Abstract
Background:
Nicotinamide has been reported to protect against liver steatosis and metabolic imbalances in nonalcoholic fatty liver disease (NAFLD) in animal models.
Objectives:
The objective was to investigate the efficacy and safety of nicotinamide supplementation in diabetic NAFLD patients.
Design:
This is a prospective randomized controlled open label study.
Methods:
Seventy diabetic NAFLD patients were randomly assigned either to the nicotinamide group (n = 35) who received nicotinamide 1000 mg once daily for 12 weeks in addition to their antidiabetic therapy or the control group (n = 35) who received their antidiabetic therapy only. The primary outcome was improvement in steatosis score, while secondary outcomes included assessment of liver stiffness, liver enzymes, lipid profile, insulin resistance, serum malondialdehyde, serum adiponectin, and patients’ quality of life (QOL).
Results:
Only 61 patients completed the study; 31 in the nicotinamide group and 30 in the control group. Comparisons between groups and within groups revealed nonsignificant changes in steatosis and fibrosis scores. However, significant reduction was observed in liver enzymes with a median decrease in alanine transaminase of 26.6% versus 0.74% in nicotinamide and control groups, respectively. After 12 weeks of treatment, the nicotinamide group showed significantly lower levels of low-density lipoprotein cholesterol (p value = 0.004), total cholesterol (p value = 0.006), and insulin resistance marker (p value = 0.005) compared with control. Serum triglycerides, malondialdehyde, and adiponectin levels were all comparable between the two groups. Regarding QOL, a significant improvement was detected in the total scores and the activity and fatigue domains scores.
Conclusion:
Nicotinamide at a dose of 1000 mg daily was tolerable, improved metabolic abnormalities and QOL of diabetic NAFLD patients with no effect on liver fibrosis or steatosis.
Trial Registration:
The study was registered at clinicaltrials.gov and given the ID number: ‘NCT03850886’. https://clinicaltrials.gov/ct2/show/NCT03850886.
Keywords: Fibroscan, nicotinamide, nonalcoholic fatty liver disease, steatosis
Introduction
Nonalcoholic fatty liver disease (NAFLD) comprises a spectrum of chronic liver diseases including simple steatosis, steatohepatitis, advanced fibrosis and cirrhosis, in the absence of excessive alcohol consumption or other causes of steatosis. 1 The global prevalence of NAFLD ranges from 24% in general population to 50–90% in obese individuals, 70% in diabetic patients and nearly 100% in individuals with both conditions.2 –4 It has become one of the most frequent causes of chronic liver disease and predictably one of the leading causes of liver transplantation. 5
The pathogenesis of NAFLD is multifactorial arising from interactions of genetics and metabolic factors, diet, oxidative stress, inflammation, and hypoadiponectinemia. 6 It is closely associated with type 2 diabetes mellitus (T2D) and insulin resistance (IR). Coexistence of NAFLD and T2D results in poor glycemic control. 7 Besides, in a biopsy-proven large study, a strong association was found between IR and the severity of liver inflammation in NAFLD patients even without diabetes. 8
Until today, there is no approved therapy for NAFLD. Despite the investigation of several treatments, including insulin sensitizers, vitamin E, lipid-lowering drugs, pentoxifylline, and angiotensin receptor blockers, none has proven efficacy yet. 9 Management of NAFLD is based on dietary and lifestyle modifications that bear the limitations of poor compliance and tedious implementation. Hence, novel strategies are required to help optimize the clinical outcomes of patients with NAFLD and ameliorate the consequences of the disease.
Nicotinamide is a natural product normally found in the body and acts as a coenzyme involved in a wide variety of energy transfer processes within the cell. It was previously used as an additive food supplement in several diseases like diabetes, skin disorders, depression, HIV, renal dysfunction, and aging. 10 Nicotinamide is the amide form of nicotinic acid (vitamin B3, Niacin) and an endogenous precursor for intracellular nicotinamide adenine dinucleotide (NAD+) biogenesis. Unlike niacin, nicotinamide lacks the side effects of flushing, deterioration of IR, and increased risk of diabetes development. These side effects have been proposed to be exclusive to niacin because of its ability to induce the niacin receptor GPR109A. 10
Nicotinamide might offer potential benefits in NAFLD patients for several reasons. Human liver is found to be depleted of NAD+ pool with increasing age. 11 This is attributed to accumulation of DNA breaks that activates poly ADP-ribose polymerases (PARPs) which consume intracellular NAD+. 12 It has been reported that NAD+ deficiency reduced β-oxidation and consequently caused the accumulation of triglycerides (TG) in the hepatocytes, enhanced oxidative stress, impaired insulin sensitivity, triggered inflammation in the liver, and hence contributed to the pathogenesis of NAFLD. 13 In mammals, salvage synthesis of NAD+ from nicotinamide rather than nicotinic acid is the predominant NAD+ synthesis pathway and nicotinamide is more powerful than nicotinic acid in sirtuins activation.14,15 Nicotinamide supplementation was found to elevate hepatic NAD+ which activates sirtuins expression and signaling pathways causing inhibition of lipogenesis and activation of fatty acid oxidation. 13 Moreover, nicotinamide riboside (NR) was found to improve glucose control, decrease hepatic steatosis, hypercholesterolemia, and liver damage in insulin-resistant mice raised on high-fat diet. 16
The American Association for the Study of Liver Disease (AASLD) has recommended initiating clinical trials to evaluate the expected therapeutic benefits of nicotinamide in NAFLD based on the results of NAD+ boosting on prevention and treatment of NAFLD in animal models. 17
To date, studies of nicotinamide use in NAFLD are limited to animal studies.18–21 Hence, this study aimed to evaluate the efficacy and safety of nicotinamide supplementation in diabetic NAFLD patients.
Materials and methods
Study design and setting
A prospective, randomized, controlled open label study was carried out at the endocrinology department of Al-Zahraa University Hospital, Cairo, Egypt, on 70 diabetic NAFLD patients.
Patients
All patients presented to the endocrinology department were screened for eligibility according to the set inclusion and exclusion criteria. Patients were included if they were from 18 to 65 years old, diagnosed with NAFLD and T2D, and treated with sulfonylurea, metformin, or both with a stable regimen for at least 4 weeks before the start of the study. The diagnosis of NAFLD was determined by the appearance of fatty liver on ultrasound with or without the presence of elevated liver enzymes in the absence of any other causes of liver diseases and confirmed by the presence of steatosis with the controlled attenuation parameter (CAP) score (>248 dB/m1).22,23 Exclusion criteria included liver cirrhosis (fibroscan result >12 Kpa), history of decompensated liver disease including encephalopathy, ascites or variceal bleeding, viral hepatitis, any other causes of liver disease (hemochromatosis, Wilson’s disease), biliary disease, congestive heart failure, hyper/hypoparathyroidism, cancer, pregnancy, lactation, or patients receiving statins, hepatotoxic medicines [nonsteroidal anti-inflammatory drugs (NSAIDs), amiodarone, and methotrexate], or medications evidenced to affect NAFLD (pioglitazone, GLP-1 analogs, dipeptidyl peptidase IV inhibitors) within 6 months before the start of the study or during the study period. Patients were also excluded if any change in their medication regimen occurred during the study period.
Eligible patients were stratified by sex and randomly assigned by permuted block randomization using randomly fixed size blocks to either the nicotinamide group (35 patients) who received one tablet (1000-mg nicotinamide) daily 2 h after lunch for 12 weeks in addition to standard antidiabetic therapy (sulfonylurea or metformin or both) or the control group (35 patients) who received standard antidiabetic therapy only. Randomization and enrollment were done by the principal investigator.
Nicotinamide was supplied as 1000-mg nicotinamide tablets under the trade name of Niacinamide manufactured by Nature’s Life Sun Food Energy, USA.
Methods
The study was conducted from January to August 2019. At baseline, all patients were subjected to a full history taking, physical examination, demographic, and clinical data assessment. Transient elastography (FibroScan) was performed at baseline and after 12 weeks to assess liver steatosis and fibrosis by measuring the CAP and liver stiffness measurement (LSM), respectively. Steatosis is expressed in dB/m1 and fibrosis is expressed in KPa. In addition, liver enzymes including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and lipid profile including low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and TG were all assessed at baseline and after 12 weeks using routine laboratory methods.
To assess IR, fasting blood sugar and fasting insulin were measured at baseline and after 12 weeks and IR was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR) according to the following equation: HOMA-IR = [fasting insulin (mU/ml 1 ) × fasting glucose (mg/dl 1 ) /405]. 24 Fasting blood sugar was assessed using routine laboratory methods, while fasting insulin was assessed using commercial enzyme-linked immunosorbent assay (ELISA) kit.
A blood sample was withdrawn from each patient at baseline and at the end of the study and serum was stored at −80°C until analysis. Malondialdehyde (MDA), a marker for lipid peroxidation, was assessed using commercial spectrophotometric kits while adiponectin, a marker of metabolic dysregulation, was assessed by ELISA technique using commercial kit.
Quality of life (QOL) was evaluated at baseline and at the end of the study using the validated Arabic, mother tongue, version of Chronic Liver Disease Questionnaire (CLDQ) supplied by contacting the questionnaire licensor. 25 The questionnaire is composed of 29 items comprising six domains: Abdominal Symptoms, Fatigue, Emotional Function, Worry, Activity, and Systemic Symptoms. Each item is scored on a seven-point Likert-type scale representing the frequency of clinical symptoms and emotional problems associated with liver diseases ranging from 1 ‘all of the time’ to 7 ‘none of the time’. Domain score was calculated using the mean score of the items included, and the overall score was calculated using the mean score of all domains. The higher the score, the better was the patient’s QOL (Supplemental material).
Patients were educated about the expected side effects of nicotinamide and were required to report their occurrence. Patients were followed up by weekly phone calls and monthly visits to assess the occurrence of adverse effect and to determine adherence to nicotinamide. Adherence was assessed by asking the patients during phone calls and by counting the remaining pills during refill. Patients were considered noncompliant if the compliance rate was less than 90% and were withdrawn from the study.
The primary outcome of the study was the evaluation of liver steatosis scores, and the secondary outcomes were assessment of liver stiffness scores, liver enzymes, IR, lipid profile, serum MDA, and adiponectin together with the QOL.
Statistical analysis
Statistical analysis was done using IBM SPSS® Statistics version 22 (IBM® Corp., Armonk, NY, USA). The analysis was performed as per protocol. Qualitative data were expressed as frequency and percentage. Pearson’s Chi-square test was used to examine the relation between qualitative variables. Numerical data were expressed as mean and standard deviation (SD) or median and range as appropriate. Percent change was calculated as follows: [(after 12 week − baseline)/baseline*100]. Quantitative data were tested for normality using the Kolmogorov–Smirnov test and Shapiro–Wilk test. Comparison between two groups was done using Student’s t test for normally distributed quantitative data. For non-normally distributed quantitative data, comparison between two groups was done using the Mann–Whitney test. Wilcoxon signed-rank test and paired t test were used to compare two consecutive measures of nonparametric and parametric numerical variables, respectively. All p values were two sided, and a value of p < 0.05 was considered significant.
Sample size calculation
Sample size calculation was done by the Approximate Sample Size Calculator (http://www.jerrydallal.com/LHSP/SIZECALC.HTM) available online. The sample size was calculated according to the primary outcome, fibroscan CAP score, to detect a mean difference of 25 units and a SD of 30 which was assumed based on a previous study. 26 Using alpha error of 5% and 80% power, a sample size of 25 for each group was estimated. Due to the expected dropout, 35 patients were considered in each group.
Results
Baseline evaluation
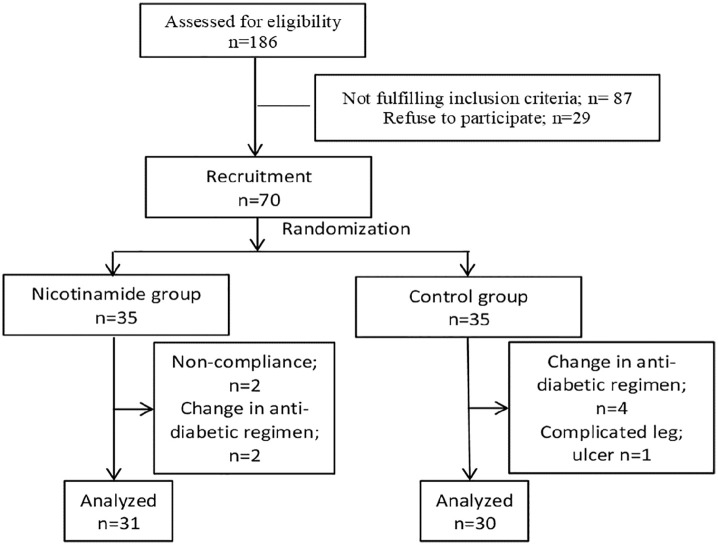
Out of a total of 186 patients assessed for eligibility, only 70 fulfilled the inclusion criteria and were included in the study. Nine patients did not complete the study due to noncompliance to nicotinamide therapy (n = 2), change in antidiabetic regimen (n = 6), and the development of complicated leg ulcer (n = 1). The study flow chart is presented in Figure 1. The age of participants ranged from 28 to 62 years old and 57.4% of them were female. Patients’ weight ranged from 65 to 130 kg and their height ranged from 145 to 186 cm with 67.2% of them were obese [body mass index (BMI) >30 kg/m2]. The diabetes mellitus duration ranged from 4 months to 10 years. There was no significant difference between the two groups regarding age, sex, weight, height, BMI, duration of diabetes, and medication received. Baseline evaluation is summarized in Table 1.
Figure 1.
The study flowchart.
Table 1.
Baseline demographics and clinical characteristics of the study groups.
| Parameter | Nicotinamide group (n = 31) | Control group (n = 30) | p value |
|---|---|---|---|
| Age (years): mean ± SD | 45.6 ± 7.3 | 47.6 ± 9.8 | 0.372 a |
| Sex: female, n (%) | 18 (58.1%) | 17 (56.7%) | 0.912 b |
| Body mass index (kg/m): mean ± SD | 32.7 ± 5.5 | 33.4 ± 4.8 | 0.585 a |
| Duration of diabetes (years): median (range) | 2 (0.3–8) | 2 (0.3–10) | 0.482c |
| Comorbidities: yes, n (%) | |||
| Dyslipidemia | 17 (54.84%) | 13 (43.33%) | 0.369 b |
| Hypertension | 9 (29.03%) | 11 (36.67%) | 0.525 b |
| Peptic ulcer | 6 (19.35%) | 9 (30%) | 0.334 b |
| Other cardiovascular conditions | 3 (9.68%) | 4 (13.33%) | 0.654 b |
| Depression | 2 (6.45%) | 0 (0) | 0.157 b |
| Medications: yes, n (%) | |||
| Glimepiride | 21 (67.74%) | 23 (76.67%) | 0.437 b |
| Metformin | 19 (61.29%) | 25 (83.33%) | 0.055 b |
| Glibenclamide | 8 (25.81%) | 4 (13.33%) | 0.221 b |
| Gliclazide | 1 (3.23%) | 1 (3.33%) | 0.981 b |
| Bisoprolol | 4 (12.90%) | 3 (10.00%) | 0.722 b |
| Captopril | 2 (6.45%) | 5 (16.67%) | 0.211 b |
| Irbesartan | 2 (6.45%) | 0 (0.00%) | d |
| Lisinopril | 1 (3.23%) | 1 (3.33%) | 0.981 b |
| Ramipril | 0 (0.00%) | 2 (6.67%) | d |
| Pregabalin | 5 (16.13%) | 2 (6.67%) | 0.246 b |
| Carbamazepine | 2 (6.45%) | 3 (10.00%) | 0.614 b |
| Gabapentin | 1 (3.23%) | 2 (6.67%) | 0.534 b |
| Ranitidine | 4 (12.90%) | 6 (20.00%) | 0.454 b |
| Omeprazole | 2 (6.45%) | 3 (10.00%) | 0.614 b |
| Aspirin | 8 (25.81%) | 9 (30.00%) | 0.715 b |
| Cilostazol | 1 (3.23%) | 0 (0.00%) | d |
| Venlafaxine | 1 (3.23%) | 0 (0.00%) | d |
| Imipramine | 1 (3.23%) | 0 (0.00%) | d |
Student’s t test.
Chi-square test.
Mann–Whitney test.
p value cannot be calculated due to small sample size in each cell.
Evaluation of efficacy of nicotinamide
There was no significant difference at baseline or after 12 weeks between the study groups and within groups regarding weight, BMI, fibrosis, and steatosis scores as presented in Table 2. Despite the nonsignificant difference in ALT and AST between groups at baseline and after 12 weeks, the within-group comparisons revealed significant changes (p < 0.001) in the nicotinamide group only with the median percent change, in ALT and AST, respectively, −26.6% and −13.3% in the nicotinamide group versus −0.74% and 2.48% in the control group, respectively.
Table 2.
Comparisons between the diagnostic and laboratory parameters at baseline and at the end of the study between study groups.
| Variable | Time of assessment | Nicotinamide group (n = 31) | Control group (n = 30) | p value |
|---|---|---|---|---|
| Weight (kg), mean ± SD | Baseline | 90.32 ± 13.13 | 95.00 ± 15.17 | 0.202 a |
| After 12 weeks | 90.65 ± 12.81 | 95.43 ± 14.42 | 0.175 a | |
| p value | 0.194 b | 0.125 b | ||
| BMI (kg/m2), mean ± SD | Baseline | 32.69 ± 5.49 | 33.41 ± 4.78 | 0.585 |
| After 12 weeks | 32.80 ± 5.41 | 33.57 ± 4.51 | 0.551 a | |
| p value | 0.201 b | 0.117 b | ||
| Steatosis (dB/m), median (range) | Baseline | 285 (246 to 377) | 283 (251 to 375) | 0.394 c |
| After 12 weeks | 280 (244 to 365) | 288 (250 to 375) | 0.269 c | |
| p value | 0.307 d | 0.665 d | ||
| % change | [−]0.38([−]3.18 to 2.36) | 0.35([−]6.25 to 5.26) | 0.644 c | |
| Fibrosis (KPa), median (range) | Baseline | 4.8 (2.5 to 8.6) | 5 (2.4 to 9.6) | 0.670 c |
| After 12 weeks | 5 (2.8 to 8.5) | 5.1 (2.5 to 9.8) | 0.718 c | |
| p value | 0.452 d | 0.084 d | ||
| % change | 0 ([−]6.67 to 16) | 2 ([−]6.67 to 8) | 0.107 c | |
| ALT (U/L) | Baseline | 33 (12 to 118) | 32 (6 to 109) | 0.925 c |
| After 12 weeks | 26 (12 to 102) | 32 (12 to 124) | 0.082 c | |
| p value | <0.001 d * | 0.740 d | ||
| % change | [−]26.6 ([−]63.7 to 8.33) | [−]0.74 ([−]16.67 to 100) | <0.001 c * | |
| AST (U/L) | Baseline | 33 (13 to 82) | 27 (12 to 78) | 0.994 c |
| After 12 weeks | 22 (13 to 60) | 28 (12 to 77) | 0.163 c | |
| p value | <0.001 d * | 0.195 d | ||
| % change | [−]13.33 ([−]65.85 to 10) | 2.84 ([−]15 to 31.58) | <0.001 c * | |
| LDL-C (mg/dl), median (range) | Baseline | 140 (78 to 221) | 137 (93 to 276) | 0.502 c |
| After 12 weeks | 114 (70 to 180) | 141 (88 to 263) | 0.004 c * | |
| p value | <0.001 d * | 0.318 d | ||
| % change | [−]15.2 ([−]50 to 17.95) | 2.5 ([−]46.2 to 34) | <0.001 c * | |
| TC (mg/dl), median (range) | Baseline | 224 (175 to 312) | 222 (170 to 352) | 0.598 c |
| After 12 weeks | 195 (160 to 269) | 230.5 (174 to 339) | 0.006 c * | |
| p value | <0.001 d * | 0.271 d | ||
| % change | [−]9.32([−]30.43 to 6.29) | 1 ([−]11.21 to 20.67) | <0.001 c * | |
| TG (mg/dl), median (range) | Baseline | 180 (90 to 284) | 167 (46 to 340) | 0.254 c |
| After 12 weeks | 176 (87 to 270) | 170 (63 to 326) | 0.312 c | |
| p value | 0.251 d | 0 .349 d | ||
| % change | [−]1.93([−]10.53 to 10.90) | 3.43 ([−]17.13 to 36.96) | 0.075 c | |
| HOMA-IR, median (range) | Baseline | 3.55 (2.04 to 10.31) | 3.59 (1.98 to 12.22) | 0.880 c |
| After 12 weeks | 3.04 (1.54 to 12.37) | 3.92 (1.68 to 11.08) | 0.137 c | |
| p value | 0.023 d * | 0.171 d | ||
| % change | [−]4.27 ([−]58.50 to 19.97) | 4.56 ([−]38.80 to 130.74) | 0.005 c * |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Independent t test.
Paired t test.
Mann–Whitney test.
Wilcoxon signed-rank test.
Indicates significance.
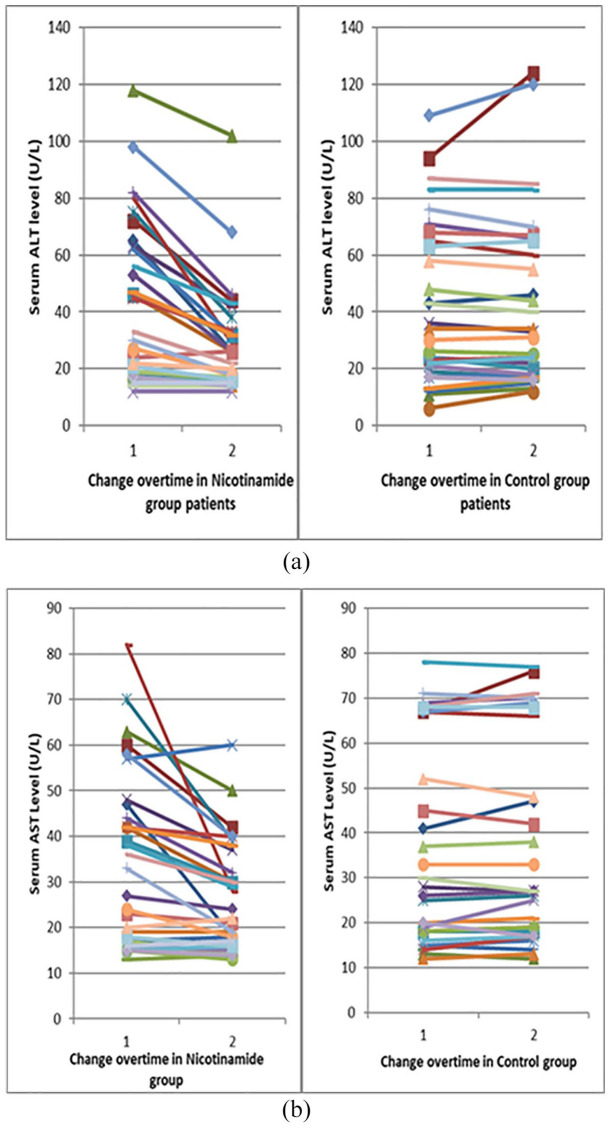
The change of ALT and AST over time is represented in Figure 2. Twenty-eight (46%) patients in this study had elevated liver enzymes at baseline. Fifteen patients were in the nicotinamide group and nine of them (60%) were normalized at the end of the study. The control group included 13 patients with elevated baseline liver enzymes that remained elevated until the end of the study.
Figure 2.
Line chart representing the change over time in ALT and AST for the study groups. (a) The change in serum ALT. (b) The change in serum AST.
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Regarding the lipid profile, both groups were comparable at baseline. However, there were significant differences between groups after 12 weeks and within the nicotinamide group with respect to LDL-C (p value = 0.004 and <0.001, respectively) and TC (p value = 0.006 and <0.001, respectively). The same was observed for HOMA-IR where significant differences were found between groups after 12 weeks and within the nicotinamide group. These data are presented in Table 2.
Evaluation of serum biomarkers
Evaluation of oxidative stress revealed nonsignificant differences at baseline and after 12 weeks regarding serum MDA with a median percent change of −7.42 in the nicotinamide group versus none in the control group. Adiponectin levels were also comparable between groups and within groups at the different time points, Table 3.
Table 3.
Comparisons between serum biomarkers at baseline and at the end of the study between study groups.
| Variable | Time of assessment | Nicotinamide group (n = 31) | Control group (n = 30) | p value |
|---|---|---|---|---|
| MDA (nmol/ml), median (range) | Baseline | 3.92 (0.71 to 12.86) | 2.86 (0.71 to 11.43) | 0.190 a |
| After 12 weeks | 3.66 (0.7 to 13.57) | 2.50 (0.49 to 11.40) | 0.466 a | |
| p value | 0.105 b | 0.971 b | ||
| % change | [−]7.42([−]50.12 to 137.65) | 0 ([−]82.94 to 200) | 0.274 a | |
| Adiponectin (mg/L), median (range) | Baseline | 2.31 (0.9 to 5.5) | 2.2 (0.9 to 4.7) | 0.756 a |
| After 12 weeks | 2.4 (0.95 to 5) | 1.93 (0.9 to 4.3) | 0.334 a | |
| p value | 0.951 b | 0.065 b | ||
| % change | 0.48 ([−]16 to 55.56) | [−]3.85 ([−]52.35 to 57.14) | 0.219 a |
MDA, malondialdehyde.
Mann–Whitney test.
Wilcoxon signed-rank test.
Evaluation of QOL
The overall score and the domain scores were not significantly different between groups at baseline. However, significant improvement was observed within the nicotinamide group and the percent change between groups with respect to overall scores and fatigue and activity domains. After 12 weeks, a significant difference was observed between groups in systemic symptoms and worry domains, yet the percent change comparisons were nonsignificant, Table 4.
Table 4.
Comparison between Chronic Liver Disease Questionnaire (CLDQ) at baseline and at the end of the study in all study groups.
| Domain | Time of assessment | Nicotinamide group (n = 31) | Control group (n = 30) | p value |
|---|---|---|---|---|
| AS domain, median (range) | Baseline | 5.7 (4.3 to 6.7) | 5.7 (4 to 6.7) | 0.780 a |
| After 12 weeks | 5.7 (4.3 to 6.7) | 5.7 (4 to 6.7) | 0.792 a | |
| p value | 0.479 b | 0.366 b | ||
| % change | 0 ([−]12.28 to 20) | 0 ([−]11.67 to 25) | 0.856 a | |
| FA domain, median (range) | Baseline | 5.4 (4 to 6.2) | 5.5 (3.6 to 6.4) | 0.942 a |
| After 12 weeks | 5.8 (4.2 to 6.4) | 5.4 (4 to 6.6) | 0.026 a * | |
| p value | <0.001 b * | 0.429 b | ||
| % change | 10.34 ([−]4.55 to 20) | 1.56 ([−]13.04 to 11.11) | 0.001 a * | |
| SS domain, median (range) | Baseline | 6 (5 to 6.8) | 5.8 (4 to 6.6) | 0.066 a |
| After 12 weeks | 6.2 (4.8 to 7) | 5.8 (4 to 6.6) | 0.037 a * | |
| p value | 0.296 b | 0.470 b | ||
| % change | 0 ([−]5.88 to 16.67) | 0 ([−]6.06 to 16) | 0.519 a | |
| AC domain, median (range) | Baseline | 5 (3.7 to 6.3) | 5.5 (4.7 to 6.3) | 0.055 a |
| After 12 weeks | 5.7 (4.7 to 6.3) | 5.5 (4.3 to 6.3) | 0.132 a | |
| p value | <0.001 b * | 0.724 b | ||
| % change | 12.77 ([−]5 to 27.03) | 5 ([−]16.67 to 14) | < 0.001 a * | |
| EF domain, median (range) | Baseline | 5.5 (4.8 to 6) | 5.4 (4.8 to 6) | 0.913 a |
| After 12 weeks | 5.5 (4.9 to 6) | 5.4 (4.6 to 6.1) | 0. 822 a | |
| p value | 0.708 b | 0.259 b | ||
| % change | 0([−]8.69 to 10.53) | 0([−]4.76 to 7.21) | 0.936 a | |
| WO domain, median (range) | Baseline | 5 (4.2 to 6.2) | 5.5 (4.2 to 6.4) | 0.068 a |
| After 12 weeks | 5.2 (4 to 6.2) | 5.5 (4 to 6.2) | 0.028 a * | |
| p value | 0.962 b | 0.857 b | ||
| % change | 0([−]100 to 9.09) | 0([−]7.69 to 8.69) | 0.971 a | |
| Total, median (range) | Baseline | 5.4 (4.9 to 6) | 5.4 (4.4 to 6.1) | 0.670 a |
| After 12 weeks | 5.5 (5.1 to 6.1) | 5.5 (4.4 to 6.1) | 0.549 a | |
| p value | 0.001 b * | 0.178 b | ||
| % change | 3.74([−]14.33 to 7.96) | 0.71([−]3.67 to 8.29) | 0.039 a * |
AC, Activity; AS, Abdominal Symptoms; EF, Emotional Function; FA, Fatigue; SS, Systemic Symptoms; WO, Worry.
Mann–Whitney test.
Wilcoxon signed-rank test.
Indicates significance.
Evaluation of tolerability
No adverse effects were reported in the control group throughout the study period. However, two patients reported nausea and vomiting, and one patient reported fatigue in the first week of the study in the nicotinamide group. All side effects were self-controlled, and none mandated study discontinuation or required interventions. None of the patients in the nicotinamide group had an increase in liver enzymes.
Discussion
Until today, this study is the first randomized controlled clinical trial assessing the effect of oral nicotinamide supplementation on diabetic NAFLD patients. This 12-week trial showed that 1000-mg nicotinamide oral supplementation had no effect on liver steatosis and fibrosis nor serum MDA and adiponectin but significantly improved liver enzymes, TC, LDL-C, IR, and patients’ QOL in diabetic NAFLD patients.
Nicotinamide has been previously used in diabetic patients with a wide range of doses from 200 mg 27 out to 3 g. 28 The postulated mechanism of action of nicotinamide in NAFLD patients is mainly based on its anti-inflammatory effects. Previous literature stated that nicotinamide in a dose range of 400–4000 mg could exert an anti-inflammatory effect.10,29 Because it was the first randomized study in NAFLD patients, and because of previous old concerns that nicotinamide might cause liver toxicity,30,31 a low dose was recommended. The 1000 mg was chosen in this study as it was the lowest available dose in the market. Despite previous study showing that improvement in fibroscan scores can occur after 4 weeks of intervention, 32 a 12-week duration was selected to allow time for detection of any changes in other parameters as lipid profile and liver enzymes.
Although liver biopsy is still the gold standard for diagnosis of NAFLD, it has several limitations including invasiveness, high chance of sampling error, interobserver, and intraobserver variability which makes it difficult and impractical to use for diagnosis and evaluation of treatment response. 33 According to the European Association for the Study of the Liver (EASL), the best noninvasive tests for the diagnosis of steatosis are the imaging ones and steatosis can be diagnosed with CAP.34,35 Fibroscan is a nonionizing and an inexpensive assessment tool with added benefits of being quantitative, machine-independent, and not subject to operator interpretation. 36 A meta-analysis found that CAP has good sensitivity and specificity for detecting liver fat. 37 An interventional study on NAFLD patients utilizing both paired biopsy and CAP as outcomes, CAP was found to be significantly associated with the grade of steatosis based on histology preintervention and postintervention. 38 Moreover, a cohort study showed that CAP significantly improved in NAFLD patients only after 4 weeks of intervention. 32 Therefore, fibroscan has been considered a suitable tool for monitoring dynamic changes in hepatic steatosis in patients with NAFLD, especially in clinical trials. 33
NAFLD is highly common in T2D patients. A majority of those T2D patients suffer from moderate/severe steatosis and 30% have significant liver stiffness, mainly due to increased IR. Hence, assessment of liver stiffness in T2D patients should be performed routinely to identify those with significant liver fibrosis. 39 Preclinical trials showed that nicotinamide has greatly reduced hepatic steatosis in mice on high-fat diet. 40 However, this study showed that the use of nicotinamide 1000 mg during the period of this study did not affect steatosis or fibrosis in diabetic NAFLD patients. The dose and duration of nicotinamide that could affect the liver steatosis parameter has not been studied yet. Nicotinamide can be used at doses up to 3000 mg with no identified adverse events. 41 Hence, higher doses or longer duration might be needed to improve steatosis and fibrosis.
Not all patients with NAFLD have elevated liver enzymes. However, high ALT enzyme activity is associated with cardiovascular risk factors in NAFLD patients, and treatment of NAFLD should aim at reducing elevated liver enzymes and hence the concomitant cardiovascular risk. 42 Moreover, patients with NAFLD and increased aminotransferase levels are at higher risk of having steatohepatitis. 34 In a preclinical study, NR was found to significantly decrease the ALT level in diabetic rats. 43 Supporting the previous evidence, this study has shown that nicotinamide significantly improved liver enzymes in the nicotinamide group.
Dyslipidemia is an established and important risk factor for cardiovascular diseases, a leading cause of morbidity and mortality worldwide. 44 In addition, correcting dyslipidemia might slow the progression to advanced liver disease. 45 In this study, nicotinamide decreased TC and LDL-C heightening the potential beneficial role of nicotinamide in diabetic NAFLD patients. Similarly, the results of an animal study of NR supplementation in high-fat fed mice showed positive effects on lipid profile without a significant effect on hepatic fat content. 46 However, nicotinamide, in the current work, had no effect on TG which might explain the lack of effect on steatosis. It has been reported that TG are the lipid profile parameter that is most often associated with fatty infiltration of the liver and showed a correlation with steatosis using computed tomography.47,48
Growing evidence showed that IR, oxidative stress, and hypoadiponectinemia might contribute to NAFLD in individuals with and without T2D.49,50 An independent relationship was found between IR evaluated by HOMA-IR, and ultrasonographic NAFLD in nondiabetic individuals. 49 Moreover, in a biopsy-based large study, a strong association was found between HOMA-IR and the severity of liver inflammation in NAFLD patients even without diabetes. 8 In this study, a significant reduction of HOMA-IR was observed in the nicotinamide group versus the control group in line with previous results of an improved insulin sensitivity and glucose tolerance, in a rodent model of obesity and T2D, with the use of nicotinamide and NR.43,51
Nicotinamide was found to attenuate oxidative stress, and hepatic damage in high fructose or high glucose consumption-induced liver steatosis in rats. 52 However, no effect was observed on serum MDA in this study. This may be attributed to the relatively low baseline levels of MDA in this study with a mean value of 3.94 nmol/ml that approximately approached normal levels. In a previous study, it was reported that normal individuals had mean serum MDA levels of 5 nmol/ml compared with diabetic NAFLD patient whose mean serum MDA levels were around 14.7 nmol/ml. 53
Adiponectin, an adipokine, was found to be plentiful in healthy volunteer plasma with levels up to 20 mg/L. Serum levels less than 4 mg/L are considered as hypoadiponectinemia. 54 In this study, diabetic NAFLD patients had a low adiponectin level with a median of 2.3 mg/L. Adiponectin is decreased in T2DM and in NAFLD patients and suggested to be a significant predictor of both conditions.55,56 Diabetic NAFLD patients were found to have the lowest adiponectin levels when compared with nondiabetic NAFLD patients and control individuals. 57 Adiponectin has been regarded as a target for NASH management with thiazolidinediones. 58 Although preclinical studies showed a significant increase in the adiponectin level upon either nicotinamide or NR supplementation,51,59 this study showed no effect of nicotinamide on the adiponectin level.
Multiple evidence has indicated that NAFLD patients have impaired QOL. Because the liver is the responsible organ for production, release, and storage of energy, metabolic and inflammatory consequences of NAFLD lead to impairment of physical and mental activities, neuromuscular dysfunction, and muscle weakness. 60 Hence, a beneficial therapeutic intervention is that which can positively affect patients’ lives and activities of daily living apart from biochemical improvement. 61 In this study, CLDQ was used in the assessment of patients’ QOL. In a large multicenter trial on biopsy-proven NAFLD patients, lobular inflammation correlated independently with QOL using CLDQ. 62 In this work, nicotinamide supplementation significantly improved the overall patients’ QOL scores in addition to activity and fatigue domains of CLDQ. Fatigue was reported as the most frequent complaint in NAFLD patients and fatigue severity negatively affected NAFLD patients’ well-being. 61 The current results showed an improvement in NAFLD-induced fatigue in the nicotinamide group.
To our knowledge and as per references, this research is the first clinical study applied with no previous clinical data in the literature; thus, a low dose of nicotinamide was used to ensure safety and tolerability of the intervention which was confirmed by the results obtained in this study showing that nicotinamide supplementation at a dose of 1000 mg was safe and tolerable in diabetic NAFLD patient. Moreover, the reported adverse effects were mild and did not require any interventions.
Conclusion
Nicotinamide provided a significant improvement in metabolic abnormalities without a significant effect on steatosis or fibrosis, in the administrated dose, in diabetic NAFLD patients. Concerning safety and tolerability issues, nicotinamide at a dose of 1000 mg daily for the study period proved to be safe and tolerable without incidence of any adverse event.
This study was limited by the small sample size, conducted at a single center with a single arm of low-dose nicotinamide used for a short duration. This was done to ensure safety and tolerability of nicotinamide use in diabetic NAFLD patients because this was the first clinical study conducted with no previous clinical data in this population.
Acknowledging the potential benefits found in this study represented in the favorable effects on IR, lipid control, liver enzyme normalization together with an improvement in patients’ activity level, nicotinamide could be considered as a promising add-on therapy in NAFLD patients. Yet, further large multicenter studies using higher doses of nicotinamide for longer duration are required to confirm the current results and to investigate the long-term effects of nicotinamide as well.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221077958 for Nicotinamide supplementation in diabetic nonalcoholic fatty liver disease patients: randomized controlled trial by Rasha R. El-Kady, Amani K. Ali, Lamia M. El Wakeel, Nagwa A. Sabri and May A. Shawki in Therapeutic Advances in Chronic Disease
Acknowledgments
A license to use the Arabic version of the Chronic Liver Disease Questionnaire (CLDQ) questionnaire was granted by Center for Outcomes Research in Liver Diseases d/b/a CLDQ. The questionnaire was provided for free.
Footnotes
Authors’ note: The authors confirm that the Principal Investigator for this article is Amani K. Ali and Rasha R. El-Kady and they had direct clinical responsibility for patients.
Author contributions: Rasha R. El-Kady: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Amani K. Ali: Formal analysis; Investigation; Methodology; Supervision; Writing – review & editing.
Lamia M. El Wakeel: Formal analysis; Methodology; Supervision; Writing – review & editing.
Nagwa A. Sabri: Formal analysis; Methodology; Supervision; Writing – review & editing.
May A. Shawki: Conceptualization; Formal analysis; Methodology; Supervision; Writing – original draft.
Availability of data and material: Data are available on reasonable request from the corresponding author.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: The study was revised and approved by the Research Ethics Committee for Experimental and Clinical Studies at the Faculty of Pharmacy, Ain Shams University (approval number: 79) which is registered and approved by the Egyptian Ministry of Health (MOH) and was conducted according to the Declaration of Helsinki 1964 and its later amendments.
Patient consent: All patients were educated about the study protocol and were required to sign a written informed consent before participation.
Clinical trial registration: The study was registered at Clinical Trial. Gov and given ID number: ‘NCT03850886’.
ORCID iD: May A. Shawki  https://orcid.org/0000-0002-8971-2332
https://orcid.org/0000-0002-8971-2332
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Rasha R. El-Kady, Department of Clinical Pharmacy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt
Amani K. Ali, Department of Internal Medicine, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt
Lamia M. El Wakeel, Department of Clinical Pharmacy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt
Nagwa A. Sabri, Department of Clinical Pharmacy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt
May A. Shawki, Department of Clinical Pharmacy, Faculty of Pharmacy, Ain Shams University, Cairo 11566, Egypt.
References
- 1. Doumas M, Imprialos K, Stavropoulos K, et al. What does the future hold for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis? Curr Vasc Pharmacol 2019; 17: 425–428. [DOI] [PubMed] [Google Scholar]
- 2. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 3. Cusi K, Sanyal AJ, Zhang S, et al. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab 2017; 19: 1630–1634. [DOI] [PubMed] [Google Scholar]
- 4. Panahi Y, Kianpour P, Mohtashami R, et al. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res (Stuttg) 2017; 67: 244–251. [DOI] [PubMed] [Google Scholar]
- 5. Pais R, Barritt AS, IV, Calmus Y, et al. NAFLD and liver transplantation: current burden and expected challenges. J Hepatol 2016; 65: 1245–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchisello S, Di Pino A, Scicali R, et al. Pathophysiological, molecular and therapeutic issues of nonalcoholic fatty liver disease: an overview. Int J Mol Sci 2019; 20: 1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Afolabi BI, Ibitoye BO, Ikem RT, et al. The relationship between glycaemic control and non-alcoholic fatty liver disease in Nigerian type 2 diabetic patients. J Natl Med Assoc 2018; 110: 256–264. [DOI] [PubMed] [Google Scholar]
- 8. Ye FZ, Liu WY, Zheng KI, et al. Homeostatic model assessment of insulin resistance closely related to lobular inflammation in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2020; 32: 80–86. [DOI] [PubMed] [Google Scholar]
- 9. Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat Commun 2018; 9: 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song SB, Park JS, Chung GJ, et al. Diverse therapeutic efficacies and more diverse mechanisms of nicotinamide. Metabolomics 2019; 15: 137. [DOI] [PubMed] [Google Scholar]
- 11. Zhou CC, Yang X, Hua X, et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol 2016; 173: 2352–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson S, Imai SI. NAD (+) biosynthesis, aging, and disease. F1000Res 2018; 7: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guarino M, Dufour JF. Nicotinamide and NAFLD: is there nothing new under the sun? Metabolites 2019; 9: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garten A, Schuster S, Penke M, et al. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 2015; 11: 535–546. [DOI] [PubMed] [Google Scholar]
- 15. Elhassan YS, Philp AA, Lavery GG. Targeting NAD+ in metabolic disease: new insights into an old molecule. J Endocr Soc 2017; 1: 816–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trammell SA, Weidemann BJ, Chadda A, et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep 2016; 6: 26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gual P, Postic C. Therapeutic potential of nicotinamide adenine dinucleotide for nonalcoholic fatty liver disease. Hepatology 2016; 63: 1074–1077. [DOI] [PubMed] [Google Scholar]
- 18. Yokouchi C, Nishimura Y, Goto H, et al. Reduction of fatty liver in rats by nicotinamide via the regeneration of the methionine cycle and the inhibition of aldehyde oxidase. J Toxicol Sci 2021; 46: 31–42. [DOI] [PubMed] [Google Scholar]
- 19. Abd-Allah H, Nasr M, Ahmed-Farid OAH, et al. Nicotinamide and ascorbic acid nanoparticles against the hepatic insult induced in rats by high fat high fructose diet: a comparative study. Life Sci 2020; 263: 118540. [DOI] [PubMed] [Google Scholar]
- 20. Shen C, Dou X, Ma Y, et al. Nicotinamide protects hepatocytes against palmitate-induced lipotoxicity via SIRT1-dependent autophagy induction. Nutr Res 2017; 40: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Dou X, Li S, et al. Nicotinamide ameliorates palmitate-induced ER stress in hepatocytes via cAMP/PKA/CREB pathway-dependent Sirt1 upregulation. Biochim Biophys Acta 2015; 1853: 2929–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017; 66: 1022–1030. [DOI] [PubMed] [Google Scholar]
- 23. Chan W-K, Nik Mustapha NR, Mahadeva S, et al. Can the same controlled attenuation parameter cut-offs be used for M and XL probes for diagnosing hepatic steatosis? J Gastroenterol Hepatol 2018; 33: 1787–1794. [DOI] [PubMed] [Google Scholar]
- 24. Matthews D, Hosker J, Rudenski A, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 25. Younossi Z, Guyatt G, Kiwi M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 1999; 45: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee YH, Kim JH, Kim SR, et al. Lobeglitazone, a novel thiazolidinedione, improves non-alcoholic fatty liver disease in type 2 diabetes: its efficacy and predictive factors related to responsiveness. J Korean Med Sci 2017; 32: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pozzilli P, Visalli N, Ghirlanda G, et al. Nicotinamide increases C-peptide secretion in patients with recent onset type 1 diabetes. Diabet Med 1989; 6: 568–572. [DOI] [PubMed] [Google Scholar]
- 28. Vague P, Picq R, Bernal M, et al. Effect of nicotinamide treatment on the residual insulin secretion in type 1 (insulin-dependent) diabetic patients. Diabetologia 1989; 32: 316–321. [DOI] [PubMed] [Google Scholar]
- 29. Kaufman W. The common form of joint dysfunction: its incidence and treatment. Brattleboro, VT: E.L. Hildreth, 1949. [Google Scholar]
- 30. Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis 2007; 11: 597–613, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rolfe HM. A review of nicotinamide: treatment of skin diseases and potential side effects. J Cosmet Dermatol 2014; 13: 324–328. [DOI] [PubMed] [Google Scholar]
- 32. Papapostoli I, Lammert F, Stokes CS. Effect of short-term vitamin D correction on hepatic steatosis as quantified by controlled attenuation parameter (CAP). J Gastrointestin Liver Dis 2016; 25: 175–181. [DOI] [PubMed] [Google Scholar]
- 33. Lee SJ, Kim SU. Noninvasive monitoring of hepatic steatosis: controlled attenuation parameter and magnetic resonance imaging-proton density fat fraction in patients with nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol 2019; 13: 523–530. [DOI] [PubMed] [Google Scholar]
- 34. Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol 2013; 58: 1007–1019. [DOI] [PubMed] [Google Scholar]
- 35. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016; 59: 1121–1140. [DOI] [PubMed] [Google Scholar]
- 36. Hasan EM, Abd Al, Aziz RA, Sabry D, et al. Genetic variants in nicotinamide-N-methyltransferase (NNMT) gene are related to the stage of non-alcoholic fatty liver disease diagnosed by controlled attenuation parameter (CAP)-fibroscan. J Gastrointestin Liver Dis 2018; 27: 265–272. [DOI] [PubMed] [Google Scholar]
- 37. Shi KQ, Tang JZ, Zhu XL, et al. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol 2014; 29: 1149–1158. [DOI] [PubMed] [Google Scholar]
- 38. Agarwal L, Aggarwal S, Shalimar, et al. Bariatric surgery in nonalcoholic fatty liver disease (NAFLD): impact assessment using paired liver biopsy and fibroscan. Obes Surg 2021; 31: 617–626. [DOI] [PubMed] [Google Scholar]
- 39. Sporea I, Mare R, Lupusoru R, et al. Liver stiffness evaluation by transient elastography in type 2 diabetes mellitus patients with ultrasound-proven steatosis. J Gastrointestin Liver Dis 2016; 25: 167–174. [DOI] [PubMed] [Google Scholar]
- 40. Mitchell SJ, Bernier M, Aon MA, et al. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metabolism 2018; 27: 667–676.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Knip M, Douek IF, Moore WP, et al. Safety of high-dose nicotinamide: a review. Diabetologia 2000; 43: 1337–1345. [DOI] [PubMed] [Google Scholar]
- 42. Schindhelm RK, Diamant M, Dekker JM, et al. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev 2006; 22: 437–443. [DOI] [PubMed] [Google Scholar]
- 43. Trammell SA, Weidemann BJ, Chadda A, et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep 2016; 6: 26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kopin L, Lowenstein CJ. Dyslipidemia. Ann Intern Med 2017; 167: ITC81–ITC96. [DOI] [PubMed] [Google Scholar]
- 45. Canbay A, Kachru N, Haas JS, et al. Patterns and predictors of mortality and disease progression among patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2020; 52: 1185–1194. [DOI] [PubMed] [Google Scholar]
- 46. Dall M, Trammell SAJ, Asping M, et al. Mitochondrial function in liver cells is resistant to perturbations in NAD(+) salvage capacity. J Biol Chem 2019; 294: 13304–13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patel V, Sanyal AJ, Sterling R. Clinical presentation and patient evaluation in nonalcoholic fatty liver disease. Clin Liver Dis 2016; 20: 277–292. [DOI] [PubMed] [Google Scholar]
- 48. Jawahar A, Gonzalez B, Balasubramanian N, et al. Comparison of correlations between lipid profile and different computed tomography fatty liver criteria in the setting of incidentally noted fatty liver on computed tomography examinations. Eur J Gastroenterol Hepatol 2017; 29: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 49. Kitade H, Chen G, Ni Y, et al. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients 2017; 9: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boutari C, Tziomalos K, Athyros VG. The adipokines in the pathogenesis and treatment of nonalcoholic fatty liver disease. Hippokratia 2016; 20: 259–263. [PMC free article] [PubMed] [Google Scholar]
- 51. Yang SJ, Choi JM, Kim L, et al. Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes. J Nutr Biochem 2014; 25: 66–72. [DOI] [PubMed] [Google Scholar]
- 52. Mejía SÁ, Gutman LAB, Camarillo CO, et al. Nicotinamide prevents sweet beverage-induced hepatic steatosis in rats by regulating the G6PD, NADPH/NADP+ and GSH/GSSG ratios and reducing oxidative and inflammatory stress. Eur J Pharmacol 2018; 818: 499–507. [DOI] [PubMed] [Google Scholar]
- 53. Narasimhan S, Gokulakrishnan K, Sampathkumar R, et al. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin Biochem 2010; 43: 815–821. [DOI] [PubMed] [Google Scholar]
- 54. Boutari C, Mantzoros CS. Adiponectin and leptin in the diagnosis and therapy of NAFLD. Metabolism 2020; 103: 154028. [DOI] [PubMed] [Google Scholar]
- 55. Banerjee A, Khemka VK, Roy D, et al. Role of serum adiponectin and vitamin D in prediabetes and diabetes mellitus. Can J Diabetes 2017; 41: 259–265. [DOI] [PubMed] [Google Scholar]
- 56. Zhang H, Niu Y, Gu H, et al. Low serum adiponectin is a predictor of progressing to nonalcoholic fatty liver disease. J Clin Lab Anal 2019; 33: e22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chellali S, Boudiba A, Griene L, et al. Incretins-adipocytokines interactions in type 2 diabetic subjects with or without non-alcoholic fatty liver disease: interest of GLP-1 (glucagon-like peptide-1) as a modulating biomarker. Ann Biol Clin 2019; 77: 261–271. [DOI] [PubMed] [Google Scholar]
- 58. Polyzos SA, Mantzoros CS. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: a systematic review. Metabolism 2016; 65: 1297–1306. [DOI] [PubMed] [Google Scholar]
- 59. Lee HJ, Hong YS, Jun W, et al. Nicotinamide riboside ameliorates hepatic metaflammation by modulating NLRP3 inflammasome in a rodent model of type 2 diabetes. J Med Food 2015; 18: 1207–1213. [DOI] [PubMed] [Google Scholar]
- 60. Gerber LH, Weinstein AA, Mehta R, et al. Importance of fatigue and its measurement in chronic liver disease. World J Gastroenterol 2019; 25: 3669–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Assimakopoulos K, Karaivazoglou K, Tsermpini EE, et al. Quality of life in patients with nonalcoholic fatty liver disease: a systematic review. J Psychosom Res 2018; 112: 73–80. [DOI] [PubMed] [Google Scholar]
- 62. Huber Y, Boyle M, Hallsworth K, et al. Health-related quality of life in nonalcoholic fatty liver disease associates with hepatic inflammation. Clin Gastroenterol Hepatol 2019; 17: 2085–2092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221077958 for Nicotinamide supplementation in diabetic nonalcoholic fatty liver disease patients: randomized controlled trial by Rasha R. El-Kady, Amani K. Ali, Lamia M. El Wakeel, Nagwa A. Sabri and May A. Shawki in Therapeutic Advances in Chronic Disease