Abstract
We have developed an inhibition enzyme immunoassay (inhibition-EIA) to monitor for the occurrence of invasive aspergillosis (IA) in sera from 45 immunocompromised (IC) patients. The test uses rabbit polyclonal antibodies and a mixture of components from Aspergillus fumigatus, containing three predominant antigens with molecular weights of 18,000, 33,000, and 56,000. Circulating antigens were found in five of seven proven cases of IA due to A. fumigatus. In two of the five positive cases, antigenemia was detected with inhibition-EIA earlier than with X ray or other biological methods. No antigens were detected in the sera from two patients with proven IA due to Aspergillus flavus and Aspergillus terreus nor in the sera from four patients with probable IA. Circulating antigens were not detected in the control group, composed of 30 healthy adult blood donors. Four of the 32 at-risk patients examined, though they displayed no definite evidence of IA, gave a positive result in this test. The sensitivity, specificity, and positive predictive value of inhibition-EIA were 71.4, 94.4, and 71.2%, respectively. The data were compared with those obtained by a latex agglutination assay of galactomannan (GM) that was positive in only one patient with probable IA. The higher sensitivity obtained by inhibition-EIA may well be due to its ability to detect circulating antigens other than GM in the sera of IC patients with IA. Detecting these antigens may improve the diagnosis of IA, as they may serve as markers of this infection.
Invasive aspergillosis (IA) has been a significant cause of life-threatening opportunistic infections in immunosuppressed hosts (9). The incidence of IA, which is the second most common cause of fungal infection in this type of patient, varies from 0.5 to 25% (10, 17, 30, 38, 42). The reported mortality mainly varies from 50% to nearly 100% (9, 10, 22, 24, 38). The diagnosis is consequential, since an early diagnosis combined with adequate therapy may improve the clinical outcome in immunosuppressed patients (1, 6). However, establishing the diagnosis continues to be a major problem for the clinician, since the clinical symptoms of IA are not pathognomonic of the disease, while histological and culture confirmations are often difficult to obtain antemortem (8, 15). Moreover, the efficient techniques of imaging do not always allow adequate discrimination among the different etiologies involved in this type of symptoms. Furthermore, the typical form of serological evidence, that is, increased antibody levels, is usually not revealed in this type of patient. The detection of circulating Aspergillus antigens and detection of Aspergillus DNA (35, 44) are two of the most promising methods to diagnose IA in at-risk patients. Many studies report the detection of circulating antigens (11, 12, 14, 21, 28, 29, 34–37, 41, 43, 46). A commercially available test, Pastorex Aspergillus (Sanofi Diagnostic Pasteur, Marnes-la-Coquette, France), can be very specific but appears to be relatively insensitive (45). In this study, we did not systematically use the Platelia Aspergillus kit, since it is more sensitive but less specific than the Pastorex system (5, 39, 40). Moreover, a recent study has suggested that heat-resistant galactomannan (GM) is not eliminated by the processes of food sterilization and may reach the circulation through damaged intestinal mucosa and cause false-positive results in tests to detect antigenemia (25). Therefore, in an effort to improve the diagnosis of IA, an inhibition enzyme immunoassay (inhibition-EIA) developed in our laboratory was selected for investigation. This system, which is thought to mainly detect antigens with Mrs of 18,000, 33,000, and 56,000, was compared to the Pastorex Aspergillus test for the detection of GM. The results obtained in each case were related to the clinical data.
Case definitions.
IA, associated with an immunodebilitated condition (i.e., prolonged neutropenia for at least 10 days within the previous 2 months, immunosuppressive therapy within the last month, or a previous episode of fungal infection) and with persistent fever (>38°C) for at least 3 days, despite a broad-spectrum antibiotherapy, was diagnosed mainly by direct isolation and culture of the organism from bronchopulmonary specimens and biopsies obtained by a sterile procedure (15). Additional diagnostic criteria included radiological disturbances (i.e., abnormal characteristic signs on chest radiography consistent with infection) obtained by the effective techniques of imaging or computed tomography.
Group I.
In the context defined above, proven IA was diagnosed by histologic evidence of the presence of hyphae in tissue specimens and in vitro growth of Aspergillus species in culture.
Group II.
Probable IA cases were defined as demonstrating at least one criterion from the context section and one major or two minor clinical criteria from an abnormal site consistent with infection and as presenting one of the following criteria: hyphae in fiber-endoscopic samples, positive Aspergillus culture from bronchoalveolar lavage fluid or bronchial aspirates, and testing positive for antigenemia with Pastorex Aspergillus.
Group III.
Patients who were at risk for IA had only clinical evidence of infection. Possible IA cases were defined as meeting at least one criterion from the context section and one microbiological or clinical criterion of infection as cited above.
In the present study, tests positive for antigenemia which were obtained by using inhibition-EIA were not considered in classifying patients, since this method was under evaluation.
Patients.
Group I contained nine patients (three women and six men, ranging from 26 to 60 years of age) with proven IA (Table 1). Group II contained four patients (all men, ranging from 62 to 72 years of age) infected by A. fumigatus, with probable IA (Table 1). Group III contained 32 patients (16 women and 16 men, ranging from 10 to 76 years of age) at risk for IA (in this group, 26 patients had hematological disorders, two had received liver transplants, and four had various pathologic disorders associated with a severely immunodebilitating condition [i.e., myeloma and cardiac disease]). The sera were obtained from all patients during a retrospective and prospective longitudinal study (up to 65 weeks).
TABLE 1.
Diagnosis criteria of IA in patients with proven or probable IA
| Patient no. | Predisposing factor | Site(s) of isolation | Result of microscopy | Species isolated | Other dataa | IA diagnosis | Outcome |
|---|---|---|---|---|---|---|---|
| Group I | |||||||
| 1 | Hodgkin's disease | Lungs | Hyphae | A. flavus | Neutropenic; corticotherapy; Amp B, then Itra | Proven | Death |
| 2 | Liver transplantation | Brain, vertebra | Hyphae | A. fumigatus | Amp B | Proven | Death |
| 3 | Acute myeloid leukemia | Lungs | Hyphae | A. fumigatus | Neutropenic, seroconversion, Amp B | Proven | Recovery |
| 4 | Aplasia | Lungs | Hyphae | A. terreus | Neutropenic, Amp B, Absidia corymbifera | Proven | Death |
| 5 | Acute myeloid leukemia | Lungs | Hyphae | A. fumigatus | Neutropenic; Amp B, then Itra | Proven | Recovery |
| 6 | Sarcoidosis | Lungs | Hyphae | A. fumigatus | Corticotherapy; Amp B and 5-flurocytosine | Proven | Death |
| 7 | Chronic respiratory disease | Lungs | Hyphae | A. fumigatus | Seroconversion, Amp B | Proven | Death |
| 8 | Liver transplantation | Brain | Hyphae | A. fumigatus | Amp B | Proven | Death |
| 9 | Acute lymphoid leukemia | Paranasal sinus, facial osteolysis | Hyphae | A. fumigatus | Amp B | Proven | Death |
| Group II | |||||||
| 10 | Bone marrow transplantation | Lungs | Hyphae | A. fumigatus | Amp B | Probable | Death |
| 11 | Chronic lymphoid leukemia | Lungs | No hyphae | A. fumigatus | Neutropenic, untreated, positive antigenemia (Pastorex) | Probable | Death |
| 12 | Chronic respiratory disease | Lungs | Hyphae | A. fumigatus | Immunosuppressive therapy, untreated | Probable | Recovery |
| 13 | Lymphoma | Lungs | No hyphae | A. fumigatus | Neutropenic, Amp B, Pneumocystis carinii | Probable | Recovery |
Amp B, amphotericin B; Itra, itraconazole.
Control group.
This group comprised 30 healthy adult blood donors (28 women and 2 men, ranging from 19 to 36 years of age) without specific antibodies to A. fumigatus in their sera, as determined by enzyme immunoassay (EIA), immunofluorescent antibody test (IFAT), and counterimmunoelectrophoresis (CIE).
Antigens.
Aspergillus fumigatus antigens from a Longbottom strain (NCPF 2109) were prepared in Panmede medium (Paines and Byrne, Greendford, United Kingdom) and were grown in a stationary 3-week culture at 27°C (CF27), 37°C (CF37), and 42°C (CF42) (31). Briefly, the mycelium was broken in the culture medium; the suspension was filtered, dialyzed, and concentrated in Amicon membrane (PM10); and was finally lyophilized. The antigens were stored at 4°C until required.
Rabbit antisera.
Antisera to CF27, CF37, and CF42 were raised in female New Zealand White rabbits. Ten milligrams of lyophilized antigens in 0.9% NaCl (wt/vol) was mixed with an equal volume of Freund's complete adjuvant and was injected intradermally at multiple sites. Two weeks later, booster injections of each antigen, to which Freund's incomplete adjuvant had been added, were administered every 2 weeks over a period of 1 to 2 months until optimum antibody levels were detected by an EIA. Sera obtained from rabbits before immunization were used as negative controls.
SDS-PAGE and immunoblotting.
The procedure described elsewhere (7) was adapted to Aspergillus antigens. Briefly, 20 mg of lyophilized CF37 in 1 ml of a solution containing 0.25 M Tris (pH 6.8), 0.25 M EDTA, 5% sodium dodecyl sulfate (SDS), and 0.05% β-mercaptoethanol was denatured for 3 min at 100°C. Following SDS-polyacrylamide gel electrophoresis (PAGE) of CF-37, using a separating gel of 12.5% (at 250 V for 45 min at 15°C), the gel was blotted with nitrocellulose sheets (NC), which were then fixed by drying at 37°C for 15 min. After saturation of the free binding sites on NC, antisera from rabbits were used at dilutions of 1:100 and 1:200 and were added to the NC at 37°C for 1 h. After four 2-min washes in phosphate-buffered saline (PBS), goat anti-rabbit immunoglobulin G (IgG) (1:1,500)-alkaline phosphatase conjugate (Miles, Puteaux, France) was added to the NC, and the mixture was incubated at 37°C for 1 h. After a new cycle of washing, the bands were visualized with 0.15 mM nitroblue tetrazolium plus 0.15 mM 5-bromo-4-chloro-3-indolylphosphate. The reaction was stopped by two 5-min washes in distilled water.
Human antibodies to A. fumigatus.
Antibodies were detected by EIA (IgG), IFAT (IgG and IgM), and CIE (19). Readings higher than the following cutoffs were considered positive: 0.44 (EIA), 1:80 (IFAT), and two arcs of precipitation (CIE).
Circulating antigen detection by inhibition-EIA.
One hundred milliliters of antigens (2 μg of lyophilized CF37/ml) in a solution containing 50 mM carbonate buffer, 25 mM EDTA, and 0.02% NaN3 (pH 9.6) was used to coat polystyrene microtiter plates (M129B; Dynatech, France) overnight at 4°C.
Two aliquots of test human serum (50 μl each) were diluted 1:5 in 145 mM PBS containing 5% nonfat milk, 0.01% Triton X-100, 25 mM EDTA, and 0.02% NaN3 (pH 7.2); one aliquot was heated at 80°C for 30 min while the other aliquot remained unheated. Antiserum from rabbit to CF27 A. fumigatus antigens and preimmune, negative serum were diluted 1:1,000 in the same solution as used above, then mixed with equal volumes of human sera, in both heated and unheated samples. The mixture was incubated at 4°C overnight.
The antigen-coated plates were blocked with a solution containing 145 mM PBS and 5% nonfat milk (pH 7.2) at 37°C for 15 min and then exposed to preincubated human and rabbit sera for 30 min at 37°C. After four 2-min washes in a solution containing 145 mM PBS and 0.01% Triton X-100, 100 μl of goat anti-rabbit IgG (1:1,500) peroxidase conjugate (CALTAG; TEBU, Le Perray en Yvelines, France) diluted in the solution of 145 mM PBS (pH 7.2), 5% nonfat milk, and 0.01% Triton X-100 were added to each well, and the mixtures were again incubated at 37°C for 30 min. After a new cycle of washing, absorbance at 492 nm (A492) was measured following incubation for 10 min in a solution of ortho-phenylenediamine–2HCl (3 mg/ml) and H2O2 (0.08%) in citrate buffer (pH 5.15), and the reaction was stopped with 2 N H2SO4. Circulating-antigen levels were estimated from a standard calibration curve plotting percentage of inhibition of antibody binding (% inh) versus amount of CF37 (0, 4, 40, 400, and 4,000 ng/ml). The inhibition cutoff values of 22.9% and 34.9%, for heated and unheated human sera, respectively, were determined from the A492 of samples from the control group by using the average reading plus twice the standard deviation. The % inh was calculated by using the following equation: % inh = (1−Ax/Ac) × 100, where Ax is the difference in A492 between the positive and negative rabbit sera in the presence of the test human serum “x” and Ac is the difference in A492 between the positive and negative rabbit sera in the presence of the pool of human sera from control group “c”.
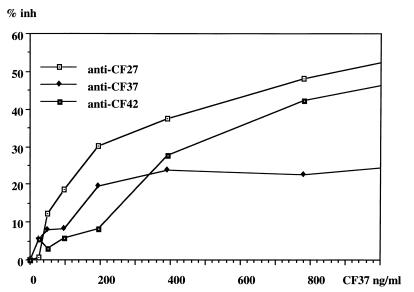
To detect circulating antigens of A. fumigatus, the parameters of inhibition-EIA were established by titration of CF37 antigen, goat anti-rabbit IgG peroxidase conjugate, and rabbit hyperimmune serum to CF27, CF37, and CF42 in the presence of a known amount of added antigen (Fig. 1). Optimal results were obtained with CF37 for coating the plates at 2 μg/ml, the peroxidase conjugate at 1:1,500, and the anti-CF27 rabbit serum at 1:1,000.
FIG. 1.
Optimization of the parameters of inhibition-EIA. Three rabbit hyperimmune sera were tested: anti-CF27, anti-CF37, and anti-CF42 (1:1,000). In this case, the plate was coated overnight with CF37 antigen at 2 μg/ml, and peroxidase conjugate was used at 1:1,500. The highest % inh was obtained experimentally with anti-CF27.
SDS-PAGE and Western blotting were carried out in order to determine which antigens were detected by inhibition-EIA. The mixture of antigens used for coating the plates contained major antigens with the following Mrs: 62,000, 56,000, 50,000, 42,000, 33,000, and 18,000 (Table 2). The anti-CF27 rabbit serum used in the inhibition-EIA mainly recognized Mrs of 56,000, 33,000, and 18,000. It is therefore probable that the inhibition-EIA, as used in this study, mainly detected these three antigens.
TABLE 2.
Mrs of A. fumigatus antigens used in inhibition-EIAa
| Mr | Rabbit serum Anti-CF42b | Rabbit serum Anti-CF37b | Rabbit serum Anti-CF27b | Control rabbit serum |
|---|---|---|---|---|
| 62,000 | ++ | + | − | − |
| 56,000 | ++ | ++ | ++ | − |
| 50,000 | ± | + | ± | − |
| 42,000 | ± | ± | − | − |
| 33,000 | ± | ++ | + | − |
| 18,000 | + | ± | + | − |
++, very positive band; +, positive band; ±, slightlly positive band; −, absent band.
Rabbit antiserum to A. fumigatus culture fluid antigens used at a dilution of 1:100. CF37 is composed of these six antigens and GM detected by latex agglutination assay. Rabbit serum anti-CF27 recognized mainly three fractions. Inhibition-EIA that used CF37 and anti-CF27 detected mainly antigens with Mrs of 56,000, 33,000, and 18,000.
The antigens of A. fumigatus used for coating the plates in inhibition-EIA were positive in the Pastorex Aspergillus test at a 1:64 dilution. If the highest sensitivity of this test is assumed to occur (2), this dilution contains approximately 15 ng of GM per ml.
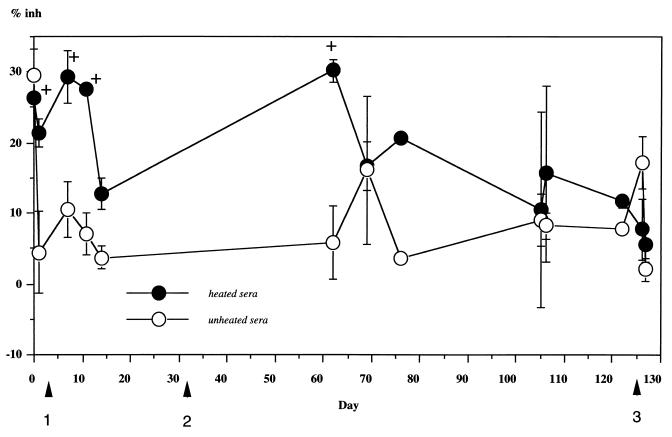
Circulating antigens of A. fumigatus were detected by inhibition-EIA only when heated serum samples from patients were used (Table 3). It is interesting to note that in the six samples obtained from patient number 1, negative inhibitions of 18 to 41.5% were observed when unheated sera were tested. In positive cases (Table 3), the antigen detection usually gave fluctuating levels of antigenemia in sera from immunodebilitated patients with IA (Fig. 2). However, in patients 3 and 8, all six samples were positive with a % inh between 27.4 and 40.3%. In two cases, antigen detection was earlier than with X ray or biological methods (Fig. 2). However, one case was diagnosed 10 days later, and the other two were diagnosed at the same time. In addition, the % inh due to circulating antigens varied from 24.9% to 40.3% (110 to 180 ng/ml) when human sera were heated before incubation with rabbit hyperimmune serum. If the data in group I are taken into account, the sensitivity, specificity, and positive predictive value of inhibition-EIA were 71.4, 94.4, and 71.2%, respectively. In group II, the sera from patients with probable IA were negative by inhibition-EIA, but one serum sample out of three was positive with the Pastorex Aspergillus test (in this group the sensitivities were 0% and 25% [n = 4], respectively). In all other cases, this last test failed to detect any circulating antigens in patients' sera.
TABLE 3.
Aspergillus antigen detection in the diagnosis of IA
Group I, nine patients (44 serum samples) with proven IA.
Group II, four patients (9 serum samples) with probable IA.
Group III, 32 patients (62 serum samples) at risk for IA.
One patient was infected with A. flavus and another one with A. terreus. The other seven were infected with A. fumigatus.
CAg, circulating antigen of A. fumigatus; H-EIA, inhibition-EIA on heating sera; LA, latex agglutination assay (Pastorex Aspergillus).
Seventeen positive serum samples.
Eight positive serum samples from four patients. Two of these patients may be classified as possible IA cases.
FIG. 2.
Detection of antigen in a patient (number 2) with proven IA (in trachea, vertebra, and brain) in terms of number of days after initial detection. Positive cultures were found first in trachea (first arrow), then in brain (second arrow), and finally in vertebra (third arrow). In this case, antigenemia detected (+) in heated sera was transient and repeated. It was positive at 4 of 13 time points and in all of the earlier stages (up to 60 days). The detection of circulating antigens in several samples may be necessary. Unheated serum samples gave negative results. The error bars represent standard deviations.
In group III, of the four cases positive by inhibition-EIA (Table 3), two were related to the transient presence of A. fumigatus in the trachea and in the bronchoalveolar lavage. In the third case, the patient had significant variations in the levels of antibodies to A. fumigatus. In the fourth case, the fungus was not found either on direct examination or in cultures. Therefore, the two first cases may be considered as possible IA, although only minor pathology was observed.
Aspergillus antigens were not detected by inhibition-EIA in the sera of patients with proven IA who were infected with Aspergillus terreus and Aspergillus flavus.
No false positive cases were obtained with the Pastorex Aspergillus test in any group.
In the present study, antigenemia detection by inhibition-EIA could be used as a method to diagnose and monitor IA in patients at risk for aspergillosis. This is supported by the data obtained from proven and suspected IA cases. However, other methods can be used in other instances to diagnose the disorder more quickly, as mortality may be reduced with adequate, timely therapy. In probable cases of IA, antigenemia was not detected by inhibition EIA, and this could be an inconvenience of this method. In group III, inhibition EIA may be useful, since it may aid in the diagnosis and monitoring of possible cases of IA after antigenemia is detected in suspected patients, as was the case for two patients in our study with transient presence of A. fumigatus in the trachea and in the bronchoalveolar lavage.
In this study, circulating antigens of A. fumigatus detected by inhibition-EIA were most likely those with Mrs of 56,000, 33,000, and 18,000, since rabbit serum anti-CF27 mainly recognized these three components on Western blots. Therefore, EIA with CF37 and anti-CF27 may detect antigens with Mrs of 56,000, 33,000, and 18,000. The 56,000-Mr antigen of CF37 may be the same as the one described by Framatico and Buckley (13) who found that sera from patients with IA reacted against an antigen with an Mr of 58,000. In that study, Framatico and Buckley noted that this antigen may also be found in culture medium. In addition, Reichard et al. (33) characterized an extracellular serine proteinase from A. fumigatus with an Mr of 32,000 to 33,000 which might be one of the components used for coating the plates in the present study. Moreover, Haynes et al. (18) detected two major antigens with Mrs of 18,000 and 11,000 in serial urine samples from patients who developed IA. Latgé et al. (23) described the antigen with an Mr of 18,000 as being secreted and present in the urine of patients with IA. In the present study, Mr determination suggests that this antigen with an Mr of 18,000 may also be part of the antigens detected by inhibition-EIA. Thus, this test may detect several antigens at the same time, which might explain the greater sensitivity of the test compared to the latex agglutination assay based solely on GM detection.
Theoretically, anti-CF37 seemed the most appropriate reagent for our test, but this proved not to be the case. This discrepancy may be due to the immunological or cytotoxic (3, 26) properties of the CF37 antigen which was involved in down regulation of anti-CF37 levels in rabbits after the last boost (data not shown). Moreover, this antigen is rich in chymotrypsin and catalase (data not shown). In addition, GM is also a component of CF37, as demonstrated by the Pastorex Aspergillus test but not by Western blotting, since this method can detect proteins or glycoproteins. Even if GM is a component of CF37, the inhibition-EIA plates were coated with approximately 1 ng of this macromolecule per ml, which is the limit of detection of a sensitive EIA (5, 39, 40). As CF37 is composed of several major antigens, GM is unlikely to be detected by the inhibition-EIA. This hypothesis is supported by the fact that the one serum sample which was positive in the Pastorex Aspergillus test was negative by inhibition-EIA.
In all of the other proven IA cases, only inhibition-EIA gave a positive result. In order to confirm the absence of GM from the sera of these patients, a sensitive EIA (Platelia) was used retrospectively in serum samples from five cases of proven IA, and only one serum sample was found to be positive by this test (data not shown). The fact that inhibition-EIA was more sensitive than GM detection may be explained by the multiple targets and by the fact that GM antigens are rapidly removed from circulation by the formation of immune complexes and by receptor-mediated endocytosis by Kupffer's cells in the liver (4). The low level of antigen detection is also thought to be due to fluctuating levels of antigenemia (35, 39, 40). These fluctuations have also been observed in the present study with CF37 antigens of A. fumigatus.
In the present study, the sensitivity of the assay is good enough, given that IA was diagnosed despite the heterogeneous underlying diseases of these patients. However, serum components and immune complexes can interfere with the detection of circulating antigens by inhibition-EIA, since antigens were detected in the samples only after heat treatment. Many laboratories use only heated serum for detecting these antigens (11, 29, 35, 39, 41, 44, 46). This may indicate that heat-sensitive molecules present in human sera are responsible for this phenomenon. The relationship between circulating antigens and these molecules may be specific, e.g., like specific antibodies, or not specific, e.g., like protein-protein interactions.
The test used in this study appears to be more specific for A. fumigatus, since it did not recognize antigens in the serum samples from group I patients infected with A. flavus and A. terreus. This might be regarded both as an inconvenience and as an advantage in diagnosing IA, since the most prevalent species is A. fumigatus.
In the present study, antigenemia was detected the earliest in two cases of proven IA, but the antigenemia was transient in these patients at that time, and its detection required the testing of several samples. The sensitivity of this test may increase when heated serum is used. However, in IA diagnosis, the antigen sensitivity may also be improved by determining the presence of antigenuria (2), by detecting antigens in cerebrospinal fluid (32), or by combining other methods (6, 16, 20, 27, 32, 39, 40). Yet, given the diversity of circulating antigens, the different methods seem to be complementary for diagnosing IA in immunocompromised patients. However, improving the sequencing of purified antigens and the identification of epitopes with high specificity for IA diagnosis is the next step in optimizing the method. Finally, even though the detection of a wide variety of antigens in patients' sera is not always proof of IA, as was indicated by the data of group III, the antigen data should be taken into account in the monitoring of patients at risk for IA.
Acknowledgments
We thank V. M. Hearn, S. Picot, and S. Durville for scientific comments and/or assistance with the preparation of the manuscript.
REFERENCES
- 1.Aisner J, Schimpff S C, Wiernik P H. Treatment of invasive aspergillosis: relation of early diagnosis and treatment to response. Ann Intern Med. 1997;86:539–544. doi: 10.7326/0003-4819-86-5-539. [DOI] [PubMed] [Google Scholar]
- 2.Ansorg R, Heintschel von Heinegg E, Rath P M. Aspergillus antigenuria compared to antigenemia in bone marrow transplant recipients. Eur J Clin Microbiol Infect Dis. 1994;13:582–589. doi: 10.1007/BF01971310. [DOI] [PubMed] [Google Scholar]
- 3.Arruda L K, Plattis-Mills T A E, Fox J W, Chapman M D. Aspergillus fumigatus allergen 1, a major IgE binding protein, is a member of the mitogillin family of cytotoxins. J Exp Med. 1992;172:1529–1532. doi: 10.1084/jem.172.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett J E, Friedmann M M, Dupont B. Receptor-mediated clearance of Aspergillus galactomannan. J Infect Dis. 1987;155:1005–1010. doi: 10.1093/infdis/155.5.1005. [DOI] [PubMed] [Google Scholar]
- 5.Bretagne S, Marmorat-Khoung A, Kuentz M, Latgé J-P, Bart-Delabesse E, Cordonnier C. Serum Aspergillus galactomannan antigen testing by sandwich ELISA: practical use in neutropenic patients. J Infect. 1997;35:7–15. doi: 10.1016/s0163-4453(97)90833-1. [DOI] [PubMed] [Google Scholar]
- 6.Caillot D, Casasnovas O, Bernard A, Couaillier J-F, Durand C, Cuisenier B, Solary E, Piard F, Petrella T, Bonnin A, Couillault G, Dumas M, Guy H. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15:139–147. doi: 10.1200/JCO.1997.15.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Chumpitazi B F F, Boussaid A, Pelloux H, Racinet C, Bost M, Goullier-Fleuret A. Diagnosis of congenital toxoplasmosis by immunoblotting and relationship with other methods. J Clin Microbiol. 1995;33:1479–1485. doi: 10.1128/jcm.33.6.1479-1485.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J. Clinical manifestations and management of aspergillosis in the compromised patients. In: Warnock D W, Richardson M D, editors. Fungal infections in immunocompromised patients. Chichester, United Kingdom: John Wiley & Sons; 1991. pp. 118–145. [Google Scholar]
- 9.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 10.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 11.De Repentigny L, Boushira M, Ste-Marie L, Bosisio G. Detection of galactomannan antigenemia by enzyme immunoassay in experimental invasive aspergillosis. J Clin Microbiol. 1987;25:863–867. doi: 10.1128/jcm.25.5.863-867.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupont B, Huber M, Kim S J, Bennett J E. Galactomannan antigenemia and antigenuria in aspergillosis: studies in patients and experimentally infected rabbits. J Infect Dis. 1987;155:1–11. doi: 10.1093/infdis/155.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Framatico P M, Buckley H R. Identification and characterization of an immunodominant 58-kilodalton antigen of Aspergillus fumigatus recognized by sera of patients with invasive aspergillosis. Infect Immun. 1991;59:309–315. doi: 10.1128/iai.59.1.309-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita S I, Mistsubar F, Matsuda T. Demonstration of antigenemia in patients with invasive aspergillosis by biotin-streptavidin enzyme-linked immunosorbent assay. J Lab Clin Med. 1988;112:464–470. [PubMed] [Google Scholar]
- 15.Grillot R, Lebeau B, Ambroise-Thomas P. L'aspergillose invasive: conduite du diagnostic mycologique. Pathol Biol. 1994;42:675–682. [PubMed] [Google Scholar]
- 16.Grundy M A, Barnes R A, Coakley W T. Highly sensitive detection of fungal antigens by ultrasound-enhanced latex agglutination. J Med Vet Mycol. 1995;33:201–203. doi: 10.1080/02681219580000411. [DOI] [PubMed] [Google Scholar]
- 17.Guillemain R, Lavarde V, Amrein C, Chevalier P, Guinvarch A, Glotz D. Invasive aspergillosis after transplantation. Transplant Proc. 1995;27:1307–1309. [PubMed] [Google Scholar]
- 18.Haynes K A, Latgé J P, Rogers T R. Detection of Aspergillus antigens associated with invasive infection. J Clin Microbiol. 1990;28:2040–2044. doi: 10.1128/jcm.28.9.2040-2044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hearn V, Donaldson G C, Healy M J H. A method to determine significant levels of immunoglobulin G Aspergillus fumigatus antigens in an ELISA system and a comparison with counter immuno-electrophoresis and double diffusion techniques. J Immunoassay. 1985;6:137–158. doi: 10.1080/01971528508063026. [DOI] [PubMed] [Google Scholar]
- 20.Hearn V, Pinel C, Blachier S, Ambroise-Thomas P, Grillot R. Specific antibody detection in invasive aspergillosis by analytical isoelectrofocusing and immunoblotting methods. J Clin Microbiol. 1995;33:982–986. doi: 10.1128/jcm.33.4.982-986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson T M, Kurup V P, Resnick A, Ash R C, Fink J N, Kalbfleisch J. Detection of circulating Aspergillus fumigatus antigen in bone marrow transplant patients. J Lab Clin Med. 1989;114:700–707. [PubMed] [Google Scholar]
- 22.Kac G, Roux P, Poirot J L, Meyohas M C, Cadranel J, Chouaid C, Watine J, Fourest F, Lancastre F. Aspergillus et aspergilloses: étude rétrospective dans deux hôpitaux parisiens. J Mycol Med. 1995;5:75–85. [Google Scholar]
- 23.Latgé J-P, Moutaouakil M, Debeaupuis J-P, Bouchara J P, Haynes K, Prevost M-C. The 18-kilodalton antigen secreted by Aspergillus fumigatus. Infect Immun. 1991;59:2586–2594. doi: 10.1128/iai.59.8.2586-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebeau B, Piens M A, Kures L, Morin O, Pospisil F, Chevrier S, Cuisenier B, Gari-Toussaint M, De Gentile L, Raberin H, Lavarde V, Grillot R. Profil actuel de l'aspergillose invasive en France. Enquête du groupe des mycoses opportunistes (GEMO) dans 19 centres hospitaliers (11 villes) J Mycol Med. 1996;6:97–102. [Google Scholar]
- 25.Lescher-Bru V, Cavalier A, Pernot-Marino E, Koenig H, Eyer D, Waller J, Condolfi E. Aspergillus galactomannan antigen detection with PlateliaRAspergillus: multiple positive antigenemia without Aspergillus infection. J Mycol Med. 1998;8:112–113. [Google Scholar]
- 26.Madan T, Arora N, Sarma U. Identification and evaluation of a major cytotoxin of A. fumigatus. Mol Cell Biochem. 1997;167:89–97. doi: 10.1023/a:1006823706119. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki T, Kohno S, Mitsutake K, Maesaki S, Tanaka K I, Ishikawa N, Hara K. Plasma (1→3)-β-d-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and crytococcosis. J Clin Microbiol. 1995;33:3115–3118. doi: 10.1128/jcm.33.12.3115-3118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson T F, Miniter P, Ryan J L, Andriole V T. Effect of immunosuppression and amphotericin B on Aspergillus antigenemia in an experimental model. J Infect Dis. 1988;156:415–422. doi: 10.1093/infdis/158.2.415. [DOI] [PubMed] [Google Scholar]
- 29.Patterson T F, Miniter P, Patterson J A, Rappeport J M, Andriole V T. Aspergillus detection in the diagnosis of invasive aspergillosis. J Infect Dis. 1995;171:1553–1558. doi: 10.1093/infdis/171.6.1553. [DOI] [PubMed] [Google Scholar]
- 30.Piens M A, Troncy J, Nicolle M C, Mercatello A, Fière D, Mojon M. Aspergilloses invasives chez des patients atteints d'hémopathies malignes. A propos de 29 observations. J Mycol Med. 1993;3:79–83. [Google Scholar]
- 31.Pinel C, Thélu J, Meunier A, Grillot R. Exo antigènes d'Aspergillus fumigatus. Étude de la spécificité des différentes fractions par ELISA. Bull Soc Fr Mycol Med. 1989;18:195–198. [Google Scholar]
- 32.Ray P, Chakrabarti A, Jatana M, Sharma B S, Pathak A. Western blot analysis of cerebrospinal fluid for detection of Aspergillus antigens. Mycopathologia. 1995;131:103–106. doi: 10.1007/BF01102887. [DOI] [PubMed] [Google Scholar]
- 33.Reichard U, Bütner S, Eiffert H, Staib F, Rüchel R. Purification and characterisation of an extracellular serine proteinase from Aspergillus fumigatus and its detection in tissue. J Med Microbiol. 1990;33:246–251. doi: 10.1099/00222615-33-4-243. [DOI] [PubMed] [Google Scholar]
- 34.Reiss E, Lehmann P F. Galactomannan antigenemia in invasive aspergillosis. Infect Immun. 1979;25:357–365. doi: 10.1128/iai.25.1.357-365.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers T R, Haynes K A, Barnes R A. Value of antigen detection in predicting invasive pulmonary aspergillosis. Lancet. 1990;ii:1210–1213. doi: 10.1016/0140-6736(90)92831-2. [DOI] [PubMed] [Google Scholar]
- 36.Sabetta J R, Miniter P, Andriole P T. The diagnosis of invasive aspergillosis by an enzyme-linked immunosorbent assay for circulating antigen. J Infect Dis. 1985;152:946–953. doi: 10.1093/infdis/152.5.946. [DOI] [PubMed] [Google Scholar]
- 37.Shaffer P J, Medoff G, Kobayashi G S. Demonstration of antigenemia by radioimmunoassay in patients with invasive aspergillosis by solid phase (protein A-rich Staphylococcus aureus) radioimmunoassay. Am J Med. 1979;67:627–630. doi: 10.1016/0002-9343(79)90245-6. [DOI] [PubMed] [Google Scholar]
- 38.Singh N, Arnow P M, Bonham A, Dominguez E, Paterson D L, Pankey G E, Wagener M M, Yu V L. Invasive aspergillosis in liver transplant recipients in the 1990s. Transplantation. 1997;64:716–720. doi: 10.1097/00007890-199709150-00009. [DOI] [PubMed] [Google Scholar]
- 39.Stynen D, Goris A, Sarfati J, Latgé J P. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J Clin Microbiol. 1995;33:497–500. doi: 10.1128/jcm.33.2.497-500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sulahian A, Tabouret M, Ribaud P, Sarfati J, Gluckman E, Latgé J P, Derouin F. Comparison of an enzyme immunoassay and latex agglutination test for detection of galactomannan in the diagnosis of invasive aspergillosis. Eur J Clin Microbiol Infect Dis. 1996;15:139–145. doi: 10.1007/BF01591487. [DOI] [PubMed] [Google Scholar]
- 41.Talbot G M, Weiner M H, Gerson S L, Provencher M, Hurwitz S. Serodiagnosis of invasive aspergillosis in patients with hematological malignancy: validation of the Aspergillus fumigatus radioimmunoassay. J Infect Dis. 1987;155:12–27. doi: 10.1093/infdis/155.1.12. [DOI] [PubMed] [Google Scholar]
- 42.Toren A, Or R, Ackerstein A, Nagler A. Invasive fungal infections in lymphoma patients receiving immunotherapy following autologous bone marrow transplantations. Bone Marrow Transplant. 1997;20:67–69. doi: 10.1038/sj.bmt.1700847. [DOI] [PubMed] [Google Scholar]
- 43.Van Cutsem J, Meulemnas L, Van Gerven F, Stynen D. Detection of circulating galactomannan by Pastorex Aspergillus in experimental invasive aspergillosis. Mycoses. 1990;33:61–69. doi: 10.1111/myc.1990.33.2.61. [DOI] [PubMed] [Google Scholar]
- 44.Verweij P E, Latgé J-P, Rijs A J M M, Melchers W J G, De Pauw B E, Hoogkamp-Korstanje J A A, Meis J F G M. Comparison of antigen detection and PCR assay using bronchoalveolar lavage fluid for diagnosing invasive pulmonary aspergillosis in patients receiving treatment for hematological malignancies. J Clin Microbiol. 1995;33:3150–3153. doi: 10.1128/jcm.33.12.3150-3153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warnock D W, Foot A B M, Johnson E M, Mitchell S B, Cornish J M, Oakhill A. Aspergillus antigen latex test for diagnosis of invasive aspergillosis. Lancet. 1991;338:1023–1024. doi: 10.1016/0140-6736(91)91890-7. [DOI] [PubMed] [Google Scholar]
- 46.Wilson E V, Hearn V M, Mackenzie D W R. Evaluation of a test to detect circulating Aspergillus fumigatus antigen in a survey of immunocompromised patients with proven or suspected invasive disease. J Med Vet Mycol. 1987;25:365–374. [PubMed] [Google Scholar]