Abstract
With antimicrobial resistance rising globally, the exploration of alternative sources of candidate molecules is critical to safeguard effective chemotherapeutics worldwide. Plant natural products are accessible, structurally diverse compounds with antimicrobial potential. The pharmacological applications of plants in medicine can be guided by the attestation of traditional use, as demonstrated in this study. In Irish ethnomedical literature, Inula helenium L. (elecampane) is often indicated for respiratory and dermal ailments. This is the first assessment of antimicrobial sesquiterpene lactones from the roots of elecampane, naturalised in Ireland. Traditional hydro-ethanolic extracts were prepared from multi-origin elecampane roots. A novel clean-up strategy facilitated the bioactivity-guided fractionation of a subset of anti-staphylococcal fractions (the compositions of which were investigated using HPLC-DAD, supported by 1H NMR). The natural products attributing to the antimicrobial activity, observed in vitro, were identified as alantolactone (1), isoalantolactone (2), igalan (3), and an unseparated mixture of dugesialactone (4) and alloalantolactone (5), as major compounds. The findings suggest that the geographical origin of the plant does not influence the anti-bacterial potency nor the chemical composition of traditional elecampane root. Considering the prevalence of staphylococci-associated infections and associated broad spectrum resistance in Irish hospitals, currently, further research is warranted into the usage of the identified compounds as potential candidates in the control of staphylococcal carriage and infection.
Keywords: Inula helenium, elecampane, antimicrobial activity, ethnobotany, compound isolation, sesquiterpene lactones, staphylococcal infection
1. Introduction
1.1. Ethnobotany in Drug Discovery
Challenges in antimicrobial chemotherapy are widespread. Most chemotherapeutic agents in clinical development today are modifications of known structures and, thus, cannot alleviate existing issues with cross- and pan-resistance among pathogens. The prevalence of antimicrobial resistance (AMR), combined with a lack of innovative leads and novel structural classes introduced to the antibacterial armamentarium in recent decades, is at the forefront of this impending crisis [1]. With ~80,000 plant species in the Amazon alone [2], the potential structural diversity in the terrestrial Plant Kingdom is enormous, albeit underutilised in medicine.
Target-directed drug discovery models have outstanding merits; however, when it comes to microbial infection, focusing on one molecule and one target could be an ineffective method over time, since microorganisms evolve at a greater rate than we can create new drugs with new targets. This is evidenced by the fact that only six new antibiotics have been approved for therapeutic use in the last 30 years and resistance has already been observed to these [3], combined with a weak pipeline for new antimicrobial agents [4]. Multi-compound (combinatorial treatment) or potentiator/adjuvant therapies [5,6] are alternatives that warrant investment.
Using an ethnobotanical approach, the pharmacological applications of plants in medicine can be guided by attesting traditional/indigenous use or by applying this knowledge to uncover natural products with new biological applications [7,8]. This approach serves as an accessible starting point and increases the probability of discovering medicinally useful compound(s), which aligns with global strategic objectives to tackle AMR [9]. Plants are a prosperous source of therapeutic chemical entities and may offer significant potential in the universal quest for effective infectious control.
1.2. Traditional Use to Modern Research
Inula helenium L. (Asteraceae family) is a distinctive perennial herb, naturalised in Ireland. The plant is a reservoir of diverse compounds, with a long history of ethnomedicinal use, with records from Minoan, Mycenaean, Egyptian, Assyrian and Serbian (Chilander Medical Codex) pharmacotherapy manuscripts circa 2700–1100 B.C. [8]. The root extract features in many traditional medical systems, including Tibetan, Ayurvedic and Traditional Chinese Medicine, where it is referred to as ‘Radix Inulae’ (Tu-Mu-Xiang, Zang-Mu-Xiang) [8]. In European pharmacopoeias it is referenced as ‘Aunée’ (France), ‘Radix Helenii’ (Netherlands), ‘Rhizoma Helenii’ (Germany) and ‘Helenii Rhizoma’ (U.K.) [8].
Cameron [10] postulated that I. helenium likely originated in Gaelic and Irish tradition, from the historic officinal name ‘Inula campana’, or ‘Helénula’ (‘Little Helen’). The plant has been referred to in traditional Irish texts as ‘áillean’, derived from ‘áille’ meaning beautiful or lovely, and ‘Ellea’ derived from the Gaelic ‘Eilidh’ meaning Helen [10]. This association is thought to be rooted from Greek Mythology, as Helen of Troy favoured the flowers of I. helenium for their aesthetic splendour [10]; as legend recites, the blossom of elecampane from her fallen tears upon abduction from her homeland [11]. The plant features in Celtic folklore, as ‘Elf-Dock’ and ‘Elf-Wort’, and other common names include ‘Horse-heal’ and ‘Scab-wort’; the latter relating to its use as a topical agent [11].
In ancient Irish literature, the medicinal use of I. helenium is often described for respiratory ailments [12]. In a translated version of Tadgh Ó Cuinn’s medieval Irish Materia Medica (circa 1415 A.D.), references to the use of I. helenium to treat respiratory organs, coughs and consumption [from tuberculosis], are documented. Respiratory preparations specified boiling the powdered herb with dilute barley water, liquorice, cinnamon and sugar, whilst digestive ailments (e.g., “ileus, colic and stranguria”) were treated with a plaster applied to the naval, comprised of the herb boiled in wine and oil [12]. Celtic ethnobotany is somewhat historically neglected, however, and as Moloney [13] recites: “[we have] relegated to oblivion many a(n) herb”. The recent establishment of digital archives (e.g., Dúchas.ie, CELT.ie) will function to preserve and facilitate further research of Irish traditional medicinal knowledge [14,15].
The British Herbal Pharmacopoeia (BHP) lists the therapeutic actions of the herb, as follows: antitussive, antiseptic expectorant, diaphoretic and bactericidal [16]. Suggested indications include respiratory mucosal catarrh (tracheal, bronchial), cough and phthisis associated with pulmonary tuberculosis, bronchitis and whooping cough in infants [16]. The root is traditionally administered as a decoction, prepared by boiling the comminuted herbal material and water, which is then strained before administration [17]. Dosage recommendations in the BHP range from 1.5–4 g decoction from the dried root/rhizome, or 1.5–4 mL liquid extract (i.e., 1:1 herbal tincture in 25% alcohol) thrice daily [16]. The plant can be combined with extracts of Marrubium, Tussilago, Asclepias or Millefolium [16], however, guidelines for such preparations are not detailed in the monograph.
In the 1980s, the German Commission E published an official monograph disproving the therapeutic use of elecampane root, based on insufficient evidence to support the efficacy of the herb and preparations thereof, and the risk of associated adverse effects [18]. It was later considered an “unapproved” medicinal herb, and consequently excluded in consecutive global monographs and compendial (Pharmacopeial) texts from thereon, including the following: the ESCOP monographs, WHO Selected Medicinal Herb monographs (Vols. 1–3), the British Herbal Compendium (Vols. 1 and 2), and the European Medicines Agency (EMA) official herbal monographs and list entries. This outcome was a likely consequence of individual case reports, documenting adverse inflammatory effects thought to be associated with I. helenium [19,20,21,22] and related species (I. conyza [23,24], I. viscosa [25,26] and I. graveolens Desf.) [27]. The findings of these earlier studies accept that the presence of causative allergens was likely family/genus related. Paulsen [28] acknowledges that while elecampane is suspected to be an inducer of Asteraceae allergic dermatitis, there is no epidemiological data available to support this. Sesquiterpene lactones (SLs) are an important group of bioactive metabolites present in elecampane root [8,29], as shown in Table 1. Paulsen [30], in agreement with Amorim [31], later cautioned towards the systemic allergic dermatitis associated with SL-containing plants in general, while emphasising the need to determine the pathogenesis of haptens, associated with the plant species, more specifically. Clinical data for the herb is limited, with one randomised, double-blind, placebo-controlled clinical trial documented for a cough syrup (KalaboTUSS®), which proved to be safe and efficacious [32]. Regarding isolated compounds, there are conflicting reports of sensitization [33,34,35] and opposing anti-allergenic properties [36,37]. More recent evidence, however, infers support for further research into the therapeutic efficacy and safety of elecampane-derived compounds.
Table 1.
Overview of known isolated sesquiterpene lactones (SLs) from I. helenium root extracts to date.
| Group | No. | Identified Compound(s) | Reference(s) |
|---|---|---|---|
| Eud- | 1 | Alantolactone | [94,95,96,97,98] |
| 2 | Isoalantolactone | [99,100,101] | |
| 3 | Dihydroalantolactone | [102,103,104,105] | |
| 4 | Dihydroisoalantolactone | [102,103,104,105] | |
| 5 | Tetrahydroalantolactone | [106] | |
| 6 | Alloalantolactone (= 1-Deoxyivangustin, = (+)-Diplophyllin) | [107] | |
| 7 | Bialantolactone | [108] | |
| 8 | Trinoralantolactone | [108] | |
| 9 | 5α-Epoxyalantolactone | [107] | |
| 10 | 4-Noralantolactone (= 4-oxo-5(6),11-eudesmadiene-8,12-olide) | [109] | |
| 11 | 4-Norisoalantolactone (= 4-oxo-11-eudesmene-8,12-olide) | [109] | |
| 12 | 1α-Hydroxy-11,13-dihydroisoalantolactone | [110] | |
| 13 | 3α-Hydroxy-11,13-dihydroalantolactone | [110] | |
| 14 | Macrophyllilactone E | [110] | |
| 14 | 4α,15α-Epoxyisoalantolactone | [108] | |
| 15 | 4,5-seco-Eudesm-11(13)-en-4,5-dioxo-8β,12-olide (=Umbellifolide) | [108] | |
| 16 | 11α-Hydroxyeudesm-5-en-8β,12-olide | [108] | |
| 17 | 3α-Hydroxyeudesma-4,11-dien-8β,12-olide | [108] | |
| 18 | Telekin | [108] | |
| 19 | 3-Oxo-eudesma-4(5),11-dien-8,12-olide (= 3-Oxoalloalantolactone) | [111] | |
| 20 | 11α,13-Dihydro-α-cyclocostunolide | [112] | |
| 21 | 11α,13-Dihydro-β-cyclocostunolide | [112] | |
| 22 | 15-Hydroxy-11βH-eudesm-4-en-8β,12-olide | [112] | |
| 23 | 3α-Hydroxy-11βH-eudesm-5-en-8β,12-olide | [112] | |
| 24 | 2β,11α-Dihydroxy-eudesm-5-en-8β,12-olide | [112] | |
| 25 | Isoheleproline | [113] | |
| 26 | 11β-Hydroxy-13-chloro-eudesm-5-en-8β,12-olide | [7] | |
| 27 | 5-epi-telekin | [7] | |
| 28 | Racemosalactone A | [7] | |
| 29 | Macrophyllilactone F | [74] | |
| El- | 30 | Igala (= 1,3,11(13)-Elematrien-8β,12-olide) | [105] |
| Er- | 31 | Dugesialactone | [114] |
| Gua- | 32 | Dehydrocostus lactone | [112] |
| 33 | 4α-Hydroxy-1β-guaia-11(13),10(14)-dien-12,8α-olide | [111] | |
| Ger- | 34 | Germacrene-D-lactone (= Germacra-1(10),4(15),5(6),11(13)-tetraen-8,12-olide) | [107] |
| 35 | 4β,5α-Epoxygermacra-1(10),11(13)-dien-12,8α-olide | [105] | |
| 36 | Isocostunolide | [79] | |
| 37 | (1(10)E)-5β-Hydroxygermacra-1(10),4(15),11(13)-trien-12,8α-olide | [109] | |
| 38 | 14-Hydroxy-11β,13-dihydrocostunolide/ 11β, 13-Dihydro-14-hydrocostunolide | [8,112] | |
| 39 | Costunolide | [112] | |
| 40 | 5β-Hydroxygermacra-1(10),4(15),11(13)-trien-12,8β-olide | [108] | |
| 41 | 4α,5α-Epoxygermacra-1(10),11(13)-dien-12,8β-olide | [108] |
Eud-: Eudesmanolides; El-: Elemanolides; Er-: Eremophilanolides; Gua-: Guaianolides; Ger-: Germacranolides.
Research interest has increased in recent years, specifically relating to the potential of the crude extract and isolated compounds (viz. alantolactone, isoalantolactone, 5α-epoxyalantolactone, (iso) costunolide and igalan) in cancer therapeutics. Antineoplastic [38] and anti-cancer activity was demonstrated in multiple cell lines, including brain [39], pancreatic [40] and breast [41] cancers. Other studies reported reduced inflammatory responses in in vivo sepsis models [42,43], rheumatoid arthritis [44] and suppressed neutrophil-mediated inflammation in acute bronchitis by down-regulating the β2-integrin [45], following exposure to the crude extract. Alantolactone has been reported to exert anti-tumour activity in a number of cancer models, including Jurkat T-lymphocyte cells [46], β-cell acute lymphoblastic leukaemia [47], chronic myelogenous leukaemia [48], gastric [49], colon [50,51], liver [52,53], lung [54,55,56], pancreatic [57] and breast cancers [58,59]. Moreover, the compound inhibited lipopolysaccharide (LPS)-induced nitric oxide synthesis in murine macrophages (RAW 264.7), the activity of which is attributed to the presence of the α-methylene-γ-lactone moiety—a key structural motif and putative pharmacophore of many identified SLs [60].
Isoalantolactone exerts diverse anticancer activity in vitro [61], including squamous cell carcinoma (head, neck) [62], oesophageal cancer [63], chronic myelogenous leukaemia [64] and breast cancer cell lines [65]. It inhibits LPS- and Phorbol-12-myristate-13-acetate (PMA)-induced inflammatory responses [66,67]. In pulmonary models, it exerts anti-inflammatory effects in acute lung injury (ALI) [68] and inhibits α-toxin, an important virulence factor secreted by S. aureus, which potentiates pneumonia pathogenesis [69]. Isoalantolactone, alantolactone and alloalantolactone have comparable anti-tumour effects in pancreatic cell lines [70]. Alantolactone [71], isoalantolactone [72] and 5α-epoxyalantolactone were reported as potential chemopreventative agents [73], while 5α-epoxyalantolactone exhibits antiproliferative effects in acute myelogenous leukaemia progenitor cells [74]. Igalan demonstrated protective [75] and anti-inflammatory activity in vivo [76]. Lastly, costunolide and isocostunolide, neither of which were identified in this study, also show promise for the anti-proliferative, anti-metastatic and neuroprotective effects in vivo [77,78,79].
1.3. Antimicrobial Potential of Elecampane SLs
Olechnowicz-Stepien and Skurska [80] first reported the antimicrobial activity of the root in vitro, while some of the earliest compounds isolated included alantopicrin [81] and dammaradienyl acetate [82]. A cascade of research followed in the late 1990s, investigating the antimicrobial activity of different extracts of elecampane root against various pathogens [43,83,84,85,86,87,88,89,90,91,92]. Methicillin- and vancomycin-resistant Staphylococcus aureus (MRSA/VRSA) are among the listed pathogens of “medium-to-high priority”, published by the WHO [1], and at a national level, Ireland has some of the highest occurrences of Staphylococci- and Enterococci-associated systemic bloodstream infections in all the European member states [93]. S. aureus was, therefore, selected as a suitable target organism in this present study, because of its priority status and clinical relevancy in Irish hospitals. Furthermore, it is in-line with ethnobotanical use and the microorganism has a known susceptibility to crude extracts of elecampane, as previously demonstrated in our laboratory [86], and among other research groups, as summarised previously [3].
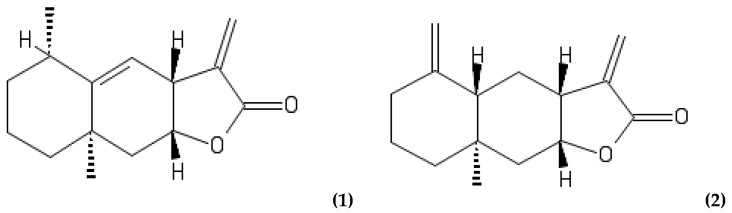
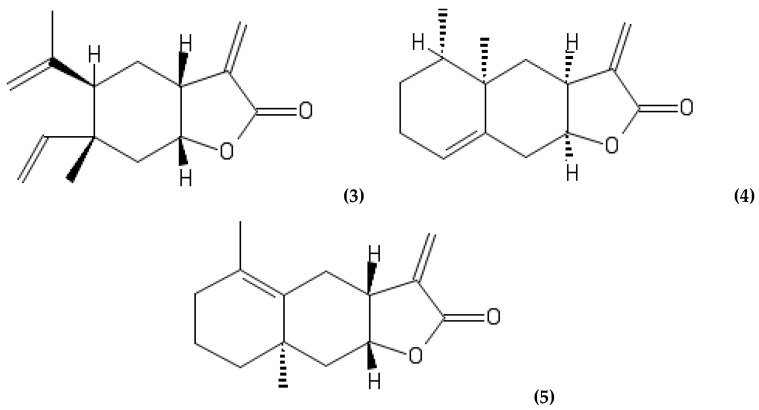
The aim of this study is to complete the first assessment of antimicrobial SLs in a traditional (hydro)ethanolic root extract of elecampane, naturalised to the Irish climate, with comparison to dried root sourced internationally. Documented here is the application of a novel one-step clean-up strategy to facilitate the bioactivity-guided fractionation of antimicrobial compounds, attributing to the anti-staphylococcal activity observed in vitro. A validated HPLC-DAD method was used to investigate the presence of alantolactone (1), isoalantolactone (2), igalan (3) and an unseparated mixture of dugesialactone (4) and alloalantolactone (5)—which could serve as potential antibiotic lead compounds. The findings suggest no observed difference in antimicrobial activity from extracts of different origin, suggesting the antimicrobial activity of elecampane is likely species-specific and is independent of the cultivation environment.
2. Results and Discussion
There is widespread acceptance that AMR presents alarming dangers, and the current clinical antimicrobial pipeline is insufficient to allay consequent threats to global infection prevention and control [1]. This reality has resulted in a united global ambition in the pursuit of new therapeutic modalities for infectious disease. Plants documented as anti-infectives in historical literature, such as I. helenium, could offer potential as mono-/poly-therapeutics and adjuvant/potentiator agents in modern medicine [5,6]. The use of plants and Ethnobotanical principles as a starting point in the drug discovery process, therefore, warrants consideration. The aim of this study was to investigate the key compounds from a traditional (hydro)ethanolic root extract of I. helenium, attributing to its in vitro anti-staphylococcal activity.
An initial preliminary screen confirmed that the crude extracts were active against the gram-positives, as follows: Group-A Streptococcus pyogenes, Group-B Streptococcus agalactiae, Listeria monocytogenes, Escherichia faecalis ATCC 29212 and Escherichia coli, as well as Mycobacterium tuberculosis H37Ra (ATCC 25177) (data not shown). S. aureus was chosen as the target organism for the bioactivity-guided fractionation in this study, based on previous results [86], and its clinical relevance in Irish hospitals currently [93].
An optimised extraction method, involving the traditional maceration of the comminuted root with the extractant solvent, was performed as before [86], to compare the activity between the root originating from a source plant naturalised to West Cork (Ireland) versus internationally sourced, commercially available dried root samples. The compounds attributing to the bioactivity, observed in vitro, were identified as alantolactone (1), isoalantolactone (2), igalan (3), and an unseparated mixture of dugesialactone and alloalantolactone (4, 5), as major constituents. Our findings suggest that the antimicrobial potency of the plant extract is not influenced by geographical origin or environmental conditions in this case, since all samples resulted in comparable activity (See Table 2) and major constituent profiles (See Figure 1 and Figure 2).
Table 2.
Average zone diameter (, mm) and total yield (mg) per bioactive fraction (n = 3).
| Extract | Bioactive Fraction No. |
Inhibitory Zone Diameter ( ± SD; mm) |
Total Yield ( mg) |
|---|---|---|---|
| CT50 | F14 | 12.2 ± 0.2 | 13.0 |
| F15 | 16.5 ± 0.3 | 110.0 | |
| F16 | 16.3 ± 0.5 | 47.7 | |
| F17 | 16.1 ± 0.3 | 56.1 | |
| F18 | 13.4 ± 0.6 | 55.7 | |
| F19 | 10.8 ± 0.5 | 56.3 | |
| F20 | 11.0 ± 0.3 | 76.3 | |
| F21 | 11.5 ± 0.6 | 82.8 | |
| F22 | 13.0 ± 0.5 | 96.0 | |
| CM50 | F16 | 15.0 ± 0.6 | 84.7 |
| F17 | 15.6 ± 1.1 | 49.0 | |
| F18 | 15.0 ± 0.1 | 42.3 | |
| F19 | 13.4 ± 0.3 | 41.5 | |
| F20 | 12.3 ± 1.2 | 44.3 | |
| F21 | 13.1 ± 0.7 | 62.4 | |
| F22 | 15.1 ± 0.8 | 43.3 | |
| F23 | 16.2 ± 0.1 | 114.7 | |
| F24 | 14.0 ± 0.9 | 134.4 | |
| CT100 | F15 | 12.2 ± 0.5 | 44.0 |
| F16 | 13.8 ± 0.1 | 57.1 | |
| F17 | 20.0 ± 0.1 | 102.5 | |
| F18 | 18.7 ± 0.4 | 165.7 | |
| F19 | 20.0 ± 0.3 | 84.3 | |
| F20 | 12.3 ± 1.0 | 54.1 | |
| F21 | 11.3 ± 0.2 | 50.5 | |
| F22 | 11.2 ± 0.3 | 57.4 | |
| F23 | 13.4 ± 0.3 | 57.6 | |
| CM100 | F16 | 18.7 ± 0.7 | 46.1 |
| F17 | 17.4 ± 0.6 | 101.3 | |
| F18 | 17.7 ± 1.6 | 61.7 | |
| F19 | 14.7 ± 0.7 | 13.9 | |
| F20 | 13.1 ± 0.1 | 9.7 | |
| F21 | 16.5 ± 0.5 | 12.7 |
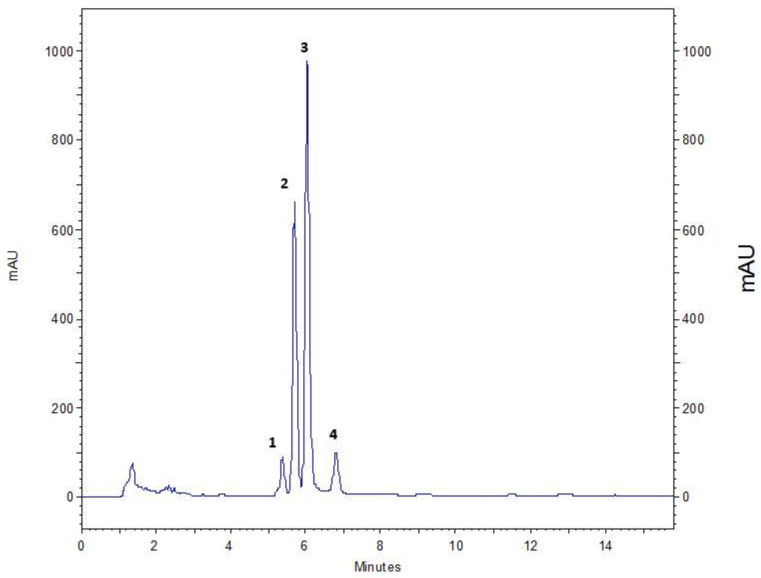
Figure 1.
Chromatographic separation of the fraction F16 (CM50) by the method proposed by Huo et al. [114], using the Zorbax® Eclipse XDB C18 column. Signals detected at 210 nm: Peak 1—igalan; 2—isoalantolactone; 3—alantolactone; 4—a mixture of dugesialactone and alloalantolactone.
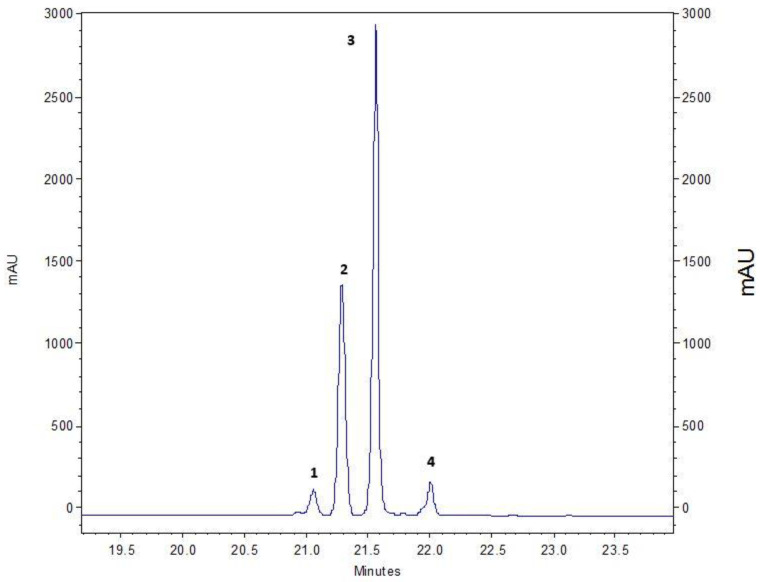
Figure 2.
Chromatographic separation of the fraction F16 (CM50) using the method proposed by Stojakowska et al. [119], using the Kinetex® 5 μm XB-C18 column. Signals detected at 205 nm: Peak 1—igalan; 2—isoalantolactone (confirmed with standard); 3—alantolactone (confirmed with standard); 4—mixture of compounds containing alloalantolactone (confirmed by 1H NMR).
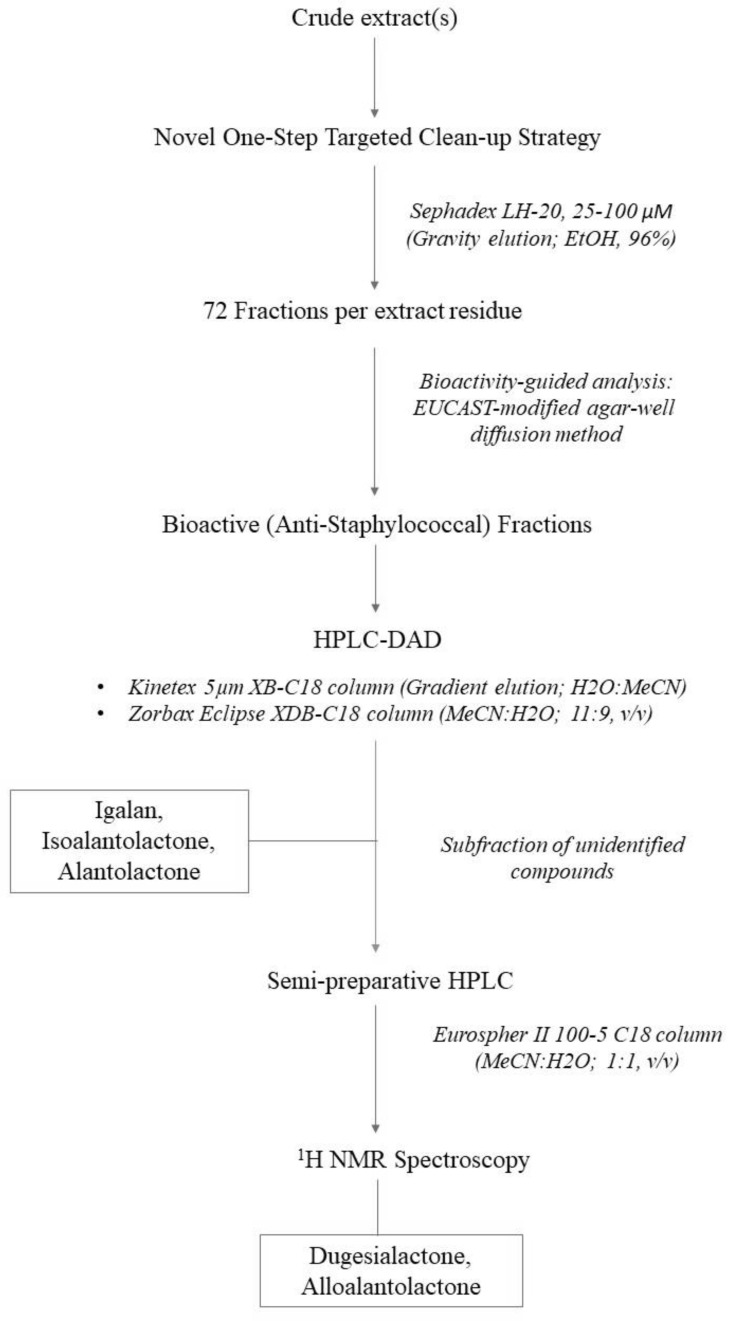
As a follow-on from our preliminary elecampane research [86], the bioactive composition of the crude extract was further explored in this study. This was achieved using an in vitro bioactivity-guided fractionation strategy, applied to fractions generated from a single chromatographic clean-up step, using Sephadex LH-20 as the stationary matrix. This model could serve as a starting point for small-scale process development, if considering the general extraction of antimicrobial compounds from this plant. Sephadex LH-20 was the matrix of choice based on the desire to minimize compound loss via in situ sorption effects/phenomena. Sephadex LH-20 has been used for the isolation of compounds from other Inula species [115,116,117,118]; however, this is the first account of its use for the initial fractionation of bioactive compounds from crude extracts of I. helenium. Results from the agar-well screening suggested that the composition of the active fractions contained a range of compounds that were physiochemically related and hence co-eluted within a narrow range, e.g., F16–24 (See Table 3).
Table 3.
Composition of bioactive fractions expressed as % of the total weight (i.e., g/100 g sample).
| Extract | Fraction | Peak 1 a | Peak 2 | Peak 3 | Peak 4 | Total Yield b |
|---|---|---|---|---|---|---|
| CT50 | F14 | 1.64 | 9.72 | 15.91 | 3.52 | 30.79 |
| F15 | 2.85 | 14.62 | 21.07 | 4.07 | 42.62 | |
| F16 | 2.89 | 12.52 | 16.32 | 3.51 | 35.24 | |
| F17 | 0.79 | 3.81 | 5.87 | 1.10 | 11.56 | |
| F18 | 1.87 | 8.93 | 14.32 | 2.66 | 27.78 | |
| F19 | 1.34 | 5.92 | 9.24 | 1.86 | 18.35 | |
| F20 | 0.20 | 1.10 | 1.75 | 0.34 | 3.40 | |
| F21 | 0.40 | 1.68 | 2.35 | 0.52 | 4.95 | |
| F22 | 1.64 | 9.72 | 15.91 | 3.52 | 30.79 | |
| CM50 | F16 | 1.21 | 11.34 | 20.73 | 1.99 | 35.27 |
| F17 | 2.42 | 20.48 | 21.16 | 2.83 | 46.89 | |
| F18 | 1.60 | 11.47 | 15.43 | 1.88 | 30.37 | |
| F19 | 1.31 | 9.06 | 10.69 | 1.44 | 22.50 | |
| F20 | 0.67 | 5.28 | 5.29 | 0.72 | 11.96 | |
| F21 | 0.49 | 3.34 | 4.23 | 0.58 | 8.64 | |
| F22 | 1.25 | 9.93 | 10.04 | 1.45 | 22.67 | |
| F23 | 0.68 | 5.50 | 4.79 | 0.73 | 11.70 | |
| F24 | 0.36 | 2.32 | 2.22 | 0.35 | 5.25 |
a 1: Igalan ([114]; not unequivocally confirmed by 1H NMR (proton nuclear magnetic resonance) due to substantial amounts of lipids in the subfraction); 2: Isoalantolactone (confirmed with standard); 3: Alantolactone (confirmed with standard); 4: unseparated mixture of dugesialactone and alloalantolactone ([114] alloalantolactone presence confirmed by 1H NMR). b Except for the eudesmanolides, fatty acids and, as it was shown by 1H NMR analysis, complex mixture of lipids without UV/Vis absorption may constitute the sample.
Further elucidation of the composition of the active fractions was performed, following methods routinely used at the Phytochemistry Department of the Maj Institute of Pharmacology, for quantification of SLs in various extracts from plants of the Inuleae tribe [119]. Figure 1 and Figure 2 outline the HPLC chromatograms for fraction F16 (CM50), following the Huo et al. [114] and Stojakowska et al. [119] methods, respectively. Since both methods produced comparable spectra, the Stojakowska et al. method [119] was used exclusively. All samples, regardless of their source location, similarly contained a mixture of closely related eudesmanolides (i.e., helenin) with alantolactone (1) and isoalantolactone (2) as major signals—annotated in Figure 1 and Figure 2 as chromatographic peaks 2 and 3—and confirmed with comparison to external standard samples as per [119]. Structures of the identified SLs are shown in Figure 3.
Figure 3.
Structures of the identified compounds: (1) alantolactone; (2) isoalantolactone; (3) igalan; (4) dugesialactone and (5) alloalantolactone.
Quantitatively, the mixture of the eudesmanolides constituted up to 50% of the sample weight (See Table 3). To confirm the identification of the partially overlapping minor lactones (Peak 4, Figure 2), 1H NMR was performed, as per Huo et al. [114], as shown in the Supplementary Data (Figure S4). Peak assignments were consistent with those available in the literature [109,114], however, 1H NMR analyses of subfractions were influenced by the presence of lipids/fatty acids (See Table 3). The content of the mixture under Peak 4 was assessed semi-quantitatively, with an assumption that the signal is generated by eudesmanolides (Compounds 4 and 5).
SLs, depending on the structure of their carbon skeleton, can be classified into several groups, including germacranolides, eudesmanolides, guaianolides and pseudoguanianolides. A comprehensive list of known SLs, identified in I. helenium root, can be found in Table 1. Several reviews discuss the extensive pharmacological potential of metabolites from the Inula genus [3,8,29,120,121].
There are, however, limitations to this study. Natural product extracts are complex, comprised of multiple compounds of unknown molecular weight and variable characteristics (e.g., polarity, solubility, viscosity, stability, toxins, fluorophores, pigments) that can cause bioassay interference in manual and automated screening platforms [122,123]. Furthermore, the unavailability of standardised antimicrobial breakpoints to guide AST of natural products, such as plant compounds, is a recognised limitation in this area of research [3]. The crude extract of I. helenium ranges from green (aqueous-ethanolic extract) to dark brown (ethanolic extract). Both the plant extract pigmentation and the extractant solvent are central to the experimental issues our lab observed when performing preliminary dilution-based antimicrobial methods, including microdilutions (i.e., MIC determination) and biofilm assays (data not reported). Pigmentation of extracts/fractions can interfere with spectral quantification of microbial turbidity and biofilm staining, irrespective of the use of colorimetric indicators. Some authors report the pre-treatment of coloured plant extracts, prior to testing, such as the multiple sequential centrifugation of the crude filtrate [124], or the addition of decolourisation steps (e.g., activated carbon) for the removal of non-polar pigments [125]. Any pre-treatment in the sample preparation phase would need to be considered, of course, when interpreting the results, to avoid adversely affecting intra- and inter-laboratory reproducibility [126].
Solvents are employed for the initial extraction, chromatographic separation and re-solubilisation of dried plant residues. In this study, EtOH was chosen as the extractant solvent, based on traditional usage [86], and similar studies evaluating EtOH extracts of I. helenium [88,109,110,127,128]. In dilution-based methods, solvents can interfere with activity—either potentiating or falsely depicting antimicrobial activity. Increased dilution ranges, using assay media as the diluent, are, therefore, necessary, which can sometimes lead to an underrepresentation of activity, since water-based diluents can be inefficient vehicles for compound solubility [129]. Solubilisation of the test articles, across a wide range of polarities, in an inert or biologically inactive solvent that is miscible with assay media is preferred [130]. CyreneTM (dihydrolevoglucosenone), an aprotic dipolar solvent, has recently been proposed as a green bio-based alternative to DMSO, offering comparable solubility properties, low toxicity and no evidence of antioxidant or radical scavenging properties, unlike DMSO [129]. Validation of its use in phytochemical research would be valuable to this field.
A negative control containing the solvent, or carrier, used to extract or dissolve the test article(s), was included in order to confirm inactivity and non-toxicity against the target microorganism. We performed a solvent tolerance test and confirmed that our target organism tolerated EtOH, up to 20% per well in the microdilution method (data not shown). With the diffusion-based assay, the EtOH content of the test articles (e.g., crude extracts, fractions or standards) did not adversely affect the assay, as the plates were left under laminar flow to evaporate the EtOH prior to incubation. Similarly, the pigment was retained within the well and did not diffuse throughout the agar. The evaluation of dilution and diffusion methods, used to determine the antimicrobial activity of plant extracts, has been reviewed [3,130]. The EUCAST guidelines, while directed for single-constituent or conventional antibiotics, encompass the core criteria to perform AST, including inoculum preparation, media preparation and dilution schemes and were, therefore, used in this current study, in the absence of any standardised methods for antimicrobial assessment of plant extracts. Outside conventional AST, methods exploring other aspects of bacterial weaponry constitute a relatively untapped market for novel targets to complement anti-infective strategies, such as methods targeting virulence factors (e.g., adhesins, toxins, effectors, ion chelators, destructive enzymes, secretory and signalling molecules), structural assembly, and biofilms compositions [131].
Our results demonstrate that in vitro anti-staphylococcal efficacy of a traditional extract of I. helenium root is in line with traditional use [12,16]. Its use in dermal (topical) applications is minimally explored in recent scientific literature, which is a likely consequence of earlier anecdotal case reports of species and family-related sensitivity. Given the prevalence of Staphylococci-associated infections in Irish hospitals, further research into the usage of the identified compounds (alone and in combination) is warranted as an alternative treatment in the prevention and control of Staphylococcal carriage and infection.
3. Materials and Methods
3.1. Crude Extract Preparation
3.1.1. Sources of Plant Material
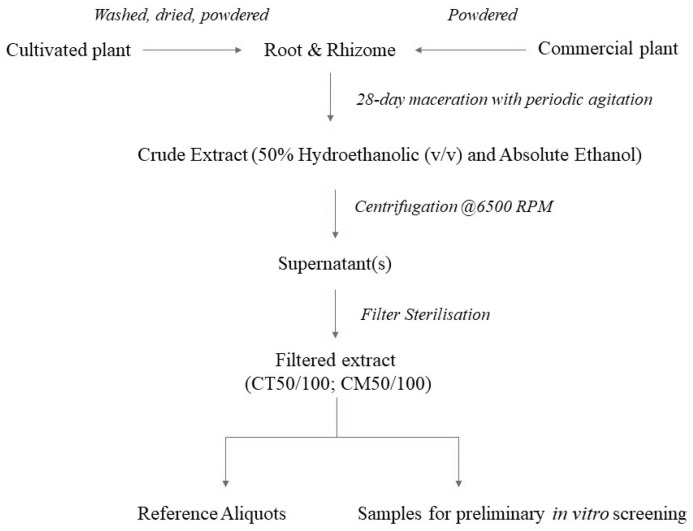
Cultivated roots (CT) were collected from Bandon Medicinal Herbs Ltd. (West Cork, Ireland) (See Figure 4), and authenticated accordingly [86]. The plant material was also identified against a voucher specimen deposited in the New York Botanical Gardens (NYBG) Steere Herbarium (Barcode: 2924501), and botanical key [132]. Commercial dried root (CM) was purchased from a registered supplier, Herbs in a Bottle© Ltd. (U.K.). Product information as per label: Code #6202b; Batch #148845; Specification: Cut; Date of Manufacture: 18/06/2015; Issue #160575; Origin: China; Identification: Conforms.
Figure 4.

Harvested roots of I. helenium L. (Elecampane).
3.1.2. Traditional Maceration
Roots and rhizomes were collected in September 2015 (Bandon Medicinal Herbs Ltd.). The harvested roots were washed with ultrapure Milli-Q water (18.2 MΩ·cm) and left to dry naturally at room temperature. The dried roots were then powdered and stored in a sterile air-tight container protected from light.
To compare cultivated versus commercially sourced plant material, a total of four plant extracts were prepared at a concentration of 100 mg mL−1 in 50% aqueous ethanol (v/v) and absolute ethanol (See Table 4), as per O’Shea et al. [86]. The extracts will be referred to as CT50 or CT100 (cultivated), and CM50 or CM100 (commercial), from herein. See Figure 5 and Figure 6 for schematic overviews of the extraction and analysis process.
Table 4.
Composition of the cultivated (CT) and commercial (CM) extracts and yields.
| Extract | Traditional Extract Composition | Total Yield * (g) |
|---|---|---|
| CT50 | Cultivated root powder in 50% ethanol (v/v). | 36.3 |
| CT100 | Cultivated root powder in absolute ethanol. | 47.4 |
| CM50 | Commercially acquired root powder in 50% ethanol (v/v). | 40.0 |
| CM100 | Commercially acquired root powder in absolute ethanol. | 38.4 |
* Yield measured by weighing the dried residue after evaporation.
Figure 5.
Schematic overview of the extraction process.
Figure 6.
Schematic overview of the phytochemical analysis methodology.
The comminuted herbal material was introduced to the vessel containing the extraction solvent as per Table 4. Extracts were periodically mixed by gentle inversion. Maceration took place for a total of 28 days at room temperature protected from light. The crude macerate(s) were centrifuged in a Thermo Scientific IEC-CL30R for 15–20 min at 6500 RPM. The decanted supernatant(s) were subsequently vacuum filtered using a Büchner funnel lined with Whatman filter paper No. 1 discs for clarification (11 µm particle retention) followed by filter sterilisation using cellulose nitrate loaded syringes (0.45 µm). Retained samples of the crude extracts were stored at −20 °C in a repository at Muster Technological University, while the test articles were stored at 2–8 °C for use as an experimental control.
3.2. Bioactivity-Guided Fractionation of Antimicrobial Compounds
3.2.1. Gravity-Eluted Size-Exclusion Chromatography
The solvent removal process was performed using a BÜCHI Rotavapor R-205 (BTX-RE2) with an attached BÜCHI vacuum controller V-800. The instrument parameters were set (40 °C; 175 mbar) and total evaporation time was approximately 90 min per 100 mL extract. Total yields are recorded in Table 4. Residues were stored at −20 °C until required for further analysis.
The CT50 extract was retrieved from −20 °C storage and left to thaw at room temperature. Methanol (MeOH) was added to resolubilise the viscous residue under sonication. The resuspended extract was transferred to an amber vial (2 mL) and the solvent was removed under a gentle flow of nitrogen. Ethanol (EtOH; extractant solvent) was used to resolubilise the extract as it is less potently toxic to cells in vitro. To maintain consistency, EtOH was further utilised as the mobile phase for subsequent chromatographic separation.
Sephadex LH-20 (25–100 µm) was prepared in MeOH and left in solution for 24 h at room temperature. All glassware was rinsed in MeOH and air-dried before use. A glass column (height 90 cm; diameter 3.2 cm) was carefully packed with the Sephadex solution and secured with parafilm to facilitate deposition. The Sephadex was prevented from drying out in the column once packed by ensuring that the apex remained submerged in EtOH. The soluble crude extract (2 mL, 100 mg) was applied to the column as a thin solvent band using a glass pipette, and the band was allowed penetrate the top layer. Once completed, a reservoir of EtOH (300 mL) was introduced as the mobile phase. The column was eluted with 96% EtOH under gravitational flow, and fractions were manually collected in 10 mL aliquots. A total of seventy-two fractions were collected per extract over a period of 12 h.
These steps were repeated for the remaining three following extracts: CT100, CM100 and CM50. All fractions were immediately screened for their antimicrobial activity against Staphylococcus aureus using a modified in vitro agar-well method, detailed below.
3.2.2. Bacterial Strains and Media Preparation
Clinical diagnostic reference strain S. aureus NCTC 6571 (cross-referenced in the American Type Culture Collection (ATCC) as ATCC 9144 [133]).
S. aureus clinical isolates from Cork University Hospital (CUH), Co. Cork, Ireland.
Culture media prepared as per manufacturer guidelines: Mueller Hinton (MH) broth (Lab M, Lancashire, U.K.; Lot: 141370/357) and agar (Lab M, Lancashire, U.K.; Lot: 144209/172). Cation-adjusted Mueller Hinton II (MHII) broth (Sigma-Aldrich, Darmstadt, Germany; Lot: BCBT9094) and agar (Sigma-Aldrich, Darmstadt, Germany; Lot: BCBV4646).
Sodium chloride (PanReac AppliChem, Barcelona, Spain; Lot: 0000893728).
Glycerol solution, 84–88% (Sigma-Aldrich, Darmstadt, Germany; Lot: SZBC010BV).
Alantolactone standard (Sigma, Darmstadt, Germany; Lot No. 125M4751V).
Isoalantolactone standard (CliniSciences; HY-N0780/CS-3635; Batch No. 20994).
3.2.3. Preparation of S. aureus Stocks
S. aureus was used as the target organism to guide the fractionation process. Stocks were maintained in glycerol at −80 °C. Briefly, overnight cultures were centrifuged at 4000 RPM for 15–20 min. The supernatant was discarded, and the pellet re-suspended in MH broth. Aliquots were combined with sterile glycerol (1:2, v/v) under laminar flow. Working stocks were stored at −20 °C and reference stocks at −80 °C.
3.2.4. In Vitro Agar-Well Screening (Modified EUCAST Disk-Diffusion Method)
Antimicrobial screening was performed following European Committee on Antimicrobial Susceptibility Testing (EUCAST) Guidelines [126,134], modified accordingly. Briefly, overnight cultures of S. aureus NCTC 6571 were adjusted to 0.5 McFarland standard in 0.85% sterile saline solution. The surfaces of MH agar plates were inoculated with the adjusted bacterial suspension using sterile cotton swabs. To prepare the wells, 8 mm holes were aseptically punched into the agar surface after inoculation. Seventy-five µL of each fraction were transferred to their corresponding well. Alantolactone (0.2–3.2 µg mL−1) and isoalantolactone (1 mg mL−1) were tested in tandem. Plates were left under laminar airflow for up to 60 min to facilitate solvent evaporation prior to incubation at 37 °C. Mupirocin and crude elecampane extracts were used as positive controls, and sterile water as a negative control. Plates were prepared in triplicate and incubated for 24 h at 37 °C. Antimicrobial activity was determined by measuring zones of inhibition (mm; (mean) ± SD (standard deviation)) using calibrated Vernier callipers (Mitutoyo).
3.2.5. Solvent Tolerance Test
To assess the possibility of EtOH potentiating antimicrobial activity in the microdilution method, a separate test was used to confirm inactivity using a solvent tolerance assay as per [135] with modifications. Eleven strains of clinical S. aureus isolates, including methicillin-resistant S. aureus (MRSA), were used as the target organism. The EtOH concentration range increased in 1% increments (1–30% EtOH). A 10% addition of AlamarBlueTM was added to the wells before measuring absorbance.
3.3. Structural Investigation of Bioactive Fractions
3.3.1. Sample Preparation
Each bioactive fraction was reduced to dryness using a ZymarkTM TurboVap® LV Concentration Evaporator system. Nitrogen flow rate was set to 20 psi for 20 min and increased incrementally to 50 psi as volume reduced. Each dried residue in the test tube(s) was resolubilised in pure EtOH, sonicated and transferred to amber HPLC vial(s) (1 mL in total). The samples were reduced under a gentle flow of nitrogen again and stored at −20 °C until required for further analysis.
3.3.2. Standards and Reagents
External standards asperilin (purity > 95%) and isoalantolactone (purity > 90%) were isolated from flowers of Telekia speciosa (Schreb.) Baumg., as it was described earlier [119]. A mixture of alantolactone and isoalantolactone of lower purity, isolated from I. helenium roots, was also used for identification purposes. Water was purified by a Milli-Q system (Millipore Corp, Bedford, Massachusetts, USA). MeOH and acetonitrile (MeCN) of gradient grade for liquid chromatography were purchased from Merck (Darmstadt, Germany).
3.3.3. HPLC-DAD Analysis
Chromatographic separations were performed using Agilent 1200 Series HPLC system (Agilent Technologies, Palo Alto, CA, USA) equipped with a Rheodyne manual sample injector, quaternary pump, degasser, column oven and a diode array detector (DAD). Analytical separations were carried out using a Kinetex® 5 μm XB-C18 column (260 × 4.6 mm, 100 A pore size) from Phenomenex (Torrance, CA, USA), at 40 °C, with a gradient mode elution, as it was described elsewhere [119]. The mobile phase consisted of H2O (A) and MeCN (B). Linear gradient from 12% B to 15% B in 5 min, 25% B in 5 min, 60% B in 5 min, 98% B in another 10 min was applied (stop time: 35 min; post time: 12 min). Flow rate was established at 1 mL min−1. Alternatively, a Zorbax® Eclipse XDB-C18 column (150 × 4.6 mm, 5 μm) (Agilent, Santa Clara, CA, USA) with a mobile phase consisting of MeCN and H2O (11:9, v/v) was used, as it was proposed by Huo et al. [114]. As similar chromatographic resolutions of the analysed compounds were achieved by both methods, the gradient elution system was used exclusively. This method is routinely used at the Phytochemistry Department of the Maj Institute of Pharmacology at the Polish Academy of Sciences (PAS), for quantification of SLs in various extracts from plants of the Inuleae tribe.
Accurately weighted aliquots of the active samples were transferred into 1.5 mL Eppendorf tubes and dissolved in 1 mL of 70% MeOH and MeCN mixture (1:1, v/v). The solution was centrifuged (11,340 g, 5 min) prior to HPLC analysis, and injected (5 μL) into the column. The detection wavelength was set at 205 nm. Quantification of compounds 1–3 was done by an external standard method (ESM), as it was described earlier [119]. The content of the mixture under Peak 4 (Figure 2) was assessed semi-quantitatively, with an assumption that the signal is generated by eudesmanolides (See Supplementary Materials).
3.3.4. Semipreparative HPLC Separation
The active fractions were dissolved in 90% MeOH and injected into the Vertex Plus column (Eurospher II 100-5 C18, 250 × 8 mm) (Knauer, Berlin, Germany). The chromatographic separations were carried out in an isocratic mode (solvent flow rate 2 mL min−1) using MeCN: H2O (1:1, v/v) as the eluent. Fractions corresponding to the four major signals that showed absorption at 205 nm were collected.
3.3.5. 1H NMR Spectroscopy
Subfractions corresponding to the signals 1 and 4, obtained by semipreparative HPLC, were subjected to 1H NMR analysis. NMR spectra were recorded in CDCl3 on a Bruker AVANCE III HD 400 (Bruker Corp., Billerica, MA, USA) (resonance frequency 400.17 MHz). Chemical shifts (δ) and coupling constants for the signals visible in the NMR spectra were compared to those of igalan (Peak 1) and alloalantolactone (Peak 4) [136,137], identified earlier by Huo et al. [114]. See Supplementary Materials (Figures S1–S5). Canonical and isomeric SMILES (Simplified Molecular-Input Line-Entry System) were derived from PubChem and input to draw the chemical structures online using the PubChem sketcher tool (Version 2.4, NCBI, Rockville, MD, USA) (Figure 3).
4. Conclusions
The natural product compounds attributing to the anti-staphylococcal activity of a traditional hydro-ethanolic extract of the root of I. helenium L. (elecampane), previously observed within our laboratory, were investigated in this study. A novel clean-up strategy resulted in a subset of (bio)active fractions, the composition of which were later analysed using a HPLC-DAD method, supported by 1H NMR. Based on the study by Huo et al. [114], the compounds identified using HPLC were the eudesmanolides alantolactone (1) and isoalantolactone (2), as major constituents, and the elemanolide (3) igalan, plus an unseparated mixture of the eremophilanolide and eudesmanolide, dugesialactone (4) and alloalantolactone (5), respectively. Alloalantolactone (5) was later confirmed, following 1H NMR analysis (see Supplementary Materials). Furthermore, our findings suggest that the geographical origin of the plant did not appear to influence either the chemical profile or the bioactivity of the root extract.
Elecampane clearly demonstrates activity against Staphylococcus spp. Considering the prevalence of MRSA and occurrence of broad-spectrum/pan-resistance in Irish hospitals amongst this Genus, further investigation into the usage of the identified SLs compounds as potential candidates in the control of staphylococcal carriage and infection is warranted. Follow-on studies could include large-scale purification or synthesis of the identified compounds, followed by in vivo analysis of the compounds, individually and in combination, as well as combinatorial experimentation to explore the possibility of utilising these compounds as potentiator or adjuvant compounds, to enhance treatment with conventional antibiotic classes.
Supplementary Materials
Figure S1: 1H NMR spectrum of isoalantolactone (in CDCl3). Figure S2: 1H NMR spectrum of isoalantolactone/alantolactone mixture isolated from roots of Inula helenium L. (in CDCl3). Figure S3: 1H NMR spectrum of the fraction corresponding to the peak 4 (CDCl3, part A expanded). Signals derived from alloalantolactone (1-deoxyivangustin) are marked with asterisks. Figure S4: 1H NMR spectrum of the fraction corresponding to the peak 4 (CDCl3, part B expanded). Signals derived from alloalantolactone (1-deoxyivangustin) are marked with asterisks. Figure S5: 1H NMR spectrum of the fraction corresponding to the peak 1 (CDCl3, part A expanded). Figure S6: 1H NMR spectrum of the fraction corresponding to the peak 1 (CDCl3, part B expanded).
Author Contributions
Conceptualization, C.-R.K. and B.L.; investigation, C.-R.K. and A.S.; methodology, C.-R.K. and A.S.; data curation, C.-R.K. and A.S.; writing—original draft, C.-R.K.; writing—review & editing, A.S., B.L. and A.F.; supervision, B.L. and A.F.; resources, A.S., B.L. and A.F., funding acquisition; C.-R.K., B.L. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
C.-R.K. is in receipt of a RÍSAM postgraduate scholarship (2015), funded by Munster Technological University (MTU), formerly Cork Institute of Technology (CIT). The authors also acknowledge the funding by the Science Foundation Ireland (SFI) Research Infrastructure Call 2012 [Proposal #12/RI/2335 (2)], and the Ministry of Science and Higher Education Poland, statutory activity funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO) Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline, Including Tuberculosis. World Health Organization; Geneva, Switzerland: 2017. (WHO/EMP/IAU/2017.12); Licence: CC BY-NC-SA 2.0 IGO. [Google Scholar]
- 2.Schultes R.E. Amazonian Ethnobotany and the Search for New Drugs. Ciba Found. Symp. 1994;185:106–112. doi: 10.1002/9780470514634.ch8. [DOI] [PubMed] [Google Scholar]
- 3.Kenny C.R., Furey A., Lucey B. A Post-Antibiotic Era Looms: Can Plant Natural Product Research Fill the Void? Br. J. Biomed. Sci. 2015;72:191–200. doi: 10.1080/09674845.2015.11665752. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) Lack of New Antibiotics Threatens Global Efforts to Contain Drug-Resistant Infections. [(accessed on 2 November 2020)]. Available online: https://www.who.int/news/item/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections.
- 5.Abreu A.C., Coqueiro A., Sultan A.R., Lemmens N., Kim H.K., Verpoorte R., Van Wamel W.J.B., Simões M., Choi Y.H. Looking to Nature for a New Concept in Antimicrobial Treatments: Isoflavonoids from Cytisus Striatus as Antibiotic Adjuvants against MRSA. Sci. Rep. 2017;7:3777. doi: 10.1038/s41598-017-03716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S.C., Liu F., Zhu K., Shen J.Z. Natural Products That Target Virulence Factors in Antibiotic-Resistant Staphylococcus aureus. J. Agri. Food Chem. 2019;67:13195–13211. doi: 10.1021/acs.jafc.9b05595. [DOI] [PubMed] [Google Scholar]
- 7.Surh Y.J. Reverse Pharmacology Applicable for Botanical Drug Development-Inspiration from the Legacy of Traditional Wisdom. J. Tradit. Complement. Med. 2011;1:5–7. doi: 10.1016/S2225-4110(16)30051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seca A., Grigore A., Pinto D.C.G.A., Silva A.M.S. The Genus Inula and Their Metabolites: From Ethnopharmacological to Medicinal Uses. J. Ethnopharmacol. 2014;154:286–310. doi: 10.1016/j.jep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Clapp R.A., Crook C. Drowning in the Magic Well: Shaman Pharmaceuticals and the Elusive Value of Traditional Knowledge. J. Environ. Dev. 2002;11:79–102. doi: 10.1177/107049650201100104. [DOI] [Google Scholar]
- 10.Cameron J. The Gaelic Names of Plants (Scottish and Irish) 1st ed. William Blackwood & Sons; Edinburgh, UK: 1883. [Google Scholar]
- 11.Grieve M. A Modern Herbal: The Medicinal, Culinary, Cosmetic, and Economic Properties, Cultivation, and Folklore of Herbs, Grasses, Fungi, Shrubs, and Trees with All Their Modern Scientific Uses. Penguin; London, UK: 1996. p. 912. [Google Scholar]
- 12.Cuinn T.Ó. An Irish Materia Medica. [(accessed on 18 December 2021)]. Available online: https://celt.ucc.ie/published/G600005/text001.html.
- 13.Moloney M.F. Irish Ethno-Botany and the Evolution of Medicine in Ireland. M.H. Gill; Dublin, Ireland: 1919. [Google Scholar]
- 14.Koay A., Shannon F., Sasse A., Heinrich M., Sheridan H. Exploring the Irish National Folklore Ethnography Database (Dúchas) for Open Data Research on Traditional Medicine Use in Post-Famine Ireland: An Early Example of Citizen Science. Front. Pharmacol. 2020;11:1–16. doi: 10.3389/fphar.2020.584595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shannon F., Sasse A., Sheridan H., Heinrich M. Are Identities Oral? Understanding Ethnobotanical Knowledge after Irish Independence (1937–1939) J. Ethnobiol. Ethnomedicine. 2017;13:1–19. doi: 10.1186/s13002-017-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.British Herbal Medicines Association (BHMA) British Herbal Pharmacopoeia. Scientific Association; Bouremouth, UK: 1983. [Google Scholar]
- 17.Hoffmann D. Medical Herbalism: The Science and Practice of Herbal Medicine. 1st ed. Simon & Schuster; New York, NY, USA: 2003. [Google Scholar]
- 18.Blumenthal M. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. 1st ed. American Botanical Council (ABC); Austin, TX, USA: 1998. [Google Scholar]
- 19.Kim S.C., Hong K.T., Kim D.H. Contact Stomatitis from a Breath Refresher (Eudan) Contact Dermat. 1988;19:309. doi: 10.1111/j.1600-0536.1988.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 20.Aberer W., Hausen B.M. Active Sensitization to Elecampane by Patch Testing with a Crude Plant Extract. Contact Dermat. 1990;22:53–55. doi: 10.1111/j.1600-0536.1990.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 21.Pazzaglia M., Venturo N., Borda G., Tosti A. Contact Dermatitis Due to a Massage Liniment Containing Inula helenium Extract. Contact Dermat. 1995;33:267. doi: 10.1111/j.1600-0536.1995.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 22.Gil Mateo M.P., Velasco M., Miquel F.J., de la Cuadra J. Erythema-multiforme-like Eruption Following Allergic Contact Dermatitis from Sesquiterpene Lactones in Herbal Medicine. Contact Dermat. 1995;33:449–450. doi: 10.1111/j.1600-0536.1995.tb02100.x. [DOI] [PubMed] [Google Scholar]
- 23.Ulbrich M., Lorenz H., Rittenbach P., Al E. Inula Conyza as a Cause of Large-Scale Poisoning in Cattle. Mon. Veterinarmed. 1966;21:896–902. [PubMed] [Google Scholar]
- 24.Reinboth W. Vergiftungen Durch Inula Conzya (Dürrqurz) Bei Rindern. Mon. Veterinarmed. 1967;11:611–612. [PubMed] [Google Scholar]
- 25.Sertoli A., Fabbri P., Campolmi P., Al E. Allergic Contact Dermatitis to Salvia Officinalus, Inula Viscosa and Conyza Bonariensis. Contact Dermat. 1978;4:314–315. doi: 10.1111/j.1600-0536.1978.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 26.Pinedo J.M., de Canales F.G., Hinojosa J.L., Llamas P., Hausen B.M. Contact Dermatitis to Sesquiterpene Lactones in Inula Viscose Aiton. Contact Dermat. 1987;17:322–323. doi: 10.1111/j.1600-0536.1987.tb01492.x. [DOI] [PubMed] [Google Scholar]
- 27.Schneider D.J., du Plessis J.L. Enteritis in Sheep Due to the Ingestion of Inula graveolens Desf. (Cape Khakiweed) J. S. Afr. Vet. Assoc. 1980;51:159–161. [PubMed] [Google Scholar]
- 28.Paulsen E. Contact Sensitization from Compositae-Containing Herbal Remedies and Cosmetics. Contact Dermat. 2002;47:189–198. doi: 10.1034/j.1600-0536.2002.470401.x. [DOI] [PubMed] [Google Scholar]
- 29.Seca A., Pinto D.C., Silva A.M. Metabolomic Profile of the Genus Inula. Chem. Biodivers. 2015;12:859–906. doi: 10.1002/cbdv.201400080. [DOI] [PubMed] [Google Scholar]
- 30.Paulsen E. Systemic Allergic Dermatitis Caused by Sesquiterpene Lactones. Contact Dermat. 2017;76:1–10. doi: 10.1111/cod.12671. [DOI] [PubMed] [Google Scholar]
- 31.Amorim M.H.R., Gil Da Costa R.M., Lopes C., Bastos M.M.S.M. Sesquiterpene Lactones: Adverse Health Effects and Toxicity Mechanisms. Crit. Rev. Toxicol. 2013;43:559–579. doi: 10.3109/10408444.2013.813905. [DOI] [PubMed] [Google Scholar]
- 32.Carnevali I., la Paglia R., Pauletto L., Raso F., Testa M., Mannucci C., Sorbara E.E., Calapai G. Efficacy and Safety of the Syrup “KalobaTUSS®” as a Treatment for Cough in Children: A Randomized, Double Blind, Placebo-Controlled Clinical Trial. BMC Pediatr. 2021;21:1–9. doi: 10.1186/s12887-020-02490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alonso Blasi N., Fraginals R., Lepoittevin J.P., Benezra C. A Murine in vitro Model of Allergic Contact Dermatitis to Sesquiterpene α-Methylene-γ-Butyrolactones. Arch. Dermatol. 1992;284:297–302. doi: 10.1007/BF00372584. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell J.C., Fritig B., Singh B., Towers G.H.N. Allergic Contact Dermatitis from Frullania and Compositae: The Role of Sesquiterpene Lactones. J. Investig. Dermatol. 1970;54:233–239. doi: 10.1111/1523-1747.ep12280310. [DOI] [PubMed] [Google Scholar]
- 35.Hausen B.M., Vieluf I.K. Allergiepflanzen Handbuch und Atlas. 2nd ed. Nikol Verlag; Hamburg, Germany: 1997. [Google Scholar]
- 36.Wang Q., Gao S., Wu G.-Z., Yang N., Zu X.-P., Li W.-C., Xie N., Zhang R.-R., Li C.-W., Hu Z.-L., et al. Total Sesquiterpene Lactones Isolated from Inula helenium L. Attenuates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-like Skin Lesions in Mice. Phytomedicine. 2018;46:78–84. doi: 10.1016/j.phymed.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 37.Lee B.K., Park S.J., Nam S.Y., Kang S., Hwang J., Lee S.J., Im D.S. Anti-Allergic Effects of Sesquiterpene Lactones from Saussurea Costus (Falc.) Lipsch. Determined Using In Vivo and In Vitro Experiments. J. Ethnopharmacol. 2018;213:256–261. doi: 10.1016/j.jep.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Dorn D., Alexenizer M., Hengstler J., Dorn A. Tumor Cell Specific Toxicity of Inula helenium Extracts. Phytother. Res. 2006;20:970–980. doi: 10.1002/ptr.1991. [DOI] [PubMed] [Google Scholar]
- 39.Koc K., Ozdemir O., Ozdemir A., Dogru U., Turkez H. Antioxidant and Anticancer Activities of Extract of Inula helenium (L.) in Human U-87 MG Glioblastoma Cell Line. J. Cancer Res. Ther. 2018;14:658–661. doi: 10.4103/0973-1482.187289. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B., Zeng J., Yan Y., Yang B., Huang M., Wang L., Zhang Q., Lin N. Ethyl Acetate Extract from Inula helenium L. Inhibits the Proliferation of Pancreatic Cancer Cells by Regulating the STAT3/AKT Pathway. Mol. Med. Rep. 2018;17:5440–5448. doi: 10.3892/mmr.2018.8534. [DOI] [PubMed] [Google Scholar]
- 41.Chun J., Song K., Kim Y.S. Sesquiterpene Lactones-Enriched Fraction of Inula helenium L. Induces Apoptosis through Inhibition of Signal Transducers and Activators of Transcription 3 Signaling Pathway in MDA-MB-231 Breast Cancer Cells. Phytother. Res. 2018;32:2501–2509. doi: 10.1002/ptr.6189. [DOI] [PubMed] [Google Scholar]
- 42.Park E., Kim Y., Park S., Kim H., Lee J., Lee D., Chang K. Induction of HO-1 Through P38 MAPK/Nrf2 Signaling Pathway by Ethanol Extract of Inula helenium L. Reduces Inflammation in LPS-Activated RAW 264.7 Cells and CLP-Induced Septic Mice. Food Chem. Toxicol. 2013;55:386–395. doi: 10.1016/j.fct.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Mazzio E.A., Li N., Bauer D., Mendonca P., Taka E., Darb M., Thomas L., Williams H., Soliman K.F.A. Natural Product HTP Screening for Antibacterial (E. coli 0157:H7) and Anti-Inflammatory Agents in (LPS from E. coli O111:B4) Activated Macrophages and Microglial Cells, Focus on Sepsis. BMC Complementary Altern. Med. 2016;16:467. doi: 10.1186/s12906-016-1429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao S., Wang Q., Tian X.-H., Li H.-L., Shen Y.-H., Xu X.-K., Wu G.-Z., Hu Z.-L., Zhang W.-D. Total Sesquiterpene Lactones Prepared from Inula helenium L. Has Potentials in Prevention and Therapy of Rheumatoid Arthritis. J. Ethnopharmacol. 2017;196:39–46. doi: 10.1016/j.jep.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Gierlikowska B., Gierlikowski W., Bekier K., Skalicka-Woźniak K., Czerwińska M.E., Kiss A.K. Inula helenium and Grindelia squarrosa as a Source of Compounds with Anti-Inflammatory Activity in Human Neutrophils and Cultured Human Respiratory Epithelium. J. Ethnopharmacol. 2020;249:112311. doi: 10.1016/j.jep.2019.112311. [DOI] [PubMed] [Google Scholar]
- 46.Dirsch V., Stuppner H., Vollmar A. Cytotoxic Sesquiterpene Lactones Mediate Their Death-Inducing Effect in Leukemia T Cells by Triggering Apoptosis. Planta Med. 2001;67:557–559. doi: 10.1055/s-2001-16478. [DOI] [PubMed] [Google Scholar]
- 47.Xu X., Huang L., Zhang Z., Tong J., Mi J., Wu Y., Zhang C., Yan H. Targeting Non-Oncogene ROS Pathway by Alantolactone in B Cell Acute Lymphoblastic Leukemia Cells. Life Sci. 2019;227:153–165. doi: 10.1016/j.lfs.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 48.Yang C., Yang J., Sun M., Yan J., Meng X., Ma T. Alantolactone Inhibits Growth of K562/Adriamycin Cells by Downregulating Bcr/Abl and P-Glycoprotein Expression. IUBMB Life. 2013;65:435–444. doi: 10.1002/iub.1141. [DOI] [PubMed] [Google Scholar]
- 49.He W., Cao P., Xia Y., Hong L., Zhang T., Shen X., Zheng P., Shen H., Zhao Y., Zou P. Potent Inhibition of Gastric Cancer Cells by a Natural Compound via Inhibiting TrxR1 Activity and Activating ROS-Mediated P38 MAPK Pathway. Free Radic. Res. 2019;53:104–114. doi: 10.1080/10715762.2018.1558448. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y., Bao Y.L., Wu Y., Yu C.L., Huang Y.X., Sun Y., Zheng L.H., Li Y.X. Alantolactone Inhibits Cell Proliferation by Interrupting the Interaction between Cripto-1 and Activin Receptor Type II A in Activin Signaling Pathway. J. Biomol. Screen. 2011;16:525–535. doi: 10.1177/1087057111398486. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y.U., Bao Y.L.I., Wu Y.I.N., Yu C.L.E.I. Alantolactone Induces Apoptosis in RKO Cells through the Generation of Reactive Oxygen Species and the Mitochondrial Pathway. Mol. Med. Rep. 2013;8:967–972. doi: 10.3892/mmr.2013.1640. [DOI] [PubMed] [Google Scholar]
- 52.Lei J.C., Yu J.Q., Yin Y., Liu Y.W., Zou G.L. Alantolactone Induces Activation of Apoptosis in Human Hepatoma Cells. Food Chem. Tox. 2012;50:3313–3319. doi: 10.1016/j.fct.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Kang X., Wang H., Li Y., Xiao Y., Zhao L., Zhang T., Zhou S., Zhou X., Yi L., Zhexing S., et al. Alantolactone Induces Apoptosis Through ROS-Mediated AKT Pathway and Inhibition of PINK1-Mediated Mitophagy in Human HepG2 Cells. Artific. Cells Nanomed. Biotechnol. 2019;47:1961–1970. doi: 10.1080/21691401.2019.1593854. [DOI] [PubMed] [Google Scholar]
- 54.Maryam A., Mehmood T., Zhang H., Li Y., Khan M., Ma T. Alantolactone Induces Apoptosis, Promotes STAT3 Glutathionylation and Enhances Chemosensitivity of A549 Lung Adenocarcinoma Cells to Doxorubicin via Oxidative Stress. Sci. Rep. 2017;7:6242. doi: 10.1038/s41598-017-06535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Zhang Y., Liu X., Wang J., Li B., Liu Y., Wang J. Alantolactone Enhances Gemcitabine Sensitivity of Lung Cancer Cells through the Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress and Akt/GSK3β Pathway. Int. J. Mol. Med. 2019;44:1026–1038. doi: 10.3892/ijmm.2019.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J., Yang Z., Kong Y., He Y., Xu Y., Cao X. Antitumor Activity of Alantolactone in Lung Cancer Cell Lines NCI-H1299 and Anip973. J. Food Biochem. 2019;43:e12972. doi: 10.1111/jfbc.12972. [DOI] [PubMed] [Google Scholar]
- 57.He R., Shi X., Zhou M., Zhao Y., Pan S., Zhao C., Guo X., Wang M., Li X., Qin R. Alantolactone Induces Apoptosis and Improves Chemosensitivity of Pancreatic Cancer Cells by Impairment of Autophagy-Lysosome Pathway via Targeting TFEB. Toxicol. Appl. Pharm. 2018;356:159–171. doi: 10.1016/j.taap.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Cui L., Bu W., Song J., Feng L., Xu T., Liu D., Ding W., Wang J., Li C., Ma C., et al. Apoptosis Induction by Alantolactone in Breast Cancer MDA-MB-231 Cells Through Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway. Arch. Pharm. Res. 2018;41:299–313. doi: 10.1007/s12272-017-0990-2. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y., Cai Q., Gao Y., Luan X., Guan Y., Lu Q., Sun P., Zhao M., Fang C. Alantolactone, a Sesquiterpene Lactone, Inhibits Breast Cancer Growth by Antiangiogenic Activity via Blocking VEGFR2 Signaling. Phytother. Res. 2018;32:643–650. doi: 10.1002/ptr.6004. [DOI] [PubMed] [Google Scholar]
- 60.Dirsch V.M., Stuppner H., Ellmerer-Müller E.P., Vollmar A.M. Structural Requirements of Sesquiterpene Lactones to Inhibit LPS-Induced Nitric Oxide Synthesis in RAW 2647. Macrophages. Bioorg. Med. Chem. 2000;8:2747–2753. doi: 10.1016/S0968-0896(00)00202-9. [DOI] [PubMed] [Google Scholar]
- 61.Lawrence N.J., McGown A.T., Nduka J., Hadfield J.A., Pritchard R.G. Cytotoxic Michael-Type Amine Adducts of α-Methylene Lactones Alantolactone and Isoalantolactone. Bioorg. Med. Chem. Lett. 2001;11:429–431. doi: 10.1016/S0960-894X(00)00686-7. [DOI] [PubMed] [Google Scholar]
- 62.Wu M., Zhang H., Hu J., Weng Z., Li C., Li H., Zhao Y., Mei X., Ren F., Li L. Isoalantolactone Inhibits UM-SCC-10A Cell Growth via Cell Cycle Arrest and Apoptosis Induction. PLoS ONE. 2013;8:2–9. doi: 10.1371/journal.pone.0076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Z., Zhang G., Zhang Y., Al E. Isoalantolactone Induces Apoptosis through Reactive Oxygen Species-Dependent Upregulation of Death Receptor 5 in Human Esophageal Cancer Cells. Toxicol. Appl. Pharmacol. 2018;352:46–58. doi: 10.1016/j.taap.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 64.Cai H., Meng X., Li Y., Yang C., Liu Y. Growth Inhibition Effects of Isoalantolactone on K562/A02 Cells: Caspase-Dependent Apoptotic Pathways, S Phase Arrest, and Downregulation of Bcr/Abl. Phytother. Res. 2014;28:1679–1686. doi: 10.1002/ptr.5182. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Cui L., Feng L., Zhang Z., Song J., Liu D., Jia X. Isoalantolactone Inhibits the Migration and Invasion of Human Breast Cancer MDA-MB-231 Cells via Suppression of the P38 MAPK/NF-ΚB Signaling Pathway. Oncol. Rep. 2016;36:1269–1276. doi: 10.3892/or.2016.4954. [DOI] [PubMed] [Google Scholar]
- 66.Hehner S.P., Heinrich M., Bork P.M., Vogt M., Ratter F., Lehmann V., Schulze-Osthoff K., Dröge W., Schmitz M.L. Sesquiterpene Lactones Specifically Inhibit Activation of NF-ΚB by Preventing the Degradation of IκB-α and IκB-β. J. Biolog. Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 67.He G., Zhang X., Chen Y., Chen J., Li L., Xie Y. Isoalantolactone Inhibits LPS-Induced Inflammation via NF-ΚB Inactivation in Peritoneal Macrophages and Improves Survival in Sepsis. Biomed. Pharmacother. 2017;90:598–607. doi: 10.1016/j.biopha.2017.03.095. [DOI] [PubMed] [Google Scholar]
- 68.Ding Y., Song Y.D., Wu Y.X., He H.Q., Yu T.H., Al E. Isoalantolactone Suppresses LPS-Induced Inflammation by Inhibiting TRAF6 Ubiquitination and Alleviates Acute Lung Injury. Acta Pharmacol. Sin. 2019;40:64–74. doi: 10.1038/s41401-018-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu J., Luo M., Wang J., Dong J., Li H., Leng B., Zhang Q., Dai X., Zhang Y., Niu X., et al. Isoalantolactone Protects against Staphylococcus aureus Pneumonia. FEMS Microbiol. Lett. 2011;324:147–155. doi: 10.1111/j.1574-6968.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- 70.Yan Y.Y., Zhang Q., Zhang B., Yang B., Lin N.M. Active Ingredients of Inula helenium L. Exhibits Similar Anti-Cancer Effects as Isoalantolactone in Pancreatic Cancer Cells. Nat. Prod. Red. 2020;34:2539–2544. doi: 10.1080/14786419.2018.1543676. [DOI] [PubMed] [Google Scholar]
- 71.Seo J.Y., Lim S.S., Kim J.R., Lim J.-S., Ha Y.R., Lee I.A., Kim E.J., Park J.H.Y., Kim J.-S. Nrf2-Mediated Induction of Detoxifying Enzymes by Alantolactone Present in Inula helenium. Phytother. Res. 2008;22:1500–1505. doi: 10.1002/ptr.2521. [DOI] [PubMed] [Google Scholar]
- 72.Seo J.Y., Park J., Kim H.J., Lee I.A., Lim J.S., Lim S.S., Choi S.J., Park J.H.Y., Kang H.J., Kim J.S. Isoalantolactone from Inula helenium Caused Nrf2-Mediated Induction of Detoxifying Enzymes. J. Med. Food. 2009;12:1038–1045. doi: 10.1089/jmf.2009.0072. [DOI] [PubMed] [Google Scholar]
- 73.Lim S.S., Im S.S., Kim J.R., Lim H.A., Jang C.H., Kim Y.K., Konishi T., Kim E.J., Park J.H.Y., Kim J.-S. Induction of Detoxifying Enzyme by Sesquiterpenes Present in Inula helenium. J. Med. Food. 2007;10:503–510. doi: 10.1089/jmf.2006.209. [DOI] [PubMed] [Google Scholar]
- 74.Ding Y., Pan W., Xu J., Wang T., Chen T., Liu Z., Xie C., Zhang Q. Sesquiterpenoids from the Roots of Inula helenium Inhibit Acute Myelogenous Leukemia Progenitor Cells. Bioorg. Chem. 2019;86:363–367. doi: 10.1016/j.bioorg.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 75.Lee K., Shin J., Chun J., Song K., Nho C., Kim Y. Igalan Induces Detoxifying Enzymes Mediated by the Nrf2 Pathway in HepG2 Cells. J. Biochem. Mol. Toxicol. 2019;33:e22297. doi: 10.1002/jbt.22297. [DOI] [PubMed] [Google Scholar]
- 76.Dao T.T.P., Song K., Kim J.Y., Kim Y.S. Igalan from Inula helenium (L.) Suppresses the Atopic Dermatitis-like Response in Stimulated HaCaT Keratinocytes via JAK/STAT3 Signaling. Inflamm. Res. 2020;69:309–319. doi: 10.1007/s00011-020-01322-4. [DOI] [PubMed] [Google Scholar]
- 77.Cai H., Li L., Jiang J., Zhao C., Yang C. Costunolide Enhances Sensitivity of K562/ADR Chronic Myeloid Leukemia Cells to Doxorubicin through PI3K/Akt Pathway. Phytother. Res. 2019;33:1683–1688. doi: 10.1002/ptr.6355. [DOI] [PubMed] [Google Scholar]
- 78.Peng S., Hou Y., Yao J., Fang J. Activation of Nrf2 by Costunolide Provides Neuroprotective Effect in PC12 Cells. Food Funct. 2019;10:4143–4152. doi: 10.1039/C8FO02249F. [DOI] [PubMed] [Google Scholar]
- 79.Chen C.-N., Huang H.-H., Wu C.-L., Lin C.P.C., Hsu J.T.A., Hsieh H.-P., Chuang S.-E., Lai G.-M. Isocostunolide, a Sesquiterpene Lactone, Induces Mitochondrial Membrane Depolarization and Caspase-Dependent Apoptosis in Human Melanoma Cells. Cancer Lett. 2007;246:237–252. doi: 10.1016/j.canlet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Olechnowicz-Stepien W., Skurska H. Studies on Antibiotic Properties of Roots of Inula helenium, Compositae. Arch. Immunol. Ther. Exp. 1960;8:179–189. [PubMed] [Google Scholar]
- 81.Von Gizycki F. Alantopicrin, a Bitter Principle from Elecampane Leaves; Contribution to the Composite Bitter Principles. Arch. Pharm. Ber. Dtsch. Pharm. Ges. 1954;287:57–62. doi: 10.1002/ardp.19542870202. [DOI] [PubMed] [Google Scholar]
- 82.Yosioka I., Yamada Y. Isolation of Dammaradienyl acetate from Inula helenium L. J. Pharm. Soc. Jpn. 1963;83:801–802. doi: 10.1248/yakushi1947.83.8_801. [DOI] [PubMed] [Google Scholar]
- 83.Cantrell C.L., Fischer N.H., Urbatsch L., McGuire M.S., Franzblau S.G. Antimycobacterial Crude Plant Extracts from South, Central, and North America. Phytomed. Int. J. Phytother. Phytopharm. 1998;5:137–145. doi: 10.1016/S0944-7113(98)80011-1. [DOI] [PubMed] [Google Scholar]
- 84.Cantrell C.L., Abate L., Fronczek F.R., Franzblau S.G., Quijano L., Fischer N.H. Antimycobacterial Eudesmanolides from Inula helenium and Rudbeckia subtomentosa. Planta Med. 1999;65:351–355. doi: 10.1055/s-1999-14001. [DOI] [PubMed] [Google Scholar]
- 85.Stojakowska A., Kędzia B., Kisiel W. Antimicrobial Activity of 10-Isobutyryloxy-8,9-Epoxythymol Isobutyrate. Fitoterapia. 2005;76:687–690. doi: 10.1016/j.fitote.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 86.O’Shea S., Lucey B., Cotter L. In Vitro Activity of Inula Helenium against Clinical Staphylococcus aureus Strains Including MRSA. Br. J. Biomed. Sci. 2009;66:186–189. doi: 10.1080/09674845.2009.11730271. [DOI] [PubMed] [Google Scholar]
- 87.Deriu A., Zanetti S., Sechi L.A., Marongiu B., Piras A., Porcedda S., Tuveri E. Antimicrobial Activity of Inula helenium L. Essential Oil against Gram-Positive and Gram-Negative Bacteria and Candida Spp. Int. J. Antimicrob. Agents. 2008;31:588–590. doi: 10.1016/j.ijantimicag.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 88.Bourrel C., Vilarem G., Perineau F. Chemical analysis, bacteriostatic and fungistatic properties of the essential oil of elecampane (Inula helenium L.) J. Essent. Oil Res. 1993;5:411–417. doi: 10.1080/10412905.1993.9698251. [DOI] [Google Scholar]
- 89.Stojanović-Radić Z., Comić L., Radulović N., Blagojević P., Denić M., Miltojević A., Rajković J., Mihajilov-Krstev T. Antistaphylococcal Activity of Inula helenium L. Root Essential Oil: Eudesmane Sesquiterpene Lactones Induce Cell Membrane Damage. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:1015–1025. doi: 10.1007/s10096-011-1400-1. [DOI] [PubMed] [Google Scholar]
- 90.Blagojević P.D., Radulović N.S. Conformational Analysis of Antistaphylococcal Sesquiterpene Lactones from Inula helenium Essential Oil. Nat. Prod. Commun. 2012;7:1407–1410. doi: 10.1177/1934578X1200701101. [DOI] [PubMed] [Google Scholar]
- 91.Gökbulut A., Ozhan O., Satilmiş B., Batçioğlu K., Günal S., Sarer E. Antioxidant and Antimicrobial Activities, and Phenolic Compounds of Selected Inula Species from Turkey. Nat. Prod. Comm. 2013;8:475–478. doi: 10.1177/1934578X1300800417. [DOI] [PubMed] [Google Scholar]
- 92.Radulović N.S., Denić M.S., Stojanović-Radić Z.Z. Synthesis of Small Combinatorial Libraries of Natural Products: Identification and Quantification of New Long-Chain 3-Methyl-2-Alkanones from the Root Essential Oil of Inula helenium L. (Asteraceae) Phytochem. Anal. 2014;25:75–80. doi: 10.1002/pca.2466. [DOI] [PubMed] [Google Scholar]
- 93.Department of Health Ireland’s National Action Plan on Antimicrobial Resistance 2017–2020. (INAP) [(accessed on 29 December 2021)]; Available online: https://assets.gov.ie/9519/afcba9bce7c54bf9bcbe9a74f49fdaf2.pdf.
- 94.Gerhardt C. Chemische Untersuchungen Über Das Helenin. Ann. Phar. 1840;34:192–204. doi: 10.1002/jlac.18400340206. [DOI] [Google Scholar]
- 95.Ruzicka L., Pieth P., Reichstein T., Ehmann L. Polyterpene Und Polyterpenoide LXXX. Zur Kenntnis Der Alantolactone. Synthese Des 1,4-Dimethyl-6-Isopropyl- Und Des 1,5-Dimethyl-7-Isopropyl-Naphtalins. Helv. Chim. 1933;16:268–275. doi: 10.1002/hlca.19330160137. [DOI] [Google Scholar]
- 96.Tsuda K., Tanabe K., Funakoshi K. On the Structure of Alantolactone. J. Am. Chem. Soc. 1957;79:1009–1010. doi: 10.1021/ja01561a074. [DOI] [Google Scholar]
- 97.Asselineau C., Bory S. The Separation and Structure of Alantolactone and Isoalantolactone. Comp. Rend. 1958;246:1874–1877. [Google Scholar]
- 98.Marshall J.A., Cohen N. The Structure of Alantolactone. J. Org. Chem. 1964;29:3727–3729. doi: 10.1021/jo01035a527. [DOI] [Google Scholar]
- 99.Kallen J. Ueber Helenin Und Alantkampher. Ber. Dtsch. Chem. Ges. 1876;9:154–157. doi: 10.1002/cber.18760090148. [DOI] [Google Scholar]
- 100.Ruzicka L., van Melsen J.A. Höhere Terpenverbindungen XLV. Zur Kenntnis Des Alantolactons Und Des Iso-Alantolactons. Helv. Chim. 1931;14:397–410. doi: 10.1002/hlca.19310140136. [DOI] [Google Scholar]
- 101.Wunderlich W. Isoalantolacton-Reindarstellung. J. Prakt. Chem. 1959;9:107. doi: 10.1002/prac.19590090304. [DOI] [Google Scholar]
- 102.Hansen K.F.W. Über Die Bitterstoffe Der Alantwurzel (II. Mitteilung Über Bitterstoffe) Ber. Dtsch. Chem. Ges. (A B Ser.) 1931;64:943–947. doi: 10.1002/cber.19310640432. [DOI] [Google Scholar]
- 103.Hansen K.F.W. Über Bitterstoffe Aus Der Alantwurzel (Vorläufige Mitteilung) Eur. J. Inorg. Chem. 1931;64:67–71. doi: 10.1002/cber.19310640111. [DOI] [Google Scholar]
- 104.Kerimov S.S., Chizhov O.S. Sesquiterpene Lactones of Inula helenium. Chem. Nat. 1974;10:267. doi: 10.1007/BF00563642. [DOI] [Google Scholar]
- 105.Konishi T., Shimada Y., Nagao T., Okabe H., Konoshima T. Antiproliferative Sesquiterpene Lactones from the Roots of Inula helenium. Biol. Pharm. Bull. 2002;25:1370–1372. doi: 10.1248/bpb.25.1370. [DOI] [PubMed] [Google Scholar]
- 106.Rosik G.G., Kotov A.G., Beskorovainyi A.A., Al E. Vapor-Phase Hydrogenation in the GLC Analysis of Sesquiterpene Lactones of the Eudesmane Series. Chem. Nat. 1991;2:703–706. doi: 10.1007/BF00629930. [DOI] [Google Scholar]
- 107.Bohlmann F., Mahanta P.K., Jakupovic J., Rastogi R.C., Natu A.A. New Sesquiterpene Lactones from Inula Species. Phytochemistry. 1978;17:1165–1172. doi: 10.1016/S0031-9422(00)94308-5. [DOI] [Google Scholar]
- 108.Jiang H.L., Chen J., Jin X.J., Yang J.L., Li Y., Yao X.J., Wu Q.X. Sesquiterpenoids, Alantolactone Analogues, and Seco-Guaiene from the Roots of Inula helenium. Tetrahedron. 2011;67:9193–9198. doi: 10.1016/j.tet.2011.09.070. [DOI] [Google Scholar]
- 109.Huo Y., Shi H., Wang M., Li X. Complete Assignments Of 1H And 13C NMR Spectral Data for Three Sesquiterpenoids from Inula helenium. Magn. Reson. Chem. 2008;46:1208–1211. doi: 10.1002/mrc.2340. [DOI] [PubMed] [Google Scholar]
- 110.Zhao Y.M., Wang Y.J., Dong M., Zhang M.L., Huo C.H., Gu Y.C., Shi Q.W. Two New Eudesmanes from Inula helenium. Chem. Nat. 2010;46:373–376. doi: 10.1007/s10600-010-9620-7. [DOI] [Google Scholar]
- 111.Huo Y., Shi H., Guo C., Li X. Chemical Constituents of the Roots of Inula helenium. Chem. Nat. 2012;48:522–524. doi: 10.1007/s10600-012-0298-x. [DOI] [Google Scholar]
- 112.Ma X.C., Liu K.X., Zhang B.J., Xin X.L., Huang J. Structural Determination of Three New Eudesmanolides from Inula helenium. Magn. Reson. Chem. 2008;46:1084–1088. doi: 10.1002/mrc.2297. [DOI] [PubMed] [Google Scholar]
- 113.Zaima K., Wakana D., Demizu Y., Kumeta Y., Kamakura H., Maruyama T., Kurihara M., Goda Y. Isoheleproline: A New Amino Acid-Sesquiterpene Adduct from Inula helenium. J. Nat. Med. 2014;68:432–435. doi: 10.1007/s11418-013-0806-8. [DOI] [PubMed] [Google Scholar]
- 114.Huo Y., Shi H., Li W., Wang M., Li X. HPLC Determination and NMR Structural Elucidation of Sesquiterpene Lactones in Inula helenium. J. Pharm. Biomed. Anal. 2010;51:942–946. doi: 10.1016/j.jpba.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 115.Zheng L.H., Hao X.J., Yuan C.M., Huang L.J., Al E. Study on Chemical Constituents of Inula Cappa. China J. Chin. Mater. Med. 2015;40:672–678. [PubMed] [Google Scholar]
- 116.Ding L.F., Wang K., Wang H.Y., Tu W.C., Al E. Chemical Constituents of Inula Japonica. Zhong Yao Cai J. Chin. Med. Mater. 2016;39:1296–1299. [PubMed] [Google Scholar]
- 117.Hua Y., Qin J., Zhang F., Cheng X., Jin H., Zhang W. Sesquiterpene Lactones from Inula Helianthus-Aquatica. China J. Chin. Mater. Med. 2012;37:1586–1589. doi: 10.4268/cjcmm20121116. [DOI] [PubMed] [Google Scholar]
- 118.Guo Q.L., Yang J.S., Liu J.X. Studies on the Chemical Constituents from Inula Cappa (II) J. Chin. Med. Mater. 2007;30:35–37. [PubMed] [Google Scholar]
- 119.Stojakowska A., Malarz J., Kisiel W. Quantitative Analysis of Sesquiterpene Lactones and Thymol Derivatives in Extracts from Telekia Speciosa. Phytochem. Lett. 2015;11:378–383. doi: 10.1016/j.phytol.2014.10.025. [DOI] [Google Scholar]
- 120.Tavares W.R., Seca A.M.L. Inula L. Secondary Metabolites Against Oxidative Stress-Related Human Diseases. Antioxidants. 2019;8:122. doi: 10.3390/antiox8050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang G.-W., Qin J.-J., Cheng X.-R., Shen Y.-H., Shan L., Jin H.-Z., Zhang W.-D. Inula Sesquiterpenoids: Structural Diversity, Cytotoxicity and Anti-Tumor Activity. Expert Opin. Investig. Drugs. 2014;23:317–345. doi: 10.1517/13543784.2014.868882. [DOI] [PubMed] [Google Scholar]
- 122.Schmid I., Sattler I., Grabley S., Thiericke R. Natural Products in High Throughput Screening: Automated High-Quality Sample Preparation. J. Biomol. Screen. 1999;4:15–25. doi: 10.1177/108705719900400104. [DOI] [PubMed] [Google Scholar]
- 123.Wilson B.A.P., Thornburg C.C., Henrich C.J., Grkovic T., O’Keefe B.R. Creating and Screening Natural Product Libraries. Nat. Prod. Rep. 2020;37:893–918. doi: 10.1039/C9NP00068B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cowan M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Petkova N., Ognyanov M., Denev P. Challenges in Chemistry. Volume 39. University of Plovdiv “Paisii Hilendarski”; Plovdiv, Bulgaria: 2014. Isolation and Characterisation of Inulin from Taproots of Common Chicory (Cichorium intybus L.) pp. 25–34. Scientific Papers. [Google Scholar]
- 126.European Committe on Antimicrobial Susceptibility Testing (EUCAST) Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Infect. 2003;9:1–7. [Google Scholar]
- 127.Trendafilova A., Chanev C., Todorova M. Ultrasound-Assisted Extraction of Alantolactone and Isoalantolactone from Inula helenium Roots. Pharmacogn. Mag. 2010;6:234–237. doi: 10.4103/0973-1296.66942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao Y.M., Wang J., Liu H.B., Guo C.Y., Zhang W.M. Microwave-Assisted Extraction of Alantolactone and Isoalantolactone from Inula helenium. Indian J. Pharm. Sci. 2015;77:116–120. doi: 10.4103/0250-474x.151594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Camp J.E., Nyamini S.B., Scott F.J. CyreneTM Is a Green Alternative to DMSO as a Solvent for Antibacterial Drug Discovery against ESKAPE Pathogens. RSC Med. Chem. 2020;11:111–117. doi: 10.1039/C9MD00341J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klančnik A., Piskernik S., Jeršek B., Možina S.S. Evaluation of Diffusion and Dilution Methods to Determine the Antibacterial Activity of Plant Extracts. J. Microbiol. Methods. 2010;81:121–126. doi: 10.1016/j.mimet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 131.Rasko D.A., Sperandio V. Anti-Virulence Strategies to Combat Bacteria-Mediated Disease. Nat. Rev. Drug Discov. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 132.Yoon J.-H., Park H.-Y., Kim T.-J., Lee S.-H., Kim J.-H., Lee G.-S., Kim H.-J., Ju Y.-S. The Identification of Aucklandiae Radix, Inulae Radix, Vladimiriae Radix and Aristolochiae Radix, Using Macroscopic, Microscopic and Physicochemical Methods. J. Korean Med. Sci. 2014;35:83–97. doi: 10.13048/jkm.14046. [DOI] [Google Scholar]
- 133.Kearns A.M., Ganner M., Holmes A. The “Oxford Staphylococcus”: A Note of Caution. J. Antimicrob. Chemother. 2006;58:480–481. doi: 10.1093/jac/dkl230. [DOI] [PubMed] [Google Scholar]
- 134.European Committee on Antimicrobial Susceptibility Testing (EUCAST) Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method. ESCMID. 2020;8:1–21. [Google Scholar]
- 135.Pando J., Pfeltz R., Cuaron J., Nagarajan V., Mishra M., Torres N., Elasri M., Wilkinson B., Gustafson J. Ethanol-Induced Stress Response of Staphylococcus aureus. Can. J. Microbiol. 2017;63:745–757. doi: 10.1139/cjm-2017-0221. [DOI] [PubMed] [Google Scholar]
- 136.Dusmatova D.E., Terent’eva E.O., Bobakulov K.M., Mukhamatkhanova R.F., Tsai E.A., Tashkhodzhaev B., Sham’yanov I.D., Azimova S.S., Abdullaev N.D. Nonpolar Constituents of Inula Grandis Roots. Cytotoxic Activity of Igalan. Chem. Nat. Compd. 2019;55:571–574. doi: 10.1007/s10600-019-02747-y. [DOI] [Google Scholar]
- 137.Marco J.A., Arno M., Carda M. Synthesis of Yomogin, 1-Deoxyivangustin, and 1-Deoxy-8-Epiivangustin. Can. J. Chem. 1987;65:630–635. doi: 10.1139/v87-108. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are contained within this article.