Key Points
Question
Are vessel density changes on optical coherence tomography angiography associated with future visual field changes over time among patients with open-angle glaucoma?
Findings
In this cohort study of 124 eyes in 82 patients, more rapid vessel density loss during an initial follow-up period was associated with greater rates of visual field loss during an extended period.
Meaning
These findings suggest that assessment of vessel density loss may be associated with visual field mean deviation loss, with optical coherence tomography angiography monitoring providing complementary information to visual field in monitoring patients with glaucoma.
This cohort study of patients with or suspected of having glaucoma assesses whether vessel density loss during initial follow-up is associated with visual field loss during extended follow-up.
Abstract
Importance
Rapid vessel density loss during an initial follow-up period may be associated with the rates of visual field loss over time.
Objectives
To evaluate the association between the rate of vessel density loss during initial follow-up and the rate of visual field loss during an extended follow-up period in patients suspected of having glaucoma and patients with primary open-angle glaucoma.
Design, Setting, and Participants
This retrospective cohort study assessed 124 eyes (86 with primary open-angle glaucoma and 38 suspected of having glaucoma) of 82 patients who were followed up at a tertiary glaucoma center for a mean of 4.0 years (95% CI, 3.9-4.1 years) from January 1, 2015, to February 29, 2020. Data analysis for the current study was undertaken in March 2021.
Main Outcomes and Measures
The rate of vessel density loss was derived from macular whole-image vessel density values from 3 optical coherence tomography angiography scans early during the study. The rate of visual field loss was calculated from visual field mean deviation during the entire follow-up period after the first optical coherence tomography angiography visit. Linear mixed-effects models were used to estimate rates of change.
Results
A total of 124 eyes from 82 patients (mean [SD] age, 69.2 [10.9] years; 41 female [50.0%] and 41 male [50.0%]; and 20 African American [24.4%], 10 Asian [12.2%], 50 White [61.0%], and 2 other race or ethnicity [2.4%]) were assessed. The annual rate of vessel density change was −0.80% (95% CI, −0.88% to −0.72%) during a mean initial follow-up of 2.1 years (95% CI, 1.9-2.3 years). Eyes with annual rates of vessel density loss of −0.75% or greater (n = 62) were categorized as fast progressors, and eyes with annual rates of less than −0.75% (n = 62) were categorized as slow progressors. The annual rate of visual field loss was −0.15 dB (95% CI, −0.29 to −0.01 dB) for the slow optical coherence tomography angiography progressors and −0.43 dB (95% CI, −0.58 to −0.29 dB) for the fast optical coherence tomography angiography progressors (difference, −0.28 dB; 95% CI, −0.48 to −0.08 dB; P = .006). The fast optical coherence tomography angiography progressor group was associated with the faster overall rate of visual field loss in a multivariable model after adjusting to include concurrent visual field mean deviation rate (−0.17 dB; 95% CI, −0.33 to −0.01 dB; P = .04).
Conclusions and Relevance
The findings of this cohort study suggest that faster vessel density loss during an initial follow-up period was associated with faster concurrent and subsequent rates of visual field loss during an extended period.
Introduction
Glaucoma is a leading cause of blindness worldwide and is characterized by progressive visual field (VF) loss.1,2 The VF test is an essential tool for following up patients with glaucoma, and progression of VF loss is monitored closely because of its significant effect on patients’ quality of life. To provide timely observation and treatment, it is beneficial to identify patients at greater risk of subsequent VF loss as early as possible.
Several diagnostic tests have been introduced to allow for more comprehensive evaluation of glaucomatous change, including optical coherence tomography angiography (OCTA).3,4,5 This diagnostic test uses a noninvasive imaging device that can assess retinal perfusion through the calculation of vessel density (VD). Patients with glaucoma have decreased retinal VD on OCTA compared with healthy controls and those suspected of having glaucoma.6
Previous studies7,8 have found that structural change often, but not always, precedes functional VF loss in glaucoma. As such, subsequent VF loss may often be associated with structural changes. For example, the retinal nerve fiber layer (RNFL) thickness measured by optical coherence tomography (OCT) has been reported to be associated with future VF loss.9,10 Recently, Swaminathan et al11 further demonstrated that rapid RNFL loss observed on OCT during a given initial follow-up period is associated with concurrent or future risk of faster VF loss.
Prior research12 on how structural parameters may be associated with functional loss has led to the hypothesis that VD may have a similar association with OCT thickness parameters. Previous studies13,14 have found that VD often decreases before detection of an apparent functional defect. In addition, decreased VD on OCTA was revealed to be associated with decreased RNFL thickness on OCT.4 However, prior studies have not examined the potential association between early VD decrease and future VF loss. In this study, we investigated the association between the rate of early VD loss and the rate of VF loss and compared it with the association between the rate of early ganglion cell complex (GCC) thinning and the rate of VF loss in patients suspected of having glaucoma and patients with primary open-angle glaucoma (POAG).
Methods
Participants
This retrospective cohort study assessed patients suspected of having glaucoma and patients with POAG enrolled in the Diagnostic Innovations in Glaucoma Study (DIGS)15,16 who underwent OCTA and spectral domain–OCT imaging using the Avanti Angiovue system (Optovue Inc). All DIGS participants were assessed longitudinally according to a standard protocol that consisted of regular follow-up visits with clinical examination, imaging, and functional tests at a tertiary glaucoma center. Patients were followed up for a mean of 4.0 years (95% CI, 3.9-4.1 years) from January 1, 2015, to February 29, 2020, and data analysis for the current study was undertaken in March 2021. All participants from DIGS who met the inclusion criteria described below were included. Written informed consent was obtained from all study participants. The University of California, San Diego, Human Subject Committee approved all protocols, and the methods described adhered to the tenets of the Declaration of Helsinki.17 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
All study participants underwent annual comprehensive ophthalmologic evaluation including best-corrected visual acuity, slitlamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, dilated fundus examination, stereoscopic optic disc photography, and ultrasonographic pachymetry in both eyes. Semiannual evaluations included intraocular pressure (IOP) measurement, OCTA imaging, and standard automated perimetry testing. This study included eyes with a minimum of 3 follow-up OCTA/OCT scans. The rates of VD loss and GCC thinning were then calculated based on 3 OCTA/OCT tests. For comparison with the rates and magnitude of VF mean deviation (MD) loss, standard automated perimetry tests were performed during follow-up, starting within 6 months after the first OCTA/OCT visit. A minimum follow-up time of 2 years and a minimum of 5 follow-up VF tests were required. The group of patients suspected of having glaucoma included eyes with elevated IOP (≥22 mm Hg) or glaucomatous-appearing optic discs (glaucomatous optic neuropathy) without the presence of repeatable glaucomatous VF damage. Eyes were classified as glaucomatous if they had repeatable (at least 2 consecutive) abnormal VF test results with evidence of glaucomatous optic neuropathy. Glaucomatous optic neuropathy was defined as excavation, the presence of focal thinning, notching of the neuroretinal rim, or localized or diffuse atrophy of the RNFL on the basis of masked grading of optic disc photographs by 2 graders or clinical examination by a glaucoma specialist. Disagreement was resolved by discussion. An abnormal VF test result was defined as a pattern SD outside the normal 95% CI or a Glaucoma Hemifield Test result outside normal limits. Glaucoma disease severity was classified as early (24-2 VF MD >−6 dB) and moderate to advanced (24-2 VF MD ≤−6 dB).
Inclusion criteria also included (1) age older than 18 years, (2) open angles on gonioscopy, (3) best-corrected visual acuity of 20/40 or better, and (4) refraction within ±5.0 diopters spherical and within ±3.0-diopters cylinder at study entry. Exclusion criteria included (1) history of trauma or intraocular surgery (except for uncomplicated cataract surgery or glaucoma surgery); (2) coexisting retinal disease, uveitis, or nonglaucomatous optic neuropathy; (3) other systemic or ocular diseases known to affect VF, such as pituitary lesions or demyelinating diseases; (4) significant cognitive impairment, Parkinson disease, Alzheimer disease, dementia, or a history of stroke; or (5) axial length of 27 mm or more. Those with unreliable VFs and poor-quality OCTA were also excluded.
OCTA and Spectral-Domain OCT
The OCTA and OCT images were acquired simultaneously, and the Angiovue system (software version 2018.1.0.43) performed automatic segmentation with exact registration of the analyzed regions.18 The OCT-based thickness measurement and OCTA-based vascular measurement were then calculated from the same scan slab. Vessel density was automatically calculated as the proportion of the measured area occupied by flowing blood, which is defined as pixels having decorrelation values (acquired by the split-spectrum amplitude-decorrelation angiography algorithm) above the threshold level. Whole-image vessel density (wiVD) from 3 × 3-mm2 scans (304 B-scans × 304 A-scans per B-scan) centered on the fovea were obtained with this system. Image quality review was completed on all scans according to the University of California, San Diego, Imaging Data Evaluation and Analysis Reading Center standard protocol. Trained graders reviewed scans and excluded poor-quality images, defined as images with (1) a scan quality less than 4, (2) poor clarity, (3) residual motion artifacts visible as irregular vessel pattern or disc boundary on the en face angiogram, (4) local weak signal, or (5) severe segmentation failures. Modifiable segmentation failure was adjusted manually if required. The GCC thickness, consisting of the RNFL, ganglion cell layer, and internal plexiform layer, was calculated from the macular cube image acquired from the OCTA scan. Each B-scan comprises 933 A-scans. The whole-image GCC (wiGCC) thickness was calculated from the same fovea-centered region where the wiVD was obtained. Three wiVD and wiGCC measurements taken on the same day were selected as the early 3 visits for this study.
Statistical Analysis
Patient and eye characteristics data are presented as mean (95% CI) for continuous variables and number (percentage) for categorical variables. Categorical variables were compared using the χ2 test. Mixed-effects modeling was used to compare ocular parameters among groups. Models were fitted with ocular measurements as the response variable and OCTA or OCT progressor groups as fixed effects. Estimates of rates of change for individual eyes were obtained by best linear unbiased prediction (BLUP). BLUPs are shrinkage estimates that take into account the results obtained by evaluating the whole sample of eyes, giving less weight to estimates obtained from eyes with fewer measurements or large intraindividual variability.19 We have previously used BLUPs to estimate individual rates of change measured by different instruments.20,21 Linear mixed-effects models estimate the mean rate of change in an outcome variable using a linear function of time, and participant- and eye-specific deviations from this mean rate are introduced by random slopes. On the basis of the median rate of the VD loss, eyes were categorized based on the annual rates of VD loss as either fast (≥−0.75%) or slow (<−0.75%) OCTA progressors. Moreover, on the basis of the annual median rate of the GCC loss, eyes were categorized as either fast (≥−0.87 μm) or slow (<−0.87 μm) OCT progressors. Adjusted rates of VF MD loss from the multivariable model were then combined with the mean total follow-up period to estimate the rates of VF MD loss during the total study period among OCTA/OCT progressor groups. The association of potential variables, such as age, mean IOP during the follow-up, and any other variable in which P < .10 in univariable analysis, with the rates of VF MD loss during the total follow-up was also introduced in the multivariable model. The concurrent VF MD rate (ie, the VF MD rate during the same period as the initial 3 OCTA/OCT scans) was also included as a covariate to assess whether OCTA progressor status significantly influenced future rates of VF loss even when accounting for concurrent rates of VF MD loss. There were no corrections of P values made for multiple comparisons. Statistical analyses were performed using Stata, version 16.0 (StataCorp LLC). A 2-sided P < .05 was considered to be statistically significant.
Results
A total of 124 eyes from 82 patients (mean [SD] age, 69.2 [10.9] years; 41 female [50.0%] and 41 male [50.0%]; and 20 African American [24.4%], 10 Asian [12.2%], 50 White [61.0%], and 2 other race or ethnicity [2.4%]) were assessed. The mean baseline wiVD was 44.0% (95% CI, 43.3%-44.8%), whereas the baseline VF MD was −3.4 dB (95% CI, −4.2 to −2.5 dB). A mean of 6.6 (95% CI, 6.4-6.9) VFs were observed during the 4.0 years of follow-up (95% CI, 3.9-4.1 years). Forty-eight eyes (38.7%) were pseudophakic at baseline, and 7 eyes underwent cataract surgery during the study. Demographic and baseline clinical characteristics of the participants are presented in Table 1.
Table 1. Demographic and Baseline Clinical Characteristics of the Study Participants.
| Characteristic | Finding (N = 124 eyes of 82 patients)a |
|---|---|
| Age, mean (SD), y | 69.2 (10.9) |
| Sex, F/M, No. | 41/41 |
| Race, No. (%) | |
| African American | 20 (24.4) |
| Asian | 10 (12.2) |
| White | 50 (61.0) |
| Other | 2 (2.4) |
| Baseline IOP, mm Hg | 14.6 (13.8 to 15.3) |
| No. of patients with glaucoma | 86 |
| No. of patients suspected of having glaucoma | 38 |
| Disease severity by baseline (24-2 VF MD), No. (%) | |
| Early glaucoma eye | 61 (70.9) |
| Moderate and advanced glaucoma eye | 25 (29.1) |
| Baseline VF MD, dB | −3.4 (−4.2 to −2.5) |
| Baseline VF PSD, dB | 4.5 (3.8 to 5.1) |
| Baseline wiGCC, μm | 91.5 (89.2 to 93.7) |
| Baseline wiVD, % | 44.0 (43.3 to 44.8) |
| SSI | 66.9 (65.6 to 68.1) |
| Follow-up, y | 4.0 (3.9 to 4.1) |
| No. of VF visits | 6.6 (6.4 to 6.9) |
Abbreviations: IOP, intraocular pressure; MD, mean deviation; PSD, pattern SD; SSI, signal strength index; VF, visual field; wiGCC, whole-image ganglion cell complex; wiVD, whole-image vessel density.
Data are presented as mean (95% CI) unless otherwise indicated.
Table 2 summarizes the characteristics of eyes categorized by the OCTA progressor group. The annual rate of VD change was −0.80% (95% CI, −0.88% to −0.72%) during initial follow-up of 2.1 years (95% CI, 1.9-2.3 years). Sixty-two eyes were classified as slow and 62 as fast OCTA progressors. The fast OCTA progressors had a faster mean annual rate of VD loss (−1.14%; 95% CI, −1.22% to −1.05%) compared with the slow progressors (−0.47%; 95% CI, −0.53% to −0.41%) (MD, −0.67%; 95% CI, −0.78% to −0.56%; P < .001). A lower proportion of African American participants were in the fast OCTA progressor group (6 [14.0%]) compared with non–African American participants (37 [86.0%]), and differences were found between the slow and fast OCTA progressor groups with respect to diagnosis group, baseline 24-2 VF MD, baseline VF pattern SD, and number of VF follow-up visits. Similarly, the characteristics of eyes categorized by OCT progressor group are summarized in the eTable in the Supplement. The eFigure in the Supplement shows representative cases from the slow and fast OCTA progressor groups.
Table 2. Characteristics of Eyes in the Optical Coherence Tomography Angiography Progressor Group.
| Characteristic | Mean (95% CI)a | P value | ||
|---|---|---|---|---|
| Slow (62 eyes of 39 patients)b | Fast (62 eyes of 43 patients)b | Difference | ||
| Initial vessel density change, %/y | −0.47 (−0.53 to −0.41) | −1.14 (−1.22 to −1.05) | −0.67 (−0.78 to −0.56) | <.001 |
| Baseline age, mean (SD), y | 67.6 (11.4) | 70.4 (10.4) | 2.0 (15.6) | .35 |
| Sex, F/M | 21/18 | 20/23 | NA | .51 |
| Race | ||||
| African American | 14 | 6 | NA | .02 |
| Asian | 2 | 8 | NA | |
| White | 23 | 27 | NA | |
| Other | 0 | 2 | NA | |
| Self-reported hypertension, No. (%) | 25 (64.1) | 22 (51.2) | NA | .24 |
| Self-reported diabetes, No. (%) | 8 (20.5) | 4 (9.3) | NA | .15 |
| MOPP, mm Hg | 53.7 (51.9 to 55.6) | 52.9 (51.0 to 54.8) | −0.8 (−3.4 to 1.7) | .53 |
| Axial length, mm | 24.3 (24.1 to 24.6) | 24.6 (24.3 to 24.8) | 0.2 (−0.2 to 0.6) | .27 |
| CCT, μm | 538.3 (529.0 to 547.6) | 525.1 (514.6 to 535.6) | −13.2 (−27.0 to 0.7) | .06 |
| Baseline IOP, mm Hg | 14.5 (13.5 to 15.5) | 14.6 (13.5 to 15.7) | 0.1 (−1.4 to 1.6) | .90 |
| Mean IOP during follow-up, mm Hg | 14.8 (13.7 to 15.8) | 14.9 (14.1 to 15.7) | −0.1 (−1.5 to 1.3) | .85 |
| No. of eyes with glaucoma | 38 | 48 | NA | .05 |
| No. of eyes suspected of having glaucoma | 24 | 14 | NA | |
| Disease severity at baseline (24-2 VF MD), No. (%) | ||||
| Early glaucoma | 31 (81.6) | 30 (62.5) | NA | .06 |
| Moderate and advanced glaucoma | 7 (18.4) | 18 (37.5) | NA | |
| Baseline VF MD, dB | −2.3 (−3.4 to −1.3) | −4.4 (−5.8 to −3.1) | −2.1 (−3.9 to −0.2) | .03 |
| Baseline VF PSD, dB | 3.5 (2.7 to 4.2) | 5.4 (4.3 to 6.5) | 1.9 (0.6 to 3.3) | .006 |
| SSI | 67.1 (65.5 to 68.7) | 66.7 (64.6 to 68.7) | −0.4 (−3.0 to 2.2) | .76 |
| Follow-up, y | 4.1 (3.9 to 4.2) | 3.9 (3.7 to 4.1) | −0.2 (−0.4 to 0.1) | .26 |
| No. of VF visits | 7.0 (6.5 to 7.4) | 6.3 (6.0 to 6.6) | −0.6 (−1.3 to 0.0) | .04 |
Abbreviations: CCT, central corneal thickness; IOP, intraocular pressure; MD, mean deviation; MOPP, mean ocular perfusion pressure; NA, not applicable; PSD, pattern SD; SSI, signal strength index; VF, visual field; wiVD, whole-image vessel density.
Data are given as mean (95% CI) unless otherwise indicated.
For rates of wiVD change, slow indicates slower than −0.75% per year, and fast indicates −0.75% per year or faster.
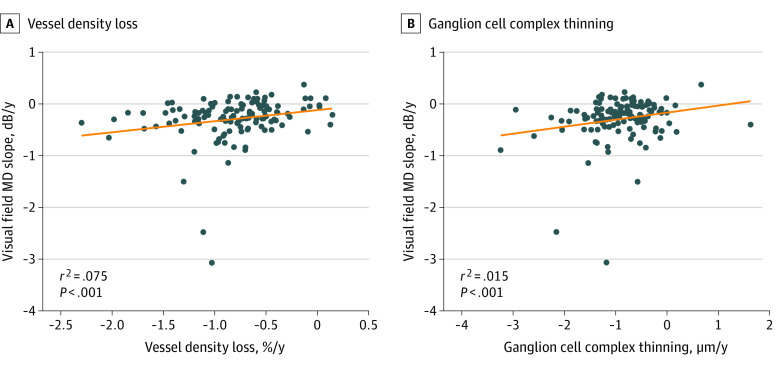
Factors that contributed to the rate of VF MD change during the total follow-up are summarized in Table 3. In the univariable model, the fast OCTA progressor group showed faster annual VF MD loss (−0.43 dB; 95% CI, −0.58 to −0.29 dB vs −0.15 dB; 95% CI, −0.29 to −0.01 dB; MD, −0.28 dB; 95% CI, −0.48 to −0.08 dB; P = .006), whereas the fast OCT progressor group showed faster annual VF MD loss (−0.39; 95% CI, −0.54 to −0.25 dB vs −0.19; 95% CI, −0.33 to −0.04 dB; MD, −0.20 dB; 95% CI, −0.41 to 0.00 dB; P = .05). Higher mean IOP during follow-up, worse baseline VF MD, and a faster concurrent VF MD rate were also associated with the overall rate of VF MD loss in a univariable model. In multivariable model 1 without concurrent VF MD rate, the fast OCTA progressor group was associated with faster annual rates of overall VF MD loss (−0.28 dB; 95% CI, −0.49 to −0.07 dB; P < .008). In multivariable model 2 with concurrent VF MD rate, the fast OCTA progressor group and concurrent VF MD rate were associated with faster annual rates of overall VF MD loss (−0.17 dB; 95% CI, −0.33 to −0.01 dB; P = .04 in the first group vs 1.44 dB; 95% CI, 0.98, 1.89 dB; P < .001 in the second group). After adjustment for covariates, the association was stronger for the OCTA model (multivariable model 1: r2 = 0.075, P < .001) than the OCT model (multivariable model 3: r2 = 0.015, P < .001). The Figure shows the association between the rates of VF MD loss and the rate of wiVD loss as well as the rates of wiGCC thinning.
Table 3. Factors Contributing to the Rate of VF MD Change Over Time by Univariable and Multivariable Mixed-Effects Model Analysis.
| Factor | Univariable model | Multivariable model 1 | Multivariable model 2 | Multivariable model 3 | Multivariable model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| Overall | ||||||||||
| Intercept | NA | NA | −7.48 (−15.17 to 0.22) | .06 | −1.73 (−7.86 to 4.30) | .57 | −8.03 (−15.80 to −0.25) | .04 | −1.72 (−7.95 to 4.50) | .59 |
| Age, per 10 y older | −0.02 (−0.11 to 0.07) | .68 | −0.02 (−0.12 to 0.08) | .71 | −0.03 (−0.12 to 0.06) | .55 | −0.03 (−0.13 to 0.08) | .59 | −0.03 (−0.13 to 0.06) | .46 |
| Female sex | 0.01 (−0.19 to 0.22) | .89 | NA | NA | NA | NA | NA | NA | NA | NA |
| African American race | 0.13 (−0.11 to 0.38) | .28 | NA | NA | NA | NA | NA | NA | NA | NA |
| Self-reported diabetes | 0.02 (−0.27 to 0.32) | .88 | NA | NA | NA | NA | NA | NA | NA | NA |
| Self-reported hypertension | 0.13 (−0.08 to 0.33) | .24 | NA | NA | NA | NA | NA | NA | NA | NA |
| MOPP, per 1 mm Hg lower | 0.01 (0.00 to 0.02) | .19 | NA | NA | NA | NA | NA | NA | NA | NA |
| Axial length, per 1 mm longer | 0.06 (−0.04 to 0.16) | .23 | NA | NA | NA | NA | NA | NA | NA | NA |
| CCT, per 100 μm thinner | −0.10 (0.18 to −0.38) | .48 | NA | NA | NA | NA | NA | NA | NA | NA |
| Baseline IOP, per 1 mm Hg higher | −0.01 (−0.03 to 0.02) | .48 | NA | NA | NA | NA | NA | NA | NA | NA |
| Mean IOP during follow-up, per 1 mm Hg higher | −0.04 (−0.07 to −0.01) | .006 | −0.04 (−0.10 to 0.02) | .16 | −0.04 (−0.10 to 0.01) | .10 | −0.03 (−0.09 to 0.02) | .22 | −0.04 (−0.10 to 0.01) | .12 |
| Concurrent VF MD rate (dB/y) | 1.50 (1.08 to 1.91) | <.001 | NA | NA | 1.44 (0.98 to 1.89) | <.001 | NA | NA | 1.51 (1.01 to 2.02) | <.001 |
| Follow-up period, per 1 y longer | −0.03 (−0.20 to 0.14) | .75 | NA | NA | NA | NA | NA | NA | NA | NA |
| No. of VF follow-up visits | −0.01 (−0.08 to 0.06) | .75 | NA | NA | NA | NA | NA | NA | NA | NA |
| SSI, per 1 higher | 0.00 (−0.01 to 0.02) | .96 | NA | NA | NA | NA | NA | NA | NA | NA |
| OCT progressor group | ||||||||||
| Intercept (baseline: slow) | −0.19 (−0.33 to −0.04) | .01 | NA | NA | NA | NA | NA | NA | NA | NA |
| Fast − slow | −0.20 (−0.41 to 0.00) | .05 | NA | NA | NA | NA | −0.14 (−0.29 to 0.01) | .06 | −0.08 (−0.20 to 0.04) | .21 |
| OCTA progressor group | ||||||||||
| Intercept (baseline: slow) | −0.15 (−0.29 to −0.01) | .03 | NA | NA | NA | NA | NA | NA | NA | NA |
| Fast − slow | −0.28 (−0.48 to −0.08) | .006 | −0.28 (−0.49 to −0.07) | .008 | −0.17 (−0.33 to −0.01) | .04 | NA | NA | NA | NA |
Abbreviations: CCT, central corneal thickness; IOP, intraocular pressure; MD, mean deviation; MOPP, mean ocular perfusion pressure; NA, not applicable; OCTA, optical coherence tomography angiography; SSI, signal strength index; VF, visual field.
Figure. Association of the Rate of Visual Field Mean Deviation (MD) Loss and the Rate of Vessel Density Loss and Ganglion Cell Complex Thinning.
The r2 and P value represent the association after adjustment for age and mean intraocular pressure during follow-up.
Discussion
This cohort study investigated the association between the rates of initial VD loss and subsequent VF loss in patients suspected of having glaucoma and patients with POAG. After dividing the included eyes into fast and slow OCTA progressors based on the median rate of VD change during the initial visits, a significantly greater rate of VF MD change during the 4-year mean follow-up period was found for fast OCTA progressors. Notably, the OCTA progressor group was associated with overall VF MD loss, even when accounting for the concurrent VF MD rate in the multivariable model. This result with OCT-measured RNFL thickness was similar to that reported in a recent study.11 Although in the earlier study11 the eyes were divided into 3 progressor groups (slow, moderate, and fast progressors), the study found that a rapid initial RNFL thinning was associated with concurrent and subsequent rates of VF loss. The similarity of these findings across different parameters than those in the current study are consistent with OCTA progression being associated with VF MD loss and also with monitoring VF in patients with glaucoma.
In our study, the included eyes were classified as fast or slow OCTA progressors based on the median annual percentage rate of change in wiVD (−0.75%). Although there is no established standard for the definition of fast VD loss, we sought to set a reasonable cutoff value for the interpretation of initial VD loss in the current study to present more clinically applicable findings. In this study, we used linear regression to model glaucomatous progression. However, progression of VD loss, VF loss, and other parameters may not necessarily occur in a linear manner, and the rate of progression of these parameters may vary at different stages of the disease. Although there may be pitfalls when using linear regression in the study of glaucoma, linear regression provides a more straightforward analysis that may allow clinicians and patients to appreciate glaucomatous progression in various clinical scenarios. Therefore, trend analysis using linear regression remains a useful method to identify glaucoma progression.
Some previous studies14,22 suggest that OCTA-measured VD may provide useful data in addition to OCT-measured RNFL thickness when evaluating for glaucoma. In a prospective study with a follow-up period of 2 to 3 years, Hou et al23 found that in POAG, the rate of macular VD loss was not only significantly faster than GCC thinning but was also more strongly associated with the severity of disease, thus supporting the usefulness of OCTA in evaluating advancing glaucoma.
Whether OCTA may be clinically useful in evaluating glaucomatous progression has been a focus of recent investigations.22,24 To the best of our knowledge, the current study is the first to examine the association between the rate of initial VD loss during a given period and subsequent rate of VF progression. Most prior studies3,4,25 have examined the association of baseline VD values or glaucomatous progression and longitudinal VD loss. Recently, Shin et al26 examined the association between VF progression and longitudinal changes in circumpapillary capillary density and circumpapillary RNFL thickness in eyes with open-angle glaucoma. They found a significantly faster rate of circumpapillary capillary density change on OCTA in progressors compared with that of nonprogressors, regardless of glaucoma stage. In contrast, no such association was found for circumpapillary RNFL thickness. Although the clinical relevance of the association between retinal VD and glaucomatous progression will require further investigation, OCTA parameters appear to be complementary to other test results, including the more established OCT parameters, in clinical glaucoma evaluation.
Limitations
This study has several limitations. First, we chose to evaluate 3 OCTA/OCT scans to determine the progressor group. A previous study27 reported that OCTA has lower reproducibility than OCT, and a larger number of scans may result in more accurate interpretation. In addition, with more studies28,29 showing a higher diagnostic accuracy for the 6 × 6-mm2 than the 3 × 3-mm2 scan, it should be kept in mind that the 3 × 3-mm2 scan may no longer be the best choice for evaluating glaucomatous macular damage or for providing the best indication of VF. Second, because the patients came to our clinic at different stages of disease, the preexisting course of glaucomatous change and progression during the initial visits may vary across patients. This study includes many eyes with early glaucoma, which should be considered when extrapolating the results to moderate to severe stages of glaucoma. Third, although eyes with nonglaucomatous VF loss were excluded, some minor cause of VF loss, such as dry eye disease and mild cataract, were not considered in this study. Although some patients (48 eyes [38.7%]) were pseudophakic at baseline, only 7 eyes underwent cataract surgery during the study. Hence, we believe the presence of media opacity would not have much influence on the data.30 Fourth, the data were collected before study design. Thus, we cannot exclude the possibility of bias associated with data selection that might affect the results. Fifth, residual confounding factors that may influence the generalizability of our study results should be noted, including patient demographic characteristics, OCT nerve thickness measurements, and the variability of OCTA measurements. With a relatively small sample size, the results should be interpreted with caution.
Conclusions
In this cohort study, rapid VD loss during an initial follow-up period was associated with higher concurrent and subsequent rates of VF MD loss during an extended period. This finding is clinically relevant if subsequent prospective studies confirm that fast progressors identified by OCTA are at higher risk of functional loss and may need more intensive observation and treatment. Taking the limitations of this investigation into account, the results support the use of OCTA for monitoring the rate of VD loss to assess VF progression in patients with glaucoma.
eTable. Characteristics of Eyes Categorized by Optical Coherence Tomography Progressor Group
eFigure. Case Examples of Fast and Slow Optical Coherence Tomography Angiography Progressor Group
References
- 1.Weinreb RN, Leung CK, Crowston JG, et al. Primary open-angle glaucoma. Nat Rev Dis Primers. 2016;2:16067. doi: 10.1038/nrdp.2016.67 [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901-1911. doi: 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoji T, Zangwill LM, Akagi T, et al. Progressive macula vessel density loss in primary open-angle glaucoma: a longitudinal study. Am J Ophthalmol. 2017;182:107-117. doi: 10.1016/j.ajo.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghimi S, Zangwill LM, Penteado RC, et al. Macular and optic nerve head vessel density and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2018;125(11):1720-1728. doi: 10.1016/j.ophtha.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 5.WuDunn D, Takusagawa HL, Sit AJ, et al. OCT angiography for the diagnosis of glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2021;128(8):1222-1235. doi: 10.1016/j.ophtha.2020.12.027 [DOI] [PubMed] [Google Scholar]
- 6.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci. 2016;57(9):OCT451-OCT459. doi: 10.1167/iovs.15-18944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harwerth RS, Carter-Dawson L, Shen F, Smith EL III, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40(10):2242-2250. [PubMed] [Google Scholar]
- 8.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688-710. doi: 10.1016/j.preteyeres.2007.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121(7):1350-1358. doi: 10.1016/j.ophtha.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu M, Lin C, Weinreb RN, Lai G, Chiu V, Leung CK. Risk of visual field progression in glaucoma patients with progressive retinal nerve fiber layer thinning: a 5-year prospective study. Ophthalmology. 2016;123(6):1201-1210. doi: 10.1016/j.ophtha.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 11.Swaminathan SS, Jammal AA, Berchuck SI, Medeiros FA. Rapid initial OCT RNFL thinning is predictive of faster visual field loss during extended follow-up in glaucoma. Am J Ophthalmol. 2021;229:100-107. doi: 10.1016/j.ajo.2021.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros FA, Lisboa R, Zangwill LM, et al. Evaluation of progressive neuroretinal rim loss as a surrogate end point for development of visual field loss in glaucoma. Ophthalmology. 2014;121(1):100-109. doi: 10.1016/j.ophtha.2013.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology. 2017;124(5):709-719. doi: 10.1016/j.ophtha.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamalipour A, Moghimi S, Jacoba CM, et al. Measurements of OCT angiography complement OCT for diagnosing early primary open-angle glaucoma. Ophthalmol Glaucoma. Published online October 9, 2021. doi: 10.1016/j.ogla.2021.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sample PA, Girkin CA, Zangwill LM, et al. ; African Descent and Glaucoma Evaluation Study Group . The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127(9):1136-1145. doi: 10.1001/archophthalmol.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girkin CA, Sample PA, Liebmann JM, et al. ; ADAGES Group . African Descent and Glaucoma Evaluation Study (ADAGES), II: ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol. 2010;128(5):541-550. doi: 10.1001/archophthalmol.2010.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Jia Y, Takusagawa HL, et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133(9):1045-1052. doi: 10.1001/jamaophthalmol.2015.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson GK. That BLUP is a good thing: the estimation of random effects. Stat Sci. 1991;6(1):15-32. doi: 10.1214/ss/1177011926 [DOI] [Google Scholar]
- 20.Medeiros FA, Zangwill LM, Weinreb RN. Improved prediction of rates of visual field loss in glaucoma using empirical Bayes estimates of slopes of change. J Glaucoma. 2012;21(3):147-154. doi: 10.1097/IJG.0b013e31820bd1fd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medeiros FA, Zangwill LM, Alencar LM, Sample PA, Weinreb RN. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol. 2010;149(6):908-915. doi: 10.1016/j.ajo.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 22.Moghimi S, Bowd C, Zangwill LM, et al. Measurement floors and dynamic ranges of OCT and OCT angiography in glaucoma. Ophthalmology. 2019;126(7):980-988. doi: 10.1016/j.ophtha.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou H, Moghimi S, Proudfoot JA, et al. Ganglion cell complex thickness and macular vessel density loss in primary open-angle glaucoma. Ophthalmology. 2020;127(8):1043-1052. doi: 10.1016/j.ophtha.2019.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HL, Kim JW, Park CK. Choroidal microvasculature dropout is associated with progressive retinal nerve fiber layer thinning in glaucoma with disc hemorrhage. Ophthalmology. 2018;125(7):1003-1013. doi: 10.1016/j.ophtha.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 25.Rao HL, Srinivasan T, Pradhan ZS, et al. Optical coherence tomography angiography and visual field progression in primary angle closure glaucoma. J Glaucoma. 2021;30(3):e61-e67. doi: 10.1097/IJG.0000000000001745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin JW, Song MK, Kook MS. Association between progressive retinal capillary density loss and visual field progression in open-angle glaucoma patients according to disease stage. Am J Ophthalmol. 2021;226:137-147. doi: 10.1016/j.ajo.2021.01.015 [DOI] [PubMed] [Google Scholar]
- 27.Manalastas PIC, Zangwill LM, Saunders LJ, et al. Reproducibility of optical coherence tomography angiography macular and optic nerve head vascular density in glaucoma and healthy eyes. J Glaucoma. 2017;26(10):851-859. doi: 10.1097/IJG.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penteado RC, Bowd C, Proudfoot JA, et al. Diagnostic ability of optical coherence tomography angiography macula vessel density for the diagnosis of glaucoma using difference scan sizes. J Glaucoma. 2020;29(4):245-251. doi: 10.1097/IJG.0000000000001447 [DOI] [PubMed] [Google Scholar]
- 29.You QS, Tan O, Pi S, et al. Effect of algorithms and covariates in glaucoma diagnosis with optical coherence tomography angiography. Br J Ophthalmol. Published online June 28, 2021. doi: 10.1136/bjophthalmol-2020-318677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musch DC, Gillespie BW, Niziol LM, et al. ; Collaborative Initial Glaucoma Treatment Study Group . Cataract extraction in the collaborative initial glaucoma treatment study: incidence, risk factors, and the effect of cataract progression and extraction on clinical and quality-of-life outcomes. Arch Ophthalmol. 2006;124(12):1694-1700. doi: 10.1001/archopht.124.12.1694 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Characteristics of Eyes Categorized by Optical Coherence Tomography Progressor Group
eFigure. Case Examples of Fast and Slow Optical Coherence Tomography Angiography Progressor Group