Abstract
Simultaneously being a non-radiative and non-invasive technique makes magnetic resonance imaging (MRI) one of the highly sought imaging techniques for the early diagnosis and treatment of diseases. Despite more than four decades of research on finding a suitable imaging agent from fluorine for clinical applications, it still lingers as a challenge to get the regulatory approval compared to its hydrogen counterpart. The pertinent hurdle is the simultaneous intrinsic hydrophobicity and lipophobicity of fluorine and its derivatives that make them insoluble in any liquids, strongly limiting their application in areas such as targeted delivery. A blossoming technique to circumvent the unfavorable physicochemical characteristics of perfluorocarbon compounds (PFCs) and guarantee a high local concentration of fluorine in the desired body part is to encapsulate them in nanosystems. In this review, we will be emphasizing different types of nanocarrier systems studied to encapsulate various PFCs and fluorinated compounds, headway to be applied as a contrast agent (CA) in fluorine-19 MRI (19F MRI). We would also scrutinize, especially from studies over the last decade, the different types of PFCs and their specific applications and limitations concerning the nanoparticle (NP) system used to encapsulate them. A critical evaluation for future opportunities would be speculated.
Keywords: magnetic resonance imaging, perfluorocarbons, imaging agent, nanosystems, nanoparticles, fluorine
1. Introduction
For an early stage detection of a disease or a routine medical checkup, it is preferable to have a non-invasive, cost-effective, and patient-friendly technique that makes it approachable and reassuring [1]. In vivo molecular imaging is one such technique that can visualize, quantify, and characterize biological processes at the cellular and molecular levels in a living entity for pretreatment planning, prognostics, and post-treatment surveillance. Screening (detecting diseases in early stages) and identifying the extent of the disease, monitoring for disease recurrence, personalized medicine (selecting patient and disease-specific therapeutic treatment), measuring molecular-specific effects of treatment, predicting and monitoring response to therapy are within the realm of its possibilities [2]. The parameters taken into consideration before choosing an imaging modality are its depth of penetration, temporal resolution (how quickly the image can be acquired/acquisition time), anatomical resolution, spatial resolution, sensitivity (ability to depict molecular features of imaging areas), multiplexing capabilities (ability to simultaneously image/visualize multiple molecular targets), etc. [3]. In most cases, the imaging modality requires the presence of an entity called a contrast agent (CA) to enhance the distinction between the target tissue and the background that overcome the issue of low sensitivity and hence obtain a good quality image. Some of the modalities for molecular imaging are optical, photoacoustic (PAI), ultrasound (US), computed tomography (CT), positron emission tomography (PET)/single-photon emission computed tomography (SPECT), X-ray, and MRI.
2. Types of Molecular Imaging
In optical imaging, the near-infrared (NIR) and visible part of the optical spectrum is feasible for detection with the help of fluorochromes [4]. Fluorochromes injected into the bloodstream emit wavelengths up to about 700 nm. Microscopic lenses are utilized throughout the near infrared spectroscopy and imaging (NIRS) ranging from 600 to 900 nm, with near-transparency of living tissue. Two modes of optical imaging are fluorescence and bioluminescence imaging [5]. Higher intensities (above approximately 1200 W/cm2) overheat the tissues, preventing deeper penetration by upheaving the light intensity used [4]. Optical imaging is an inexpensive technique with temporal resolution in minutes and spatial resolution in millimeters (mm) and is well-suited for diagnostic and microscopic studies of cells and tissue sections in real-time scans. However, the penetration depth, which is limited to a few millimeters, autofluorescence, and poor spatial resolution at greater depths limits the present applicability of optical molecular imaging in clinical practice [6,7].
Photoacoustic imaging (PAI), also known as optoacoustic or thermoacoustic imaging, is a modality for non-invasive visualization based on converting laser into heat [8]. Known for good penetration depth (mm–cm), it can image semi-transparent objects, soft biological tissue, and biological samples. Imaging agents are frequently used, including methylene blue, gold NPs, etc. that have a superior ability to absorb light to produce vivid photoacoustic images [9]. The technique still suffers from certain technical hurdles like the indispensable coupling of the instrument to the subject and the possibility to image only soft tissues and not bones or air structure, and due to the moderate laser energy, a small part of the body can only be imaged at a time.
Ultrasound (US) is a rapid, real-time, soft-tissue imaging technique that is rather inexpensive [10]. However, the spatial resolution (mm–cm) is inconsistent depending upon the required penetration of depth, and it is unsuitable for adult brain imaging as it does not penetrate air gaps or bone. Currently, US is used in the clinic and has an excellent sensitivity [11]. Unsuitable for multiplexing, and its imaging is limited to soft tissues with the unavoidable physical coupling of the device to the subject.
Computed tomography (CT), positron emission tomography (PET)/single-photon emission computed tomography (SPECT) imaging, often used in sequence, uses ionizing radiation. Notwithstanding the use of radiations, CT is the most commonly used clinical imaging modality with its advantage of limitless depth penetration. CT provides mm–cm resolution and a good contrast between hard and soft tissues with a typical scan taking up to 3–4 min to acquire [12]. CT provides mainly anatomical information but has poor sensitivity, specificity, and temporal resolution [13]. PET/SPECT is a radionuclide molecular imaging technique that allows for whole-body imaging of molecular targets or processes, has 1–2 mm resolution, and typically has scan time in minutes. Yet, the need for freshly prepared radioactive chemicals makes it a costly and complex technique. Even though this technique has excellent sensitivity, it has a poor spatial resolution [7,14,15]. PET/SPECT has the great advantage of identifying diseases at early stages since it visualizes molecular targets affected by changes at an earlier stage than that occurring in structural tissue.
The state-of-the-art X-ray imaging uses an X-ray source to get the images and have an inherent high spatial resolution. The instrumentation and use of it are relatively inexpensive, though the imaging process should be precisely monitored. The absorption of X-rays is directly proportional to the atomic number of the absorptive element [16]. A contrast medium is used elsewise soft tissues will not be visible in the image. Except for using an ionizing radiation source like X-rays which can cause radioactive damages in the human body if exceeded a safe dosage, it is essentially a very economic diagnostic technique with a straightforward image acquisition [4]. Refer to the review by Gambhir et al. [3] and Debbage et al. [4], for further explanations on each of the previous techniques.
MRI is an extremely versatile anatomical, functional, and diagnostic imaging technique which excels at deep soft tissue imaging and provides disease information [17]. It can stipulate finer distinctions between soft tissues at higher resolution (mm range) than the previously mentioned imaging techniques without the need for ionizing radiation [18]. In 2010, the Food and Drug Administration (FDA) center for devices and radiological health started an initiative to reduce unnecessary radiation exposure from medical imaging [19], favoring the use of techniques that do not require the use of radioactive sources. Compared to other imaging techniques, limitless sample penetration, the possibility to manipulate contrast between tissues of interest by altering the scan acquisition parameters, and a better differentiation among fat, water, muscle, and other soft tissues make MRI one of the most sought imaging techniques despite low sensitivity (including coil sensitivity), lower temporal resolution (scan time depends on the required resolution and the field of view size) and a time-consuming data acquisition process [3]. Its safety profile allows repetitive imaging sessions, an exigent aspect for prolonged, chronic disorders [20].
Since every imaging technique has its unique benefits and drawbacks, combining imaging modalities (multimodal imaging) can offer synergistic advantages over a single modality to compensate for each imaging method’s inherent limitations, subsequently to obtain more accurate and informative images. In fact, in most studies, multimodal imaging has become a trend both in research and clinic applications for meticulous examinations [21,22,23]. Table 1 compares the different parameters of all the imaging modalities discussed including optical imaging, PAI, US, CT, PET, SPECT, X-ray, MRI.
Table 1.
Features of in vivo imaging modalities including their emission source, technique’s requirement of a contrast agent, their penetration depth, acquisition time and the targeted region for imaging.
| Technique | Emission Source | Need of Contrast Agents | Spatial Resolution | Acquisition Time | Target |
|---|---|---|---|---|---|
| Optical Imaging | Visible and Near-Infrared Light | ✓ | millimeters (mm) | Seconds (S) to Minutes (Min) | Soft tissues |
| Photoacoustic (PAI) | Laser | ✓ | centimeters (cm) | Soft tissues | |
| Ultrasound (US) | Sound Waves | ✓ | cm | S | Soft tissues |
| Computed Tomography (CT), Positron Emission Tomography (PET) Single-Photon Emission Computed Tomography (SPECT) | Gamma Rays | ✓ | mm | Min | Hard tissues and soft tissues |
| X-ray | X-rays | ✓ | micrometer (µm) | S | Hard tissues and soft tissues |
| Magnetic Resonance Imaging (MRI) | Radiofrequency Waves | ✓ | µm–mm | Seconds (S) to Hours | Deep soft tissue |
3. Principles of NMR and MRI
Depending on the appropriately tuned amplifiers and transceiver coils, in theory, any nuclear magnetic resonance (NMR) active nucleus can be used for imaging by MRI [24]. A nucleus with a spin quantum number of ½ (e.g., 1H, 3He, 13C, 14N, 15N, 19F, 19O, 31P, etc.) is designated to be in two spin states and the direction of spin alignment depends on the sign (+/−) of the gyromagnetic ratio, one of the two spin states will align along the magnetic field (ground state, lower energy), whereas the other one will align against it (excited state, higher energy). When an external magnetic field is applied, the spins in the ground state can be promoted to the excited state after absorbing the energy [25]. Upon the termination of the external magnetic field, the spin returns to its equilibrium state (ground state) by a process called relaxation. There are two processes involved, each with an exponential time constant (Ti, i = 1,2): ‘T1’ (longitudinal or spin-lattice) or ‘T2’ (transverse or spin-spin) relaxation times [26]. These parameters help in determining the signal/contrast-to-noise ratio (SNR) and the image resolution.
The distinctiveness in the color density of the images of the biological tissue obtained in the MRI (which is the contrast) is fundamentally due to the difference in the rate of relaxations of the nucleus under study. Standard proton MRI (1H MRI) imaging relies upon the detection of differences in relaxation of water protons to their ground state (relaxation rates) among tissue types, whose signal strengths are reconstructed to give a well-defined distinctive final image [27]. While conventional MRI does not necessarily require the addition of an external CA, there are circumstances when there is not sufficient difference in the relaxation rates of protons among the tissue types (bones, bodily fluids (soft tissues), fat, etc.) to produce a decent contrast. In such cases, an external CA is administered to alter the endogenous proton relaxation times (T1/T2) to obtain highly enhanced tissue contrast signals. Gadolinium(III)-based CAs (GBCA) are among the widely used examples of inorganic substances used for 1H MRI. Currently, a few others are also being explored as potential MRI CAs, including perfluorocarbon (PFC) compounds and fluorinated molecules, which will be extensively considered in this review.
3.1. Gadolinium Based Contrast Agents (GBCAs)
GBCAs are paramagnetic coordination complexes comprising of a Gadolinium-III (Gd(III)/Gd3+) ion and a chelator that independently do not emit MR signals but can bring about a significant reduction of the T1 of nearby water protons [28]. Annually, millions of patients globally undergo MRI scans who receive GBCAs. The lanthanides like Gd are highly coveted CAs due to their intrinsic paramagnetic properties, favorable relaxation time, [29], and stable shelf life. GBCAs permit the imaging of tissues that are less sensitive to motion (hence better quality images) and higher throughput by shortening T1 of the proton [28]. The contrast enhancement function comes from Gd3+ that has seven unpaired electrons. After administering the CA, the diagnostic image is procured while the patient is in the scanner. Generally, the diagnostic and prognostic information attained from MRI predominates the information given from other techniques. Several GBCAs have gained regulatory approvals, including Eovist® (gadoxetate disodium), Omniscan® (a gadodiamide), Gadavist® (gadobutrol), Optimark® (gadoversetamide), etc. [30,31]. The free Gd3+ ion is toxic since its ionic radius is relatively close to zinc, calcium, or iron [32]. Likely interference with calcium ion channels in the living entity is plausible. Gd3+, therefore, needs to be cocooned within chelator (most often used is organic ligands) to avoid those toxicity issues [33,34]. Two classes of chelates developed to complex Gd: linear or macrocyclic organic ligands evade the release of free Gd3+ and make the resulting complexes kinetically and thermodynamically stable [35].
However, in 2006, GBCAs were associated with a devastating and potentially fatal condition called nephrogenic systemic fibrosis [36], recurrently reported in patients suffering from renal deficiency, and its onset can occur months after the last GBCA administration [28]. Furthermore, it is prevailing that some fraction of the residual Gd3+ can remain in the body for long periods, although the chemical form or its whole-body distribution is still obscure [28]. Because of the low sensitivity of MRI, formulation stipulates a high concentration of Gd3+, typically 0.1 mmol kg−1 body weight (approximately 0.5 M aqueous solution) that is hypertonic relative to body fluids [37]. Notwithstanding this, some macrocyclic GBCAs are still sanctioned and can be administered to the patients but in the lowest possible doses. Together, these conclusions have led to renewed interest in finding alternatives to using Gd3+ for MR contrast [38,39]. Further, in 2017, the European medicines agency (EMA) and FDA confirmed the necessity of restricting the use of some linear GBCAs because they tend to release Gd ions in the biological environment [40,41]. For a deeper understanding of GBCAs, the reader is referred to the following reviews [27,28,34,39].
3.2. Fluorine as a Contrast Agent
There is variation among different elements of an NMR active nucleus for their relative natural abundance and response to a magnetic field, meaning that the NMR signal per mole of the compound varies from element to element [24]. Choosing an imaging nucleus from the different NMR active elements depends on its properties entailing to its inherent physical, chemical, and biological properties. In 1977, shortly after the invention of 1H MRI, Holland et al. [42], have demonstrated the feasibility of fluorine-19 suited for fluorine-MRI (19F MRI), which paved the way for new research avenues in molecular and cellular imaging. 19F MRI is anticipated to be a promising imaging tool in the future due to unambiguous detection, acceptable in vivo acquisition times, and relatively high spatial resolution [43]. The external addition of a suitable fluorinated compound (also called a probe/tracer/label) is a prerequisite for 19F MRI/magnetic resonance spectroscopy (MRS).
Only insignificant amounts of endogenous fluorine are embedded in the teeth and bone matrix of the human body. This immobilized fluorine (<10–6 M) has only a very short T2 relaxation as they are in the solid-state and cannot be detected by 19F MRI (that is below the detection limit), which extinguishes the possibility of intrinsic background signals, implying potentially high SNR [44]. Using the same scanner and the receiver electronics of 1HMRI with retuned radiofrequency coils/dual-tuned coils, 19F-images can be superimposed on anatomical, high-resolution 1H images, generating hotspot 19F-images (hybrid 1H/19F MRI) [45,46,47]. The MR effect of the additional element (19F here) does not disturb the local magnetic field either and adds a second colored layer of complementary information to the corresponding grayscale 1H image, hence called “hot spot” [48,49]. Aside from that, 19F is a natural halogen, non-radioactive stable isotope of fluorine [50], unlike the radioactive isotope 18F used in PET imaging [51], and thus it is not necessary to have advanced synthetic skills to introduce fluorine into a probe.
3.3. Similarity between Fluorine and Hydrogen
19F exhibits the NMR phenomenon like 1H, which has one unpaired proton and no unpaired neutrons, and thus with a net spin of ½. Many fluorinated compounds that are non-toxic and chemically inert provide a non-invasive means to study biological systems. When an NMR-active nucleus is placed in an external magnetic field of strength B, it can absorb a photon of frequency ν that depends on the gyromagnetic ratio (γ) of the particle.
| (1) |
In Equation (1), B is the strength of the applied magnetic field (in Tesla [T]), and γ is the gyromagnetic ratio of the nucleus (in MHzT−1). The similarity of 19F’s gyromagnetic ratio to 1H is another strong suit that makes 19F the second most sensitive stable nuclei for MRI followed by 1H (Table 1) [52,53]. At 3T, the typical field strength for clinical MRI scanners, ν is 128 MHz for 1H and 120 MHz for 19F [37]. These frequencies (commonly known as ‘resonance frequencies’) lie in the radiofrequency (RF) range, and hence, MRI signals are RF signals. 19F resonates at a resonant frequency of 94% that of 1H [54]. A huge advantage of MRI over other imaging methods is that RF pulse is non-ionizing radiation and per se can penetrate deep into soft tissues [18]. Once the wave packet of frequency (in this case RF pulse) is applied, as already disclosed, the ground state spins obtain the energy to transition to the excited state, whose energy can be posited by Equation (2)
| (2) |
where h is Planck’s constant (6.626 × 10−34 joules (J)-second (S)). Denoting the population of the ground state as NG and that of the excited state as NE, the MR signal intensity is proportional to the population excess between the two states that can be secured by Equation (3) [37]
| (3) |
At thermal equilibrium, the distribution of spins between the two states obeys Boltzmann’s law. The population ratio, which is the ratio between the spins in the excited state to the ground state, (NE/NG), is obtained by Equation (4) which is 0.9999802 for 1H and 0.9999814 for 19F [37].
| (4) |
where ∆ϵ is the energy difference between the excited and ground state, k the Boltzmann constant (1.381 × 10−23 JK−1), and T, the absolute temperature in kelvin (K). Hence, the MR signal is the output of a tiny population difference between the two states as only 9–10 spins out of almost 10 lakhs contribute to the sequel. It sums to the fact that in the absence of CAs, MRI is an intrinsically low-sensitive technique. NMR receptivity is the absolute NMR sensitivity of a nucleus at its natural abundance [24]. 1H has the most distinguished receptivity of any nucleus. To identify an absolute value of receptivity for other nuclei, it is represented relative to 1H, with 1H having a receptivity of 1. 19F atom with a natural abundance of 100%, has a receptivity of 0.834 relative to 1H, and the fact that it is not a particularly rare (or expensive) element [52] makes it exemplary suitor for replacing 1H. It has a relative sensitivity of 83% compared to 1H and is essentially devoid in biological tissues [52]. Table 2 compares the properties of hydrogen and fluorine that present a large extent of similarity between them except for the chemical shift, for which fluorine is electron-rich, so possesses a high chemical shift.
Table 2.
Comparative properties between hydrogen and fluorine.
| Parameter | 1H | 19F |
|---|---|---|
| Natural abundance (%) | 99.98 | 100 |
| Spin | 1/2 | 1/2 |
| Gyromagnetic ratio (γ) in MHz/T | 42.576 | 40.076 |
| Relative sensitivity | 1.0 | 0.834 |
| Van de Waals’ radius (in Å) | 1.2 (H–C) | 1.35 (F–C) |
| The population ratio (NE/NG) | 0.9999802 | 0.9999814 |
| ∆ϵ/kT at 3T | 1.98 × 10−5 | 1.86 × 10−5 |
| Lattice spacing | 4.97 Å (Hydrocarbon) |
5.9 Å (fluorocarbon) |
| Chemical shifts in ppm (NMR) | 0 to 15 | >350 |
4. Perfluorocarbons (PFCs) as Contrast Agents for 19F MRI
4.1. Physicochemical and Biological Properties of Perfluorocarbon (PFC) Molecules
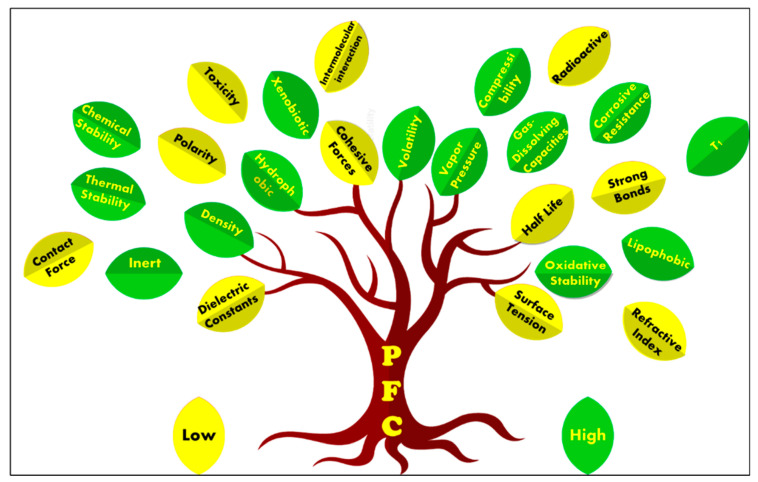
The signal from 1HMRI originates from nearly two-thirds of all protons present in the body, and for 19F MRI to produce an equivalent image, a very high density of 19F nuclei needs to be comprised in the CA to reach an optimal concentration. One way around the prerequisite of high F-concentration is by using PFCs and their derivatives, where all the protons (H’s) of the hydrocarbon chain are switched to 19F nuclei [54,55,56,57]. Other options would be fluorine-rich macromolecules, nanosystems, and paramagnetic metal-containing agents. PFCs are one of the most biologically inert organic molecules ever known and have been under scrutiny for the last few decades [55]. Usually, fluorination enhances the bioavailability of the new drug by increasing lipophilicity [58]. Since fluorine is the highest electronegative element in the periodic table, the covalent C–F bonds are among the strongest known bonds that attribute to the high thermal, chemical, and oxidative stability [44,59].
In addition, they have higher compressibility, higher gas-dissolving capacities, extreme corrosive resistance, high density, high vapor pressure, high fluidity, low cohesive forces, lower dielectric constants, low refractive indices, low polarity, weak intermolecular interactions, and lower surface tension [44,57,60]. The high density and compressibility enable PFCs to reduce the contact force. Even at very high in vivo doses, this class of molecules is biologically compatible with no toxicity partly because of their high hydrophobicity and significant lipophobicity that gives them the tendency to segregate from the surrounding environments [55,60,61].
Between the degree of hydrophobicity and lipophobicity, the former outstands the latter [62,63]. Furthermore, they are xenobiotic, there are no known enzymes that metabolize PFCs in vivo [62], and are degradation resistant [50] at typical lysosomal pH values or in the volatile form such as Freon®. Furthermore, most PFCs, within the molecular weight range of 460–520 Da, exhibit no significant toxicological risks, carcinogenicity, teratogenicity, or mutagenicity [53,54]. The notable properties of PFCs are represented graphically as a tree and its leaves in Figure 1.
Figure 1.
The unique properties of perflurocarbon compounds represented by a tree and its leaves. The green leaves represent the properties of PFC in which the values are higher, and the yellow leaves represent lower values.
4.2. PFC Molecules in a Nanoparticle Formulation
The bottleneck factor for manipulation of most PFCs, bearing in mind its high fluorinated nature, is their simultaneously hydrophobic and lipophobic character, which makes them, in most cases, insoluble in any medium [61,64]. This peculiar feature has an ultimate implication for the design and formulation of MR imaging agents. One way around is to encapsulate/hide PFCs inside a biocompatible coating or capsule to optimize their biopharmaceutical properties. These formulations are accomplished by making nanoemulsions or microemulsions stabilized by surfactants whose employment might also influence cellular uptake [65]. Some of the frequently used surfactants are pluronics and phospholipids, the surface-active agents that can form a film around the dispersed globules of PFC by adsorbing at PFC–water interfaces and reducing the interfacial surface tension (water–PFC interfacial tension is around 60 mN/m) [54]. In many instances, one or more surfactants are blended to get the desired characteristics. By way of alternative, nanoscale micelle had been reported that can self-assemble without the need of surfactants in an aqueous solution like amphiphilic poly-fluorinated polymers [66,67]. It is a pragmatic choice since the emulsification process with a surfactant is adding yet another complexity to the system in addition to equipment and different reagents, and practically the outcome is the formation of a heterogeneous system with disparate NP size.
There are various preparation procedures reported for formulating a stable emulsion with longer shelf life. The techniques are identified as a top-down and bottom-up approach [68]. The commonly used methods are from the bottom-up approach, including emulsion-solvent evaporation method, double emulsion, and evaporation method, the emulsions-diffusion method using a homogenizer or a sonicator, nanoprecipitation (solvent displacement), salting out method, microfluidization, etc. [69]. A perfect nanoemulsion would depend on the definitive desired application, and it is always a balance of emulsion stability, the desired outcome, and body clearance. The therapeutic effect of the nanosystem can be further reduced after administration into the body by proteins adsorbed to the nanosystems surface by so-called ‘protein corona’ formation [70]. There are camouflaging ways to prolong the nanosystems circulation in the blood, including modification of its surface with polyethylene glycol (PEG) [71] shell, dextrose, polysaccharide (chitosan, hyaluronic acid, fucoidan), albumin, or zwitterion, etc. [72]. Research has found that PFC emulsions have no adverse renal toxicity [73], thereupon might be the best alternative for people with kidney complications.
The nanosystems can be chemically/physically modified with a targeting ligand (antibodies and peptides) to amplify their accumulation to the target site [74,75]. Compared to non-targeted nanosystems, targeted nanosystems seem to stay longer in the blood circulation. The former immediately accumulates in the liver and spleen post-injection. Active targeting involves conjugating the NPs with ligands that can specifically bind to cellular antigens in the pathological site of interest. On the other hand, a passive targeting strategy exploits the abnormalities of tumour vasculature that cause leakage of macromolecular agents and NPs into the tumour interstitium, the phenomenon known as the enhanced permeability and retention (EPR) effect [76]. Concomitantly it is possible to equip the nanosystems with other payloads (drugs, genes, protein, etc.) to craft them as a therapeutic agent. Likewise, a theragnostic agent could potentially combine an imaging and a therapeutic agent into a single formulation [77]. There are also ‘‘smart’’ systems that can respond to the biochemical or physiological abnormalities (pH, temperature, the concentration of ions or metabolites, hypoxia, enzyme, etc.) to modulate their SNR by their physical-chemical properties [78,79,80].
The primary clearance system of the majority of the nanosystems in humans is the immune system. The first line of defense that they encounter within the body is the reticuloendothelial system/mononuclear phagocyte system (RES), which can, later on, undergo opsonization (surface adsorption of serum proteins to the nanosystem), and phagocytosis (engulfing and destruction/removal of foreign materials from the bloodstream into organs like liver and spleen) [81]. In principle, the duration of PFCs remaining in the body/exact clearance depends on their chemical structure, individual intrinsic properties, the mode of administration (intravenously, orally, or inhalation), molecular weight, and vapor pressure/volatility [73]. The half-life of the PFCs has an inverse relation to their volatility, which can range from minutes to years. Due to the hydrophobicity of PFCs, they have slow diffusion in their natural form that can prolong their stay in the target site.
After being drawn up by RES, PFC emulsions are diffused back into the blood, where they dissolve in plasma lipids and are carried to the lungs to be expelled out mainly through exhalation by the lungs [56,73,82]. Even though PFC is intrinsically inert, there are reports of severe retention of PFCs in the liver, spleen, and lungs [83,84,85], and the effect of the PFC when stayed too long in vivo or their intracellular fate is currently undetermined [53,86]. In general, nanosystems of size less than 10 nm, exceedingly are devoured by the renal clearance system, 20 to 100 nm by far, is the optimal size range to avoid physiological barriers, 100 to 200 nm particles have extended potential for prolonged circulation, and size greater than 200 nm is retreated almost certainly to spleen and liver and has the possibility for capillary clogging [87]. Frequently, a formulation contrived by design considerations including droplet sizes ranging from 10 to 200 nm (to take advantage of passive targeting by EPR effect), a low polydispersity index (narrow size range) of less than 0.2, and a high fluorine concentration [88]. So far, reported, PFCs have a half-life for blood clearance ranging from 3 h (h) to 42 h and tissue half-life ranging from 4 to 8 days for PFOB, up to 65 days for PFTA, and over 100 days for PFCE [53,89,90].
4.3. Types of PFC Molecules
One of the critical aspects of fabricating an optimized 19F MRI CA using PFC is the chemical structure of the CA itself. The sensitivity of PFCs as MRI CA is highly dependent on the number of fluorine atoms present in the CA, and to increase the signal intensity, the number of fluorine atoms per imaging agent molecule is a vital parameter. In addition, the dosage, magnetic field strength, detector design, etc. affect the sensitivity. PFCs can be detected and quantified directly by 19F NMR, an excellent technique for preliminary studies in 19F MRI. One of the colossal benefits of using PFCs is that their 19FNMR has an extensive chemical shift range (~400 ppm), which asserts the marginal possibility of signal overlap when multiple agents are simultaneously studied [91].
PFCs are clear colorless dense liquids, and their molecular structures generally fall into several classes, including, aromatic–hexafluorobenzene (HFB) [92], trans-1,2-bis(perfluorbutyl)-ethylene (TBPE), 2,3,4,5,6-pentafluorostyrene (PFS)), saturated linear–perfluoro-tert-butanol (PFTB), perfluoropropane (PFP), perfluorohexane (PFH), perfluorononane (PFN), perfluorooctyl bromide (PFOB), perfluorooctanoic acid (PFOA)), saturated ring system–perfluorodecalin (PFD), perfluoro-1,3-dimethylcyclohexane (PFDCH), perfluoroperhydrophenanthrene (PFPHP), perfluoroethers and polyether–perfluoro-15-crown-5 ether (PFCE), perfluoro-2,2,2’,2’-tetramethyl-4,4’-bis(1,3-dioxolane) (PTBD) [93], fluorescent ‘blended’ PFPE amides (FBPA) [94], superfluorinated probe (PERFECTA), perfluoropolyether (PFPE), perfluoroamines–perfluorotriethylamine (PFTA), perfluorotributylamine (FC-43), 19F imaging tracer (19FIT) [95] and perflurosilanes -(pentafluorophenyl)triethoxysilane (PFPTS), 1H,1H,2H,2H-Perfluorooctyltriethoxysilane (PFOTS), Trichloro(1H,1H,2H,2H-perfluorooctyl)silane (TCPFOS) [65] (refer to Table 3 for detailed information on each of the PFCs mentioned). Depending on its structure, the same could be classified as cyclic, branched, linear, and non-linear. Currently, even though not all of the described PFCs are applied in 19F MRI, most of them hold the potential to be trialed for biomedical applications and then sieve them for clinical trials. There is a variety of PFC’s presently available, in which some of them are commercially used for applications such as an ultrasound probe or cell tracking agent.
Table 3.
Survey of PFC molecules for potential MRI applications. The MF stands for the molecular formula/chemical formula, Mw is the molecular weight in g/mol, B.P is the boiling point, the density (D) is expressed in g/mL at 25 °C (lit.), the FNMR signals are estimated based on the molecular structure and based on the fluorine environment: S—Singlet, M—multiple peaks.
| Aromatic PFCs |
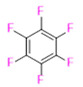
Hexafluorobenzene (HFB) MF = C6F6 Mw = 186.05 B.P = 80.2 °C FNMR signals = S |
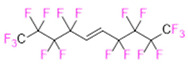 Trans-1,2-bis(perfluoro-N-butyl)ethylene (TBPE) MF = C10H2F18 Mw = 464.09 B.P = 64,3 °C D = 1.675 FNMR signals = M |
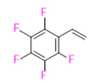
2,3,4,5,6-Pentafluorostyrene (PFS) MF = C8H3F5 Mw = 194.10 B.P = 139–140 °C D = 1.406 FNMR signals = 3 major peaks with spitting |
| Saturated Linear PFCs |
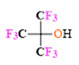
Perfluoro-tert-butanol (PFTB) MF = C4HF9O Mw = 236.04 B.P = 45.0 °C FNMR signals = S |
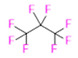
Perfluoropropane (PFP) MF = C3F8 Mw = 188.02 B.P = −36.6 °C FNMR signals = 2 major peaks with spitting |
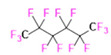
Perfluorohexane (PFH) MF = C6F14 Mw = 338.04 B.P = 56.6–57.2 °C FNMR signals = 3 major peaks with spitting |
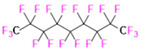
Perfluorononane (PFN) MF = C9F20 Mw = 488.06 B.P = 125–126 °C D = 1.799 FNMR signals = 3 major peaks with spitting |
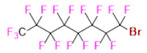
Perfluorooctyl bromide (PFOB) MF = C8BrF17 Mw = 498.96 B.P = 142 °C D = 1.93 FNMR signals = M |
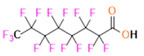
Perfluorooctanoic acid (PFOA) MF = C8HF15O2 Mw = 414.07 B.P = 189.0–192 °C D = 1.792 FNMR signals = M |
|
| Saturated Ring System PFCs |
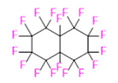
Perfluorodecalin (PFD) MF = C10F18 Mw = 462.08 B.P = 142 °C D = 1.908 FNMR signals = M |
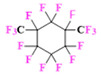
Perfluoro-1,3-dimethylcyclohexane (PFDCH) MF = C8F16 Mw = 400.06 B.P = 101–102 °C D = 1.828 FNMR signals = M |
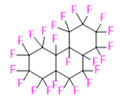
Perfluoroperhydrophenanthrene (PFPHP) MF = C14F24 Mw = 624.11 B.P = 212–218 °C D = 2.03 FNMR signals = M |
| Perfluoroethers and Polyethers |
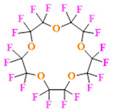
Perfluoro-15-crown-5 ether (PFCE) MF = C10F20O5 Mw = 580.07 B.P = 145 °C D = 1.780 FNMR signals = S |
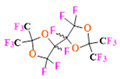
Perfluoro-2,2,2’,2’-tetramethyl-4,4’-bis(1,3-dioxolane) (PTBD) MF = C10F18O4 Mw = 526.08 B.P = ~ 160 °C D = ~1.9 FNMR signals = M |
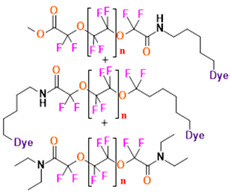
Fluorescent ‘blended’ PFPE Amides (FBPA) MF, Mw, B.P = Depends on repeat unit and the dye attached FNMR signals = 1 major peak, 4 minor peaks |
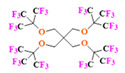
Superfluorinated probe (PERFECTA) MF = C21H8F36O4 Mw = 1008.23 FNMR signals = S |
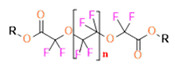
Perfluoropolyether (PFPE) MF, Mw, B.P = Depends on repeat unit and the R-group attached FNMR signals = 1 major peak and 1 minor peak |
||
| Perfluoroamines |
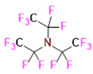 Perfluorotriethylamine(PFTA) MF = C6F15N Mw = 371.05 B.P = 68–69 °C D = 1.736 FNMR signals = 2 major peaks with spitting |
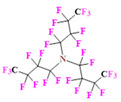
Perfluorotributylamine (FC-43) MF = C12F27N Mw = 671.09 B.P = 178.0 °C D = 1.884 FNMR signals = M |
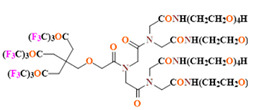
19 F Imaging Tracer (19FIT) MF = C63H94F27N7O27 Mw = 1894.41 FNMR signals = S |
| Perflurosilanes |
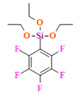
(Pentafluorophenyl)triethoxysilane (PFPTS) MF = C12H15F5O3Si Mw = 330.32 B.P = 69 °C D = 1.242 FNMR signals = 3 major peaks with spitting |
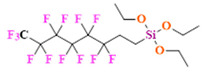
1 H,1H,2H,2H-Perfluorooctyltriethoxysilane (PFOTS) MF = C14H19F13O3Si Mw = 510.36 B.P = 220 °C D = 1.329 FNMR signals = M |
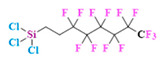
Trichloro(1H,1H,2H,2H-perfluorooctyl)silane (TCPFOS) MF = C8H4Cl3F13Si Mw = 481.54 B.P = 192 °C D = 1.30 FNMR signals = M |
Perfluorooctyl bromide (PFOB/perflubron) is one of the most used PFC materials in biomedicine [96,97]. It is a tasteless and odourless liquid and is extensively adapted for artificial oxygen carriers [73]. It is a dense liquid with a low diffusion coefficient inside the blood, has a longer blood circulation time, and excreted out faster than most other PFCs. It has a linear structure, low surface tension, high specific gravity, finite lipophilicity due to a covalent bond to bromine which enhances its clearance rates from the body [98]. PFCs with additional chemical elements, such as a bromine atom in PFOB, tend to have a short biologic half-life value [99]. They are scarcely absorbed in the gastrointestinal tract, wherefore could be ingested in large doses for bowel imaging [62]. Albeit PFOB displays multiple 19F peaks (eight peaks, one for each CFn moiety) that compromises its sensitivity, it is possible to minimize undesired resonance peaks by including pre-saturation RF pulses with MRI pulse sequences before readout [98,100].
Perfluoropolyether (PFPE) is the simplest linear polymer that is an excellent 19F MRI probe as they provide a single sharp resonance for hassle-free identification, maximizing the SNR and eradicating any chemical shift artifact of the PFC [101]. This class of molecule is known for its remarkable thermal stability and high molecular mobility that improves 19F MRI sensitivity [102]. Linear PFPE possesses end groups susceptible to chemical modification by synthetic strategies [103,104] to bolster additional scope in multimodal imaging. This polymer has short T1 (600 ms) and adequately long T2 (160 ms), the desired trait for an imaging agent. They own a linear structure and high content of MR equivalent 19F nuclei per molecule, with >40 chemically equivalent fluorine [88] that should theoretically give them single resonance. The carbon-oxygen bonds stipulate an increased bond rotation that aids them to be better biodegradable [59].
Using macrocyclic perfluoropolyethers (PFPEs), e.g., the 12, 15, or 18 crown ethers with their high number of equivalent fluorine atoms (16, 20, and 24, respectively) assure an outstanding NMR performance, notably of chemical shift artifacts, SNR, single sharp resonance peak that enable for unambiguous identification, etc. Macrocyclic PFCs such as the perfluoro-15-crown-5 ether (PFCE) assure a substantial improvement in MRI sensitivity with 20 chemically equivalent fluorines (NMR resonance at around ~−92.5 ppm) [98] and is one of the most explored PFC [105,106,107,108,109,110,111,112].
PERFECTA (suPERFluorinatEdContrasT Agent) has 36 chemically equivalent fluorine atoms per molecule, which gives them a single major resonance in FNMR [113]. Unlike other PFCs, they have a polar hydrocarbon core. They are found to have reliable cellular compatibility from the preliminary in vivo F-MRI experiments [113,114].
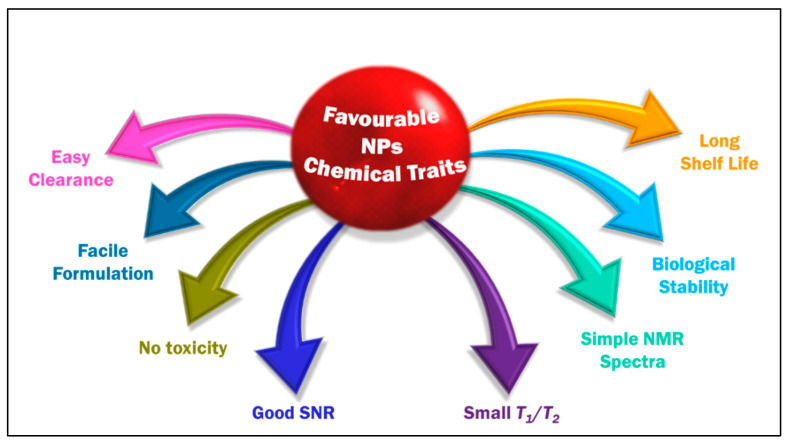
4.4. The Sine Qua Non of Fluorinating Agents in 19F MRI for Clinical Translation—Chemical, Physical and Biological Traits
Even after four decades into the research of 19F MRI, none of the PFC formulations have gained clinical approval [83]. Umpteen requisites should be actualized to extend a formulation into the clinic. Until August 2021, as many as six PFC 19F MRI agents (phase 1) are in clinical trials, mainly employing PFP for cell labelling and lung imaging [115]. There are several parameters and requirement norms for any CA formulation to be spanned to the clinic in 19F MRI. In a nutshell, some of the particulars taken into consideration for the optimal formulation of a probe with PFCs or fluorinated molecules are depicted in Figure 2. In short,
Figure 2.
The favorable characteristics of a CA for 19F MRI.
Significant biological stability and possessing desired chemical traits [91]. The probe must be chemically inert to such an extent that it can endure all of the omnifarious chemicals in the biological milieu until it performs its mission and will be degraded. Most organofluoride compounds easily match this precondition given the strong C–F bond.
An ideal tracer should possess a restrained T1 relaxation time (reduce acquisition times and increase the number of scans per unit time) and an adequately long T2 relaxation time (to avoid signal intensity loss) [116]. A constant relaxation is anticipated in the complex biological environment. T2/T1 ratio close to unity is desirable for a better SNR. One of the drawbacks of PFC is their long T1. When a PFC has intrinsically long T1 relaxation (PFOB and PFCE have T1 relaxations > 1 s), it will severely limit the rate of data acquisition and its sensitivity [98]. Invariably, in the literature, T2 is an easily manipulable parameter, and this is inspected by modulating the length of fluorinated chains in the probe.
High number of magnetically equivalent 19F-content: 19FNMR spectrum, a characterization technique used in the initial analysis, for an ideal CA should be simple, preferably with a single, sharp, narrow resonance and intense peak to maximize sensitivity and prevent chemical shift imaging artifacts. The integral of an NMR signal is quantitative [117,118], directly proportional to the imaging agent concentration. The probe should also have a high fluorine content to give a single dominant signal and a good sensitivity, customarily accomplished employing PFCs. One of the undesired attributes of PFCs is that some of them lack proper symmetry, so they have a split signal in the NMR due to the disparate chemical environment of the fluorine in the molecule. This issue is surmounted by methodically applying 19F MRI probes with high symmetry like PFCE/PFPE or polymeric species like dendrimers.
Prominent SNR enhancement: 19F MRI often suffers from low SNR [119]. The commonly performed strategies to enhance the SNR are to use a CA, modulate the magnetic field strength [120], to improve pulse sequences [121] or hyperpolarization techniques such as dynamic nuclear polarization, chemically induced dynamic nuclear polarization, spin-exchange optical pumping, and parahydrogen-induced polarization that can achieve the same goal [122]. In the case of 19F MRI, using the fluorinated component CA with a considerable amount of fluorine in the probe is also one of the commonly used approaches. Howbeit, high concentrations of CAs might potentially result in toxicity issues.
Nominal/no in vitro and in vivo toxicity: neither should it modify any biological functions nor degrade to give by-products detrimental for other tissues/organs and hence should possess low immunogenicity.
Easy and scalable synthesis and formulation of CA: a reproducible synthesis that can sustain the purity of the formulation with as simple as a single-step reaction and adeptness of scaling up.
Water solubility would be an advantageous feature that would help in the easy application of fluorine. The approach to effectuate water-soluble fluorinated moiety is by chemically modifying the system with hydrophilic compounds or employing hydrophilic hyperfluorinated organofluorine molecules [123]. One requirement for such a probe is possessing a suitable conjugation site. One of the most explored PFC in this regard is PFPE.
A long shelf life is favoured for a probe (at least six months).
Finally, it is always preferred to have an easy clearance from the living system to be approved for clinical application.
4.5. Biomedical Applications of PFC Molecules
PFC emulsions are flourishing in an array of biomedical applications, including molecules with high oxygen solubility for respiration and blood substitution, anaesthetics, chemotherapeutic agents, etc. Time after time it is being used in inflammation studies. Some of the early biomedical applications of PFCs include approved use as artificial oxygen transport vehicles and blood substitutes for human use, as PFC can readily dissolve oxygen and, at a constant temperature, the concentration of O2 in the liquid PFC linearly correlates with the partial pressure of O2 [124]. Hence, the safety profile of PFCs inside the human body is vastly explored. It is also capable of dissolving carbon dioxide and nitrogen. Over and above, incorporating other imaging agents like fluorescein isothiocyanate (FITC), Alexa647, and boron-dipyrromethene (BODIPy) can further extend their application in the field of multimodal imaging. The unique characteristics of PFCs in unison with their hydrophobic nature favored them to be suitable for US imaging as injectable emulsions of PFCs [125]. Some of the PFC-containing formulations are approved by the FDA for CAs in the ultrasound. They are Definity® and Optison® [126,127], both of which avail PFP/perflutren in the gaseous state [30]. Alike, there are two commercially available PFPE emulsions used for in vivo 19F imaging in cell tracking studies–cell sense and V-Sense [128,129,130,131]. Perftoran®, rebranded under the name Vidaphor TM, is a drug approved for clinical application in Russia, Mexico, Kazakhstan, Kyrgyzstan, and Ukraine, and is in the progression to be introduced in the US and European markets [132,133], to be used as a blood substitute [134]. It consists of PFD and perfluoromethyl cyclohexyl piperidine as PFCs and is stabilized by proxanol-268-polymeric surfactant and electrolyte mixture [85,133,135].
A plethora of studies are underway to evaluate the various properties of PFCs. They are found feasible for applications such as hypothermic total or partial liquid ventilation of the lungs [135,136,137,138,139], in vivo visualization of the effects of antibiotic therapy [140], oligonucleotide therapeutics [141], cell tracking [142], including stem cells [143,144,145], specific detection of organ rejection [146], identifying penumbra in stroke patient [147], quantifying immune cells (tumour-associated macrophages) in the tumour microenvironment [148,149,150], organ preservation [151], quantifying renal vascular damage [152], 19F-oximetry [153], inflammation imaging of various diseases [154,155,156,157,158,159], gasification-enhanced photoacoustic cavitation [160] etc. There are some excellently written reviews for further reference on PFCs used for oxygen delivery [55,63,73,124,161,162,163,164], imaging inflammation [48,91,165,166], and cell tracking [65,98,129,167,168,169]. Refer to the reviews for profound understanding on 19F MRI used in biomedicine [44,47,56,170,171,172], PFCs used for various applications [53,74,84,89,173,174,175,176] and fluorinated compounds including PFCs used for imaging and/or drug delivery [17,24,54,177,178,179,180,181,182,183]. Being in the tight grip of SARS-CoV-2, PFCs have been proposed as a source of gas exchange in patients in critical conditions and be employed to protect blood cells [184].
5. Examples of Nanosystems Used for 19F MRI Studies
A miscellaneous collection of NP probes has evolved and is employed to overcome the present limitations and drawbacks of the 19F MRI CA. These nanosystems will be reviewed scrupulously, in particular, nanosystems loaded with PFCs besides fluorinated molecules like dendrimers and polymers will be considered in dept. With the intention to make the study more comprehensible and coherent, the nanosystems have been broadly classified as organic, inorganic, and hybrid systems. The organic nanosystem comprises polymeric, hyperbranched, dendrimer, hydrogel, lipid, and micelle systems. The inorganic system consists of metal and carbon-based nanoparticles. The hybrid or the mixed system consists of a fusion of organic and inorganic systems.
5.1. Organic NPs
5.1.1. Polymeric NPs
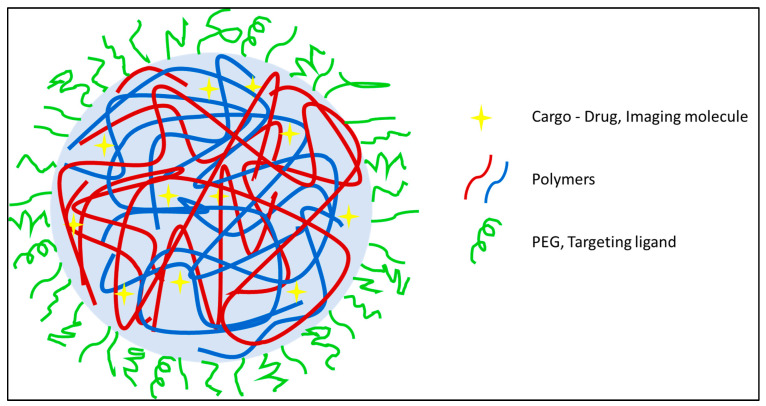
Due to the sparsity of 19F nuclei per molecule, 19F MRI possesses a low sensitivity which subsists as the major stumbling block. These, in turn, can help in increasing the sensitivity, thus reducing chemical shift artifacts. Figure 3 represents a general polymeric nanosystem where the NPs are pieced together with either one polymer or a combination of two or more polymers that can encapsulate the payload with a manipulable outer surface. Such a system increases the practicability to make it chemically fine-tuned, smart NPs that hold the possibility to add drugs moieties or target ligands compliant with its physicochemical properties.
Figure 3.
Polymeric nanoparticle complex with random polymer (blue and maroon) coils and a modifiable surface PEGylated surface here (green)). They can encapsulate/hold the payload (yellow star) in the polymer matrix.
The additional advantages of using polymeric species include high stability, manipulative chemical synthesis based on the desired properties, and depending on the polymers, they can be easily eliminated from the body [185,186]. In polymeric NPs, generally, two types of system are encountered-nanosystems made of CA-modified polymers that are fluorinated polymers and physical encapsulation of CA (mainly using PFCs) into nanosystems.
Geared towards overcoming the quandary faced by typical PFC encapsulated NPs–emulsion localized in diseased tissue and long-term accumulation in off-target tissue, Wallet et al. prepared low-molecular-weight fluorous polymeric colloidal NPs [187]. The NPs from the copolymer synthesized by atom transfer radical polymerization (ATRP) using an azide-terminated initiator consisted of trifluoroethyl methacrylate and oligo (ethylene glycol) methyl ether methacrylate, and they proved effective for breast and ovarian cancer models with little off-target accumulation. NPs have been prepared from 1H,H-perfluoro-n-octyl acrylate, N-vinylformamide, (1,5-N-vinylformamido) ethyl ether, and (E)-2,20-(diazene-1,2-diyl)bis(2,4-dimethylpentanenitrile as an initiator by one step, free radical polymerization technique [188]. The NPs appear to be promising carriers for 19F MRI CAs, and their in vivo and ex vivo studies are yet to be carried out to confirm the results. A salient amalgamation trio of the fluorophilic−lipophilic−hydrophilic system was developed by Kaberov et al. using poly(2-oxazoline) block copolymers [189] to be an imminent 19F MRI CA. Di- and tri-block-low molecular weight copolymers were synthesized based on 2-(1H,1H,2H,2H-perfluorooctyl)-2-oxazoline, 2-methyl-2-oxazoline, and 2-n-octyl-2-oxazoline, which self-assembled in aqueous solution and DMSO to micelles like structure and polymersomes possessing a core–shell structure.
A new class of fluorinated MRI agents, synthesized by one-pot reversible addition-fragmentation chain transfer (RAFT) polymerization of 19F-containing functional copolymer poly(oligo(ethylene glycol) methyl ether methacrylate-co-2,2,2-trifluoroethyl acrylate-b-poly(styrene-co-3-vinylbenzaldehyde) (poly(OEGA-co-TFEA)-b-poly(St-co-VBA)) was introduced to study the effect of morphology on different properties of NPs [190]. The core−shell structured particles formed from the copolymers proved that polymeric nano-objects of varied morphologies could be potential 19F MRI agents potential 19F MRI agents. The prepared NPs were of spherical, worm-like, or vesicle particles morphologies. Comparative studies showed that worm-like NPs had the highest uptake, and vesicle NPs were less likely to be taken up by the cells as the former has a higher aspect ratio. Levels of cytotoxicity (Chinese hamster ovarian cells) were in the order of spherical NPs > vesicle NPs > worm-like NPs, although none of them were toxic, while 19F MRI sensitivity was in the order of spheres > worm-like > vesicles (which depends on the fluorinated segment’s motion in the corona). Interestingly, there was not any variation in the T2. This study showed the influential role of morphologies in NPs.
Fu et al. developed novel 19F polymeric imaging agents activated by reactive oxygen species (ROS) such as H2O2 or low pH [191]. The monomers (thioether- and fluorine-containing methacrylate) were gathered from a PEG-based initiator by ATRP. The NPs formation was ensued in an aqueous solution by self-assembly with compact hydrophobic cores to give core–shell structured nanoaggregates. The imaging agent acted as a molecular switch by variation of T2 relaxation in the presence of ROS, depending on the oxidation of the hydrophobic thioether group of the agent into hydrophilic sulfoxide group. These were pertinently useful for imaging cancer cells as the environment is hypoxic. For specific imaging of bio-thiols using 19F MRI, Huang et al. developed intracellular reducing microenvironment-induced amino-activatable nanoprobe [192]. The copolymers for the nanoprobe acquired by RAFT polymerization from 2-((2,4-dinitro-N-(3,3,3-trifluoropropyl)-phenyl)sulfonamido)-ethyl methacrylate monomers. This nanoprobe could specifically detect bio-thiols, including cysteine, homocysteine, and glutathione. Initially, the fluorinated segments immobilized in the hydrophobic core quenched the MRI signal (OFF state). When encountered a sulfhydryl moiety, a change induced in the molecular substitution of the nanoprobe ultimately dissembled the nanoprobe, and MRI signals were regained (ON state).
To evaluate the influence of NP’s charge on the stability of 19F MRI CAs, a fluorinated multifunctional monomer was used to prepare cationic NPs. The study started up with the development of six forms of NPs with poly(methyl methacrylate) as the hydrophobic block and differing in hydrophilic block [PEG, mannose, fructose, two different 2,2,2-trifluoroethylamide L-arginine methacrylamide ratios (10 and 20 mol%)]. This study concluded that the choice of the hydrophilic copolymer had an immense implication on stabilizing the NP corona, thereupon the performance of the CA. The conclusion stemmed from the finding that PEG with bulky side chains prevented the aggregation of the fluorinated moieties in the NP corona, hence exhibiting extended T2. Other studies in the polymeric system include Nafion (sulfonated tetrafluoroethylene based fluoropolymer-copolymer) based nanocarriers that were experimented with for 19F MRI [193] together with poly-L-lysine and pegylated poly-L-glutamic. Cascaded, multi-responsive, self-assembled nanoprobe was identified for sensing and imaging by the sequential redox-triggered and NIR irradiation-induced 19F MR signal activation/amplification [66]. The nanoprobe consisted of amphiphilic polymers containing monodisperse PEG (mPEG2k) and 19F bearing moiety with NIR-absorbing indocyanine green (ICG). The ICG NPs dissociated in a reductive environment leading to the formation of ultrasmall NPs that could further dissociate to small and water-soluble molecules under the response to photothermal therapy.
Srinivas and co-workers have harnessed the benefits of poly(D, L-lactide-co-glycolide) (PLGA) (Resomer RG 502H, lactide: glycolide molar ratio 48:52–52:48) particles of sizes ranging from 200 nm to 2000 nm formulated by single and double emulsion techniques under sonication [194]. The effects of the moiety confined inside the NPs—different PFCs, and the surface coating (targeting agent, antibody)—have been studied and shown that the NPs were exceedingly flexible in terms of encapsulated contents (imaging agent, fluorescent dye, drug), particle size, charge (−40 to 30 mV), and the bound moiety. These resulted in a versatile system with the capability to optimize parameters depending on the application. PLGA NPs were already documented for the detection of the labeled cells and direct quantification of cell migration in a diabetes model, using the PFPE by the cellular MRI method with the anticipated expansion of the PFPE-imaging platform to a wide range of cell and disease models. It was also proposed that the PFPE imaging platform could be outstretched to a wide range of cell and disease models [195].
PFCE/perfluron with different fluorescent dyes were encapsulated inside the PLGA NPs for simultaneous imaging of distinct cell populations [196]. The PLGA-PFCE-NPs with ICG or fluorescite fluorescent dye was applied simultaneously to obtain the images in less than ten minutes, making it expeditious. The slow processing and poor resolution images from unsatisfactory penetration of MRI were prevailed over by fluorescence imaging. PFCE encapsulated PLGA NPs were recognized to have many applications such as imaging using 19F MRI in conjunction with the US discerning the NPs were stable on exposure to high-pressure ultrasound [197]. With ICG dye incorporated in the formulation, its application was extended to PAI and fluorescent imaging [198] and to obtain cardiac 19F MRI using PFCE labeled cells [199]. These PLGA-PFCE-NPs were used alongside gold NPs to assess the bone fillers and images using MRI and CT [200].
Srinivas et al. did an exhaustive study with PLGA (resomer RG 502H, lactide: glycolide molar ratio 50:50) for the formulation of triphasic NPs containing PFCs applicable to clinical imaging [201]. Exploring different parameters such as surfactant type and concentration, polymer concentration, and solvent type affecting the miniemulsion formation of PLGA NPs loaded with PFCE, such as their size, stability, release properties, and cell viability. The insight on the ultrastructure of the NPs is crucial for determining their exclusion from the body. The PLGA-PFCE NPs established to have a multicore structure in contrast to the anticipated simple core one, which helped in the easy clearance of NPs from the body, as proven by in vivo studies conducted on mice [202]. The simultaneous loading of two PFC agents (PFCE and PERFECTA) yielded a two-color MRI probe [203]. When modified with 111In-DTPA to the PFCE-PLGA NP, they had the aptness for combined SPECT/PET and 19F MRI in vivo cell tracking [204]. Since these particles are powerful theranostic agents evidenced from the previous discussions, their production was aspired to be scaled up. It was achieved by a modular microfluidic system, with sufficient yields for clinical use [205].
Chitosan-coated PLGA-PFOB NPs (RG Resomer 504H) attained by homogenization under emulsion evaporation method followed by sonication was applicative for tracking in vivo cell migration [206]. The encapsulation efficiency of PFOB was 67.1% ± 10 (w/w). Cyanine dyes like IR Dye 800CW used are advantageous for biomolecule labeling and in vivo clinical diagnostic. NPs derived using single emulsion and solvent extraction methods with NIR fluorophores and PFCE give an entrapment efficiency of PFCE of around 240 μg/mg. The same group prepared PLGA-PEG-folate polymer, encapsulated with PFOB and either ICG (for NIRS) or the chemotherapeutic agent doxorubicin, showed enhanced uptake on human nasopharyngeal epidermal carcinoma (KB) cells, and in vitro cytotoxic studies showed that folate-targeted NPs were able to kill cancer cells more efficiently than non-folate conjugated counterpart [207]. With an encapsulation efficiency of 80% PFOB, the same NPs had been taken advantage of rheumatoid arthritis diagnosis [208]. Poly(styrene sulfonate), an ionic polyelectrolyte polymer, was used to modify the PLGA-PFOB NPs, to be used for cell labeling [209]. It was demonstrated from the in vivo and in vitro studies that the prepared NPs could be effective for cell tracking studies with MRI, least affecting any cellular functions.
PEGylation of PLGA polymer is a widely used approach for increasing the half-life of the NPs in the bloodstream, and it was reestablished with PLGA-PEG nanocapsule encapsulated with PFOB [210]. For probing ultrasound-triggered drug release, PFOB loaded PLGA-PEG NPs encapsulated with Nile red had been investigated, which proved that the mentioned NPs are least suitable for the function due to the requirement of robust inertial cavitation [211]. Cruz et al. manipulated PEGylated PLGA NPs for the detection and monitoring of ischemic diseases and traumatic brain injury, using optical microscopy and 19F MRI [212]. Tumour-associated macrophages (TAM) are tumour-promoting inflammations that could be potential biomarkers for diagnosis, prognosis, and therapeutic targets for cancer [213]. Zambito et al. used PLGA-PEG-mannose NPs encapsulated with PFCE to visualize TAMs by optical imaging and 19F MRI [214] with higher specificity and robust signal strength. NIR dye encapsulated PLGA-PEG NPs were adapted for monitoring and imaging in osteoarthritis by modifying the NPs with trifluoroacetamide [215]. In vitro, in vivo and, ex vivo 19F MRI and optical imaging studies proved their prospect to be multi-modal nanoprobes.
The combined effect of PFCs capability to diffuse oxygen into the tumour tissue and the possibility to modify the surface of PLGA NPs have been exploited for the enhanced antitumour efficacy in colon cancer using PLGA NPs functionalized with epidermal growth factor and co-loaded with 5-fluorouracil (chemotherapeutic drug) and PFC [216]. The aforementioned system proved to be more fitting in accumulating in tumours via ligand-targeting interactions and amended the hurdle of hypoxia-induced chemotherapy resistance. A very distinct approach was adopted by Neri et al. for the PLGA polymers-they had been fluorinated with two different fluorinated amine ligands (coupling reaction) to form F3-PLGA and F9-PLGA that contained three and nine equivalent fluorine atoms, respectively [217]. They displayed a higher efficacy to load hydrophobic drugs. Preliminary in vitro studies of F9-PLGA NPs were done using the drug (dexamethasone) loaded NPs to assess their cellular availability and drug release showed a greater efficacy.
The linear PFPEs possess a functional group, unlike most PFCs, to allow for facile chemical modification, and this trait was maneuvered to achieve the desired effect. A nanoemulsion with tyramide modified PFCE with NIR dye, surfactants, and hydrocarbon oil was designed for hydrophobic drugs delivery and dual imaging [88]. By inhibiting the function of the cyclooxgenase-2 enzyme by selective inhibitor-Celecoxib is an anticancer strategy to reduce cancer risk and suppress tumour growth. Janjic et al. have reported the PFPE nanoemulsions loaded with Celecoxib and NIR dye for theranostic application including, three complementary imaging modalities-fluorescence, NIRS, and 19F MRI [218,219]. The application of the developed nanoemulsions has been extended for in vivo monitoring and modulating tumour-infiltrating immune cells [218]. The authors were the first to show the two-color PFC nanoemulsion [220]. PFPE was modified with oligo(ethylene glycol) methyl ether acrylate by RAFT polymerization to form a CA that had high imaging sensitivity and was hydrophilic [221]. To investigate the aggregation behavior of nanosystems that can have a role in the interaction between the NPs and living entities, doxorubicin-loaded polymeric PFPE-based NPs were reported [222]. Evaluation of fluorinated NPs on 3D spheroids concluded that for greater efficacy of drug delivery, it was efficacious for the NPs to have a smaller fluorinated core and the fluorinated segments to have greater exposure to the external environment.
RAFT polymerization intended to combine 2,2,2-trifluoroethyl acrylate with 2-(methylsulfinyl)ethylacrylate resulted in an MRI CA that was exceedingly hydrophilic and displayed intense in vitro/in vivo MRI signals [223]. With the solid-phase peptide synthesis of disordered fluorinated peptides by sequential addition of amino acid–trifluoroacetylated lysine, a platform conceivable for in vivo targeting applications was made [224]. Copolymers developed from perfluoropolyether methacrylate and oligo(ethylene glycol)methacrylate and modified with a green fluorescence dye–N-(5-fluoresceinyl)maleimide, had a hydrodynamic size around 12 nm and molecular weight ~75,000 gmol−1 [225]. From the in vivo studies, it was acknowledged to have favorable non-phagocytic cells uptake profiles and outstanding MRI performance. With the widely used PEG, a novel, low cost, hydro-soluble, highly flexible, easily tunable with a facile synthetic route, PEG-based fluorinated esters were built up using 2-(trifluoromethyl)-3,3,3-trifluoro-propanoic acid [226] and PFTB [227]. Polydispersity in PEG is an inherent trait of the polymer, and recent years have seen the development of mPEG with improved biodegradability [228]. A thermoresponsive imaging probe with fine-tunable lower critical solution temperature pioneered from peptidic mPEG combs [229] was explored for their smart drug-carrying ability using doxorubicin.
5.1.2. Hyperbranched
Coupled with linear, cross-linked, and branched-chain polymers, dendritic polymers are the fourth subclass of polymers that are invariably branched irregularly [230]. Hyperbranched polymers (as shown in Figure 4) are a subclass of dendritic polymers whose polymeric structures are bestowed with abundant functional groups, intramolecular cavities, low viscosity, and high solubility [231]. This class of molecules has been ventured mainly to overcome the intrinsic drawback of PFC formulations like low stability, limited aqueous dispersibility, and a limited possibility to functionalize.
Figure 4.
Hyperbranched polymers structure - the polymer structure is a randomly branched polymer with circles (blue) representing the branching points and rhombus shapes(green) for surface groups.
As already highlighted regarding the benefits of multimodal imaging–combining the high resolution, 3D anatomic images for soft tissues with MRI, and high spatial resolution for hard tissue by CT, a more accurate diagnosis is guaranteed, facilitating treatments. Multifunctional hyperbranched polymers containing iodine and fluorine were synthesized by initially using a hyperbranched iodopolymer via RAFT polymerization. The 2-(2′,3′,5′-triiodobenzoyl)ethyl methacrylate was incorporated to provide X-ray opacity along with poly-(ethylene glycol) methyl ether methacrylate (PEGMA) to provide hydrophilicity, and bis2-(methacryloyl)oxyethyl disulfide was chosen as a crosslinker to achieve biodegradability [232]. HBIP was chain extended with 2,2,2-trifluoroethyl acrylate (TFEA) and PEGMA to obtain hyperbranched iodopolymer containing 19F (HBIPF). From the in vivo degradation studies, the polymers were proven to be biodegradable. Thereby, this study demonstrated that multifunctional hyperbranched polymers were promising molecular imaging agents for CT/19F MRI bimodal imaging.
To boost the local fluorine concentration, segmental mobility of the fluorine-containing moieties, and for active and specific targeting of diseased tissues, a multifunctional PFPE-based NPs conjugated with a peptide aptamer, Hsp70, as targeting ligand was pioneered (Hsp70–specifically to target the heat shock protein 70 overexpressed in breast cancer cells) [233]. They were attained from RAFT polymerization with hydrophobic PFPE segments and oligo(ethylene glycol) methyl ether acrylate (OEGA) as the hydrophilic monomer. The poly(OEGA)3-PFPE polymer, further chain extended with OEGA and ethylene glycol dimethylacrylate (EGDMA) monomers gave rise to a hyperbranched PFPE-based polymer. After polymerization, fluorescence dye molecules, Cy5.5 were conjugated by reduction at the termini and aptamer peptide by click chemistry. The series of outcomes from the in vivo detection of breast cancer on a murine tumour model indicated that PFPE based NPs are efficacious theranostic agents for the specific detection of in vivo breast cancer. The explored properties included scrutinizing the 19F NMR and MRI properties, in vivo and ex vivo molecular imaging, in vitro cell uptake, intracellular distribution, and trafficking, elimination of polymers from the body alongside tumour-penetration analysis.
Self-assembled colloids prepared using fluorinated hyperbranched polyglycerols were macromolecules germane to therapeutic functions [234]. By ring-opening multibranching polymerization (ROMBP) of glycidol followed by copolymerization with a fluorinated glycidyl ether (2-[(2,2,2-trifluoroethoxy)methyl]oxirane), hyperbranched polyglycerols were formed and explored for their ability to perform both as 19F-MRI nanoprobes and drug-loaded nanocarrier. A synthetic steroidal anti-inflammatory drug–dexamethasone, was used as the model drug. The formation of the micelles gave a narrow size distribution after the drugs were incorporated inside.
5.1.3. Dendrimers
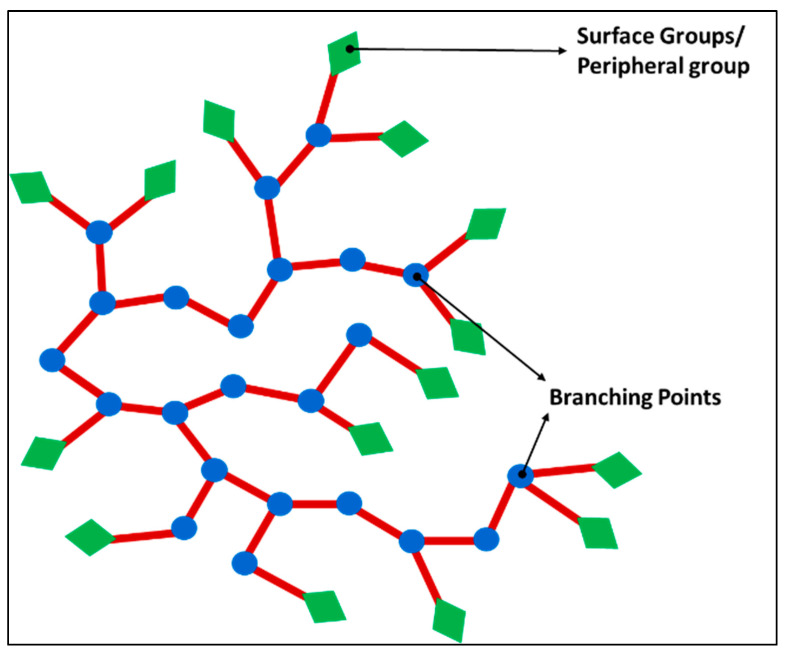
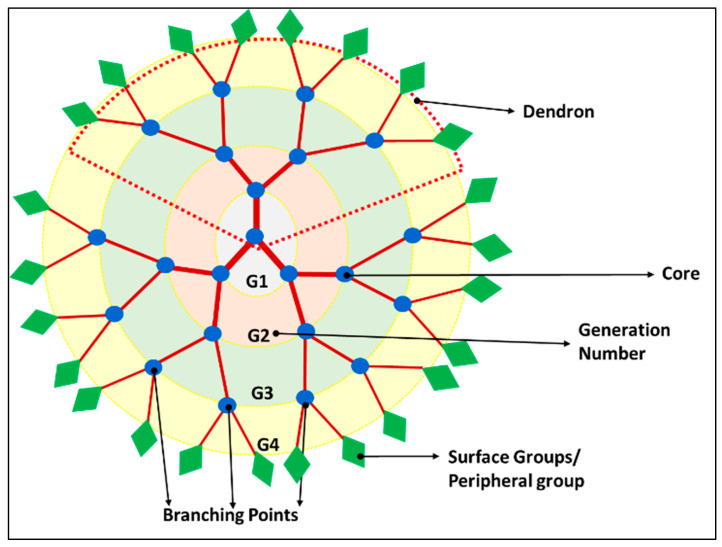
Even though multiple fluorines could be incorporated into a single molecule in fluorinated polymer, they suffer from pitfalls that often split signals are obtained in the FNMR. A group of macromolecules belonging to the family of dendritic polymer is of great use in enhancing the 19F signal intensity per imaging agent molecule since they possess a spherical symmetry that can provide an identical chemical environment to the multiple fluorine atoms [235]. Frequently obtained from convergent synthesis methods, they self-organize to form well-defined 3D structures called dendrimers, with radically distributed branches, growing out from a focal point as illustrated in Figure 5. As they flare out to wide branches, the ‘generation’, as well as the number of peripheral groups of a dendrimer can be recognized from each subsequent branching unit. Even though both hyperbranched polymer and dendritic polymer have a 3-dimensional (3-D) macromolecular structure, the difference between them is that the latter has a regular topology as pictorially represented in Figure 5 with a multistep synthesis, while the former has an irregular topology as shown in Figure 4 with relatively facile one-step preparation [230].
Figure 5.
Dendrimers structural components include the core, branching points (blue circles), surface/peripheral groups (green rhombus), and the a dendron segment in a dotted red triangle.
Dendrimers have unique properties including monodispersity, multi-valence, uniform and well-controlled size and shape, modifiable peripheral surface groups, and available internal cavities that make them a strong candidate for both imaging and drug delivery [236]. Their internal cavity can incorporate other imaging agents or drugs and have high intrinsic payload capability. When these polymer chains are fluorinated, they are adequate for 19F MRI. The first fluorinated dendrimer studied for MRI was a small Janus dendrimer, a polymer assembly with a core attached to two different side chains [237]. Multiple studies had been carried out to study the dendrimers as nanocarriers.
The intricacy of 19F MRI is their high T1, nuclear anisotropy, and frequently, NPs made by emulsions result in a size greater than 200 nm that can hardly pass through the capillaries of the blood vessel [238]. To evade these demerits a bifunctional Gd3+ chelate (DOTA—1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) was prepared and characterized to be employed as dendrimers. The dendrons, synthesized using fluorinated amino acids (BOC-L-4-trifluoromethylphenylalanine and 3,5-bis(trifluoromethyl)-DL-phenylalanine) along with carboxylic acids of the repeat branch unit. Different dendrimers with a size around 3 nm and T1 decreasing with increasing dendrimer generation, were tested with animal studies (Sprague Dawley female rats), and they exhibited less toxicity (KB cells) and had better SNR [239]. Kolmël et al. described the synthesis of polyfluorinated second-generation dendrons consisting of 72 magnetically equivalent fluorine atoms and displaying a single sharp resonance in its 19F NMR spectrum. The polymer was prepared by repeating iteratively Sonogashira coupling, alkyne deprotection, and copper-catalyzed azide-alkyne cycloaddition (CuAAC) for the generation build-up [240]. For a plenitude of pseudo symmetrical fluorines and excellent 19F MRI properties, the target dendrimer was convergently synthesized on a gram scale over 11 steps with an overall yield of 8%. Through assembling of the building block, the acidic bis(trifluoromethyl)carbinols, 540 fluorines were symmetrically distributed on each spherical layer, in unison emitted a single 19F peak with high signal intensity and therefore had high 19F MRI sensitivity [241].
Fluorinated self-assembled dendrimers were observed as promising 19F NMR/MRI-traceable drug-delivery vehicles for in vivo tracing and quantifying drugs, detecting drug microenvironments, and weak interactions [242]. It was established that co-self-assembly of fluorinated amphiphile dendrimers could determine weak interactions between the drug and the drug-delivery vehicle because of the changes in the self-assembling profile (π–π stacking, hydrophobic interactions, etc.) that sensitively effectuated corresponding 19FMR responses. To study drug-amphiphile interactions in micelle- and liposome-based drug-delivery systems, a total of 15 model molecules with structural diversity such as (R)-carvone, cholesterol, the anesthetic propofol, and the anticancer drug doxorubicin, were chosen. In comparison to the per-hydrogenated dendrimers, fluorinated counterparts had different traits due to the fluorophobic effect relative to solubility and micro-segregation effect. Like PFCs, their degradation pathway and toxicities were still ambiguous after being retained for a longer time in the body [242].
Although dendrimers seem like a scintillating prospect, the enigma faced by this class of molecules is their arduous synthetic procedure and use of organic solvents that limit them from being an easily approachable technique. Often, for the formation of dendrimers, different chemical groups can be fine-tuned depending on the outcome. Its cytocompatibility, biodegradability, cellular toxicity, and cellular uptake are complex and abstruse, and on top, it requires further investigations and inferences. There is also a condition called “hydrophobic aggregation-induced signal attenuation” that happens when the 19F-content in the molecular structure is greater than 10 wt.% [243]. The nanoprobes cannot exceed a threshold concentration of fluorine, for stimulation in biological systems, as exceeding a base concentration result in hydrophobic aggregation of fluorinated segments.
5.1.4. Nanohydrogel
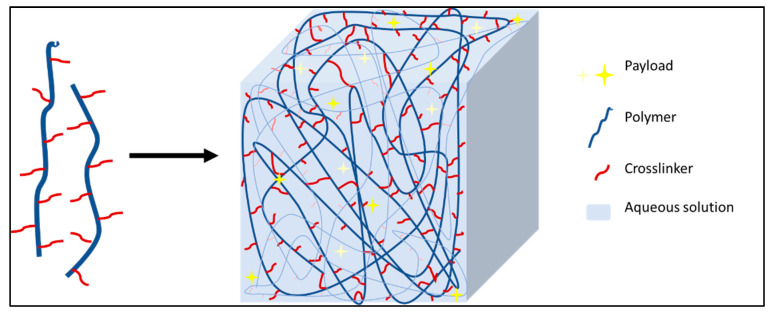
Hydrogels are 3D hydrophilic cross-linked or self-assembled polymer networks (Figure 6) that have high loading capacities of payloads (30% wt.), self-healing ability, viscoelastic behavior, ample stability, and can be triggered to release the payload through swelling in response to environmental changes in pH, ionic strength, or temperature [244,245]. The payloads can be encapsulated in nanohydrogels through various means, such as (i) passive/diffusion-based, (ii) covalent conjugation to either the interior or exterior, (iii) physical entrapment within the polymer network [246].
Figure 6.
Nanohydrogel matrices are formed by polymers that can form the 3D network with the help of crosslinkers (red) that act as linking ligands. The payloads (yellow star) can be trapped inside the 3D matrix.
Designing NPs targeting the lymphatic system (a vital part of the immune and circulatory system), both PEGylated and fluorinated chitosan were synthesized to fathom their application for encapsulation of probes and MRI lymphography (relating to the body’s lymphatic system) experiments [247]. The nanohydrogels were prepared by ionic gelation, the spontaneous supramolecular assembly of cationic chitosan with anionic compounds. After resolving the dilemma of determining the degree of substitution of PEGylated and fluorinated derivatives with chitosan, in vivo experiments affirmed good biocompatibility and prospective use of nanohydrogel for the relevant applications. Similarly, a thermoresponsive hydrogel was reported by Kolouchova et al. where the structure of the nanohydrogel was based on amphiphilic copolymers containing two blocks: one hydrophilic biocompatible block–poly[N-(2-hydroxypropyl)methacrylamide] (PHPMA) or poly(2-methyl-2-oxazoline) (PMeOx) and one fluorinated thermoresponsive block–poly[N(2,2difluoroethyl)acrylamide] with excellent sensitivity and non-cytotoxic for cell lines like human cervical carcinoma, murine monocyte/macrophage, HF-primary fibroblasts, and human B lymphoblast cell lines [248].
To prevail over the crucial challenge of aggregation in fluorocarbon substitutions that induced the segments of polymers hydrophobic, Munkhbat et al. had used an intelligent chemical play using nanohydrogels [249]. It facilitated in fully realizing the potential of polymeric tracers. Firstly, polymeric assembly was constructed with degradable hydrocarbon moieties and a high fluorocarbon core, and by chemical cross-linking, preserved the morphology of assembly. Eventually, segmental mobilities were amplified within the nanohydrogel interior by triggered degradation of cleavable hydrocarbon parts that decreased the density of the assembly’s interior. That prompted escalated T2 relaxation time and propelled signal intensities enhancement in 19F NMR and 19F MRI phantom imaging.
To delve into the controlled release of bioactive agents, 19F MRI was used to quantify the degradation rate of implantable or injectable hydrogels and provide the precise location in a real-time and non-invasive manner, without interruption of endogenous background signals and limitation of penetration depth. Traditionally, gravimetric methods are being used to provide this information in vitro but offer limited insight on the in vivo fate and sequential tracking [250]. Ergo, a zwitterionic, fluorinated and alkynyl 19F MRI molecular CA was designed, namely N-(carboxymethyl)-N-methyl-N-(3,3,3-trifluoropropyl) prop-2-yn-1-aminium (termed PA-CBF3), with zwitterionic carboxybetaine structure, which was superhydrophilic and had superior resistance to protein adsorption and was capable to tether with different hydrogels [243]. The probed nanohydrogels included polyacrylamide hydrogel, injectable alginate hydrogel, thermosensitive poloxamer hydrogel, and poly(ethylene glycol)-b-poly(L-valine) polypeptide hydrogel.
Besides manoeuvring of PFPE modified polymeric nanoemulsion for various applications [65,88,94,163,218,219,220,251], Janjic et al. had extended their use in hydrogels too. Anti-tumour necrosis factor-alpha (anti-TNFα) therapy had been a proven strategy for treating inflammatory bowel disease, where TNFα-binding lactococci bacterium can also act as infrared fluorescent protein. For localized delivery of anti-TNFα therapy, the PFPE nanoemulsion loaded with theranostic TNF α-binding lactococci (Lactococcus lactis) was incorporated into a thermoresponsive polymer (Pluorinic®F127) hydrogel [77]. The resulting nanoemulsion-based hydrogel (nanoemulgel) was 19F MRI and NIRS visible. The same group had used a slightly modified hydrogel for increasing its ability to load different payloads (fluorescent dyes, pH sensors, chelators, drugs, and antibodies) and therefore adapt the hydrogel for a broad range of biomedical imaging and delivery applications. PFPE nanoemulsions were crosslinked with polyethylenimine to form hydrogels hence ridding the necessity of any energy utilizing the emulsification step [252].
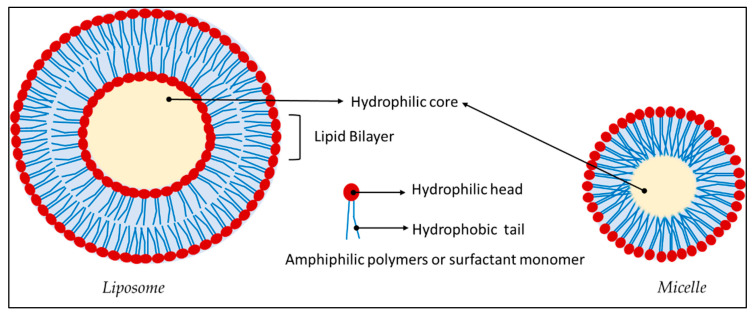
5.1.5. Lipids
The primary component for the vaccine technologies (Pfizer/BioNTech and Moderna) used during the outbreak of the novel coronavirus causing severe acute respiratory syndrome is lipid NPs [253]. This system helped in the translocation of the mRNA/self-replicating RNA (responsible for producing the immune response) across the plasma membrane. Typically, liposomes are derived by the self-assembly of the phospholipids like phosphatidylethanolamine, phosphatidylcholine, phosphatidylglycerol, or cholesterol. As portrayed in Figure 7, they possess a hydrophilic core and a lipid bilayer, thereupon have the stupendous advantage over other NPs in encapsulating hydrophilic (in the core), hydrophobic (between the bilayer), and even amphiphilic drugs in addition to the possibility of surface modification [175]. They are one of the widely used systems with less known toxicity compared to conventional drugs. For instance, they are the primary component of the first FDA-approved nanodrug Doxil® for the treatment of Kaposi’s sarcoma, ovarian, and breast cancer [254].
Figure 7.
Liposome and micelle consist of an assembly of amphiphilic polymers or surfactant monomers that possess a hydrophobic tail (blue) and a hydrophilic head (maroon).
Cellular therapeutic dendritic cells (DC)-based vaccination is an ex vivo modified DC with tumour-associated antigens and had been used to initiate anti-tumour immune responses. Hence, it is vitally substantial to track the fate and location of the injected DC. Intending to collectively load antigenic proteins into DC and enable high-resolution tracking of the antigen-loaded cells 19F-MRI CAs, antigen-coated PFC particles for DCs were prepped. The cationic particle consisted of 1,2-dioleoyl-3-trimethylammonium-propane, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000], and cholesterol. The PFC component was either PFH or PFCE, and it was emulsified in the presence of excess lipid solution through high frequency shaking. Particles were loaded electrostatically by negatively charged ovalbumin (commonly used model antigen) [255]. The same particles were used for in vivo imaging the transplantation of pancreatic islets and tracking the autoreactive T-cell migration in the pancreatic region [256]. Liposomes were formulated with hydrophilic organofluorine molecules with a fluorine encapsulation up to 22.7 mg/mL [257] that could concomitantly image multiple targets without any chemical shift artifacts.
In an attempt to mimic the temperature range in tumours (37–39 °C) that has a different microenvironment than normal cells, Lima et al. demonstrated the change of the 19F NMR signal of F—containing compound in thermally responsive lipid nano-emulsion particles, mainly the T1 and T2 values, depending on the temperature change (37–42 °C). The carriers were tripalmitin, tristearin, and triarachidin, favored based on high melting point neutral saturated fatty acid, and the fluorine compound was a modified α-tocopherol. The study concluded that T2 changed more than T1, and the change in T2 was mainly given by the increased molecular motion of the modified α-tocopherol, highlighting that the local temperature might impact the 19F NMR signal intensity [258]. With MRI multimodal imaging, it was demonstrated that PFC NPs were prospective to be delivered for lung cancer [259]. The PFCE emulsions with rhodamine-phospholipid surfactants consisted of 20% (v/v) PFCE, 2% (w/v) of a surfactant commixture, 1.7% (w/v) glycerin and water. Surfactant commixture consisted of dipalmitoylphosphatidylcholine, cholesterol, Gd-diethylenetriaminepentaacetic acid-phosphatidylethanolamine, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissaminerhodamine B sulfonyl), and 1,2-dipalmitoyl-sn-glycero-3-phospho-(1’-rac-glycerol). The PFCE emulsions were exposed to human bronchial epithelial (BEAS-2B) and human lung squamous carcinoma (H520) cell lines following intratracheal or intravenous administration. This study established evidence that with minimal extratumour systemic exposure, PFC NPs can be locally delivered into lung cancers intratracheally (reported for the first time) in high concentrations.
5.1.6. Micelle
Typically, micelles are obtained via the self-assembly of the amphiphilic molecules similar to liposomes. They engineer into the core–shell architectures that possess outer hydrophilic surfaces that can impart steric stability, and prolong their circulation lifetimes while the entire interior of the NP is hydrophobic, which portends that only hydrophobic drugs could be encapsulated as depicted in Figure 7 [175]. This scenario leaves hydrophilic cargo to be attached to the surface. They are smaller (10–100 nm) than liposomes, making them suitable for leaky vasculature of tumours. A biosynthesized fluorinated protein was presented by Hill et al. [260] as a “fluorinated thermoresponsive assembled protein” (F-TRAP) that could encapsulate small-molecule chemotherapeutic doxorubicin in its self-assembled micelle structure and release them in response to temperature and concentration, owing to its inherent stimuli-responsive properties. They bore a coiled-coil pentamer corona and a hydrophobic, thermoresponsive elastin-like polypeptide core. When exposed to increased concentration and temperature, they assembled into nanoscale micelles characterized by nearly a constant 19F T1 relaxation times and a remarkable decrease in 19F T2 relaxation. Furthermore, through thermally induced in vitro coacervation of the proteins at 45 °C, free doxorubicin was collected in the supernatant. The therapeutic efficacy of the precedent had been assessed in mammalian tumour cells, MCF-7 human breast adenocarcinoma cells, and discerned that it was significantly effective at reducing cell viability.
5.2. Inorganic NPs
Inorganic NPs such as metallic, magnetic, quantum dots consist of a central core made of inorganic material, as illustrated in Figure 8, which defines their unique characteristics. We would be focusing on metal, silica, and carbon-based NPs in particular, due to the vast literature published for exploring them. Hequet and coworkers [35] had prepared a CA containing a paramagnetic center and chemically equivalent fluorine atoms using a cycloaddition reaction for dual 1H/19F MRI. T1 agents (mostly lanthanide complexes) are called positive agents because of the hyperintense signal they engender in the accumulation areas, whereas T2 agents (usually iron oxide NPs) are “negative” CAs since they induce darker contrast in the accumulation area. A series of cyclen derivative lanthanide (Gd(III), dysprosium (III), terbium (III), and europium (III)) complexes associated with nine chemically and magnetically equivalent fluorine atoms were synthesized, and the study showed that gadolinium, dysprosium, and terbium complexes were promising transition metals for future use in 19F MRI in terms of their relaxation time.
Figure 8.
General representation of inorganic NPs where payloads (red star) are attached to the surface of the nanosystem. They include gold, silica, iron oxide, quantum dots, nanotubes etc.
Lanthanide-based upconversion (UC) NPs have potential applications in MRI or drug targeting or carriers due to their luminescent properties, such as large anti-stokes shifts, photostability, narrow emission peaks, and low toxicity. NaYF4 is an efficient well-known UC host material where Y3+ can be replaced in any ratio by rare-earth ions, of which Gd3+ is the most attractive one for their intrinsic magnetic properties. To track down the possibility of their UC application in the biological field, water-soluble NaGdF4:Yb3+/Tm3+ nanorods were prepared using the hydrothermal method [261]. The samples were conductive to visible light, and luminescence images were obtained in laser diode excitation. PLGA was reported to encapsulate doxorubicin and inorganic nanocrystals–NaYF4:Yb,Er@NaGdF4 used for cancer cell imaging and exhibited pH-responsive drug-releasing behavior [262].
Another inorganic material of interest is nanofluoride (calcium fluoride (CaF2))-based inorganic nanocrystals. The unique characteristics like controllable content, sizes, and shapes, are often outperformed with the disadvantage of the restricted mobility of the elements within the crystal that leads to NMR line broadening and impedes their use as MRI tracers. Ashur et al. established a synthetic, water-soluble, small (<10 nm) fluoride-based nanocrystals that average out homonuclear dipolar interactions and thus allow high-resolution 19F NMR spectroscopy of the nanocrystals in aqueous solutions. The formulated PEG-coated CaF2 nanocrystals were used as an imaging tracer, combining the advantages of nanocrystals (small, high 19F equivalency, surface modifiability, maximal 19F density) with the merits of 19F MRI tracer after being used for in vivo 19F MRI in mouse models [263]. To increase the relaxivity of NPs that could help improve the SNR, fluoride doped iron oxide (ɣ-Fe2O3) NPs were considered [264]. Doping citric acid- on the mentioned NPs chemically induced intensification of magnetic anisotropy, unaffecting either its crystal structure or electronic configuration.
5.2.1. Metal NPs
Metal NPs are picked apart for their unique electrical, optical, and mechanical properties [265]. Widely explored for their size, shape, surface chemistry, and optical properties, this class of NPs had opened a broad array of applications when stumbled upon the possibility of obtaining advanced materials with the required properties by various modifications.
The combination of metal NPs with fluorinated ligands anchored on its surface is suitable for 19F MRI as this combination of NPs has superior properties in terms of their function. Boccalon et al. [266] prepared gold NPs (F-MPC) for dual-mode imaging, whose surfaces were grafted with fluorinated organic monolayer and ligands ending with a fluorescent dye. The exclusive properties included a central fluorinated chain to generate the 19F signal, the ability to solubilize/disperse in water (hence in biological media) without any additives and impart solubility in many solvents due to the presence of terminal hydrophilic triethylene oxide or PEG550 chain. Further, the outer monolayer capable to solubilize small hydrophobic molecules laid the groundwork for the development of drug nanovectors. Thanks to these features of the gold NPs that made them a novel imaging platform. The gold core had less than 2 nm size, and the overall size with attached ligands less than 10 nm, permitted them to penetrate smaller blood vessels. F-MPC-cell interactions were evaluated with human cervical carcinoma cells (HeLa), showed that more than 95% of the cells were viable after the uptake.
The aforesaid group had also functionalized the gold NPs with Gd(III) chelates [267] in an endeavor to develop a probe for dual imaging. Some of the impediments considered to get around were the NPs’ detectability and the usual size range of emulsions (200–300 nm), which limit NPs in vivo applications. Increasing the number of fluorine reinforces the odds of making it hydrophobic, hence the development of probes containing enough fluorine to reach detectability in 19F MRI is one of the captious challenges in the field. The Gd chelating unit of the ligand was based on the DOTA scaffold, deeply embedded in the monolayer of water-soluble gold NPs that gave good quality MRI images at a 20 mg/mL concentration with Gd(III) units. In pursuance of the ligands with fluorine atoms in the same chemical environment, PFTB ligands were set up for the preparation of gold NPs. Out of the prepared NPs (fluorinated/non-fluorinated, attaching PEG/thiolate to its side chain, etc.), long-chain PEGylated compounds were proven to be the best option to obtain colloidally stable NPs in vitro and hence obtain a single chemical shift, narrow 19F-NMR signal, and high fluorine loading [268].
5.2.2. Silica NPs
Another widely used metal for NPs preparation is mesoporous silica. Massive surface areas (1000 m2/g), tunable pore sizes (2–20 nm), facile surface modification capability via various synthetic approaches, and controlled release of numerous drugs from its pore make mesoporous silica NPs (MSN) unique for diversified applications [269]. Hollow mesoporous silica particles filled with PFCE were put to use to demonstrate the type of cargo in the mesoporous silica drug vectors may have substantial influences on the biodistribution [270]. Since the outer surface of these particles is mesoporous as illustrated in Figure 9, the PFCE will be exposed at every stage to the biological environment. Subsequentially, the fate of the NPs can be accurately deciphered from the protein adsorption behavior and how cargo is affecting the protein adsorption. Protein adsorption studies and in vivo 19F quantification results proved that adsorbed amount of protein (apolipoprotein A-1 and A-2) was much higher for the PFCE-filled NPs as compared to the native particles and that PFCE-loaded particles were eliminated by liver 72 h post-injection.
Figure 9.
Mesoporous silica nanoparticles structure represented by a blue sphere and the darker small circles shows the pores present on its surface.
Lee et al. have communicated the PFC-loaded ultraporous mesostructured silica NPs (PERFUMNs) for 19F MRI detectable oxygen-sensing probes [271]. They had used a post-synthetic loading method for experimenting with three different PFCs (PFCE, PFD, and perfluoro(tert-butylcyclohexane)) which made it possible to encapsulate more PFCs, around five times more than usually encapsulated by MSN. Post-synthetic loading methods were explored to find their influence on the loading yield and efficiency, and it was pinpointed that sonication time is a crucial factor [272]. It was revealed that for silica NPs, as the leaching of PFCs was typical due to their porous structure, it was better to graft fluorine probes onto its surface by covalent bonds. To put this into practice, Bouchoucha et al. [273] reported the synthesis of mesoporous silica NPs with covalent modification with either fluorosilane or polyfluorosiloxane together with a paramagnetic Gd chelate grafted at the surface. NPs (MCM-48-type) were synthesized and functionalized with fluorine-containing molecules and Gd chelates (Gd-DTPA). It helped in the production of imaging probes which induced a strong “positive” contrast enhancement effect, and NPs with the potential for dual 1H and 19F MRI. Even though the metal Gd on NPs surface aided improvement of the 19F relaxation time, a strong effect on T2 relaxation had the possibility to prevent the detection of probes. This dual detection represented a potential alternative to MRI−PET or in MRI−SPECT and hence the use of radioactive molecules in them.
Kikuchi et al. [274] developed novel multifunctional core–shell NPs utilizing the PRE effect to detect enzymatic (Caspase-3) activity and to use 19F MRI for detecting gene expression. Paramagnetic relaxation enhancement (PRE) is the liaison between two magnetic moments of paramagnetic nuclei and observed nuclei, resulting in efficient curtailment of T1 and T2 of the nuclei under observation [275,276]. A probe was designed by connecting a 19F containing moiety with a Gd3+ complex via an enzyme cleavable linker. Anticipating the T2 of 19F would be shrunk by the existing PRE effect in the enzyme reaction (hence low/no signal) as 19F containing moiety was close to the Gd3+ complex, and when the enzyme cleaved the substrate, 19F MRI signal would emanate as T2 increased in the guise of no longer effective PRE (distance between 19F and Gd3+ became infinite). However, in mouse experiments with small molecule-based 19F MRI probes, there were sensitivity issues, and to prevail over, PFCE inclusive silica-NPs were synthesized with surface modified with PEG.
Being mindful of the ingrained downside of predominant 19F MRI probes—arduous modifiability of the surface of nanoemulsions and low sensitivity of small molecule-based probes—multifunctional core–shell silica NPs were introduced for successful detection of gene expression in living cells and tumour tissue in living mice by 19F MRI. The biological inertness, favorable colloidal properties, and ease of surface modification of silica with the acquiescence of PFCE resulted in the formation of fluorine accumulated silica NP for MRI contrast enhancement (FLAME) [277]. The surfactant was n-cetyltrimethylammonium bromide (CTAB), and the surface was tailored with a folate receptor. The identic system had been modified for 19F MRI traceable silica NPs as drug carriers (mFLAME) by improving the silica coverage of the PFC core, reforming the mesoporous silica shell with a NIR dye (Cy5), and functionalizing it with a folate receptor. By this, it was possible to extend its application to dual-modal imaging (NIRS/19F MRI) and drug delivery [278]. Flow cytometric analysis confirmed mFLAME internalized by KB cells (HeLa or cervical adenocarcinoma). Further loading it with doxorubicin had a top-tier cytotoxic effect on KB cells. The combination of fluorine atoms with a paramagnetic ion reduced the 19F relaxation times, which was attributed to the PRE effect.
In like manner, to create an OFF/ON switching ability of Ln3+ complexes by PRE effect, FLAME NPs were attached to Gd3+ diethylenetriamine pentaacetate (DTPA) complexes on its surface by disulfide linkers (FLAME-SS-Gd3+). The study had been engaged on the FLAME-DTPA complex after treating with a reducing agent–tris(2-carboxyethyl)-phosphine. This inspection facilitated to show for the first time that the PRE effect of surface Gd3+ complexes was effective for fluorine compounds in NPs over 50 Å [279] that were contradictory to the study of De Vries et al., who previously observed that the distance between Gd3+ complexes and the fluorine core was less than 22 Å for the PRE of PFCE in Gd3+ modified nanoemulsions [280]. FLAME NPs were also reported for their use in multicolor MRI probes (PFC@SiO2, FLAME). Five different types of PFCs were employed–PFCE, PFOB, FC-43, perfluorodichlorooctane (PFDCO), and 1,1,1-tris(perfluorotert-butoxymethyl)ethane (TPFBME) and to render multicolor fluorescence imaging capabilities, rhodamine B isothiocyanate, sulfo-cyanine 5, and fluorescein-4-isothiocyanate were covalently modified to silica shells [281]. Nanoprobes (PFCE@SiO2, TPFBME@SiO2, and FC-43@SiO2) enabled the triple-color 19F MR imaging in vivo for the first time.
5.2.3. Carbon Based
Carbon nanotubes are nanoscale hollow cylindrical tubes consisting of rolled-up sheets of layer (single-walled or multi-walled) of graphene as sketched in Figure 10 [282]. The unique 1D structure, high mechanical tensile strength, high surface area, high thermal conductivity, chemical stability, effective resistance to any chemical impact, rich electronic polyaromatic structure, lightweight, possible surface functionalization, and the possibility to stuff the hollow interior with various imaging agents/drugs/molecules of interest renders them with manifold potential for applications [283,284].
Figure 10.
Carbon nanotubes with payload (blue circles).
Among the ROS, H2O2 plays an indispensable physiological role. Monitoring its pathological level gives valuable information on the multiple abnormalities occurring in the living entity. Fluorinated halloysite nanotube (HNT) was used to detect the low concentration of H2O2 by 19F NMR probe prepared using halloysite nanotubes ((Al2Si2O5(OH)4·nH2O)) and 3,5-bis(trifluoromethyl) benzeneboronic acid [285]. The halloysite nanotube with minor modifications uncovered its implementation as a fluorescent probe for selective and sensitive response to hyperoxide using 1-pyrenylboronic acid [286] in conjunction with a smart halloysite-based hydrogel prepared for H2O2-responsive drug delivery system [287].
5.3. Mixed/Hybrid NPs
To impart multifunctionality to an NP-based system, the convergence of organic and inorganic components is often pivotal, resulting in hybrid theranostic NPs. Ingenious and intelligent combinations of discrete functional nanostructured materials will enable the development of versatile nanomedical platforms for multimodal imaging or simultaneous diagnosis and therapy.
‘FETRIS’/Iron(III) tris-β-diketonate with PFPE were prepared with the idea that high-spin paramagnetic metal ions can profoundly alter the relaxation times T1 and T2 for the cell detection via 19F MRI [288]. The PFPE-based ligand–fluorinated β-diketones (FDK) was made using Claisen condensation between PFPE and p-methoxyacetophenone. Formed FDK was blended with an assortment of PFC derivatives (PFPE, PFPE diethylamide, PFOB, short PFPE oligomer perfluorotetraglyme), and the obtained blended oils were formulated into lipid-based paramagnetic nanoemulsions using microfluidization. Cytocompatible FETRIS agents were formed as FDK efficiently and irreversibly extracted Fe3+ ions from an aqueous solution into the fluorous phase in the PFPE-in-water nanoemulsions. The system had stretched out its application for intravenous injection and in vivo inflammation imaging [289]. To forge a multifunctional nanoprobe, Chen et al. used the strategy of the one-pot encapsulation method, where the PFCE was anchored through hydrophobic–hydrophobic interactions to Cu1.75S NPs and then trapped within the silica shell (Cu1.75S–19F@OFP–SiO2) [290]. Co-encapsulation agents were oleylamine-functionalized polysuccinimide and trimethoxy(octadecyl)silane. The resulting nanoprobes contained up to ~2.0 × 108 fluorine atoms per particle with an ultrahigh 19F MRI signal.
Star polymers were contrived with a polyhedral oligomeric silsesquioxanes (POSS) core and partly fluorinated arms expecting the star’s arm to be visible by 19F MRI, while the POSS core to load drug molecules [291]. The arm of the star polymer consisted of TFEA and PEGMA monomers. The formation of eight partly fluorinated copolymer arms and a POSS core, with different sizes of NPs, molded depending on the length of the chain. The fabricated NPs were dissolved in the water, and the characterization showed a singlet for 19F NMR and augmented T2. Hybrid functionalized 2D carbon nanomaterials like graphene oxide and iron oxide (Fe3O4) NPs blended the magnetic characteristics of Fe3O4 and the photoluminescence of graphene oxide in MRI and fluorescence imaging [292]. Fluorinated graphite polymer was oxidized by strong oxidizing agents, to give highly fluorinated graphene oxide. Hybrid of fluorinated graphene oxide and iron oxide were prepared by co-precipitation technique using iron sulfate and iron chloride.
Another nanocomposite based on multifunctional Cu7S4−Au@PSI−19F/PEG was reported by Cui et al. [293]. Plasmonic nanostructures, including gold (Au) with a localized surface plasmon resonance (LSPR) peak in the transparent window (800−900 nm), had been recognized as a promising agent for photothermal therapy. LSPR in metals is due to free electrons whereas, for doped semiconductors, it is the cation vacancies (holes) that can be tampered with doping. Heterodimers (combining plasmonic nanostructures with chalcogenide) can aid in tuning the LSPR peak position. To attune the LSPR (~808 nm), the nanocomposite was prepared by growing a small Au domain on a heavily doped Cu7S4 to form a Cu7S4−Au heterodimer. Furthermore, by click chemistry, adding the fluorine component that had an inconsequential background, good sensitivity combined with high spatial resolution enhanced photothermal efficacy and decreased the optical damage to normal tissues.
A multifunctional hybrid vesicle was synthesized from PEGylated magnetite/PFOB–loaded organic/inorganic hybrid that could be used in dual-modality US/MR imaging and intensified image-guided high intensity focused ultrasound ablation [294]. The organic component was amphiphilic block copolymer–polystyrene-block-poly(acrylic acid). The hybrid shell layer was formed by shell cross-linking of the micelle of Fe3O4 NPs and PFOB using thiol-silane (3-mercaptopropyltrimethoxysilane). Hu et al. used copper sulfide (Cu7S4) coated with oleylamine functionalized 3,5-bis(trifluoromethyl)benzaldehyde fluorinated ligands for 19F MRI and photothermal ablation [295]. Ionic liquids (IL) based on 1-butyl-2,3-dimethyl-imidazolium (BMMI, BMMIBF4), 1-ethyl-3-methyl-imidazolium (EMI, EMIOTf), and 1-ethylpyridinium (EPy, EPyBF4) were explored with fluorinated anions, such as tetrafluoroborate (BF4) and trifluoromethanesulfonate (OTf) engendering a fluorinated IL-based activaTable 19F MRI platform (FILAMP) [80]. At 50 to 60 °C, these ILs confined inside the hollow mesoporous silica, and the pores sealed by stimuli-responsive copolymers could respond and release free IL upon biological stimulation.
A promising dual 1H/19F MRI probe was derived from lipid NPs, whose surface was modified with paramagnetic calcium-responsive Gd-chelates and encapsulated with PFCE [79]. The surfactants were dipalmitoylphosphatidylcholine with 5% of PEGylated phospholipid. Calcium helped in the contrast enhancement of 1H MRI. Water-soluble fluorinated NPs with metal-core and fluorinated ligands were reported by Arango et al. [116] with interesting MR features. A simple phase transfer method was used to bind gold NPs with fluorinating building blocks, and in vivo mice studies showed that they are suitable for 19F MRI/MRS. Magnetite (Fe3O4) NPs were modified with oleic acid, and this system formed self-assembled magneto-micelles with amphiphilic copolymers [296]. The fluorine-containing copolymers were synthesized using 2,2,3,4,4,4-hexafluorobutyl methacrylate (for holding hydrophobic drugs) and PEGMA (increased the hydrophilicity of the hybrid system). 5-fluorouracil was used to study the capability of the magneto-micelles to carry and load drugs and for in vitro release studies.
Some of the other hybrid systems include fluorinated polymer and manganese-layered double hydroxide NPs (Mn-LDH NPs) that benefitted the specific and sensitive detection of breast cancer [297]. Layered double hydroxides (LDHs) are 2D nanomaterials consisting mainly of divalent and trivalent cations in layers with anionic species intercalated between the layers. The cations could be metals, and the anions could be drug/polymers/siRNA, etc. pH-activated 19F MRI agents based on the PRE were specifically activated within the acidic tumour environment and were assembled with PFPE-based polymer and Mn-LDH NPs. Furthermore, Wang et al. reported the function of fluorine and nitrogen co-doped carbon dot complexed with Fe(III) put together as T1 CA in 19F MRI [298]. Using HeLa as a model cell line, the CA exhibited the lowest T1 relaxation, and in BALB/c mice, it displayed an accurate tumour image effect in addition to efficient renal clearance, low toxicity, high relativity, and bright luminescence.
A recent review by Mali et al. discusses recent advances in 19F-nanoparticles for 19F MRI [299], with a detailed analysis on the nanotechnologies employed for the design and applications of 19F-based nanoprobes. Table 4 gives information on most of the nanosystems discussed so far, including their preparation technique, fluorine component, characterization technique, their interesting pros and cons, and their applications.
Table 4.
The selected examples of studied fluorinated and PFC nanosystems explaining their preparation technique, fluorine component, characterisation techniques used for studying various aspects of the nanosystems, pros and cons based on the synthesis/preparation and the practicality, and applications. * The abbreviations are expanded at the ‘Abbreviation’ session.
| Type | Name | Preparation Technique | Fluorine Component | Characterisation * | Pros and Cons | Application | Ref |
|---|---|---|---|---|---|---|---|
| POLYMERIC | Fluorous colloidal NPs | Copolymer by ATRP. NPs formation by self-assembly to micelle | Trifluoroethyl methacrylate | DLS (260 nm), TEM, FMRI, FC, CM, UV-Vis, CyA–on macrophage cells, animal studies–female athymic NCR nude mice for breast cancer | Simple preparation of copolymer | Immune cell tracking and systemic disease monitoring | [187] |
| No surfactant | |||||||
| Little off target accumulation | |||||||
| Tumour-homing | |||||||
| Poly(OEGA-co-TFEA)-b-poly(St-co-VBA) | Polymerisation by RAFT and NPs by PISA | 2,2,2-trifluoroethyl acrylate | FMRI and NMR, DLS, TEM, CM | Little or no cytotoxicity–Chinese Hamster Ovarian cells | In vivo cell tracking | [190] | |
| Multiple NPs morphologies by controlling reaction time and polymer chain length in one preparation (spherical, worm, vesicle) | |||||||
| ROS-responsive fluorinated polymers | Polymer by ATRP and NPs by self-assembly | 2,2,2-trifluoroethyl methacrylate | H and F NMR, FMRI, DLS (62, 32 and 18 nm), UV-Vis | Enhanced sensitivity for acidic microenvironment and the presence of ROS | ROS/pH dual-responsive 19F MRI agent | [191] | |
| The concentration of H2O2 studied (~1 M) were higher than biological levels (50–100 μM) | |||||||
| 6-step synthesis that requires purification | |||||||
| “OFF–ON” regulation of NPs to acidic environment | |||||||
| Amino activable nanoprobe- p(mPEGMA)-co-poly(AMA-DNBS-F) (PEDF nanoprobe) | Copolymers by RAFT polymerisation and nanoprobe by nanoprecipitation | Trifluoromethyl-containing segments | H and F NMR, DLS (33 nm), FMRI, TEM, FTIR, CLSM, in vivo imaging in tumours–xenograft tumour models in mice | 2 step preparations for monomers | In vivo bio-thiols imaging | [192] | |
| Highly sensitive to bio-thiols | |||||||
| Water soluble | |||||||
| Fluorinated block copolymers NPs | RAFT for the block polymers and NPs by self-assembly in aqueous solution | 2,2,2-trifluoroethylamide L-arginine methacrylamide | H, F- NMR, DLS (25 to 60 nm), TEM | Fluorinated functionalities in the hydrophilic shell | MRI Imaging | [300] | |
| Increased T2 | |||||||
| 19F MRI-detectable drug delivery system | Layer-by-layer technique deposition of polyelectrolyte shells on nanoemulsion drops | Polyelectrolyte Nafion–fluorinated anionic polymer | DLS (170 nm), LDV, NTA, C-SEM, QCM, FMRI | Sufficient SNR ratio | Passive tumour targeting and drug delivery | [193] | |
| Highly cationic particle (+68 ± 5 mV) | |||||||
| Self-assembled 19F nanoprobes | Self-assembly of amphiphilic redox-responsive 19F-containing polymers and NIR-absorbing ICG molecules | 3,5-Bis(trifluoromethyl) benzoic acid part in the polymer | TEM, DLS (40 nm), UV–Vis, FNMR and MRI, TEM | Water-soluble | Accurate sensing and imaging of tumours | [66] | |
| In vivo and in vitro studies–HepG2 tumour-bearing cells and mice | |||||||
| High SNR ratio | |||||||
| Good biocompatibility | |||||||
| 5 steps for preparation with purification requirement and moderate yield | |||||||
| Novel system which has potential to be extended for imaging other tumour targets | |||||||
| Multi-functional fluorocarbon NPs | Single and double emulsion | PFD, PFH, perfluorooctane, PFOB, PFCE | DLS (200 nm–200 µm), SEM, CM, FC, FI, FMRI, Cell viability–primary human dendritic cells, histology |
Customizable NPs, minimal toxicity | In vivo imaging and targeting applications | [194] | |
| Size smaller than 200 nm is not formed by this NP formation | |||||||
| PLGA PFPE | Emulsification (Sonicator)–1:1 molar ratio of autoclaved PFPE and sterile filtered Pluronic | PFPE | DLS (103 nm), FNMR and MRI, FM, cellular viability–diabetogenic mice T cells | Specificity for the labelled cells | Non-invasive monitoring the trafficking of cellular therapeutics | [195] | |
| Reliable estimates of the apparent number of cells from image data | |||||||
| PFCE encapsulated PLGA | Single emulsion | PFCE | DLS, FNMR and MRI, SANS, animal studies–male Wistar rats, mouse, mice, cell studie–primary murine/human dendritic cells | Biocompatible NPs | US and 19F MRI | [197] | |
| Better acquisition time | Murine cardiac 19F MRI/MRS | [199] | |||||
| Obtains complimentary information when in combination with other imaging agents | In vivo PAI, 19F MRI and fluorescent imaging (FI) | [198] | |||||
| NPs loaded with chemotherapeutic drugs could give it a theranostic effect, Resomer RG 502 H, lactide: glycolide molar ratio 48:52 to 52:48 is the mostly used PLGA. The other ratios of lactide: glycolide and also their end group might give interesting results. The encapsulation efficiency of PFC could be studied each time to better understand the sensitivity | FMRI and CT (with gold NPs) | [200] | |||||
| SPECT/PET and 19F MRI | [204] | ||||||
| Chitosan coated PLGA -PFOB NPs | Single emulsion by homogenisation followed by sonication using 1.5% sodium cholate | PFOB | DLS (170 nm), CLSM, FC, FNMR and FMRI, TEM | Background-free signal compared to Gd (III) and super paramagnetic iron oxides NPs | Labelling and tracking therapeutic cells in vivo | [206] | |
| As chitosan coating is just a physical adsorption, the stability of it has to be verified in biological environment | |||||||
| Size of NPs is increased (200–400 nm) after the chitosan coating | |||||||
| PEGylated PLGA NPs (PLGA NP (NIR700 + PFC)-PEG-800 CW | O/W emulsion and solvent evaporation-extraction method | PFCE | DLS (240–250 nm), TEM, FMRI, TEM, FM, histology, cell culture–murine breast carcinoma cell line | Quantitative 3D information from deeper tissues | In vivo imaging | [212] | |
| Rapid qualitative optical monitoring | |||||||
| PLGA–PEG folate-receptor-targeted NPs | Single emulsion-evaporation (1.5% sodium cholate surfactant) | PFOB | DLS (150 nm), FC, CLSM, F MRI, NIRS, CyA-KB cells | Encapsulate imaging agent and drug | Theranostic NP | [207] | |
| Insufficient SNR in vivo for FMRI | |||||||
| The loading capacity of the NPs is low for doxorubicin and ICG (0.04% and 0.127%) | |||||||
| Doxorubicin-conjugated PFPE NPs | Polymers by RAFT polymerization | PFPE | DLS (8.1, 9.3 and 8.3), FNMR, MD | Improved cellular uptake | Improved therapeutic efficacy | [222] | |
| Deep tumour penetration | |||||||
| Studies done using 3D tumour spheroids | |||||||
| F3-PLGA and F9-PLGA | Nanoprecipitation–surfactant free | Fluorinated PLGA (2,2,2-trifluoroethanolamine, nonafluoro-t-butoxyethylamine) | DLS (~54 nm and 58 nm), TEM, F NMR, FM, CyA–immortalized human glomerular endothelial cells and podocytes | No surfactant used | Theranostic NPs | [217] | |
| Encapsulate hydrophobic drugs | |||||||
| The reaction yield of the fluorinated polymer is not understood | |||||||
| HYPERBRANCHED | Multifunctional hyperbranched polymers containing 19F | RAFT polymerization for polymer, NPs by self-assembly in water | 2,2,2-trifluoroethylacrylate | DLS (∼13 nm), GPC, TEM, FNMR and MRI, CT | Direct dissolution in water | Quantitative 19F MRI CA | [232] |
| Biodegradable | |||||||
| 3 step preparation and the final product is not pure (3 mixture products) | |||||||
| FNMR with multiple peaks | |||||||
| T2 shortened | |||||||
| PFPE based hyperbranched NPs conjugated with targeting aptamers | RAFT polymerization–for NPs, click chemistry for aptamers attaching | PFPE | F-DOSY (<10 nm), FM, FC, CrM, MD, FNMR and MRI | Superior MR imaging sensitivity and fluorine content -breast cancer cells | Quantitative 19F MRI CA | [233] | |
| Low-cost fluorescence imaging | |||||||
| Unsuitable for long term studies due to faster clearance from the body | |||||||
| Accumulation of polymer in the liver was observed after 48 h and the 19F signal could be still detected in the liver | |||||||
| Fluorinated hyperbranched polyether copolymers | ROMBP and copolymerization for polymers and self-assembly of the colloids | 2-[(2,2,2-trifluoroethoxy) methyl]oxirane/epifluorohydrin | DLS (160–200 nm), H NMR and F MRI, FM, HPLC, cytotoxicity studies - immortalized human glomerular endothelial cells and immortalized human podocytes | Repair damaged kidney glomerular cells in vitro | New generation 19F MRI nanotheranostics | [234] | |
| Negligible cytotoxicity | |||||||
| Narrow size distribution | |||||||
| Relatively long T1 | |||||||
| Higher amount of F gives less SNR | |||||||
| DENDRIMERS | Fluorinated Gd(III)-DOTA complexes | Convergent synthesis for polymer and self-assembly for NPs | Fluorinated amino acid group | F NMR, DOSY, H and F MRI, KB cells for in vitro cytotoxicity study, animal imaging—Sprague Dawley female rats | Substantial improvement in relaxation rate and SNR ratio | CA for high field imaging | [239] |
| Easily cleared through the kidneys | |||||||
| The fluorine in the surface layer of dendrimers is toxic which can be diminished by burying the fluorine further into the dendrimer interior | |||||||
| Second-generation dendron | Sonogashira coupling, alkyne deprotection and CuAAC | PFTB group attached to the dendron | FNMR | Higher number of equivalent fluorine than commercially available 19F MRI probes | Probes for 19F MRI | [240] | |
| Too unpolar to be water-soluble | |||||||
| Just one characterisation technique used | |||||||
| Pseudo-symmetrical fluorines dendrimers | Polymer prep–bromination and Williamson ether synthesis, NPs by self-assembly | Bis(4-fluorophenyl) trifluoromethyl carbinol group | FNMR and MRI | Large amount of fluorine with a single NMR peak | 19F MRI-guided drug therapy | [241] | |
| Optimize 19F relaxation time | |||||||
| High sensitivity | |||||||
| Reliable quantification | |||||||
| Comparatively low yield (8%) for 11 synthesis steps | |||||||
| Self-assembled fluorinated amphiphiles | Convergent way–Sonogashira coupling and Williamson ether synthesis for polymer, NPs by self-assembly | Fluorinated benzyl group | FNMR, DLS (6.3 nm), TEM | Quantifying drugs, detecting drug microenvironments and weak interactions | 19F NMR/MRI guided drug therapy. | [242] | |
| Several synthetic step for the preparation with most of them requiring separation | |||||||
| NANOHYDROGELS | Chitosan | Ionic gelation using hyaluronic acid and tripolyphosphate | 4,4,4-trifluorobutyric acid | DLS (274 nm), ELS (+30 mV), FNMR (−66 ppm), HNMR, TGA, DOSY, IR | Good biocompatibility toward murine macrophages cell line | Chitosan drug delivery systems for MRI lymphography | [247] |
| Degree of substitution is comparatively low (0.3% and 20%) and varies between different substitutes, and determination is laborious | |||||||
| Diblock polymers | Self-assembly by heating in aqueous solution | Poly[N(2,2 difluoroethyl)acrylamide] | SLS (100 and 67 nm), TEM, C-TEM, FNMR | Good sensitivity | 19F MR imaging–angiogenesis imaging or the labelling of pancreatic islets | [248] | |
| Non-cytotoxic for several cell lines | |||||||
| Long synthesis steps for preparation of polymers | |||||||
| Fluorinated amphiphilic polymers | Self-assembly of polymers–direct dissolution of amphiphilic polymers in PBS buffer | -CF3 groups attached to the chains of polymer | DLS (6- 14 nm), FNMR, FMRI–phantom and animal imaging, CM, CyA-HeLa cells | Enhancement in T2 relaxation times by increasing the segment mobility | Multimodal imaging and therapeutic applications. | [249] | |
| Superhydrophilic 19F MRI CA | Hydrogel matrix attached to zwitterionic, fluorinated and alkynyl molecule by click chemistry | The fluorine atoms on trifluoromethyl groups | HMRS, FTIR, GPC, CD, Rheometer, SEM, FMRI, degradation study–female BALB/c mice, CyA-Dendritic cells, NIH 3T3 cells | Gelation properties of hydrogels unaffected by labelling CA | Real-time FMRI to precisely locate and quantify the degradation rate of hydrogel scaffolds in vivo | [250] | |
| 3D-stereoscopic and 2D-anatomical information | |||||||
| LIPIDS | Antigen-loaded PFC particles | High-frequency mixing of the liquid PFC with a cationic lipid mixture-particles coat with PEG | PFH or PFCE | CM, TEM, F NMR, Cytotoxicity in transplanted pancreatic islets and beta cell-like cells and T-cell proliferation assay | Improving pancreatic islets transplantation technique | Theranostic PFC NPs | [255,256] |
| Good cell viability and no change in cells’ phenotypical properties | |||||||
| High resolution localization of transplanted cells | |||||||
| The use of PFCE is better than PFH because the latter have 3 peaks in FNMR which reduces its sensitivity | |||||||
| Thermally responsive lipid nano-emulsion | Nano-emulsion | Modified α-tocopherol | FNMR, DLS (50 nm), ZP | Proved that T2 changes more than T1 due to variation in temperature for FNMR | Potential tumour diagnosis | [258] | |
| The temperature studied is extreme (37 and 42 °C) compared to real tumour | |||||||
| Multifunctional paramagnetic PFC NP | Microfluidization | PFCE | DLS (132 nm), AFM, UV–vis, FM, cellular toxicity on bronchial epithelium, FC, clinical pathology, FMRI | Enhanced intratumoural penetration | PFC NP delivery from intravenous applications to intratracheal use (for lung cancer) | [259] | |
| NPs stored under very special condition | |||||||
| The lipid surfactant used have a laborious preparation | |||||||
| The studied NPs contain Gd3+ as Gd-lipid chelates | |||||||
| MICELLE | Fluorinated thermoresponsive assembled protein (F-TRAP) | Self-assembly micelle | Fluorinated amino acids within a protein (5,5,5-DL-trifluoroleucines) | DLS (30 nm), FA, SLS, CD, MALDI-TOF-MS, TEM, turbidometry, FNMR, FMRI, Animal studies-mouse xenograft model of human breast cancer | No change in T1 | Thermoresponsive 19F MRI/MRS-traceable theranostic agents | [260] |
| Doxorubicin encapsulation and thermoresponsive release | |||||||
| Zero echo time 19F MRI was used to get the direct imaging of protein as after micelle formation, there is a reduction in T2 | |||||||
| The release of drug is at 45 °C (usually tumour temperature range is 37 °C to 39 °C) | |||||||
| INORGANIC | Gold NPs protected by fluorinated ligands (F- MPC) | Homogeneous phase synthesis | Fluorinated tetraethylene glycol part of the ligand | DLS (10 nm), TEM, HAADF-STEM, FNMR, UV-Vis, ESR, CLSM, cell interaction with HeLa cells | Elimination of the use of surfactants | Nanovector | [266] |
| Size may help to reach small vasculature vessels | |||||||
| Soluble in many organic solvents | |||||||
| The preparation of fluorinated ligands contains 6 steps, most of them requiring purification | |||||||
| Functionalized gold NPs | Homogeneous phase synthesis | Fluorinated tetraethylene glycol part of the ligand | DLS, HNMR, TEM (1.5–2 nm), UV-Vis, FNMR and MRI | Water-soluble | Dual 1H/19F MRI | [267] | |
| Good quality MRI images | |||||||
| Same as ref [266] + Gd(III) is embedded deep in the layer of Au NPs that causes reduction in T1 relaxation times of bulk water proton | |||||||
| Gold NP functionalised with fluorine atoms | Reduction of HAuCl4 in the presence of NaBH4 | PFTB | ICP-MS, F-NMR/MRI, UV-Vis, TEM, Cell viability and apoptosis assays -MDA-MB-231, C33-A and MDA-MB-435S cell lines, MTS CyA | Colloidal stability in water and other solvents | 19F MR imaging | [268] | |
| Single chemical shift | |||||||
| Long storage | |||||||
| High fluorine loading | |||||||
| Long preparation and purification procedure for the fluorine ligands | |||||||
| The position of fluorine in the NPs is not established | |||||||
| Hollow mesoporous silica NPs (HMSN-PFCE) | Modified protocol from [301] | PFCE | DLS, SEM (290 nm), TEM, MRI, NMR, PAGE | Prolonged circulation time | Dual MRI (1H and 19F) | [270] | |
| Helps in understanding the effect of loading agent on the biodistribution of NPs | |||||||
| Better biodistribution of NPs | |||||||
| The study is majorly applicable to systems whose cargo is on the outer surface | |||||||
| Fluorinated mesoporous silica NPs (FMSNs and polyFMSNs) | Repeated impregnation-calcination process | Fluorosilane or polyfluorosiloxane | TGA, TEM (140 nm), DLS, FNMR and MRI, XPS, relaxometric properties | Colloidal stability | Dual MRI (1H and 19F) | [273] | |
| Increase in 19F relaxivities | |||||||
| Meticulous NPs preparation | |||||||
| Contains Gd3+ | |||||||
| The detection of probe might be impeded by the strong reduction of T2 after NPs formation | |||||||
| PEG modified silica NP | Dehydration polymerizing reaction–PFCE including micelle as a platform | PFCE | TEM, DLS (50 nm), 1H/19F MRI | High sensitivity | Tumour imaging | [274] | |
| Water stability | |||||||
| Information on long term stability, encapsulation efficiency of PFCE is deficient | |||||||
| Silica multifunctional core–shell NPs (FLAMEs) | PFCE-phospholipid nanoemulsion by sol-gel process using a novel surfactant, PAP | PFCE | DMS (76 nm), F NMR and MRI, TEM, biocompatibility by MTT assay-colon-26 cells, Passive targeting, and accumulation- mice bearing a tumour |
High sensitivity | Detection of gene expression and in vivo tumour imaging | [277] | |
| Modifiability of the surface, biocompatibility | |||||||
| In vivo stability | |||||||
| The FLAME NPs needs to be PEGylated as naked NPs is trapped immediately by the RES | |||||||
| The information on long term stability of NPs is lacking | |||||||
| Mesoporous FLAME (mFLAME) | PFCE emulsion by Sol–gel process | PFCE | DLS (165 nm), TEM, FNMR and MRI, CLSM, FC, MTT CyA-KB cells, FM | Ample cellular uptake and drug release in folate receptor-overexpressing tumour cells | Theranostic cancer treatment | [278] | |
| Drug release abilities at lower pH. (pH 5) | |||||||
| Efficient tumour cell internalization | |||||||
| Gd3+ complexes on FLAME NPs surface (FLAME-SS-Gd3+) | Gd3+ complexes were attached to the FLAME surface by disulfide linkers | PFCE | DLS (53.4 nm), FNMR and MRI, ICP-AES | Smart nanoprobe–based on PRE effect | Novel 19F MRI probes that visualize reducing environments | [279] | |
| In vivo imaging | |||||||
| High SNR ratio | |||||||
| PFC based 19F MRI nanoprobes (PFC@SiO2, FLAME) | PFC emulsion by sol–gel process | PFCE, PFOB, FC-43, PFN, PFDCO, TPFBME | DLS, TEM (40–120 nm), FI-RAW264.7 cells, H MRI and FMRI, hepatic uptake in mouse | T2 values -relatively longer than polymer-based or inorganic 19F MRI nanoprobes | Multicolour MRI probes | [281] | |
| In vivo triple-colour 19F MRI | |||||||
| The shelf-life information is lacking for the NPs | |||||||
| Fluorinated paramagnetic CAs | Multistep synthesis–cycloaddition reaction | Nonafluorinated carboxylic acid | FNMR, relaxivity measurements, MD | Relaxation times depending on the lanthanide ion | 19F MRI | [35] | |
| Low solubility in aqueous media | |||||||
| Hexagonal-phase NaGdF4:Yb3+/Tm3+ NPs | Hydrothermal method | NH4F/NaF | XRD, SEM, EDX, UV, photoluminescence spectra, EPR | Conducive to the UV light | IR tomography and MRI | [261] | |
| Good water solubility | |||||||
| Lanthanide-based upconversion NPs | |||||||
| Inorganic nanocrystals-PEG-coated CaF2 nanocrystals | Solvothermal approach | CaF2 | H and C and F-NMR, DLS (<10 nm), TEM, XRD, EDX, FTIR, TGA, mouse model of inflammation | Maximal 19F density | Imaging tracers for in vivo 19F MRI | [263] | |
| Average out homonuclear dipolar interactions | |||||||
| Direct and real-time in vivo 19F MRI | |||||||
| Chemically surface modifiable | |||||||
| Long T2 | |||||||
| Halloysite nanotubes- benzeneboronic acids (HNTs-6FBB) | One-pot synthesis | 3,5-bis(trifluoromethyl) benzeneboronic acid |
FNMR (−60 ppm), XRD, FTIR, XPS, TEM, EA (0.31% F) | Relatively long T2 | Selective response toward H2O2 | [285] | |
| Water dispersibility | |||||||
| Detection of H2O2 is based on a very minute shift in FNMR (0.2 ppm) | |||||||
| Low cell cytotoxicity | |||||||
| MIXED/HYBRID | Fe(III) tris-β-diketonate with PFPE (‘FETRIS’) | Microfluidization –metal-binding β-diketones conjugated to PFPE using pluronic surfactant | PFPE and PFPE derivatives, PFOB | DLS (140 nm to 200 nm), FNMR and FMRI, cell labelling-rodent glioma cell line | Ability to tune T1 by Fe concentration | In vivo detection of cell therapies and inflammatory cells | [288] |
| Low cytotoxicity | |||||||
| Small rates of metal leakage in the presence of EDTA in vitro and after cell labelling | |||||||
| Cu1.75S–19F@OFP–SiO2 | One-pot encapsulation method-PFCE anchored to Cu1.75S NPs and trapped within the silica shell | PFCE | DLS (20.8 nm), TEM, FMRI, PTT | Ultrahigh F signal | Ablation and sensitive multimodal imaging | [290] | |
| Biocompatible | |||||||
| Capable of both in vivo imaging (F-MRI) and photothermal ablation | |||||||
| Presence of excess of metals in a single probe! | |||||||
| The degradation of this complex should be evaluated since without the SiO2 coating it is cytotoxic | |||||||
| Fluorinated POSS-star polymers | Synthesis of star polymers by RAFT polymerization and polymer formation in water | 2,2,2-Trifluoroethyl acrylate in the ligands attached to POSS | DLS (8–10 nm), FNMR and FMRI | High imaging intensity | Theranostic agents for cancer diagnosis and treatment | [291] | |
| No surfactants | |||||||
| The yield for the formation of star polymers is low and extreme conditions for preparation | |||||||
| Hybrid of fluorinated graphene oxide and iron oxide (IFGO) | Graphene oxide-Hummer’s method. Hybrid–co-precipitation | Fluorinated graphene | DLS (8–10 nm), FMRI, XRD, XPS, SEM and HRTEM, FTIR, MTT CyA-benign breast epithelial cell line, Raman, UV-Vis, hysteresis | Additional imaging modality–magnetic targeted drug delivery | Superior CAs for MRI and fluorescent imaging | [292] | |
| Increased magnetic saturation-better contrast | |||||||
| Cu7S4−Au heterodimer Cu7S4−Au@PSI−19F/PEG nanocomposites | Wet-chemical method for Cu7S4-Au nano seeds followed by click chemistry | 2,2,2-trifluoro-N-2-propyn-1-yl-acetamide | DLS, HRTEM (27 nm), XRD, EDX, HAADF-STEM, XPS, STEM, F NMR and MRI, CT, cell viability-4T1 cell lines, PTT–liver of female mice | Deep penetration | Multimodal imaging guided photothermal therapy | [293] | |
| High spatial resolution | |||||||
| Enhances the photothermal efficacy | |||||||
| Long preparation for the nanocomposite | |||||||
| Mn-LDH@PFPE NPs | Composite system by conjugating a PFPE onto the surface of manganese-incorporated layered double hydroxide | PFPE | NMR and MRI, DLS (10 nm), TEM, GPC, CM, MTT assay–MDA-MB- 468 breast cancer cells, histopathologic examination | High specificity to breast cancer cells | Potential “smart” 19F MRI agent for detection of cancer diseases | [297] | |
| Fe3+@F,N-CD (fluorine and nitrogen co-doped carbon dot) | Simple microwave-assisted thermal decomposition method–from glucose and levofloxacin | Levofloxacin | DLS (16 nm), TEM, GPC, FTIR, XPS, FM, ESR, cytotoxic studies–HeLa cells, In vivo experiments -4T1 tumour bearing BALB/c mice, FMRI, CLSM | High T1 relaxivity | T1-weighted MRI CA | [298] | |
| Strong photoluminescence | |||||||
| Low synthetic cost | |||||||
| Low toxicity | |||||||
| Cannot be used for long term imaging in the body as they are excreted in a very short time from the body |
6. Conclusions and Future Perspectives
Even though we see massive progress in the field of 19F MRI with fluorinated compounds, and in particular with PFCs, its clinical translation requires an in-depth understanding of their exact behavior from its intake until its complete degradation. PFCs are atypical molecules and overcoming specific barriers can shorten the distance to its clinical reality. Even though emulsion is one of the most used techniques to encapsulate PFC compounds, there are still various shortcomings that must be subjugated, including instability, heterogeneity, complex formulation procedure, split 19F signals, excessive retention of the agent within organs for months or longer are some of them [226]. The bottleneck attributes for PFC compounds are that they are immiscible with water or lipids, besides their organ retention and inefficacious chemical modification. It is simultaneously intriguing as well as exciting. These will open new approaches to drug delivery besides making scientists think differently. It is quintessential to comprehend the degradation of PFCs at extreme (pH/temperature/anoxic/hypoxic) conditions and the effects on high doses of PFCs as the knowledge of this is scarce considering PFCs are not naturally occurring compounds. Storage conditions need to be ascertained as long-term storage of PFC NPs remains a persisting setback.
Reviewing the recent works on nanosystems using fluorinated ligands and PFCs for 19F MRI, we see a prodigious stride in our understanding of formulation of the nanosystems and bringing novel ways of producing 19F signals. Not restricted to the fact that PFC is a unique liquid, the expedient means explored to encapsulate PFCs, above and beyond uncovering ways to produce molecules with a strong and single 19F signal, have been remarkable. The frequently confronted issues when attempting to make molecules equal to or better than PFCs is the complexity of the molecules’ preparation itself, in addition to the required number of synthetic steps, utilization of organic solvents, purification contingency, and reproducibility of the same. We can always see that the products that ultimately manage to pass to clinical stages are straightforward and, typically, would not require complex synthetic skills. One of the concerning issues is the usage of Gd3+ yet in these nanosystems, especially for making hybrid 1H/19F MRI. The unreserved fact that Gd has a plethora of advantageous properties cannot be denied. An effectual CA from another element can pave the way for unexplored possibilities and conceivably higher quality images.
The aerial perspectives for the future use of PFCs in an emulsion would be to experiment with the use of fluoro-surfactants instead of commonly used surfactants [302], to find a carrier system that has an affinity to PFCs and could hold PFCs inside with some interactions, to explore the therapeutic effect of PFCs, to make PFCs more hydrophilic by modifying them or to make them less hydrophobic so that they could be soluble in an organic solvent and to study the effect of using more than two PFCs concurrently. Similarly, one of the emerging and compelling nanosystems would be to combine different classes of NPs to produce a complementary system with a synergic effect. It is like a jigsaw puzzle where pieces are fit together to create a serendipitous combination.
The anticipated challenges faced when the chemical modification is performed, as already pointed out, is the usage of organic solvents, the increase in the number of reaction steps that decreases the overall yield of the final compound, which can hinder its progress to the clinic. Formulating a synthesis/preparation towards a ‘green’ approach can make its way to the clinic swifter. PFCs have some inimitable exclusive characteristics, and not only could this be utilized in imaging but also in other research areas like cell tracking, 19F-oximetry, inflammation probing, etc. Some of the PFC-based compounds are already FDA-approved for ultrasound-based CAs and it is only a matter of time before discovering the most befitting CA for 19F MRI, which would open a floodgate of applications.
Abbreviations
| 19F MRI/FMRI | Fluorine-19 Magnetic Resonance Imaging |
| 19F NMR | Fluorine-19 Nuclear Magnetic Resonance |
| 1H MRI/HMRI | Proton Magnetic Resonance Imaging |
| AFM | Atomic Force Microscopy |
| ATRP | Atom Transfer Radical Polymerization |
| BALB/c | Albino, laboratory-bred strain of the house mouse |
| BODIPy | Boron-dipyrromethene |
| C NMR | Carbon-13 Nuclear Magnetic Resonance |
| CA | Contrast Agent |
| CD | Circular Dichroism Spectroscopy |
| CLSM | Confocal Laser Scanning Microscopy |
| CM | Confocal Microscopy |
| CrM | Correlation Microscopy |
| C-SEM | Scanning Cryo-Electron microscopy |
| CT | Computed Tomography |
| C-TEM | Cryo-Transmission Electron Microscopy |
| CuAAC | Copper-Catalyzed Azide-Alkyne Cycloaddition |
| CyA | Cytotoxicity Assays. The usually used assays are
|
| DC | Dendritic cellsCells |
| DLS | Dynamic Light Scattering |
| DOSY | Diffusion Ordered 2D-NMR Spectroscopy |
| EA | Elemental Analyzer |
| EDX | Energy-Dispersive X-Ray Spectroscopy |
| EGDMA | Ethylene Glycol Dimethylacrylate |
| ELS | Electrophoretic Light Scattering |
| EMA | European Medicines Agency |
| EPR | Electron Paramagnetic Resonance Spectrum |
| FA | Fluorescence Anisotropy |
| FC | Flow Cytometry |
| FDA | The Food and Drug Administration |
| FDK | Fluorinated β-diketones |
| Fe3O4 | Iron Oxide/Magnetite |
| FI | Fluorescence Imaging |
| FITC | Fluorescein Isothiocyanate |
| FLAME | Fluorine Accumulated Silica NP for MRI Contrast Enhancement |
| FM | Fluorescence Microscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GBCAs | Gadolinium Based Contrast Agents |
| Gd(III)/Gd3+ | Gadolinium-III |
| GPC | Gel Permeation Chromatography |
| HAADF-STEM | High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy |
| HBIPF | Hyperbranched Iodopolymer Containing 19F |
| HepG2 Cells | Hepatocellular carcinoma cells |
| HNMR | Proton Nuclear Magnetic Resonance |
| HNT | Halloysite Nanotube |
| HPLC | High-Performance Liquid Chromatography |
| HRTEM | High Resolution Transmission Electron Microscopy |
| ICG | Indocyanine Green |
| ICP-AES | Inductively Coupled Plasma Atomic Emission Spectrometry |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| KB cells | Human Nasopharyngeal Epidermal Carcinoma |
| LDV | Laser Doppler Velocimetry |
| LSPR | Localized Surface Plasmon Resonance |
| MALDI-TOF-MS | Matrix-Assisted Laser Desorption Ionization-Time-Of-Flight Mass Spectrometry |
| MD | Atomistic Molecular Dynamic Simulations |
| mPEG | Monodisperse Poly (ethylene-glycol) |
| MR | Magnetic Resonance |
| MRI | Magnetic Resonance Imaging |
| MRS | Magnetic Resonance Spectroscopy |
| NIR | Near Infrared |
| NIRS | Near Infrared Spectroscopy and Imaging |
| NMR | Nuclear Magnetic Resonance |
| NP | Nanoparticle |
| NTA | Nanoparticle Tracking Analysis |
| OEGA | Oligo(Ethylene Glycol) Methyl Ether Acrylate |
| PAGE | Polyacrylamide Gel Electrophoresis |
| PAI | Photoacoustic Imaging |
| PEG | Poly (ethylene-glycol) |
| PEGMA | Poly-(ethylene glycol) methyl ether methacrylate |
| PET | Positron Emission Tomography |
| PFC | Perfluorocarbon |
| PFCE | Perfluoro-15-crown-5 ether |
| PFDCO | Perfluorodichlorooctane |
| PFOB | Perfluorooctyl Bromide |
| PFP | Perfluoropropane |
| PFPE | Perfluoropolyether |
| PFTB | Perfluoro-tert-butanol |
| PLGA | Poly (lactic-co-glycolic acid) |
| PRE | Paramagnetic Relaxation Enhancement |
| PTT | Photothermal Therapy |
| QCM | Quartz Crystal Microbalance |
| RAFT | Reversible Addition−Fragmentation Chain-Transfer Polymerization |
| RF | Radiofrequency |
| ROMBP | Ring-Opening Multibranching Polymerization |
| ROS | Reactive Oxygen Species |
| SANS | Small Angle Neutron Scattering |
| SEC | Size Exclusion Chromatography |
| SEM | Scanning Electron Microscope |
| SLS | Static Light Scattering |
| SNR | Signal/Contrast-to-Noise Ratio |
| SPECT | Single-Photon Emission Computed Tomography |
| STEM | Scanning Transmission Electron Microscope |
| TAM | Tumour-associated macrophages |
| TEM | Transmission Electron Microscope |
| TFEA | 2,2,2-trifluoroethyl Acrylate |
| TGA | Thermogravimetric Analysis |
| TM | Turbidometry |
| TPFBME | 1,1,1-tris(perfluorotert- butoxymethyl)ethane |
| UC | Upconversion |
| US | Ultrasound |
| UV–Vis | UV/Vis Absorption Spectra |
| XPS | X-Ray Photoelectron Spectroscopy |
| XRD | X-Ray Diffraction |
| ZP | Zeta Potential |
Author Contributions
Conceptualization, J.M.J., R.C., and P.D.M.; writing—original draft preparation, J.M.J.; writing—review and editing, J.M.J., M.R.G., B.M.F., R.C., and P.D.M..; All authors have read and agreed to the published version of the manuscript.
Funding
This research was granted by European Commission (H2020-MSCA-RISE-2017-CANCER grant number 777682; H2020-MSCA-ITN-2019-NOVA-MRI grant number 859908, H2020-MSCA-ITN-2019-CAST grant number 857894; H2020-MSCA-IF-2021-GELNANODEP grant number 101029908).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McMahon M.T., Chan K.W. Developing MR probes for molecular imaging. Adv. Cancer Res. 2014;124:297–327. doi: 10.1016/B978-0-12-411638-2.00009-4. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z.Y., Wang Y.X., Lin Y., Zhang J.S., Yang F., Zhou Q.L., Liao Y.Y. Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. Biomed. Res. Int. 2014;2014:819324. doi: 10.1155/2014/819324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James M.L., Gambhir S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 4.Debbage P., Jaschke W. Molecular imaging with nanoparticles: Giant roles for dwarf actors. Histochem. Cell Biol. 2008;130:845–875. doi: 10.1007/s00418-008-0511-y. [DOI] [PubMed] [Google Scholar]
- 5.Schulz R.B., Semmler W. Molecular Imaging I. Handbook of Experimental Pharmacology. Volume 185. Springer; Berlin/Heidelberg, Germany: 2008. Fundamentals of Optical Imaging; pp. 3–22. [DOI] [PubMed] [Google Scholar]
- 6.Hong G., Antaris A.L., Dai H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017;1:0010. doi: 10.1038/s41551-016-0010. [DOI] [Google Scholar]
- 7.Kwon Y.D., Byun Y., Kim H.K. 18F-labelled BODIPY dye as a dual imaging agent: Radiofluorination and applications in PET and optical imaging. Nucl. Med. Biol. 2021;93:22–36. doi: 10.1016/j.nucmedbio.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Beard P. Biomedical photoacoustic imaging. Interface Focus. 2011;1:602–631. doi: 10.1098/rsfs.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg I., Huland D.M., Vermesh O., Frostig H.E., Tummers W.S., Gambhir S.S. Photoacoustic clinical imaging. Photoacoustics. 2019;14:77–98. doi: 10.1016/j.pacs.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells P.N. Ultrasound imaging. Phys. Med. Biol. 2006;51:R83–R98. doi: 10.1088/0031-9155/51/13/R06. [DOI] [PubMed] [Google Scholar]
- 11.Alcazar J.L., Aubá M., Olartecoechea B. Three-dimensional ultrasound in gynecological clinical practice. Rep. Med. Imaging. 2012;2012:1–13. doi: 10.2147/RMI.S21963. [DOI] [Google Scholar]
- 12.Kramme R., Hoffmann K., Pozos R.S., Buzug T.M. Springer Handbook of Medical Technology. Volume 413. Springer; Berlin/Heidelberg, Germany: 2011. p. 1497. [Google Scholar]
- 13.Beik J., Jafariyan M., Montazerabadi A., Ghadimi-Daresajini A., Tarighi P., Mahmoudabadi A., Ghaznavi H., Shakeri-Zadeh A. The benefits of folic acid-modified gold nanoparticles in CT-based molecular imaging: Radiation dose reduction and image contrast enhancement. Artif. Cells Nanomed. Biotechnol. 2017;8:1–9. doi: 10.1080/21691401.2017.1408019. [DOI] [PubMed] [Google Scholar]
- 14.Rahmim A., Zaidi H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z., Lu Z.R. Molecular imaging of the tumor microenvironment. Adv. Drug Deliv. Rev. 2017;113:24–48. doi: 10.1016/j.addr.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Molteni R. Micro-Computed Tomography (Micro-CT) in Medicine and Engineering. Springer; Cham, Switzerland: 2020. X-ray; pp. 7–25. [DOI] [Google Scholar]
- 17.Cho M.H., Shin S.H., Park S.H., Kadayakkara D.K., Kim D., Choi Y. Targeted, Stimuli-Responsive, and Theranostic 19F Magnetic Resonance Imaging Probes. Bioconjug. Chem. 2019;30:2502–2518. doi: 10.1021/acs.bioconjchem.9b00582. [DOI] [PubMed] [Google Scholar]
- 18.Kissane J., Neutze J.A., Singh H. Radiology Fundamentals, Introduction to Imaging & Technology. 6th ed. Springer; Cham, Switzerland: 2020. 431p [Google Scholar]
- 19.Initiative to Reduce Unnecessary Radiation Exposure from Medical Imaging. Center for Devices and Radiological Health, U.S. Food and Drug Administration; Silver Spring, MD, USA: 2010. [Google Scholar]
- 20.Balducci A., Helfer B.M., Ahrens E.T., O’Hanlon C.F., 3rd, Wesa A.L. Visualizing arthritic inflammation and therapeutic response by fluorine-19 magnetic resonance imaging (19F MRI) J. Inflamm. 2012;9:24. doi: 10.1186/1476-9255-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Liu H., Yao D., Li J., Yang S., Zhang C., Chen W., Wang D. (18)F-labeled magnetic nanoparticles for monitoring anti-angiogenic therapeutic effects in breast cancer xenografts. J. Nanobiotechnol. 2019;17:105. doi: 10.1186/s12951-019-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belderbos S., Gonzalez-Gomez M.A., Cleeren F., Wouters J., Pineiro Y., Deroose C.M., Coosemans A., Gsell W., Bormans G., Rivas J., et al. Simultaneous in vivo PET/MRI using fluorine-18 labeled Fe3O4@Al(OH)3 nanoparticles: Comparison of nanoparticle and nanoparticle-labeled stem cell distribution. EJNMMI Res. 2020;10:73. doi: 10.1186/s13550-020-00655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S., Jiang W., Yuan Y., Sui M., Yang Y., Huang L., Jiang L., Liu M., Chen S., Zhou X. Delicately Designed Cancer Cell Membrane-Camouflaged Nanoparticles for Targeted 19F MR/PA/FL Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces. 2020;12:57290–57301. doi: 10.1021/acsami.0c13865. [DOI] [PubMed] [Google Scholar]
- 24.Knight J.C., Edwards P.G., Paisey S.J. Fluorinated contrast agents for magnetic resonance imaging; a review of recent developments. RSC Adv. 2011;1 doi: 10.1039/c1ra00627d. [DOI] [Google Scholar]
- 25.Katti G., Ara S.A., Shireen A. Magnetic Resonance Imaging (MRI)—A Review. Int. J. Dent. Clin. 2011;3:65–70. [Google Scholar]
- 26.Neil J., Ackerman J.J.H. Encyclopedia of the Neurological Sciences. Volume 6. Elsevier; Amsterdam, The Netherlands: 2014. Magnetic Resonance (MR); Overview; pp. 971–972. [DOI] [Google Scholar]
- 27.Hao D., Ai T., Goerner F., Hu X., Runge V.M., Tweedle M. MRI contrast agents: Basic chemistry and safety. J. Magn. Reson. Imaging. 2012;36:1060–1071. doi: 10.1002/jmri.23725. [DOI] [PubMed] [Google Scholar]
- 28.Wahsner J., Gale E.M., Rodriguez-Rodriguez A., Caravan P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019;119:957–1057. doi: 10.1021/acs.chemrev.8b00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Y.D., Paudel R., Liu J., Ma C., Zhang Z.S., Zhou S.K. MRI contrast agents: Classification and application (Review) Int. J. Mol. Med. 2016;38:1319–1326. doi: 10.3892/ijmm.2016.2744. [DOI] [PubMed] [Google Scholar]
- 30.Index to Drug-Specific Information. [(accessed on 2 January 2022)]; Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/index-drug-specific-information.
- 31.Lohrke J., Frenzel T., Endrikat J., Alves F.C., Grist T.M., Law M., Lee J.M., Leiner T., Li K.C., Nikolaou K., et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016;33:1–28. doi: 10.1007/s12325-015-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Idee J.M., Port M., Raynal I., Schaefer M., Le Greneur S., Corot C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: A review. Fundam. Clin. Pharmacol. 2006;20:563–576. doi: 10.1111/j.1472-8206.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 33.Stockman J.A. Oral Prednisolone for Preschool Children with Acute Virus-Induced Wheezing. Yearb. Pediatr. 2010;2010:515–518. doi: 10.1016/S0084-3954(09)79361-3. [DOI] [Google Scholar]
- 34.De León-Rodríguez L.M., Martins A.F., Pinho M.C., Rofsky N.M., Sherry A.D. Basic MR relaxation mechanisms and contrast agent design. J. Magn. Reson. Imaging. 2015;42:545–565. doi: 10.1002/jmri.24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hequet E., Henoumont C., Djouana Kenfack V., Lemaur V., Lazzaroni R., Boutry S., Vander Elst L., Muller R.N., Laurent S. Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI. Magnetochemistry. 2020;6:8. doi: 10.3390/magnetochemistry6010008. [DOI] [Google Scholar]
- 36.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y.B. Fluorinated dendrimers as imaging agents for 19F MRI. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013;5:646–661. doi: 10.1002/wnan.1239. [DOI] [PubMed] [Google Scholar]
- 38.FDA Gadolinium Retention after Gadolinium Based Contrast Magnetic Resonance Imaging in Patients with Normal Renal Function. U.S. Food and Drug Administration; Silver Spring, MD, USA: 2017. pp. 127–131. [Google Scholar]
- 39.Do C., DeAguero J., Brearley A., Trejo X., Howard T., Escobar G.P., Wagner B. Gadolinium-Based Contrast Agent Use, Their Safety, and Practice Evolution. Kidney360. 2020;1:561–568. doi: 10.34067/KID.0000272019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dekkers I.A., Roos R., van der Molen A.J. Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European Medicines Agency. Eur. Radiol. 2018;28:1579–1584. doi: 10.1007/s00330-017-5065-8. [DOI] [PubMed] [Google Scholar]
- 41.FDA . Warns that Gadolinium-Based Contrast Agents (GBCAs) are Retained in the Body; Requires New Class Warnings. FDA; Silver Spring, MR, USA: 2017. FDA Drug Safety Communication: FDA. [Google Scholar]
- 42.Holland G.N., Bottomley P.A., Hinshaw W.S. 19F magnetic resonance imaging. J. Magn. Reson. Imaging. 1977;28:133–136. doi: 10.1016/0022-2364(77)90263-3. [DOI] [Google Scholar]
- 43.Weise G., Basse-Luesebrink T.C., Wessig C., Jakob P.M., Stoll G. In vivo imaging of inflammation in the peripheral nervous system by 19F MRI. Exp. Neurol. 2011;229:494–501. doi: 10.1016/j.expneurol.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Tirotta I., Dichiarante V., Pigliacelli C., Cavallo G., Terraneo G., Bombelli F.B., Metrangolo P., Resnati G. 19F magnetic resonance imaging (MRI): From design of materials to clinical applications. Chem. Rev. 2015;115:1106–1129. doi: 10.1021/cr500286d. [DOI] [PubMed] [Google Scholar]
- 45.Otake Y., Soutome Y., Hirata K., Ochi H., Bito Y. Double-tuned radiofrequency coil for 19F and 1H imaging. Magn. Reson. Med. Sci. 2014;13:199–205. doi: 10.2463/mrms.2013-0094. [DOI] [PubMed] [Google Scholar]
- 46.Keupp J., Rahmer J., Grasslin I., Mazurkewitz P.C., Schaeffter T., Lanza G.M., Wickline S.A., Caruthers S.D. Simultaneous dual-nuclei imaging for motion corrected detection and quantification of 19F imaging agents. Magn. Reson. Med. 2011;66:1116–1122. doi: 10.1002/mrm.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolters M., Mohades S.G., Hackeng T.M., Post M.J., Kooi M.E., Backes W.H. Clinical Perspectives of Hybrid Proton-Fluorine Magnetic Resonance Imaging and Spectroscopy. Investig. Radiol. 2013;48:341–350. doi: 10.1097/RLI.0b013e318277528c. [DOI] [PubMed] [Google Scholar]
- 48.Bouvain P., Temme S., Flogel U. Hot spot 19 F magnetic resonance imaging of inflammation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020;12:e1639. doi: 10.1002/wnan.1639. [DOI] [PubMed] [Google Scholar]
- 49.Liu W., Frank J.A. Detection and quantification of magnetically labeled cells by cellular MRI. Eur. J. Radiol. 2009;70:258–264. doi: 10.1016/j.ejrad.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grapentin C., Barnert S., Schubert R. Monitoring the Stability of Perfluorocarbon Nanoemulsions by Cryo-TEM Image Analysis and Dynamic Light Scattering. PLoS ONE. 2015;10:e0130674. doi: 10.1371/journal.pone.0130674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vatsadze S.Z., Eremina O.E., Veselova I.A., Kalmykov S.N., Nenajdenko V.G. 18F-Labelled catecholamine type radiopharmaceuticals in the diagnosis of neurodegenerative diseases and neuroendocrine tumours: Approaches to synthesis and development prospects. Russ. Chem. Rev. 2018;87:350–373. doi: 10.1070/RCR4752. [DOI] [Google Scholar]
- 52.Harris R.K., Becker E.D., de Menezes C., Sonia M., Goodfellow R., Granger P. NMR nomenclature. Nuclear spin properties and conventions for chemical shifts (IUPAC Recommendations 2001) Pure Appl. Chem. 2001;73:1795–1818. doi: 10.1351/pac200173111795. [DOI] [PubMed] [Google Scholar]
- 53.Schmieder A.H., Caruthers S.D., Keupp J., Wickline S.A., Lanza G.M. Recent Advances in 19Fluorine Magnetic Resonance Imaging with Perfluorocarbon Emulsions. Engineering. 2015;1:475–489. doi: 10.15302/J-ENG-2015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruiz-Cabello J., Barnett B.P., Bottomley P.A., Bulte J.W. Fluorine 19F MRS and MRI in biomedicine. NMR Biomed. 2011;24:114–129. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riess J.G. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif. Cells Blood Substit. Immobil. Biotechnol. 2005;33:47–63. doi: 10.1081/BIO-200046659. [DOI] [PubMed] [Google Scholar]
- 56.Chen J., Lanza G.M., Wickline S.A. Quantitative magnetic resonance fluorine imaging: Today and tomorrow. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010;2:431–440. doi: 10.1002/wnan.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger R., Resnati G., Metrangolo P., Weber E., Hulliger J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011;40:3496–3508. doi: 10.1039/c0cs00221f. [DOI] [PubMed] [Google Scholar]
- 58.Shah P., Westwell A.D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 2007;22:527–540. doi: 10.1080/14756360701425014. [DOI] [PubMed] [Google Scholar]
- 59.Barres A.R., Molugu S.K., Stewart P.L., Mecozzi S. Droplet Core Intermolecular Interactions and Block Copolymer Composition Heavily Influe.ence Oil-In-Water Nanoemulsion Stability. Langmuir. 2019;35:12765–12772. doi: 10.1021/acs.langmuir.9b01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krafft M.P., Riess J.G. Highly fluorinated amphiphiles and colloida systems, and their applications in the biomedical field. A contribution. Biochimie. 1998;80:489–514. doi: 10.1016/S0300-9084(00)80016-4. [DOI] [PubMed] [Google Scholar]
- 61.Gladysz J.A., Curran D.P., Horvath I.T. Handbook of Fluorous Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; Weiheim, Germany: 2004. [DOI] [Google Scholar]
- 62.Krafft M.P., Riess J.G. Perfluorocarbons: Life sciences and biomedical usesDedicated to the memory of Professor Guy Ourisson, a true RENAISSANCE man. J. Polym. Sci. Part A Polym. Chem. 2007;45:1185–1198. doi: 10.1002/pola.21937. [DOI] [Google Scholar]
- 63.Jagers J., Wrobeln A., Ferenz K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Pflug. Arch. 2021;473:139–150. doi: 10.1007/s00424-020-02482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dalvi V.H., Rossky P.J. Molecular origins of fluorocarbon hydrophobicity. Proc. Natl. Acad. Sci. USA. 2010;107:13603–13607. doi: 10.1073/pnas.0915169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janjic J.M., Ahrens E.T. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:492–501. doi: 10.1002/wnan.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang X., Gong X., Li A., Lin H., Peng C., Zhang X., Chen X., Gao J. Cascaded Multiresponsive Self-Assembled 19F MRI Nanoprobes with Redox-Triggered Activation and NIR-Induced Amplification. Nano Lett. 2020;20:363–371. doi: 10.1021/acs.nanolett.9b04016. [DOI] [PubMed] [Google Scholar]
- 67.Bo S., Song C., Li Y., Yu W., Chen S., Zhou X., Yang Z., Zheng X., Jiang Z.X. Design and Synthesis of Fluorinated Amphiphile as 19F MRI/Fluorescence Dual-Imaging Agent by Tuning the Self-Assembly. J. Org. Chem. 2015;80:6360–6366. doi: 10.1021/acs.joc.5b00810. [DOI] [PubMed] [Google Scholar]
- 68.Baig N., Kammakakam I., Falath W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021;2:1821–1871. doi: 10.1039/D0MA00807A. [DOI] [Google Scholar]
- 69.Pal S.L., Jana U., Manna P.K., Mohanta G.P., Manavalan R. Nanoparticle: An overview of preparation and characterization. J. Appl. Pharm. Sci. 2011;1:228–234. [Google Scholar]
- 70.Nel A.E., Madler L., Velegol D., Xia T., Hoek E.M., Somasundaran P., Klaessig F., Castranova V., Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 71.Gref R., Lück M., Quellec P., Marchand M., Dellacherie E., Harnisch S., Blunk T., Müller R.H. ‘Stealth’ Corona-Core Nanoparticles Surface Modified by Polyethylene Glycol (PEG): Influences of the Corona (PEG Chain Length and Surface Density) and of the Core Com-position on Phagocytic Uptake and Plasma Protein Adsorption. Colloids Surf. B Biointerfaces. 2000;18:301–313. doi: 10.1016/S0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 72.Han X., Xu K., Taratula O., Farsad K. Applications of nanoparticles in biomedical imaging. Nanoscale. 2019;11:799–819. doi: 10.1039/C8NR07769J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spahn D.R. Blood substitutes. Artificial oxygen carriers: Perfluorocarbon emulsions. Crit. Care. 1999;3:R93–R97. doi: 10.1186/cc364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaneda M.M., Caruthers S., Lanza G.M., Wickline S.A. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann. Biomed. Eng. 2009;37:1922–1933. doi: 10.1007/s10439-009-9643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Southworth R., Kaneda M., Chen J., Zhang L., Zhang H., Yang X., Razavi R., Lanza G., Wickline S.A. Renal vascular inflammation induced by Western diet in ApoE-null mice quantified by 19F NMR of VCAM-1 targeted nanobeacons. Nanomedicine. 2009;5:359–367. doi: 10.1016/j.nano.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bailey M.M., Kline S.R., Anderson M.D., Staymates J.L., Berkland C. Chemically modifiable fluorinated copolymer nanoparticles for 19F-MRI contrast enhancement. J. Appl. Polym. Sci. 2012;126:1218–1227. doi: 10.1002/app.36889. [DOI] [Google Scholar]
- 77.Achilefu S., Raghavachari R., Janjic J.M., Berlec A., Bagia C., Liu L.S., Jeric I., Gach M., Janjic B.M., Strukelj B. NIR and MR imaging supported hydrogel based delivery system for anti-TNF alpha probiotic therapy of IBD; Proceedings of the Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications VIII; San Francisco, CA, USA. 13–18 February 2016. [Google Scholar]
- 78.Bonnet C.S., Toth E. Smart Contrast Agents for Magnetic Resonance Imaging. Chimia. 2016;70:102–108. doi: 10.2533/chimia.2016.102. [DOI] [PubMed] [Google Scholar]
- 79.Gambino G., Gambino T., Angelovski G. Combination of bioresponsive chelates and perfluorinated lipid nanoparticles enables in vivo MRI probe quantification. Chem. Commun. 2020;56:9433–9436. doi: 10.1039/D0CC04416D. [DOI] [PubMed] [Google Scholar]
- 80.Zhu X., Tang X., Lin H., Shi S., Xiong H., Zhou Q., Li A., Wang Q., Chen X., Gao J. A Fluorinated Ionic Liquid-Based Activatable 19F MRI Platform Detects Biological Targets. Chem. 2020;6:1134–1148. doi: 10.1016/j.chempr.2020.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Behzadi S., Serpooshan V., Tao W., Hamaly M.A., Alkawareek M.Y., Dreaden E.C., Brown D., Alkilany A.M., Farokhzad O.C., Mahmoudi M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017;46:4218–4244. doi: 10.1039/C6CS00636A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castro O., Nesbitt A.E., Lyles D. Effect of a perfluorocarbon emulsion (Fluosol-DA) on reticuloendothelial system clearance function. Am. J. Hematol. 1984;16:15–21. doi: 10.1002/ajh.2830160103. [DOI] [PubMed] [Google Scholar]
- 83.Wu T., Li A., Chen K., Peng X., Zhang J., Jiang M., Chen S., Zheng X., Zhou X., Jiang Z.X. Perfluoro-tert-butanol: A cornerstone for high performance fluorine-19 magnetic resonance imaging. Chem. Commun. 2021;57:7743–7757. doi: 10.1039/D1CC02133H. [DOI] [PubMed] [Google Scholar]
- 84.Mayer D., Ferenz K.B. Perfluorocarbons for the treatment of decompression illness: How to bridge the gap between theory and practice. Eur. J. Appl. Physiol. 2019;119:2421–2433. doi: 10.1007/s00421-019-04252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kosenkov A.V., Gulyaev M.V., Anisimov N.V., Lobyshev V.I., Pirogov Y.A. Investigation of the distribution of heavy nuclei in laboratory animals using multinuclear magnetic resonance imaging. Phys. Wave Phenom. 2015;23:311–315. doi: 10.3103/S1541308X15040093. [DOI] [Google Scholar]
- 86.Bouvain P., Flocke V., Kramer W., Schubert R., Schrader J., Flogel U., Temme S. Dissociation of 19F and fluorescence signal upon cellular uptake of dual-contrast perfluorocarbon nanoemulsions. Magn. Reson. Mater. Phys. Biol. Med. 2019;32:133–145. doi: 10.1007/s10334-018-0723-7. [DOI] [PubMed] [Google Scholar]
- 87.Banik B.L., Fattahi P., Brown J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8:271–299. doi: 10.1002/wnan.1364. [DOI] [PubMed] [Google Scholar]
- 88.O’Hanlon C.E., Amede K.G., O’Hear M.R., Janjic J.M. NIR-labeled perfluoropolyether nanoemulsions for drug delivery and imaging. J. Fluor. Chem. 2012;137:27–33. doi: 10.1016/j.jfluchem.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu L., Liu F., Liu S., Xu X., Liu Z., Sun X. Perfluorocarbons-Based 19F Magnetic Resonance Imaging in Biomedicine. Int. J. Nanomed. 2020;15:7377–7395. doi: 10.2147/IJN.S255084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacoby C., Temme S., Mayenfels F., Benoit N., Krafft M.P., Schubert R., Schrader J., Flögel U. Probing different perfluorocarbons forin vivoinflammation imaging by19F MRI: Image reconstruction, biological half-lives and sensitivity. NMR Biomed. 2014;27:261–271. doi: 10.1002/nbm.3059. [DOI] [PubMed] [Google Scholar]
- 91.Stoll G., Basse-Lusebrink T., Weise G., Jakob P. Visualization of inflammation using 19 F-magnetic resonance imaging and perfluorocarbons. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2012;4:438–447. doi: 10.1002/wnan.1168. [DOI] [PubMed] [Google Scholar]
- 92.Mason R.P., Rodbumrung W., Antich P.P. Hexafluorobenzene: A sensitive 19F NMR indicator of tumor oxygenation. NMR Biomed. 1996;9:125–134. doi: 10.1002/(SICI)1099-1492(199605)9:3<125::AID-NBM405>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 93.Sotak C.H., Hees P.S., Huang H.N., Hung M.H., Krespan C.G., Raynolds S. A new perfluorocarbon for use in fluorine-19 magnetic resonance imaging and spectroscopy. Magn. Reson. Med. 1993;29:188–195. doi: 10.1002/mrm.1910290206. [DOI] [PubMed] [Google Scholar]
- 94.Janjic J.M., Srinivas M., Kadayakkara D.K.K., Ahrens E.T. Self-delivering nanoemulsions for dual fluorine-19 MRI and fluorescence detection. J. Am. Chem. Soc. 2008;130:2832–2841. doi: 10.1021/ja077388j. [DOI] [PubMed] [Google Scholar]
- 95.Jiang Z.X., Liu X., Jeong E.K., Yu Y.B. Symmetry-guided design and fluorous synthesis of a stable and rapidly excreted imaging tracer for 19F MRI. Angew. Chem. Int Ed. Engl. 2009;48:4755–4758. doi: 10.1002/anie.200901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goette M.J., Keupp J., Rahmer J., Lanza G.M., Wickline S.A., Caruthers S.D. Balanced UTE-SSFP for 19F MR imaging of complex spectra. Magn. Reson. Med. 2015;74:537–543. doi: 10.1002/mrm.25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nienhaus F., Colley D., Jahn A., Pfeiler S., Flocke V., Temme S., Kelm M., Gerdes N., Flogel U., Bonner F. Phagocytosis of a PFOB-Nanoemulsion for 19F Magnetic Resonance Imaging: First Results in Monocytes of Patients with Stable Coronary Artery Disease and ST-Elevation Myocardial Infarction. Molecules. 2019;24:2058. doi: 10.3390/molecules24112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahrens E.T., Zhong J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed. 2013;26:860–871. doi: 10.1002/nbm.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kramer W., Schubert R., Massing U. Small-scale preparation of perfluorocarbon-nanoemulsions utilizing dual centrifugation. Int. J. Pharm. 2019;572:118753. doi: 10.1016/j.ijpharm.2019.118753. [DOI] [PubMed] [Google Scholar]
- 100.Parak W.J., Osinski M., Yamamoto K.I., Diou O., Fattal E., Payen T., Bridal S.L., Valette J., Tsapis N. Nanocapsules of perfluorooctyl bromide for theranostics: From formulation to targeting; Proceedings of the Colloidal Nanoparticles for Biomedical Applications IX; San Francisco, CA, USA. 1–6 February 2014. [Google Scholar]
- 101.Kadayakkara D.K., Damodaran K., Hitchens T.K., Bulte J.W.M., Ahrens E.T. 19F spin–lattice relaxation of perfluoropolyethers: Dependence on temperature and magnetic field strength (7.0–14.1T) J. Magn. Reson. 2014;242:18–22. doi: 10.1016/j.jmr.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gerhardt G.E., Lagow R.J. Synthesis of the perfluoropoly(ethylene glycol) ethers by direct fluorination. J. Org. Chem. 1978;43:4505–4509. doi: 10.1021/jo00417a026. [DOI] [Google Scholar]
- 103.Sansotera M., Talaeemashhadi S., Gambarotti C., Pirola C., Longhi M., Ortenzi M.A., Navarrini W., Bianchi C.L. Comparison of Branched and Linear Perfluoropolyether Chains Functionalization on Hydrophobic, Morphological and Conductive Properties of Multi-Walled Carbon Nanotubes. Nanomaterials. 2018;8:176. doi: 10.3390/nano8030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonneaud C., Howell J., Bongiovanni R., Joly-Duhamel C., Friesen C.M. Diversity of Synthetic Approaches to Functionalized Perfluoropolyalkylether Polymers. Macromolecules. 2021;54:521–550. doi: 10.1021/acs.macromol.0c01599. [DOI] [Google Scholar]
- 105.Lemaire L., Bastiat G., Franconi F., Lautram N., Duong Thi Dan T., Garcion E., Saulnier P., Benoit J.P. Perfluorocarbon-loaded lipid nanocapsules as oxygen sensors for tumor tissue pO(2) assessment. Eur. J. Pharm. Biopharm. 2013;84:479–486. doi: 10.1016/j.ejpb.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 106.Constantinides C., McNeill E., Carnicer R., Al Haj Zen A., Sainz-Urruela R., Shaw A., Patel J., Swider E., Alonaizan R., Potamiti L., et al. Improved cellular uptake of perfluorocarbon nanoparticles for in vivo murine cardiac 19F MRS/MRI and temporal tracking of progenitor cells. Nanomedicine. 2019;18:391–401. doi: 10.1016/j.nano.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 107.Flogel U., Schluter A., Jacoby C., Temme S., Banga J.P., Eckstein A., Schrader J., Berchner-Pfannschmidt U. Multimodal assessment of orbital immune cell infiltration and tissue remodeling during development of graves disease by 1 H19 F MRI. Magn. Reson. Med. 2018;80:711–718. doi: 10.1002/mrm.27064. [DOI] [PubMed] [Google Scholar]
- 108.Prinz C., Delgado P.R., Eigentler T.W., Starke L., Niendorf T., Waiczies S. Toward 19F magnetic resonance thermometry: Spin-lattice and spin-spin-relaxation times and temperature dependence of fluorinated drugs at 9.4 T. Magn. Reson. Mater. Phys. Biol. Med. 2019;32:51–61. doi: 10.1007/s10334-018-0722-8. [DOI] [PubMed] [Google Scholar]
- 109.Weise G., Basse-Lusebrink T.C., Kleinschnitz C., Kampf T., Jakob P.M., Stoll G. In vivo imaging of stepwise vessel occlusion in cerebral photothrombosis of mice by 19F MRI. PLoS ONE. 2011;6:e28143. doi: 10.1371/journal.pone.0028143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shin S.H., Park E.J., Min C., Choi S.I., Jeon S., Kim Y.H., Kim D. Tracking Perfluorocarbon Nanoemulsion Delivery by 19F MRI for Precise High Intensity Focused Ultrasound Tumor Ablation. Theranostics. 2017;7:562–572. doi: 10.7150/thno.16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu X., Yan Y., Liu F., Wu L., Shao M., Wang K., Sun X., Li Y., Beinpuo E.S.W., Shen B. Folate receptor-targeted 19 F MR molecular imaging and proliferation evaluation of lung cancer. J. Magn. Reson. Imaging. 2018;48:1617–1625. doi: 10.1002/jmri.26177. [DOI] [PubMed] [Google Scholar]
- 112.Saini S., Korf H., Liang S., Verbeke R., Manshian B., Raemdonck K., Lentacker I., Gysemans C., De Smedt S.C., Himmelreich U. Challenges for labeling and longitudinal tracking of adoptively transferred autoreactive T lymphocytes in an experimental type-1 diabetes model. Magn. Reson. Mater. Phys. Biol. Med. 2019;32:295–305. doi: 10.1007/s10334-018-0720-x. [DOI] [PubMed] [Google Scholar]
- 113.Tirotta I., Mastropietro A., Cordiglieri C., Gazzera L., Baggi F., Baselli G., Bruzzone M.G., Zucca I., Cavallo G., Terraneo G., et al. A superfluorinated molecular probe for highly sensitive in vivo19F-MRI. J. Am. Chem. Soc. 2014;136:8524–8527. doi: 10.1021/ja503270n. [DOI] [PubMed] [Google Scholar]
- 114.Chirizzi C., De Battista D., Tirotta I., Metrangolo P., Comi G., Bombelli F.B., Chaabane L. Multispectral MRI with Dual Fluorinated Probes to Track Mononuclear Cell Activity in Mice. Radiology. 2019;291:351–357. doi: 10.1148/radiol.2019181073. [DOI] [PubMed] [Google Scholar]
- 115.19F MRI Contrast Agents. [(accessed on 21 September 2021)]; Available online: Clinicaltrials.gov.
- 116.Arango J.M., Padro D., Blanco J., Lopez-Fernandez S., Castellnou P., Villa-Valverde P., Ruiz-Cabello J., Martin A., Carril M. Fluorine Labeling of Nanoparticles and In Vivo 19F Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces. 2021;13:12941–12949. doi: 10.1021/acsami.1c01291. [DOI] [PubMed] [Google Scholar]
- 117.Weise G., Stoll G. Magnetic resonance imaging of blood brain/nerve barrier dysfunction and leukocyte infiltration: Closely related or discordant? Front. Neuurol. 2012;3:178. doi: 10.3389/fneur.2012.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bharti S.K., Roy R. Quantitative 1H NMR spectroscopy. TrAC Trends Anal. Chem. 2012;35:5–26. doi: 10.1016/j.trac.2012.02.007. [DOI] [Google Scholar]
- 119.Liang S., Dresselaers T., Louchami K., Zhu C., Liu Y., Himmelreich U. Comparison of different compressed sensing algorithms for low SNR 19 F MRI applications-Imaging of transplanted pancreatic islets and cells labeled with perfluorocarbons. NMR Biomed. 2017;30 doi: 10.1002/nbm.3776. [DOI] [PubMed] [Google Scholar]
- 120.Waiczies S., Rosenberg J.T., Kuehne A., Starke L., Delgado P.R., Millward J.M., Prinz C., Dos Santos Periquito J., Pohlmann A., Waiczies H., et al. Fluorine-19 MRI at 21.1 T: Enhanced spin-lattice relaxation of perfluoro-15-crown-5-ether and sensitivity as demonstrated in ex vivo murine neuroinflammation. Magn. Reson. Mater. Phys. Biol. Med. 2019;32:37–49. doi: 10.1007/s10334-018-0710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Heeswijk R.B., Colotti R., Darcot E., Delacoste J., Pellegrin M., Piccini D., Hernando D. Chemical shift encoding (CSE) for sensitive fluorine-19 MRI of perfluorocarbons with complex spectra. Magn. Reson. Med. 2018;79:2724–2730. doi: 10.1002/mrm.26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Plaumann M., Bommerich U., Trantzschel T., Lego D., Dillenberger S., Sauer G., Bargon J., Buntkowsky G., Bernarding J. Parahydrogen-induced polarization transfer to 19F in perfluorocarbons for 19F NMR spectroscopy and MRI. Chemistry. 2013;19:6334–6339. doi: 10.1002/chem.201203455. [DOI] [PubMed] [Google Scholar]
- 123.Tanifum E.A., Devkota L., Ngwwa C., Badachhape A.A., Ghaghada K.B., Romero J., Pautler R.G., Annapragada A.V. A Hyperfluorinated Hydrophilic Molecule for Aqueous 19F MRI Contrast Media. Contrast Media Mol. Imaging. 2018;2018:1693513. doi: 10.1155/2018/1693513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferenz K.B., Steinbicker A.U. Artificial Oxygen Carriers-Past, Present, and Future-a Review of the Most Innovative and Clinically Relevant Concepts. J. Pharmacol. Exp. Ther. 2019;369:300–310. doi: 10.1124/jpet.118.254664. [DOI] [PubMed] [Google Scholar]
- 125.Stenzel M., Mentzel H.J. Ultrasound elastography and contrast-enhanced ultrasound in infants, children and adolescents. Eur. J. Radiol. 2014;83:1560–1569. doi: 10.1016/j.ejrad.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 126.Paefgen V., Doleschel D., Kiessling F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front. Pharmacol. 2015;6:197. doi: 10.3389/fphar.2015.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Podell S., Burrascano C., Gaal M., Golec B., Maniquis J., Mehlhaff P. Physical and biochemical stability of Optison, an injectable ultrasound contrast agent. Biotechnol. Appl. Biochem. 1999;30:213–223. [PubMed] [Google Scholar]
- 128.Saucedo A.M., De La Cerda J., Suami H., Serda R.E. Multimodal imaging of the tumor microenvironment and biological responses to immune therapy. Biomed. Microdevices. 2018;20:105. doi: 10.1007/s10544-018-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fox M.S., Gaudet J.M., Foster P.J. Fluorine-19 MRI Contrast Agents for Cell Tracking and Lung Imaging. Magn. Reson. Insights. 2015;8:53–67. doi: 10.4137/MRI.S23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.O’Hanlon C.F., Fedczyna T., Eaker S., Shingleton W.D., Helfer B.M. Integrating a 19F MRI Tracer Agent into the Clinical Scale Manufacturing of a T-Cell Immunotherapy. Contrast Media Mol. Imaging. 2017;2017:9548478. doi: 10.1155/2017/9548478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rizzo S., Padelli F., Rinaldi E., Gioeni D., Aquino D., Brizzola S., Acocella F., Spaggiari L., Baggi F., Bellomi M., et al. 7-T MRI tracking of mesenchymal stromal cells after lung injection in a rat model. Eur. Radiol. Exp. 2020;4:54. doi: 10.1186/s41747-020-00183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pavlova O.S., Gulyaev M.V., Anisimov N.V., Silachev D.N., Gervits L.L., Pirogov Y.A. New Aspects of Biodistribution of Perfluorocarbon Emulsions in Rats: Thymus Imaging. Appl. Magn. Reson. 2020;51:1625–1635. doi: 10.1007/s00723-020-01242-w. [DOI] [Google Scholar]
- 133.Latson G.W. Perftoran (Vidaphor)-Introduction to Western Medicine. Shock. 2019;52:65–69. doi: 10.1097/SHK.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 134.Maevsky E., Ivanitsky G., Bogdanova L., Axenova O., Karmen N., Zhiburt E., Senina R., Pushkin S., Maslennikov I., Orlov A., et al. Clinical results of Perftoran application: Present and future. Artif. Cells Blood Substit. Immobil. Biotechnol. 2005;33:37–46. doi: 10.1081/BIO-200046654. [DOI] [PubMed] [Google Scholar]
- 135.Scholz A.W., Eberle B., Heussel C.P., David M., Schmittner M.D., Quintel M., Schreiber L.M., Weiler N. Ventilation-perfusion ratio in perflubron during partial liquid ventilation. Anesth. Analg. 2010;110:1661–1668. doi: 10.1213/ANE.0b013e3181d3e1d5. [DOI] [PubMed] [Google Scholar]
- 136.Chenoune M., De Rochefort L., Bruneval P., Lidouren F., Kohlhauer M., Seemann A., Ghaleh B., Korn M., Dubuisson R.-M., Ben Y.A., et al. Evaluation of lung recovery after static administration of three different perfluorocarbons in pigs. BMC Pharmacol. Toxicol. 2014;15 doi: 10.1186/2050-6511-15-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kohlhauer M., Boissady E., Lidouren F., de Rochefort L., Nadeau M., Rambaud J., Hutin A., Dubuisson R.M., Guillot G., Pey P., et al. A new paradigm for lung-conservative total liquid ventilation. EBioMedicine. 2020;52:102365. doi: 10.1016/j.ebiom.2019.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weigel J.K., Steinmann D., Emerich P., Stahl C.A., v Elverfeldt D., Guttmann J. High-resolution three-dimensional 19F-magnetic resonance imaging of rat lung in situ: Evaluation of airway strain in the perfluorocarbon-filled lung. Physiol. Meas. 2011;32:251–262. doi: 10.1088/0967-3334/32/2/008. [DOI] [PubMed] [Google Scholar]
- 139.André Dias S., Berdeaux A., Darrasse L., Demanesse M., de Rochefort L., Filoche M., Ghaleh B., Hutin A., Isabey D., Kunc T., et al. ABYSS: Therapeutic hypothermia by total liquid ventilation following cardiac arrest and resuscitation. IRBM. 2015;36:110–117. doi: 10.1016/j.irbm.2015.01.011. [DOI] [Google Scholar]
- 140.Hertlein T., Sturm V., Jakob P., Ohlsen K. 19F magnetic resonance imaging of perfluorocarbons for the evaluation of response to antibiotic therapy in a Staphylococcus aureus infection model. PLoS ONE. 2013;8:e64440. doi: 10.1371/journal.pone.0064440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Rochambeau D., Barłóg M., Edwardson T.G.W., Fakhoury J.J., Stein R.S., Bazzi H.S., Sleiman H.F. “DNA–Teflon” sequence-controlled polymers. Polym. Chem. 2016;7:4998–5003. doi: 10.1039/C6PY00532B. [DOI] [Google Scholar]
- 142.Temme S., Baran P., Bouvain P., Grapentin C., Kramer W., Knebel B., Al-Hasani H., Moll J.M., Floss D., Schrader J., et al. Synthetic Cargo Internalization Receptor System for Nanoparticle Tracking of Individual Cell Populations by Fluorine Magnetic Resonance Imaging. ACS Nano. 2018;12:11178–11192. doi: 10.1021/acsnano.8b05698. [DOI] [PubMed] [Google Scholar]
- 143.Boehm-Sturm P., Mengler L., Wecker S., Hoehn M., Kallur T. In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS ONE. 2011;6:e29040. doi: 10.1371/journal.pone.0029040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sehl O.C., Makela A.V., Hamilton A.M., Foster P.J. Trimodal Cell Tracking In Vivo: Combining Iron- and Fluorine-Based Magnetic Resonance Imaging with Magnetic Particle Imaging to Monitor the Delivery of Mesenchymal Stem Cells and the Ensuing Inflammation. Tomography. 2019;5:367–376. doi: 10.18383/j.tom.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Muhammad G., Jablonska A., Rose L., Walczak P., Janowski M. Effect of MRI tags: SPIO nanoparticles and 19F nanoemulsion on various populations of mouse mesenchymal stem cells. Acta Neurobiol. Exp. (Wars) 2015;75:144–159. [PMC free article] [PubMed] [Google Scholar]
- 146.Flogel U., Su S., Kreideweiss I., Ding Z., Galbarz L., Fu J., Jacoby C., Witzke O., Schrader J. Noninvasive detection of graft rejection by in vivo 19 F MRI in the early stage. Am. J. Transplant. 2011;11:235–244. doi: 10.1111/j.1600-6143.2010.03372.x. [DOI] [PubMed] [Google Scholar]
- 147.Deuchar G.A., Brennan D., Griffiths H., Macrae I.M., Santosh C. Perfluorocarbons enhance a T2*-based MRI technique for identifying the penumbra in a rat model of acute ischemic stroke. J. Cereb. Blood Flow Metab. 2013;33:1422–1428. doi: 10.1038/jcbfm.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khurana A., Chapelin F., Xu H., Acevedo J.R., Molinolo A., Nguyen Q., Ahrens E.T. Visualization of macrophage recruitment in head and neck carcinoma model using fluorine-19 magnetic resonance imaging. Magn. Reson. Med. 2018;79:1972–1980. doi: 10.1002/mrm.26854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shin S.H., Park S.H., Kang S.H., Kim S.W., Kim M., Kim D. Fluorine-19 Magnetic Resonance Imaging and Positron Emission Tomography of Tumor-Associated Macrophages and Tumor Metabolism. Contrast Media Mol. Imaging. 2017;2017:4896310. doi: 10.1155/2017/4896310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Makela A.V., Gaudet J.M., Foster P.J. Quantifying tumor associated macrophages in breast cancer: A comparison of iron and fluorine-based MRI cell tracking. Sci. Rep. 2017;7:42109. doi: 10.1038/srep42109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hosgood S.A., Nicholson M.L. The role of perfluorocarbon in organ preservation. Transplantation. 2010;89:1169–1175. doi: 10.1097/TP.0b013e3181da6064. [DOI] [PubMed] [Google Scholar]
- 152.Moore J.K., Chen J., Pan H., Gaaut J.P., Jain S., Wickline S.A. Quantification of vascular damage in acute kidney injury with fluorine magnetic resonance imaging and spectroscopy. Magn. Reson. Med. 2018;79:3144–3153. doi: 10.1002/mrm.26985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baete S.H., Vandecasteele J., Colman L., De Neve W., De Deene Y. An oxygen-consuming phantom simulating perfused tissue to explore oxygen dynamics and 19F MRI oximetry. Magn. Reson. Mater. Phys. Biol. Med. 2010;23:217–226. doi: 10.1007/s10334-010-0219-6. [DOI] [PubMed] [Google Scholar]
- 154.Jacoby C., Borg N., Heusch P., Sauter M., Bonner F., Kandolf R., Klingel K., Schrader J., Flogel U. Visualization of immune cell infiltration in experimental viral myocarditis by 19F MRI in vivo. Magn. Reson. Mater. Phys. Biol. Med. 2014;27:101–106. doi: 10.1007/s10334-013-0391-6. [DOI] [PubMed] [Google Scholar]
- 155.van Heeswijk R.B., De Blois J., Kania G., Gonzales C., Blyszczuk P., Stuber M., Eriksson U., Schwitter J. Selective in vivo visualization of immune-cell infiltration in a mouse model of autoimmune myocarditis by fluorine-19 cardiac magnetic resonance. Circ. Cardiovasc. Imaging. 2013;6:277–284. doi: 10.1161/CIRCIMAGING.112.000125. [DOI] [PubMed] [Google Scholar]
- 156.Darcot E., Colotti R., Pellegrin M., Wilson A., Siegert S., Bouzourene K., Yerly J., Mazzolai L., Stuber M., van Heeswijk R.B. Towards Quantification of Inflammation in Atherosclerotic Plaque in the Clinic—Characterization and Optimization of Fluorine-19 MRI in Mice at 3 T. Sci. Rep. 2019;9:17488. doi: 10.1038/s41598-019-53905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rothe M., Jahn A., Weiss K., Hwang J.H., Szendroedi J., Kelm M., Schrader J., Roden M., Flogel U., Bonner F. In vivo 19F MR inflammation imaging after myocardial infarction in a large animal model at 3 T. Magn. Reson. Mater. Phys. Biol. Med. 2019;32:5–13. doi: 10.1007/s10334-018-0714-8. [DOI] [PubMed] [Google Scholar]
- 158.Ebner B., Behm P., Jacoby C., Burghoff S., French B.A., Schrader J., Flogel U. Early assessment of pulmonary inflammation by 19F MRI in vivo. Circ. Cardiovasc. Imaging. 2010;3:202–210. doi: 10.1161/CIRCIMAGING.109.902312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Flogel U., Ding Z., Hardung H., Jander S., Reichmann G., Jacoby C., Schubert R., Schrader J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cao B., Lyu X., Wang C., Lu S., Xing D., Hu X. Rational collaborative ablation of bacterial biofilms ignited by physical cavitation and concurrent deep antibiotic release. Biomaterials. 2020;262:120341. doi: 10.1016/j.biomaterials.2020.120341. [DOI] [PubMed] [Google Scholar]
- 161.Riess J.G. Perfluorocarbon-based oxygen delivery. Artif. Cells Blood Substit. Immobil. Biotechnol. 2006;34:567–580. doi: 10.1080/10731190600973824. [DOI] [PubMed] [Google Scholar]
- 162.Castro C.I., Briceno J.C. Perfluorocarbon-based oxygen carriers: Review of products and trials. Artif. Organs. 2010;34:622–634. doi: 10.1111/j.1525-1594.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 163.Lambert E., Gorantla V.S., Janjic J.M. Pharmaceutical design and development of perfluorocarbon nanocolloids for oxygen delivery in regenerative medicine. Nanomedicine. 2019;14:20. doi: 10.2217/nnm-2019-0260. [DOI] [PubMed] [Google Scholar]
- 164.Goh F., Sambanis A. In vivo noninvasive monitoring of dissolved oxygen concentration within an implanted tissue-engineered pancreatic construct. Tissue Eng. Part C Methods. 2011;17:887–894. doi: 10.1089/ten.tec.2011.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Temme S., Bonner F., Schrader J., Flogel U. 19F magnetic resonance imaging of endogenous macrophages in inflammation. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2012;4:329–343. doi: 10.1002/wnan.1163. [DOI] [PubMed] [Google Scholar]
- 166.Palekar R.U., Jallouk A.P., Lanza G.M., Pan H., Wickline S.A. Molecular imaging of atherosclerosis with nanoparticle-based fluorinated MRI contrast agents. Nanomedicine. 2015;10:1817–1832. doi: 10.2217/nnm.15.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Srinivas M., Heerschap A., Ahrens E.T., Figdor C.G., de Vries I.J. 19F MRI for quantitative in vivo cell tracking. Trends Biotechnol. 2010;28:363–370. doi: 10.1016/j.tibtech.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Srinivas M., Boehm-Sturm P., Figdor C.G., de Vries I.J., Hoehn M. Labeling cells for in vivo tracking using 19F MRI. Biomaterials. 2012;33:8830–8840. doi: 10.1016/j.biomaterials.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 169.Yahyapour R., Farhood B., Graily G., Rezaeyan A., Rezapoor S., Abdollahi H., Cheki M., Amini P., Fallah H., Najafi M., et al. Stem Cell Tracing Through MR Molecular Imaging. Tissue Eng. Regen. Med. 2018;15:249–261. doi: 10.1007/s13770-017-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Heerschap A. In Vivo 19F Magnetic Resonance Spectroscopy. eMagRes. 2016;5:1283–1290. doi: 10.1002/9780470034590.emrstm1445. [DOI] [Google Scholar]
- 171.Bober Z., Aebisher D., Tabarkiewicz J., Guz W., Tutka P., Bartusik-Aebisher D. Investigation of pharmaceuticals by nuclear magnetic resonance imaging and spectroscopy. Eur. J. Clin. Exp. Med. 2017;15:99–108. doi: 10.15584/ejcem.2017.2.2. [DOI] [Google Scholar]
- 172.Waiczies S., Srinivas M., Flogel U., Boehm-Sturm P., Niendorf T. Special issue on fluorine-19 magnetic resonance: Technical solutions, research promises and frontier applications. Magn. Reson. Mater. Phys. Biol. Med. 2019;32:1–3. doi: 10.1007/s10334-019-00741-7. [DOI] [PubMed] [Google Scholar]
- 173.Diaz-Lopez R., Tsapis N., Fattal E. Liquid perfluorocarbons as contrast agents for ultrasonography and 19F-MRI. Pharm. Res. 2010;27:1–16. doi: 10.1007/s11095-009-0001-5. [DOI] [PubMed] [Google Scholar]
- 174.Cosco D., Fattal E., Fresta M., Tsapis N. Perfluorocarbon-loaded micro and nanosystems for medical imaging: A state of the art. J. Fluor. Chem. 2015;171:18–26. doi: 10.1016/j.jfluchem.2014.10.013. [DOI] [Google Scholar]
- 175.Zhang T., Zhang Q., Tian J.-H., Xing J.-F., Guo W., Liang X.-J. Perfluorocarbon-based nanomedicine: Emerging strategy for diagnosis and treatment of diseases. MRS Commun. 2018;8:303–313. doi: 10.1557/mrc.2018.49. [DOI] [Google Scholar]
- 176.Amiri H., Srinivas M., Veltien A., van Uden M.J., de Vries I.J., Heerschap A. Cell tracking using 19F magnetic resonance imaging: Technical aspects and challenges towards clinical applications. Eur. Radiol. 2015;25:726–735. doi: 10.1007/s00330-014-3474-5. [DOI] [PubMed] [Google Scholar]
- 177.Riess J.G. The design and development of improved fluorocarbon-based products for use in medicine and biology. Artif. Cells Blood Substit. Immobil. Biotechnol. 1994;22:215–234. doi: 10.3109/10731199409117416. [DOI] [PubMed] [Google Scholar]
- 178.Riess J.G., Pierre K.M. Fluorinated materials for in vivo oxygen transport (blood substitutes), diagnosis and drug delivery. Biomaterials. 1998;19:1529–1539. doi: 10.1016/S0142-9612(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 179.Bartusik D., Aebisher D. 19F applications in drug development and imaging—A review. Biomed. Pharmacother. 2014;68:813–817. doi: 10.1016/j.biopha.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 180.Peterson K.L., Srivastava K., Pierre V.C. Fluorinated Paramagnetic Complexes: Sensitive and Responsive Probes for Magnetic Resonance Spectroscopy and Imaging. Front. Chem. 2018;6:160. doi: 10.3389/fchem.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jirak D., Galisova A., Kolouchova K., Babuka D., Hruby M. Fluorine polymer probes for magnetic resonance imaging: Quo vadis? Magn. Reson. Mater. Phys. Biol. Med. 2019;32:173–185. doi: 10.1007/s10334-018-0724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hequet E., Henoumont C., Muller R.N., Laurent S. Fluorinated MRI contrast agents and their versatile applications in the biomedical field. Future Med. Chem. 2019;11:1157–1175. doi: 10.4155/fmc-2018-0463. [DOI] [PubMed] [Google Scholar]
- 183.Bartusik-Aebisher D., Bober Z., Aebisher D. Selected applications of fluorinated MR contrast agents and fluorine-containing drugs in medicine. Acta Pol. Pharm.—Drug Res. 2020;77:403–410. doi: 10.32383/appdr/118742. [DOI] [Google Scholar]
- 184.Moroz V.V., Chernysh A.M., Kozlova E.K. Coronavirus SARS-CoV-2: Hypotheses of Impact on the Circulatory System, Prospects for the Use of Perfluorocarbon Emulsion, and Feasibility of Biophysical Research Methods. Gen. Reanimatol. 2020;16:4–13. doi: 10.15360/1813-9779-2020-3-0-1. [DOI] [Google Scholar]
- 185.Begines B., Ortiz T., Perez-Aranda M., Martinez G., Merinero M., Arguelles-Arias F., Alcudia A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials. 2020;10:1403. doi: 10.3390/nano10071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zielinska A., Carreiro F., Oliveira A.M., Neves A., Pires B., Venkatesh D.N., Durazzo A., Lucarini M., Eder P., Silva A.M., et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules. 2020;25:3731. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wallat J.D., Czapar A.E., Wang C., Wen A.M., Wek K.S., Yu X., Steinmetz N.F., Pokorski J.K. Optical and Magnetic Resonance Imaging Using Fluorous Colloidal Nanoparticles. Biomacromolecules. 2017;18:103–112. doi: 10.1021/acs.biomac.6b01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bailey M.M., Mahoney C.M., Dempah K.E., Davis J.M., Becker M.L., Khondee S., Munson E.J., Berkland C. Fluorinated copolymer nanoparticles for multimodal imaging applications. Macromol. Rapid Commun. 2010;31:87–92. doi: 10.1002/marc.200900505. [DOI] [PubMed] [Google Scholar]
- 189.Kaberov L.I., Verbraeken B., Riabtseva A., Brus J., Radulescu A., Talmon Y., Stepanek P., Hoogenboom R., Filippov S.K. Fluorophilic–Lipophilic–Hydrophilic Poly(2-oxazoline) Block Copolymers as MRI Contrast Agents: From Synthesis to Self-Assembly. Macromolecules. 2018;51:6047–6056. doi: 10.1021/acs.macromol.8b00957. [DOI] [Google Scholar]
- 190.Zhao W., Ta H.T., Zhang C., Whittaker A.K. Polymerization-Induced Self-Assembly (PISA)—Control over the Morphology of 19F-Containing Polymeric Nano-objects for Cell Uptake and Tracking. Biomacromolecules. 2017;18:1145–1156. doi: 10.1021/acs.biomac.6b01788. [DOI] [PubMed] [Google Scholar]
- 191.Fu C., Herbst S., Zhang C., Whittaker A.K. Polymeric 19F MRI agents responsive to reactive oxygen species. Polym. Chem. 2017;8:4585–4595. doi: 10.1039/C7PY00986K. [DOI] [Google Scholar]
- 192.Huang P., Guo W., Yang G., Song H., Wang Y., Wang C., Kong D., Wang W. Fluorine Meets Amine: Reducing Microenvironment-Induced Amino-Activatable Nanoprobes for 19F-Magnetic Resonance Imaging of Biothiols. ACS Appl. Mater. Interfaces. 2018;10:18532–18542. doi: 10.1021/acsami.8b03764. [DOI] [PubMed] [Google Scholar]
- 193.Szczech M., Lopuszynska N., Tomal W., Jasinski K., Weglarz W.P., Warszynski P., Szczepanowicz K. Nafion-Based Nanocarriers for Fluorine Magnetic Resonance Imaging. Langmuir. 2020;36:9534–9539. doi: 10.1021/acs.langmuir.0c01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Srinivas M., Cruz L.J., Bonetto F., Heerschap A., Figdor C.G., de Vries I.J. Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials. 2010;31:7070–7077. doi: 10.1016/j.biomaterials.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 195.Srinivas M., Morel P.A., Ernst L.A., Laidlaw D.H., Ahrens E.T. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn. Reson. Med. 2007;58:725–734. doi: 10.1002/mrm.21352. [DOI] [PubMed] [Google Scholar]
- 196.Mangala S., Jurjen T., Gerty S., Fernando B., Luis-Javier C., Houshang A., Arend H., Carl G.F., de Vries J.M. PLGA-encapsulated perfluorocarbon nanoparticles for simultaneous visualization of distinct cell populations by 19F MRI. Nanomedicine. 2015;10:2339–2348. doi: 10.2217/NNM.15.76. [DOI] [PubMed] [Google Scholar]
- 197.Koshkina O., Lajoinie G., Bombelli F.B., Swider E., Cruz L.J., White P.B., Schweins R., Dolen Y., van Dinther E.A.W., van Riessen N.K., et al. Multicore Liquid Perfluorocarbon-Loaded Multimodal Nanoparticles for Stable Ultrasound and 19F MRI Applied to In Vivo Cell Tracking. Adv. Funct. Mater. 2019;29:1806485. doi: 10.1002/adfm.201806485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Swider E., Daoudi K., Staal A.H.J., Koshkina O., van Riessen N.K., van Dinther E., de Vries I.J.M., de Korte C.L., Srinivas M. Clinically-Applicable Perfluorocarbon-Loaded Nanoparticles For In vivo Photoacoustic, 19F Magnetic Resonance And Fluorescent Imaging. Nanotheranostics. 2018;2:258–268. doi: 10.7150/ntno.26208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Constantinides C., Maguire M., McNeill E., Carnicer R., Swider E., Srinivas M., Carr C.A., Schneider J.E. Fast, quantitative, murine cardiac 19F MRI/MRS of PFCE-labeled progenitor stem cells and macrophages at 9.4T. PLoS ONE. 2018;13:e0190558. doi: 10.1371/journal.pone.0190558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mastrogiacomo S., Dou W., Koshkina O., Boerman O.C., Jansen J.A., Heerschap A., Srinivas M., Walboomers X.F. Perfluorocarbon/Gold Loading for Noninvasive in Vivo Assessment of Bone Fillers Using 19F Magnetic Resonance Imaging and Computed Tomography. ACS Appl. Mater. Interfaces. 2017;9:22149–22159. doi: 10.1021/acsami.7b04075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Swider E., Staal A.H.J., Koen van Riessen N., Jacobs L., White P.B., Fokkink R., Janssen G.-J., van Dinther E., Figdor C.G., de Vries I., et al. Design of triphasic poly(lactic-co-glycolic acid) nanoparticles containing a perfluorocarbon phase for biomedical applications. RSC Adv. 2018;8:6460–6470. doi: 10.1039/C7RA13062G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Staal A.H.J., Becker K., Tagit O., Koen van Riessen N., Koshkina O., Veltien A., Bouvain P., Cortenbach K.R.G., Scheenen T., Flogel U., et al. In vivo clearance of 19F MRI imaging nanocarriers is strongly influenced by nanoparticle ultrastructure. Biomaterials. 2020;261:120307. doi: 10.1016/j.biomaterials.2020.120307. [DOI] [PubMed] [Google Scholar]
- 203.Koshkina O., White P.B., Staal A.H.J., Schweins R., Swider E., Tirotta I., Tinnemans P., Fokkink R., Veltien A., van Riessen N.K., et al. Nanoparticles for “two color” 19F magnetic resonance imaging: Towards combined imaging of biodistribution and degradation. J. Colloid Interface Sci. 2020;565:278–287. doi: 10.1016/j.jcis.2019.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Krekorian M., Van Riessen K., Sandker G., Swider E., Staal A., Koshkina O., Heskamp S., Srinivas M., Aarntzen E. PLGA nanoparticles for combined SPECT/PET and 19F MRI in vivo cell tracking. Nucl. Med. Biol. 2019;72–73:S42–S43. doi: 10.1016/S0969-8051(19)30305-1. [DOI] [Google Scholar]
- 205.Hoogendijk E., Swider E., Staal A.H.J., White P.B., van Riessen N.K., Glasser G., Lieberwirth I., Musyanovych A., Serra C.A., Srinivas M., et al. Continuous-Flow Production of Perfluorocarbon-Loaded Polymeric Nanoparticles: From the Bench to Clinic. ACS Appl Mater. Interfaces. 2020;12:49335–49345. doi: 10.1021/acsami.0c12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vu-Quang H., Vinding M.S., Xia D., Nielsen T., Ullisch M.G., Dong M., Nielsen N.C., Kjems J. Chitosan-coated poly(lactic-co-glycolic acid) perfluorooctyl bromide nanoparticles for cell labeling in 19F magnetic resonance imaging. Carbohydr. Polym. 2016;136:936–944. doi: 10.1016/j.carbpol.2015.09.076. [DOI] [PubMed] [Google Scholar]
- 207.Vu-Quang H., Vinding M.S., Nielsen T., Ullisch M.G., Nielsen N.C., Kjems J. Theranostic tumor targeted nanoparticles combining drug delivery with dual near infrared and 19F magnetic resonance imaging modalities. Nanomedicine. 2016;12:1873–1884. doi: 10.1016/j.nano.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 208.Vu-Quang H., Vinding M.S., Jakobsen M., Song P., Dagnaes-Hansen F., Nielsen N.C., Kjems J. Imaging Rheumatoid Arthritis in Mice Using Combined Near Infrared and 19F Magnetic Resonance Modalities. Sci. Rep. 2019;9:14314. doi: 10.1038/s41598-019-50043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Quang H.V., Chang C.C., Song P., Hauge E.M., Kjems J. Caveolae-mediated mesenchymal stem cell labelling by PSS-coated PLGA PFOB nano-contrast agent for MRI. Theranostics. 2018;8:2657–2671. doi: 10.7150/thno.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Diou O., Tsapis N., Giraudeau C., Valette J., Gueutin C., Bourasset F., Zanna S., Vauthier C., Fattal E. Long-circulating perfluorooctyl bromide nanocapsules for tumor imaging by 19FMRI. Biomaterials. 2012;33:5593–5602. doi: 10.1016/j.biomaterials.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 211.Somaglino L., Mousnier L., Giron A., Urbach W., Tsapis N., Taulier N. In vitro evaluation of polymeric nanoparticles with a fluorine core for drug delivery triggered by focused ultrasound. Colloids Surf. B Biointerfaces. 2021;200:111561. doi: 10.1016/j.colsurfb.2021.111561. [DOI] [PubMed] [Google Scholar]
- 212.Cruz L.J., Que I., Aswendt M., Chan A., Hoehn M., Löwik C. Targeted nanoparticles for the non-invasive detection of traumatic brain injury by optical imaging and fluorine magnetic resonance imaging. Nano Res. 2016;9:1276–1289. doi: 10.1007/s12274-016-1023-z. [DOI] [Google Scholar]
- 213.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zambito G., Deng S., Haeck J., Gaspar N., Himmelreich U., Censi R., Lowik C., Di Martino P., Mezzanotte L. Fluorinated PLGA-PEG-Mannose Nanoparticles for Tumor-Associated Macrophage Detection by Optical Imaging and MRI. Front. Med. 2021;8:712367. doi: 10.3389/fmed.2021.712367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zerrillo L., Gupta K., Lefeber F., Da Silva C.G., Galli F., Chan A., Veltien A., Dou W., Censi R., Di Martino P., et al. Novel Fluorinated Poly (Lactic-Co-Glycolic acid) (PLGA) and Polyethylene Glycol (PEG) Nanoparticles for Monitoring and Imaging in Osteoarthritis. Pharmaceutics. 2021;13:235. doi: 10.3390/pharmaceutics13020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu P., Zhou Q., Zhu H., Zhuang Y., Bao J. Enhanced antitumor efficacy in colon cancer using EGF functionalized PLGA nanoparticles loaded with 5-Fluorouracil and perfluorocarbon. BMC Cancer. 2020;20:354. doi: 10.1186/s12885-020-06803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Neri G., Mion G., Pizzi A., Celentano W., Chaabane L., Chierotti M.R., Gobetto R., Li M., Messa P., Campo F.D., et al. Fluorinated-PLGA Nanoparticles for Enhanced Drug Encapsulation and 19F-NMR Detection. Chem.—A Eur. J. 2020;26:10057–10063. doi: 10.1002/chem.202002078. [DOI] [PubMed] [Google Scholar]
- 218.Achilefu S., Janjic J.M., Patel S.K., Patrick M.J., Pollock J.A., DiVito E., Cascio M., Raghavachari R. Suppressing inflammation from inside out with novel NIR visible perfluorocarbon nanotheranostics; Proceedings of the Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications V; San Francisco, CA, USA. 2–7 February 2013. [Google Scholar]
- 219.Patel S.K., Zhang Y., Pollock J.A., Janjic J.M. Cyclooxgenase-2 inhibiting perfluoropoly (ethylene glycol) ether theranostic nanoemulsions-in vitro study. PLoS ONE. 2013;8:e55802. doi: 10.1371/journal.pone.0055802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Patel S.K., Patrick M.J., Pollock J.A., Janjic J.M. Two-color fluorescent (near-infrared and visible) triphasic perfluorocarbon nanoemuslions. J. Biomed. Opt. 2013;18:101312. doi: 10.1117/1.JBO.18.10.101312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang C., Moonshi S.S., Han Y., Puttick S., Peng H., Magoling B.J.A., Reid J.C., Bernardi S., Searles D.J., Král P., et al. PFPE-Based Polymeric 19F MRI Agents: A New Class of Contrast Agents with Outstanding Sensitivity. Macromolecules. 2017;50:5953–5963. doi: 10.1021/acs.macromol.7b01285. [DOI] [Google Scholar]
- 222.Zhang C., Liu T., Wang W., Bell C.A., Han Y., Fu C., Peng H., Tan X., Kral P., Gaus K., et al. Tuning of the Aggregation Behavior of Fluorinated Polymeric Nanoparticles for Improved Therapeutic Efficacy. ACS Nano. 2020;14:7425–7434. doi: 10.1021/acsnano.0c02954. [DOI] [PubMed] [Google Scholar]
- 223.Fu C., Zhang C., Peng H., Han F., Baker C., Wu Y., Ta H., Whittaker A.K. Enhanced Performance of Polymeric 19F MRI Contrast Agents through Incorporation of Highly Water-Soluble Monomer MSEA. Macromolecules. 2018;51:5875–5882. doi: 10.1021/acs.macromol.8b01190. [DOI] [Google Scholar]
- 224.Kirberger S.E., Maltseva S.D., Manulik J.C., Einstein S.A., Weegman B.P., Garwood M., Pomerantz W.C.K. Synthesis of Intrinsically Disordered Fluorinated Peptides for Modular Design of High-Signal 19 F MRI Agents. Angew. Chem. Int. Ed. Engl. 2017;56:6440–6444. doi: 10.1002/anie.201700426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Moonshi S.S., Zhang C., Peng H., Puttick S., Rose S., Fisk N.M., Bhakoo K., Stringer B.W., Qiao G.G., Gurr P.A., et al. A unique 19F MRI agent for the tracking of non phagocytic cells in vivo. Nanoscale. 2018;10:8226–8239. doi: 10.1039/C8NR00703A. [DOI] [PubMed] [Google Scholar]
- 226.Rossi S., Benaglia M., Ortenzi M., Micotti E., Perego C., De Simoni M.G. Poly(ethylene-glycol)-based fluorinated esters: A readily available entry for novel 19F-MRI agents. Tetrahedron Lett. 2011;52:6581–6583. doi: 10.1016/j.tetlet.2011.09.133. [DOI] [Google Scholar]
- 227.Biaggi C., Benaglia M., Ortenzi M., Micotti E., Perego C., De Simoni M.-G. Easily available, low cost 19F MRI agents: Poly(ethylene-glycol)-functionalized fluorinated ethers. J. Fluor. Chem. 2013;153:172–177. doi: 10.1016/j.jfluchem.2013.04.010. [DOI] [Google Scholar]
- 228.Wang J., Deng T., Liu Y., Chen K., Yang Z., Jiang Z.X. Monodisperse and Polydisperse PEGylation of Peptides and Proteins: A Comparative Study. Biomacromolecules. 2020;21:3134–3139. doi: 10.1021/acs.biomac.0c00517. [DOI] [PubMed] [Google Scholar]
- 229.Zhu J., Xiao Y., Zhang H., Li Y., Yuan Y., Yang Z., Chen S., Zheng X., Zhou X., Jiang Z.X. Peptidic Monodisperse PEG "combs" with Fine-Tunable LCST and Multiple Imaging Modalities. Biomacromolecules. 2019;20:1281–1287. doi: 10.1021/acs.biomac.8b01693. [DOI] [PubMed] [Google Scholar]
- 230.Zheng Y., Li S., Weng Z., Gao C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015;44:4091–4130. doi: 10.1039/C4CS00528G. [DOI] [PubMed] [Google Scholar]
- 231.Gao C., Yan D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004;29:183–275. doi: 10.1016/j.progpolymsci.2003.12.002. [DOI] [Google Scholar]
- 232.Wang K., Peng H., Thurecht K.J., Puttick S., Whittaker A.K. Multifunctional hyperbranched polymers for CT/19F MRI bimodal molecular imaging. Polym. Chem. 2016;7:1059–1069. doi: 10.1039/C5PY01707F. [DOI] [Google Scholar]
- 233.Zhang C., Moonshi S.S., Wang W., Ta H.T., Han Y., Han F.Y., Peng H., Kral P., Rolfe B.E., Gooding J.J., et al. High F-Content Perfluoropolyether-Based Nanoparticles for Targeted Detection of Breast Cancer by 19F Magnetic Resonance and Optical Imaging. ACS Nano. 2018;12:9162–9176. doi: 10.1021/acsnano.8b03726. [DOI] [PubMed] [Google Scholar]
- 234.Celentano W., Neri G., Distante F., Li M., Messa P., Chirizzi C., Chaabane L., De Campo F., Metrangolo P., Baldelli Bombelli F., et al. Design of fluorinated hyperbranched polyether copolymers for 19F MRI nanotheranostics. Polym. Chem. 2020;11:3951–3963. doi: 10.1039/D0PY00393J. [DOI] [Google Scholar]
- 235.Hernández-Ainsa S., Barberá J. Fluorinated liquid crystalline dendrimers. J. Fluor. Chem. 2015;177:37–45. doi: 10.1016/j.jfluchem.2015.02.018. [DOI] [Google Scholar]
- 236.Ray S., Li Z., Hsu C.H., Hwang L.P., Lin Y.C., Chou P.T., Lin Y.Y. Dendrimer- and copolymer-based nanoparticles for magnetic resonance cancer theranostics. Theranostics. 2018;8:6322–6349. doi: 10.7150/thno.27828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Caminade A.-M., Laurent R., Delavaux-Nicot B., Majoral J.-P. “Janus” dendrimers: Syntheses and properties. New J. Chem. 2012;36:217–226. doi: 10.1039/C1NJ20458K. [DOI] [Google Scholar]
- 238.Setyawati M.I., Tay C.Y., Docter D., Stauber R.H., Leong D.T. Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem. Soc. Rev. 2015;44:8174–8199. doi: 10.1039/C5CS00499C. [DOI] [PubMed] [Google Scholar]
- 239.Huang Z., Sengar R.S., Nigam A., Abadjian M.C., Potter D.M., Grotjahn D.B., Wiener E.C. A Fluorinated Dendrimer-Based Nanotechnology Platform New Contrast Agents for High Field Imaging. Investig. Radiol. 2010;45:641–654. doi: 10.1097/RLI.0b013e3181ee6e06. [DOI] [PubMed] [Google Scholar]
- 240.Kölmel D.K., Niegerb M., Bräse S. Highly efficient synthesis of polyfluorinated dendrons suitable for click chemistry. RSC Adv. 2015;5:36762–36765. doi: 10.1039/C5RA02804C. [DOI] [Google Scholar]
- 241.Yu W., Yang Y., Bo S., Li Y., Chen S., Yang Z., Zheng X., Jiang Z.-X., Zhou X. Design and Synthesis of Fluorinated Dendrimers for Sensitive 19F MRI. J. Org. Chem. 2015;80:4443–4449. doi: 10.1021/acs.joc.5b00294. [DOI] [PubMed] [Google Scholar]
- 242.Liu X., Yuan Y., Bo S., Li Y., Yang Z., Zhou X., Chen S., Jiang Z.-X. Monitoring Fluorinated Dendrimer-Based Self-Assembled Drug-Delivery Systems with 19F Magnetic Resonance. Eur. J. Org. Chem. 2017;2017:4461–4468. doi: 10.1002/ejoc.201700566. [DOI] [Google Scholar]
- 243.Feng Z., Li Q., Wang W., Ni Q., Wang Y., Song H., Zhang C., Kong D., Liang X.-J., Huang P. Superhydrophilic fluorinated polymer and nanogel for high-performance 19F magnetic resonance imaging. Biomaterials. 2020;256:120184. doi: 10.1016/j.biomaterials.2020.120184. [DOI] [PubMed] [Google Scholar]
- 244.Soni K.S., Desale S.S., Bronich T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release. 2016;240:109–126. doi: 10.1016/j.jconrel.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dong R., Pang Y., Su Y., Zhu X. Supramolecular hydrogels: Synthesis, properties and their biomedical applications. Biomater. Sci. 2015;3:937–954. doi: 10.1039/C4BM00448E. [DOI] [PubMed] [Google Scholar]
- 246.Eckmann D.M., Composto R.J., Tsourkas A., Muzykantov V.R. Nanogel Carrier Design for Targeted Drug Delivery. J. Mater. Chem. B. 2014;2:8085–8097. doi: 10.1039/C4TB01141D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Belabassi Y., Moreau J., Gheran V., Henoumont C., Robert A., Callewaert M., Rigaux G., Cadiou C., Vander Elst L., Laurent S., et al. Synthesis and Characterization of PEGylated and Fluorinated Chitosans: Application to the Synthesis of Targeted Nanoparticles for Drug Delivery. Biomacromolecules. 2017;18:2756–2766. doi: 10.1021/acs.biomac.7b00668. [DOI] [PubMed] [Google Scholar]
- 248.Kolouchova K., Sedlacek O., Jirak D., Babuka D., Blahut J., Kotek J., Vit M., Trousil J., Konefal R., Janouskova O., et al. Self-Assembled Thermoresponsive Polymeric Nanogels for 19F MR Imaging. Biomacromolecules. 2018;19:3515–3524. doi: 10.1021/acs.biomac.8b00812. [DOI] [PubMed] [Google Scholar]
- 249.Munkhbat O., Canakci M., Zheng S., Hu W., Osborne B., Bogdanov A.A., Thayumanavan S. 19F MRI of Polymer Nanogels Aided by Improved Segmental Mobility of Embedded Fluorine Moieties. Biomacromolecules. 2019;20:790–800. doi: 10.1021/acs.biomac.8b01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li Q., Feng Z., Song H., Zhang J., Dong A., Kong D., Wang W., Huang P. 19F magnetic resonance imaging enabled real-time, non-invasive and precise localization and quantification of the degradation rate of hydrogel scaffolds in vivo. Biomater. Sci. 2020;8:3301–3309. doi: 10.1039/D0BM00278J. [DOI] [PubMed] [Google Scholar]
- 251.Patrick M.J., Janjic J.M., Teng H., O’Hear M.R., Brown C.W., Stokum J.A., Schmidt B.F., Ahrens E.T., Waggoner A.S. Intracellular pH measurements using perfluorocarbon nanoemulsions. J. Am. Chem. Soc. 2013;135:18445–18457. doi: 10.1021/ja407573m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Herneisey M., Salcedo P.F., Domenech T., Bagia C., George S.S., Tunney R., Velankar S., Hitchens T.K., Janjic J.M. Design of Thermoresponsive Polyamine Cross-Linked Perfluoropolyether Hydrogels for Imaging and Delivery Applications. ACS Med. Chem. Lett. 2020;11:2032–2040. doi: 10.1021/acsmedchemlett.0c00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kisby T., Yilmazer A., Kostarelos K. Reasons for success and lessons learnt from nanoscale vaccines against COVID-19. Nat. Nanotechnol. 2021;16:843–850. doi: 10.1038/s41565-021-00946-9. [DOI] [PubMed] [Google Scholar]
- 254.Barenholz Y. Doxil(R)--the first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 255.Dewitte H., Geers B., Liang S., Himmelreich U., Demeester J., De Smedt S.C., Lentacker I. Design and evaluation of theranostic perfluorocarbon particles for simultaneous antigen-loading and 19F-MRI tracking of dendritic cells. J. Control. Release. 2013;169:141–149. doi: 10.1016/j.jconrel.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 256.Liang S., Louchami K., Holvoet B., Verbeke R., Deroose C.M., Manshian B., Soenen S.J., Lentacker I., Himmelreich U. Tri-modal In vivo Imaging of Pancreatic Islets Transplanted Subcutaneously in Mice. Mol. Imaging Biol. 2018;20:940–951. doi: 10.1007/s11307-018-1192-0. [DOI] [PubMed] [Google Scholar]
- 257.Tanifum E.A., Patel C., Liaw M.E., Pautler R.G., Annapragada A.V. Hydrophilic fluorinated molecules for spectral 19F MRI. Sci. Rep. 2018;8:2889. doi: 10.1038/s41598-018-21178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Iima R., Takegami S., Konishi A., Tajima S., Minematsu N., Kitade T. Thermal Behavior of 19F Nuclear Magnetic Resonance Signal of 19F-Containing Compound in Lipid Nano-Emulsion for Potential Tumor Diagnosis. AAPS PharmSciTech. 2018;19:2679–2686. doi: 10.1208/s12249-018-1102-4. [DOI] [PubMed] [Google Scholar]
- 259.Wu L., Wen X., Wang X., Wang C., Sun X., Wang K., Zhang H., Williams T., Stacy A.J., Chen J., et al. Local Intratracheal Delivery of Perfluorocarbon Nanoparticles to Lung Cancer Demonstrated with Magnetic Resonance Multimodal Imaging. Theranostics. 2018;8:563–574. doi: 10.7150/thno.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hill L.K., Frezzo J.A., Katyal P., Hoang D.M., Ben Youss Gironda Z., Xu C., Xie X., Delgado-Fukushima E., Wadghiri Y.Z., Montclare J.K. Protein-Engineered Nanoscale Micelles for Dynamic 19F Magnetic Resonance and Therapeutic Drug Delivery. ACS Nano. 2019;13:2969–2985. doi: 10.1021/acsnano.8b07481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Liu J., Zhang J.-Y., Liu K., Gao H.-J., Yu X.-L., Cao Y., Liu Z.-X. Upconversion luminescent property and EPR study of NaGdF4:Yb3+/Tm3+ synthesized by the hydrothermal method. Front. Mater. Sci. 2015;9:241–246. doi: 10.1007/s11706-015-0287-7. [DOI] [Google Scholar]
- 262.Zhao J., Yang H., Li J., Wang Y., Wang X. Fabrication of pH-responsive PLGA(UCNPs/DOX) nanocapsules with upconversion luminescence for drug delivery. Sci. Rep. 2017;7:18014. doi: 10.1038/s41598-017-16948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ashur I., Allouche-Arnon H., Bar-Shir A. Calcium Fluoride Nanocrystals: Tracers for In Vivo 19 F Magnetic Resonance Imaging. Angew. Chem. Int. Ed. Engl. 2018;57:7478–7482. doi: 10.1002/anie.201800838. [DOI] [PubMed] [Google Scholar]
- 264.Jones N.E., Burnett C.A., Salamon S., Landers J., Wende H., Lazzarini L., Gibbs P., Pickles M., Johnson B.R.G., Evans D.J., et al. Fluoride doped gamma-Fe2O3 nanoparticles with increased MRI relaxivity. J. Mater. Chem. B. 2018;6:3665–3673. doi: 10.1039/C8TB00360B. [DOI] [PubMed] [Google Scholar]
- 265.Singh P., Kumari K., Vishvakrma V.K., Mehrotra G.K., Chandra R., Kumar D., Patel R., Shahare V.V. Green Technologies and Environmental Sustainability. Springer; Berlin/Heidelberg, Germany: 2017. Metal NPs (Au, Ag, and Cu): Synthesis, Stabilization, and Their Role in Green Chemistry and Drug Delivery; pp. 309–337. [DOI] [Google Scholar]
- 266.Boccalon M., Franchi P., Lucarini M., Delgado J.J., Sousa F., Stellacci F., Zucca I., Scotti A., Spreafico R., Pengo P., et al. Gold nanoparticles protected by fluorinated ligands for 19F MRI. Chem. Commun. 2013;49:8794–8796. doi: 10.1039/c3cc44572k. [DOI] [PubMed] [Google Scholar]
- 267.Sologan M., Padelli F., Giachetti I., Aquino D., Boccalon M., Adami G., Pengo P., Pasquato L. Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI. Nanomaterials. 2019;9:879. doi: 10.3390/nano9060879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Michelena O., Padro D., Carrillo-Carrion C., Del Pino P., Blanco J., Arnaiz B., Parak W.J., Carril M. Novel fluorinated ligands for gold nanoparticle labelling with applications in 19F-MRI. Chem. Commun. 2017;53:2447–2450. doi: 10.1039/C6CC08900C. [DOI] [PubMed] [Google Scholar]
- 269.Croissant J.G., Butler K.S., Zink J.I., Brinker C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020;5:886–909. doi: 10.1038/s41578-020-0230-0. [DOI] [Google Scholar]
- 270.Pochert A., Vernikouskaya I., Pascher F., Rasche V., Lindén M. Cargo-influences on the biodistribution of hollow mesoporous silica nanoparticles as studied by quantitative 19 F-magnetic resonance imaging. J. Colloid Interface Sci. 2017;488:1–9. doi: 10.1016/j.jcis.2016.10.085. [DOI] [PubMed] [Google Scholar]
- 271.Lee A.L., Gee C.T., Weegman B.P., Einstein S.A., Juelfs A.R., Ring H.L., Hurley K.R., Egger S.M., Swindlehurst G., Garwood M., et al. Oxygen Sensing with Perfluorocarbon-Loaded Ultraporous Mesostructured Silica Nanoparticles. ACS Nano. 2017;11:5623–5632. doi: 10.1021/acsnano.7b01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee A.L., Lee S.H., Nguyen H., Cahill M., Kappel E., Pomerantz W.C.K., Haynes C.L. Investigation of the Post-Synthetic Confinement of Fluorous Liquids Inside Mesoporous Silica Nanoparticles. Langmuir. 2021;37:5222–5231. doi: 10.1021/acs.langmuir.1c00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bouchoucha M., van Heeswijk R.B., Gossuin Y., Kleitz F., Fortin M.A. Fluorinated Mesoporous Silica Nanoparticles for Binuclear Probes in 1H and 19F Magnetic Resonance Imaging. Langmuir. 2017;33:10531–10542. doi: 10.1021/acs.langmuir.7b01792. [DOI] [PubMed] [Google Scholar]
- 274.Kikuchi K. 19F MRI Probes with Tunable Switches and Highly Sensitive 19F MRI Nanoprobes. Bull. Chem. Soc. Jpn. 2015;88:518–521. doi: 10.1246/bcsj.20140392. [DOI] [Google Scholar]
- 275.Helm L. Relaxivity in paramagnetic systems: Theory and mechanisms. Prog. Nucl. Magn. Reson. Spectrosc. 2006;49:45–64. doi: 10.1016/j.pnmrs.2006.03.003. [DOI] [Google Scholar]
- 276.Xie D., Yu M., Kadakia R.T., Que E.L. 19F Magnetic Resonance Activity-Based Sensing Using Paramagnetic Metals. Acc. Chem. Res. 2020;53:2–10. doi: 10.1021/acs.accounts.9b00352. [DOI] [PubMed] [Google Scholar]
- 277.Matsushita H., Mizukami S., Sugihara F., Nakanishi Y., Yoshioka Y., Kikuchi K. Multifunctional core-shell silica nanoparticles for highly sensitive 19F magnetic resonance imaging. Angew. Chem. Int. Ed. Engl. 2014;53:1008–1011. doi: 10.1002/anie.201308500. [DOI] [PubMed] [Google Scholar]
- 278..Nakamura T., Sugihara F., Matsushita H., Yoshioka Y., Mizukami S., Kikuchi K. Mesoporous silica nanoparticles for 19F magnetic resonance imaging, fluorescence imaging, and drug delivery. Chem. Sci. 2015;6:1986–1990. doi: 10.1039/C4SC03549F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Nakamura T., Matsushita H., Sugihara F., Yoshioka Y., Mizukami S., Kikuchi K. Activatable 19F MRI nanoparticle probes for the detection of reducing environments. Angew. Chem. Int. Ed. Engl. 2015;54:1007–1010. doi: 10.1002/anie.201409365. [DOI] [PubMed] [Google Scholar]
- 280.de Vries A., Moonen R., Yildirim M., Langereis S., Lamerichs R., Pikkemaat J.A., Baroni S., Terreno E., Nicolay K., Strijkers G.J., et al. Relaxometric studies of gadolinium-functionalized perfluorocarbon nanoparticles for MR imaging. Contrast Media Mol. Imaging. 2014;9:83–91. doi: 10.1002/cmmi.1541. [DOI] [PubMed] [Google Scholar]
- 281.Akazawa K., Sugihara F., Nakamura T., Matsushita H., Mukai H., Akimoto R., Minoshima M., Mizukami S., Kikuchi K. Perfluorocarbon-Based 19 F MRI Nanoprobes for In Vivo Multicolor Imaging. Angew. Chem. Int. Ed. Engl. 2018;57:16742–16747. doi: 10.1002/anie.201810363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Holban A.M., Grumezescu A.M., Andronescu E. Surface Chemistry of Nanobiomaterials. William Andrew; Norwich, NY, USA: 2016. Inorganic nanoarchitectonics designed for drug delivery and anti-infective surfaces; pp. 301–327. [DOI] [Google Scholar]
- 283.He H., Pham-Huy L.A., Dramou P., Xiao D., Zuo P., Pham-Huy C. Carbon nanotubes: Applications in pharmacy and medicine. Biomed. Res. Int. 2013;2013:578290. doi: 10.1155/2013/578290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rao C.N.R., Govindaraj A. Nanotubes and Nanowires. RSC Publishing; London, UK: 2011. Chapter 1. Carbon Nanotubes; pp. 1–242. [DOI] [Google Scholar]
- 285.Gao W., Zhang Y., Zheng Y., Zhang H., Wang X., Bai L., Wu Y., Ba X. Development of a halloysite nanotube-based 19F NMR probe as a promising detection tool for H2O2. J. Nanoparticle Res. 2020;22:342. doi: 10.1007/s11051-020-05073-5. [DOI] [Google Scholar]
- 286.Zhang H., Ren T., Ji Y., Han L., Wu Y., Song H., Bai L., Ba X. Selective Modification of Halloysite Nanotubes with 1-Pyrenylboronic Acid: A Novel Fluorescence Probe with Highly Selective and Sensitive Response to Hyperoxide. ACS Appl. Mater. Interfaces. 2015;7:23805–23811. doi: 10.1021/acsami.5b08600. [DOI] [PubMed] [Google Scholar]
- 287.Cheng C., Gao Y., Song W., Zhao Q., Zhang H., Zhang H. Halloysite nanotube-based H2O2-responsive drug delivery system with a turn on effect on fluorescence for real-time monitoring. Chem. Eng. J. 2020;380 doi: 10.1016/j.cej.2019.122474. [DOI] [Google Scholar]
- 288.Kislukhin A.A., Xu H., Adams S.R., Narsinh K.H., Tsien R.Y., Ahrens E.T. Paramagnetic fluorinated nanoemulsions for sensitive cellular fluorine-19 magnetic resonance imaging. Nat. Mater. 2016;15:662–668. doi: 10.1038/nmat4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rho J., Stares E., Adams S.R., Lister D., Leach B., Ahrens E.T. Paramagnetic Fluorinated Nanoemulsions for in vivo F-19 MRI. Mol. Imaging Biol. 2020;22:665–674. doi: 10.1007/s11307-019-01415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chen H., Song M., Tang J., Hu G., Xu S., Guo Z., Li N., Cui J., Zhang X., Chen X., et al. Ultrahigh 19F Loaded Cu1.75S Nanoprobes for Simultaneous 19F Magnetic Resonance Imaging and Photothermal Therapy. ACS Nano. 2016;10:1355–1362. doi: 10.1021/acsnano.5b06759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wang K., Peng H., Thurecht K.J., Whittaker A.K. Fluorinated POSS-Star Polymers for 19F MRI. Macromol. Chem. Phys. 2016;217:2262–2274. doi: 10.1002/macp.201600084. [DOI] [Google Scholar]
- 292.Radhakrishnan S., Sudeep P.M., Park J.H., Woellner C.F., Maladonado K., Galvao D.S., Kaipparettu B.A., Tiwary C.S., Ajayan P.M. Multifunctional Hybrids Based on 2D Fluorinated Graphene Oxide and Superparamagnetic Iron Oxide Nanoparticles. Part. Part. Syst. Charact. 2017;34:1700245. doi: 10.1002/ppsc.201700245. [DOI] [Google Scholar]
- 293.Cui J., Jiang R., Guo C., Bai X., Xu S., Wang L. Fluorine Grafted Cu7S4-Au Heterodimers for Multimodal Imaging Guided Photothermal Therapy with High Penetration Depth. J. Am. Chem. Soc. 2018;140:5890–5894. doi: 10.1021/jacs.8b00368. [DOI] [PubMed] [Google Scholar]
- 294.Niu D., Wang X., Li Y., Zheng Y., Li F., Chen H., Gu J., Zhao W., Shi J. Facile synthesis of magnetite/perfluorocarbon co-loaded organic/inorganic hy.ybrid vesicles for dual-modality ultrasound/magnetic resonance imaging and imaging-guided high-intensity focused ultrasound ablation. Adv. Mater. 2013;25:2686–2692. doi: 10.1002/adma.201204316. [DOI] [PubMed] [Google Scholar]
- 295.Hu G., Tang J., Bai X., Xu S., Wang L. Superfluorinated copper sulfide nanoprobes for simultaneous 19F magnetic resonance imaging and photothermal ablation. Nano Res. 2016;9:1630–1638. doi: 10.1007/s12274-016-1057-2. [DOI] [Google Scholar]
- 296.Li X., Li H., Liu G., Deng Z., Wu S., Li P., Xu Z., Xu H., Chu P.K. Magnetite-loaded fluorine-containing polymeric micelles for magnetic resonance imaging and drug delivery. Biomaterials. 2012;33:3013–3024. doi: 10.1016/j.biomaterials.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 297.Zhang C., Li L., Han F.Y., Yu X., Tan X., Fu C., Xu Z.P., Whittaker A.K. Integrating Fluorinated Polymer and Manganese-Layered Double Hydroxide Nanoparticles as pH-activated 19 F MRI Agents for Specific and Sensitive Detection of Breast Cancer. Small. 2019;15:e1902309. doi: 10.1002/smll.201902309. [DOI] [PubMed] [Google Scholar]
- 298.Wang J., Hu X., Ding H., Huang X., Xu M., Li Z., Wang D., Yan X., Lu Y., Xu Y., et al. Fluorine and Nitrogen Co-Doped Carbon Dot Complexation with Fe(III) as a T1 Contrast Agent for Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces. 2019;11:18203–18212. doi: 10.1021/acsami.9b03644. [DOI] [PubMed] [Google Scholar]
- 299.Mali A., Kaijzel E.L., Lamb H.J., Cruz L.J. 19F-nanoparticles: Platform for in vivo delivery of fluorinated biomaterials for 19F-MRI. J. Control. Release. 2021;338:870–889. doi: 10.1016/j.jconrel.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 300.Piloni A., Simonutti R., Stenzel M.H. The effect of cationic groups on the stability of 19F MRI contrast agents in nanoparticles. J. Polym. Sci. Part. A Polym. Chem. 2019;57:1994–2001. doi: 10.1002/pola.29387. [DOI] [Google Scholar]
- 301.Pochert A., Ziller S., Kapetanovic S., Neusser G., Kranz C., Lindén M. Intermediate pickering emulsion formation as a means for synthesizing hollow mesoporous silica nanoparticles. New J. Chem. 2016;40:4217–4222. doi: 10.1039/C5NJ02855H. [DOI] [Google Scholar]
- 302.Astafyeva K., Somaglino L., Desgranges S., Berti R., Patinote C., Langevin D., Lazeyras F., Salomir R., Polidori A., Contino-Pepin C., et al. Perfluorocarbon nanodroplets stabilized by fluorinated surfactants: Characterization and potentiality as theranostic agents. J. Mater. Chem. B. 2015;3:2892–2907. doi: 10.1039/C4TB01578A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.