Abstract
A 61-year-old patient with end-stage liver cirrhosis was admitted for cataract surgery with corrected distance visual acuities (CDVAs) of 0.3, in both eyes. His international correction ratio (INR) for blood coagulation was 2.1 without any anticoagulants, and general anesthesia was contraindicated. He was deemed inoperable for liver transplantation. Two weeks after uneventful phacoemulsification in his right eye under topical anesthesia, he underwent phacoemulsification for the cataract in the left eye. However, during surgery, extensive zonular dialysis was noted and the surgery proceeded with extracapsular cataract extraction and anterior vitrectomy, during which a rapid suprachoroidal hemorrhage (SCH) was noted. The incisions were then rapidly sutured. Intravenous 150 cc of 18% mannitol and 2 mg midazolam and sublingual 5 drops of nifedipine were given, and he was placed in the slightly reverse-trendelenburg position. Following suturation of the incision, the globe was left aphakic, slightly hypertonic with no loss of vitreous through the incisions. The postoperative treatment regimen of topical prednisolone and moxifloxacin eye drops of each per hour, cyclopentolate three times a day, and peroral prednisolone 40 mg was commenced. Despite no retinal reflex on the first day and no light perception for 2 weeks, transscleral SCH evacuation with limited pars plana vitrectomy was performed in the postoperative third week. Despite recurrent hemorrhage and intravitreal inflammatory bands, choroidal detachments regressed slowly with the improvement of CDVA up to 0.6 with aphakic contact lens correction at 3 months. The patient passed away due to complications of liver cirrhosis at 6 months.
Keywords: Expulsive hemorrhage, phacoemulsification, suprachoroidal hemorrhage
Introduction
Suprachoroidal hemorrhage (SCH) is one of the most frightening and devastating complications of ophthalmic surgeries. It occurs as a result of rapid blood accumulation in the suprachoroidal space due to increased tension and rupture of the posterior ciliary arteries or vortex veins (1). Acute expulsive SCH might occur during surgery, while a delayed SCH might take place hours or days after the surgery (2,3). SCH presents with severe eye pain, marked decrease in visual acuity, shallow anterior chamber, and severe increase in intraocular pressure (IOP) (4). Intraoperative SCH is thought to be a result of acute hypotonia or fluctuation of the IOP (5). Other reasons include choroidal vascular congestion (due to peribulbar or retrobulbar anesthesia) and atherosclerotic vasculature (6).
Although SCH is one of the most sight-threatening complications of cataract surgery, its incidence is fortunately low (7). Herein, we aim to present a case of intraoperative SCH that can be managed rapidly and appropriately. A satisfactory good visual acuity can be gained after proper intervention and medical treatment with patience.
Case Report
A 61-year-old male patient was admitted to our clinic with decreased vision in both eyes. Corrected distance visual acuity (CDVA) was 0.3 bilaterally in Snellen lines with –2.75 (–1.25@110) in the right eye and –2.50 (–1.25@45) in the left eye. His slit-lamp examination, IOP measurements, fundus examination, and optical coherence tomography findings were within normal limits, except for bilateral stage 3 nuclear cataracts. The patient had a history of alcohol-induced liver failure and hypertension. He was deemed inoperable for liver transplantation, and general anesthesia was contraindicated. The international correction ratio (INR) was 2.1 without any anticoagulants. Other preoperative laboratory results of the patient are shown in Table 1. Uncomplicated phacoemulsification and intraocular lens (IOL) implantation were performed with topical anesthesia in the right eye, by an experienced cataract surgeon (CAU). The postoperative visual acuity was 1.0 without any spectacle correction.
Table 1.
Preoperative laboratory results of the patient
| Result | Unit | Reference | |
|---|---|---|---|
| AST | 72 | U/L | 0–50 |
| ALT | 23 | U/L | 0–50 |
| GGT | 38 | U/L | 0–55 |
| ALP | 120 | U/L | 30–120 |
| Albumin | 2.53 | g/dL | 3.5–5.2 |
| Total bilirubin | 7.20 | mg/dL | 0.3–1.2 |
| Direct bilirubin | 2.82 | mg/dL | 0–0.2 |
| WBC | 8.6 | 103 µL-1 | 4–10.3 |
| Hb | 13.3 | g/dL | 13.5–17.5 |
| PLT | 173 | 103 µL-1 | 156–373 |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: Gamma-glutamyl transferase; ALP: Alkaline phosphatase; WBC: White blood cell; Hb: Hemoglobin; PLT: Platelet.
Two weeks later, during phacoemulsification surgery of the left eye under topical anesthesia, up to 180° zonular dialysis was noted, and the surgery proceeded with extracapsular cataract surgery and anterior vitrectomy. However, a rapidly progressing black shadow from the back of the eye was noted indicating a SCH. Interestingly, the patient did not notice any pain. The incisions were then rapidly sutured (Fig. 1a), and at the same time, arterial blood pressure was reduced by the administration of intravenous 150 cc of 18% mannitol, 2 mg midazolam, and sublingual 5 drops of nifedipine. The patient was placed in a slightly reverse-trendelenburg position, while sutures were placed on the incisions. The globe was left mildly hypertonic with no prolapse of the vitreous through corneal incisions. Postoperatively, topical prednisolone acetate and moxifloxacin one drop of each per hour, cyclopentolate three times a day, and peroral prednisolone 40 mg treatment was commenced.
Figure 1.
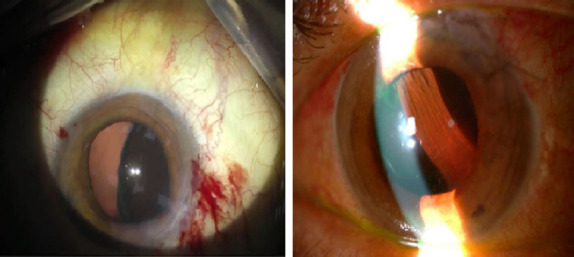
Intraoperative and postoperative images of loss of retinal reflex and development of suprachoroidal hemorrhage during the phacoemulsification surgery.
The retinal red reflex could not be obtained on the postoperative first day (Fig. 1b). Despite visual acuity of no light perception at the second week due to severe hemorrhage, transscleral SCH evacuation and a limited pars plana vitrectomy were performed at postoperative third week (Fig. 2) (FHÖ). A recurrent hemorrhage occurred postoperatively, but the CDVA increased to counting fingers at 10 cm. Nasal and temporal choroidal detachments (Fig. 3a) and intravitreal inflammatory bands (Fig. 3b) regressed slowly following subtenon triamcinolone acetonide injection, which was performed 2 weeks after the vitreoretinal surgery. CDVA then progressively improved to 0.6 with aphakic correction, at the postoperative third month (Fig. 4). Secondary IOL implantation was not planned due to high-risk characteristics; instead, an aphakic contact lens correction was performed and adopted by the patient. Unfortunately, the patient passed away due to liver failure complications at postoperative sixth month.
Figure 2.
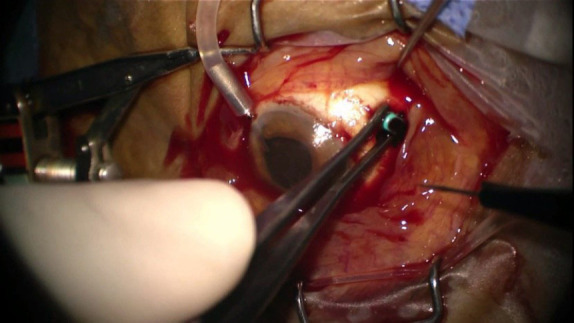
Transscleral evacuation of suprachoroidal hemorrhage 3 weeks after the incident.
Figure 3.
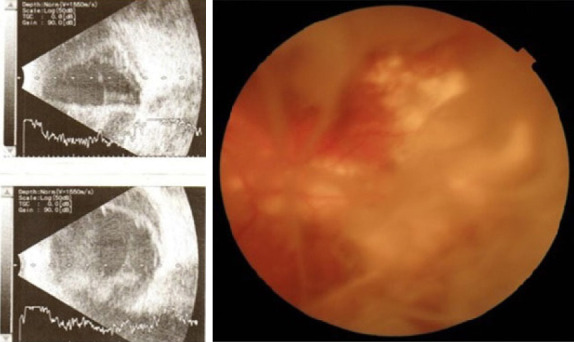
Nasal and temporal choroidal detachment, choroidal infarct areas at the vascular arcades, and intravitreal inflammatory bands, at postoperative first month.
Figure 4.
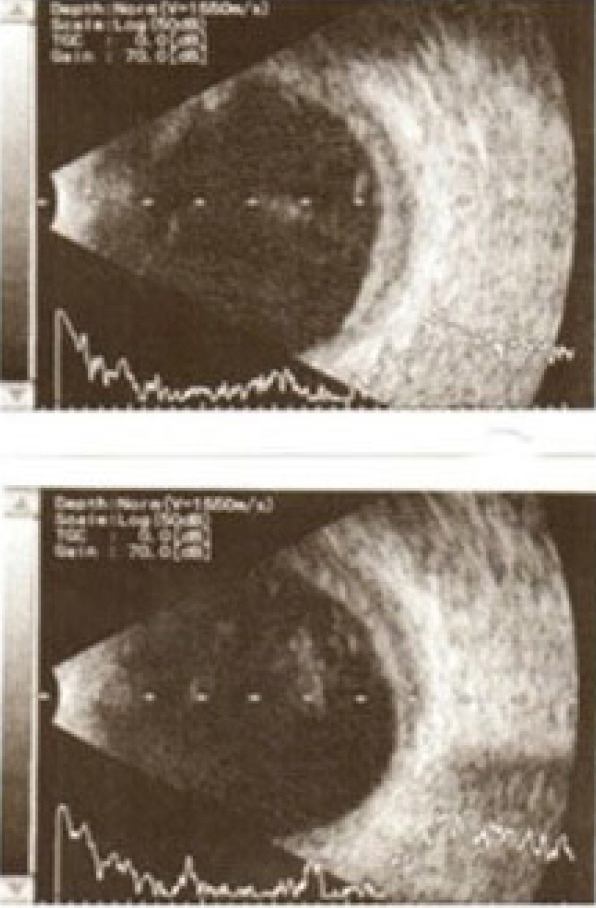
B-mode ultrasound at postoperative third month.
Discussion
SCH is characterized by a rapid and massive accumulation of blood in the suprachoroidal space (2). Tightening and rupture of the choroidal vessels due to rapid reduction or fluctuation of IOP precipitates the event. SCH can occur with any intraocular surgery, mostly after glaucoma surgery and rarely after vitrectomy and keratoplasty surgeries (8–10). Hypotonia after glaucoma surgery, aphakia, systemic hypertension, anticoagulant medication, high myopia and high axial length, presence of retinal detachment, previous history of vitrectomy, and postoperative emesis are among risk factors for SCH development (11,12).
The incidence of SCH after cataract surgery ranges from 0.03% to 0.13% (1,11). Phacoemulsification technique is advantageous as it necessitates smaller incisions, provides closed irrigation-aspiration system and IOP control, and allows faster postoperative wound healing. It has been shown that the use of smaller incisions or scleral tunnels is more beneficial for SCH control (13), demonstrating the advantages of a closed system and less reduction in IOP.
Findings indicating the development of SCH during surgery are shallow anterior chamber (78%), increased IOP (59.6%), posterior capsule stretching (39.4%), loss of red reflex (36.7%), and prolapse of intraocular structures (iris, lens, vitreous, and retina). In these cases, early diagnosis and rapid suturation of incisions without vitreous and iris prolapse are important to achieve optimal visual acuity. When SCH is detected, it is important to place the patient in a slightly reverse-trendelenburg position to decrease the central venous pressure and reduce the fluid passage to the choroidal layer. In addition, the osmotic effects of SCH should be controlled by giving intravenous 150 cc of 18% mannitol. Administering 1–3 mg midazolam and/or sublingual 5 drops of nifedipine helps to reduce systemic blood pressure. The pain and anxiety of the patient should be relieved (14). If effective closure cannot be achieved or if high IOP threatens the vitality of the optic nerve, posterior sclerotomy is indicated for drainage of hemorrhage (6). If the globe can be effectively sutured and left normotonic or slightly hypertonic, SCH drainage should be delayed to decrease the risk of rebleeding. Conservative management includes topical steroids, cycloplegia, and antibiotics, as well as systemic steroids.
SCH drainage can be performed through posterior sclerotomy incisions. If the intraoperative IOP is severely high to threaten the vitality of the optic nerve, drainage can be done intraoperatively. Otherwise, the drainage process is recommended to be performed 10–14 days after SCH, to allow blood liquefaction to occur (15). In some cases, a combined limited vitrectomy with sclerotomy can be performed (16). Fei et al. reported a patient with massive SCH during cataract surgery who underwent an early successful tissue plasminogen activator assisted vitrectomy and attained a good visual outcome (visual acuity increased to 0.5 from light perception in this case) (17). In our case, the patient had no light perception immediately after the surgery, not because of any threat to the viability of the optic nerve but due to the thick blood layer that had accumulated at the suprachoroidal area and hindered the light to reach the posterior pole. This was also apparent as the vital and preserved optic disk and macular anatomy after the evacuation of the blood allowed increased postoperative visual acuity.
The underlying disease for bleeding diathesis in our case was end-stage liver failure. Hemostasis deficiency in liver disease occurs with mixed mechanisms. Thrombocytopenia, thrombocyte dysfunction, dysfibrinogenemia, vitamin K deficiency, and hyperfibrinolysis coexist in these patients. Bleeding risk should be thoroughly assessed before the surgery to prevent the development of SCH. In cases with low-to-intermediate risk, it is sometimes sufficient to increase the platelet (PLT) count above 50,000 μL–1 with a single PLT concentrate infusion. In high-risk surgical situations, it is aimed to keep the PLT level above 100,000 μL–1 (18). Prophylaxis is not required before minor and moderate surgical interventions in patients with an INR value < 2.0. Fresh frozen plasma, thrombocyte suspensions, deamino-delta-D-arginine vasopressin infusion, and 5–10 mg vitamin K can be used prior to high-risk surgeries or in patients with severe hemostasis deficiency (19,20). PLT levels of our patient were 173,000 μL–1 preoperatively and 149,000 μL–1 postoperatively. Therefore, prophylaxis was not indicated.
Cataract surgery is generally considered an elective procedure except for specific conditions such as phacomorphic glaucoma or traumatic cataract. The patients who were recommended to have cataract surgery should be evaluated for systemic conditions in advance to prevent intraoperative/postoperative complications. Complete blood count and coagulation panel should be performed and blood values, especially the INR values, should be stabilized before the operation to avoid hemorrhagic complications like SCH.
Although SCH is not a common complication of cataract surgery, there are several similar cases like ours in the literature. In addition, relatively short follow-up time and lack of late-phase colored fundus images due to the patient’s passing can be considered as the limitations of this report.
In conclusion, SCH occurs due to increased tension and rupture of choroidal vessels as a result of the sudden drop or fluctuation in IOP during cataract surgery. Although it is one of the serious and sight-threatening complications of cataract surgery, fortunately, its incidence is low. Although there are several similar case reports in the literature, this case is unique in having no history of anticoagulant use and no indications for bleeding prophylaxis due to liver deficiency, but the patient experienced intraoperative SCH with absolutely no pain. With rapid diagnosis and prompt intraoperative management, as well as accurate postoperative intervention and medical treatment with patience, it is possible to achieve good visual acuity after SCH.
Acknowledgment
We owe our former head of department, Prof. Dr. Ferit Hakan Öner, a debt of gratitude for his generous support for the management of this challenging case. Unfortunately, he passed away in August 2020, but his legacy will live with us forever.
Footnotes
Disclosures
Informed consent: Written informed consent was obtained from the patient for the publication of the case report and the accompanying images.
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
Authorship Contributions: Involved in design and conduct of the study (CAU); preparation and review of the study (CAU, SK); data collection (MK).
References
- 1.Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology. 2011;118:1716–23. doi: 10.1016/j.ophtha.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu TG, Green RL. Suprachoroidal hemorrhage. Surv Ophthalmol. 1999;43:471–86. doi: 10.1016/s0039-6257(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 3.Gressel MG, Parrish RK 2nd, Heuer DK. Delayed nonexpulsive suprachoroidal hemorrhage. Arch Ophthalmol. 1984;102:1757–60. doi: 10.1001/archopht.1984.01040031421015. [DOI] [PubMed] [Google Scholar]
- 4.Tuli SS, WuDunn D, Ciulla TA, Cantor LB. Delayed suprachoroidal hemorrhage after glaucoma filtration procedures. Ophthalmology. 2001;108:1808–11. doi: 10.1016/s0161-6420(01)00763-1. [DOI] [PubMed] [Google Scholar]
- 5.Speaker MG, Guerriero PN, Met JA, Coad CT, Berger A, Marmor M. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology. 1991;98:202–9. doi: 10.1016/s0161-6420(91)32316-9. [DOI] [PubMed] [Google Scholar]
- 6.Learned D, Eliott D. Management of delayed suprachoroidal hemorrhage after glaucoma surgery. Semin Ophthalmol. 2018;33:59–63. doi: 10.1080/08820538.2017.1353814. [DOI] [PubMed] [Google Scholar]
- 7.Ling R, Cole M, James C, Kamalarajah S, Foot B, Shaw S. Suprachoroidal haemorrhage complicating cataract surgery in the UK:epidemiology, clinical features, management, and outcomes. Br J Ophthalmol. 2004;88:478–80. doi: 10.1136/bjo.2003.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig SB. Delayed massive suprachoroidal hemorrhage after descemet stripping automated endothelial keratoplasty. Cornea. 2011;30:818–9. doi: 10.1097/ICO.0b013e318201255a. [DOI] [PubMed] [Google Scholar]
- 9.Qian CX, Harissi-Dagher M. Delayed suprachoroidal haemorrhage following Boston Keratoprosthesis in two aniridic patients. Br J Ophthalmol. 2011;95:436–7. doi: 10.1136/bjo.2010.189571. [DOI] [PubMed] [Google Scholar]
- 10.Mantopoulos D, Hariprasad SM, Fine HF. Suprachoroidal hemorrhage:risk factors and diagnostic and treatment options. Ophthalmic Surg Lasers Imaging Retina. 2019;50:670–4. doi: 10.3928/23258160-20191031-01. [DOI] [PubMed] [Google Scholar]
- 11.Song W, Zhang Y, Chen H, Du C. Delayed suprachoroidal hemorrhage after cataract surgery:A case report and brief review of literature. Medicine (Baltimore) 2018;97:e8697. doi: 10.1097/MD.0000000000008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akkan AydoğmuşFS, Serdar K, Kalayci D, Çelik A. Spontaneous suprachoroidal hemorrhage associated with iatrogenic coagulopathy. Retin Cases Brief Rep. 2019;13:174–5. doi: 10.1097/ICB.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 13.Davison JA. Acute intraoperative suprachoroidal hemorrhage in extracapsular cataract surgery. J Cataract Refract Surg. 1986;12:606–22. doi: 10.1016/s0886-3350(86)80075-x. [DOI] [PubMed] [Google Scholar]
- 14.Savastano A, Rizzo S, Savastano MC, Piccirillo V, Forte R, Sbordone S, et al. Choroidal effusion and suprachoroidal hemorrhage during phacoemulsification:intraoperative management to prevent expulsive hemorrhage. Eur J Ophthalmol. 2016;26:338–41. doi: 10.5301/ejo.5000707. [DOI] [PubMed] [Google Scholar]
- 15.Chu TG, Cano MR, Green RL, Liggett PE, Lean JS. Massive suprachoroidal hemorrhage with central retinal apposition. A clinical and echographic study. Arch Ophthalmol. 1991;109:1575–81. doi: 10.1001/archopht.1991.01080110111047. [DOI] [PubMed] [Google Scholar]
- 16.Jin W, Xing Y, Xu Y, Wang W, Yang A. Management of delayed suprachoriodal haemorrhage after intraocular surgery and trauma. Graefes Arch Clin Exp Ophthalmol. 2014;252:1189–93. doi: 10.1007/s00417-013-2550-x. [DOI] [PubMed] [Google Scholar]
- 17.Fei P, Jin HY, Zhang Q, Li X, Zhao PQ. Tissue plasminogen activator-assisted vitrectomy in the early treatment of acute massive suprachoroidal hemorrhage complicating cataract surgery. Int J Ophthalmol. 2018;11:170–1. doi: 10.18240/ijo.2018.01.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reding MT, Key NS. Hematologic problems in the surgical patient:Bleeding and thrombosis. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, editors. Hematology basic principals and practice. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 2613–28. [Google Scholar]
- 19.Tosetto A, Balduini CL, Cattaneo M, De Candia E, Mariani G, Molinari AC, Rossi E, et al. Italian Society for Haemostasis and Thrombosis. Management of bleeding and of invasive procedures in patients with platelet disorders and/or thrombocytopenia:Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) Thromb Res. 2009;124:e13–8. doi: 10.1016/j.thromres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Chongsrisawat V, Suprajitporn V, Kittikalayawong Y, Poovorawan Y. Platelet count in predicting bleeding complication after elective endoscopy in children with portal hypertension and thrombocytopenia. Asian Biomedicine. 2009;3:731–4. [Google Scholar]


