Abstract
Background
Diphtheria is uncommon in the World Health Organization (WHO) European Region. Nevertheless, sporadic cases, sometimes fatal, continue to be reported.
Aim
To report on diphtheria cases and coverage with first and third doses of diphtheria, tetanus and pertussis vaccines (DTP1 and DTP3, respectively) for 2010–19 in the Region with a focus on 2019.
Methods
Data on diphtheria cases were obtained from WHO/United Nations International Children's Emergency Fund (UNICEF) Joint Reporting Forms submitted annually by the Region’s Member States. WHO/UNICEF Estimates of National Immunization Coverage for DTP1 and DTP3 were summarised for 2010–19. For 2019, we analysed data on age, and vaccination status and present data by country on DTP1 and DTP3 coverage and the percentage of districts with ≥ 90% and < 80% DTP3 coverage.
Results
For 2010–19, 451 diphtheria cases were reported in the Region. DTP1 and DTP3 coverage was 92–96% and 95–97%, respectively. For 2019, 52 cases were reported by 11 of 48 countries that submitted reports (including zero reporting). Thirty-nine countries submitted data on percentage of their districts with ≥ 90% and < 80% DTP3 coverage; 26 had ≥ 90% districts with ≥ 90% coverage while 11 had 1–40% districts with < 80% coverage.
Conclusion
Long-standing high DTP3 coverage at Regional level probably explains the relatively few diphtheria cases reported in the Region. Suboptimal surveillance systems and inadequate laboratory diagnostic capacity may also be contributing factors. Still, the observed cases are of concern. Attaining high DTP3 coverage in all districts and implementing recommended booster doses are necessary to control diphtheria and prevent outbreaks.
Keywords: diphtheria, Corynebacterium diphtheriae, public health surveillance, epidemiology
Introduction
Diphtheria is an acute bacterial disease caused by Corynebacterium species. The most common type of diphtheria is classic respiratory diphtheria caused by toxin-producing Corynebacterium diphtheriae. The disease is characterised by a membranous inflammation of the upper respiratory tract, with widespread damage to other organs, primarily the myocardium and peripheral nerves. C. diphtheriae is transmitted by physical contact via respiratory secretions from a patient or a carrier. Most diphtheria-related deaths result from the effects of the toxin and include acute systemic toxicity, myocarditis and neurologic complications. The case fatality of respiratory diphtheria is 5–10% even with treatment [1]. Non-toxigenic strains may cause a sore throat but do not produce membranous lesions. Less commonly, diphtheria affects the skin (cutaneous diphtheria) and mucous membranes at other non-respiratory sites, such as genitalia and conjunctivae [2]. Two other potentially toxigenic species, Corynebacterium ulcerans and Corynebacterium pseudotuberculosis, are primarily zoonotic infections but can also cause disease in humans. C. ulcerans infection is associated with disease indistinguishable from that caused by toxigenic strains of C. diphtheriae [3,4].
Following the massive re-emergence of diphtheria in the newly independent states of the former Soviet Union (NIS) in the 1990s [5], the disease is currently considered uncommon in the World Health Organization (WHO) European Region. Nevertheless, sporadic cases, sometimes resulting in death, continue to be reported.
We hereby present data on diphtheria for 2010–19 with a focus on 2019. We also report on coverage with a diphtheria-containing vaccine represented by first and third doses of diphtheria, tetanus and pertussis vaccines (DTP1 and DTP3, respectively). Data on vaccination schedules, and school-based screening and vaccination activity for 2019 are also presented.
Methods
Data on the number of reported diphtheria cases for 2010–19 (as at 6 April 2021) were obtained from WHO/United Nations International Children's Emergency Fund (UNICEF) Joint Reporting Forms (JRFs) submitted to the WHO Regional Office for Europe. This form has been in use since 1998 and is intended to collect countries’ annual immunisation data through a standard questionnaire sent to all 53 Member States of the WHO European Region [6]. Only the reported total cases of diphtheria were considered. These comprised laboratory-confirmed cases, epidemiologically linked cases and clinical cases; suspected cases of diphtheria were not included in the analysis. Since 2018 the JRF requests that all toxigenic diphtheria cases should be reported. It specified that asymptomatic, mild, cutaneous, and mucosal and respiratory cases should be included if laboratory confirmed as toxigenic diphtheria and that non-toxigenic diphtheria cases should be excluded. Also, since 2018, the JRFs allows for the collection of data on cases by age group and vaccination status.
For 2019, we analysed the data by age and vaccination status obtained in the JRF for that year (as at 6 April 2021). We also report on data (as at 6 April 2021) on vaccination schedules, and school-based screening and delivery of routine doses of vaccines on the national immunisation schedule to children at school (excluding doses of vaccine given in supplementary immunisation activities or other vaccination campaigns) obtained in the same JRF. Where no data on school-based screening and vaccination activity for 2019 was provided, we used data from the JRF for 2018. School-based screening refers to the routine checks of a child’s vaccination status at the time of enrolment to or during primary and secondary school. We considered primary school to begin at 5–7 years of age, with a typical duration of 4 to 6 years, and secondary school to begin usually around 14–15 years of age, with a typical duration of 4 years [7].
Data on diphtheria-related deaths were obtained for 2010–19, except for 2016, for which no request had been made in the JRF for that year. Case fatality was calculated on diphtheria-related deaths as a percentage of the number of cases reported for the 9 years for which data on deaths had been requested in the JRFs.
WHO/UNICEF Estimates of National Immunization Coverage (WUENIC) for DTP1 and DTP3 coverage (as at 4 October 2021) [8] were summarised for 2010–19. In addition, DTP1 and DTP3 coverage, and the percentage of districts with ≥ 90% and < 80% DTP3 coverage for 2019 were presented by country. We considered the first three doses of DTP-containing vaccine as the primary series; subsequent doses were considered booster doses. Percentages were rounded to the nearest whole number.
Ethical statement
We did not seek an ethical evaluation of this work as no personal data were collected. The data presented in this article are based on WHO/UNICEF JRFs submitted annually by Member States of the WHO European Region.
Results
Diphtheria cases
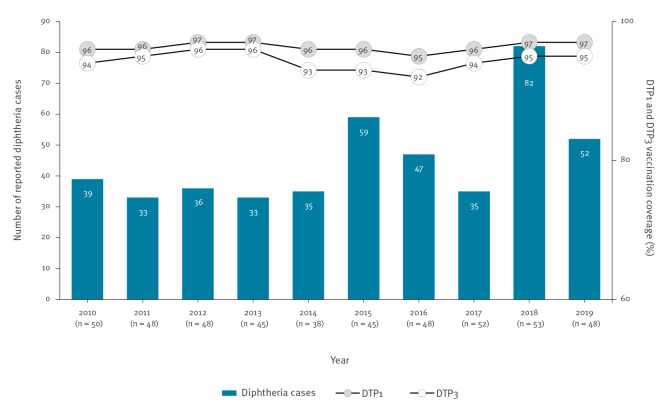
For 2010–19, the number of countries that submitted reports (including zero reporting) ranged from 38 in 2014 to 53 in 2018. During this 10-year period, there were 451 cases of diphtheria reported in the Region (Figure 1). For 2019, the number of countries that submitted reports (including zero reporting) was 48 of which 11 countries, altogether, reported 52 diphtheria cases: Germany (n = 15), United Kingdom (UK) (n = 12), Belgium (n = 6), Russian Federation (n = 5), Sweden (n = 4), Latvia (n = 2), Norway (n = 2), Slovakia (n = 2), Spain (n = 2), Georgia (n = 1) and Greece (n = 1). For the 9 years for which data on deaths was available, there were 12 diphtheria-related deaths reported from six countries: Latvia (n = 5 deaths), France (n = 2), UK (n = 2), Greece (n = 1), Spain (n = 1) and Turkey (n = 1). This gives a case fatality in the Region of 3%.
Figure 1.
Number of reported diphtheria casesa (n = 451) and DTP1 and DTP3 coverage in the WHO European Region, 2010–2019
DTP1: first dose of diphtheria, tetanus and pertussis vaccine; DTP3: third dose of diphtheria, tetanus and pertussis vaccine; WHO: World Health Organization.
a The number of countries that submitted reports (including zero reporting) on diphtheria cases are shown in parentheses below the year.
Of the total 52 reported cases in 2019, 26 cases had data on age: one case was aged < 1 year, two were aged 1–4 years, seven were aged 5–14 years, one was aged 15–29 years and 15 were aged ≥ 30 years. The vaccination status was known for 24 cases. Of these, seven were unvaccinated, five received one dose, two received three doses and 10 received > three doses. For the remaining 28 cases, the vaccination status was unknown and included 17 cases that also had missing data on age. Figure 2 shows the age distribution of cases by vaccination status.
Figure 2.
Diphtheria cases by age and DTP vaccination status in the WHO European Region, 2019 (n = 26)a
DTP: diphtheria, tetanus and pertussis; WHO: World Health Organization.
a Excluded from the graph are the 26 cases with no data on age. These include 17 cases that also had no data on vaccination status.
There were no reported diphtheria cases with two DTP doses.

Of the total cases, 46 cases (88%) were laboratory-confirmed. These were reported by nine countries and were mostly from Germany (n = 15) and the UK (n = 12) (Table).
Table. Number of diphtheria cases, DTP1 and DTP3 coverage, district level DTP3 coverage, timing of third dose, number of booster doses for diphtheria-containing vaccine, and school-based screening and vaccination activity by country, WHO European Region, 2019.
| Country (n = 53) |
Diphtheria cases | Vaccine coverage (%) | Per cent of districts with DTP3 coverage: | Age at third vaccine dose (months) | Booster doses (n) | Routine screening of vaccination status at: | Delivery of routine doses of vaccines at school | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 52) |
Laboratory-confirmed (n = 46) |
DTP1 | DTP3 | ||||||||
| ≥ 90% | < 80% | Primary school | Secondary school | ||||||||
| Albania | 0 | 0 | 99 | 99 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | NDa | No |
| Andorra | 0 | 0 | 99 | 99 | NA | NA | > 6 | 2 | Yes | Yes | No |
| Armenia | 0 | 0 | 96 | 92 | 84 | 0 | ≤ 6 | ≥ 3 | No | No | No |
| Austria | 0 | 0 | 90 | 85 | ND | ND | > 6 | 1 | NDa | NDa | Yes |
| Azerbaijan | 0 | 0 | 96 | 94 | 87 | 5 | ≤ 6 | 2 | Yes | Yesc | No |
| Belarus | 0 | 0 | 98 | 98 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
| Belgium | 6 | 6 | 99 | 98 | ND | ND | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
| Bosnia and Herzegovina | 0 | 0 | 89 | 73 | 65 | 12 | ≤6 | 2–3b | Yesc | Yesc | Yesc |
| Bulgaria | 0 | 0 | 96 | 93 | 86 | 4 | ≤ 6 | ≥ 3 | Yes | Yes | No |
| Croatia | 0 | 0 | 98 | 94 | 90 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
| Cyprus | 0 | 0 | 98 | 96 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
| Czechia | 0 | 0 | 99 | 97 | 99 | 0 | > 6 | 2 | No | Noc | No |
| Denmark | 0 | 0 | 97 | 97 | 100 | 0 | > 6 | 1 | Yes | No | No |
| Estonia | 0 | 0 | 92 | 91 | 73 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
| Finland | 0 | 0 | 98 | 91 | ND | ND | > 6 | ≥ 3 | Yes | Yes | Yes |
| France | ND | ND | 99 | 96 | ND | ND | > 6 | ≥ 3 | NDa | NDa | NDa |
| Georgia | 1 | 0 | 99 | 94 | 82 | 0 | ≤ 6 | ≥ 3 | Noc | Noc | No |
| Germany | 15 | 15 | 98 | 93 | 56 | 3 | ≤ 6 | ≥ 3 | Yes | Noc | No |
| Greece | 1 | 1 | 99 | 99 | ND | ND | ≤ 6 | ≥ 3 | Yes | Yes | No |
| Hungary | 0 | 0 | 99 | 99 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
| Iceland | 0 | 0 | 95 | 92 | ND | ND | > 6 | 2 | Yes | No | Yes |
| Ireland | 0 | 0 | 98 | 94 | 97 | 0 | ≤ 6 | 2 | No | No | Yes |
| Israel | 0 | 0 | 99 | 98 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | No | Yes |
| Italy | 0 | 0 | 98 | 96 | 95 | 0 | > 6 | ≥ 3 | Yes | No | No |
| Kazakhstan | 0 | 0 | 99 | 97 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | Yesc | Yes |
| Kyrgyzstan | 0 | 0 | 99 | 95 | 95 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
| Latvia | 2 | 2 | 99 | 99 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | No | No |
| Lithuania | 0 | 0 | 96 | 92 | 84 | 2 | ≤ 6 | ≥ 3 | Yes | No | No |
| Luxembourg | ND | ND | 99 | 99 | ND | ND | ≤ 6 | ≥ 3 | Yesc | Yesc | Noc |
| Malta | 0 | 0 | 98 | 98 | NA | NA | ≤ 6 | 2 | Yes | Yes | Yes |
| Monaco | 0 | 0 | 99 | 99 | NA | NA | > 6 | 1 | Yes | Yes | No |
| Montenegro | ND | ND | 94 | 85 | ND | ND | ≤ 6 | ≥ 3 | Yesc | Noc | Yesc |
| Netherlands | 0 | 0 | 98 | 94 | 90 | 4 | < 6 to > 6d | 2 | NDa | NDa | NDa |
| North Macedonia | ND | ND | 98 | 92 | ND | ND | ≤ 6 | ≥ 3 | Yesc | Yesc | Yesc |
| Norway | 2 | 2 | 99 | 97 | 95 | 0 | > 6 | 2 | No | No | Yes |
| Poland | ND | ND | 99 | 95 | ND | ND | ≤ 6 | ≥ 3 | Noc | Noc | Noc |
| Portugal | 0 | 0 | 99 | 99 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | No |
| Republic of Moldova | 0 | 0 | 91 | 91 | 68 | 11 | ≤ 6 | ≥ 3 | Yes | Yes | No |
| Romania | 0 | 0 | 96 | 88 | 45 | 10 | > 6 | 2 | No | No | No |
| Russian Federation | 5 | 0 | 97 | 97 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
| San Marino | 0 | 0 | 90 | 88 | NA | NA | > 6 | ≥ 3 | Yes | Yes | No |
| Serbia | 0 | 0 | 99 | 97 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | No | No |
| Slovakia | 2 | 2 | 99 | 97 | 100 | 0 | > 6 | ≥ 3 | Yes | No | No |
| Slovenia | 0 | 0 | 98 | 95 | 100 | 0 | ≤ 6 | 2 | Yes | Yes | No |
| Spain | 2 | 2 | 98 | 96 | 95 | 0 | > 6 | ≥ 3 | No | No | Noe |
| Sweden | 4 | 4 | 98 | 98 | 93 | 0 | > 6 | 2 | Yes | Yes | Yes |
| Switzerland | 0 | 0 | 98 | 96 | 41 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
| Tajikistan | 0 | 0 | 98 | 97 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | No |
| Turkey | 0 | 0 | 99 | 99 | 91 | 1 | ≤ 6 | ≥ 3 | Yesc | Noc | Yes |
| Turkmenistan | 0 | 0 | 99 | 99 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | No |
| Ukraine | 0 | 0 | 89 | 80 | 16 | 40 | ≤ 6 | ≥ 3 | Yes | Yes | No |
| United Kingdom | 12 | 12 | 97 | 93 | 81 | 1 | ≤ 6 | 2 | Yes | Yes | Yes |
| Uzbekistan | 0 | 0 | 96 | 96 | 100 | 0 | ≤ 6 | ≥ 3 | Yes | Yes | Yes |
DTP1: first dose of diphtheria, tetanus and pertussis vaccine; DTP3: third dose of diphtheria, tetanus and pertussis vaccine; NA: country that is not divided into subnational levels; ND: no data; WHO: World Health Organization.
a No data was provided for 2019 and 2018.
b Number of booster doses varies between the country’s three entities.
c Based on 2018 data as no data for 2019 was provided.
d In the Netherlands, the third primary dose is given at 4–11 months of age.
e However, some regions of Spain administer the booster dose at 14 years of age in a school setting.
DTP1 and DTP3 coverage
All 53 countries reported on DTP1 and DTP3 coverage for 2010–19. Coverage at Regional level was relatively stable during this period and, in 2019, was at 97% and 95%, respectively (Figure 1). For 2019, 32 countries reported ≥ 95% DTP3 coverage. The remaining 21 countries reported < 95% DTP3 coverage and included six countries reporting < 90% DTP3 coverage: Austria (85%), Bosnia and Herzegovina (73%), Montenegro (85%), Romania (88%), San Marino (88%) and Ukraine (80%).
For 2019, 39 countries submitted data on the percentage of districts with ≥ 90% and < 80% DTP3 coverage (Table). Of the remaining 14 countries, four countries did not report on these variables because they are not divided into subnational levels. Twenty-six countries achieved ≥ 90% DTP3 coverage in ≥ 90% of their districts. Twenty-eight countries reported none of their districts with < 80% DTP3 coverage. Of the remaining 11 countries: seven countries had 1–5% of their districts with < 80% DTP3 coverage, three countries had 10–12%, and one country had 40%.
Vaccination schedules
In 2019, all 53 countries offered a primary series of three doses of diphtheria-containing vaccine. Fifteen countries recommended the third dose after 6 months of age (Table). One country recommended the third dose at 4–11 months of age.
All 53 countries gave at least one booster dose, with 37 countries providing the recommended three or more booster doses, 12 countries provided two booster doses and three countries provided one booster dose. One other country provided two to three booster doses depending on the subnational level.
School-based screening and vaccination activity
In 2019, most of the 53 countries (n = 42) reported that they routinely check the vaccination status of children at primary school. Of these, 30 countries also routinely check the vaccination status at secondary school (Table). Twenty-four countries reported the delivery of routine doses of vaccines on the national immunisation schedule to children at school.
Discussion
Prior to the widespread use of diphtheria immunisation, the disease was a major cause of death among children [2]. Diphtheria is now considered uncommon in the WHO European Region; of the over 87,500 diphtheria cases reported globally in 2010–19 [9], only 451 cases were from the Region. For 2019, there was a decline in the total reported cases from 82 cases in 2018 to 52 cases. We assume that the 46 laboratory-confirmed cases of the 52 total cases in 2019 were all toxigenic diphtheria since, from 2018, the JRF specifically requested all such diphtheria cases to be reported, including cases presenting with respiratory, cutaneous and mucosal forms of the disease. Despite this decline, the reported cases are still of concern, highlighting the need for more efforts to address diphtheria in the Region.
The WHO Regional Office for Europe had set two targets related to diphtheria in its European Vaccine Action Plan (EVAP) [10]. The first (EVAP goal 4) was to achieve ≥ 95% DTP3 coverage at national level in 48 of the 53 countries (90%). The second (EVAP objective 4) was on geographical equity within the countries in which ≥ 90% districts (or equivalent administrative units) achieve ≥ 90% DTP3 coverage. Despite the reported high coverage at Regional level, these coverages were not consistent across the Region as for 2019, 21 countries had < 95% DTP3 coverage and included six countries reporting < 90% coverage. In addition, of the 39 countries in the Region that reported data by district, 11 countries had districts with < 80% DTP3 coverage, indicating geographical inequities in vaccination uptake that need to be addressed. One factor in preventing a major outbreak in a community is the herd immunity threshold which, for diphtheria, has been estimated at 80–85%, based on average age of infection in the pre-vaccine era [2]. To minimise the potential for diphtheria to re-emerge, population immunity through vaccination should be maintained at high levels in all areas. Screening of vaccination status at school entry can provide an effective opportunity to catch up on any missed vaccinations. Immunisation programmes targeting school-age children are increasingly important and particularly relevant for booster doses of diphtheria toxoid-containing vaccine.
Most diphtheria cases were reported in adults aged 30 years and older. This finding concurs with that reported in recent reviews of diphtheria epidemiology that showed an age distribution shift, with cases mostly occurring in adolescents and adults [11,12]. In countries where diphtheria has been well controlled and the disease has become sporadic, immunity is known to wane in late childhood or adolescence depending on the schedule of immunisation [2]. With diphtheria becoming uncommon, it can be assumed that there is little chance of exposure to infection that would provide natural boosting of immunity in adults following that induced by childhood immunisation. Most countries (n = 37) in the Region provide the recommended three or more booster doses of diphtheria toxoid during childhood and adolescence to compensate for the loss of natural boosting after completion of the primary immunisation series during infancy. However, nearly a third of the countries (n = 17) in the Region provided less than the three recommended booster doses. People living in low incidence or non-endemic areas may require booster doses of diphtheria toxoid at about 10-year intervals to sustain immunity following a three-dose primary and three-dose booster schedule before adolescence [13]. However, more recent data suggest that the administration of decennial booster doses following this schedule may not be necessary through middle age [14,15]. Nevertheless, this needs to be monitored in the long term given the increasing life expectancy worldwide [16].
Long-standing high coverage with DTP3 at Regional level is probably the main reason why there are relatively few diphtheria cases reported in the Region. Still, the cases observed in the Region are of concern, and may be also partly attributed to suboptimal surveillance systems and inadequate or lack of specialised laboratory diagnostic capacity. Indeed, sustaining the required laboratory capacity in countries particularly with zero or low incidence of diphtheria is a major challenge and significant gaps in this field of work has been reported in the Region [17,18]. The areas with significant gaps are related to training and surveillance of all three potentially toxigenic corynebacteria – C. diphtheriae, C. ulcerans and C. pseudotuberculosis. Surveillance systems should be in place for the three pathogens, with appropriate methods to determine toxigenicity. Early and accurate laboratory diagnosis of each suspected case is essential to inform proper treatment of a case and management of close contacts. In recent years, the WHO Collaborating Centre for Reference and Research on Diphtheria and Streptococcal Infections in collaboration with the WHO Regional Office for Europe [19] and the European Centre for Disease Prevention and Control (ECDC) have organised training workshops to improve diphtheria diagnostic capacity in 26 countries in the Region including 11 NIS.
Limitations
Comparisons between countries should be made with caution because apart from potential differences in the quality of diphtheria surveillance, importation potential of cases may also vary among countries. Moreover, the JRF does not stipulate a common case definition and classification for countries to use. However, there are WHO-recommended surveillance standards for vaccine-preventable diseases in place to serve as a guide to good practice and may help to harmonise surveillance activities [20]. Furthermore, the data collected in the JRFs does not distinguish between respiratory diphtheria and non-respiratory presentations of the disease. We can only assume that the cases reported in the JRFs before 2018 were cases of respiratory diphtheria, since the WHO-recommended surveillance standards for diphtheria [21] – before their revision in 2018 – focused specifically on this disease presentation. However, we cannot exclude the possibility that, although less common, non-respiratory presentations such as cutaneous disease may have been included among the reported cases especially since, in the last decade, cutaneous forms of the disease have been reported more frequently [4]. Indeed, collecting data on all clinical presentations of diphtheria is important to monitor changes in the epidemiology of the disease. The revised WHO-recommended surveillance standards invite countries to expand the case definition of suspected diphtheria cases to include non-healing ulcers in a person with a travel history to countries with endemic disease or countries with diphtheria outbreaks. It also recommends the collection of clinical data elements including cutaneous lesions and other non-respiratory involvement.
The lack of data on Corynebacterium species type limits the description of diphtheria epidemiology as does the lack of information on whether cases have been imported from abroad or acquired indigenously. The revised WHO surveillance standards recommend the collection of data on Corynebacterium species type, i.e. C. diphtheria, C. ulcerans and C. pseudotuberculosis, as well as travel history within 10 days of onset of illness.
The relatively small number of cases reported annually cautions against interpreting significant epidemiological trends. Data on age and vaccination status was only available for 15 cases out of the total 52 reported cases in 2019. Another limitation is that data on diphtheria-related deaths was restricted to the number of reported fatal cases without information on key variables such as age and vaccination status. In addition, a request for data on diphtheria-related deaths for 2016 had not been made in the JRF for that year so the case fatality rate of 3% could only be calculated using 9 of the 10 years of the study period. An additional case of diphtheria reported to WHO Regional Office from Belgium in 2016 and later published in the literature [22] was therefore excluded from the calculation.
Recommendations
Although reported DTP1 and DTP3 coverage rates at Regional level were maintained at a high level throughout 2010–19, for 2019, 11 countries had districts with < 80% DTP3 coverage. Countries should strive to ensure strong national immunisation programmes that address geographical inequities in vaccination uptake.
While all countries offered a primary series of three doses of diphtheria-containing vaccine, 17 countries provided less than the three recommended booster doses. Immunisation programmes should ensure that three primary doses and three booster doses of diphtheria toxoid-containing vaccine of age-appropriate formulations with respect to potency are provided during childhood and adolescence. Possible options for giving booster doses are: at the age of 12 months, at the age of primary school entry and a third booster dose on completion of primary school or start of secondary school [15]. A WHO-convened expert group on the use of reduced diphtheria toxoid (≥ 2–5 IU) has concluded that tetanus-diphtheria with reduced diphtheria toxoid (Td) vaccines currently licensed for ages 7 years and older, can be given in ages 4–7 years, as a second booster dose. This use of Td would be beneficial for immunisation programmes in many low- and middle-income countries [23].
To monitor the epidemiology of toxigenic diphtheria more closely, data collection on different clinical presentations and Corynebacterium species causing the disease is recommended. For the WHO European Region, a request for data collection on these variables will be included in future JRFs.
Although reports of diphtheria are uncommon in the Region, both clinicians and laboratory personnel should maintain a high index of suspicion in patients presenting with signs and symptoms of respiratory or cutaneous diphtheria, particularly after being in countries endemic for the disease. Indeed, surveillance systems for this disease including laboratory diagnostic capacity need to be adequate to ensure that cases are not missed. All countries are urged to undertake national surveillance primarily to monitor disease burden and identify outbreaks. The WHO surveillance standards for vaccine-preventable diseases provides guidelines that countries should consider in establishing and improving existing surveillance of such diseases, including that of diphtheria [20]. All providers identifying cases should be required to report them and, if possible, laboratory testing of all suspected cases should be conducted for case confirmation. An adequate surveillance of diphtheria requires that laboratories are equipped with the appropriate materials and that all isolates of potentially toxigenic corynebacteria should ideally be submitted to a reference/specialist laboratory for confirmation of identification and toxigenicity testing. A revised WHO manual for laboratory diagnosis of diphtheria and related infections has recently been published to assist laboratory workers in the correct procedures to diagnose diphtheria cases and to guide clinicians in treatment options [24].
Conclusion
The relatively few diphtheria cases reported in the Region are probably the result of overall long-standing high DTP3 coverage at Regional level. However, attaining high DTP3 coverage in all districts and implementing recommended booster doses are necessary to maintain control of diphtheria and prevent outbreaks. At the same time, surveillance systems for this disease also need to be optimal and laboratory diagnostic capacity adequate to ensure that cases of toxigenic diphtheria are not missed.
Conflict of interest: None declared.
Authors’ contributions: MM and BG contributed equally in this article. MM contributed to data analysis and wrote the manuscript. BG managed the database, provided technical support and contributed to manuscript conceptualisation. AE critically reviewed the manuscript. SD critically reviewed the manuscript. DD contributed to data analysis, provided technical and methodological support and critically reviewed the manuscript. All authors have seen and approved the final manuscript.
References
- 1.Heymann DL, editor. Control of Communicable Diseases Manual. 20th edition. Washington: American Public Health Association; 2015. [Google Scholar]
- 2.Tiwari T, Wharton M. Diphtheria Toxoid. In: Plotkin SA, Orenstein WA, Offit PA, (eds). Vaccines, 7th edn. Elsevier: Philadelphia, Pennsylvania, 2017: 261-275. [Google Scholar]
- 3. Tiwari TS, Golaz A, Yu DT, Ehresmann KR, Jones TF, Hill HE, et al. Investigations of 2 cases of diphtheria-like illness due to toxigenic Corynebacterium ulcerans. Clin Infect Dis. 2008;46(3):395-401. 10.1086/525262 [DOI] [PubMed] [Google Scholar]
- 4. Gower CM, Scobie A, Fry NK, Litt DJ, Cameron JC, Chand MA, et al. The changing epidemiology of diphtheria in the United Kingdom, 2009 to 2017. Euro Surveill. 2020;25(11):1900462. 10.2807/1560-7917.ES.2020.25.11.1900462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hardy IR, Dittmann S, Sutter RW. Current situation and control strategies for resurgence of diphtheria in newly independent states of the former Soviet Union. Lancet. 1996;347(9017):1739-44. 10.1016/S0140-6736(96)90811-9 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO). Immunization, vaccines and biologicals. WHO/UNICEF joint reporting process: new web-based application provides faster and easier reporting of annual data on immunization. Geneva: WHO. [Accessed: 1 Aug 2021]. Available from: https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/who-unicef-joint-reporting-process
- 7.World Health Organization (WHO). School-based immunization. Geneva: WHO. [Accessed: 6 Apr 2021]. Available from: https://immunizationdata.who.int/pages/indicators-by-category/policy.html?ISO_3_CODE=&YEAR=
- 8.World Health Organization (WHO). Immunization dashboard. Last update: 4 October 2021. Geneva: WHO; 2021. Available from: https://immunizationdata.who.int/index.html
- 9.World Health Organization (WHO). Diphtheria reported cases and incidence. Geneva: WHO. [Accessed: 6 April 2021]. Available from: https://immunizationdata.who.int/pages/incidence/DIPHTHERIA.html?CODE=Global&YEAR=
- 10.World Health Organization (WHO) Regional Office for Europe. European Vaccine Action Plan 2015-2020. Copenhagen: WHO/Europe; 2014. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/2014/european-vaccine-action-plan-20152020-2014
- 11. Clarke KEN. Review of the Epidemiology of Diphtheria – 2000-2016. Atlanta: United States Centers for Disease Control and Prevention; 2017. Available from: http://www.who.int/immunization/sage/meetings/2017/april/1_Final_report_Clarke_april3.pdf?ua=1
- 12. Clarke KEN, MacNeil A, Hadler S, Scott C, Tiwari TSP, Cherian T. Global Epidemiology of Diphtheria, 2000-20171. Emerg Infect Dis. 2019;25(10):1834-42. 10.3201/eid2510.190271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization (WHO) . Outbreak news. Avian influenza, Indonesia. Wkly Epidemiol Rec. 2006;81(3):21-32. English, French. Available from: https://www.who.int/wer/2006/wer8103.pdf?ua=1 [PubMed] [Google Scholar]
- 14. Swart EM, van Gageldonk PGM, de Melker HE, van der Klis FR, Berbers GA, Mollema L. Long-term protection against diphtheria in the Netherlands after 50 years of vaccination: results from a seroepidemiological study. PLoS One. 2016;11(2):e0148605. 10.1371/journal.pone.0148605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization (WHO) . Diphtheria vaccine: WHO position paper – August 2017. Wkly Epidemiol Rec. 2017;92(31):417-35. English, French. 28776357 [Google Scholar]
- 16.World Health Organization (WHO). Diphtheria vaccine. Review of evidence on vaccine effectiveness and immunogenicity to assess the duration of protection ≥10 years after the last booster dose. Geneva: WHO; 2017. Available from: https://www.who.int/immunization/sage/meetings/2017/april/2_Review_Diphtheria_results_April2017_final_clean.pdf?ua=1
- 17.World Health Organization (WHO) Regional Office for Europe. Gaps found in the Region’s capacity to diagnose diphtheria. Copenhagen: WHO/Europe; 2017. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/news/news/2017/07/gaps-found-in-the-regions-capacity-to-diagnose-diphtheria
- 18.European Centre for Disease Prevention and Control (ECDC). Gap analysis on securing diphtheria diagnostic capacity and diphtheria antitoxin availability in the EU/EEA. Stockholm: ECDC; 2017. Available from: https://www.ecdc.europa.eu/en/publications-data/gap-analysis-securing-diphtheria-diagnostic-capacity-and-diphtheria-antitoxin
- 19.World Health Organization (WHO) Regional Office for Europe. Workshop on Laboratory diagnosis of diphtheria. Copenhagen: WHO/Europe; 2017. Available from: http://www.euro.who.int/__data/assets/pdf_file/0005/365414/diphtheria-workshop-2017-eng.pdf?ua=1
- 20.World Health Organization (WHO). Diphtheria. Vaccine-preventable diseases surveillance standards. Geneva: WHO; 2018. Available from: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/WHO_SurveillanceVaccinePreventable_04_Diphtheria_R2.pdf?ua=1
- 21.World Health Organization (WHO). WHO-recommended standards for surveillance of selected vaccine-preventable diseases. Geneva: WHO; 1999. Available from: https://apps.who.int/iris/handle/10665/64165
- 22. Martini H, Soetens O, Litt D, Fry NK, Detemmerman L, Wybo I, et al. Diphtheria in Belgium: 2010-2017. J Med Microbiol. 2019;68(10):1517-25. 10.1099/jmm.0.001039 [DOI] [PubMed] [Google Scholar]
- 23. Desai S, Scobie HM, Cherian T, Goodman T, Expert group on the use of Td vaccine in childhood . Use of tetanus-diphtheria (Td) vaccine in children 4-7 years of age: World Health Organization consultation of experts. Vaccine. 2020;38(21):3800-7. 10.1016/j.vaccine.2020.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO). Diphtheria. WHO laboratory manual for the diagnosis of diphtheria and other related infections. Third Revision. Geneva: WHO; 2022. Available from: https://cdn.who.int/media/docs/default-source/immunization/diphtheria_lab_manual_v2.pdf?sfvrsn=3e1d6f8f_3