Key Points
Question
Is COVID-19 vaccination associated with sudden sensorineural hearing loss (SSNHL)?
Findings
In this cross-sectional study and case series involving 555 cases of SSNHL among adults reported to the Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System, no increase in the rate of hearing loss after COVID-19 vaccination was found compared with the incidence in the general population. Assessment of 21 adult patients who presented to tertiary care centers with SSNHL after COVID-19 vaccination did not reveal any apparent associations with respect to clinical or demographic factors.
Meaning
These results suggest that there is no association between vaccination and the development of SSNHL among adults who received a COVID-19 vaccine.
Abstract
Importance
Emerging reports of sudden sensorineural hearing loss (SSNHL) after COVID-19 vaccination within the otolaryngological community and the public have raised concern about a possible association between COVID-19 vaccination and the development of SSNHL.
Objective
To examine the potential association between COVID-19 vaccination and SSNHL.
Design, Setting, and Participants
This cross-sectional study and case series involved an up-to-date population-based analysis of 555 incident reports of probable SSNHL in the Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System (VAERS) over the first 7 months of the US vaccination campaign (December 14, 2020, through July 16, 2021). In addition, data from a multi-institutional retrospective case series of 21 patients who developed SSNHL after COVID-19 vaccination were analyzed. The study included all adults experiencing SSNHL within 3 weeks of COVID-19 vaccination who submitted reports to VAERS and consecutive adult patients presenting to 2 tertiary care centers and 1 community practice in the US who were diagnosed with SSNHL within 3 weeks of COVID-19 vaccination.
Exposures
Receipt of a COVID-19 vaccine produced by any of the 3 vaccine manufacturers (Pfizer-BioNTech, Moderna, or Janssen/Johnson & Johnson) used in the US.
Main Outcomes and Measures
Incidence of reports of SSNHL after COVID-19 vaccination recorded in VAERS and clinical characteristics of adult patients presenting with SSNHL after COVID-19 vaccination.
Results
A total of 555 incident reports in VAERS (mean patient age, 54 years [range, 15-93 years]; 305 women [55.0%]; data on race and ethnicity not available in VAERS) met the definition of probable SSNHL (mean time to onset, 6 days [range, 0-21 days]) over the period investigated, representing an annualized incidence estimate of 0.6 to 28.0 cases of SSNHL per 100 000 people per year. The rate of incident reports of SSNHL was similar across all 3 vaccine manufacturers (0.16 cases per 100 000 doses for both Pfizer-BioNTech and Moderna vaccines, and 0.22 cases per 100 000 doses for Janssen/Johnson & Johnson vaccine). The case series included 21 patients (mean age, 61 years [range, 23-92 years]; 13 women [61.9%]) with SSNHL, with a mean time to onset of 6 days (range, 0-15 days). Patients were heterogeneous with respect to clinical and demographic characteristics. Preexisting autoimmune disease was present in 6 patients (28.6%). Of the 14 patients with posttreatment audiometric data, 8 (57.1%) experienced improvement after receiving treatment. One patient experienced SSNHL 14 days after receiving each dose of the Pfizer-BioNTech vaccine.
Conclusions and Relevance
In this cross-sectional study, findings from an updated analysis of VAERS data and a case series of patients who experienced SSNHL after COVID-19 vaccination did not suggest an association between COVID-19 vaccination and an increased incidence of hearing loss compared with the expected incidence in the general population.
This cross-sectional study and case series used data from the Vaccine Adverse Events Reporting System and a selected sample of patients at multiple institutions to assess the association between COVID-19 vaccination and sudden sensorineural hearing loss among adults during the first 7 months of the US vaccination campaign.
Introduction
Anecdotal reports of sudden sensorineural hearing loss (SSNHL) occurring after COVID-19 vaccination have emerged in otolaryngologic professional societies and have important public health implications. Tinnitus, dizziness, and vertigo have also been reported within 2 weeks of vaccination in a recent single-institution case series.1 Otolaryngologists encounter increasing challenges to promoting public health conduct recommended during the pandemic when they are counseling and evaluating patients who have developed SSNHL and reported a temporal association with COVID-19 vaccination.
Other large-scale vaccination campaigns, such as those for the measles-mumps-rubella and influenza vaccines, have previously been investigated after anecdotal reports of SSNHL emerged among vaccinated individuals. In each campaign, epidemiologic studies2,3 did not show an association between vaccination and SSNHL. Although data from similar epidemiologic studies are not yet available for COVID-19 vaccination, a preliminary analysis4 of incident reports from the Centers for Disease Control and Prevention (CDC) Vaccine Adverse Events Reporting System (VAERS) during the early phase of public COVID-19 vaccination did not identify an association between vaccination and SSNHL. However, as vaccination campaigns have expanded across the US and currently include vaccines from 3 manufacturers (Pfizer-BioNTech [BNT162b2], Moderna [mRNA-1273], and Janssen/Johnson & Johnson [Ad26.COV2.S]), questions remain regarding whether an association exists between COVID-19 vaccination and SSNHL. In addition, VAERS does not provide detailed patient-level clinical data that may be valuable in evaluating specific patient cofactors.
The purposes of the present study were to (1) extend the preliminary incidence estimate of SSNHL after COVID-19 vaccination4 to the present phase of vaccination across 3 manufacturers and (2) examine whether emerging patterns in VAERS incident reports suggest an association between COVID-19 vaccination and SSNHL. In addition, we sought to augment this public database evaluation with an in-depth analysis of clinical characteristics among a multi-institutional series of patients who presented with confirmed SSNHL after COVID-19 vaccination.
Methods
This study was approved by the institutional review boards of Johns Hopkins University School of Medicine and the Massachusetts Eye and Ear Infirmary/Harvard Medical School. Because the VAERS records review obtained data from a publicly available deidentified database, this portion of the study was deemed exempt from review; similarly, the case series was deemed exempt because the patients’ files did not contain identifiable data.
The study was performed in 2 phases. In the first phase, VAERS was queried for reports of SSNHL after COVID-19 vaccination between December 14, 2020, and July 16, 2021. Cases deemed to represent probable SSNHL were compiled for analysis using previously dfescribed methods.4 In brief, the search terms sudden hearing loss, deafness, deafness neurosensory, deafness unilateral, deafness bilateral, and hypoacusis were selected as adverse events (AEs) for data extraction. Because multiple symptoms could be selected for each incident report, deduplication was performed to ensure there was only 1 unique VAERS identification number per report. Narratives and laboratory data from all reports were reviewed to assess the likelihood of a report representing probable SSNHL. Inclusion criteria for probable SSNHL consisted of a temporal association with COVID-19 vaccination (defined as onset within 21 days after vaccination) and a high credibility of reporting. A report was deemed credible if it could demonstrate at least 1 of the following: (1) reference to an audiographic test result confirming hearing loss, (2) evaluation by an otolaryngologist, audiologist, or other physician resulting in a diagnosis of sudden hearing loss, or (3) evaluation by an otolaryngologist resulting in treatment with systemic steroid or intratympanic steroid medications, performance of magnetic resonance imaging, or any combination thereof. Incident reports were excluded if they did not reference evaluation by a physician or audiologist leading to a diagnosis of hearing loss, did not contain details within the report or laboratory results section to indicate that a diagnosis of sudden hearing loss was provided (eg, no mention of audiologic testing, no receipt of systemic or intratympanic steroid medications, or no magnetic resonance imaging scan), or indicated that hearing loss onset occurred more than 21 days after vaccination. In addition, reports that described the discovery of an alternative origin for hearing loss (eg, vestibular schwannoma or stroke) were excluded. Examples of narratives and their classifications are shown in eTable 1 in the Supplement.
The number of vaccine doses administered in the US during the study period was obtained from the CDC.5 An incidence estimate of probable SSNHL on a per-person basis during the study period was obtained and annualized. To account for intrinsic uncertainties, such as the number of unique individuals receiving a vaccine relative to the number of doses administered, the true case numbers of SSNHL based on VAERS incident reports, and potential underreporting bias in VAERS, we conducted a sensitivity analysis that adjusted these assumptions to achieve a range estimate of the incidence of SSNHL. The maximum incidence estimate was produced based on the assumptions that (1) all reports submitted to VAERS represented true cases of SSNHL (eTable 1 in the Supplement); (2) the number of reports submitted to VAERS was likely subject to a 50% underreporting bias based on previous studies of VAERS sensitivity for rare AEs, such as Guillain-Barré syndrome and anaphylaxis6; and (3) each vaccinated individual received 2 doses, resulting in the smallest possible population size given the number of vaccine doses administered (ie, the highest possible incidence).
Because VAERS reports are unverified and lack detailed clinical data,6 an in-depth record review of a multi-institutional consecutive series of all adult patients with audiometrically confirmed SSNHL after COVID-19 vaccination was performed in the second phase of the study. The study sites comprised 2 large academic neurotologic centers and 1 community otolaryngological practice. Cases were included if audiometrically confirmed SSNHL occurred within 3 weeks of vaccination and was contemporaneous with VAERS reports of SSNHL (ie, occurring between January 1 and June 30, 2021). Patients with a history of Ménière disease were excluded.
Statistical Analysis
Reports of SSNHL were exported from VAERS into Excel software, version 16.57 (Microsoft Corporation). Simple descriptive statistics (means, ranges, and percentages) were calculated using this software for both the VAERS reports and the case series.
Results
Between December 14, 2020, and July 16, 2021, 185 424 899 COVID-19 vaccine doses were administered in the US across the 3 manufacturers.5 After deduplication, 2170 VAERS reports of hearing loss based on search criteria and occurring within 21 days of vaccination were extracted and compiled. In total, 555 of the 2170 reports met our definition of probable SSNHL. A total of 305 incidents (55.0%) occurred among women, and 250 incidents (45.0%) occurred among men, with a mean age of 54 years (range, 15-93 years) (Table 1). Data on race and ethnicity were not available in VAERS. Overall, 305 incidents (55.0%) involved the Pfizer-BioNTech vaccine, 222 (40.0%) involved the Moderna vaccine, and 28 (5.0%) involved the Janssen/Johnson & Johnson vaccine.
Table 1. Demographic and Clinical Characteristics of Hearing Loss Incidents After COVID-19 Vaccination Reported in VAERS Database Between December 14, 2020, and July 16, 2021a.
| Characteristic | No. (%) |
|---|---|
| Total incidents, No. | 555 |
| Age, mean (range), y | 54 (15-93) |
| Sex | |
| Female | 305 (55.0) |
| Male | 250 (45.0) |
| Manufacturer | |
| Pfizer-BioNTech | 305 (55.0) |
| Moderna | 222 (40.0) |
| Janssen/Johnson & Johnson | 28 (5.0) |
| Time to SSNHL onset after vaccine dose, mean (range), d | 6 (0-21) |
Abbreviations: SSNHL, sudden sensorineural hearing loss; VAERS, Vaccine Adverse Events Reporting System.
A total of 185 424 899 vaccine doses were administered. Incidents were classified as most probable based on temporal association and high credibility of reporting.
A sensitivity analysis was then performed to estimate the incidence range on an annualized basis, revealing 0.6 to 28.0 cases of SSNHL per 100 000 people per year (Table 2). In comparison, the annual incidence of idiopathic SSNHL was estimated to be 11 to 77 cases per 100 000 people per year, depending on age.7 Because speculation has occurred regarding the novel lipid nanoparticle delivery vehicle and the messenger RNA (mRNA) technologies that underlie the Moderna and Pfizer-BioNTech vaccines, we next investigated whether vaccines produced by these 2 manufacturers accounted for a disproportionate number of reports of SSNHL. A total of 186.88 million doses of the Pfizer-BioNTech vaccine were administered, 136.48 million doses of the Moderna vaccine were administered, and 12.97 million doses of the Janssen/Johnson & Johnson vaccine were administered over the period examined. The VAERS reporting rate of probable SSNHL was similar across manufacturers, with 0.16 cases per 100 000 doses administered for both the Pfizer-BioNTech and Moderna vaccines, and 0.22 cases per 100 000 doses administered for the Janssen/Johnson & Johnson vaccine (eTable 2 in the Supplement).
Table 2. Sensitivity Analysis for Incidence Estimates of Sudden Sensorineural Hearing Loss After COVID-19 Vaccination.
| Source | Incidence | |
|---|---|---|
| Minimuma | Maximumb | |
| Cases | 555 | 2170 |
| Population size | 185 424 899c | 92 712 450d |
| VAERS underreporting bias, % | 0 | 50 |
| Incidencee | 0.6 | 28.0 |
Abbreviations: SSNHL, sudden sensorineural hearing loss; VAERS, Vaccine Adverse Events Reporting System.
Estimates of minimum incidence were based on the 555 cases deemed to be probable SSNHL, a population size that included 1 vaccine dose per person, and the assumption that all cases were reported to VAERS.
Estimates of maximum incidence (the most inclusive estimates) were based on all 2170 reports of SSNHL reported to VAERS using search terms described in the Methods section, a population size that included 2 vaccine doses per person (ie, 2 opportunities to experience a COVID-19 vaccination–associated SSNHL), and the assumption that only 50% of cases were reported to VAERS.
One dose per person.
Two doses per person.
Per 100 000 people per year.
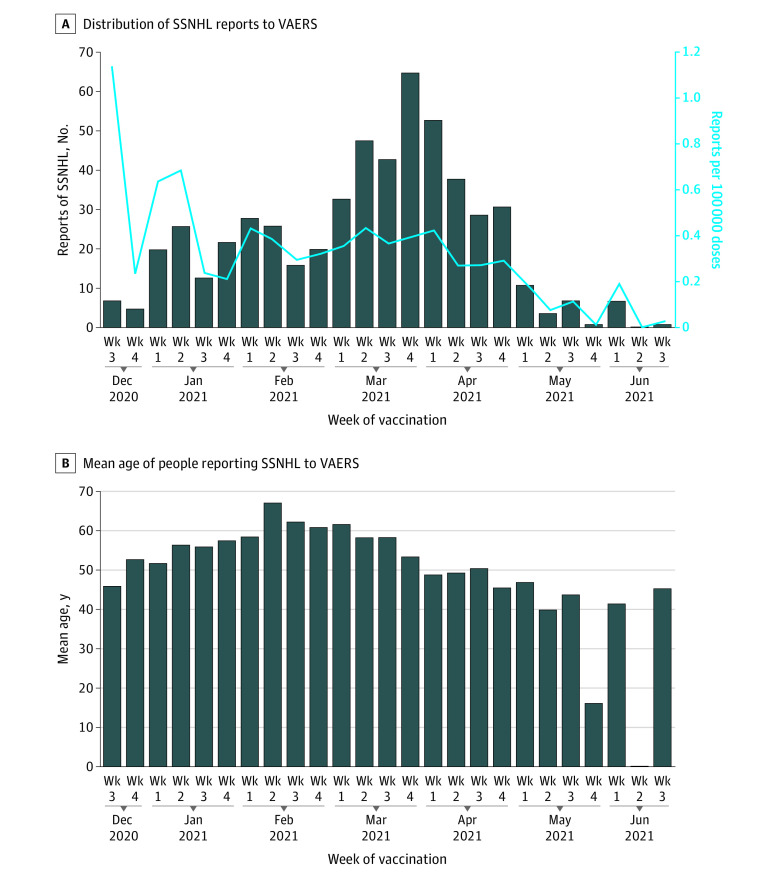
To further investigate whether reports of SSNHL were associated with COVID-19 vaccination, we examined the total number of reports of the condition submitted to VAERS over each weekly period from the beginning of the public vaccination campaign (Figure 1A). The number of submitted reports peaked in the last week of March 2021, which corresponded to the largest number of vaccine doses (16 177 521) administered during a 1-week period since the vaccination campaign began.5 However, over each weekly period, the relative number of SSNHL reports decreased when accounting for the number of doses administered nationally, from 1.10 reports per 100 000 doses at the beginning of the campaign in December 2020 to 0.01 reports per 100 000 doses by June 2021.
Figure 1. Reports of Sudden Sensorineural Hearing Loss (SSNHL) by Week of COVID-19 Vaccination.
A, Distribution of reports of SSNHL by week of vaccination and time to onset after vaccination. The rate of SSNHL reports per 100 000 vaccine doses (blue line) is overlaid. B, Mean age of people reporting SSNHL to VAERS according to the time period reported who met the definition of probable SSNHL (n = 555). Note that the weekly time periods are identical in A and B. VAERS indicates Vaccine Adverse Events Reporting System.
Because the risk of idiopathic SSNHL is highly dependent on age,7 we specifically examined the mean ages of patients who submitted reports of probable SSNHL, which remained relatively stable over the study period (eg, mean age, 45.9 years [range, 34.0-79.0 years] in December 2020 and 41.6 years [range, 19.0-54.0 years] in June 2021) (Figure 1B). We also estimated the age of the overall vaccinated population using publicly available data from the CDC8 (eFigure 3 in the Supplement). In the early phases of the vaccination campaign, no preponderance of older individuals (who may have been at higher risk of idiopathic SSNHL) receiving vaccine doses was apparent. In addition, in the later phases of the campaign, no preponderance of younger individuals (who may have been at lower risk of idiopathic SSNHL) was seen.
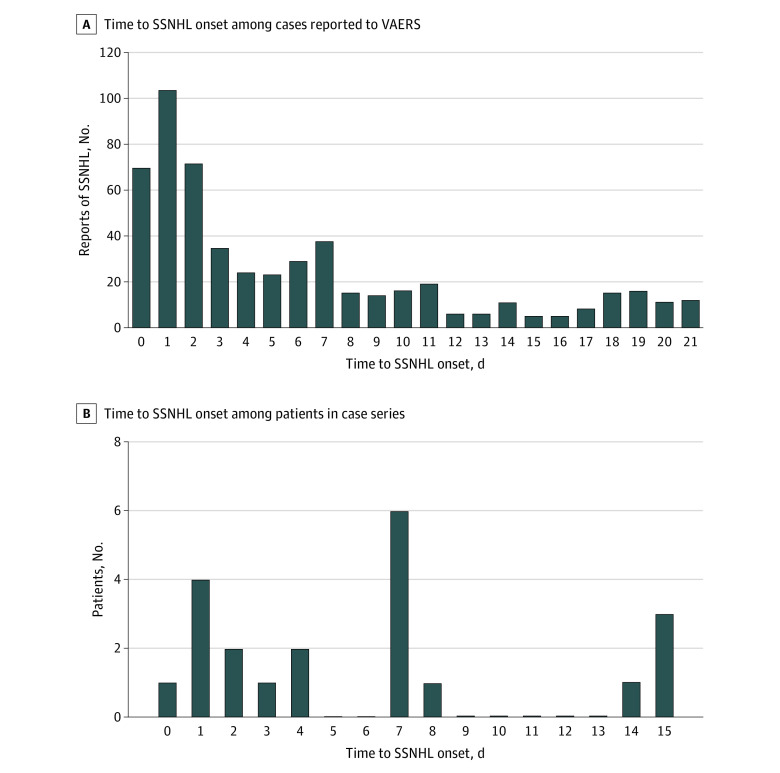
We then evaluated the possible temporal association between COVID-19 vaccination and the onset of idiopathic SSNHL as documented in VAERS incident reports (Figure 2A). The mean time to onset of SSNHL was 6 days (range, 0-21 days), with the highest incidence occurring at 0 days (70 reports), 1 day (104 reports), and 2 days (72 reports) after vaccination and a smaller second peak occurring at 7 days (38 reports) after vaccination.
Figure 2. Time to Onset of Sudden Sensorineural Hearing Loss (SSNHL) After COVID-19 Vaccination.
A, Includes 555 cases reported to the VAERS database that met the definition of probable SSNHL during the period examined. B, Includes 21 patients in multi-institutional case series. The x-axis extends to only 15 days after vaccination because no new cases were observed after day 15. VAERS indicates Vaccine Adverse Events Reporting System.
To better understand the clinical profiles of patients who reported SSNHL after COVID-19 vaccination, we examined the detailed clinical characteristics of patients with confirmed hearing loss occurring after COVID-19 vaccination in a multi-institutional case series. A total of 21 patients were identified across study sites, with a mean age of 61 years (range, 23-92 years; 13 women [61.9%]). Demographic, clinical, and audiometric characteristics of patients are shown in Table 3. Six patients (28.6%) had a history of autoimmune disease, including eczema, episcleritis, Hashimoto thyroiditis, multiple sclerosis, and rheumatoid arthritis. The mean time to onset of SSNHL was 6 days (range, 0-15 days) after vaccination, with the highest number of cases (6) occurring at 7 days after vaccination (Figure 2B). Overall, 18 of 21 patients (85.7%) received treatment; of those, 9 patients (50.0%) received intratympanic steroids, 5 (27.8%) received oral corticosteroids, and 4 (22.2%) received both. No adjuvant therapies were prescribed. Complete posttreatment audiometric data were available for 14 patients, 8 of whom (57.1%) experienced audiometric improvement (Table 3; eFigure 1 in the Supplement).
Table 3. Patients Presenting to 3 Neurotologic Practices With Sudden Sensorineural Hearing Loss After COVID-19 Vaccination.
| Sex | Age range, y | Vaccine manufacturer | Medical/otologic history | Symptoms after first or second vaccine dose | Time to symptom onset, da | Pretreatment | Treatment | Posttreatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure tone average, dBHLb | Word recognition score, % | Course | Response | Pure tone average, dBHLb | Word recognition score, % | ||||||
| Female | 60-69 | Unknown | Tympanoplasty; previous chemoradiotherapy for NPC | First | 3 | 81 | 50 | OCS; IT | NA | NA | NA |
| Female | 60-69 | Pfizer-BioNTech | Eczema | First | 7 | 33 | 98 | OCS | No | 33 | 98 |
| Female | 70-79 | Moderna | Left-sided SSNHL 5 y before treated with prednisone with resolution | First | 15 | 20 | 96 | OCS; IT | No | 33 | 78 |
| Male | 50-59 | Moderna | None | First | 2 | 13 | 100 | IT | NA | NA | NA |
| Male | 50-59 | Pfizer-BioNTech | None | First | 7 | 28 | 92 | OCS | NA | NA | NA |
| Male | ≥90 | Pfizer-BioNTech | None | First | 7 | 86 | 54 | IT | Yes | 59 | 70 |
| Female | 70-79 | Pfizer-BioNTech | Hashimoto thyroiditis; lichen planus | Second | 15 | 44 | 98 | OCS | Yes | 34 | 92 |
| Female | 80-89 | Moderna | Eczema | First | 8 | 96 | 79 | Declined | NA | NA | NA |
| Male | 50-59 | Pfizer-BioNTech | None | First | 4 | 73 | 24 | OCS | Yes | 11 | 96 |
| Female | 40-49 | Pfizer-BioNTech | None | Second | 15 | NA | 84 | Did not receive | NA | NA | NA |
| Male | 20-29 | Pfizer-BioNTech | Benign paroxysmal positional vertigo | First | 0 | 29 | 88 | OCS | Yes | 11 | 96 |
| Female | 30-39 | Pfizer-BioNTech | Hypothyroidism; 32-wk pregnant | First | 1 | NA | 100 | IT | NA | NA | NA |
| Male | 70-79 | Pfizer-BioNTech | Hypothyroidism; type 2 diabetes | Second | 1.5 | 43 | 96 | IT | Yes | 33 | 100 |
| Female | 60-69 | Pfizer-BioNTech | Multiple sclerosis; type 2 diabetes | First | 1 | 65 | 24 | IT | Yes | 15 | 88 |
| Male | 50-59 | Pfizer-BioNTech | None | First | 4 | 23 | 92 | IT | Yes | 19 | 96 |
| Female | 60-69 | Moderna | Rheumatoid arthritis, previous right-sided labyrinthitis with right-sided SSNHL | First | 1 | 54 | 44 | OCS; IT | No | 60 | 20 |
| Female | 30-39 | Pfizer-BioNTech | None | First and second | 14, 14 | 16, 19 | 92 | OCS; IT | Yes | 9; NA | 92 |
| Female | 80-89 | Janssen/Johnson & Johnson | None | First | 7 | 46 | 100 | IT | Yes | 40 | 90 |
| Male | 60-69 | Moderna | Episcleritis | First | 7 | 31 | 100 | IT | No | 33 | 100 |
| Female | 60-69 | Pfizer-BioNTech | None | First | 1 | 26 | 100 | IT | Yes | 21 | 100 |
| Female | 70-79 | Moderna | None | Second | 7 | 34 | 100 | None | NA | NA | NA |
Abbreviations: dBHL, decibel hearing level; IT, intratympanic steroid; NA, not available or not applicable; NPC, nasopharyngeal cancer; OCS, oral corticosteroid; SSNHL, sudden sensorineural hearing loss.
After administration of COVID-19 vaccine.
Pure tone average obtained at 500, 1000, 2000, and 4000 Hz.
One patient without any history of Ménière disease or autoimmune inner ear disease experienced new-onset low-frequency (250-500 Hz) SSNHL at 14 days after the first vaccine dose; the condition improved with oral and intratympanic steroid treatment but worsened again at 14 days after the second vaccine dose (15-dB threshold increase in hearing loss at 500 Hz) (eFigure 2 in the Supplement).
Discussion
This comprehensive cross-sectional study of CDC VAERS reports of SSNHL after COVID-19 vaccination during the first 7 months of the national vaccination campaign included 185 million doses across all 3 manufacturers. Although VAERS reports contain raw data that are unverified, they present a national snapshot of potential AEs occurring after vaccination. Our analysis found that, based on VAERS reports, the estimated incidence of SSNHL after COVID-19 vaccination did not exceed the reported incidence of idiopathic SSNHL in the general population.7 Furthermore, despite the novel delivery vehicle and immunologic mechanism of the mRNA-based vaccines manufactured by Pfizer-BioNTech and Moderna, we did not find an increased reporting rate of SSNHL associated with lipid nanoparticle mRNA vaccines compared with the adenoviral platform used in the Janssen/Johnson & Johnson vaccine.
We also hypothesized that if an association existed between COVID-19 vaccination and SSNHL, we would find an association between the number of reports of SSNHL submitted to VAERS and the number of vaccine doses administered. However, we found the rate of reports per 100 000 doses decreased across the vaccination period, despite large concomitant increases in the absolute number of vaccine doses administered per week (Figure 1A).
We also tested the hypothesis that the increased rate of reports of SSNHL in the initial vaccination phase could be associated with older individuals being vaccinated first9; our analysis of the mean ages of people reporting SSNHL after vaccination to VAERS (Figure 1B) and the CDC COVID-19 tracking data on the number of individuals vaccinated in each age group over time (eFigure 3 in the Supplement) did not support this hypothesis. Given that health care professionals were also included in the first phase of vaccination, one might assume that this group would be more attuned to AEs and more likely to report SSNHL; however, the relative number of health care professionals who initially experienced SSNHL was impossible to ascertain based on VAERS data. Taken together, these data suggest that an association between COVID-19 vaccination and SSNHL during the first 7 months of vaccination was unlikely at the population level.
Because VAERS incident reports lack clinical detail, conclusions regarding specific risk factors associated with SSNHL after COVID-19 vaccination cannot be reached. Narrative information within VAERS is self-reported and highly variable, ranging from no information on medical history to detailed information on both medical history and medication use. Thus, we assessed the clinical characteristics of patients with confirmed SSNHL at 3 large otolaryngological practices. The demographic and clinical characteristics of patients examined in our multi-institutional case series (Table 3) did not clearly identify any specific cofactors among those experiencing SSNHL after vaccination, and patient characteristics appeared similar to the highly heterogeneous profiles observed among those with idiopathic SSNHL and those included in case series conducted at other institutions.1 A previous study suggested that autoimmune disease may increase the risk of idiopathic SSNHL,10 and we observed that autoimmune disease was present in 28.6% of the 21 patients in the case series reporting SSNHL after COVID-19 vaccination. Autoimmune disease as a risk factor for SSNHL with or without vaccination remains speculative, and further research is needed.
Both the mRNA payload and the lipid nanoparticle delivery vehicle have been suggested to be potential mechanisms of autoimmunogenicity.11 Notably, the patient in the case series who reported having normal hearing before vaccination (no prevaccination audiometric data were available) and no history of autoimmune disease (Table 3) was found to have low-frequency unilateral SSNHL at 14 days after the first vaccine dose. The patient received treatment with a course of oral steroid medication and experienced partial recovery of hearing; however, the patient subsequently reported new hearing deficit at 14 days after the second vaccine dose and was found to have a 15-dB threshold increase in hearing loss at 500 Hz (eFigure 2 in the Supplement). Although not meeting the American Academy of Otolaryngology–Head and Neck Surgery criteria for SSNHL,12 the observed audiometric changes were nonetheless concerning. Sudden sensorineural hearing loss after each COVID-19 vaccine dose was also reported among 3 patients in a recent case series, although 2 of those 3 patients had autoimmune inner ear disease, Ménière disease, or both.1 Thus, our findings suggested that although no association between COVID-19 vaccination and SSNHL was found at the population level, an association among some individuals cannot be excluded without further research.
We also considered the timing of SSNHL after COVID-19 vaccination because this timing may have offered insight into the mechanistic basis of any potential biological association. For instance, Wichova et al1 hypothesized that otologic symptoms, such as dizziness or SSNHL occurring 10 to 14 days after vaccination, could coincide with the production of immunoglobulin G at 10 to 14 days after vaccine administration. In both the national VAERS reports and our multi-institutional case series, we found that the mean time to onset of SSNHL was 6 days, with the highest incidence at 0 to 2 days and 7 days after vaccination (Figure 2A and B). These temporal patterns were consistent with the timing of onset for other COVID-19 vaccine–associated AEs, such as myocarditis (2-4 days)13,14,15 and vaccine-induced immune thrombotic thrombocytopenia (7-10 days).16 In a large epidemiologic study, Baxter et al3 reported that the mean time to onset of reported SSNHL after influenza vaccination was also 2 days.
Observed peaks in reports of SSNHL at 1 and 7 days after vaccination in both VAERS and our case series could be partly accounted for by recall bias, which has been well documented in studies of passive vaccine AE reporting.17,18 For example, an analysis of AEs associated with the hepatitis B vaccine, in which patient self-reports were cross-referenced with specific vaccination records, found substantial recall bias that produced an inaccurate association between vaccination and the development of multiple sclerosis.19 The VAERS data may have been especially sensitive to recall bias because a substantial number of reports were submitted in a delayed manner, sometimes weeks to months after the onset of SSNHL. In particular, it is possible that patients, or health care professionals reporting on their behalf, may have estimated “about 1 day” or “about 1 week” when asked about the timing to onset of hearing loss because these are intuitive intervals for estimation. Bias in the perception of vaccine-associated AEs has substantial implications for an individual’s decision to receive a vaccine, as Betsch et al20 reported in a study of a simulated online social network. Participants in that study were more likely to overestimate true vaccine-associated AE rates if presented with narratives from others that suggested a higher risk of experiencing a vaccine-associated AE, and they were subsequently less likely to receive a vaccine.20 Notably, narrative information included in reports of AEs was more meaningful in influencing participants’ decisions to receive a vaccine than were statistical summaries.20
Similar to recommendations provided by other reports of AE clusters, including cerebral venous sinus thrombosis21 and myocarditis,13,14,15 after COVID-19 vaccination, long-term epidemiologic and vaccine safety studies supported by mechanistic research are needed to more definitively address any potential association between COVID-19 vaccination and SSNHL. Reports of recovery of SARS-CoV-2 RNA in the middle ear of individuals who died of COVID-1922 and recent findings of the ability of SARS-CoV-2 to directly infect human vestibular hair and Schwann cells23 provide plausible biological mechanisms for COVID-19–associated hearing loss and may open avenues of investigation into immune mechanisms in the inner ear.
Limitations
This study has several limitations. One limitation of the case series is its lack of a comparison group (eg, a group of patients who did not receive a COVID-19 vaccine but experienced SSNHL within the same period examined). Nonetheless, the detailed patient data in this series may serve as a supplement to the national patterns identified through analysis of SSNHL reports in the VAERS database.
Although an important tool for systematic vaccine safety studies,24 the VAERS incident reports used in the present study are not yet verified by the CDC and therefore need to be interpreted with caution. We specifically focused on SSNHL, which is a well-defined clinical condition with a known population-level incidence, in contrast to other otolaryngological conditions, such as tinnitus or Ménière disease. To account for inherent uncertainties associated with raw report data, we developed a standardized case definition for probable SSNHL to identify the most credible incident reports. Few data exist to guide selection of the risk interval for SSNHL after vaccination. The 3-week interval used in the present study was designed to be longer than the primary interval used in previous studies3 to balance considerations of temporal association with the risk of overexclusion.
It was also not possible to apply American Academy of Otolaryngology–Head and Neck Surgery criteria for SSNHL (loss of 30 dB over 3 consecutive frequencies)12 to VAERS reports given the lack of numerical audiometric testing results contained within those reports. Using a sensitivity analysis, the maximum incidence estimate was produced based on the assumptions that (1) all submitted reports represented true SSNHL, which was unlikely (eTable 1 in the Supplement), and (2) reports were subject to an additional 50% underreporting bias based on previous studies of VAERS sensitivity to detect rare AEs, such as Guillain-Barré syndrome and anaphylaxis.6 Therefore, our calculated maximum incidence is likely an overestimate of the true incidence of SSNHL, especially given that our 3-week time to onset interval was substantially longer than the interval of 0 to 72 hours endorsed by the American Academy of Otolaryngology–Head and Neck Surgery.12 In the absence of incident report verification and large-scale vaccine safety studies using verified reports, the estimation strategies used in this study nonetheless provide a snapshot and a potential tool that can be used by otolaryngologists challenged by this difficult clinical issue and its important public health implications.
Conclusions
This cross-sectional study and case series used an up-to-date analysis of VAERS case reports during the first 7 months of the US COVID-19 vaccination campaign across 3 vaccine manufacturers along with retrospective data from a series of patients with confirmed SSNHL, finding no population-level association between COVID-19 vaccination and SSNHL. Assessment of verified cases of SSNHL revealed heterogeneity in patient demographic characteristics, risk factors, and audiologic patterns. Further prospective investigation is needed to identify any potential associations between COVID-19 vaccination and SSNHL in some individuals. It is important that clinicians report all suspected COVID-19 vaccine–associated AEs rigorously and accurately to VAERS to allow verification and future performance of systematic vaccine safety studies.
eTable 1. Representative Examples of VAERS Incident Reports Meeting Criteria for Probable SSNHL Compared With Those Unlikely to Represent True SSNHL
eTable 2. Rate of SSNHL Reports in VAERS by Vaccine Manufacturer
eFigure 1. Scattergrams of Pretreatment and Posttreatment Hearing Results
eFigure 2. Audiogram Revealing Unilateral Sensorineural Hearing Loss Occurring 14 Days After Each of 2 COVID-19 Vaccine Doses in 1 Patient
eFigure 3. Number of People in the US With at Least 1 COVID-19 Vaccine Dose According to Age Group at 3 Points During the Initial COVID-19 Vaccination Rollout
References
- 1.Wichova H, Miller ME, Derebery MJ. Otologic manifestations after COVID-19 vaccination: the House Ear Clinic experience. Otol Neurotol. 2021;42(9):e1213-e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asatryan A, Pool V, Chen RT, Kohl KS, Davis RL, Iskander JK; VAERS Team . Live attenuated measles and mumps viral strain–containing vaccines and hearing loss: Vaccine Adverse Event Reporting System (VAERS), United States, 1990-2003. Vaccine. 2008;26(9):1166-1172. doi: 10.1016/j.vaccine.2007.12.049 [DOI] [PubMed] [Google Scholar]
- 3.Baxter R, Lewis N, Bohrer P, Harrington T, Aukes L, Klein NP. Sudden-onset sensorineural hearing loss after immunization: a case-centered analysis. Otolaryngol Head Neck Surg. 2016;155(1):81-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Formeister EJ, Chien W, Agrawal Y, Carey JP, Stewart CM, Sun DQ. Preliminary analysis of association between COVID-19 vaccination and sudden hearing loss using US Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System data. JAMA Otolaryngol Head Neck Surg. 2021;147(7):674-676. doi: 10.1001/jamaoto.2021.0869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . COVID data tracker. 2021. Accessed July 23, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 6.Miller ER, McNeil MM, Moro PL, Duffy J, Su JR. The reporting sensitivity of the Vaccine Adverse Event Reporting System (VAERS) for anaphylaxis and for Guillain-Barré syndrome. Vaccine. 2020;38(47):7458-7463. doi: 10.1016/j.vaccine.2020.09.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander TH, Harris JP. Incidence of sudden sensorineural hearing loss. Otol Neurotol. 2013;34(9):1586-1589. doi: 10.1097/MAO.0000000000000222 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Demographic trends of people receiving COVID-19 vaccinations in the United States. 2021. Accessed December 28, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- 9.McClung N, Chamberland M, Kinlaw K, et al. The Advisory Committee on Immunization Practices’ ethical principles for allocating initial supplies of COVID-19 vaccine—United States, 2020. Am J Transplant. 2021;21(1):420-425. doi: 10.1111/ajt.16437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong J, Lim H, Lee K, Hong CE, Choi HS. High risk of sudden sensorineural hearing loss in several autoimmune diseases according to a population-based national sample cohort study. Audiol Neurootol. 2019;24(5):224-230. doi: 10.1159/000502677 [DOI] [PubMed] [Google Scholar]
- 11.Tsilingiris D, Vallianou NG, Karampela I, Liu J, Dalamaga M. Potential implications of lipid nanoparticles in the pathogenesis of myocarditis associated with the use of mRNA vaccines against SARS-CoV-2. Metabol Open. 2022;13:100159. doi: 10.1016/j.metop.2021.100159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. 2019;161(1 suppl):S1-S45. [DOI] [PubMed] [Google Scholar]
- 13.Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144(6):502-505. doi: 10.1161/CIRCULATIONAHA.121.055891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148(3):e2021052478. doi: 10.1542/peds.2021-052478 [DOI] [PubMed] [Google Scholar]
- 15.Jain SS, Steele JM, Fonseca B, et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148(5):e2021053427. doi: 10.1542/peds.2021-053427 [DOI] [PubMed] [Google Scholar]
- 16.Perry RJ, Tamborska A, Singh B, et al. ; CVT After Immunisation Against COVID-19 (CAIAC) Collaborators . Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021;398(10306):1147-1156. doi: 10.1016/S0140-6736(21)01608-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen RT, DeStefano F. Vaccine adverse events: causal or coincidental? Lancet. 1998;351(9103):611-612. doi: 10.1016/S0140-6736(05)78423-3 [DOI] [PubMed] [Google Scholar]
- 18.Andrews NJ. Statistical assessment of the association between vaccination and rare adverse events post-licensure. Vaccine. 2001;20(suppl 1):S49-S53. doi: 10.1016/S0264-410X(01)00280-8 [DOI] [PubMed] [Google Scholar]
- 19.Ascherio A, Zhang SM, Hernan MA, et al. Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med. 2001;344(5):327-332. doi: 10.1056/NEJM200102013440502 [DOI] [PubMed] [Google Scholar]
- 20.Betsch C, Renkewitz F, Haase N. Effect of narrative reports about vaccine adverse events and bias-awareness disclaimers on vaccine decisions: a simulation of an online patient social network. Med Decis Making. 2013;33(1):14-25. doi: 10.1177/0272989X12452342 [DOI] [PubMed] [Google Scholar]
- 21.Ostergaard SD, Schmidt M, Horvath-Puho E, Thomsen RW, Sorensen HT. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet. 2021;397(10283):1441-1443. doi: 10.1016/S0140-6736(21)00762-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frazier KM, Hooper JE, Mostafa HH, Stewart CM. SARS-CoV-2 virus isolated from the mastoid and middle ear: implications for COVID-19 precautions during ear surgery. JAMA Otolaryngol Head Neck Surg. 2020;146(10):964-966. doi: 10.1001/jamaoto.2020.1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong M, Ocwieja KE, Han D, et al. Direct SARS-CoV-2 infection of the human inner ear may underlie COVID-19–associated audiovestibular dysfunction. Commun Med (London). 2021;1(1):44. doi: 10.1038/s43856-021-00044-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo EJ, Moro PL. Postmarketing safety surveillance of quadrivalent recombinant influenza vaccine: reports to the Vaccine Adverse Event Reporting System. Vaccine. 2021;39(13):1812-1817. doi: 10.1016/j.vaccine.2021.02.052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Representative Examples of VAERS Incident Reports Meeting Criteria for Probable SSNHL Compared With Those Unlikely to Represent True SSNHL
eTable 2. Rate of SSNHL Reports in VAERS by Vaccine Manufacturer
eFigure 1. Scattergrams of Pretreatment and Posttreatment Hearing Results
eFigure 2. Audiogram Revealing Unilateral Sensorineural Hearing Loss Occurring 14 Days After Each of 2 COVID-19 Vaccine Doses in 1 Patient
eFigure 3. Number of People in the US With at Least 1 COVID-19 Vaccine Dose According to Age Group at 3 Points During the Initial COVID-19 Vaccination Rollout