hype? Eur J Clin Nutr 2019; 73: 24–37. [DOI] [PubMed] [Google Scholar]
- 73.Sanders ME. Impact of probiotics on colonizing microbiota of the gut. J Clin Gastroenterol 2011; 45(Suppl): S115–S119. [DOI] [PubMed] [Google Scholar]
- 74.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 1997; 390: 88–91. [DOI] [PubMed] [Google Scholar]