Abstract
Background
This case reviews the cardiac involvement of myotonic dystrophy type 2 in terms of ventricular arrhythmias (VAs) and individual myocardial scar formation as target for catheter ablation.
Case summary
A 62-year-old woman with myotonic dystrophy type 2 and a severely reduced left ventricular ejection fraction (25%) presented with recurrent episodes of VAs and consecutive implantable cardioverter-defibrillator therapies. The patient already underwent two VA ablation attempts focusing on an ischaemia-related arrhythmia substrate in the left ventricle. The patient was scheduled for repeat ablation after the progression of coronary artery disease was ruled out. Interestingly bipolar voltage as well as activation mapping revealed an arrhythmia substrate along with the basal and inferior aspects of the right ventricle (RV). Catheter ablation of this scarred area in the RV resulted in specific termination of the VAs. Due to end-stage heart failure, key heart transplant criteria were met. The patient was evaluated for heart transplantation and added to the waiting list. Hitherto, no further VAs were documented during follow-up.
Discussion
As these patients present with specific dystrophia-related arrhythmia substrates, we propose pre-procedural visualization of dystrophy-associated arrhythmia substrates using cardiac magnetic resonance imaging allowing for personalized ablation approaches in these patients.
Keywords: Myotonic dystrophy, Ventricular tachyarrhythmia, Catheter ablation, Cardiac magnetic resonance imaging, Arrhythmia substrate, Case report
For the podcast associated with this article, please visit https://academic.oup.com/ehjcr/pages/podcast
Learning points.
Arrhythmia substrates between ischaemia and dystrophia differ in terms of localization and distribution of scar and fibrosis.
Both ventricles might be responsible for ventricular arrhythmias (VAs) in patients with ischaemia and dystrophia and arrhythmia substrates might have negative synergistic effects.
Substrate modification for VA elimination was safe and effective.
MRI might have a role in approaching the best management plan for patients with dystrophy and ischaemia.
Introduction
Hereditary muscle diseases can affect the heart muscle leading to cardiomyopathies and arrhythmias.1 Myotonic dystrophy type 2 [proximal myotonic myopathy (PROMM)] is a multi-systemic disease with autosomal dominant inheritance characterized by myotonia, skeletal muscle weakness, diabetes, early cataracts, central nervous system involvement, systolic dysfunction, and conduction defects including higher degree atrioventricular blocks.2 Due to the wide clinical spectrum of PROMM, the precise diagnosis is often delayed.2 The underlying genetic defect is a CCTG repeat expansion (75–11 000 repeats) in intron 1 of the CNBP/ZFNF9 gene.3 Due to the large size and somatic instability of the expansion mutation, a complex genotyping diagnostic procedure is required.2
Although life-threatening ventricular arrhythmia (VA) and sudden cardiac death (SCD) can be the first clinical manifestation,1 there is very limited data reporting on disease-specific electrophysiological conditions and approaches for catheter ablation of VA substrates in this specific cohort of patients.
Timeline
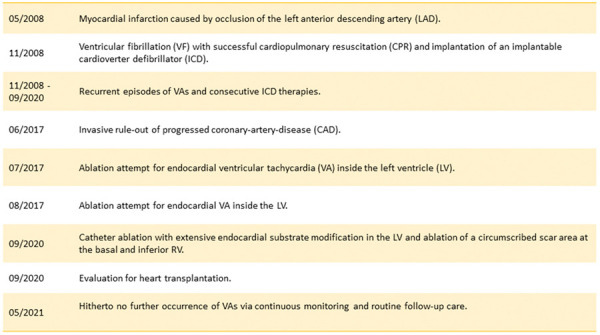
Myotonic dystrophy type 2, multiple episodes of symptomatic ventricular tachyarrhythmia, advanced stage of left ventricular dysfunction and heart failure symptoms, severe first-degree atrioventricular block (380 ms), cataracts, and pre-diabetes (for further details see Timeline).
Case presentation
We report on a 62-year-old woman with genetically confirmed PROMM with a severely reduced left ventricular ejection fraction (LVEF 25%).
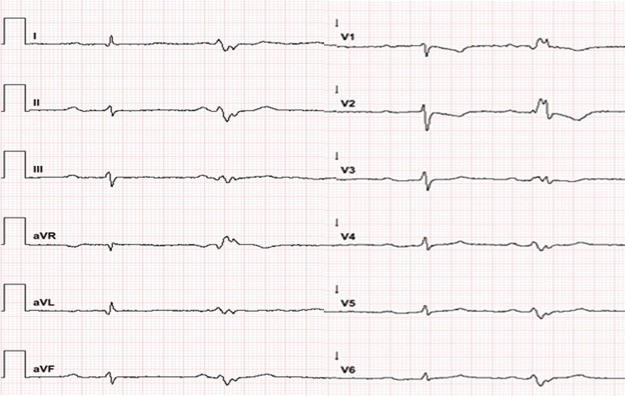
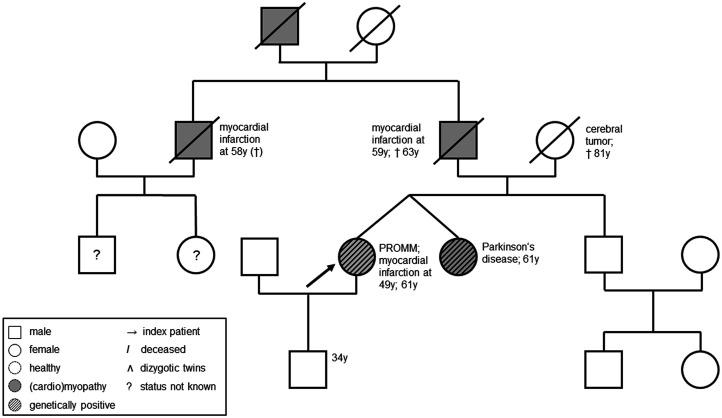
In line with aforementioned typical symptoms of PROMM, the patient showed a severe first-degree atrioventricular block (380 ms) (Figure 1), cataracts, and pre-diabetes. A one-vessel coronary artery disease was diagnosed with a history of myocardial infarction. The patient’s pedigree revealed that her father, uncle, and grandfather had also suffered from cardiomyopathies with fatal outcome before the age of 64 (Figure 2).
Figure 1.
Resting electrocardiogram documenting a long first-degree atrioventricular block (380 ms) and a ventricular extrasystole originating from the apical wall of the left ventricle however without delivering a hint for right ventricle pathology.
Figure 2.
Patient’s pedigree.
After ventricular fibrillation (VF) with successful cardiopulmonary resuscitation in 2008, the patient received an implantable cardioverter-defibrillator (ICD). Meanwhile multiple episodes of symptomatic sustained and non-sustained ventricular tachyarrhythmias (VTs) were documented. Due to palpitations and dyspnoea [New York Heart Association (NYHA) Class III)] after invasive rule-out of progressed coronary artery disease, the patient underwent two endocardial VA ablation attempts for inside the left ventricle (LV) in another centre, as a left-sided ischaemia-related arrhythmia substrate was suspected due to previous myocardial infarction caused by occlusion of the left anterior descending artery (LAD). Adherent to the guidelines,4 the patient was treated with a maximum dose of a beta-blocker (95 mg metoprolol twice daily) and amiodarone (200 mg).
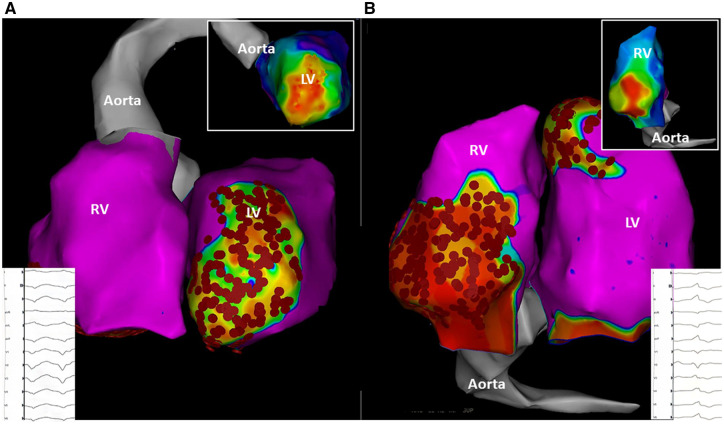
As VA recurrence accompanied by palpitations and dyspnoea (NYHA Class III) was observed, the patient was referred to our centre for repeat ablation and evaluation for an urgent heart transplantation (HTX). In the meantime, she developed acute cardiac decompensation requiring inpatient recompensation with i.v. diuretics and levosimendan therapy. Catheter ablation was performed under general anaesthesia. Two diagnostic catheters were introduced via the femoral veins and positioned in the coronary sinus (6 Fr, Webster®, Biosense Webster, Inc., Diamond Bar, CA, USA) and the right ventricle (RV) (5 Fr, Webster®, Biosense Webster, Inc., Diamond Bar, CA, USA). For an antegrade approach, venous access was achieved via the right femoral vein. A single transseptal puncture was performed under fluoroscopic guidance using a modified Brockenbrough technique and an 8.5-Fr transseptal sheath (Carto VIZIGOTM Bi-Directional Guiding Sheath). As the LV summit area as well as anterior aspects of the LV may sometimes be reached more easily using the retrograde approach, an additional retrograde access to the LV was achieved via the right femoral artery with insertion of an 8.5-Fr sheath (SL1®, St. Jude Medical, Inc., St. Paul, MN, USA). Endocardial mapping was performed using a 3D-mapping system (CARTO3®, Biosense Webster, Inc., Diamond Bar, CA, USA) and a multipolar mapping catheter (PentaRay®, Biosense Webster, Inc., Diamond Bar, CA, USA). Ultra-high density electro-anatomical reconstruction of the RV and LV was conducted aiming for >1000 mapping points. A low-voltage area suggestive for myocardial scar was defined as bipolar voltage of ≤1.5 mV. Interestingly, voltage mapping found the pre-described ischaemia-induced arrhythmia substrate in the anterior segments and apex of the LV, but it also revealed a second low-voltage area along with the basal and inferior aspects of the RV (Figure 3). Programmed ventricular stimulation induced a sustained VA with right bundle branch block and a tachycardia cycle length (CL) of 380 ms suggestive for a LV arrhythmia substrate (Figure 3). Activation mapping revealed the earliest activation of the myocardium during VA at the anterior segments of the LV, in line with the abovementioned bipolar low-voltage area (Figure 3). Catheter ablation (40 W; irrigation: 30 mL/min) at this specific certain anatomical area resulted in deceleration and specific termination of the VA. Afterwards, extensive endocardial substrate modification was performed in the LV aiming for the elimination of all late and fractionated potentials in the zones of bipolar low voltage and at the border between normal and scarred tissue. Repeat ventricular stimulation induced another VA with a left bundle branch block and a tachycardia CL of 440 ms (Figure 3). Of note, tachycardia CL was in the range of the previously documented VA episodes stored in the ICD (Figure 3). Activation mapping revealed the earliest activation during sustained VA at a circumscribed low-voltage area at the basal and inferior RV (Figure 3). Catheter ablation (40 W; irrigation: 30 mL/min) at the inferior RV resulted in specific termination of the second VA. At the end of the ablation procedure, no VA could be induced with programmed stimulation. As data on the efficacy of VA ablations in PROMM are scarce and alternative therapy options were exhausted, the patient was evaluated for HTX. In case of recurrent VAs after previous VT ablation, we actually consider and recommend an epicardial approach, especially with respect to dilated cardiomyopathy (DCM) or right ventricular involvement. Hitherto, no further VAs were documented during follow-up. LVEF was unchanged, palpitations improved, but dyspnoea persisted.
Figure 3.
Patient-specific visualization of myocardial scar tissue in the right and left ventricle (A: left anterior oblique (LAO)-view; B: inferior view). Besides co-existing left ventricle myocardial scar tissue due to ischaemia (A) biventricular bipolar voltage mapping (cut-off value for ventricular scar tissue ≤1.5 mV) revealed an individual dystrophia-related arrhythmia substrate in the right ventricle. (B). Programmed ventricular stimulation induced a sustained ventricular arrhythmia with a tachycardia cycle length of 380 (A) suggestive for a left ventricular arrhythmia substrate and another ventricular arrhythmia with a tachycardia cycle length of 440 (B) origination from the right ventricle. Activation mapping revealed the earliest activation of the myocardium during ventricular arrhythmia at the anterior segments of the left ventricle (A) and a circumscribed low-voltage area at the basal and inferior right ventricle (B).
Discussion
Proximal myotonic myopathy is a rare disease, and knowledge on dystrophia-related scar pattern is scarce. With reference to the current ESC guidelines, recommendations concerning cardiac involvement in muscular dystrophies are limited to the consensus of opinion among experts.4 Patients with neuromuscular disorders suffering from VAs should be treated in the same way as patients without (IC recommendation).4 To improve treatment strategies in these patients, more experience has to be gained.
This case report has four major findings. First, arrhythmia substrates between ischaemia and dystrophia differ in terms of localization and distribution of scar and fibrosis. Second, both ventricles might be responsible for VAs in patients with ischaemia and dystrophia and arrhythmia substrates might have negative synergistic effects. Third, substrate modification for VA elimination was safe and effective. Fourth, MRI (even with an implanted ICD5) might play a role in finding the best management plan for patients with dystrophy and ischaemia.
Ventricular arrhythmias are relevant in myotonic dystrophy as they account for an increased risk of SCD.6 Until today, no data are available describing the amount and distribution of VA substrates in PROMM. As complete elimination of all inducible VAs is desirable and associated with an improved long-term success in ischaemic and non-ischaemic-dilated cardiomyopathy,7 further information on arrhythmia substrates in PROMM is of special interest since the coexistence of dystrophia and ischaemia might lead to negative-synergistic effects resulting in early and severe cardiac dysfunction. As illustrated in our case, PROMM patients present with specific dystrophia-related arrhythmia substrates besides coexisting fibrosis and scar tissue due to ischaemic heart disease (Figure 3). Magnetic resonance imaging was not performed because the device was not MRI compatible. However, the voltage map was useful to define obviously non-ischaemic substrate in basal RV. Both ventricles should always be evaluated using voltage mapping in patients with VAs and myotonic dystrophia. Ventricular scar patterns differed between ischaemia and dystrophia in terms of localization and distribution. Personalized ablation strategies targeting both arrhythmia substrates were safe and effective and avoided an urgent need for HTX.
Lead author biography

Denise Guckel, MD is a cardiologist. In 2014, she graduated from the University of Münster. She works at the Clinic for Electrophysiology at the Herz- und Diabeteszentrum NRW, University Hospital of the Ruhr-Universität Bochum, Bad Oeynhausen, Germany. Her clinical research focus is electrophysiology including VT ablation in cardiomyopathies.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
Funding: We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidelines.
Conflict of interest: none declared.
Funding: We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
References
- 1.Arbustini E, Di Toro A, Giuliani L, Favalli V, Narula N, Grasso M.. Cardiac phenotypes in hereditary muscle disorders: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2485–2506. [DOI] [PubMed] [Google Scholar]
- 2.Meola G, Cardani R.. Myotonic dystrophies: an update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochim Biophys Acta 2015;1852:594–606. [DOI] [PubMed] [Google Scholar]
- 3.Schoser BG, Kress W, Walter MC, Halliger-Keller B, Lochmüller H, Ricker K.. Homozygosity for CCTG mutation in myotonic dystrophy type 2. Brain 2004;127:1868–1877. [DOI] [PubMed] [Google Scholar]
- 4.Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 5.Strom JB, Whelan JB, Shen C, Zheng SQ, Mortele KJ, Kramer DB.. Safety and utility of magnetic resonance imaging in patients with cardiac implantable electronic devices. Heart Rhythm 2017;14:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahbi K, Meune C, Bécane HM, Laforêt P, Bassez G, Lazarus A. et al. Left ventricular dysfunction and cardiac arrhythmias are frequent in type 2 myotonic dystrophy: a case control study. Neuromuscul Disord 2009;19:468–472. [DOI] [PubMed] [Google Scholar]
- 7.Dinov B, Fiedler L, Schönbauer R, Bollmann A, Rolf S, Piorkowski C. et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014;129:728–736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.