Abstract
Introduction
Several endoscopic methods can be employed to manage post-bariatric leaks. However, endoluminal vacuum therapy (EVT) and endoscopic internal drainage (EID) are relatively new methods, and studies regarding these methods are scarce. We performed a systematic review of the literature and a meta-analysis to evaluate the efficacy of EVT and EID.
Methods
Databases were searched for eligible studies. The clinical success of leak closure was the primary outcome of interest. A proportional meta-analysis was performed for pooling the primary outcome using a fixed-effects model. A meta-analysis or descriptive analysis of other outcomes was performed based on the data availability.
Results
Data from 3 EVT and 10 EID studies (n = 279) were used for evidence synthesis. The leak closure rates (95% confidence interval [CI]) of EVT and EID were 85.2% (75.1%–95.4%) and 91.6% (88.1%–95.2%), respectively. The corresponding mean treatment durations (95% CI) were 28 (2.4–53.6) and 78.4 (50.1–106.7) days, respectively. However, data about other outcomes were extremely limited; thus, a pooled analysis could not be performed.
Conclusions
Both EVT and EID were effective when used as the first-line treatment for post-bariatric leaks. However, larger studies must be conducted to compare the efficacy of the 2 interventions.
Keywords: Post-bariatric leak, Endoluminal vacuum therapy, Endoscopic internal drainage
Introduction
Bariatric surgery is effective against obesity and metabolic syndrome. However, previous studies have reported several life-threatening complications associated with this procedure. That is, approximately 1.1% and 0.6% of patients who underwent sleeve gastrectomy (SG) [1] and Roux-en-Y gastric bypass (RYGB) [2] experienced staple line and anastomotic leakage, respectively. If leakage occurs, infection control must be prioritized, which can be achieved with prompt surgical or percutaneous drainage. The mortality rate of individuals with post-bariatric leaks is approximately 0.8%–9% [2, 3].
To date, advanced therapeutic endoscopy plays an essential role in post-bariatric leak management. Among available interventions, self-expandable metallic stent (SEMS) placement has been most commonly examined. Meta-analyses have shown that the leak closure rates of SEMS placement are 82%–93% [4, 5]. However, approximately 15%–32% of patients experience stent migration [4, 5], and the use of >1 stent, which is expensive, might be required during treatment.
Recently, endoluminal vacuum therapy (EVT) and endoscopic internal drainage (EID) have gained popularity. Two meta-analyses have shown that EVT resulted in 16%–21% increase of the orifice closure rate compared with SEMS in nonbariatric anastomotic leakage and transmural defect [6, 7]. After EID was introduced in 2012, it became the first-line management method for post-bariatric leaks at some centers [8, 9, 10, 11]. Successful fistula closure (90%) following EID was also reported outside the bariatric field [12]. However, relatively few studies have assessed EVT and EID. Most of those studies assessed the treatment effect in a small number of patients. Because of the limited evidence, we systematically reviewed publications that used both procedures as the first-line modality for post-bariatric leakage. Nevertheless, pooling of the treatment effect of EVT versus EID could not be performed owing to the lack of original studies comparing both techniques. Thus, the effects of each treatment were meta-analyzed separately.
Methods
This study is registered at PROSPERO (CRD42021236024) and is reported in accordance with the PRISMA [13], MOOSE [14], and AMSTAR-2 [15] guidelines.
Search Strategies and Study Selection
The following search terms were used: “bariatric surgery,” “sleeve gastrectomy,” “Roux-en-Y gastric bypass,” “leak,” “fistula,” “endoluminal vacuum therapy,” “endoscopic internal drainage,” and “septotomy.” See online suppl. Table 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000518946) for more details. Septotomy was included as a search term because it involves drainage of leaks, similar to EVT and EID, which are the interventions of interest in the current study. There was no language restriction when searching the MEDLINE (via PubMed), Scopus, and Embase databases to identify relevant studies from inception to February 8, 2021. The cited literature of the included studies was assessed for additional articles.
Studies that reported the clinical success rate of post-bariatric leak/fistula treatment from the interventions of interest were eligible. However, studies that reported interventions that are not the first-line endoscopic treatment were excluded from this study. Case series, nonrandomized comparative studies, and randomized clinical trials were reviewed. However, small case series (<5 patients treated with the intervention of interest) and case reports were not considered. Studies were selected for further analysis by 2 independent reviewers (I.L. and W.K.). Any disagreement was resolved by a third reviewer (A.T.).
Data Extraction and Quality Assessment
The following data were extracted from the eligible articles: interventions, patients' mean (or median) age and BMI, percentage of male patients, comorbidities, time from index bariatric surgery to initial endoscopic treatment, follow-up duration, and co-intervention (i.e., surgical, radiological, or endoscopic procedures). For further analysis, information about the total number of patients, clinical success rate, mean (or median) treatment duration and endoscopy sessions, duration of hospital stay, complications, and mortality was extracted. Cross-tabulated data of interventions and outcomes were collected from comparative studies (i.e., randomized and nonrandomized studies). However, none of the comparative studies were eligible after a full-text screening. Equivocal data were clarified by contacting the author.
The quality of the included studies was assessed using the National Institutes of Health tool for case series if the full-text article was available. This tool can be used to rate the quality of studies as follows: good, fair, and poor. Both processes were independently performed by 2 reviewers (I.L. and T.A.). Any disagreement was resolved by a third reviewer (A.T.).
Statistical Analysis
A random-effects model was used to pool data on clinical success rates, mean treatment duration, and mean endoscopy sessions across studies if heterogeneity was observed (i.e., I2 > 25 or Cochrane's Q test, p < 0.1). Otherwise, a fixed-effects model was used. In case data were presented as the median, the value was transformed to mean using Wan's method before pooling [16]. A meta-regression was considered for the assessment of heterogeneity. However, it was not performed due to the lack of covariate data. As no comparative study was included, publication bias assessment was not required. Sensitivity analysis was conducted excluding studies with poor quality. All statistical analyses were performed using STATA 16. Other outcomes with limited data that were not appropriate for pooling were reported descriptively.
Results
Characteristics and Quality of the Included Studies
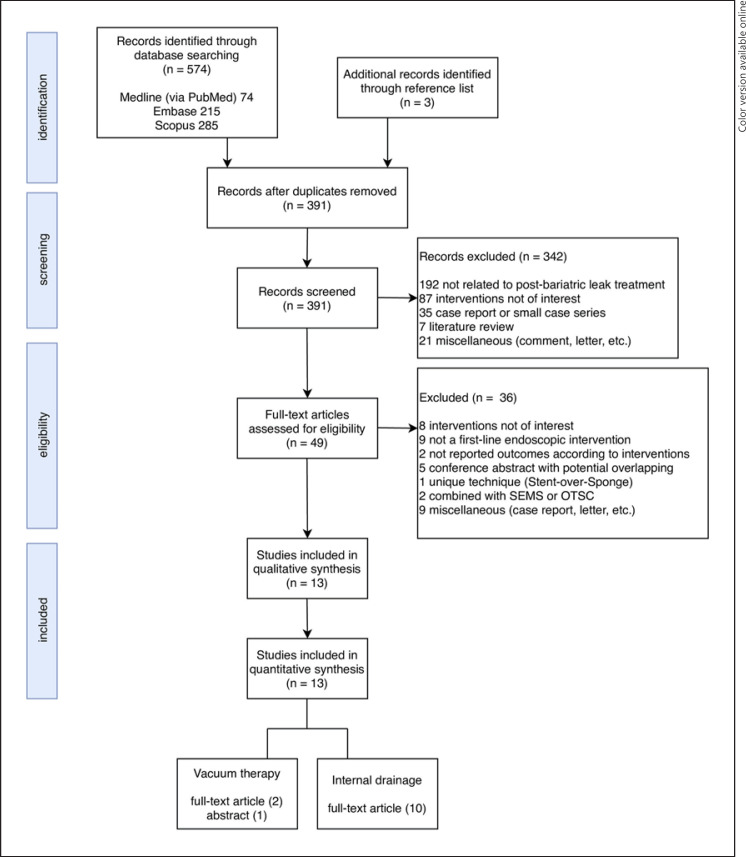
Of 577 articles, 13 case series [9, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28] (n = 279) were eligible (Fig. 1). One study [22] was only presented as an abstract, without the full text, at a conference. At least 230 and 35 patients who underwent SG and RYGB, respectively, were analyzed. Among studies that reported patients' characteristics, age, BMI, and percentage of men ranged from 32.4 to 46.6 years, 33–53 kg/m2, and 7.1%–60.6%, respectively (Table 1). Most of the leaks following SG occurred at the proximal staple line. Unfortunately, most studies did not report the type of leak (i.e., acute, early, late, and chronic) at the time of presentation and radiologic findings. Seven studies included the duration from the index bariatric procedure to the initial endoscopic treatment, which ranged from 4 to 60.5 days [17, 19, 20, 25, 26, 27, 28]. However, only few studies included information regarding the follow-up duration (57 days to 1 year) [17, 19, 20, 24, 25]. To prevent infection, pre-endoscopic surgical or percutaneous drains were used according to each center's treatment protocol. The percentage of drain placement varied across studies, of which one routinely used pre-endoscopic drainage [25] (Table 1).
Fig. 1.
Study selection diagram.
Table 1.
Study characteristics
| Study | Age, years (mean ± SD, range) | Male sex, % |
BMI, kg/m2 (mean ± SD, range) | Bariatric surgery (n) | Major leak site (n) | Intervention | Time to endoscopic treatment, days (mean ± SD, range) | Follow-up time, days (mean, range) | Additional surgery* or interventional radiology (%) | Additional endoscopic treatment (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Christogianni et al. [22] | n/a | n/a | n/a | SG (21) | Prox SL (21) | EVT | n/a | n/a | Surgery | − |
| Mencio et al. [23] | 46.6 | n/a | 33 | SG (17), RYGB (1) | SL (17) | EVT | n/a | n/a | −− | −− |
| Archid et al. [26] | 40.2±15.6 | 12.5 | 53±11.3 | SG (8) | Prox SL (5) | EVT | 32.3±20 | n/a | Surgery (37.5), IR (12.5) | Botox injection (50) |
| Donatelli et al. [17] | 43 (23–70) | 14.9 | n/a | SG (64) | Prox SL (56) | EID | 60.5 (4–1460) | 316 (20–600) | Surgery (62.7) | −− |
| Nedelcu et al. [18] | 32.4 (21–45) | 33 | 46 (35–62) | SG (9) | Prox SL (9) | EID | n/a | n/a | −− | SEMS for sleeve stenosis (33) |
| Bouchard et al. [19] | 42±11.2 | 21.2 | 41.2±7.1 | SG, RYGB (unknown) | n/a | EID | 14 (5–49) | Median 358 (111–1712) | Surgery (28.6), IR (12) | − |
| Rebibo et al. [9] | 39.7±11 | 9.3 | 45.2±7.5 | SG (47) | Prox SL (46) | EID | n/a | n/a | Surgery | −− |
| Donatelli et al. [20] | 43 (20–65) | 60.6 | n/a | RYGB (33) | Cardia (30) | EID | 10 (4–35) | 300 (30–990) | Surgery (60) | −− |
| Talbot et al. [21] | n/a | n/a | n/a | SG (7) | n/a | EID | n/a | n/a | −− | Balloon dilation (28.6) |
| Dammaro et al. [25] | Median 38 (28–69) |
7.1 | Median 40.4 (34.9–44.2) | SG (14) | Prox SL (14) | EID | Median 4 (0–17) | 1 year | Surgery (100) | SEMS for sleeve stenosis (21.4) |
| Sportes et al. [24] | 41.3 (18–60) | 24.2 | 46.2 (35–70) | SG (33) | n/a | EID | n/a | 57 (13–450) | Surgery | −− |
| Lazzarin et al. [27] | 28–57 | 20 | n/a | SG (5) | n/a | EID | 4–13 | n/a | −− | − |
| Fuentes–Valenzuela et al. [28] | 49.5 | 16.7 | n/a | SG (5), RYGB (1) | Prox SL (5) | EID | Median 14 (IQR 7–34) | n/a | − | − |
BMI, body mass index; EG, esophagogastric; EID, endoscopic internal drainage; EVT, endoluminal vacuum therapy; IQR, interquartile range; IR, interventional radiology; n/a, not available; Prox SL, proximal staple line; RYGB, Roux-en-Y gastric bypass; SD, standard deviation; SEMS, self-expandable metallic stent; SG, sleeve gastrectomy.
Not included definite surgery such as total gastrectomy and conversion from SG to RYGB.
To comply with the review question, we did not consider studies that included cases in which other endoscopic modalities for leak closure were unsuccessful. For example, studies of patients who underwent unsuccessful SEMS placement were excluded [11, 29, 30]. However, this review included studies that involved some adjunct endoscopic management procedures, such as SEMS placement, balloon dilation, and botulinum toxin injection, which aim to correct sleeve stenosis or pyloric spasm. Four studies reported the additional use of endoscopic procedures (21.4%–50%) [18, 21, 25, 26]. There were 7 studies about septotomy [31, 32, 33, 34, 35, 36, 37]. However, most reports involved late or chronic leak, thereby making the first-line endoscopic management equivocal. Thus, these studies were not included in the full-text assessment phase. The quality of the included studies, which was assessed only if the full text was available, was rated as good (2), fair (9), and poor (1) (online suppl. Table 2).
Endoscopic Techniques
In EVT studies, the EVT systems were delivered via the orifice into the leak cavity (intracavitary). However, intraluminal vacuum (a system placed inside the stomach lumen instead of the leak cavity) was applied in one study [26]. The vacuum systems used were either commercially available systems or homemade versions. Homemade systems could be constructed using foam or open-pore film drainage wrapped around the catheter. The entire vacuum system was delivered under endoscopic guidance or using a guide wire. In 2 EVT studies [23, 26], the vacuum system was changed every 5–7 days. With the use of EVT, continuous collection drainage could be provided, and granulation tissue growth could be promoted.
EID involved bridging the orifice with double-pigtail plastic stents with or without a nasocystic catheter. Multiple stents could be inserted simultaneously. The rationale of bridging with the plastic stent is to drain the fluid collection, prevent further leaks by occluding the orifice, and induce fistula re-epithelialization. In one study, follow-up endoscopy was performed at 4-week intervals until leak closure was validated [25], whereas pigtail stents were changed every 3 weeks in another study [24]. In other studies [9, 18], follow-up endoscopy was performed 6–8 weeks after double-pigtail stent insertion.
Clinical Success
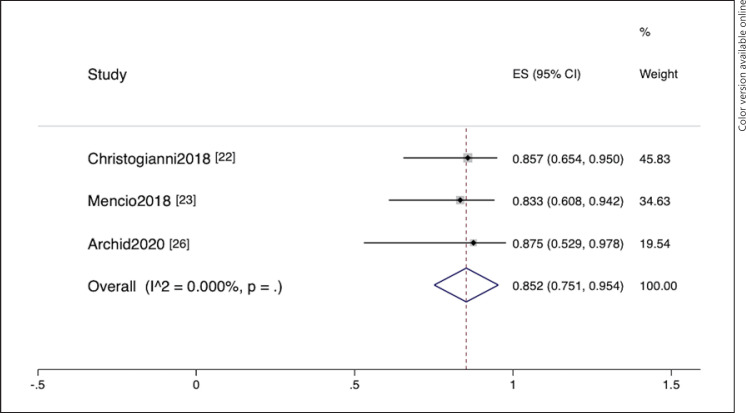
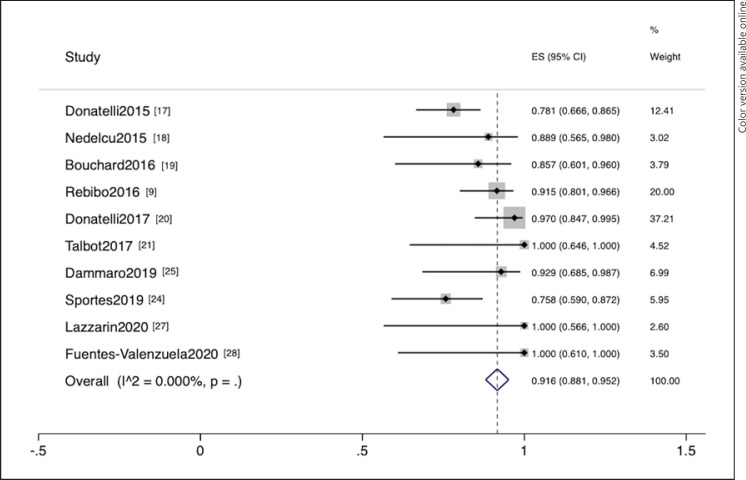
This meta-analysis included 3 EVT [22, 23, 26] and 10 EID [9, 17, 18, 19, 20, 21, 24, 25, 27, 28] studies. EVT was employed in 47 patients. The summary data are provided in online suppl. Table 3. Clinical success was defined as clinical plus endoscopic or radiologic resolution. The pooled clinical success rates (95% confidence interval [CI]) of EVT and EID were 85.2% (75.1%–95.4%) and 91.6% (88.1%–95.2%), respectively (Fig. 2, 3). No heterogeneity was observed in both pooling (I2 = 0%). When the EID study [21] with poor quality was excluded, the pooled success rate did not significantly change (clinical success rate = 91.2% [87.6%–94.9%]).
Fig. 2.
Forest plot of successful leak closure from endoluminal vacuum therapy studies.
Fig. 3.
Forest plot of successful leak closure from endoscopic internal drainage studies.
Treatment Duration and Endoscopy Sessions
Information about the treatment duration was available for pooling in 9 studies [9, 17, 20, 22, 23, 25, 26, 27, 28]. The mean pooled treatment durations (95% CI) of EVT and EID were 28 (2.4–53.6) and 78.4 (50.1–106.7) days, respectively (online suppl. Fig. 1, 2). The results revealed extremely high heterogeneity (I2 = 97.1% and 97.9%, respectively). Nevertheless, a meta-regression analysis was not performed due to unavailability of covariate data.
The pooling of endoscopy sessions was achieved in 6 EID studies [17, 18, 19, 20, 25, 28]. The mean corresponding pooled endoscopy session (95% CI) was 2.9 (2.4–3.3) times (online suppl. Fig. 3). The results showed high heterogeneity (I2 = 76.2%), and a meta-regression analysis was not performed.
Duration of Hospital Stay, Complications, and Mortality
The mean duration of hospital stay was 19 ± 15.1 days in one EVT study [26], and it ranged from 8 to 21 days in 3 EID studies [9, 19, 28]. No procedure-related death was reported. In one EVT study, 1 patient experienced bleeding [26]. Four EID studies recorded complications including stenoses (n = 8), perforation (n = 1), esophageal ulceration (n = 3), bleeding (n = 2), and splenic hematoma (n = 1) [17, 19, 24, 28].
Discussion
This meta-analysis used data from 3 EVT and 10 EID studies, and the results showed a high rate of successful leak closure after both interventions (85.2% and 91.6%, respectively). However, the number of studies on EVT for the management of post-bariatric leaks is extremely limited. Hence, data of only 47 patients were included in this analysis. Additionally, 2 EVT studies [29, 38] were identified via title and abstract screening. However, these studies were excluded because they did not meet the eligibility criteria. Considering the lack of data, more studies with a larger number of patients must be performed to confirm the efficacy of EVT for post-bariatric leak management. Nevertheless, the present study is the only meta-analysis conducted in a clinical setting. The pooled success rate of this study is comparable to that of a previous meta-analysis on EVT for leakage after esophageal and gastric resection [7].
The success rate of EID was slightly higher in the present study than in previous meta-analyses. In a previous meta-analysis, gastric leaks after SG with EID were successfully managed in 84.7% of patients [39]. However, there were several overlapping studies included in that meta-analysis, and the management of these overlaps was not sufficiently explained. Our review excluded overlapping studies, and only the most updated publication from the same group of patients was included. Studies that recruited patients with unsuccessful endoscopic treatments were excluded from our review. The reasons for excluding some key studies [8, 10, 11, 29, 30, 38, 40] are explained in Table 2. In addition, more recent studies were incorporated in the current meta-analysis.
Table 2.
Reasons of not including some key studies
| Study | Intervention | Patients, n | Reason |
|---|---|---|---|
| Pequignot et al. [8] | EID | 7 | Overlapping with Rebibo et al. [9] |
| Christophorou et al. [40] | EID | 30 | Not reported the number of EID used as a first-line treatment and a number of successful first-line EID cases |
| Gonzalez et al. [10] | EID | 22 | Success rate included using second-line endoscopic interventions (SEMS and OTSC) |
| Lorenzo et al. [11] | EID | 22 | Included previously failed endoscopic treatment (31% of the cases), overlapping with Gonzalez et al. [10] |
| Siddique et al. [30] | EID | 20 | Included previously failed endoscopic treatment (85% of the cases) |
| Leeds et al. [29] | EVT | 9 | Included previously failed endoscopic treatment (5/9) |
| Morell et al. [38] | EVT | 6 | Unique technique; SoS |
EID, endoscopic internal drainage; EVT, endoluminal vacuum therapy; OTSC, over-the-scope clip; SEMS, self-expandable metallic stent; SoS, Stent-over-Sponge.
Initially, we planned to review septotomy for post-bariatric leak management because it is similar to EVT and EID in terms of the treatment concept. That is, it is employed for drainage of collected fluid. However, most septotomy studies involved late or chronic leaks, which commonly evolved from early leaks. Hence, septotomy is more likely to be performed after failure of other endoscopic treatments. The inclusion of septotomy in this study may be in conflict with the review question. Therefore, an independent analysis should be performed.
The present study pooled the clinical success rates of each first-line intervention and described the outcomes separately. A better endoscopic intervention was not indicated in this meta-analysis due to the lack of original studies that performed direct comparison. Comparative studies should be conducted in the future. However, enrollment in a randomized clinical trial is unlikely to be successful due to the limited number of post-bariatric leak cases. Therefore, retrospective studies using data from a more extensive database with appropriate statistical adjustment should be conducted.
The present study did not assess the intervention effect of salvage procedures. Data from studies about unsuccessful management are required for the assessment of salvage interventions. The number of such studies is limited, and they are challenging to identify. The variations between the EVT and EID techniques were not analyzed individually considering the small number of eligible studies.
Reports of the duration of treatment and endoscopy sessions were missing from some studies. High heterogeneities were observed from pooling, and they may be explained by differences in the treatment protocol among centers.
Not all treatment outcomes were reported in original articles. Most studies did not include information about the length of hospital stay. Moreover, procedure-related complications were not thoroughly explained. Only 1 patient presented with bleeding associated with EVT. Meanwhile, there were various complications correlated with EID. Among all complications, splenic injury from the proximity of stents may be caused by EID [41, 42, 43], and only 1 patient presented with this complication.
This study had some strengths. It is the first meta-analysis to assess the efficacy of EVT for post-bariatric leaks. Moreover, it can be considered an updated meta-analysis about EID. The number of participants was higher when pooling is performed in a meta-analysis, thereby making the assessment of effect size more precise than small individual studies. However, the present study also had several limitations. Only few studies were included in evidence synthesis. The reason for this was that there are only a few studies in this area, especially if only post-bariatric leaks were considered. Fewer studies could be included as a result of the strict eligibility criteria. If the inclusion criteria were more lenient, all successful closures might have been considered regardless if the patients were required to undergo one or more endoscopic procedures. The lack of covariate data is another problem that can inhibit the evaluation of heterogeneity via a meta-regression analysis.
In conclusion, both EVT and EID were feasible and effective for leak closure after bariatric surgery. Nevertheless, whether one intervention is more effective than the other should be evaluated in large comparative studies, which could be conducted using real-world information from a registered database. This meta-analysis should be updated when more data are available.
Statement of Ethics
An ethical statement was not required for this study type as no human or animal subjects or materials were used.
Conflict of Interest Statement
All authors declare no conflicts of interest.
Funding Sources
This study was funded by the Faculty of Medicine Vajira Hospital, Navamindradhiraj University Research Fund.
Author Contributions
Amarit Tansawet contributed to conceptualization. Issaree Laopeamthong, Thanita Akethanin, and Wisit Kasetsermwiriya contributed to data curation. Amarit Tansawet contributed to formal analysis. Suphakarn Techapongsatorn contributed to funding acquisition. Amarit Tansawet contributed to methodology. Amarit Tansawet contributed to project administration. Suphakarn Techapongsatorn contributed to supervision. Wisit Kasetsermwiriya contributed to validation. Thanita Akethanin contributed to visualization. Issaree Laopeamthong contributed to writing − original draft. Amarit Tansawet and Wisit Kasetsermwiriya contributed to writing − review and editing.
Data Availability Statement
All data are available from previously published articles.
Supplementary Material
Supplementary data
verified
References
- 1.Rosenthal RJ, Diaz AA, Diaz AA, Arvidsson D, Baker RS, Basso N, et al. International Sleeve Gastrectomy Expert Panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8((1)):8–19. doi: 10.1016/j.soard.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Vidarsson B, Sundbom M, Edholm D. Incidence and treatment of leak at the gastrojejunostomy in Roux-en-Y gastric bypass: a cohort study of 40,844 patients. Surg Obes Relat Dis. 2019;15((7)):1075–9. doi: 10.1016/j.soard.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27((1)):240–5. doi: 10.1007/s00464-012-2426-x. [DOI] [PubMed] [Google Scholar]
- 4.Hamid HKS, Emile SH, Saber AA, Dincer M, de Moura DTH, Gilissen LPL, et al. Customized bariatric stents for sleeve gastrectomy leak: are they superior to conventional esophageal stents? A systematic review and proportion meta-analysis. Surg Endosc. 2021;35((3)):1025–38. doi: 10.1007/s00464-020-08147-6. [DOI] [PubMed] [Google Scholar]
- 5.Rogalski P, Swidnicka-Siergiejko A, Wasielica-Berger J, Zienkiewicz D, Wieckowska B, Wroblewski E, et al. Endoscopic management of leaks and fistulas after bariatric surgery: a systematic review and meta-analysis. Surg Endosc. 2021;35((3)):1067–87. doi: 10.1007/s00464-020-07471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.do Monte Junior ES, de Moura DTH, Ribeiro IB, Hathorn KE, Farias GFA, Turiani CV, et al. Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: systematic review and meta-analysis. Dig Endosc. 2020 doi: 10.1111/den.13813. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Tavares G, Tustumi F, Tristão LS, Bernardo WM. Endoscopic vacuum therapy for anastomotic leak in esophagectomy and total gastrectomy: a systematic review and meta-analysis. Dis Esophagus. 2021;34((5)):doaa132. doi: 10.1093/dote/doaa132. [DOI] [PubMed] [Google Scholar]
- 8.Pequignot A, Fuks D, Verhaeghe P, Dhahri A, Brehant O, Bartoli E, et al. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes Surg. 2012;22((5)):712–20. doi: 10.1007/s11695-012-0597-0. [DOI] [PubMed] [Google Scholar]
- 9.Rebibo L, Bartoli E, Dhahri A, Cosse C, Robert B, Brazier F, et al. Persistent gastric fistula after sleeve gastrectomy: an analysis of the time between discovery and reoperation. Surg Obes Relat Dis. 2016;12((1)):84–93. doi: 10.1016/j.soard.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez JM, Lorenzo D, Guilbaud T, Bège T, Barthet M. Internal endoscopic drainage as first line or second line treatment in case of postsleeve gastrectomy fistulas. Endosc Int Open. 2018;6((6)):E745–750. doi: 10.1055/s-0044-101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzo D, Guilbaud T, Gonzalez JM, Benezech A, Dutour A, Boullu S, et al. Endoscopic treatment of fistulas after sleeve gastrectomy: a comparison of internal drainage versus closure. Gastrointest Endosc. 2018;87((2)):429–37. doi: 10.1016/j.gie.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Donatelli G, Dumont JL, Cereatti F, Dhumane P, Tuszynski T, Vergeau BM, et al. Endoscopic internal drainage as first-line treatment for fistula following gastrointestinal surgery: a case series. Endosc Int Open. 2016;4((6)):E647–51. doi: 10.1055/s-0042-105206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162((11)):777–84. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283((15)):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donatelli G, Dumont JL, Cereatti F, Ferretti S, Vergeau BM, Tuszynski T, et al. Treatment of leaks following sleeve gastrectomy by endoscopic internal drainage (EID) Obes Surg. 2015;25((7)):1293–301. doi: 10.1007/s11695-015-1675-x. [DOI] [PubMed] [Google Scholar]
- 18.Nedelcu M, Manos T, Cotirlet A, Noel P, Gagner M. Outcome of leaks after sleeve gastrectomy based on a new algorithm adressing leak size and gastric stenosis. Obes Surg. 2015;25((3)):559–63. doi: 10.1007/s11695-014-1561-y. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard S, Eisendrath P, Toussaint E, Le Moine O, Lemmers A, Arvanitakis M, et al. Trans-fistulary endoscopic drainage for post-bariatric abdominal collections communicating with the upper gastrointestinal tract. Endoscopy. 2016;48((9)):809–16. doi: 10.1055/s-0042-108726. [DOI] [PubMed] [Google Scholar]
- 20.Donatelli G, Dumont JL, Dhumane P, Dritsas S, Tuszynski T, Vergeau BM, et al. Double pigtail stent insertion for healing of leaks following Roux-en-Y gastric bypass. Our experience (with videos) Obes Surg. 2017;27((2)):530–5. doi: 10.1007/s11695-016-2465-9. [DOI] [PubMed] [Google Scholar]
- 21.Talbot M, Yee G, Saxena P. Endoscopic modalities for upper gastrointestinal leaks, fistulae and perforations. ANZ J Surg. 2017;87((3)):171–6. doi: 10.1111/ans.13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christogianni V, Bemponis P, Buesing M, Dukovska R. The endoscopic-vacuum assisted closure-technique in the treatment of staple line leaks after sleeve gastrectomy. Obes Surg. 2018;28((1)):S48–S49. [Google Scholar]
- 23.Mencio MA, Ontiveros E, Burdick JS, Leeds SG. Use of a novel technique to manage gastrointestinal leaks with endoluminal negative pressure: a single institution experience. Surg Endosc. 2018;32((7)):3349–56. doi: 10.1007/s00464-018-6055-x. [DOI] [PubMed] [Google Scholar]
- 24.Sportes A, Aireini G, Kamel R, Pratico C, Raynaud JJ, Sabate JM, et al. Efficacy of endoscopic treatment of post-sleeve gastrectomy fistulas according to the radiological type. Obes Surg. 2019;29((7)):2217–24. doi: 10.1007/s11695-019-03825-4. [DOI] [PubMed] [Google Scholar]
- 25.Dammaro C, Lainas P, Dumont JL, Tranchart H, Donatelli G, Dagher I. Endoscopic internal drainage coupled to prompt external drainage mobilization is an effective approach for the treatment of complicated cases of sleeve gastrectomy. Obes Surg. 2019;29((9)):2929–35. doi: 10.1007/s11695-019-03933-1. [DOI] [PubMed] [Google Scholar]
- 26.Archid R, Wichmann D, Klingert W, Nadiradze G, Hönes F, Archid N, et al. Endoscopic vacuum therapy for staple line leaks after sleeve gastrectomy. Obes Surg. 2020;30((4)):1310–5. doi: 10.1007/s11695-019-04269-6. [DOI] [PubMed] [Google Scholar]
- 27.Lazzarin G, Di Furia M, Romano L, Di Sibio A, Di Giacomo C, Lombardi L, et al. Endoscopic double-pigtail catheter (EDPC) internal drainage as first-line treatment of gastric leak: a case series during laparoscopic sleeve gastrectomy learning curve for morbid obesity. Minim Invasive Surg. 2020;2020:8250904. doi: 10.1155/2020/8250904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuentes-Valenzuela E, García-Alonso FJ, Tejedor-Tejada J, Najera-Muñoz R, De Benito Sanz M, Sánchez-Ocaña R, et al. Endoscopic internal drainage using transmural double-pigtail stents in leaks following upper gastrointestinal tract surgery. Rev Esp Enferm Dig. 2020 doi: 10.17235/reed.2020.7514/2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis. 2016;12((7)):1278–85. doi: 10.1016/j.soard.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Siddique I, Alazmi W, Al-Sabah SK. Endoscopic internal drainage by double pigtail stents in the management of laparoscopic sleeve gastrectomy leaks. Surg Obes Relat Dis. 2020;16((7)):831–8. doi: 10.1016/j.soard.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Baretta G, Campos J, Correia S, Alhinho H, Marchesini JB, Lima JH, et al. Bariatric postoperative fistula: a life-saving endoscopic procedure. Surg Endosc. 2015;29((7)):1714–20. doi: 10.1007/s00464-014-3869-z. [DOI] [PubMed] [Google Scholar]
- 32.Campos JM, Ferreira FC, Neto MG, Silva LB, Alhinho HC, Godoy ES, et al. Septotomy to treatment of late and chronic fistula after sleeve gastrectomy and duodenal switch: a novel endoscopic approach. Gastrointest Endosc. 2017;85((5)):AB83–4. [Google Scholar]
- 33.Diaz R, Welsh LK, Perez JE, Narvaez A, Davalos G, Portenier D, et al. Endoscopic septotomy as a treatment for leaks after sleeve gastrectomy: meeting presentations: digestive disease week 2019. Endosc Int Open. 2020;8((1)):E70–5. doi: 10.1055/a-1027-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferraz ÁAB, Feitosa PHF, Santa-Cruz F, Aquino MAR, Dompieri LT, Santos EM, et al. Gastric fistula after sleeve gastrectomy: clinical features and treatment options. Obes Surg. 2021 Mar;31((3)):1196–1203. doi: 10.1007/s11695-020-05115-w. [DOI] [PubMed] [Google Scholar]
- 35.Fishman S, Shnell M, Gluck N, Santo E. Endoscopic septotomy is a novel treatment for chronic leaks complicating laparoscopic sleeve gastrectomy. United European Gastroenterol J. 2016;4((5)):A199–200. [Google Scholar]
- 36.Mahadev S, Kumbhari V, Campos JM, Galvao Neto M, Khashab MA, Chavez YH, et al. Endoscopic septotomy: an effective approach for internal drainage of sleeve gastrectomy-associated collections. Endoscopy. 2017;49((5)):504–8. doi: 10.1055/s-0042-122012. [DOI] [PubMed] [Google Scholar]
- 37.Shnell M, Gluck N, Abu-Abeid S, Santo E, Fishman S. Use of endoscopic septotomy for the treatment of late staple-line leaks after laparoscopic sleeve gastrectomy. Endoscopy. 2017;49((1)):59–63. doi: 10.1055/s-0042-117109. [DOI] [PubMed] [Google Scholar]
- 38.Morell B, Murray F, Vetter D, Bueter M, Gubler C. Endoscopic vacuum therapy (EVT) for early infradiaphragmal leakage after bariatric surgery-outcomes of six consecutive cases in a single institution. Langenbecks Arch Surg. 2019;404((1)):115–21. doi: 10.1007/s00423-019-01750-9. [DOI] [PubMed] [Google Scholar]
- 39.Giuliani A, Romano L, Marchese M, Necozione S, Cianca G, Schietroma M, et al. Gastric leak after laparoscopic sleeve gastrectomy: management with endoscopic double pigtail drainage. A systematic review. Surg Obes Relat Dis. 2019;15((8)):1414–9. doi: 10.1016/j.soard.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Christophorou D, Valats JC, Funakoshi N, Duflos C, Picot MC, Vedrenne B, et al. Endoscopic treatment of fistula after sleeve gastrectomy: results of a multicenter retrospective study. Endoscopy. 2015;47((11)):988–96. doi: 10.1055/s-0034-1392262. [DOI] [PubMed] [Google Scholar]
- 41.Donatelli G, Airinei G, Poupardin E, Tuszynski T, Wind P, Benamouzig R, et al. Double-pigtail stent migration invading the spleen: rare potentially fatal complication of endoscopic internal drainage for sleeve gastrectomy leak. Endoscopy. 2016;48((Suppl 1 UCTN)):E74–5. doi: 10.1055/s-0042-102446. [DOI] [PubMed] [Google Scholar]
- 42.Genser L, Pattou F, Caiazzo R. Splenic abscess with portal venous gas caused by intrasplenic migration of an endoscopic double pigtail drain as a treatment of post-sleeve gastrectomy fistula. Surg Obes Relat Dis. 2016;12((1)):e1–3. doi: 10.1016/j.soard.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Romano L, Giuliani A, Cianca G, Di Sibio A, Carlei F, Amicucci G, et al. A case of intrasplenic displacement of an endoscopic double-pigtail stent as a treatment for laparoscopic sleeve gastrectomy leak. Int J Surg Case Rep. 2018;53:367–9. doi: 10.1016/j.ijscr.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data are available from previously published articles.