Summary
Nuclear deubiquitinase BAP1 (BRCA1-associated protein 1) is a core component of multiprotein complexes that promote transcription by reversing the ubiquitination of histone 2A (H2A). BAP1 is a tumor suppressor whose germline loss-of-function variants predispose to cancer. To our knowledge, there are very rare examples of different germline variants in the same gene causing either a neurodevelopmental disorder (NDD) or a tumor predisposition syndrome. Here, we report a series of 11 de novo germline heterozygous missense BAP1 variants associated with a rare syndromic NDD. Functional analysis showed that most of the variants cannot rescue the consequences of BAP1 inactivation, suggesting a loss-of-function mechanism. In T cells isolated from two affected children, H2A deubiquitination was impaired. In matching peripheral blood mononuclear cells, histone H3 K27 acetylation ChIP-seq indicated that these BAP1 variants induced genome-wide chromatin state alterations, with enrichment for regulatory regions surrounding genes of the ubiquitin-proteasome system (UPS). Altogether, these results define a clinical syndrome caused by rare germline missense BAP1 variants that alter chromatin remodeling through abnormal histone ubiquitination and lead to transcriptional dysregulation of developmental genes.
Keywords: BAP1, BRCA1, intellectual disability, ubiquitin, deubiquitination, ubiquitin-proteasome system, UPS, histone 2A, chromatin remodeling, neurodevelopment, cancer, tumor
Main text
Protein degradation by the ubiquitin-proteasome system (UPS) is essential for the maintenance of proteostasis in eukaryotic cells.1 It prevents the accumulation of potentially cytotoxic misfolded or short-lived proteins whose functional conformation can no longer be restored by chaperones.2,3 Before being transported to the proteasome for hydrolysis, proteins destined to be degraded are specifically tagged by the addition of ubiquitin molecules through a cascade of reactions involving activating-, conjugating-, and ligating-enzymes.4,5 However, the ubiquitination process can be reversed by deubiquitinases (DUBs), which are able to cleave and disassemble the polyubiquitin chains of tagged substrates, thus avoiding their degradation by the proteasome.6 This action of DUBs is important for recycling the ubiquitin, avoiding proteasome overload and regulating protein turnover. Approximately a hundred DUBs are divided into four main families, including the ubiquitin C-terminal hydrolases (UCH) to which BRCA1-associated protein 1 (BAP1) belongs.7,8
BAP1 is a nuclear DUB recognized for its tumor-suppressor properties that was proposed to depend on its ability to bind to the RING finger domain of BRCA1.9 Nonetheless, later studies have shown that BAP1 acts as a tumor-suppressor gene independently of BRCA1 (MIM: 113705). BAP1 (MIM: 603089) is frequently inactivated in tumors by somatic loss-of-function (LoF) variants and its germline variants predispose to a tumor syndrome (BAP1-TPDS [MIM: 614327]) that encompasses various cancers, notably uveal melanoma, malignant pleural mesothelioma, and cutaneous melanoma.10,11 In the nucleus, BAP1 acts as a chromatin-associated protein exerting its deubiquitinating function through the multiprotein complexes formed with transcription factors and co-factors. A prominent role of BAP1 is the modulation of chromatin through the complexes formed with the additional sex comb-like proteins ASXL1, ASXL2, and ASXL3 (ASXL1/2/3).12 BAP1 complexes remove mono-ubiquitin from lysine 119 of histone H2A (Ub-H2A) previously added by Polycomb-repressive complex 1 (PRC1), thus antagonizing gene silencing mediated by PRC1 and activating expression of genes that contribute in particular to embryonic development or differentiation.10,13,14 In addition, still in association with ASLX proteins that stabilize it,13 BAP1 has been shown to regulate a wide range of other cellular processes via its interactions with partner proteins involved in DNA damage response (BRCA1, BARD1), cell cycle control and proliferation (HCF1, YY1, FoxK1/K2), ferroptosis (SLC7A11), apoptosis (IP3R3), or even the immune response.10,15, 16, 17, 18
We show herein that the consequences of BAP1 germline variants are not limited to cancer predisposition but also extend to developmental disabilities. In the frame of an international collaborative effort initiated by the Western France consortium HUGODIMS, we compiled the clinical findings for a series of 11 unrelated individuals exhibiting a syndromic form of intellectual disability (ID) and/or developmental delay (DD) due to de novo heterozygous missense single-nucleotide variants (SNVs) in BAP1. The identification of the cases was partly facilitated by the web-based tool GeneMatcher.19 The variants were identified by subject-parents trio-based exome or genome sequencing (ES/GS) in diagnostic or research settings. In this study, which was approved by the local ethics committee of the University Hospital Center (CHU) of Nantes (number CCTIRS: 14.556), all participants were clinically assessed by at least one expert clinical geneticist from each of the centers involved. Written informed consent was obtained from the parents or legal guardians of all study participants and written authorization for the publication of photographs shown in Figure 1B.
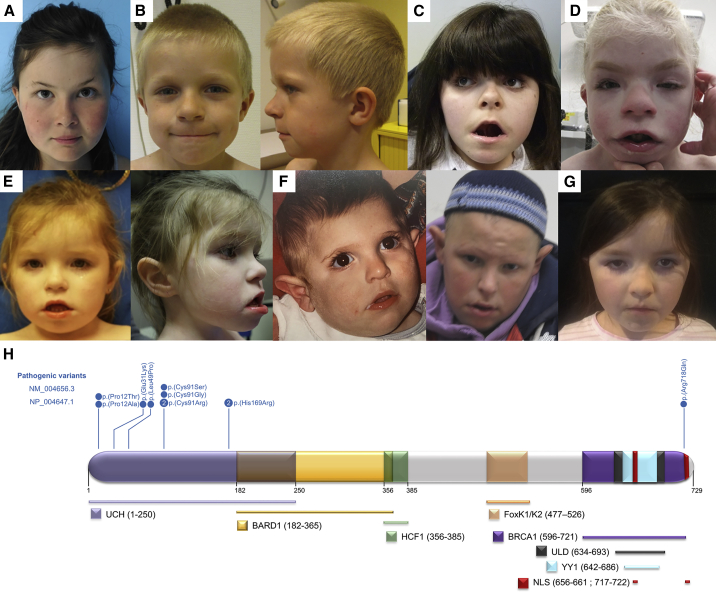
Figure 1.
BAP1 variants: Associated facial features and localization in the protein structure
(A–F) Facial photographs showing dysmorphic features in individuals 1 (A), 4 (B), 5 (C), 6 (D), 8 (E), 9 (F), and 10 (G).
(H) The majority of variants (10/11) affect only five different codons located in the UCH domain. By contrast, variant p.Arg718Gln is located in the nuclear-localization signal (NLS). Protein structure refers to the one previously described elsewhere.10 UCH, ubiquitin C-terminal hydrolase (UCH) domain; ULD, UCH37-like domain; BARD1, HCF1, BRCA1, and YY1, domains of interaction with BARD1 (BRCA1-associated RING domain 1), HCF1, BRCA1, and YY1; NLS, bipartite nuclear localization signal.
All affected individuals harboring a de novo BAP1 variant had DD or ID (11/11) characterized notably by speech (11/11) and motor delay (6/11) (Table 1; Figure 1A). Most of them had hypotonia (7/11), seizures (6/11), and abnormal behavior (8/10), including autism spectrum disorder, attention deficit hyperactivity disorder, and hypersensitivity. Almost all individuals showed dysmorphic facial features (10/11), and more than half (6/11) had skeletal malformations (involving the hands [4/11], feet [3/11], or spine [2/11],). Most of the individuals had growth failure (9/11), including four individuals with a very short stature (ranging from −3.18 to −6 SD). Organ abnormalities were inconsistent and heterogeneous and involved the eye (5/10), heart (3/10), and kidney or urogenital system (2/10). Other findings included sleep disorders, reported in 3/5 individuals, frequent episodes of otitis media in 4/11 (followed by hearing loss in two individuals), hypertrichosis in 3/11 and alopecia in 1/11, and feeding difficulties in 4/8. It is noteworthy that we could clinically distinguish two subgroups of affected individuals: one is represented by individuals 5 to 9 with a very syndromic phenotype who exhibit the most severe symptoms (severe ID, very short stature, facial dysmorphism, and congenital malformations), whereas the second subgroup with the six remaining individuals has a less syndromic phenotype with generally milder symptoms. Of note, the initial diagnostic impression was Cornelia de Lange syndrome 1 (MIM: 122470) for individuals 5 and 6 and Smith-Magenis syndrome (MIM: 182290) for individual 3.20
Table 1.
Phenotype of affected individuals with BAP1 de novo variants
| Individual (family) | 1 (F1) | 2 (F2) | 3 (F3)a | 4 (F4) | 5 (F5) | 6 (F6) | 7 (F7) | 8 (F8) | 9 (F9) | 10 (F10) | 11 (F11) | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant nomenclature Chr3: (GRCh37; GenBank:NM_004656.3) | g.52443861G>T | g.52443861G>C | g.52443601C>T | g.52442599A>G | g.52442077C>G | g.52442078A>C | g.52442078A>G | g.52442078A>G | g.52441264T>C | g.52441264T>C | g.52436341C>T | 11 variants de novo | |
| c.34C>A | c.34C>G | c.91G>A | c.146T>C | c.272G>C | c.271T>G | c.271T>C | c.271T>C | c.506A>G | c.506A>G | c.2153G>A | |||
| p.Pro12Thr | p.Pro12Ala | p.Glu31Lys | p.Leu49Pro | p.Cys91Ser | p.Cys91Gly | p.Cys91Arg | p.Cys91Arg | p.His169Arg | p.His169Arg | p.Arg718Gln | |||
| ClinVar accession number | SCV001572228 | SCV001738368 | SCV001738369 | SCV001738370 | SCV001738372 | SCV001738373 | SCV001738374 | SCV001738374 | SCV001738375 | SCV001738375 | SCV001738376 | ||
| Variant annotation | MobiDetailsb | 22880 | 22881 | 22882 | 22883 | 22865 | 22884 | 22885 | 22885 | 22886 | 22886 | 22888 | |
| CADD (GRCh37-v1.6) | 26.5 | 25.7 | 29.4 | 29.7 | 26.1 | 28.5 | 29.1 | 29.1 | 25.7 | 25.7 | 24.4 | ||
| gnomAD v2.1.1 | absent | absent | absent | absent | absent | absent | absent | absent | absent | absent | present (x1):4.7E−6 | ||
| Metadome (tolerance score) | HI (0.13) | HI (0.13) | I (0.26) | HI (0.11) | I (0.35) | I (0.35) | I (0.35) | I (0.35) | I (0.34) | I (0.34) | SI (0.94) | ||
| Method of variant detection | ES | ES | ES | ES | ES | ES | ES | ES | ES | ES | ES and GS | ||
| Sex | female | male | female | male | female | female | male | female | male | female | female | 7 F/4 M | |
| Age at last investigation | 10 years | 3 years | 14 years | 6 years | 11 years 5 months | 15 years | 1 years 11 months | 4 years | 16 years | 8 years 4 months | 12 years 1 month | 1 year 11 month to 15 years | |
| Growth failure | − | − | + | + | + | + | + | + | + | + | + | 9/11 | |
| Weight at age last investigation (kg/SD) | 36.2/+1.5 | 12.3/−1.5 | 48.6/+0.5 | 20/−0.99 | 26.5/−2 | 30/−4.35 | 12.17/+0.03 | 13.7/−1.28 | 50/−1.17 | 30/+1 | 26/−2.5 | −4.35 to +1 | |
| Height at age last investigation (cm/SD) | 128.5/−1.5 | 88.9/−1.5 | 149.4/−2.5 | 109.7/−2.44 | 116/−4.5 | 125.7/−6 | 78/−3.18 | 90/−2.71 | 142/−3.69 | 117.4/−2 | 135.3/−2 | −6 to −1.5 | |
| Head circumference at age last investigation (cm/SD) | 51/−1 | 47.9/−2 | 56/+1 | 54.7/+1.67 | 52/−1 | 50/−2.86 | 49/+0.56 | 50/−0.01 | 53/−1.41 | 48/+0 at 27 months | 54.5/+1 | −2.86 to +1.67 | |
| Developmental delay or ID | + | + | +; mild | + | +; severe | +; severe | + | + | + | + | + | 11/11 | |
| Age of walking | 18 months | 19 months | 18 months | 2 years | not walking | not achieved | 17 months | 2.5 years | 2 years | 23 months | 15 months | not applicable | |
| Speech delay | + | + | + | + | + | + | + | + | + | + | + | 11/11 | |
| Hypotonia | − | + | + | + | + | + | − | + | − | + | − | 7/11 | |
| Seizures | + | − | − | − | + | − | − | + | + | + | + | 6/11 | |
| Behavioral anomalies | +; ADHD | +; ASD | +; ADHD | +; ASD | N/A | +; ASD | − | + | + | + | +; ADHD | 9/10 | |
| Cardiac anomalies | − | + | + | − | − | − | N/A | − | − | +; VSD | − | 3/10 | |
| Eye anomalies | − | + | + | − | − | + | N/A | + | − | + | − | 5/10 | |
| Urogenital/kidney anomalies | − | − | − | − | + | − | N/A | − | − | + | − | 2/10 | |
| Hands | − | + | − | + | − | + | − | − | + | − | − | 4/11 | |
| Feet | − | − | − | − | + | + | − | − | + | − | − | 3/11 | |
| Feeding difficulties | − | − | + | − | N/A | + | N/A | + | − | + | N/A | 4/8 | |
| Facial dysmorphism | + | + | + | + | + | + | + | + | + | + | − | 10/11 | |
| Other recurrent signs | − | hearing loss | frequent otitis | hypertrichosis | hypertrichosis | hypertrichosis | recurrent otitis | − | hearing loss | − | − | not applicable | |
HI, highly intolerant; I, intolerant; SI, slightly tolerant; ES, exome sequencing; GS, genome sequencing; N/A, not analyzed; VSD, ventricular septal defect; ASD, autism spectrum disorder; ADHD, attention deficit hyperactivity disorder; VUR, vesicoureteral reflux.
Individual 3 was first reported by Berger et al., 201720 in a Smith-Magenis syndrome (SMS)-like cohort.
The detailed predictions for variants can be accessed via MobiDetails21 (see web resources for the URL for variant 22880; to access other variants’ information, replace “22880” with the relevant variant number in the URL).
In total, we found 11 missense SNVs, two of them recurrent (c.271T>C [p.Cys91Arg] and c.506A>G [p.His169Arg]) and eight restricted to three codons: 12 (2/11), 91 (4/11), and 169 (2/11) (Table 1; Figure 1H). Almost all variants were absent in public variant databases, and bioinformatics predictions were in favor of their pathogenicity; only c.2153G>A (p.Arg718Gln) was present in one heterozygote in gnomAD. According to gnomAD,22 BAP1 is intolerant of LoF variants (probability of being loss-of-function intolerant [pLI] = 0.99; observed/expected variants (o/e) = 0.12 [0.06–0.28]) and moderately intolerant to missense variants across the entire gene (Z score = 2.64; o/e = 0.64 [0.58–0.71]). Yet, the analysis by missense tolerant ratio (MTR) Gene Viewer23 shows that the region encoding the UCH domain, where 10/11 variants in the study reside (Figure 1H), is much less tolerant to missense variants than the rest of the protein (Figure S1). All amino acid residues affected by the variants are highly conserved across the species (to Caenorhabditis elegans for the variants harbored by individuals 1–10 and to Drosophila melanogaster for c.2153G>A [p.Arg718Gln]). Most variants are localized to a small region of the protein within the catalytic domain. Several alter critically important functional residues, including Cys91, which is the critical active site residue of the enzyme and whose substitutions disrupt BAP1 activity, and His169, which is also part of the enzyme active site and interacts directly with Cys9124 (supplemental information). The only variant not located in the catalytic domain,c.2153G>A (p.Arg718Gln) (GenBank: NM_004656.3), affects an amino acid within the nuclear-localization signal (NLS) domain. However, no clear genotype-phenotype correlation could be established that supports the observation of two distinct phenotypes made during clinical assessment. Secondary molecular findings were made in exome data for some of the affected individuals (Table S1), but without any evidence of their pathogenicity.
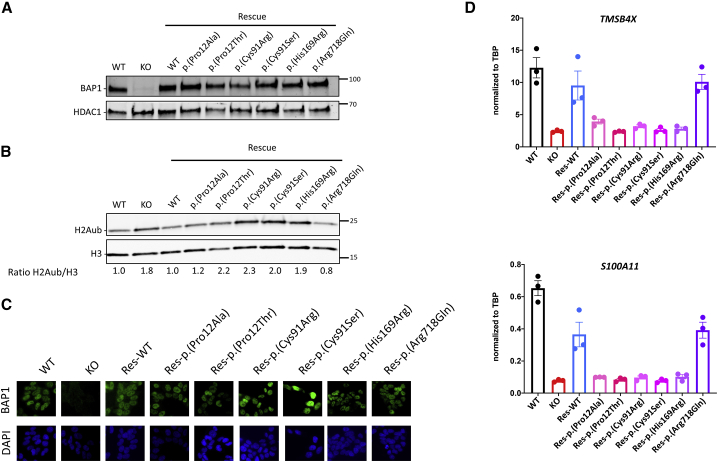
In order to evaluate directly whether the mutations described above could affect the activity of BAP1, we used a previously characterized model cell line (HAP1) in which we knocked out BAP1 by genome editing and then rescued its expression through expression of a retrovirus carrying BAP1 cDNA.13 We generated rescued cell lines for six variants and first checked by immunoblot that the mutant proteins are expressed and stable (Figure 2A). H2AK119ub is a major substrate for BAP1 enzymatic activity,14 so we therefore used its quantification as a proxy for BAP1 activity. As expected, H2AK119ub level increases by about 2-fold in the BAP1 knockout cells and reaches the original level in the BAP1-WT rescue line (Figure 2B). In contrast, four out of the six variants, namely p.Pro12Thr, p.Cys91Arg, p.Cys91Ser, and p.His169Arg are unable to rescue H2AK119ub levels. The level of H2AK119ub with the variant p.Pro12Ala rescue is near that of wild type (WT), suggesting that this variant is either partially or fully enzymatically active, at least on this substrate. The p.Arg718Gln variant displays a normal deubiquitinase activity toward H2AK119ub. We also analyzed the subcellular localization of BAP1 in the cell lines rescued with the six different variants. All showed prominent nuclear staining (Figure 2C), even the variant located in the NLS domain, p.Arg718Gln. This finding suggests that this domain is not strictly required for the nuclear localization and rules out the possibility that p.Arg718Gln could compromise the function of BAP1 by causing its cytosolic accumulation.7 Finally, we investigated whether the regulation of H2AK119ub correlates with BAP1-mediated transcriptional regulation. We had shown that TMSB4X (MIM: 300159) and S100A11 (MIM: 603114) expression are regulated by BAP1 in HAP1 cells.13 Indeed, RT-qPCR experiments confirmed that their expression decreases in the absence of BAP1 and is restored in the WT rescue line. However, five out of six variants did not rescue the expression of those genes, including the p.Pro12Ala variant, although it is enzymatically active on H2AK119ub (Figure 2D). Only the p.Arg718Gln variant behaves similarly to the WT, suggesting that this might not be an LoF variant; further investigation will be necessary to determine whether it is pathogenic. In the meantime, we consider it as a variant of uncertain significance (VUS).
Figure 2.
Assessment of BAP1 variants effect in model cell lines
(A) Nuclear extract of HAP1 cells WT, BAP1 knockout or BAP1-knockout-rescued with the different variants indicated on top were probed by immunoblot with anti-BAP1 antibody (top) or anti-HDAC1 (bottom, loading control). Representative result.
(B) Same as in (A), but this time probed with anti-H2AK119ub antibody (top) or anti-Histone H3 (bottom, loading control). Immunoblots were quantified, the ratio between H2AK119ub and H3 is indicated below after normalization to 1 for HAP1 WT cells. Representative result.
(C) Detection of BAP1 by immunofluorescence microscopy (top), nuclear staining with DAPI (bottom), representative experiment.
(D) Gene expression was analyzed by RT-qPCR in the different cell lines described in (A). TMSB4X (MIM: 300159) and S100A11 (MIM: 603114) levels normalized to TBP (MIM: 600075) expression are shown (n = 3, biological replicates).
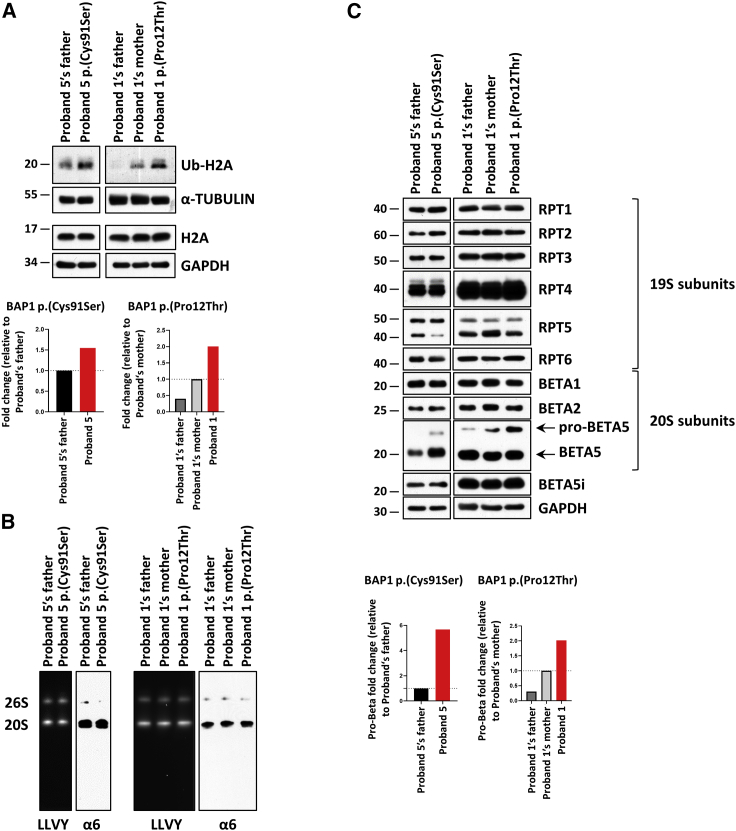
We assessed T cells isolated from affected children (individuals 1 and 5) carrying BAP1 variants c.34C>A (p.Pro12Thr) and c.272G>C (p.Cys91Ser) for their contents of ubiquitinated H2A (Ub-H2A) by immunoblotting. As shown in Figure 3A, although the amounts of H2A were similar between control and affected individuals, the steady-state level of Ub-H2A was substantially increased in cells of the affected children when compared to those of their respective related controls (i.e., father and/or mother of the probands). Specifically, densitometry analysis of the band intensities revealed that Ub-H2A was 1.5-fold and 2.0-fold enriched in the subjects bearing the p.Cys91Ser and p.Pro12Thr BAP1 variants, respectively (Figure 3A, bottom). These data are fully in line with our previous observation that both of these variants were unable to rescue H2A deubiquitination in BAP1-knockout cells (Figure 2) and confirm that the missense variants p.Pro12Thr and p.Cys91Ser cause LoF.
Figure 3.
Subjects with BAP1 variants exhibit increased amounts of Ub-H2A and pro-β5 proteasome subunit
(A) Top: whole-cell lysates from T cells of affected individuals carrying the p.Pro12Thr or p.Cys91Ser BAP1 variants (labeled probands 1 and 5) and control T cells (subjects’ father and/or mother) were subjected to protein extraction and SDS-PAGE/immunoblotting with antibodies directed against Ub-H2A, H2A as well as α-tubulin and GAPDH (loading control), as indicated. Bottom: densitometry analysis of the shown immunoblots (top) depicting the Ub-H2A contents detected in control (black) and BAP1 subjects (red). The y axis represents the fold changes of normalized Ub-H2A (Ub-H2A/α-tubulin) in densitometry measurements, which were set as 1 for subjects’ father or mother, as indicated.
(B) Native-PAGE analysis from control and affected individuals’ T cells with proteasome bands being visualized by exposing the gel to 0.1 mM of the LLVY fluorescent peptide (left) and staining the gels with a monoclonal antibody specific for the α6 subunit (right), as indicated.
(C) Top: whole-cell lysates from control and affected individuals’ T cells were separated by SDS-PAGE and analyzed by immunoblotting with antibodies directed against, RPT1, RPT2, RPT3, RPT4, RPT5, RPT5, β1, β2, β5, and β5i, as indicated. Equal loading was ensured by probing the membranes with an anti-GAPDH antibody, as indicated. Bottom: densitometry analysis of the shown immunoblots (top) depicting the pro-β5 contents detected in control (black) and affected individuals (red). The y axis represents the fold changes of normalized pro-β5 (pro-β5/GAPDH) in densitometry measurements, which were set as 1 for subjects’ father or mother, as indicated.
Because several recent studies have suggested the role of deubiquitination in the regulation of the UPS,25,26 we next sought to determine whether these BAP1 variants were associated with impaired proteasome expression and/or function. To this end, T cells from affected children carrying the p.Pro12Thr and p.Cys91Ser BAP1 variants were subjected to a non-denaturing cell lysis prior to analysis of their proteasome complexes by native-PAGE and immunoblotting. As illustrated in Figure 3B, the chymotrypsin-like activity and amounts of the 20S and 26S proteasomes did not substantially vary between control and affected children’s cells, as determined by in-gel overlay assay and α6 staining. To further ascertain the impact of BAP1 variants on proteasomes, we next compared the proteasome subunit composition between control and affected children’s T cells by SDS-PAGE and immunoblotting. Our data show that the proteasome expression profile in T cells of affected individuals was similar to that detected in control cells with no major changes in the abundance of the AAA+-ATPase subunits and most of the catalytic β-subunits (Figure 3C). Strikingly, the β5 subunit precursor, however, consistently accumulated in probands’ T cells, as determined by immunoblotting (Figure 3C). Importantly, it is highly unlikely that such pro-β5 upregulation reflects greater amounts of proteasomes in affected individuals’ T cells, as our analysis of the 20S/26S native complexes failed to show any quantitative differences between control and affected children (Figure 3B). The enrichment of the β5 precursor protein (pro-β5), which was confirmed by densitometry analysis in both probands (Figure 3C, bottom), strongly suggests that BAP1 LoF is associated with β5 processing defect. Altogether, the elevated levels of ubiquitinated H2A and pro-β5 proteasome subunit demonstrate that the identified substitutions p.Pro12Thr and p.Cys91Ser behave as LoF variants in affected individuals’ T cells impairing H2A deubiquitination and altering some features of the UPS.
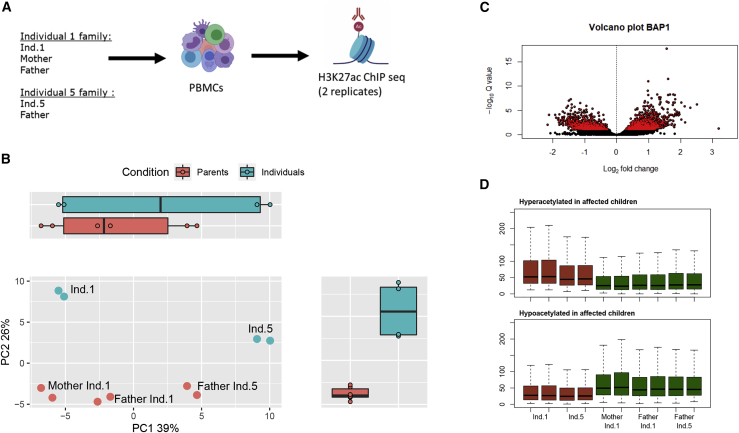
To gain additional insights into the molecular consequences of BAP1 variants, we profiled the histone acetylation (histone H3 K27 acetylation) chromatin state in the same two families of affected individuals 1 and 5. Histone acetylation marks active enhancers and promoters throughout the genome and is thus a good proxy for identifying alterations in gene regulatory mechanisms. This approach is highly sensitive, as one can quantify chromatin alterations caused by single-nucleotide variations27 and detect epigenetic mechanisms associated with complex diseases.28,29 We have previously used this approach in affected individuals harboring gene deletions of the chromatin factors SIN3B (MIM: 607777) and SIN3A (MIM: 607776) and showed that they lead to chromatin state alterations.30 Because BAP1 alters H2A-Ub in the affected individuals (Figure 3A), we decided to use this epigenomics approach to identify putative gene regulatory alterations by comparing the parents to affected individuals (Figure 4A).
Figure 4.
ChIP-seq analyses in individuals with BAP1 variants c.34C>A (p.Pro12Thr) and c.272G>C (p.Cys91Ser)
(A) Diagram of the epigenetic analysis performed, ChIP-seq H3K27ac in technical replicates on peripheral blood mononuclear cells (PBMCs) of affected individuals compared to healthy members of their family.
(B) PCA (principal-component analysis) graph of the 500 acetylated peaks most variable between all control individuals compared to affected individuals. Peak heights were estimated and transformed with regularized log transform (rlog) of the normalized counts.
(C) Volcano plot of differential peaks between affected individuals and their family. Significantly differentially acetylated peaks (FDR < 0.1) are shown in red.
(D) Boxplots of hyperacetylated and hypoacetylated peaks in normalized counts of affected individuals (red) and their family members (green).
Using peripheral blood mononuclear cells from affected individuals, we performed H3K27ac ChIP-seq in technical replicates and sequenced the ChIP libraries to a median of 45M reads. After quality controls and peak calling, we defined a common peak set in all individuals and quantified peak heights (as a function of normalized read counts in peaks) for each individual sample separately (supplemental methods). Principal-component analysis of histone acetylation peak heights in the two families reveals that variance associated to principal component (PC) 1 separates the two families (39% variance explained; Figure 4B). Interestingly, PC2 separated the affected individuals from their parents (26% variance explained), suggesting that they may bear distinct histone acetylation states. To investigate this further, we performed a differential peak height analysis and found 1,492 downregulated and 1,190 upregulated peaks when comparing the affected individuals 1 and 5 to their parents (Figure 4C).
We also evaluated whether these differential acetylated peaks (DAPs) were shared between the two affected individuals. For this, we compared the heights of up- and downregulated peaks in the individuals and their parents (Figure 4D). Peaks were similarly up- and downregulated in the replicates of the individuals, indicating that the chromatin state alterations are shared between the individuals carrying BAP1 variants p.Pro12Thr and p.Cys91Ser.
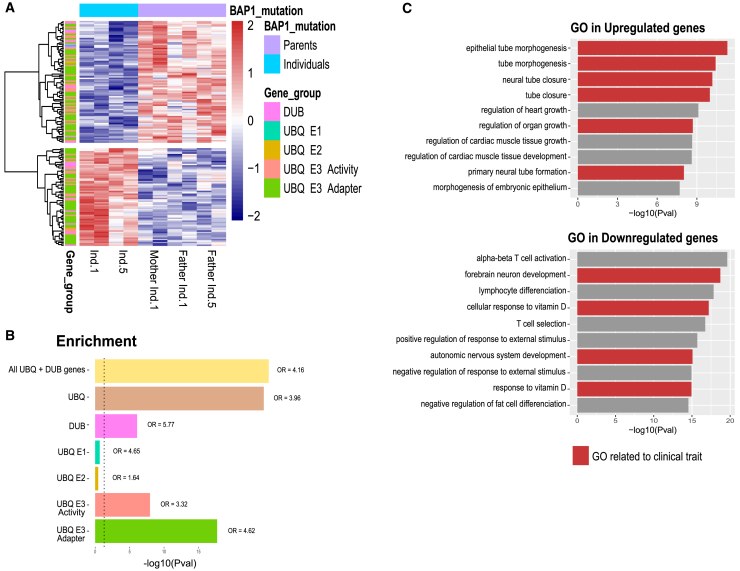
We next investigated whether these BAP1 variants affect the gene regulatory mechanisms of the ubiquitin proteasome pathway by testing whether DAPs are enriched for ubiqutin/proteasome genes. For this, we first assigned enhancers to nearest genes by using the GREAT analysis tool31 and then employed a literature-curated list of ubiquitin-related and deubiquitinase genes covering 665 genes within our dataset.32 Interestingly, we found a 4-fold enrichment of ubiquitin-related genes (Fisher’s exact test p value 1.5E−25, odds ratio [OR] = 4.16) in DAP among all genes (Table S2). The DAPs associated to the ubiquitin-related genes are shown in Figure 5A. We found a specific enrichment for DUBs as well as ubiquitin E3 genes and adapters (Figure 5B). Nevertheless, the changes are consistent between the affected individuals, suggesting that BAP1 variants might induce compensatory gene regulatory alterations of the ubiquitin-proteasome pathway, as revealed here by changes in chromatin state of enhancers. To gain additional insights on the gene regulatory alterations, we performed Gene Ontology (GO) enrichment analysis by comparing DAP to all acetylation peaks by using GREAT (Figure 5C). Interestingly, the enriched GO terms matched with the observed phenotypes of affected individuals (malformations, developmental and behavioral anomalies, and short stature) (Figure 5C). However, we remain very cautious about such inferences because they are merely hypotheses derived from associations of epigenetic traits in blood cells of affected individuals, their GO, and the derived postulations on their association with clinical traits in these individuals.
Figure 5.
Gene Ontology enrichment analysis of ChIP-seq data related to variants c.34C>A (p.Pro12Thr) and c.272G>C (p.Cys91Ser)
(A) Heatmap of a selected set of peaks related to the ubiquitin-proteasome system with rlog transformed counts.
(B) Enrichment analysis of each subgroup performed with Fisher’s exact test (see also Table S2).
(C) Gene Ontologies (GOs) of down- and upregulated peaks in affected individuals performed with the GREAT tool. Relevant GOs that are conceivably associated to the clinical traits observed in affected individuals are shown in red.
In this work, we provide evidence that BAP1 p.Pro12Thr and p.Cys91Ser are LoF variants that prevent BAP1 from removing ubiquitin from H2A (Figure 3A). The failure to deubiquitinate H2A was accompanied by a rise of the pro-β5 proteasome catalytic subunit in affected individuals’ T cells, as determined by immunoblotting (Figure 3C). This observation is intriguing considering the fact that the abundance of the other catalytic β-subunits (i.e., β1, β2, β5i) remains unchanged between affected individuals’ and controls’ T cells (Figure 3C) and suggests the existence of a specific link between BAP1 and β5. A similar relationship has been recently reported in a study showing that BAP1 depletion in tumor cells leads to decreased sensitivity to bortezomib,33 a proteasome inhibitor that specifically binds to the β5 and β5i subunits.34 Here, the upregulation of pro-β5 detected in subjects with BAP1 LoF variants was not associated with a modulation of the β5-mediated chymotrypsin-like activity (Figure 3B). This apparent discrepancy could be explained by T cells’ expression of large amounts of immunoproteasomes containing β5i (Figure 3C), which are likely to mask the contribution of standard proteasomes carrying β5 to the chymotrypsin-like activity measured in these cells. Together with OTUD6B and OTUD7A,25,26 BAP1 is one of the very few ubiquitin hydrolases able to regulate proteasome function. The cellular mechanisms by which BAP1 influences proteasome subunit expression however remain unclear and warrant further investigations.
The impairment of BAP1 catalytic properties by LoF variants primarily found in the catalytic domain is likely to negatively impact the function of all BAP1 complexes. The increased levels of Ub-H2A in T cells of affected individuals particularly reflect the dysfunction of PR-DUB complexes in which BAP1 is bound to ASXL proteins. Interestingly, pathogenic variants in all three ASXL genes result in syndromic ID disorders: Bohring-Opitz (MIM: 605039), Shashi-Pena (MIM: 617190), and Bainbridge-Ropers (MIM: 615485) syndromes are caused by ASXL1 (MIM: 612990), ASXL2 (MIM: 612991), and ASXL3 (MIM: 615115) variants, respectively.35 Incidentally, it was the elevated Ub-H2A levels observed in fibroblasts from individuals harboring ASXL3 variants that allowed the identification of the BAP1/ASXL3 PR-DUB complex.12 Further investigations would be required to determine how germline variants of the same gene can cause either a neurodevelopmental or tumor syndrome. Although all of the variants have an LoF effect, we noted that, in this short series, all variants are missense, whereas the anomalies that predispose to the tumor syndrome are splice or truncating variants or gene deletions. It is worth noting that several of these missense variants occur at known active site residues, suggesting that these LoF missense variants may disrupt or eliminate deubiquitinase activity, while maintaining protein-protein interactions with and binding to complexes (e.g., interactions with BARD1 or BRCA1), resulting in different cellular effects compared to nonsense and other severely disruptive variants. Despite the absence of tumors in our series, we cannot exclude the possibility that individuals with NDD are also predisposed to cancer, given that the older subject is 16 years old.
Many genes are known to be involved in cancer through recurrent somatic mutations and developmental disorders through germline variants.36 Fewer cases are known where different germline variants of the same gene can cause either a neurodevelopmental disorder or a tumor predisposition syndrome. The best examples certainly come from the SWI/SNF-related matrix-associated actin-dependent regulator of chromatin (SMARC) family, whose members SMARCA4 (MIM: 603254) and SMARCB1 (MIM: 601607) are involved in Coffin-Siris syndromes (MIM: 614609 and 614608, respectively) and rhabdoid tumor predisposition syndromes (MIM: 613325 and 609322, respectively), while SMARCE1 (MIM: 603111) can cause either susceptibility to familial meningioma or neurodevelopmental delay (MIM: 616938 and 607174). Interestingly, these three genes contribute to chromatin remodeling, like BAP1, and their protein products can bind BRCA1. Moreover, it was shown that nonsense SMARCA4 variants might induce concomitantly developmental and cancerous symptoms.37
In conclusion, we describe a neurodevelopmental disorder caused by rare de novo germline heterozygous missense variants of BAP1, most located in the region encoding the catalytic UCH domain. These variants affect the deubiquitinase activity of the BAP1 complexes and disrupt chromatin remodeling by inducing abnormally high levels of ubiquitinated H2A. They are also associated with perturbations of proteasome assembly by increasing the production of the pro-β5 proteasome catalytic subunit. To our knowledge, BAP1 is one of the rare genes in which different germline variants cause either an NDD or a tumor predisposition syndrome.
Consortia
The members of the Undiagnosed Diseases Network are Mercedes E. Alejandro, Mahshid S. Azamian, Carlos A. Bacino, Ashok Balasubramanyam, Lindsay C. Burrage, Hsiao-Tuan Chao, Gary D. Clark, William J. Craigen, Hongzheng Dai, Shweta U. Dhar, Lisa T. Emrick, Alica M. Goldman, Neil A. Hanchard, Fariha Jamal, Lefkothea Karaviti, Seema R. Lalani, Brendan H. Lee, Richard A. Lewis, Ronit Marom, Paolo M. Moretti, David R. Murdock, Sarah K. Nicholas, James P. Orengo, Jennifer E. Posey, Lorraine Potocki, Jill A. Rosenfeld, Susan L. Samson, Daryl A. Scott, Alyssa A. Tran, Tiphanie P. Vogel, Michael F. Wangler, Shinya Yamamoto, Hugo J. Bellen, Christine M. Eng, Pengfei Liu, Patricia A. Ward, Edward Behrens, Matthew Deardorff, Marni Falk, Kelly Hassey, Kathleen Sullivan, Adeline Vanderver, David B. Goldstein, Heidi Cope, Allyn McConkie-Rosell, Kelly Schoch, Vandana Shashi, Edward C. Smith, Rebecca C. Spillmann, Jennifer A. Sullivan, Queenie K.-G. Tan, Nicole M. Walley, Pankaj B. Agrawal, Alan H. Beggs, Gerard T. Berry, Lauren C. Briere, Laurel A. Cobban, Matthew Coggins, Cynthia M. Cooper, Elizabeth L. Fieg, Frances High, Ingrid A. Holm, Susan Korrick, Joel B. Krier, Sharyn A. Lincoln, Joseph Loscalzo, Richard L. Maas, Calum A. MacRae, J. Carl Pallais, Deepak A. Rao, Lance H. Rodan, Edwin K. Silverman, Joan M. Stoler, David A. Sweetser, Melissa Walker, Chris A. Walsh, Cecilia Esteves, Isaac S. Kohane, Athena Hantzaridis, Kimberly LeBlanc, Alexa T. McCray, Anna Nagy, Shilpa N. Kobren, Amelia L.M. Tan, Surendra Dasari, Brendan C. Lanpher, Ian R. Lanza, Eva Morava, Devin Oglesbee, Guney Bademci, Deborah Barbouth, Stephanie Bivona, Olveen Carrasquillo, Ta Chen Peter Chang, Irman Forghani, Alana Grajewski, Rosario Isasi, Byron Lam, Roy Levitt, Xue Zhong Liu, Jacob McCauley, Ralph Sacco, Mario Saporta, Judy Schaechter, Mustafa Tekin, Fred Telischi, Willa Thorson, Stephan Zuchner, Heather A. Colley, Jyoti G. Dayal, David J. Eckstein, Laurie C. Findley, Donna M. Krasnewich, Laura A. Mamounas, Teri A. Manolio, John J. Mulvihill, Grace L. LaMoure, Madison P. Goldrich, Tiina K. Urv, Argenia L. Doss, Maria T. Acosta, Carsten Bonnenmann, Precilla D’Souza, David D. Draper, Carlos Ferreira, Rena A. Godfrey, Catherine A. Groden, Ellen F. Macnamara, Valerie V. Maduro, Thomas C. Markello, Avi Nath, Donna Novacic, Barbara N. Pusey, Camilo Toro, Colleen E. Wahl, Eva Baker, Elizabeth A. Burke, David R. Adams, William A. Gahl, May Christine V. Malicdan, Cynthia J. Tifft, Lynne A. Wolfe, John Yang, Bradley Power, Bernadette Gochuico, Laryssa Huryn, Lea Latham, Joie Davis, Deborah Mosbrook-Davis, Francis Rossignol, Ben Solomon, John MacDowall, Audrey Thurm, Wadih Zein, Muhammad Yousef, Margaret Adam, Laura Amendola, Michael Bamshad, Anita Beck, Jimmy Bennett, Beverly Berg-Rood, Elizabeth Blue, Brenna Boyd, Peter Byers, Sirisak Chanprasert, Michael Cunningham, Katrina Dipple, Daniel Doherty, Dawn Earl, Ian Glass, Katie Golden-Grant, Sihoun Hahn, Anne Hing, Fuki M. Hisama, Martha Horike-Pyne, Gail P. Jarvik, Jeffrey Jarvik, Suman Jayadev, Christina Lam, Kenneth Maravilla, Heather Mefford, J. Lawrence Merritt, Ghayda Mirzaa, Deborah Nickerson, Wendy Raskind, Natalie Rosenwasser, C. Ron Scott, Angela Sun, Virginia Sybert, Stephanie Wallace, Mark Wener, Tara Wenger, Euan A. Ashley, Gill Bejerano, Jonathan A. Bernstein, Devon Bonner, Terra R. Coakley, Liliana Fernandez, Paul G. Fisher, Jason Hom, Yong Huang, Jennefer N. Kohler, Elijah Kravets, Beth A. Martin, Shruti Marwaha, Archana N. Raja, Chloe M. Reuter, Maura Ruzhnikov, Jacinda B. Sampson, Kevin S. Smith, Shirley Sutton, Holly K. Tabor, Brianna M. Tucker, Matthew T. Wheeler, Diane B. Zastrow, Chunli Zhao, William E. Byrd, Andrew B. Crouse, Matthew Might, Mariko Nakano-Okuno, Jordan Whitlock, Gabrielle Brown, Manish J. Butte, Esteban C. Dell’Angelica, Naghmeh Dorrani, Emilie D. Douine, Brent L. Fogel, Irma Gutierrez, Alden Huang, Deborah Krakow, Hane Lee, Sandra K. Loo, Bryan C. Mak, Martin G. Martin, Julian A. Martínez-Agosto, Elisabeth McGee, Stanley F. Nelson, Shirley Nieves-Rodriguez, Christina G.S. Palmer, Jeanette C. Papp, Neil H. Parker, Genecee Renteria, Rebecca H. Signer, Janet S. Sinsheimer, Jijun Wan, Lee-kai Wang, Katherine Wesseling Perry, Jeremy D. Woods, Justin Alvey, Ashley Andrews, Jim Bale, John Bohnsack, Lorenzo Botto, John Carey, Laura Pace, Nicola Longo, Gabor Marth, Paolo Moretti, Aaron Quinlan, Matt Velinder, Dave Viskochil, Pinar Bayrak-Toydemir, Rong Mao, Monte Westerfield, Anna Bican, Elly Brokamp, Laura Duncan, Rizwan Hamid, Jennifer Kennedy, Mary Kozuira, John H. Newman, John A. Phillips III, Lynette Rives, Amy K. Robertson, Emily Solem, Joy D. Cogan, F. Sessions Cole, Nichole Hayes, Dana Kiley, Kathy Sisco, Jennifer Wambach, Daniel Wegner, Dustin Baldridge, Stephen Pak, Timothy Schedl, Jimann Shin, and Lilianna Solnica-Krezel (see Table S3).
Acknowledgments
We would like to thank all the families for participating in this study. We acknowledge HUGODIMS (Western France exome-based trio approach project to identify genes involved in intellectual disability); funding for HUGODIMS (individual 5) is supported by a grant from the French Ministry of Health and from the Health Regional Agency from Poitou-Charentes (HUGODIMS, 2013, RC14_0107). We thank Frédérique Allaire from the Health Regional Agency of Poitou-Charentes for supporting this project. We thank Léa Ferrand and Emilie Le Blanc for grant and data management. We are most grateful to the GenoBiRD core facility in Nantes (Biogenouest Genomics) for its technical support. Research reported in this manuscript was supported by the NIH Common Fund through the Office of Strategic Coordination/Office of the NIH Director under award number U01HG007672 to V.S. Further support was obtained by funding from the German Research Foundation (SFBTR 167 A4, GRK2719 B4) to E.K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of interests
The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing completed at Baylor Genetics Laboratory. K.Mc., R.E.S., and I.M.W. are employees of GeneDx, Inc.
Published: January 19, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2021.12.011.
Contributor Information
Sébastien Küry, Email: sebastien.kury@chu-nantes.fr.
Bertrand Isidor, Email: bertrand.isidor@chu-nantes.fr.
Data and code availability
The sequence variants in BAP1 (GenBank: NM_004656.3) reported in the paper have been deposited in ClinVar database. Their respective accession numbers are reported in Table 1. ChIP-seq raw data are deposited and available at GEO (GEO: GSE190394). Most exome sequencing data were generated in a diagnostic setting and can therefore not be shared for privacy. Only exome raw data related to individual 1 were obtained in a research setting and are available upon request.
Web resources
GeneMatcher, https://genematcher.org/
MobiDetails, https://mobidetails.iurc.montp.inserm.fr/MD/
MobiDetails variant 22880, https://mobidetails.iurc.montp.inserm.fr/MD/variant/22880
Missense Tolerance Ratio (MTR) Gene Viewer, http://mtr-viewer.mdhs.unimelb.edu.au/
OMIM, https://www.omim.org/
Supplemental information
References
- 1.Chiti F., Dobson C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 2.Franić D., Zubčić K., Boban M. Nuclear Ubiquitin-Proteasome Pathways in Proteostasis Maintenance. Biomolecules. 2021;11:54. doi: 10.3390/biom11010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samant R.S., Livingston C.M., Sontag E.M., Frydman J. Distinct proteostasis circuits cooperate in nuclear and cytoplasmic protein quality control. Nature. 2018;563:407–411. doi: 10.1038/s41586-018-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickart C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 5.Pickart C.M., Eddins M.J. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Meyer-Schwesinger C. The ubiquitin-proteasome system in kidney physiology and disease. Nat. Rev. Nephrol. 2019;15:393–411. doi: 10.1038/s41581-019-0148-1. [DOI] [PubMed] [Google Scholar]
- 7.Szczepanski A.P., Wang L. Emerging multifaceted roles of BAP1 complexes in biological processes. Cell Death Discov. 2021;7:20. doi: 10.1038/s41420-021-00406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masclef L., Ahmed O., Estavoyer B., Larrivée B., Labrecque N., Nijnik A., Affar E.B. Roles and mechanisms of BAP1 deubiquitinase in tumor suppression. Cell Death Differ. 2021;28:606–625. doi: 10.1038/s41418-020-00709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen D.E., Rauscher F.J., 3rd Defining biochemical functions for the BRCA1 tumor suppressor protein: analysis of the BRCA1 binding protein BAP1. Cancer Lett. 1999;143(Suppl 1):S13–S17. doi: 10.1016/s0304-3835(99)90004-6. [DOI] [PubMed] [Google Scholar]
- 10.Louie B.H., Kurzrock R. BAP1: Not just a BRCA1-associated protein. Cancer Treat. Rev. 2020;90:102091. doi: 10.1016/j.ctrv.2020.102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popova T., Hebert L., Jacquemin V., Gad S., Caux-Moncoutier V., Dubois-d’Enghien C., Richaudeau B., Renaudin X., Sellers J., Nicolas A., et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am. J. Hum. Genet. 2013;92:974–980. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava A., Ritesh K.C., Tsan Y.C., Liao R., Su F., Cao X., Hannibal M.C., Keegan C.E., Chinnaiyan A.M., Martin D.M., Bielas S.L. De novo dominant ASXL3 mutations alter H2A deubiquitination and transcription in Bainbridge-Ropers syndrome. Hum. Mol. Genet. 2016;25:597–608. doi: 10.1093/hmg/ddv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campagne A., Lee M.K., Zielinski D., Michaud A., Le Corre S., Dingli F., Chen H., Shahidian L.Z., Vassilev I., Servant N., et al. BAP1 complex promotes transcription by opposing PRC1-mediated H2A ubiquitylation. Nat. Commun. 2019;10:348. doi: 10.1038/s41467-018-08255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheuermann J.C., de Ayala Alonso A.G., Oktaba K., Ly-Hartig N., McGinty R.K., Fraterman S., Wilm M., Muir T.W., Müller J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bononi A., Giorgi C., Patergnani S., Larson D., Verbruggen K., Tanji M., Pellegrini L., Signorato V., Olivetto F., Pastorino S., et al. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature. 2017;546:549–553. doi: 10.1038/nature22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao W., Steinfeld J.B., Liang F., Chen X., Maranon D.G., Jian Ma C., Kwon Y., Rao T., Wang W., Sheng C., et al. BRCA1-BARD1 promotes RAD51-mediated homologous DNA pairing. Nature. 2017;550:360–365. doi: 10.1038/nature24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ladanyi M., Zauderer M.G., Krug L.M., Ito T., McMillan R., Bott M., Giancotti F. New strategies in pleural mesothelioma: BAP1 and NF2 as novel targets for therapeutic development and risk assessment. Clin. Cancer Res. 2012;18:4485–4490. doi: 10.1158/1078-0432.CCR-11-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao L., Testa J.R., Yang H. The roles of chromatin-remodelers and epigenetic modifiers in kidney cancer. Cancer Genet. 2015;208:206–214. doi: 10.1016/j.cancergen.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger S.I., Ciccone C., Simon K.L., Malicdan M.C., Vilboux T., Billington C., Fischer R., Introne W.J., Gropman A., Blancato J.K., et al. Exome analysis of Smith-Magenis-like syndrome cohort identifies de novo likely pathogenic variants. Hum. Genet. 2017;136:409–420. doi: 10.1007/s00439-017-1767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baux D., Van Goethem C., Ardouin O., Guignard T., Bergougnoux A., Koenig M., Roux A.F. MobiDetails: online DNA variants interpretation. Eur. J. Hum. Genet. 2021;29:356–360. doi: 10.1038/s41431-020-00755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traynelis J., Silk M., Wang Q., Berkovic S.F., Liu L., Ascher D.B., Balding D.J., Petrovski S. Optimizing genomic medicine in epilepsy through a gene-customized approach to missense variant interpretation. Genome Res. 2017;27:1715–1729. doi: 10.1101/gr.226589.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen D.E., Proctor M., Marquis S.T., Gardner H.P., Ha S.I., Chodosh L.A., Ishov A.M., Tommerup N., Vissing H., Sekido Y., et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 25.Santiago-Sim T., Burrage L.C., Ebstein F., Tokita M.J., Miller M., Bi W., Braxton A.A., Rosenfeld J.A., Shahrour M., Lehmann A., et al. Biallelic Variants in OTUD6B Cause an Intellectual Disability Syndrome Associated with Seizures and Dysmorphic Features. Am. J. Hum. Genet. 2017;100:676–688. doi: 10.1016/j.ajhg.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garret P., Ebstein F., Delplancq G., Dozieres-Puyravel B., Boughalem A., Auvin S., Duffourd Y., Klafack S., Zieba B.A., Mahmoudi S., et al. Report of the first patient with a homozygous OTUD7A variant responsible for epileptic encephalopathy and related proteasome dysfunction. Clin. Genet. 2020;97:567–575. doi: 10.1111/cge.13709. [DOI] [PubMed] [Google Scholar]
- 27.del Rosario R.C., Poschmann J., Rouam S.L., Png E., Khor C.C., Hibberd M.L., Prabhakar S. Sensitive detection of chromatin-altering polymorphisms reveals autoimmune disease mechanisms. Nat. Methods. 2015;12:458–464. doi: 10.1038/nmeth.3326. [DOI] [PubMed] [Google Scholar]
- 28.Sun W., Poschmann J., Cruz-Herrera Del Rosario R., Parikshak N.N., Hajan H.S., Kumar V., Ramasamy R., Belgard T.G., Elanggovan B., Wong C.C.Y., et al. Histone Acetylome-wide Association Study of Autism Spectrum Disorder. Cell. 2016;167:1385–1397.e11. doi: 10.1016/j.cell.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Marzi S.J., Leung S.K., Ribarska T., Hannon E., Smith A.R., Pishva E., Poschmann J., Moore K., Troakes C., Al-Sarraj S., et al. A histone acetylome-wide association study of Alzheimer’s disease identifies disease-associated H3K27ac differences in the entorhinal cortex. Nat. Neurosci. 2018;21:1618–1627. doi: 10.1038/s41593-018-0253-7. [DOI] [PubMed] [Google Scholar]
- 30.Latypova X., Vincent M., Mollé A., Adebambo O.A., Fourgeux C., Khan T.N., Caro A., Rosello M., Orellana C., Niyazov D., et al. Haploinsufficiency of the Sin3/HDAC corepressor complex member SIN3B causes a syndromic intellectual disability/autism spectrum disorder. Am. J. Hum. Genet. 2021;108:929–941. doi: 10.1016/j.ajhg.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLean C.Y., Bristor D., Hiller M., Clarke S.L., Schaar B.T., Lowe C.B., Wenger A.M., Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge Z., Leighton J.S., Wang Y., Peng X., Chen Z., Chen H., Sun Y., Yao F., Li J., Zhang H., et al. Integrated Genomic Analysis of the Ubiquitin Pathway across Cancer Types. Cell Rep. 2018;23:213–226.e3. doi: 10.1016/j.celrep.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirosawa T., Ishida M., Ishii K., Kanehara K., Kudo K., Ohnuma S., Kamei T., Motoi F., Naitoh T., Selaru F.M., Unno M. Loss of BAP1 expression is associated with genetic mutation and can predict outcomes in gallbladder cancer. PLoS ONE. 2018;13:e0206643. doi: 10.1371/journal.pone.0206643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Škrott Z., Cvek B. Linking the activity of bortezomib in multiple myeloma and autoimmune diseases. Crit. Rev. Oncol. Hematol. 2014;92:61–70. doi: 10.1016/j.critrevonc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Shashi V., Pena L.D.M., Kim K., Burton B., Hempel M., Schoch K., Walkiewicz M., McLaughlin H.M., Cho M., Stong N., et al. De Novo Truncating Variants in ASXL2 Are Associated with a Unique and Recognizable Clinical Phenotype. Am. J. Hum. Genet. 2016;99:991–999. doi: 10.1016/j.ajhg.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi H., Dong C., Chung W.K., Wang K., Shen Y. Deep Genetic Connection Between Cancer and Developmental Disorders. Hum. Mutat. 2016;37:1042–1050. doi: 10.1002/humu.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Errichiello E., Mustafa N., Vetro A., Notarangelo L.D., de Jonge H., Rinaldi B., Vergani D., Giglio S.R., Morbini P., Zuffardi O. SMARCA4 inactivating mutations cause concomitant Coffin-Siris syndrome, microphthalmia and small-cell carcinoma of the ovary hypercalcaemic type. J. Pathol. 2017;243:9–15. doi: 10.1002/path.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence variants in BAP1 (GenBank: NM_004656.3) reported in the paper have been deposited in ClinVar database. Their respective accession numbers are reported in Table 1. ChIP-seq raw data are deposited and available at GEO (GEO: GSE190394). Most exome sequencing data were generated in a diagnostic setting and can therefore not be shared for privacy. Only exome raw data related to individual 1 were obtained in a research setting and are available upon request.