Abstract
Background
Recurrence after resection of pancreatic cancer occurs in up to 80% of patients in the first 2 years after complete resection. While most patients are not eligible for surgical treatment due to disseminated disease, a certain group of patients can be evaluated for re-resection of local recurrence. This review summarizes the current literature on surgical treatment of recurrent pancreatic cancer and potential prognostic factors.
Summary
Re-resection of recurrent pancreatic cancer provides a significant survival benefit to selected patients with acceptable procedure-related mortality. Median overall survival after re-resection of recurrent pancreatic cancer is up to 28 months. The most relevant clinical parameters associated with a prognostic benefit are young patient age (<65 years), time to initial resection (>10 months), and preoperative chemotherapy before re-resection. Molecular markers are currently under investigation and might help to improve patient selection in the future.
Key Message
Re-resection of recurrent pancreatic cancer is safe and feasible in experienced hands. Selected patients benefit from surgical treatment, but future studies are needed to identify reliable prognostic markers predicting survival.
Keywords: Pancreatic cancer, Local recurrence, Surgical resection, Chemotherapy, Prognostic factors
Introduction
Pancreatic cancer is one of the most aggressive and fatal malignancies, accounting for almost 500,000 deaths per year worldwide [1]. While most patients are diagnosed in an advanced tumor stage without any options of surgical treatment, around 30% of patients can be treated by radical resection. Over the past 2 decades, adjuvant treatment regimens have been introduced into clinical routine based on the findings of randomized-controlled trials such as those by the ESPAC group.
Since the publication of first results from the ESPAC-1 trial, advances in adjuvant therapy have been constantly increasing overall survival. Postoperative therapy with gemcitabine and subsequently with gemcitabine and capecitabine combined has been shown to significantly increase prognosis [2, 3]. The percentage of patients who can be offered surgical resection has increased as well since the introduction of neoadjuvant treatment for locally advanced tumors [4]. The FOLFIRINOX scheme has significantly improved resectability rates in a neoadjuvant setting and has become a clinical standard for adjuvant treatment after complete resection [5].
Along with improvement in adjuvant and neoadjuvant treatment, surgical resection techniques have been tremendously enhanced and remain the backbone of any curative therapy for pancreatic cancer [6]. Main surgery-related factors that determine oncologic outcome are clear resection margins and, given the key prognostic significance of positive lymph nodes, likely also the extent of lymphadenectomy [7, 8, 9]. In all clinical trials investigating different adjuvant treatment regimens, patients with R0 resection consistently had better outcomes than those with an R1 resection. This applies independently of primary tumor localization (head, body, or tail of pancreas), and R0 resection should thus be the main goal of surgical procedures in pancreatic cancer.
The Role of Neoadjuvant Treatment
Based on the data from the ESPAC trials, surgical resection followed by intense adjuvant chemotherapy is the current standard of care for resectable pancreatic cancer. As described above, neoadjuvant therapy is the standard treatment for borderline-resectable or locally advanced tumors. The implementation of standard neoadjuvant treatment is currently the focus of controversial discussion. Several studies have advocated standard neoadjuvant treatment for resectable pancreatic cancer in the presence of certain risk factors for early recurrence [10, 11]. However, these studies have certain limitations [12] that should be considered when interpreting the results. Alternatively, high-quality studies on this topic do not show any significant survival benefit between patients with resectable pancreatic cancer who received neoadjuvant therapy and those who underwent upfront surgery [13, 14]. In summary, the question which patients with resectable pancreatic cancer benefit from neoadjuvant therapy has not been answered sufficiently yet, and prospective randomized-controlled trials are needed to generate the evidence required for implementation of standard neoadjuvant treatment into the clinical routine.
The Role of Surgery in Preventing Local Recurrence
While the studies cited above failed to show any significant reduction in local recurrence by neoadjuvant treatment, recent studies suggest a critical role of surgery in prevention of local recurrence. In the past few years, new developments and modifications of established surgical techniques have improved the oncologic quality of resection, in particular for locally advanced tumors [6]. While venous infiltration has never been considered a contraindication for resection, arterial infiltration is still regarded as a contraindication for resection by many surgeons. However, with sufficient surgical experience and after a certain learning curve, arterial resection can be performed with appropriate morbidity and increase survival [15]. As the extent of arterial infiltration determines the extent of surgery, the introduction of different “artery-first” approaches has reduced overall morbidity [16], along with an increased rate of R0 resections and prolonged overall survival. After neoadjuvant treatment, artery-sparing resection of locally advanced pancreatic cancer can be achieved by clearing all potentially tumor-infiltrated nerve and lymph tissue from the anatomic triangle in between the superior mesenteric artery, the celiac trunk, and the portomesenteric venous axis [17, 18], or by divestment of devitalized tumor tissue from the major arteries [19, 20]. Albeit clinical evidence from randomized trials is still lacking, these innovative surgical techniques have the potential to prevent local recurrence and to increase survival.
Surgery for Recurrent Pancreatic Cancer: Current Options and Obstacles
80% of local recurrences occur within 2 years after resection. About 30% of patients that develop recurrence show isolated local recurrence in the absence of distant metastasis [21]. Recurrence patterns of pancreatic cancer have been investigated on the basis of the ESPAC-4 data. 730 patients were followed for a median time of 43 months, and 65% of them were diagnosed with tumor recurrence [22]. In the group of patients with tumor recurrence, 49% had local-only recurrence, while 40% patients had distant-only recurrence. 10% of patients were diagnosed with simultaneous local and distant recurrence. Local recurrence occurred at a median of 11.6 months, which differed significantly from patients who were diagnosed with distant metastasis (median 9.4 months). The median time from the diagnosis of local recurrence to death was 9.3 months, and there was no significant difference in the time of diagnosis of recurrence to death in patients with local and distant recurrence. When looking closer at survival data from the 238 patients suffering local recurrence included in this trial, 17 of those undergoing palliative treatment were still alive after 24 months, and 6 survived more than 36 months. These data raise the question which potential treatment options should be chosen in patients with local-only recurrence and, in particular, which fraction of patients benefits best from the aggressive local treatment. While patients with disseminated distant metastasis do clearly not benefit from local therapy, patients with limited (“oligometastatic”) recurrence, and especially those with limited local recurrence should be evaluated for potential multidisciplinary treatment. This includes systemic therapy, innovative options for targeted therapy in combination with radiotherapy [23], and surgical resection in cases where complete tumor resection seems achievable.
Surgical Resection of Recurrent Pancreatic Cancer
The most important goal in the surgical resection of recurrent pancreatic cancer is, as in initial resection, achieving clear resection margins and complete removal of the recurrent tumor respecting the general principles of surgical oncology. True local recurrence usually is defined as the bed of the pancreatic margin, the pancreatic remnant, or the mesenteric root [24] and can thus be distinguished from lymph node recurrence. Before considering surgical resection, all patients should undergo complete CT staging and analysis of CA 19-9 levels. Surgical resection of recurrent pancreatic cancer should be performed in high-volume centers with sufficient surgical expertise to minimize complications and reduce mortality [25].
Even if the current level of evidence is low, several studies have been published on resection of pancreatic cancer recurrence. First reports of successful resection range back to the 1980s, but are mainly limited to case reports or small case series [26]. Beginning with the 2000s, first series from larger cohorts have been analyzed and published. One of the first studies by Kleeff et al. [27] retrospectively analyzed 30 patients with isolated recurrence of pancreatic cancer. Fifteen patients underwent resection, while 15 patients underwent exploration without resection. Median overall survival in resected patients was 17 months, compared to 9 months in patients without surgical resection. The authors identified several prognostic factors such as time to initial operation, younger patient age, and low CA 19-9 levels. However, due to the small cohort, a significant impact of these parameters could not be established. Seelig et al. [28] presented a series of 17 patients who underwent re-exploration for suspected local recurrence after initial resection of pancreatic cancer. In this cohort, only 12 patients had histologically proven recurrence, while chronic pancreatitis or benign structures had been mistaken for recurrence in the remaining case. Re-resection was only possible in 2 of the 12 patients with cancer recurrence, and all others received palliative surgery. Based on these results, the authors suggested that resection of “true” recurrence is rarely feasible, but that patients with suspected recurrence should be explored to rule out chronic pancreatitis or benign structures as a potential reason. A study reporting promising results of surgical resection in combination with intraoperative radiation therapy was presented by Roeder et al. [29]. Eighteen out of 36 patients with isolated local recurrence of pancreatic cancer could be resected, and the others received exploration or palliative debulking only. In the group of patients that underwent resection, a 2-year survival of 26% was observed and 17% of the patients lived more than 3 years. Of note, in this study, all patients received intraoperative radiation with a total dosage of 15 Gy [29]. Strobel et al. [30] from the same group presented the first and to our knowledge largest dataset from a prospective cohort study, including 57 patients with isolated local recurrence of pancreatic cancer after initial curative resection. In this cohort, 72% of all patients undergoing surgical exploration received successful re-resection, resulting in median survival of 26 months with low procedure-related morbidity (25%) and mortality (1.8%). Prognostic factors for survival were complete resection of recurrence and low preoperative CA 19-9 values. The most frequent surgical procedures for re-resection were completion pancreatectomy (resection of the remaining head or tail, 32%), but in a majority of the cases, no further pancreatic resection was needed (56%). In 32% of cases, multivisceral resection including the mesenteric axis, the celiac trunk, or adjacent organs was necessary to achieve complete resection [30]. Indeed, since pancreatic ductal adenocarcinoma frequently infiltrates autonomous nerves alongside major arteries, local recurrence commonly affects the celiac axis or superior mesenteric artery, potentially necessitating arterial resection to achieve tumor clearance (see Fig. 1). These technical obstacles demand proper selection of patients who benefit from aggressive local therapy even in the scenario of tumor recurrence.
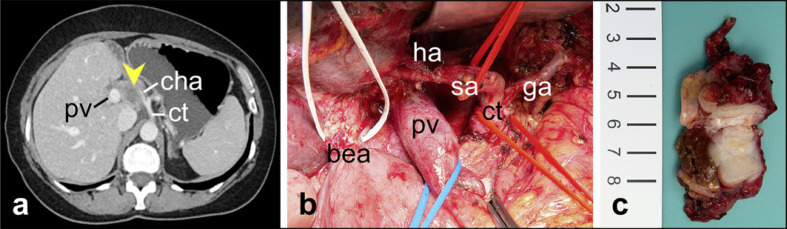
Fig. 1.
Resection of locally recurrent pancreatic cancer 3 years after partial pancreatoduodenectomy for primary tumor resection. a CT scan revealing tumor recurrence (arrowhead) with infiltration of hepatoduodenal ligament and cha. ct, celiac trunk; pv, portal vein; cha, common hepatic artery. b Surgical field after tumor resection with segmental resection of pv, resection of common hepatic artery, completion pancreatectomy, and transposition of sa to proper ha just proximal to its bifurcation. bea preserved from prior partial pancreatoduodenectomy; sa, splenic artery; ha, hepatic artery; pv, portal vein; bea, biliary-enteric anastomosis; ct, celiac trunk; ga, left gastric artery. c Resection specimen.
Several smaller or retrospective series, likewise revealing promising results of surgical resection, have been published in the past decade. Miyazaki et al. [31] published a cohort of 11 patients with repeat resection of pancreatic cancer. The vast majority of these patients underwent completion pancreatectomy after initial resection of the distal pancreas, whereas patients with extra-pancreatic recurrence were not included in this collective [31]. Patients who underwent successful resection of cancer recurrence had a significant survival benefit, with a 2-year survival rate of 61% compared to only 19% in patients who received palliative chemotherapy. Comparable results were published by Boone et al. [32] who analyzed a cohort of 1,707 patients with periampullary adenocarcinoma that were diagnosed with either local recurrence or isolated metastatic disease, and identified 22 patients treated with surgical resection. Out of these patients, 10 had isolated local recurrence and 12 were diagnosed with distant recurrence mainly in the liver and lung. The authors reported a median overall survival of 28 months in the group of resected patients with a morbidity of 32% and a mortality of 0% [32]. The largest series based on a retrospective analysis was published by Yamada et al. [33] on behalf of the Japanese Society of Hepato-Biliary Surgery, representing a multicenter study of 40 Japanese centers. They included patients with recurrence in the remnant pancreas only and identified 90 patients treated by surgery. They, too, observed a significant survival benefit in patients undergoing resection with a median 2-year survival of 49% (vs. 7% in the group of patients with recurrence who did not undergo re-resection) and a 5-year survival of 14% (compared to 3%). The authors identified several factors associated with a beneficial prognostic impact, among them patient age under 65 years, a body mass index over 20 kg/m2, tumor size below 2 cm, and large distance of tumor recurrence from the pancreatic stump. A recent study by Kim et al. [34] reported outcomes from a cohort of 48 patients who underwent re-resection of recurrent pancreatic cancer, representing 3.6% of all patients with recurrence. The authors pooled patients with local recurrence and liver, lung, and other organ metastasis. Median overall survival was 23.5 months in the group of resected patients compared to 12 months in patients that did not undergo surgical resection. In this cohort, factors associated with a prognostic benefit were time to recurrence (more than 10 months), tumor differentiation, a positive lymph node status, and chemotherapy for tumor recurrence [34].
A recent systematic review and meta-analysis pooled the results from 6 of the larger studies on the topic and analyzed the overall survival of resected patients compared to those who did not undergo surgery. All studies were designed retrospectively, and the total number of included patients was 431 (176 patients who underwent resection vs. 255 who received nonsurgical treatment). In the pooled analysis, patients who underwent re-resection had a median overall survival benefit of 28.7 months and a median survival benefit of 15.2 months after re-resection [35]. A limitation of these, as of all data outlined above, is that they are exclusively derived from retrospective studies and therefore carry a relevant risk of selection bias. Future prospectively designed studies are needed to generate more solid evidence on the benefit of surgical resection in recurrent pancreatic cancer.
Perioperative Chemo- and Radiation Therapy for Recurrent Pancreatic Cancer
As described above, the introduction of perioperative chemotherapy has significantly improved outcomes after pancreatic cancer resection. Though there is no evidence for a prognostic benefit of standard neoadjuvant chemotherapy in resectable pancreatic cancer, preoperative chemotherapy before resection of tumor recurrence seems to be associated with increased survival. Kim et al. [34] have shown that completion of the adjuvant chemotherapy regimen after initial resection as well as preoperative chemotherapy before resection of tumor recurrence is associated with significantly increased overall survival. The role of pre- or intraoperative radiation therapy is controversially discussed. While some authors described a potential benefit of perioperative radiation especially in situations with potential R1 resection, Strobel et al. [30] observed significantly decreased overall survival in patients who received resection with intraoperative radiotherapy compared to those undergoing resection without intraoperative radiotherapy. This observation is, however, affected by potential selection bias, since intraoperative radiation may have preferably been applied in cases with suspected positive resection margins. Of note, this study was published prior to introduction of intensive perioperative chemotherapy regimens such as FOLFIRINOX. Even if there are no head-to-head studies comparing intraoperative radiotherapy and postoperative FOLFIRINOX therapy, retrospective studies suggest that the latter is superior to intraoperative radiation [36].
Who Benefits? Prognostic Factors Associated with Increased Survival
Selection of patients who benefit from re-resection remains the crucial aspect in surgical treatment of recurrent pancreatic cancer. The studies cited and discussed above identified several clinical parameters associated with a prognostic benefit after resection of recurrent pancreatic cancer. First, successful complete resection is one of the most important factors associated with increased survival. Second, time from initial resection to occurrence of recurrence is significantly associated with oncologic outcome. An interval of 9 to 10 months after initial resection seems to be a cutoff value with prognostic relevance. Third, chemotherapy before the re-resection (and tumor response to it) seems to be extremely relevant for postoperative outcome, while the impact of peri- or intraoperative radiotherapy remains unclear. Other clinical parameters associated with increased survival are, like in other cancer entities, patient age and performance status, tumor grading, and CA 19-9 levels. Concerning location of tumor recurrence, lung metastases and isolated local recurrence seem to have better prognosis than isolated metastases to the liver or other organs. A summary of clinical markers associated with increased survival is provided in Table 1.
Table 1.
Prognostic factors associated with oncologic outcome after resection of pancreatic cancer recurrence according to retrospective studies
| Clinical parameters associated with prognostic benefit | Molecular markers associated with prognostic benefit |
|---|---|
| Patient age, <65 years [33] | IPMN-associated primary carcinoma [37] |
| Preoperative chemotherapy [33] | KRAS-wild-type tumor [42] |
| Completion of adjuvant chemotherapy [34] | SMAD4-wild-type tumor [45] |
| Time from initial resection, >10 months [34] | |
| Low CA 19–9 level [30] | |
| BMI > 20 kg/m2 [33] | |
| R0 resection of tumor recurrence [30] | |
| Recurrence in the remnant pancreas or in the lung [34] |
While these clinical parameters may help to estimate a putative benefit of re-resection in the individual case, distinct and reliable tumor-dependent markers are urgently needed to better predict outcomes based on tumor biology. To our knowledge, no studies are available investigating the prognostic significance of specific molecular markers or profiles in the setting of recurrent pancreatic cancer. Given this limitation, we would estimate that prognostic markers that are associated with early recurrence and impaired prognosis in patients undergoing resection of the primary tumor apply in the recurrent situation as well. For instance, patients who developed pancreatic cancer arising from intraductal papillary mucinous neoplasm (IPMN) have a better prognosis than tumors arising without any precursor lesions [37]. Therefore, patients suffering tumor recurrence in the remnant pancreas after resection of IPMN-associated carcinoma should be offered surgical re-resection. Distinction between tumor recurrence or secondary IPMN-associated lesions in the remnant pancreas is challenging and therefore these patients should be thoroughly evaluated for surgical treatment [38, 39].
Besides IPMN-associated pancreatic cancer, several molecular markers have been shown to be associated with prognosis. Alterations in the KRAS gene represent one of the most common mutations in pancreatic cancer and are detected in more than 90% of cases [40]. Patients with mutations in this gene have been shown to have significantly shortened survival compared to patients without KRAS mutations [41]. Hashimoto et al. [42] investigated the role of KRAS mutations in the setting of cancer recurrence in the remnant pancreas following primary cancer resection. Analyzing specific sequences of the KRAS gene in 8 patients with tumor recurrence, they found that only 50% of recurrent tumors presented the same mutations as the primary, while the other 50% represented genetically “new” tumors. Another molecular marker that has attracted attention is the SMAD4/DPC4 protein, a central mediator of the TGF-ß pathway. For almost 2 decades, this protein has been known to be inactivated in more than 50% of patients with pancreatic cancer [43]. A recent meta-analysis confirmed an association between SMAD4 inactivation and decreased overall patient survival [44]. Similar to KRAS mutations, no clinical data investigating the significance of SMAD4 in the setting of tumor recurrence have been published yet. Given its role in primary tumors, it can be assumed that patients without SMAD4 inactivation are more likely to benefit from re-resection of recurrent pancreatic cancer than those carrying tumors with a mutated SMAD4 protein. Indeed, both mutations were correlated with clinical outcome in a study including 356 patients and were confirmed to be significantly associated with reduced survival and early recurrence [45]. This study identified 2 additional mutations, namely, alterations in the CDKN2A and TP53 gene. Another marker of potential interest might be RUNX3. Applying genetically engineered mouse models, previous studies have shown that RUNX3 expression is significantly correlated with tumor cell proliferation and metastatic spread in pancreatic ductal adenocarcinoma, which makes it conceivable that it can likewise predict prognosis upon pancreatic cancer recurrence [46]. However, all of these data were generated in the setting of primary resection or in experimental models and have not been validated in the clinical scenario of tumor recurrence. However, the molecular status of these genes is most likely associated with prognosis in the situation of tumor recurrence as well, and analysis of mutations in these genes could thus be of interest for clinical decision-making in patients with recurrent pancreatic cancer.
Summary and Outlook
Taken together, clinical and scientific evidence as to which patients benefit from re-resection of recurrent pancreatic cancer is limited. All hitherto published studies are retrospective and carry relevant risks of bias but reveal a significant survival benefit upon surgical re-resection in selected patients. Perioperative morbidity and mortality are acceptable in experienced centers and patients who are potential candidates for surgical treatment of recurrent pancreatic cancer should be carefully evaluated by an interdisciplinary team of surgeons and oncologist. In contrast to primary resection, patients should undergo intense chemotherapy before re-resection as this significantly improves survival. Other clinical parameters associated with improved prognosis after resection of recurrent pancreatic cancer are patient age and performance status as well as time to initial resection and tumor differentiation. The prognostic significance of molecular markers such as KRAS and SMAD4 has not been investigated specifically in the setting of recurrent pancreatic cancer but could potentially offer additional information for patient selection. Since the introduction of intense chemotherapy regimens and the improvement of surgical resection techniques have markedly increased the prognosis of pancreatic cancer patients, the question who benefits from re-resection will gain importance in the upcoming years. Prospective clinical trials are needed to answer this question and to identify patients who will benefit best from aggressive local treatment of tumor recurrence.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There was no specific funding source for this article, but resources of the University Hospital of Heidelberg were used for the preparation of the manuscript.
Author Contributions
M.S. concepted the manuscript. H.N., M.W.B., and M.S. wrote and revised the manuscript.
verified
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71((3)):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350((12)):1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389((10073)):1011–24. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 4.Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264((3)):457–63. doi: 10.1097/SLA.0000000000001850. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379((25)):2395–406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 6.Schneider M, Hackert T, Strobel O, Büchler MW. Technical advances in surgery for pancreatic cancer. Br J Surg. 2021 Jul 23;108((7)):777–85. doi: 10.1093/bjs/znab133. [DOI] [PubMed] [Google Scholar]
- 7.Niesen W, Hank T, Büchler M, Strobel O. Local radicality and survival outcome of pancreatic cancer surgery. Ann Gastroenterol Surg. 2019;3((5)):464–75. doi: 10.1002/ags3.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghaneh P, Kleeff J, Halloran CM, Raraty M, Jackson R, Melling J, et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg. 2019;269((3)):520–9. doi: 10.1097/SLA.0000000000002557. [DOI] [PubMed] [Google Scholar]
- 9.Strobel O, Hinz U, Gluth A, Hank T, Hackert T, Bergmann F, et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg. 2015;261((5)):961–9. doi: 10.1097/SLA.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 10.Guo SW, Shen J, Gao JH, Shi XH, Gao SZ, Wang H, et al. A preoperative risk model for early recurrence after radical resection may facilitate initial treatment decisions concerning the use of neoadjuvant therapy for patients with pancreatic ductal adenocarcinoma. Surgery. 2020;168((6)):1003–14. doi: 10.1016/j.surg.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Izumo W, Higuchi R, Furukawa T, Yazawa T, Uemura S, Shiihara M, et al. Evaluation of preoperative prognostic factors in patients with resectable invasive intraductal papillary mucinous carcinoma. Surgery. 2020;168((6)):994–1002. doi: 10.1016/j.surg.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Schneider M, Neoptolemos JP, Büchler MW. Commentary: neoadjuvant treatment of resectable pancreatic cancer: lack of level III evidence. Surgery. 2020;168((6)):1015–6. doi: 10.1016/j.surg.2020.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC Trial. J Clin Oncol. 2020;38((16)):1763–73. doi: 10.1200/JCO.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (prep-02/JSAP05) Jpn J Clin Oncol. 2019;49((2)):190–4. doi: 10.1093/jjco/hyy190. [DOI] [PubMed] [Google Scholar]
- 15.Loos M, Kester T, Klaiber U, Mihaljevic AL, Mehrabi A, Müller-Stich BM, et al. Arterial resection in pancreatic cancer surgery: effective after a learning curve. Ann Surg. 2020 Jun 12; doi: 10.1097/SLA.0000000000004054. [DOI] [PubMed] [Google Scholar]
- 16.Ironside N, Barreto SG, Loveday B, Shrikhande SV, Windsor JA, Pandanaboyana S. Meta-analysis of an artery-first approach versus standard pancreatoduodenectomy on perioperative outcomes and survival. Br J Surg. 2018;105((6)):628–36. doi: 10.1002/bjs.10832. [DOI] [PubMed] [Google Scholar]
- 17.Hackert T, Strobel O, Michalski CW, Mihaljevic AL, Mehrabi A, Müller-Stich B, et al. The TRIANGLE operation - radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB. 2017;19((11)):1001–7. doi: 10.1016/j.hpb.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Schneider M, Strobel O, Hackert T, Büchler MW. Pancreatic resection for cancer-the heidelberg technique. Langenbecks Arch Surg. 2019;404((8)):1017–22. doi: 10.1007/s00423-019-01839-1. [DOI] [PubMed] [Google Scholar]
- 19.Diener MK, Mihaljevic AL, Strobel O, Loos M, Schmidt T, Schneider M, et al. Periarterial divestment in pancreatic cancer surgery. Surgery. 2021;169((5)):1019–25. doi: 10.1016/j.surg.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Cai B, Lu Z, Neoptolemos JP, Diener MK, Li M, Yin L, et al. Sub-adventitial divestment technique for resecting artery-involved pancreatic cancer: a retrospective cohort study. Langenbecks Arch Surg. 2021;406((3)):691–701. doi: 10.1007/s00423-021-02080-5. [DOI] [PubMed] [Google Scholar]
- 21.Groot VP, van Santvoort HC, Rombouts SJ, Hagendoorn J, Borel Rinkes IH, van Vulpen M, et al. Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and SBRT. HPB. 2017;19((2)):83–92. doi: 10.1016/j.hpb.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Jones RP, Psarelli EE, Jackson R, Ghaneh P, Halloran CM, Palmer DH, et al. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial. JAMA Surg. 2019;154((11)):1038–48. doi: 10.1001/jamasurg.2019.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Cao Y, Liu W, Ju X, Zhao X, Jiang L, et al. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2021;22((8)):1093–102. doi: 10.1016/S1470-2045(21)00286-2. [DOI] [PubMed] [Google Scholar]
- 24.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16((4)):836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lidsky ME, Sun Z, Nussbaum DP, Adam MA, Speicher PJ, Blazer DG., 3rd Going the extra mile: improved survival for pancreatic cancer patients traveling to high-volume centers. Ann Surg. 2017;266((2)):333–8. doi: 10.1097/SLA.0000000000001924. [DOI] [PubMed] [Google Scholar]
- 26.Menke-Pluymers MB, Klinkenbijl JH, Tjioe M, Jeekel J. Treatment of locoregional recurrence after intentional curative resection of pancreatic cancer. Hepatogastroenterology. 1992;39((5)):429–32. [PubMed] [Google Scholar]
- 27.Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007;245((4)):566–72. doi: 10.1097/01.sla.0000245845.06772.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seelig MH, Janot M, Chromik AM, Herzog T, Belyaev O, Weyhe D, et al. Redo-surgery following curative resection of pancreatic carcinoma: the difference between true and suspected recurrence. Dig Surg. 2009;26((3)):222–8. doi: 10.1159/000219332. [DOI] [PubMed] [Google Scholar]
- 29.Roeder F, Timke C, Uhl M, Habl G, Hensley FW, Buechler MW, et al. Aggressive local treatment containing intraoperative radiation therapy (IORT) for patients with isolated local recurrences of pancreatic cancer: a retrospective analysis. BMC Cancer. 2012;12:295. doi: 10.1186/1471-2407-12-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strobel O, Hartwig W, Hackert T, Hinz U, Berens V, Grenacher L, et al. Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol. 2013;20((3)):964–72. doi: 10.1245/s10434-012-2762-z. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki M, Yoshitomi H, Shimizu H, Ohtsuka M, Yoshidome H, Furukawa K, et al. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: is it worthwhile? Surgery. 2014;155((1)):58–66. doi: 10.1016/j.surg.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 32.Boone BA, Zeh HJ, Mock BK, Johnson PJ, Dvorchik I, Lee K, et al. Resection of isolated local and metastatic recurrence in periampullary adenocarcinoma. HPB. 2014;16((3)):197–203. doi: 10.1111/hpb.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada S, Kobayashi A, Nakamori S, Baba H, Yamamoto M, Yamaue H, et al. Resection for recurrent pancreatic cancer in the remnant pancreas after pancreatectomy is clinically promising: results of a project study for pancreatic surgery by the Japanese society of hepato-biliary-pancreatic surgery. Surgery. 2018;164((5)):1049–56. doi: 10.1016/j.surg.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 34.Kim YI, Song KB, Lee YJ, Park KM, Hwang DW, Lee JH, et al. Management of isolated recurrence after surgery for pancreatic adenocarcinoma. Br J Surg. 2019;106((7)):898–909. doi: 10.1002/bjs.11144. [DOI] [PubMed] [Google Scholar]
- 35.Serafini S, Sperti C, Friziero A, Brazzale AR, Buratin A, Ponzoni A, et al. Systematic review and meta-analysis of surgical treatment for isolated local recurrence of pancreatic cancer. Cancers. 2021;13((6)):1277. doi: 10.3390/cancers13061277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weibel P, Pavic M, Lombriser N, Gutknecht S, Weber M. Chemoradiotherapy after curative surgery for locally advanced pancreatic cancer: a 20-year single center experience. Surg Oncol. 2021;36:36–41. doi: 10.1016/j.suronc.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Yopp AC, Katabi N, Janakos M, Klimstra DS, D'Angelica MI, DeMatteo RP, et al. Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg. 2011;253((5)):968–74. doi: 10.1097/SLA.0b013e318214bcb4. [DOI] [PubMed] [Google Scholar]
- 38.Kim HS, Han Y, Kang JS, Choi YJ, Byun Y, Kim H, et al. Fate of patients with intraductal papillary mucinous neoplasms of pancreas after resection according to the pathology and margin status: continuously increasing risk of recurrence even after curative resection suggesting necessity of lifetime surveillance. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004478. [DOI] [PubMed] [Google Scholar]
- 39.Miyasaka Y, Ohtsuka T, Tamura K, Mori Y, Shindo K, Yamada D, et al. Predictive factors for the metachronous development of high-risk lesions in the remnant pancreas after partial pancreatectomy for intraductal papillary mucinous neoplasm. Ann Surg. 2016;263((6)):1180–7. doi: 10.1097/SLA.0000000000001368. [DOI] [PubMed] [Google Scholar]
- 40.Singhi AD, George B, Greenbowe JR, Chung J, Suh J, Maitra A, et al. Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers. Gastroenterology. 2019;156((8)):2242–e4. doi: 10.1053/j.gastro.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 41.Kinugasa H, Nouso K, Miyahara K, Morimoto Y, Dohi C, Tsutsumi K, et al. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer. 2015;121((13)):2271–80. doi: 10.1002/cncr.29364. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto D, Chikamoto A, Ohmuraya M, Sakata K, Miyake K, Kuroki H, et al. Pancreatic cancer in the remnant pancreas following primary pancreatic resection. Surg Today. 2014;44((7)):1313–20. doi: 10.1007/s00595-013-0708-0. [DOI] [PubMed] [Google Scholar]
- 43.Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7((12)):4115–21. [PubMed] [Google Scholar]
- 44.Shugang X, Hongfa Y, Jianpeng L, Xu Z, Jingqi F, Xiangxiang L, et al. Prognostic value of SMAD4 in pancreatic cancer: A Meta-analysis. Transl Oncol. 2016;9((1)):1–7. doi: 10.1016/j.tranon.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian ZR, Rubinson DA, Nowak JA, Morales-Oyarvide V, Dunne RF, Kozak MM, et al. Association of alterations in main driver genes with outcomes of patients with resected pancreatic ductal adenocarcinoma. JAMA Oncol. 2018;4((3)):e173420. doi: 10.1001/jamaoncol.2017.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittle MC, Izeradjene K, Rani PG, Feng L, Carlson MA, DelGiorno KE, et al. RUNX3 controls a metastatic switch in pancreatic ductal adenocarcinoma. Cell. 2015;161((6)):1345–60. doi: 10.1016/j.cell.2015.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]