Abstract
The gene encoding the pneumococcal surface adhesin A (PsaA) protein, psaA, was confirmed in all Streptococcus pneumoniae serotypes by a newly developed PCR (psaA PCR) assay. Eighty-nine of the 90 serotypes amplified produced an 838-bp fragment; the exception was a serotype 16F strain acquired from the American Type Culture Collection (ATCC). Analysis of 20 additional 16F strains from the United States and Brazil showed that the gene was amplified in all 16F strains, implying that the serotype 16F ATCC strain must be a variant. The specificity of the assay was verified by the lack of signal from analysis of heterologous bacterial species (n = 30) and genera (n = 14), including viridans group streptococci. The potential of the assay for clinical application was shown by its ability to detect pneumococci in culture-positive nasopharyngeal specimens. Demonstration of psaA in all 90 serotypes and lack of amplification of heterologous organisms suggest that this assay could be a useful tool for detection of pneumococci and diagnosis of disease.
Streptococcus pneumoniae is frequently isolated from the young, the elderly, and the immunocompromised as the etiologic agent of a broad range of diseases, including meningitis, community-acquired pneumonia, and otitis media (2). A number of diagnostic assays have been developed and are described in the literature, but none are used routinely because they are not sufficiently definitive, reliable, or sensitive (3, 15). The existence of 90 different serotypes of pneumococci increases the challenge of diagnosis and further complicates assay development and vaccine development.
A major area of focus in pneumococcal disease research has been in vaccine development. The failure of the licensed 23-valent polysaccharide vaccine to provide protection in young children (<2 years of age), the elderly, or the immunocompromised (4) led to development of a second-generation protein-conjugate vaccine, soon to be licensed. This vaccine, composed of the seven most frequent invasive disease-causing capsular serotypes, may overcome the problems of poor immunogenicity associated with the 23-valent vaccine. However, there are indications that this protein-conjugate vaccine may not prevent replacement carriage of serotypes not contained in the vaccine (9). These concerns, along with reports of an increase in antibiotic-resistant pneumococci (2), have shifted interest towards the development of a vaccine based on immunogenic pneumococcal species-common proteins of S. pneumoniae (5). The most promising of these proteins include pneumolysin (10), pneumococcal surface protein (PspA) (1), and of particular focus in this study, pneumococcal surface adhesin A (PsaA) (13).
PsaA, a 37-kDa surface protein first identified by Russell et al. (12), is under study both as a vaccine immunogen and as a reagent for diagnostic assay development (15). Monoclonal antibody studies suggest that PsaA is expressed in all 90 serotypes of S. pneumoniae (3), and PCR-restriction fragment length polymorphism analysis of the 23 vaccine serotypes demonstrated the conservation of the gene (psaA) (14). We sought to genetically confirm that psaA is present and detectable by PCR assay in all 90 S. pneumoniae serotypes and to take the first steps in developing, evaluating, and demonstrating the potential of this psaA PCR as a specific and sensitive species-specific diagnostic assay.
Bacterial strains.
The 90 S. pneumoniae serotypes as outlined by Henrichsen (6) were previously obtained from the Statens Seruminstitut, Copenhagen, Denmark, the American Type Culture Collection (ATCC), and the Streptococcal Reference Laboratory, Centers for Disease Control and Prevention (CDC), Atlanta, Ga. The Streptococcal Reference Laboratory provided clinical isolates of the heterologous species Streptococcus mitis, S. oralis, S. mitior, S. parasanguinis, S. sanguinis, S. crista, S. gordonii, S. vestibularis, S. salivarius, and various B-hemolytic strains of S. pyogenes as well as 10 clinical isolates of S. pneumoniae serotype 16F. In addition, heterologous genera, Staphylococcus aureus, Enterococcus faecalis, Corynebacterium minutissimum, Corynebacterium pseudodiphtheriticum, Corynebacterium xerosis, Corynebacterium pseudotuberculosis, Staphylococcus epidermidis, Klebsiella pneumoniae, Escherichia coli, Moraxella catarrhalis, and Haemophilus influenzae, were also obtained from the Streptococcal Reference Laboratory. An additional 10 clinical strains of serotype 16F were provided by Maria Christina de C. Brandileone and Claudio Sacchi, Instituto Adolfo Lutz, Sao Paulo, Brazil. Eleven multidrug-resistant strains of S. pneumoniae were provided by the Pneumococcal Molecular Epidemiology Network. Mycobacterium fortuitum, Norcardia farcinica, and Rhodococcus equi were provided by the Actinomycetes Reference Laboratory, Meningitis and Special Pathogens Branch, CDC; Chlamydia pneumoniae, Pseudomonas aeruginosa, and Mycoplasma pneumoniae were provided by the Respiratory Diseases Laboratory, Respiratory Diseases Branch, CDC.
PCR.
Bacterial strains were grown for isolation on Trypticase soy agar plates supplemented with 5% defibrinated sheep blood for 16 h at 37°C in CO2. For PCR amplification, approximately 5 CFU were placed directly into the PCR mixture and allowed to lyse in the thermocycler. If this method failed to produce an amplified product, whole cells were boiled in 200 μl of filtered water for 10 min and then cooled on ice for at least 5 min. An aliquot of boiled lysate was then used in the PCR mixture.
We obtained nasopharyngeal secretions collected from children under 5 years of age attending a clinic or emergency room in the United States, China, or Israel. These specimens had no identifiers and were unlinked. The secretions had been inoculated into skim milk-tryptone-glucose-glycerol (STGG) transport medium and were prepared by placing a 10-μl aliquot of the specimen into 2.0 ml of Todd-Hewitt broth and incubating the suspension in a tightly capped test tube for 3.5 h in a 37°C water bath. The suspension was then centrifuged at 14,000 × g for 10 min in a microcentrifuge (Eppendorf model 5415C). The pellet was retained and resuspended in 100 μl of ultrafiltered water, and the suspension was centrifuged again. The final pellet was resuspended in 50 μl of filtered water and boiled for 10 min. After boiling, the suspension was cooled on ice for at least 5 min and then used in the psaA PCR mixture as described below.
Primers.
The sequences of the primers used to amplify psaA were as follows: 5′CTTTCTGCAATCATTCTTG3′ (P1) or 5′AGGATCTAATGAAAAAATTAG3′ (P3) as the forward primer and 3′GCCTTCTTTACCTTGTTCTGC5′ (P2) as the reverse primer. All primers were designed from the nucleic acid sequence data from serotype 6B (GenBank accession no. U53509) (14). Primers P1 and P2 yield a 838-bp fragment; primers P3 and P2 yield a 930-bp fragment. Broad-range PCR primers were designed from bacterial 16S ribosomal DNA (rDNA) sequences. All primers were prepared at the Biotechnology Core Facility, CDC.
The PCR mixture (100 μl) contained 100 ng of each primer (P1 and P2, or P3 and P2), 2.0 μl of 10 mM deoxynucleoside triphosphates (Boehringer Mannheim, Indianapolis, Ind.), 10.0 μl of 10 mM MgCl2, 50.0 μl of PCR Master Mix (Boehringer Mannheim), and approximately 5 colonies of bacteria or 10 μl of boiled lysate. Amplification was performed in a Thermal Cycler 480 (Perkin-Elmer, Norwalk, Conn.) for 35 cycles (95°C for 0.5 min, 52°C for 0.5 min, and 72°C for 2.0 min for denaturing, annealing, and extension, respectively), with a final extension at 72°C for 8.0 min. Negative controls contained the PCR mixture without the template DNA. The positive control contained serotype 6B DNA as the template DNA.
Approximately 10 μl of each PCR amplicon was electrophoresed on a 1.0% agarose gel and subsequently stained with ethidium bromide and visualized with a UV transilluminator. Amplified product size was determined by comparison with a 1-kb DNA ladder molecular marker (Life Technologies, Rockville, Md.).
Assay results.
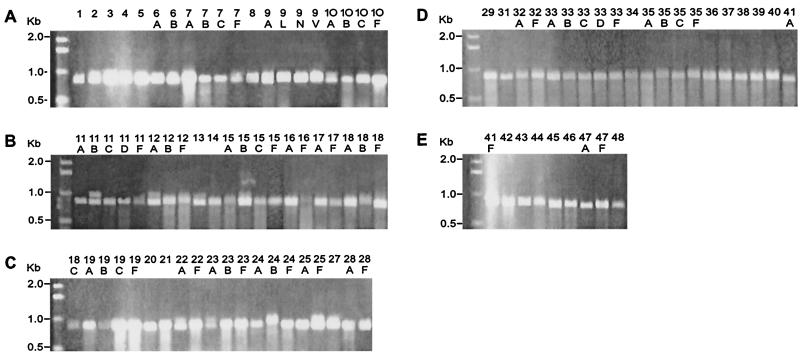
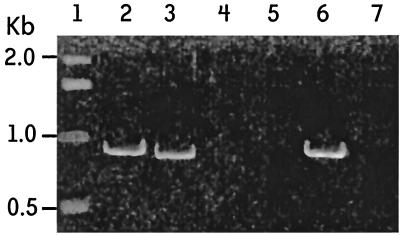
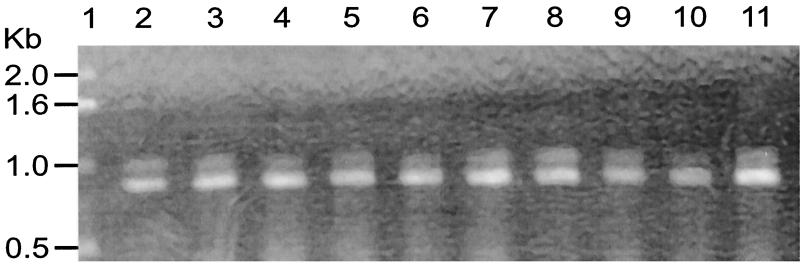
Initial assays showed that psaA in 89 of the 90 S. pneumoniae serotypes was amplified by using primers P1 and P2 (Fig. 1). Amplification resulted in an 838-bp fragment as expected. This fragment is slightly smaller than that of the gene, which is 930 bp. However, for successful amplification of a serotype 16F ATCC strain (ATCC 6316), a different forward primer (P3) had to be used (Fig. 2). This primer was determined from a very conserved region of the N terminus of the psaA sequence (14). To determine if the inability of P1 and P2 primers to amplify 16F was common to all strains of serotype 16F, 10 clinical strains of serotype 16F from the United States and 10 clinical 16F isolates from Brazil were tested with the primers. All 20 clinical strains were amplified by using primers P1 and P2 (Fig. 3), indicating that lack of amplification of the one isolate was peculiar to that ATCC strain of serotype 16F and not to all serotype 16F strains.
FIG. 1.
Amplification of 90 S. pneumoniae serotypes using primers P1 and P2. Agarose gel electrophoresis of PCR-amplified products from serotypes (838-bp fragment) is shown. Serotype designations are indicated above the lanes. (A) Serotypes 1 to 10F; (B) serotypes 11A to 18F; (C) serotypes 18C to 28F; (D) serotypes 29 to 41A; (E) serotypes 41F to 48.
FIG. 2.
Amplification of ATCC serotype 16F using primers P1 and P2. Lane 1, DNA marker; lane 2, serotype 6B amplified with P3 and P2; lane 3, serotype 6B amplified with P1 and P2; lane 4, negative control P3 and P2; lane 5, negative control P1 and P2; lane 6, serotype 16F with P2 and P3; lane 7, serotype 16F with P1 and P2.
FIG. 3.
Amplification of 16F clinical isolates using P1 and P2. Agarose gel electrophoresis of PCR-amplified products from S. pneumoniae serotype 16F clinical isolates is shown. Lane 1, DNA marker; lanes 2 to 11, amplified clinical strains.
To ascertain if there were significant differences within the sequence of the ATCC serotype 16F isolates compared with that of serotype 6B, the DNA sequence of the ATCC serotype 16F psaA gene was determined (B. De, unpublished data) and compared with the sequences of primers P1 and P2. There was a six-base region of variability between the 16F sequence and 6B sequence within the 19-base P1 primer binding site. Therefore, ATCC serotype 16F may be a genetic variant which may have acquired mutations within the gene through recombination with other species. This area of mutation does not occur within the known important functional areas of the gene such as the three monoclonal binding sites (epitopes) previously mapped by Zeiler et al. (J. Zeiler, J. S. Sampson, G. M. Carlone, E. W. Ades, J. A. Tharpe, and M. A. J. Westerink, 1st Internatl. Conf. on Emerg. Infect. Dis., slide session 7, no. 4, 1998.) and the metal binding region of the protein identified by Paton (14).
Novak et al. (8), who examined the effects of mutations in psaA, suggested that PsaA is involved in autolysis and that tolerance to penicillin may be related to loss of PsaA or PsaA function. Therefore, we examined 11 multidrug-resistant strains defined by the Pneumococcal Molecular Epidemiology Network (all penicillin resistant) by the psaA PCR assay. All strains produced the expected DNA fragment of 838 bp upon amplification, indicating that the gene was present in these strains and that no major alterations or deletions occurred within the gene.
The specificity of the primers used in the PCR assay was tested by using various heterologous upper respiratory bacteria as well as other bacteria (Table 1). All bacteria tested failed to amplify with the psaA primers. Broad-range 16S bacterial rDNA primers were used to confirm that there was DNA present in the samples.
TABLE 1.
Heterologous bacteria tested (all were PCR negative)
| Organism | No. of strains tested |
|---|---|
| Streptococcal species | |
| S. mitis | 7 |
| S. oralis | 5 |
| S. parasanguinis | 3 |
| S. sanguinis | 3 |
| S. crista | 4 |
| S. gordonii | 5 |
| S. vestibularis | 2 |
| S. salivarius | 2 |
| Beta-hemolytic streptococci | |
| S. pyogenes | 1 |
| Group C | 3 |
| Group F | 1 |
| Group G | 5 |
| Nonstreptococcal organisms | |
| Pseudomonas aeruginosa | 1 |
| Chlamydia pneumoniae | 1 |
| Mycoplasma pneumoniae | 1 |
| Mycobacterium fortuitum | 1 |
| Nocardia farcinica | 1 |
| Rhodococcus equi | 1 |
| Staphylococcus aureus | 8 |
| Staphylococcus epidermidis | 1 |
| Enterococcus faecalis | 2 |
| Corynebacterium minutissimum | 1 |
| Corynebacterium pseudodiphtheriticum | 1 |
| Corynebacterium xerosis | 1 |
| Corynebacterium pseudotuberculosis | 1 |
| Klebsiella pneumoniae | 1 |
| Escherichia coli | 2 |
| Moraxella catarrhalis | 1 |
| Haemophilus influenzae | 1 |
The sensitivity of the PCR was evaluated by using pneumococcal DNA as a target in fivefold dilutions. The sensitivity was about 1,400 fg, as determined by visualization of the 838-bp band in agarose gels (data not shown).
To evaluate the potential usefulness of the psaA PCR assay as a possible diagnostic method, clinical nasopharyngeal secretions obtained in a blinded manner from children under 5 years of age were tested by both culture and the psaA PCR assay. To improve sensitivity because our specimen size was small (20 μl), we incorporated an enrichment step before the PCR assay and found that positive results could be obtained without purification of the DNA template. Three of four specimens were determined to be positive for S. pneumoniae by culture. Of these three specimens, two were amplified by the psaA PCR assay. These specimens contained ≥25 colonies per 0.1 ml. The third specimen, determined by culture to have fewer than 25 organisms per 0.1 ml, was not detected by the psaA PCR. The fourth specimen was negative by both culture and the psaA PCR. These results demonstrate the potential usefulness of this assay in epidemiologic studies to determine carriage or to confirm the presence of S. pneumoniae. The use of this short enrichment step is ideal for maximal enhancement of potential template when a sample is limited and allows for testing without performing stringent DNA purification procedures. On the other hand, many pneumococcal disease patients have received antibiotics for treatment and any available specimens would contain only nonviable organisms. In this case, enrichment of sample would not be of value and sample preparation procedures via DNA extraction would be preferable since it would make available DNA from both viable and nonviable organisms. Future studies using different types of clinical samples (ear fluid, sputum, and tracheal aspirates) will include investigation of adequate sample sizes and the most effective methods for sample preparation.
This study confirms by PCR analysis the genetic presence of psaA in all 90 S. pneumoniae serotypes. It supports and expands on the previous immunoblot studies by Crook et al. (3), which demonstrated the presence of PsaA epitopes among the 90 serotypes by using monoclonal antibodies. The generation of amplified products of comparable size implies similarity in the gene size and, in turn, gene product and is indicative of a common and highly similar protein.
A protein common to all 90 serotypes with genetic and immunologic similarity has implications both for vaccine studies and diagnostic development. The use of an immunogenic common protein as a vaccine immunogen or carrier could eliminate the need for multiple capsular types in a pneumococcal vaccine and additionally elicit a memory response, which occurs only with protein-based vaccines.
Amplification of the gene from all 90 serotypes also has clinical significance because of the potential to design assays to detect all S. pneumoniae isolates regardless of serotype, eliminating the need for assay reagents representing 90 different components as are used in serologic assays such as latex agglutination and counterimmunoelectrophoresis. Pneumococcal diagnostic assays are based primarily on detection of pneumococci, pneumococcal antigens, DNA, or RNA in blood or body fluids. Although blood culture is currently the most accurate method by which to diagnose pneumococcal disease, only approximately 30% of specimens test positive with this method (7). Conventional serologic methods lack uniform diagnostic sensitivity and specificity and are time-consuming because they require reagents in which all serotypes are represented. Therefore, there is a need for a highly sensitive and highly species-specific method of detection that will make diagnosis of pneumococcal disease less complicated. Our studies suggest that the psaA PCR assay is highly specific, as indicated by the lack of amplification of heterologous bacteria, even viridans group streptococci. Other PCRs reported in the literature (16) have not been widely adapted for use in the clinical laboratory because they have not been shown to be consistently and uniformly sensitive. Toikka et al. (16), for example, described the necessity of testing three different blood fractions to maximize sensitivity. They also suggested that a combination of other diagnostic methods is needed for diagnosis of invasive pneumococcal disease. The sensitivity (sensitivity, 1,400 fg of purified DNA) of the psaA PCR assay needs further evaluation and improvement before use of the assay in broad clinical applications (i.e., with blood or lung aspirates). Although we have not addressed the problems of specimen size, type, or preparation, a more sensitive assay should be achievable by making modifications in components and/or conditions of the assay and by definitively determining the appropriate sample or specimen type.
In conclusion, by demonstrating the presence of the gene (psaA) among the 90 serotypes, we offer an additional candidate toward which to direct development of assays. We also illustrate the potential of the psaA PCR assay for use in epidemiologic studies for identification of S. pneumoniae and for diagnosis of pneumococcal disease. Future studies will include improvement of the sensitivity of the assay as well as examination of clinical samples (i.e., blood, sputum, and lung aspirates) to validate diagnostic usefulness of psaA amplification. It should prove to be a valuable method of S. pneumoniae identification and diagnosis for use in clinical and epidemiologic studies.
Acknowledgments
We thank Maria Christina de C. Brandileone and Claudio Sacchi of the Bacteriology Department, Instituto Adolfo Lutz, and Melinda Brondson of the Navaho Study for providing strains, and the following laboratories at the Centers for Disease Control and Prevention: the Streptococcal Reference Laboratory, the Actinomyces Laboratory, and the Meningococcal Typing Laboratory. We also acknowledge the Centers for Disease Control and Prevention Biotechnology Core Facility for making the oligonucleotides that were used in the study.
REFERENCES
- 1.Briles D E, Yother J, McDaniels L S. Role of the pneumococcal surface protein A in the virulence of Streptococcus pneumoniae. Rev Infect Dis. 1988;10(Suppl. 2):372–374. doi: 10.1093/cid/10.supplement_2.s372. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbid Mortal Weekly Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 3.Crook J, Tharpe J A, Johnson S E, Williams D B, Stinson A R, Facklam R R, Ades E W, Carlone G M, Sampson J S. Immunoreactivity of five monoclonal antibodies against the 37-kilodalton common cell wall protein (PsaA) of Streptococcus pneumoniae. Clin Diagn Lab Immunol. 1998;5:205–210. doi: 10.1128/cdli.5.2.205-210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forrester H L, Jahnigen D W, LaForce F M. Inefficacy of pneumococcal vaccine in a high-risk population. Am J Med. 1987;83:425–430. doi: 10.1016/0002-9343(87)90751-0. [DOI] [PubMed] [Google Scholar]
- 5.Hammerschmidt S, Talay S R, Brandtzeg P, Chhatwal G S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 6.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalin M, Lindberge A A. Diagnosis of pneumococcal pneumonia: a comparison between microscopic examination of expectorate, antigen detection, and cultural procedures. Scand J Infect Dis. 1983;15:247–255. doi: 10.3109/inf.1983.15.issue-3.04. [DOI] [PubMed] [Google Scholar]
- 8.Novak R, Baun J S, Charpentier E, Tuomanen E. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex Psa. Mol Microbiol. 1998;29:1285–1296. doi: 10.1046/j.1365-2958.1998.01016.x. [DOI] [PubMed] [Google Scholar]
- 9.Obaro S K, Adegbola R A, Banya W A S, Greenwood B M. Carriage of pneumococci after pneumococcal vaccination. Lancet. 1996;348:271–272. doi: 10.1016/s0140-6736(05)65585-7. [DOI] [PubMed] [Google Scholar]
- 10.Paton J C. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996;4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 11.Rudolph K M, Parkinson A J, Black C M, Mayer L W. Evaluation of polymerase chain reaction for diagnosis of pneumococcal pneumonia. J Clin Microbiol. 1993;31:2661–2666. doi: 10.1128/jcm.31.10.2661-2666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell H, Tharpe J A, Wells D E, White E H, Johnson J E. Monoclonal antibody recognizing a species-specific protein from Streptococcus pneumoniae. J Clin Microbiol. 1990;28:2192–2195. doi: 10.1128/jcm.28.10.2191-2195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampson J S, O'Connor S P, Stinson A R, Tharpe J A, Russell H. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect Immun. 1994;62:319–324. doi: 10.1128/iai.62.1.319-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson J S, Furlow Z, Whitney A M, Williams D, Facklam R, Carlone G M. Limited diversity of Streptococcus pneumoniae psaA among pneumococcal vaccine serotypes. Infect Immun. 1997;65:1967–1971. doi: 10.1128/iai.65.5.1967-1971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tharpe J A, Russell H, Leionen M, Plikaytis B D, Breiman R F, Carlone G M, Ades E W, Sampson J S. Comparison of a pneumococcal common protein (PsaA) antibody ELISA and a PsaA immune complex ELISA for detection of pneumococcal serum antibody. Pathobiology. 1998;66:77–83. doi: 10.1159/000028000. [DOI] [PubMed] [Google Scholar]
- 16.Toikka P, Nikkari S, Ruuskanen O, Leinonen M, Mertsola J. Pneumolysin PCR-based diagnosis of invasive pneumococcal infection in children. J Clin Microbiol. 1998;37:633–637. doi: 10.1128/jcm.37.3.633-637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]