Key Points
Question
Is concomitant use of oxycodone with selective serotonin reuptake inhibitors (SSRIs) that are potent inhibitors of oxycodone metabolism via the cytochrome-P450 2D6 (CYP2D6) enzyme (fluoxetine and paroxetine) associated with opioid overdose?
Findings
In this cohort study of more than 2 million US adults, use of SSRIs that are potent inhibitors of oxycodone metabolism at the time of oxycodone therapy initiation was associated with a small but significantly higher risk of opioid overdose compared with the use of other, noninhibiting SSRIs.
Meaning
These findings suggest that concomitant use of oxycodone with potent CYP2D6-inhibiting SSRIs may increase the risk of opioid overdose; other SSRIs should be considered for patients undergoing SSRI and oxycodone therapy.
This cohort study compares opioid overdose rates in patients initiating oxycodone while taking selective serotonin reuptake inhibitors (SSRIs) that are potent inhibitors of the cytochrome-P450 2D6 enzyme (CYP2D6) vs SSRIs that are not.
Abstract
Importance
Some selective serotonin reuptake inhibitors (SSRIs) inhibit the enzymes responsible for the metabolism of oxycodone, a potent prescription opioid. The clinical consequences of this interaction on the risk of opioid overdose have not been elucidated.
Objective
To compare opioid overdose rates in patients initiating oxycodone while taking SSRIs that are potent inhibitors of the cytochrome-P450 2D6 enzyme (CYP2D6) vs SSRIs that are not.
Design, Setting, and Participants
This cohort study included adults who initiated oxycodone while receiving SSRI therapy between 2000 and 2020 whose data were included in 3 US health insurance databases.
Exposures
Use of SSRIs that strongly inhibit CYP2D6 enzyme (fluoxetine or paroxetine) vs use of other SSRIs at the time of oxycodone initiation.
Main Outcomes and Measures
Opioid overdose hospitalization or emergency department visit. Outcomes were assessed within 365 days of oxycodone initiation; in primary analyses, patients were followed up until the discontinuation of either oxycodone or their index SSRI group. Propensity score matching weights were used to adjust for confounding. Crude and weighted (adjusted) incidence rates and hazard ratios were estimated using Cox regression models, separately within each database and overall, stratifying on database.
Results
A total of 2 037 490 initiated oxycodone while taking SSRIs (1 475 114 [72.4%] women; mean [SD] age, 50.1 [15.3] years). Most (1 418 712 [69.6%]) were receiving other SSRIs at the time of oxycodone initiation. In the primary analysis, we observed 1035 overdose events (0.05% of the study cohort). The adjusted incidence rate of opioid overdose in those using inhibiting SSRIs at the time of oxycodone initiation (9.47 per 1000 person-years) was higher than in those using other SSRIs (7.66 per 1000 person-years), indicating a greater risk of overdose among patients using CYP2D6-inhibiting SSRIs (adjusted hazard ratio, 1.23; 95% CI, 1.06-1.31). Results were consistent across multiple subgroup and sensitivity analyses.
Conclusions and Relevance
In this cohort study of US adults, initiating oxycodone in patients treated with paroxetine or fluoxetine was associated with a small increased risk of opioid overdose.
Introduction
Opioid-related overdose deaths continue to impose an extensive and unabated public health burden in the United States.1 Between 1999 and 2019, more than half of all drug overdose deaths in the United States were attributed to opioids, with a substantial number involving prescription opioids.2,3,4 In 2019, nearly a third of all opioid overdose deaths occurred in people taking prescription opioids.5,6 Among the multiple risk factors associated with opioid overdose, a 2018 Surgeon General advisory warned that, even when opioid medications are used as prescribed, patients may be at higher risk of accidental overdose when using medications that can interact with opioids.7 The advisory specifically highlighted sedatives, such as benzodiazepines; however, opioids have the potential to interact, either pharmacokinetically or pharmacodynamically, with many other medications.7,8,9,10 For the most part, the clinical impact of pharmacological drug-drug interactions on opioid-related adverse events, including overdose, is unknown.
Selective serotonin reuptake inhibitors (SSRIs) are commonly coprescribed with opioids.11,12 Many conditions that cause pain, such as neuropathy, rheumatoid arthritis, and fibromyalgia, are also associated with depression.13 Studies also suggest that individuals with depression are more likely to develop new chronic pain that is subsequently treated with an opioid.13,14,15,16,17 SSRIs are the most commonly used class of antidepressants and one of the most commonly used drug classes overall in the United States.18 Some SSRIs, however, are potent inhibitors of liver enzymes responsible for metabolism of many medications, including oxycodone, a potent opioid. Oxycodone is metabolized by the cytochrome P450 (CYP) enzyme system, and 2 SSRIs, fluoxetine and paroxetine, are potent inhibitors of CYP2D6 enzymes.19,20,21,22,23 Oxycodone is one of the most commonly used opioids implicated in overdose deaths.10 Increased oxycodone plasma concentrations resulting from the interaction with fluoxetine or paroxetine could potentially lead to accidental overdose.24,25,26
Although pharmacokinetic studies have found increased plasma concentrations of oxycodone in the presence of CYP2D6 or CYP3A4 inhibition, no population-based study has examined whether concomitant use of oxycodone and CYP-inhibiting SSRIs is associated with opioid overdose.27,28,29 In this study, we investigated the comparative risk of opioid overdose in a large cohort of US patients who initiated oxycodone while taking SSRIs, comparing oxycodone initiation in the presence of SSRIs known to be potent or strong CYP2D6 inhibitors (fluoxetine and paroxetine) to oxycodone initiation in the presence of other SSRIs.
Methods
We conducted a cohort study following the International Society for Pharmacoepidemiology Guidelines for Good Pharmacoepidemiology Practice.30 The article complies with the Reporting of Studies Conducted Using Observational Routinely Collected Health Data Statement for Pharmacoepidemiology (RECORD-PE) and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.31,32 The study was approved by the Brigham and Women’s Hospital’s Institutional Review Board, including a waiver for informed consent owing to the use of deidentified data.
Data Sources and Settings
We used data from 3 large US health care claims databases covering individuals who received health insurance from commercial and public payers from 2000 to 2020. We specifically assembled deidentified data from Optum Clinformatics Data Mart (2004-2020), IBM Truven MarketScan (2003-2018), and Medicaid Analytic eXtract (MAX; 2000-2014). The deidentified Optum and MarketScan databases contain longitudinal medical and pharmacy claims for commercially insured and nationally representative US population. MAX includes medical and pharmacy claims for persons eligible for Medicaid in 49 US states and the District of Columbia.
Study Design and Population
Figure 1 presents a visual depiction of the study design, and Figure 2 depicts the study cohort selection process. The study population comprised adults aged 18 years or older who initiated oxycodone therapy while receiving SSRIs between 2000 and 2020. We defined initiation of oxycodone by requiring that patients have at least 180 days of oxycodone-free continuous enrollment in the database before their first prescription for oxycodone. We defined an active SSRI prescription as a dispensation before or on the date of oxycodone initiation with days’ supply overlapping the date of oxycodone initiation. We excluded Medicaid patients with incomplete capture of claims, such as those in managed care plans, restricted benefits, or benefits administered through a private plan.
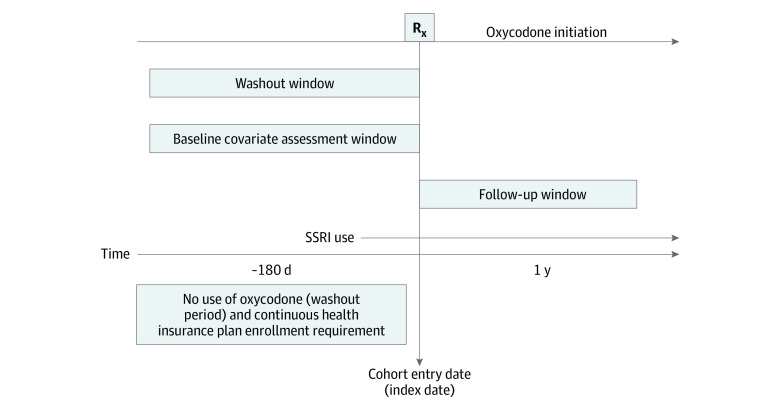
Figure 1. Visual Depiction of the Study Design.
Individuals taking selective serotonin reuptake inhibitors (SSRIs) with no oxycodone prescription in 180 days before their first oxycodone prescription were followed up from the day of oxycodone initiation. Patients were censored at the end of insurance enrollment, end of the database-specific study period, when switching to the other SSRI exposure group, when discontinuing either oxycodone or index SSRI exposure (defined as the end of days’ supply with no subsequent refilling within 14 days), or end of 1-year follow-up, whichever comes first. Rx indicates oxycodone prescription.
Figure 2. Cohort Selection Process.
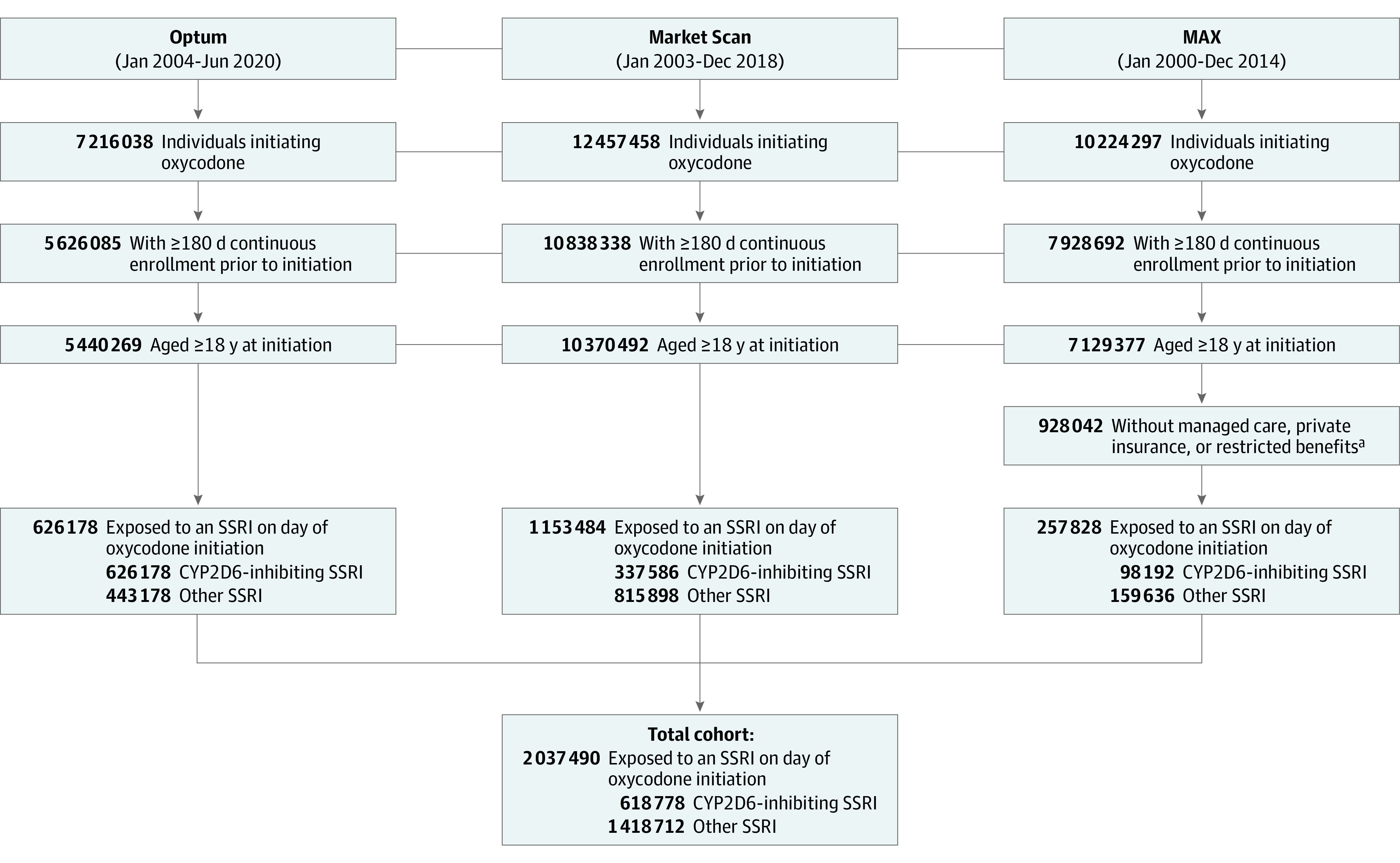
CYP2D6 indicates cytochrome-P450 2D6; MAX, Medicaid Analytic eXtract; and SSRI, selective serotonin reuptake inhibitor.
aMedicaid patients with incomplete capture of claims, which encompasses patients with managed care plans, restricted benefits, or benefits administered through a private plan, were excluded.
Exposure
We assessed medication exposure based on pharmacy-recorded dispensed fills, which included the date of dispensing, quantity dispensed, and days’ supply. Patients were divided into 2 exposure groups based on SSRI exposure on the date of oxycodone initiation. CYP2D6-inhibiting SSRIs included fluoxetine and paroxetine, SSRIs known to be potent inhibitors of CYP2D6.19,20,21,22 Other SSRIs included citalopram, escitalopram, fluvoxamine, and sertraline.
Outcome and Follow-up
The outcome was hospitalization or emergency department visit for opioid overdose. We identified outcomes based on International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes for poisoning by opiates and related narcotics (eTable 1 in the Supplement). ICD-9 codes used to define overdose, together with the code for poisoning by heroin, have been found to have a specificity of 99.9%.33
In the primary analysis, we followed up patients from the day of oxycodone initiation while receiving concomitant SSRI therapy until outcome occurrence, the end of insurance enrollment, end of data availability in the specific database, switching to the other SSRI exposure group, discontinuation of either oxycodone or index SSRI exposure (defined as the end of days’ supply with no subsequent refilling within 14 days), or end of 1 year of follow-up, whichever came first.
Covariates
In total, we assessed 115 covariates that we believed to be risk factors, or proxies for risk factors, for opioid overdose. We assessed demographic characteristics (age, sex, year, and region in the United States) on the date of oxycodone initiation (index date). Comorbidities, prior medications, and health care utilization measures were assessed during the baseline period, defined as 180 days before the date of oxycodone initiation. Comorbidities included pain conditions, psychiatric disorders, depression, neurological disorders, cancer, diabetes, liver disease, and kidney dysfunction as well as opioid abuse or dependence, intentional self-harm, and any drug use disorder or drug dependence. We assessed prior use of antidepressants other than SSRIs, benzodiazepines, muscle relaxants, antipsychotics, anticonvulsants, dementia drugs, triptans, and nonsteroidal anti-inflammatory drugs. Measures of health care utilization included number of physician visits and number of hospitalizations as well as hospitalization in the 30 days prior to oxycodone initiation and the number of unique drugs dispensed during the baseline period. We estimated the amount of prior nonoxycodone opioid exposure using morphine milligram equivalents (MMEs) as well as total oxycodone amount and nonoxycodone opioid amount dispensed on the index date (also in MMEs). Finally, we assessed exposure to other drugs that are known to affect CYP2D6 and CYP3A4 enzymes (eTable 2 in the Supplement) on the index date and estimated chronic disease burden with the combined comorbidity score (a combination of conditions in the Charlson and Elixhauser measures that better estimates patient mortality).34
Statistical Analysis
To control for confounding due to nonrandom allocation of patients to treatment groups, we used propensity score (PS) matching weights (MW). The MW approach represents an extension of the inverse probability of treatment weighting (IPTW) that mimics PS matching and standardizes the distribution of covariates to those of patients in empirical equipoise.35 Weights are bound between 0 and 1, reducing the consequences of extreme weights in comparison to IPTW.36 We used logistic regression models to estimate patients’ probabilities of exposure to inhibiting SSRIs vs other SSRIs on the index date as a function of all pretreatment covariates.37,38 We fit a separate PS model within each database. Following PS estimation, we calculated MWs as the smaller of the estimated probabilities of receiving or not receiving inhibiting SSRIs divided by the estimated probability of being assigned to the exposure group a patient was actually in.36 We assessed covariate balance between treatment groups before and after weighting, using standardized differences.39 A standardized difference greater than 0.1 was considered an imbalance.39
We report crude (unweighted) and adjusted (weighted) numbers of events and incidence rates (IR) per 1000 person-years of follow-up for each treatment group. We used Cox proportional hazards models40 to estimate the crude and weighted (ie, adjusted) hazard ratios (HRs) and 95% CIs, comparing potent CYP2D6-inhibiting SSRIs with other SSRIs. We conducted these analyses within each database and in all data pooled from the 3 databases using a Cox regression model, stratified by database. Statistical significance was established by the 95% CIs: if the 95% CI did not include 1, it was considered significant; if it did include 1, it was considered not significant. All analyses were performed using R version 4.1.0. (R Project for Statistical Computing).
To examine the robustness of our findings and account for potential differential informative censoring, we conducted a sensitivity analysis using an intention-to-treat (ITT) approach.41,42 In this analysis, we followed up patients for a maximum of 60 days from oxycodone initiation, regardless of changes in therapy. Patients were followed from the day of oxycodone initiation until the first of outcome occurrence, the end of insurance enrollment, the end of data availability in the specific database, or end of 60 days follow-up.
Additionally, to assess potential effect modification, we conducted subgroup analyses, stratified by pertinent patient characteristics, such as age (<65 years vs ≥65), sex, total MME dispensed on the index date (categorized as less than the median value vs the median or greater), and prior opioid use (defined as any nonoxycodone opioid dispensing during the baseline period). In these analyses, we reestimated database-specific PSs and MWs within each specific subgroup of interest and pooled the data from the 3 databases, as described previously. Unless otherwise noted, sensitivity and subgroup analyses of the outcome model were conducted with the same statistical approaches used in the primary analysis.
Results
Study Population
We identified 2 037 490 individuals who were receiving SSRI therapy at the time of oxycodone initiation; 618 778 (30.4%) were receiving inhibiting SSRIs (paroxetine, 243 787 [12.0%]; fluoxetine, 374 991 [18.4%]) and 1 418 712 (69.6%) were receiving other SSRIs (citalopram, 407 501 [20.0%]; escitalopram, 449 549 [22.1%]; fluvoxamine, 9142 [0.4%]; sertraline, 552 520 [27.1%]). Baseline characteristics were well balanced between the 2 treatment groups before and after PS weighting (Table 1). eTable 3 in the Supplement presents the complete list of baseline characteristics with standardized differences, and eTables 4 to 6 in the Supplement present database-specific characteristics. The mean (SD) age of patients was 50.1 (15.3) years, and 1 475 114 (72.4%) were women.
Table 1. Selected Patient Baseline Characteristics Before and After Propensity Score Matching Weighting.
| Patient characteristic | Unweighted | Weighted | ||||
|---|---|---|---|---|---|---|
| Patients by SSRI group, No. (%) | Standardized differencea | Patients by SSRI group, No. (%) | Standardized differencea | |||
| CYP2D6-inhibiting SSRI (n = 618 778) | Other SSRI (n = 1 418 712) | CYP2D6-inhibiting SSRI (n = 614 385) | Other SSRI (n = 614 336) | |||
| Demographic characteristics | ||||||
| Age, mean (SD), y | 50.09 (14.66) | 50.03 (15.54) | 0.004 | 50.13 (14.68) | 50.08 (14.75) | 0.003 |
| Women | 457 181 (73.9) | 1 017 933 (71.8) | 0.049 | 453 481 (73.8) | 453 318 (73.8) | <0.001 |
| Men | 161 396 (26.1) | 400 463 (28.2) | 160 714 (26.2) | 160 829 (26.2) | ||
| Comorbidities | ||||||
| Combined comorbidity score, mean (SD) | 0.85 (1.82) | 0.97 (2.01) | 0.063 | 0.85 (1.82) | 0.85 (1.83) | 0.001 |
| Alcohol use disorder or dependence | 7534 (1.2) | 17 995 (1.3) | 0.005 | 7454 (1.2) | 7478 (1.2) | <0.001 |
| Anxiety | 145 133 (23.5) | 368 942 (26.0) | 0.059 | 144 172 (23.5) | 144 379 (23.5) | 0.001 |
| Back and neck pain | 213 302 (34.5) | 480 401 (33.9) | 0.013 | 211 468 (34.4) | 211 825 (34.5) | 0.001 |
| Bipolar disorder | 29 533 (4.8) | 55 819 (3.9) | 0.041 | 29 156 (4.7) | 29 530 (4.8) | 0.001 |
| Back pain | ||||||
| Without radiculopathy | 193 753 (31.3) | 433 119 (30.5) | 0.017 | 191 986 (31.2) | 192 272 (31.3) | 0.001 |
| With radiculopathy | 56 462 (9.1) | 127 185 (9.0) | 0.006 | 56 116 (9.1) | 56 221 (9.1) | 0.001 |
| Bone fracture | 14 378 (2.3) | 41 851 (2.9) | 0.039 | 14 377 (2.3) | 14 385 (2.3) | <0.001 |
| Cancer | 50 887 (8.2) | 128 400 (9.1) | 0.029 | 50 656 (8.2) | 50 570 (8.2) | <0.001 |
| COPD, asthma, or oxygen use | 92 373 (14.9) | 207 671 (14.6) | 0.008 | 91 458 (14.9) | 91 499 (14.9) | <0.001 |
| Dementia | 11 966 (1.9) | 42 181 (3.0) | 0.067 | 11 954 (1.9) | 11 957 (1.9) | <0.001 |
| Dental pain | 12 352 (2.0) | 23 015 (1.6) | 0.028 | 12 006 (2.0) | 12 052 (2.0) | 0.001 |
| Depression | 178 850 (28.9) | 434 992 (30.7) | 0.038 | 177 706 (28.9) | 178 363 (29.0) | 0.002 |
| Diabetes | 94 277 (15.2) | 215 056 (15.2) | 0.002 | 93 587 (15.2) | 93 498 (15.2) | <0.001 |
| Diabetic neuropathy | 14 476 (2.3) | 35 465 (2.5) | 0.01 | 14 399 (2.3) | 14 413 (2.3) | <0.001 |
| Epilepsy or convulsions | 15 649 (2.5) | 35 218 (2.5) | 0.003 | 15 404 (2.5) | 15 459 (2.5) | 0.001 |
| Fibromyalgia | 42 235 (6.8) | 87 819 (6.2) | 0.026 | 41 791 (6.8) | 42 011 (6.8) | 0.001 |
| Headache | 87 642 (14.2) | 195 453 (13.8) | 0.011 | 86 759 (14.1) | 87 054 (14.2) | 0.001 |
| Intentional self-harm | 1684 (0.3) | 4036 (0.3) | 0.002 | 1676 (0.3) | 1680 (0.3) | <0.001 |
| Liver disease | 37 041 (6.0) | 89 621 (6.3) | 0.014 | 36 761 (6.0) | 36 812 (6.0) | 0.001 |
| Musculoskeletal injury | 76 285 (12.3) | 175 443 (12.4) | 0.001 | 75 766 (12.3) | 75 906 (12.4) | 0.001 |
| Opioid use disorder or dependence | 7582 (1.2) | 14 835 (1.0) | 0.017 | 7445 (1.2) | 7466 (1.2) | <0.001 |
| Osteoarthritis | 107 848 (17.4) | 248 164 (17.5) | 0.002 | 107 272 (17.5) | 107 210 (17.5) | <0.001 |
| Other arthritis, arthropathies, and musculoskeletal pain | 283 302 (45.8) | 659 259 (46.5) | 0.014 | 281 350 (45.8) | 281 579 (45.8) | 0.001 |
| Other neuropathic pain | 109 214 (17.6) | 249 634 (17.6) | 0.001 | 108 505 (17.7) | 108 727 (2.3) | 0.001 |
| Other drug use disorder or dependence | 14 612 (2.4) | 28 573 (2.0) | 0.024 | 14 345 (2.3) | 14 410 (2.3) | 0.001 |
| Postherpetic neuralgia | 830 (0.1) | 2162 (0.2) | 0.005 | 823 (0.1) | 816 (0.1) | <0.001 |
| Previous overdose | 891 (0.1) | 1699 (0.1) | 0.007 | 870 (0.1) | 868 (0.1) | <0.001 |
| Psychosis | 14 132 (2.3) | 29 951 (2.1) | 0.012 | 13 856 (2.3) | 13 931 (2.3) | 0.001 |
| Kidney dysfunction | 38 050 (6.1) | 99 132 (7.0) | 0.034 | 37 934 (6.2) | 37 890 (6.2) | <0.001 |
| Rheumatoid arthritis | 12 434 (2.0) | 27 110 (1.9) | 0.007 | 12 319 (2.0) | 12 342 (2.0) | <0.001 |
| Tobacco use | 71 124 (11.5) | 167 210 (11.8) | 0.009 | 70 807 (11.5) | 70 901 (11.5) | 0.001 |
| Urinary calculus | 31 176 (5.0) | 75 031 (5.3) | 0.011 | 31 026 (5.1) | 31 100 (5.1) | 0.001 |
| Opioid-related medication use, mean (SD) | ||||||
| Total oxycodone dispensed on index date, MME | 423.94 (554.70) | 413.14 (539.64) | 0.020 | 429.77 (595.47) | 430.12 (598.12) | 0.001 |
| Total nonoxycodone dispensed on index date, MME | 25.64 (136.46) | 23.89 (130.97) | 0.013 | 28.52 (160.72) | 28.56 (160.60) | <0.001 |
| Total dispensed 60 d prior to index date, MME | 559.93 (1464.62) | 484.04 (1364.58) | 0.054 | 583.96 (1644.74) | 584.43 (1655.84) | <0.001 |
| Total dispensed 180 d prior to index date, MME | 1571.82 (4243.76) | 1357.44 (3947.98) | 0.052 | 1633.31 (4723.77) | 1635.05 (4759.39) | <0.001 |
| Other prior medications | ||||||
| Other antidepressants | 162 188 (26.2) | 344 128 (24.3) | 0.045 | 160 391 (26.1) | 161 212 (26.2) | 0.003 |
| Benzodiazepines | 229 768 (37.1) | 508 449 (35.8) | 0.027 | 227 523 (37.0) | 228 132 (37.1) | 0.002 |
| Muscle relaxants | 140 775 (22.8) | 299 501 (21.1) | 0.040 | 139 431 (22.7) | 139 735 (22.7) | 0.001 |
| Other sedatives or hypnotics | 83 542 (13.5) | 198 287 (14.0) | 0.014 | 83 046 (13.5) | 83 354 (13.6) | 0.001 |
| NSAID | 238 226 (38.5) | 522 889 (36.9) | 0.034 | 235 713 (38.4) | 235 789 (38.4) | <0.001 |
| Lithium | 5580 (0.9) | 9210 (0.6) | 0.029 | 5436 (0.9) | 5516 (0.9) | 0.001 |
| Antipsychotics | ||||||
| Atypical | 54 791 (8.9) | 105 438 (7.4) | 0.052 | 54 107 (8.8) | 54 632 (8.9) | 0.003 |
| Typical | 4717 (0.8) | 8682 (0.6) | 0.018 | 4549 (0.7) | 4577 (0.7) | 0.001 |
| Barbiturates | 3311 (0.5) | 5700 (0.4) | 0.020 | 3233 (0.5) | 3235 (0.5) | <0.001 |
| Agents for dementia | 5786 (0.9) | 22 194 (1.6) | 0.057 | 5782 (0.9) | 5783 (0.9) | <0.001 |
| Anticonvulsants | 63 887 (10.3) | 136 013 (9.6) | 0.025 | 63 305 (10.3) | 63 908 (10.4) | 0.003 |
| Gabapentinoids | 81 383 (13.2) | 183 276 (12.9) | 0.007 | 80 791 (13.1) | 81 117 (13.2) | 0.002 |
| Triptans | 29 940 (4.8) | 62 457 (4.4) | 0.021 | 29 667 (4.8) | 29 850 (4.9) | 0.001 |
| CYP3A4, on index date | ||||||
| Inhibitors | 52 695 (8.5) | 119 390 (8.4) | 0.004 | 52 234 (8.5) | 52 192 (8.5) | <0.001 |
| Inducers | 29 604 (4.8) | 62 288 (4.4) | 0.019 | 29 329 (4.8) | 29 583 (4.8) | 0.002 |
| CYP2D6, on index date | ||||||
| Inhibitors | 6769 (1.1) | 16 020 (1.1) | 0.003 | 6707 (1.1) | 6674 (1.1) | <0.001 |
| Inducers | 9539 (1.5) | 18 944 (1.3) | 0.017 | 9355 (1.5) | 9334 (1.5) | <0.001 |
| Health care utilization, mean (SD) | ||||||
| Distinct drugs, No. | 9.44 (5.86) | 9.15 (5.78) | 0.049 | 9.41 (5.85) | 9.43 (5.86) | 0.002 |
| Physician visits, No. | 5.33 (4.31) | 5.44 (4.34) | 0.026 | 5.33 (4.31) | 5.34 (4.27) | 0.003 |
| Hospitalizations, No. | 0.34 (0.77) | 0.37 (0.85) | 0.033 | 0.34 (0.77) | 0.34 (0.76) | 0.001 |
| Hospitalization in 30 d before index, No. (%) | 118 630 (19.2) | 285 570 (20.1) | 0.024 | 117 942 (19.2) | 117 540 (19.1) | 0.002 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CYP, cytochrome P450; MME, morphine milligram equivalent; NSAID, nonsteroidal anti-inflammatory drugs; SSRI, selective serotonin reuptake inhibitor.
Standardized differences greater than 0.1 indicate lack of balance between exposure groups.
Opioid Overdose
The mean duration of concomitant exposure to both oxycodone and an SSRI was 23 days, regardless of exposuire group, and did not differ substantially between the 2 exposure groups (eTable 7 in the Supplement). Most patients (1 507 730 [77.1%]) were censored because they discontinued oxycodone; 482 507 patients (27.2%) were censored because of SSRIs discontinuation (eTable 8 in the Supplement). In the primary analysis, we observed 1035 overdose events (0.05% of the study cohort). We observed 654 crude overdose events during 90 125 person-years of follow-up in the group exposed to other SSRIs and 381 events during 40 053 person-years of follow-up in the group exposed to inhibiting SSRIs, yielding an unadjusted HR of 1.20 (95% CI, 1.06-1.36) for inhibiting SSRIs vs other SSRIs (Table 2). The adjusted incidence rate of opioid overdose for CYP2D6-inhibiting SSRIs (9.47 per 1000 person-years) was higher compared with other SSRIs (7.66 per 1000 person-years). The pooled adjusted analysis revealed that coadministration of oxycodone with CYP2D6-inhibiting SSRIs was associated with a significantly greater hazard of opioid overdose compared with coadministration with other SSRIs (adjusted HR, 1.23; 95% CI, 1.06-1.31). The rate of opioid overdose in Optum and MarketScan databases were comparable, while that in Medicaid population was higher (eTable 9 in the Supplement). All database-specific estimates suggested concordant direction of the association.
Table 2. Risk of Opioid Overdose Associated With Concomitant Use of Oxycodone and CYP2D6-Inhibiting SSRIs vs Oxycodone and Other SSRIs.
| Exposure | No. | Follow-up, person-years | Incidence rate, per 1000 person-years | HR (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Patients | Events | Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Primary analysisa | |||||||
| Other SSRI | 1 418 712 | 654 | 90 125 | 7.26 | 7.66 | 1 [Reference] | 1 [Reference] |
| CPY2D6-inhibiting SSRI | 618 778 | 381 | 40 053 | 9.51 | 9.47 | 1.20 (1.06-1.36) | 1.23 (1.06-1.31) |
| Intention-to-treat analysis | |||||||
| Other SSRI | 1 418 712 | 996 | 224 766 | 4.43 | 4.82 | 1 [Reference] | 1 [Reference] |
| CPY2D6-inhibiting SSRI | 618 778 | 559 | 98 219 | 5.69 | 5.66 | 1.18 (1.06-1.31) | 1.17 (1.05-1.30) |
Abbreviations: CYP, cytochrome P450; HR, hazard ratio; SSRI, selective serotonin reuptake inhibitor.
Patients were censored on discontinuation of either oxycodone or index SSRI exposure group and on switch to the other treatment group.
Sensitivity and Subgroup Analyses
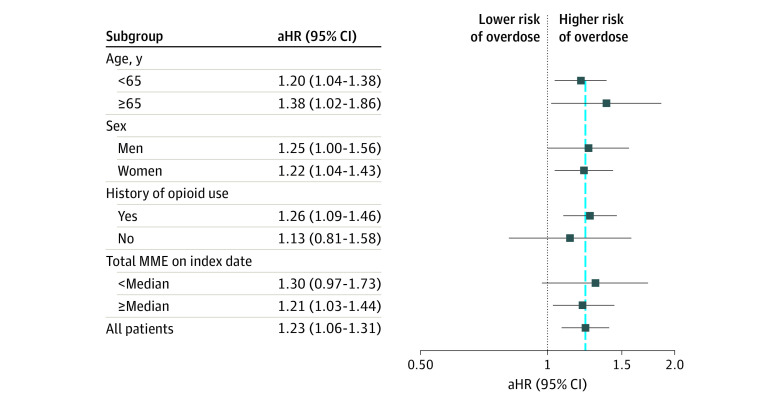
In the 60-day ITT analysis in which patients were not censored on treatment changes, the overall pooled estimate was consistent with the primary analysis estimate (adjusted HR, 1.17; 95% CI, 1.05-1.30). Subgroup analyses showed consistent results. Among older adults (≥65 years), there was an adjusted HR of 1.38 (95% CI, 1.02-1.86) for CYP2D6-inhibiting SSRIs vs other SSRIs (Figure 3).
Figure 3. Association Between Concomitant Exposure to Selective Serotonin Reuptake Inhibitors and Oxycodone and Opioid Overdose Among Subgroups.
The vertical blue line shows the overall measure of association in all patients. aHR indicates adjusted hazard ratio; MME, morphine milligram equivalent.
Discussion
In this large cohort study of a representative sample of more than 2 million patients, starting oxycodone while receiving a CYP2D6-inhibiting SSRI (fluoxetine or paroxetine) vs other SSRIs was associated with a small but significant increase in the risk of opioid overdose. The overall occurrence of outcomes was low, with only 0.05% of patients experiencing an overdose that led to an emergency department visit or hospitalization while receiving concomitant oxycodone-SSRI therapy. Nevertheless, these findings add to our knowledge regarding factors that may contribute to opioid overdoses in adults taking multiple medications.
Prior studies examining the potential for pharmacokinetic drug interaction between CYP2D6-inhibiting SSRIs and oxycodone have produced mixed findings.29,43 Although some pharmacokinetic studies have found that inhibiting the CYP2D6 enzyme can increase oxycodone plasma concentration, others have not observed such an increase.43,44,45 To our knowledge, the current study is the first population-based, real-world study assessing the clinical consequences of this potential interaction. A study that examined outcomes of prescribing hydrocodone, another commonly used opioid analgesic, recommended that for patients taking CYP2D6-inhibiting SSRIs, prescribers select oxycodone or morphine as a more effective alternative, because the authors found that inhibiting CYP2D6 was associated with suboptimal pain control with hydrophone.14 However, the current study’s findings suggest that oxycodone may not be an optimal substitute in the presence of CYP2D6-inhibiting SSRIs.
Given that most studies suggest the central effects of oxycodone on both analgesia and respiratory depression are attributed more to parent oxycodone than to its metabolites,46,47 the higher overdose rate found in our study may have been caused, at least partially, by increased oxycodone plasma concentration from the inhibition of its metabolism by paroxetine or fluoxetine. In addition to strongly inhibiting CYP2D6, both paroxetine and fluoxetine mildly inhibit CYP3A4, which may further increase oxycodone concentration in blood.20,23,48 The narrow therapeutic index of oxycodone may have contributed to the increased risk of overdose.49,50,51 It has been demonstrated that concomitant use of potent inhibitors of CYP2D6 with substrates that have a narrow therapeutic index is more likely to be associated with clinically relevant adverse health outcomes than substrates with a wide therapeutic index.52
Of note, the magnitude of the observed potential interaction in our study is small. Our results suggest that the interaction does not appear to manifest in detrimental clinical outcomes for most patients. Moreover, the overall incidence of overdoses was very low. However, we may have not captured all overdose events that occurred in our cohort because of a highly specific outcome definition that required a hospitalization or emergency department visit. In addition, our study focused on overdoses only; it is possible that the interaction might lead to higher incidence of other, more common oxycodone adverse events, such as drowsiness, confusion, or constipation. While the use of oxycodone has generally decreased from 2014, it is still relatively high.53 Moreover, the rate of overdoses that involve oxycodone has remained relatively high.54 Given that most SSRIs have similar antidepressant effectiveness profiles, it is possible that unintentional opioid overdose events can be prevented by considering an SSRI that is not a potent inhibitor of CYP2D6.
Our findings of a potentially more pronounced increase in overdose risk in persons aged 65 years and older have important implications for routine clinical practice. Possible age-related decreases in clearance of oxycodone55 might increase the risk of overdose in this population. Also, older adults may be more susceptible to adverse effects of opioids and may be taking many other medications that further increase the risk of overdose from drug interactions. Further studies are needed to confirm that older adults indeed have a heightened risk of overdose or other adverse events because of oxycodone drug interactions.
Limitations
This study’s findings should be interpreted with the following limitations in mind. First, administrative claims data do not provide information on clinical parameters that may affect outcomes, such as CYP2D6 genotype, which may act as an important effect modifier.52,56 We also did not have information on some important overdose risk factors, such as illicit drug use and socioeconomic status. It is worth noting, however, that unmeasured risk factors would only confound the association in our study if they were associated with the choice of an SSRI, once the measured variables are controlled for. Given that even before adjustment, the measured baseline characteristics were well balanced between the 2 exposure groups, it is unlikely that the choice of an SSRI was driven by characteristics that would represent a major risk factor for opioid overdose. Moreover, prior studies have shown that adjusting for numerous clinical and health care utilization variables that are available in administrative health care claims databases often balances important, but unmeasured, confounders by proxy.57 Nevertheless, as in any observational study, residual confounding cannot be excluded.
Misclassification of exposure is possible in claims-based analyses because pharmacy claim records provide accurate information about reimbursed drugs dispensed to patients but do not provide information on whether patients actually took the medications as prescribed. Claims-based outcome definitions are also subject to potential misclassification. While we used a highly specific outcome definition,33 which ensures an unbiased measure of relative risk, our definition may have missed events that did not result in contact with health care system, and thus, our incidence rates may be underestimated. ICD-based classification also precludes evaluation of opioid-specific overdoses. Thus, it is possible that some of the outcomes in our cohort were caused by opioids other than oxycodone, including illicit drugs. Moreover, the duration of follow-up was relatively short in our study, as most people only had short oxycodone therapy. Future studies could investigate how the risk changes with longer concomitant duration. The data use agreement with Optum prevents us from knowing the exact degree of potential overlap in the deidentified sample of patients between Optum and MarketScan databases. Furthermore, it should also be noted that because the absolute magnitude of the association observed in our study is statistically significant but small with a narrow confidence interval, we cannot completely rule out a chance finding. Future research may be needed to confirm our findings.
Conclusions
In this cohort study of US adults, initiation of oxycodone while receiving paroxetine or fluoxetine therapy was associated with an increased risk of opioid overdose compared with oxycodone initiation while receiving other SSRIs. The absolute magnitude of the observed association in our study is small, and further research is needed to corroborate our findings.
eTable 1. Outcome Definitions
eTable 2. List of Drugs That Inhibit or Induce Cytochrome P450 2D6 or 3A4 Enzymes Adjusted for in the Propensity Score Model
eTable 3. Patient Baseline Characteristics Before and After Propensity Score Weighting for the Combined Database (Optum, MarketScan, and MAX)
eTable 4. Patient Baseline Characteristics Before and After Propensity Score Weighting for MarketScan Database
eTable 5. Patient Baseline Characteristics Before and After Propensity Score Weighting for MAX Database
eTable 6. Patient Baseline Characteristics Before and After Propensity Score Weighting for Optum Database
eTable 7. Follow-up Duration
eTable 8. Reasons for Censoring by Treatment Group Based on Primary Analysis
eTable 9. Results for Each Database
References
- 1.Baldwin GT, Seth P, Noonan RK. Continued increases in overdose deaths related to synthetic opioids: implications for clinical practice. JAMA. 2021;325(12):1151-1152. doi: 10.1001/jama.2021.1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson N, Kariisa M, Seth P, Smith H IV, Davis NL. Drug and opioid-involved overdose deaths—United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi: 10.15585/mmwr.mm6911a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi: 10.15585/mmwr.mm675152e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi: 10.15585/mmwr.mm7006a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Centers for Disease Control and Prevention (CDC) . Wide-ranging online data for epidemiologic research (WONDER). Accessed July 15, 2021. http://wonder.cdc.gov
- 6.US Centers for Disease Control and Prevention (CDC) . Prescription opioid overdose death maps. Accessed on July 15, 2021. https://www.cdc.gov/drugoverdose/deaths/prescription/maps.html
- 7.HHS.gov . US Surgeon General’s advisory on naloxone and opioid overdose. Accessed July 29, 2021. https://www.hhs.gov/surgeongeneral/priorities/opioids-and-addiction/naloxone-advisory/index.html
- 8.Babu KM, Brent J, Juurlink DN. Prevention of opioid overdose. N Engl J Med. 2019;380(23):2246-2255. doi: 10.1056/NEJMra1807054 [DOI] [PubMed] [Google Scholar]
- 9.Warner M, Trinidad JP, Bastian BA, Minino AM, Hedegaard H. Drugs most frequently involved in drug overdose deaths: United States, 2010-2014. Natl Vital Stat Rep. 2016;65(10):1-15. [PubMed] [Google Scholar]
- 10.Hedegaard H, Bastian BA, Trinidad JP, Spencer M, Warner M. Drugs most frequently involved in drug overdose deaths: United States, 2011-2016. Natl Vital Stat Rep. 2018;67(9):1-14. [PubMed] [Google Scholar]
- 11.Molina KC, Fairman KA, Sclar DA. Concomitant use of opioid medications with triptans or serotonergic antidepressants in US office-based physician visits. Drug Healthc Patient Saf. 2018;10:37-43. doi: 10.2147/DHPS.S151073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perananthan V, Buckley N. Opioids and antidepressants: which combinations to avoid. Aust Prescr. 2021;44:41-44. doi: 10.18773/austprescr.2021.004 [DOI]
- 13.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433-2445. doi: 10.1001/archinte.163.20.2433 [DOI] [PubMed] [Google Scholar]
- 14.Parthipan A, Banerjee I, Humphreys K, et al. Predicting inadequate postoperative pain management in depressed patients: a machine learning approach. PLoS One. 2019;14(2):e0210575. doi: 10.1371/journal.pone.0210575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll LJ, Cassidy JD, Côté P. Depression as a risk factor for onset of an episode of troublesome neck and low back pain. Pain. 2004;107(1-2):134-139. doi: 10.1016/j.pain.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 16.Currie SR, Wang J. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychol Med. 2005;35(9):1275-1282. doi: 10.1017/S0033291705004952 [DOI] [PubMed] [Google Scholar]
- 17.Larson SL, Clark MR, Eaton WW. Depressive disorder as a long-term antecedent risk factor for incident back pain: a 13-year follow-up study from the Baltimore Epidemiological Catchment Area sample. Psychol Med. 2004;34(2):211-219. doi: 10.1017/S0033291703001041 [DOI] [PubMed] [Google Scholar]
- 18.IQVIA Institute for Human Data Science . Medicine use and spending in the US: a review of 2017 and outlook to 2020. Accessed July 29, 2021. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/medicine-use-and-spending-in-the-us-a-review-of-2017-and-outlook-to-2022.pdf?_=1549160094009
- 19.Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drug Metab. 2002;3(1):13-37. doi: 10.2174/1389200023338017 [DOI] [PubMed] [Google Scholar]
- 20.Brown CH. Overview of drug-drug interactions with SSRIs. US Pharmacist. January 23, 2008. Accessed January 25, 2022. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
- 21.Overholser BR, Foster DR. Opioid pharmacokinetic drug-drug interactions. Am J Manag Care. 2011;17(suppl 11):S276-S287. [PubMed] [Google Scholar]
- 22.Donneyong MM, Bykov K, Bosco-Levy P, Dong YH, Levin R, Gagne JJ. Risk of mortality with concomitant use of tamoxifen and selective serotonin reuptake inhibitors: multi-database cohort study. BMJ. 2016;354:i5014. doi: 10.1136/bmj.i5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Indiana University . Drug interactions Flockhart table. Accessed December 30, 2021. https://drug-interactions.medicine.iu.edu/MainTable.aspx
- 24.Kinnunen M, Piirainen P, Kokki H, Lammi P, Kokki M. Updated clinical pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacokinet. 2019;58(6):705-725. doi: 10.1007/s40262-018-00731-3 [DOI] [PubMed] [Google Scholar]
- 25.Gudin J. Opioid therapies and cytochrome p450 interactions. J Pain Symptom Manage. 2012;44(6)(suppl):S4-S14. doi: 10.1016/j.jpainsymman.2012.08.013 [DOI] [PubMed] [Google Scholar]
- 26.Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-S35. doi: 10.1111/j.1526-4637.2011.01134.x [DOI] [PubMed] [Google Scholar]
- 27.Samer CF, Daali Y, Wagner M, et al. The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone. Br J Pharmacol. 2010;160(4):907-918. doi: 10.1111/j.1476-5381.2010.00673.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Söderberg Löfdal KC, Andersson ML, Gustafsson LL. Cytochrome P450-mediated changes in oxycodone pharmacokinetics/pharmacodynamics and their clinical implications. Drugs. 2013;73(6):533-543. doi: 10.1007/s40265-013-0036-0 [DOI] [PubMed] [Google Scholar]
- 29.Grönlund J, Saari TI, Hagelberg NM, Neuvonen PJ, Olkkola KT, Laine K. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010;70(1):78-87. doi: 10.1111/j.1365-2125.2010.03653.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Public Policy Committee, International Society of Pharmacoepidemiology . Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol Drug Saf. 2016;25(1):2-10. doi: 10.1002/pds.3891 [DOI] [PubMed] [Google Scholar]
- 31.Langan SM, Schmidt SAJ, Wing K, et al. The Reporting of Studies Conducted Using Observational Routinely Collected Health Data Statement for Pharmacoepidemiology (RECORD-PE). BMJ. 2018;363:k3532. doi: 10.1136/bmj.k3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 33.Rowe C, Vittinghoff E, Santos GM, Behar E, Turner C, Coffin PO. Performance measures of diagnostic codes for detecting opioid overdose in the emergency department. Acad Emerg Med. 2017;24(4):475-483. doi: 10.1111/acem.13121 [DOI] [PubMed] [Google Scholar]
- 34.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida K, Hernández-Díaz S, Solomon DH, et al. Matching weights to simultaneously compare three treatment groups: comparison to three-way matching. Epidemiology. 2017;28(3):387-395. doi: 10.1097/EDE.0000000000000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657. doi: 10.1136/bmj.l5657 [DOI] [PubMed] [Google Scholar]
- 37.Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637-1638. doi: 10.1001/jama.2015.13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316(17):1818-1819. doi: 10.1001/jama.2016.16435 [DOI] [PubMed] [Google Scholar]
- 39.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolles J, Lewis RJ. Time-to-event analysis. JAMA. 2016;315(10):1046-1047. doi: 10.1001/jama.2016.1825 [DOI] [PubMed] [Google Scholar]
- 41.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766-779. doi: 10.1097/EDE.0b013e3181875e61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gagne JJ, Polinski JM, Avorn J, Glynn RJ, Seeger JD. Standards for causal inference methods in analyses of data from observational and experimental studies in patient-centered outcomes research. Patient-Centered Outcome Research Institute. March 15, 2012. Accessed January 25, 2022. https://www.pcori.org/sites/default/files/Standards-for-Causal-Inference-Methods-in-Analyses-of-Data-from-Observational-and-Experimental-Studies-in-Patient-Centered-Outcomes-Research1.pdf [Google Scholar]
- 43.Grönlund J, Saari TI, Hagelberg NM, Neuvonen PJ, Laine K, Olkkola KT. Effect of inhibition of cytochrome P450 enzymes 2D6 and 3A4 on the pharmacokinetics of intravenous oxycodone. Clin Drug Investig. 2011;31(3):143-153. doi: 10.2165/11539950-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 44.Kummer O, Hammann F, Moser C, Schaller O, Drewe J, Krähenbühl S. Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Eur J Clin Pharmacol. 2011;67(1):63-71. doi: 10.1007/s00228-010-0893-3 [DOI] [PubMed] [Google Scholar]
- 45.Hoffelt C, Gross T. A review of significant pharmacokinetic drug interactions with antidepressants and their management. Ment Health Clin. 2016;6(1):35-41. doi: 10.9740/mhc.2016.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther. 2006;79(5):461-479. doi: 10.1016/j.clpt.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 47.Kaiko RF, Benziger DP, Fitzmartin RD, Burke BE, Reder RF, Goldenheim PD. Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther. 1996;59(1):52-61. doi: 10.1016/S0009-9236(96)90024-7 [DOI] [PubMed] [Google Scholar]
- 48.Feng XQ, Zhu LL, Zhou Q. Opioid analgesics-related pharmacokinetic drug interactions: from the perspectives of evidence based on randomized controlled trials and clinical risk management. J Pain Res. 2017;10:1225-1239. doi: 10.2147/JPR.S138698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elder NM, Atayee RS, Best BM, Ma JD. Observations of urinary oxycodone and metabolite distributions in pain patients. J Anal Toxicol. 2014;38(3):129-134. doi: 10.1093/jat/bku007 [DOI] [PubMed] [Google Scholar]
- 50.Australian Government Department of Health Therapeutic Goods Administration . Australian public assessment report for oxycodone/naloxone. Accessed June 1, 2021. https://www.tga.gov.au/sites/default/files/auspar-oxycodone-170607.docx
- 51.OxyContin Package Insert. Purdue Pharma. Accessed June 1, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020553s059lbl.pdf
- 52.Bernard SA, Bruera E. Drug interactions in palliative care. J Clin Oncol. 2000;18(8):1780-1799. doi: 10.1200/JCO.2000.18.8.1780 [DOI] [PubMed] [Google Scholar]
- 53.Bykov K, He M, Gagne JJ. Trends in utilization of prescribed controlled substances in US commercially insured adults, 2004-2019. JAMA Intern Med. 2020;180(7):1006-1008. doi: 10.1001/jamainternmed.2020.0989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hedegaard H, Miniño AM, Spencer MR, Warner M. Drug overdose deaths in the United States, 1999–2020.NCHS Data Brief, No. 428. National Center for Health Statistics. December 2021. doi: 10.15620/cdc:112340 [DOI] [Google Scholar]
- 55.Dücker CM, Brockmöller J. Genomic variation and pharmacokinetics in old age: a quantitative review of age- vs. genotype-related differences. Clin Pharmacol Ther. 2019;105(3):625-640. doi: 10.1002/cpt.1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stamer UM, Zhang L, Book M, Lehmann LE, Stuber F, Musshoff F. CYP2D6 genotype dependent oxycodone metabolism in postoperative patients. PLoS One. 2013;8(3):e60239. doi: 10.1371/journal.pone.0060239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patorno E, Gopalakrishnan C, Franklin JM, et al. Claims-based studies of oral glucose-lowering medications can achieve balance in critical clinical variables only observed in electronic health records. Diabetes Obes Metab. 2018;20(4):974-984. doi: 10.1111/dom.13184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Outcome Definitions
eTable 2. List of Drugs That Inhibit or Induce Cytochrome P450 2D6 or 3A4 Enzymes Adjusted for in the Propensity Score Model
eTable 3. Patient Baseline Characteristics Before and After Propensity Score Weighting for the Combined Database (Optum, MarketScan, and MAX)
eTable 4. Patient Baseline Characteristics Before and After Propensity Score Weighting for MarketScan Database
eTable 5. Patient Baseline Characteristics Before and After Propensity Score Weighting for MAX Database
eTable 6. Patient Baseline Characteristics Before and After Propensity Score Weighting for Optum Database
eTable 7. Follow-up Duration
eTable 8. Reasons for Censoring by Treatment Group Based on Primary Analysis
eTable 9. Results for Each Database