Key Points
Question
What is the cost-effectiveness of pertuzumab, trastuzumab, and chemotherapy for patients with metastatic breast cancer?
Findings
In this economic evaluation, pertuzumab treatment was associated with survival benefit but was not cost-effective based on conventional willingness-to-pay threshold ($100 000/quality-adjusted life-year), even after reducing the price of pertuzumab to $0. This finding supports previous model-based results from the UK National Institute for Health and Care Excellence that suggested pertuzumab would not be cost-effective even at a price of $0, using the conventional threshold.
Meaning
Results of this study suggest that, based on the conventional willingness-to-pay threshold, pertuzumab, trastuzumab, and chemotherapy would not be considered cost-effective, even if pertuzumab was free.
Abstract
Importance
The initial assessment of pertuzumab use for treatment of metastatic breast cancer by health technology assessment agencies suggested that pertuzumab was not cost-effective. In Ontario, Canada, pertuzumab became funded in November 2013 based on the substantial clinical benefit. To date, there is a paucity of analysis of pertuzumab using real-world data for cost-effectiveness.
Objective
To assess the cost-effectiveness of pertuzumab, trastuzumab, and chemotherapy vs trastuzumab and chemotherapy for patients with metastatic breast cancer.
Design, Setting, and Participants
A population-based retrospective economic evaluation was conducted in Ontario, Canada. Patients who received first-line treatments for metastatic breast cancer from January 1, 2008, to March 31, 2018, were identified. Patients were followed up from the start of treatment up to 5 years, with maximum follow-up to March 31, 2019. Patients were identified from the Ontario Cancer Registry and linked to the New Drug Funding Program database to identify receipt of first-line treatment (N = 1158).
Interventions
Treatment with pertuzumab, trastuzumab, and chemotherapy after public funding (November 25, 2013) compared with treatment with trastuzumab and chemotherapy before funding.
Main Outcomes and Measures
Cost-effectiveness, from a public payer perspective, was estimated from administrative data with a 5-year time horizon, adjusted for censoring, and discounted (1.5%). Incremental cost-effectiveness ratios for life-years gained and quality-adjusted life year (QALY) with bootstrapped 95% CIs were calculated. Sensitivity analysis with price reduction of pertuzumab alone or in combination with trastuzumab was conducted.
Results
A total of 579 pairs of matched patients receiving pertuzumab and controls were included. The mean (SD) age of the matched study cohort was 58 (12.97) years; 1151 were women (99.4%). Pertuzumab resulted in 0.61 life-years gained and 0.44 QALYs gained at an incremental cost of $192 139 (all costs measured in Canadian dollar values, CAD) with an incremental cost-effectiveness ratio of $316 203 per life-year gained and $436 679 per QALY. The main factors associated with cost included the cost of pertuzumab (60%), outpatient cancer treatment delivery (24%), and trastuzumab (15%). With 100% price reduction of pertuzumab, the incremental cost-effectiveness ratio was $174 027 per QALY. When the price of pertuzumab and trastuzumab were both reduced by more than 71%, the incremental cost-effectiveness ratio decreased below $100 000 per QALY.
Conclusions and Relevance
The findings of this population-based study suggest that pertuzumab may increase survival for patients with metastatic breast cancer but would not be considered cost-effective, even after 100% price reduction, under conventional thresholds.
This economic evaluation examines the cost-effectiveness of pertuzumab, trastuzumab, and chemotherapy vs trastuzumab and chemotherapy for patients with metastatic breast cancer.
Introduction
The economic burden of cancer care is increasing around the world.1,2 In Canada, the national net expenditure in Canadian dollars (CAD) for cancer care more than doubled from $2.9 billion in 2005 to $7.5 billion in 2012.3 The estimated lifetime total cost for patients with breast cancer, which accounts for 25% of new cancer diagnoses in women in Canada,4 is one of the highest among different tumor types.5 One study reported that, during a period of 10 years, the 1-year treatment cost incurred after breast cancer diagnosis increased by 2-fold.6 One factor in this increasing cancer care cost is use of chemotherapy, which has grown more than 3-fold from 2005 to 2012.3
With the changing treatment landscape in breast cancer, patients diagnosed with late-stage cancer often incur higher cancer-related drug costs.7 For ERBB2 (formerly HER2)-positive breast cancer, the cancer-related drug costs for ERBB2-positive cancer can be 6- to 10-fold higher than those for ERBB2-negative breast cancers.7,8 For patients with metastatic breast cancer that is ERBB2-positive, the standard first-line treatment in Canada was trastuzumab, an ERBB2-targeted therapy, plus taxane in the earlier years, and since 2013, the addition of pertuzumab to trastuzumab with taxane became routinely available.9
In 2013, the Canadian Agency for Drugs and Technologies in Health (CADTH) conducted a health technology assessment on pertuzumab.9 At the initial health technology assessment review, the CADTH indicated the landmark CLEOPATRA trial demonstrated that the addition of pertuzumab to trastuzumab and docetaxel in patients with ERBB2-positive metastatic breast cancer substantially improved progression-free survival (hazard ratio [HR], 0.62; P < .001) and overall survival (HR, 0.66; P < .001).9,10 According to the manufacturer’s economic model, pertuzumab was projected to provide an incremental clinical benefit of 0.64 life-years (LY) gained (LYG) or 0.51 quality-adjusted life-years (QALY) gained, along with an incremental cost of $120 287 (dollar amounts presented herein as CAD unless otherwise indicated).9,11 Based on these estimates, the submitted incremental cost-effectiveness ratio (ICER) was $187 376/LYG and $238 014/QALY.9 The economic guidance panel at CADTH conducted a reanalysis and estimated the ICER was between $262 263/QALY and $303 726/QALY, which was not considered cost-effective.9
Similar to the CADTH evaluation, the UK National Institute for Health and Care Excellence (NICE) also suggested that the base-case ICERs generated by the manufacturer’s model had a 0% probability of being cost-effective at a willingness-to-pay threshold of £30 000 per QALY (with 1 British sterling pound equal to approximately USD $1.36).12,13 The NICE Decision Support Unit examined pertuzumab and found that the factor associated with this relatively high ICER appeared to be use of pertuzumab in combination with trastuzumab and any additional progression-free survival was accompanied by the costs of both pertuzumab and trastuzumab; thus, pertuzumab would not be considered cost-effective even at a price of $0.12,13,14 After the initial NICE review in 2013, pertuzumab became available through the Cancer Drug Fund and, following a subsequent review in 2018, pertuzumab was approved for routine use.15 Despite deeming pertuzumab as not cost-effective, the CADTH issued a conditional recommendation for the funding of pertuzumab based on the improved clinical benefit in 2013, conditional on pricing arrangements to improve the cost-effectiveness.9 Within the same year, pertuzumab became publicly funded in Ontario for patients with ERBB2-positive metastatic breast cancer.9
Economic models developed by researchers across the globe have ranged between USD $183 901/QALY to $593 741/QALY.16,17,18,19,20 Along with the wide range of variations, the estimated ICERs were all above conventional willingness-to-pay thresholds, suggesting potentially inefficient investment in pertuzumab. Moreover, the model-based cost-effectiveness studies derived the estimate of clinical benefit of pertuzumab from the CLEOPATRA trial. Similar to other literature that demonstrated the gap between the efficacy in the clinical trial and the outcomes in the real-world setting,21,22,23 pertuzumab has also been reported to have lower benefit in the real world compared with the efficacy observed in the trial.24,25,26
Given these uncertainties regarding the clinical benefit and the wide variation in the economic estimates, there is an impetus for evaluating the cost-effectiveness of pertuzumab using real-world population-based data. Moreover, with the growing interest toward life-cycle health technology assessment by health technology agencies, such as CADTH and NICE, cost-effectiveness studies can also inform the reassessment of funded drugs.27 To address these gaps, we conducted a population-based study to evaluate the cost-effectiveness of pertuzumab, trastuzumab, and chemotherapy, compared with trastuzumab plus chemotherapy in patients with metastatic breast cancer from the public payer perspective. Using population-based administrative data sets, we estimated the incremental cost per LYG and per QALY.
Methods
Study Design and Setting
A population-based retrospective economic evaluation was conducted using data from Ontario, Canada, which has a population of approximately 14 million people.28 Treatments for breast cancer are administered in cancer clinics and are reimbursed by the provincial government. We identified adults diagnosed with incident breast cancer from the Ontario Cancer Registry using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision site codes C50.0 to C50.9. The cohort was linked to the New Drug Funding Program database to ascertain treatment records for patients with metastatic breast cancer between January 1, 2008, and March 31, 2018 (N = 1158). The treatments of interest included first-line use of trastuzumab and chemotherapy with or without pertuzumab administered under the respective palliative-intent funding policies.
Pertuzumab became publicly funded in Ontario on November 25, 2013. The pertuzumab group consisted of patients who received first-line treatment for metastasis comprising pertuzumab, trastuzumab, and chemotherapy after pertuzumab funding, and the control group included patients who received treatment for metastasis with trastuzumab and chemotherapy before the funding was available. We excluded patients who had a date of first dose of pertuzumab before the funding date, received treatment before the cancer diagnosis in the cancer registry, or were not an Ontario resident at the time of diagnosis. The index date of treatment was the first record of pertuzumab administration for metastatic intent for the pertuzumab case group, and the first record of trastuzumab administration for metastatic intent for the control group. The study cohort was followed up until March 31, 2019. The cohort creation process and study design are shown in eFigure 1 in the Supplement.
Data Sources and Reporting
Data were retrieved from administrative databases and were linked using unique encoded identifiers and analyzed at ICES, an independent, nonprofit research institute funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The databases used to create the study cohort include Registered Persons Database, Ontario Cancer Registry, Ontario Health Insurance Plan database, Canadian Institute of Health Information Discharge Abstract Database, Canadian Institute of Health Information National Ambulatory Care Reporting System, and Activity Level Reporting Systems. Baseline demographic and clinical characteristics were ascertained from linked administrative data sets (eMethods in the Supplement). As a prescribed entity under Ontario’s privacy legislation, ICES is authorized to collect and use health care data for the purposes of health system analysis, evaluation, and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario. This study was designed, analyzed, and reported in accordance with the RECORD-PE and Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guidelines.29,30 In accordance with the policies of ICES, small cell counts were suppressed and are reported as less than 6 to limit the risk of patient reidentification. This study was approved by the Sunnybrook Research Ethics Board, which waived informed consent for deidentified data.
Treatment Effectiveness
Treatment effectiveness was measured as life-years (LY) and QALY. Life-years were defined as 5-year survival calculated from the index date until death date or the end of the 5-year follow up. Patients who were alive at the end of the study period on March 31, 2019, or who had lost their Ontario Health Insurance Plan eligibility were censored. Quality-adjusted life-years were obtained by adjusting 5-year survival with different utilities for patients who were receiving ERBB2-directed treatment vs those who discontinued treatment. Utility weights were obtained from existing literature and were also used by the initial CADTH drug review.31 The time that patients were receiving initial ERBB2-directed treatment was weighted with the utility value of 0.79, and the time that patients had discontinued treatment was weighted with the utility value of 0.5.31,32
Cost Analysis
We adopted the public payer perspective when estimating the 5-year total costs for each patient. The total cost from the index date to death or the end of the 5-year follow-up period was estimated using a validated costing algorithm available at ICES.33 Costs were censored for patients who were alive at the maximum follow-up or had lost their Ontario Health Insurance Plan eligibility. The total costs for each patient were the sum of health care services from the following categories: outpatient visits, ambulatory hospital care visits, acute inpatient hospitalizations, chronic and rehabilitation care, and drug costs (eMethods in the Supplement). All costs were adjusted to the 2018 CAD amount using the Statistics Canada Consumer Price Index.34
Statistical Analysis
The study cohort was characterized using descriptive statistics. The pertuzumab case and control groups were matched using propensity score methods including variables listed in the eMethods in the Supplement. The pertuzumab and control groups were matched 1:1, with a caliper width equal to 0.2.35 Standardized differences between the adjusted covariates were calculated, and differences less than or equal to 0.1 are generally considered to represent acceptable balance.36 Findings were considered statistically significant with a 2-sided test at P < .05. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Cost-effectiveness Analysis
Inverse probability censoring weighting was applied to the costs and effectiveness (LY or QALY), partitioned into 30-day intervals to adjust for administrative censoring.37 All costs and effectiveness (LY and QALY) were discounted using a rate of 1.5% annually as a base case as per the CADTH guidelines.38
Cost-effectiveness was estimated by computing the ICER and net monetary benefit methods.39,40,41 The ICER was determined by the difference in mean total costs between the pertuzumab case and control group and divided by the difference in mean total effectiveness (LYG or QALY gained) between the pertuzumab case and control groups. The 95% CIs for the ICERs were calculated by generating 1000 bootstrap resamples. Cost-effectiveness acceptability curves were plotted for the distribution of bootstrapped ICERS that are below each willingness-to-pay threshold. The net benefit (NB), for each person (i) was calculated for the willingness-to-pay threshold (λ) between $100 000 and $700 000 as NB = (effecti × λ) − costi.39,42 Two scenarios were conducted as part of the sensitivity analysis. The first scenario varied the base discount rate (1.5%) to no discounting (0%). The second scenario examined the outcome of reducing the price of pertuzumab (from 0% to 100%) alone and in combination with trastuzumab on the ICER.
Results
Study Population and Baseline Characteristics
A total of 1823 patients with incident breast cancer diagnosis received trastuzumab and chemotherapy with or without pertuzumab for treatment of metastasis between January 1, 2008, and March 31, 2018 (eFigure 1 in the Supplement); of these, 912 were in the pertuzumab case group and 911 were in the control group. Propensity score matching identified 579 pairs of the pertuzumab and control groups. Overall, the mean (SD) age of the matched study cohort was 58 (12.97) years, 1151 were women (99.4%), 7 were men (0.6%), and 1012 (87.4%) lived in urban regions (Table 1). All variables were balanced between the propensity score–matched pertuzumab case and control groups.
Table 1. Baseline Characteristics of Study Population.
| Variablea | Crude cohort | Propensity score–matched cohort | ||||
|---|---|---|---|---|---|---|
| Pertuzumab (n = 912) | Control (n = 911) | P value | Pertuzumab (n = 579) | Control (n = 579) | SDif | |
| Age at index date, mean (SD), y | 57.7 (12.7) | 58.1 (12.8) | .50 | 58.3 (12.5) | 58.2 (13.0) | 0.01 |
| LHIN, No. (%) | ||||||
| Region 1 | 38 (4.2) | 52 (5.7) | .08 | 30 (5.2) | 28 (4.8) | 0.02 |
| Region 2 | 92 (10.1) | 83 (9.1) | 56 (9.7) | 54 (9.3) | 0.01 | |
| Region 3 | 41 (4.5) | 41 (4.5) | 27 (4.7) | 29 (5.0) | 0.02 | |
| Region 4 | 81 (8.9) | 73 (8.0) | 52 (9.0) | 56 (9.7) | 0.02 | |
| Region 5 | 56 (6.1) | 45 (4.9) | 28 (4.8) | 27 (4.7) | 0.01 | |
| Region 6 | 93 (10.2) | 95 (10.4) | 58 (10.0) | 58 (10.0) | 0 | |
| Region 7 | 76 (8.3) | 90 (9.9) | 53 (9.2) | 48 (8.3) | 0.03 | |
| Region 8 | 95 (10.4) | 121 (13.3) | 73 (12.6) | 77 (13.3) | 0.02 | |
| Region 9 | 88 (9.6) | 92 (10.1) | 56 (9.7) | 53 (9.2) | 0.02 | |
| Region 10 | 42 (4.6) | 42 (4.6) | 29 (5.0) | 29 (5.0) | 0 | |
| Region 11 | 136 (14.9) | 103 (11.3) | 70 (12.1) | 75 (13.0) | 0.03 | |
| Region 12 | 35 (3.8) | 20 (2.2) | <20b | 16 (2.8) | 0.01 | |
| Region 13 | 33 (3.6) | 41 (4.5) | 25 (4.3) | 22 (3.8) | 0.03 | |
| Region 14 | 6 (0.7) | 13 (1.4) | <5b | 7 (1.2) | 0.03 | |
| Neighborhood income quintile, No. (%) | ||||||
| 1 (lowest) | 155 (17.0) | 154 (16.9) | .64 | 99 (17.1) | 99 (17.1) | 0 |
| 2 | 202 (22.1) | 191 (21.0) | 135 (23.3) | 133 (23.0) | 0.01 | |
| 3 | 189 (20.7) | 195 (21.4) | 122 (21.1) | 124 (21.4) | 0.01 | |
| 4 | 174 (19.1) | 195 (21.4) | 113 (19.5) | 114 (19.7) | 0 | |
| 5 (highest) | 192 (21.1) | 175 (19.2) | 110 (19.0) | 109 (18.8) | 0 | |
| Urban residence, No. (%) | 801 (87.8) | 797 (87.5) | .82 | 505 (87.2) | 507 (87.6) | 0.01 |
| Charlson comorbidity index score | ||||||
| 0 | 505 (55.4) | 522 (57.3) | .07 | 333 (57.5) | 327 (56.5) | 0.02 |
| 1 | 61 (6.7) | 72 (7.9) | 41 (7.1) | 45 (7.8) | 0.03 | |
| ≥2 | 16 (1.8) | 28 (3.1) | 13 (2.2) | 17 (2.9) | 0.04 | |
| No hospitalization | 330 (36.2) | 289 (31.7) | 192 (33.2) | 190 (32.8) | 0.01 | |
| Time between diagnosis to index date, mean (SD), y | 2.75 (4.1) | 3.10 (3.6) | .06 | 2.71 (4.14) | 2.72 (3.75) | 0 |
| Cancer stage at diagnosis, No. (%) | ||||||
| I | 50 (5.5) | 36 (4.0) | <.001 | 30 (5.2) | 33 (5.7) | 0.02 |
| II | 177 (19.4) | 99 (10.9) | 85 (14.7) | 84 (14.5) | 0 | |
| III | 222 (24.3) | 156 (17.1) | 127 (21.9) | 120 (20.7) | 0.03 | |
| IV | 325 (35.6) | 284 (31.2) | 224 (38.7) | 222 (38.3) | 0.01 | |
| Missing/unknown | 138 (15.1) | 336 (36.9) | 113 (19.5) | 120 (20.7) | 0.03 | |
| Prior therapy, No. (%) | ||||||
| Hormonal | 136 (14.9) | 166 (18.2) | .06 | 85 (14.7) | 93 (16.1) | 0.04 |
| Bisphosphonate | 88 (9.6) | 155 (17.0) | <.001 | 69 (11.9) | 67 (11.6) | 0.01 |
| Adjuvant trastuzumab | 313 (34.3) | 236 (25.9) | <.001 | 160 (27.6) | 160 (27.6) | 0 |
| Any adjuvant | 209 (22.9) | 238 (26.1) | .11 | 128 (22.1) | 118 (20.4) | 0.04 |
| Neoadjuvant | 122 (13.4) | 82 (9.0) | .003 | 53 (9.2) | 60 (10.4) | 0.04 |
| Adjuvant radiotherapy | 326 (35.7) | 326 (35.8) | .99 | 187 (32.3) | 185 (32.0) | 0.01 |
| Prior cancer | ||||||
| Breast | 66 (7.2) | 17 (1.9) | <.001 | 16 (2.8) | 17 (2.9) | 0.01 |
| Other | 43 (4.7) | 31 (3.4) | .16 | 23 (4.0) | 25 (4.3) | 0.02 |
| Estrogen receptor, No. (%)c | ||||||
| Negative (20) | 189 (39.0) | 116 (48.9) | <.001 | 117 (50.6) | 111 (48.3) | 0.03 |
| Positive (10) | 295 (61.0) | 121 (51.1) | 114 (49.4) | 119 (51.7) | 0.02 | |
| Progesterone receptor, No. (%)c | ||||||
| Negative (20) | 252 (52.3) | 152 (64.4) | <.001 | 149 (64.5) | 146 (63.7) | 0.01 |
| Positive (10) | 230 (47.7) | 84 (35.6) | 82 (35.5) | 83 (36.3) | 0 | |
Abbreviations: LHIN, Local Health Integration Network; SDif, standardized difference.
In accordance with the patient privacy policies of ICES, the numbers and percentage values for male and female populations in the data are not reported to avoid the possibility of back calculation of populations less than 5.
Small cells are suppressed in compliance with ICES policy to limit the risk of patient identification.
Percentages are based on known cases.
Cost Distribution
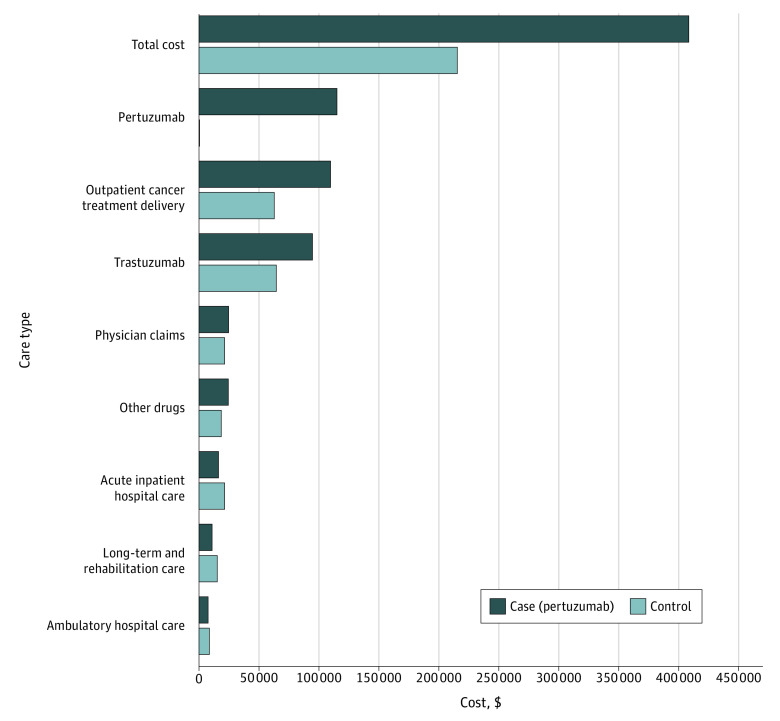
Figure 1 presents the breakdown of the 5-year total costs by the major resource categories. The main categories included pertuzumab drug cost (pertuzumab: 28% of total costs), outpatient cancer treatment delivery (pertuzumab: 27%; control: 29%), trastuzumab drug costs (pertuzumab: 23%; control: 30%), physician claims (pertuzumab: 6%; control 10%), other drugs (pertuzumab: 6%; control: 9%), acute inpatient hospital care (pertuzumab: 4%; control: 10%), chronic rehabilitation care costs (pertuzumab: 3%; control: 7%), and ambulatory hospital care (pertuzumab: 2%; control: 5%). The main factors associated with cost for both groups included trastuzumab cost, pertuzumab costs, and outpatient cancer delivery cost. For the pertuzumab group, the cost of pertuzumab was the main factor. The incremental cost difference between the 2 groups was $192 139. The main contributors of the incremental cost differences included the costs of pertuzumab (60%), outpatient cancer treatment delivery (24%), and trastuzumab (15%).
Figure 1. Disaggregated Cost by Cases and Controls After Accounting for Censoring.
All costs reported in Canadian dollars and adjusted for censoring and 1.5% discounted.
Incremental Cost-effectiveness
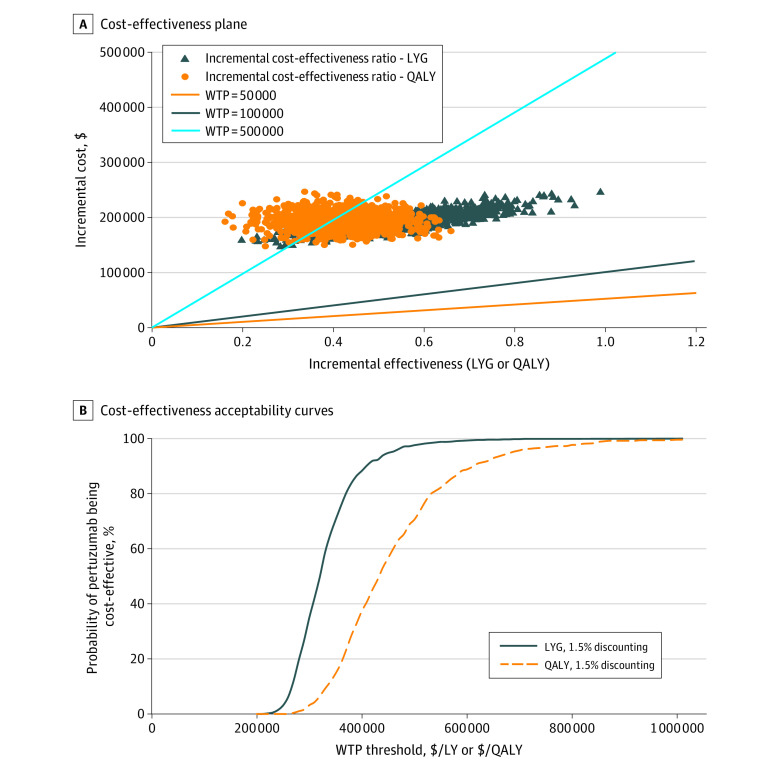
Table 2 summarizes the mean inverse probability censoring weighting–adjusted 5-year LY, QALY, total costs, and ICERs. Mean total health care cost was higher for the pertuzumab group compared with the control group. Mean LY was estimated as 3.08 for the pertuzumab group and 2.48 for the control group, with an incremental LY gained of 0.61. Mean QALYs were 2.11 for the pertuzumab group and 1.67 for the control group, with an incremental QALY gained of 0.44. The ICER for the pertuzumab group vs the control group was $316 203 per LYG and $436 679 per QALY. The scenario for no discounting was similar to the 1.5% discounting case with slightly lower ICERs. All bootstrapped samples comparing the pertuzumab case and control groups were in the northeast quadrant of the cost-effectiveness plane (Figure 2A). The cost-effectiveness acceptability curve noted that the probability of pertuzumab being cost-effective at a willingness-to-pay threshold of $50 000 or $100 000 was 0 (Figure 2B). eFigure 2 in the Supplement presents the incremental net benefit. At a willingness-to-pay threshold of $50 000 and $100 000, the incremental net benefit for both LYG and QALY gained were negative (ie, pertuzumab was not cost-effective at these thresholds).
Table 2. Cost, Cost-effectiveness, and ICERa.
| Variable | Cost, mean 5-y total, $ | Mean LY | Mean QALY | Incremental difference (95% CI) | ICER, $ (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| Cost, $ | LYG | QALY | Per mean LYG | Per mean QALY gained | ||||
| 1.5% Discounting | ||||||||
| Controls | 215 648 | 2.48 | 1.67 | 192 139 (160 715-224 736) | 0.61 (0.33-0.87) | 0.44 (0.23-0.63) | 316 203 (247 725-498 153) | 436 679 (288 990-833 190) |
| Pertuzumab | 407 787 | 3.08 | 2.11 | |||||
| Scenario: no discounting | ||||||||
| Controls | 219 417 | 2.53 | 2.00 | 196 622 (180 774-219 172) | 0.63 (0.48-0.84) | 0.50 (0.26-0.71) | 312 147 (260 753-375 492) | 393 244 (273 866-759 238) |
| Pertuzumab | 416 039 | 3.16 | 2.50 | |||||
Abbreviations: ICER, incremental cost-effectiveness ratio; LY, life-year; LYG, LY gained; QALY, quality-adjusted life-year.
All costs reported in Canadian dollars.
Figure 2. Incremental Cost-effectiveness Ratios (ICERs) for Life-years Gained (LYG) and Quality-Adjusted Life-years (QALY).
Cost-effectiveness plane (A) and acceptability curve (B) for LYG and QALY. WTP indicates willingness to pay. All costs reported in Canadian dollars.
Price Reduction
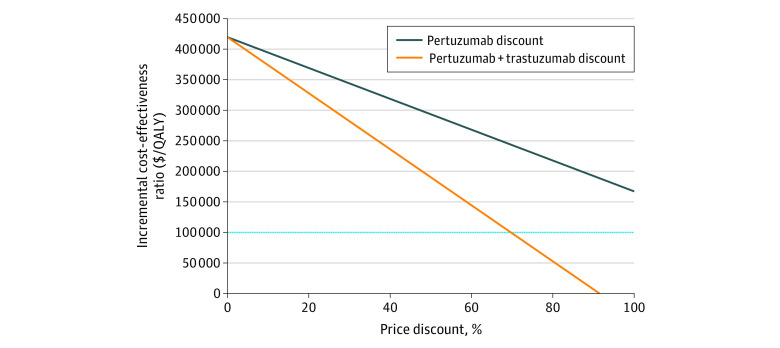
At 100% price reduction of pertuzumab, that is, when the price of pertuzumab is $0, the ICER was calculated to be $174 027/QALY. Figure 3 illustrates the outcome of reducing the price of pertuzumab alone or in combination with trastuzumab associated with the calculated ICER. The ICER was reduced to less than $100 000/QALY when the combination of pertuzumab and trastuzumab was reduced by 71% and to less than $50 000/QALY when the combination was reduced by 81%.
Figure 3. Price Reduction of Pertuzumab Alone and in Combination With Trastuzumab.
QALY indicates quality-adjusted life-years. All costs reported in Canadian dollars.
Discussion
In this population-based, propensity score–matched economic evaluation, we examined the cost-effectiveness of pertuzumab for treatment of patients with metastatic breast cancer. The results largely support that pertuzumab was associated with improved clinical benefit but was not cost-effective at the common willingness-to-pay thresholds from a public payer’s perspective. We found that the average 5-year total cost incurred by the pertuzumab group was 89% higher than that of the control group. The main cost contributors in both groups were the costs associated with outpatient cancer treatment delivery (eg, cancer clinics) and ERBB2-directed therapy (pertuzumab and trastuzumab). The ICERs ($316 203/LYG and $436 679/QALY) were higher than the initial economic model ($187 376/LYG and $238 014/QALY). This difference may be due to higher incremental drug cost ($192 139) compared with the initial model ($117 932). Moreover, although we limited the time horizon in our study to 5 years, the initial economic review projected the ICERs over 10 years.11 It is expected that the ICERs might commonly decrease over a longer time horizon as the costs for the drugs may be larger during the earlier years while the survival benefit may continue to accrue.
The ICERs from this study lie within the range calculated from model-based models (USD $183 901/QALY to USD $593 741/QALY).16,17,18,19,20 Our finding also largely supports the evaluation by the NICE Decision Support Unit during the initial appraisal of pertuzumab that there is 0% probability of pertuzumab being cost-effective at £30 000 and that it would not be cost-effective at any price.12,13,43 In the real world, even at the price of $0, the ICER of pertuzumab is greater than the conventional willingness-to-pay threshold of $100 000/QALY. NICE suggested that, because pertuzumab was given in combination with trastuzumab and chemotherapy, any additional survival benefit would be accompanied by cost of both pertuzumab and companion drugs.13 We observed that the cost of trastuzumab was 44% higher in the pertuzumab group compared with the control group, thus supporting previous projections that the longer survival in the pertuzumab group led to a substantial increase in treatment and management costs in addition to the cost of pertuzumab.13,17
Strengths and Limitations
This study has limitations. First, inherent to the nature of observational studies are possible selection biases due to nonrandom treatment assignment. Although we used propensity score methods to minimize potential biases between groups, given that we used historical controls, there may be treatment and/or practice pattern changes over time resulting in potential bias. Second, we were only able to capture 5-year total costs in both groups owing to the public funding date. This short period limits the ability to compare with model-based estimates that estimate these outcomes based on a lifetime horizon. For example, the initial CADTH model was projected using a 10-year time horizon and the NICE model was projected using a 25-year time horizon.11,12 Third, the approval of a novel subcutaneous formulation of pertuzumab and trastuzumab by the US Food and Drug Administration in 2020 may potentially affect the cost-effectiveness of the combination drug.44 Although the drug acquisition cost may be higher, the reduction of nondrug costs, such as chair time, may partly offset the higher drug acquisition cost.45 Future cost-effectiveness analysis could explore this further after approval of the subcutaneous formulation in Canada. Despite these limitations, using real-world data allowed us to avoid some of the assumptions around the parameters required for the model, such as estimating both the effectiveness from clinical trials using parametric methods or estimating costs using published data.
To our knowledge, this was the first economic evaluation of pertuzumab use for patients with metastatic breast cancer using population-based patient-level data. Although other economic studies have explored the cost-effectiveness of pertuzumab, the strength of our study was that we used data on survival and cost accrued by the same patients who received public funding under examination. By evaluating the cost-effectiveness, we noted the initial estimation that pertuzumab would not be cost-effective at any price. Our study also suggests the feasibility of conducting economic evaluations using patient-level data that are routinely collected in Ontario. The lessons learned from this study will be important for the larger work of the CanREValue Collaboration or other initiatives that aim to develop frameworks for the reassessment of publicly funded drugs as part of life-cycle health technology management.46,47,48 With the increasing costs of new drugs, life-cycle health technology management with reassessment allows decision-makers to consider alternative funding approaches, such as conditional approval contingent on collection of data or performance-based agreement.49,50 Our study suggests the feasibility of conducting population-based economic evaluation that can support these alternative funding approaches to allow decision-makers to renegotiate drug prices that can achieve lower drug costs and improve the long-term sustainability of health care systems.
Conclusions
We conducted a population-based economic evaluation to examine the cost-effectiveness of adding pertuzumab to the treatment regimen for patients with metastatic breast cancer. Although pertuzumab is associated with clinical benefit, it comes with substantial increases in cost. The main factors associated with cost are targeted therapies and outpatient oncologic treatment delivery. Our findings largely support the results from the initial health technology assessments that the ICERs for pertuzumab would not be considered cost-effective, at any price, using commonly accepted willingness-to-pay thresholds. This finding is mostly owing to the added cost of pertuzumab in the case group, as well as longer duration of trastuzumab incurred because of longer survival after adding pertuzumab to the combination. In addition, we noted the feasibility of deriving ICERs using population-based patient-level data, which may be used to inform life cycle health technology assessment.
eFigure 1. Cohort Creation and Study Design
eMethods. Detailed Methods
eReferences
eFigure 2. Incremental Net Benefit
References
- 1.Saluja R, Arciero VS, Cheng S, et al. Examining trends in cost and clinical benefit of novel anticancer drugs over time. J Oncol Pract. 2018;14(5):e280-e294. doi: 10.1200/JOP.17.00058 [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. doi: 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Oliveira C, Weir S, Rangrej J, et al. The economic burden of cancer care in Canada: a population-based cost study. CMAJ Open. 2018;6(1):E1-E10. doi: 10.9778/cmajo.20170144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Cancer Statistics Advisory Committee . Canadian Cancer Statistics 2019. Canadian Cancer Society. [Google Scholar]
- 5.de Oliveira C, Pataky R, Bremner KE, et al. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer. 2016;16(1):809. doi: 10.1186/s12885-016-2835-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Oliveira C, Bremner KE, Pataky R, et al. Trends in use and cost of initial cancer treatment in Ontario: a population-based descriptive study. CMAJ Open. 2013;1(4):E151-E158. doi: 10.9778/cmajo.20130041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittmann N, Liu N, Cheng SY, et al. Health system costs for cancer medications and radiation treatment in Ontario for the 4 most common cancers: a retrospective cohort study. CMAJ Open. 2020;8(1):E191-E198. doi: 10.9778/cmajo.20190114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seung SJ, Traore AN, Pourmirza B, Fathers KE, Coombes M, Jerzak KJ. A population-based analysis of breast cancer incidence and survival by subtype in Ontario women. Curr Oncol. 2020;27(2):e191-e198. doi: 10.3747/co.27.5769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee (pERC) final recommendation. 2013. Accessed June 3, 2021. https://cadth.ca/sites/default/files/pcodr/pcodr-perjetacp-mbc-fn-rec.pdf
- 10.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461-471. doi: 10.1016/S1470-2045(13)70130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan-Canadian Oncology Drug Review . Pan-Canadian Oncology Drug Review final economic guidance report—pertuzumab (Perjeta) for metastatic breast cancer. December 3, 2013. Accessed June 3, 2021. https://cadth.ca/sites/default/files/pcodr/pcodr-perjetacp-mbc-fn-egr.pdf
- 12.Fleeman N, Bagust A, Beale S, et al. Pertuzumab in combination with trastuzumab and docetaxel for the treatment of HER2-positive metastatic or locally recurrent unresectable breast cancer. Pharmacoeconomics. 2015;33(1):13-23. doi: 10.1007/s40273-014-0206-2 [DOI] [PubMed] [Google Scholar]
- 13.Davis S. Assessing technologies that are not cost-effective at a zero price: report by the Decision Support Unit. July 2014. Accessed June 15, 2021. http://nicedsu.org.uk/wp-content/uploads/2016/03/Not_CE_at_zero_price_FINAL_14.07.14.pdf
- 14.National Institute for Health and Care Excellence . Single technology appraisal pertuzumab in combination with trastuzumab and docetaxel for treating HER2-positive metastatic or locally recurrent unresectable breast cancer [ID523] committee papers—Appraisal Committee meeting 3. August 2, 2017. https://www.nice.org.uk/guidance/ta509/documents/committee-papers-2
- 15.National Institute for Health and Care Excellence . Pertuzumab with trastuzumab and docetaxel for treating HER2-positive breast cancer. March 7, 2018. Accessed June 24, 2021. https://www.nice.org.uk/guidance/ta509/chapter/1-Recommendations
- 16.Moriwaki K, Uechi S, Fujiwara T, Hagino Y, Shimozuma K. Economic evaluation of first-line pertuzumab therapy in patients with HER2-positive metastatic breast cancer in Japan. Pharmacoecon Open. 2021;5(3):437-447. doi: 10.1007/s41669-020-00254-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao B, Garrison LP Jr. The challenge of value-based pricing in combination therapy: the case of trastuzumab and pertuzumab in HER2+ metastatic breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):497-504. doi: 10.1080/14737167.2021.1896968 [DOI] [PubMed] [Google Scholar]
- 18.Cheng LJ, Loke L, Lim EH, Pearce F, Aziz MIA, Ng K. Cost-effectiveness of pertuzumab and trastuzumab biosimilar combination therapy as initial treatment for HER2-positive metastatic breast cancer in Singapore. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):449-456. doi: 10.1080/14737167.2021.1880323 [DOI] [PubMed] [Google Scholar]
- 19.Leung HWC, Chan ALF, Muo C-H, Leung JH. Cost-effectiveness of pertuzumab combined with trastuzumab and docetaxel as a first-line treatment for HER-2 positive metastatic breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2018;18(2):207-213. doi: 10.1080/14737167.2018.1386559 [DOI] [PubMed] [Google Scholar]
- 20.Durkee BY, Qian Y, Pollom EL, et al. Cost-effectiveness of pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2016;34(9):902-909. doi: 10.1200/JCO.2015.62.9105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips CM, Parmar A, Guo H, et al. Assessing the efficacy-effectiveness gap for cancer therapies: a comparison of overall survival and toxicity between clinical trial and population-based, real-world data for contemporary parenteral cancer therapeutics. Cancer. 2020;126(8):1717-1726. doi: 10.1002/cncr.32697 [DOI] [PubMed] [Google Scholar]
- 22.Chan KKW, Guo H, Cheng S, et al. Real-world outcomes of FOLFIRINOX vs gemcitabine and nab-paclitaxel in advanced pancreatic cancer: a population-based propensity score-weighted analysis. Cancer Med. 2020;9(1):160-169. doi: 10.1002/cam4.2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai WF, Beca JM, Croxford R, et al. Real-world comparative effectiveness of second-line ipilimumab for metastatic melanoma: a population-based cohort study in Ontario, Canada. BMC Cancer. 2020;20(1):304. doi: 10.1186/s12885-020-06798-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong IY, Yan AT, Earle CC, Trudeau ME, Eisen A, Chan KKW. Comparison of outcomes in a population-based cohort of metastatic breast cancer patients receiving anti-HER2 therapy with clinical trial outcomes. Breast Cancer Res Treat. 2020;181(1):155-165. doi: 10.1007/s10549-020-05614-5 [DOI] [PubMed] [Google Scholar]
- 25.Christensen T, Berg T, Nielsen LB, Andersson M, Jensen M-B, Knoop A. Dual HER2 blockade in the first-line treatment of metastatic breast cancer—a retrospective population-based observational study in Danish patients. Breast. 2020;51:34-39. doi: 10.1016/j.breast.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai WF, Beca JM, Nagamuthu C, et al. Comparative effectiveness and safety of pertuzumab and trastuzumab plus chemotherapy vs trastuzumab plus chemotherapy for treatment of metastatic breast cancer. JAMA Netw Open. 2022;5(1):e2145460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CADTH . Procedures for CADTH drug reimbursement reviews—December 2021. Accessed January 21, 2022. https://cadth.ca/sites/default/files/Drug_Review_Process/CADTH_Drug_Reimbursement_Review_Procedures.pdf.
- 28.Statistics Canada . Population estimates on July 1st, by age and sex. Table 17-10-0005-01. September 29, 2021. Accessed June 3, 2021. https://www150.statcan.gc.ca/n1/en/catalogue/1710000501
- 29.Langan SM, Schmidt SA, Wing K, et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ. 2018;363:k3532. doi: 10.1136/bmj.k3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husereau D, Drummond M, Petrou S, et al. ; ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231-250. doi: 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 31.Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683-690. doi: 10.1038/sj.bjc.6603326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute for Health and Care Excellence . Single technology appraisal. Pertuzumab in combination with trastuzumab and docetaxel for treating HER2-positive metastatic or locally recurrent unresectable breast cancer [ID523]: committee papers—appraisal committee meeting 3 (08/02/17). November 11, 2016. Accessed November 26, 2021. https://www.nice.org.uk/guidance/ta509/documents/committee-papers-2
- 33.Wodchis WP, Bushmeneva K, Nikitovic M, McKillop I. Guidelines on person level costing using administrative databases in Ontario. University of Toronto. May 2013. Accessed June 10, 2021. https://tspace.library.utoronto.ca/handle/1807/87373
- 34.Statistics Canada . Consumer price index, annual average, not seasonally adjusted. January 19, 2022. Accessed June 10, 2021. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501.
- 35.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87(2):329–343. doi: 10.1093/biomet/87.2.329 [DOI] [Google Scholar]
- 38.Guidelines for the Economic Evaluation of Health Technologies. 4th Edition. Canadian Agency for Drugs and Technologies in Health; 2017. [Google Scholar]
- 39.Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ. 2002;11(5):415-430. doi: 10.1002/hec.678 [DOI] [PubMed] [Google Scholar]
- 40.Hoch JS, Rockx MA, Krahn AD. Using the net benefit regression framework to construct cost-effectiveness acceptability curves: an example using data from a trial of external loop recorders versus Holter monitoring for ambulatory monitoring of “community acquired” syncope. BMC Health Serv Res. 2006;6:68. doi: 10.1186/1472-6963-6-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen DJ, Reynolds MR. Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol. 2008;52(25):2119-2126. doi: 10.1016/j.jacc.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaranuwatchai W, Markle-Reid M, Hoch JS. Adjusting for baseline covariates in net benefit regression: how you adjust matters. Pharmacoeconomics. 2015;33(10):1083-1090. doi: 10.1007/s40273-015-0287-6 [DOI] [PubMed] [Google Scholar]
- 43.The National Institute for Health and Care Excellence . Breast Cancer (HER2 Positive, Metastatic)—Pertuzumab (With Trastuzumab and Docetaxel): Appraisal Consultation Document. The National Institute for Health and Care Excellence; 2013. [Google Scholar]
- 44.Gao JJ, Osgood CL, Gong Y, et al. FDA approval summary: pertuzumab, trastuzumab, and hyaluronidase-zzxf injection for subcutaneous use in patients with HER2-positive breast cancer. Clin Cancer Res. 2021;27(8):2126-2129. doi: 10.1158/1078-0432.CCR-20-3474 [DOI] [PubMed] [Google Scholar]
- 45.Manevy F, Filkauskas G, Levy P, Fredriksson J, Sussell J. Potential non-drug cost differences associated with the use of the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection (PH FDC SC) in the treatment of HER2-positive early breast cancer patients in Western Europe and the United States [abstract]. J Clin Oncol. 2021;39(15 suppl):544. doi: 10.1200/JCO.2021.39.15_suppl.544 [DOI] [Google Scholar]
- 46.Chan K, Nam S, Evans B, et al. Developing a framework to incorporate real-world evidence in cancer drug funding decisions: the Canadian Real-world Evidence for Value of Cancer Drugs (CanREValue) collaboration. BMJ Open. 2020;10(1):e032884. doi: 10.1136/bmjopen-2019-032884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai WF, Arciero V, Craig E, et al. ; On Behalf of the CanREValue Collaboration Reassessment and Uptake Working Group . Considerations for developing a reassessment process: report from the Canadian Real-World Evidence for Value of Cancer Drugs (CanREValue) Collaboration’s Reassessment and Uptake Working Group. Curr Oncol. 2021;28(5):4174-4183. doi: 10.3390/curroncol28050354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai WF, Craig E, Fraser B, et al. ; On Behalf of the CanREValue Collaboration . Building a national reassessment process for oncology drugs: lessons learned by the Canadian Real-World Evidence for Value of Cancer Drugs (CanREValue) collaboration through a simulated reassessment exercise. Curr Oncol. 2021;28(6):4645-4654. doi: 10.3390/curroncol28060392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garrison LP Jr, Towse A, Briggs A, et al. Performance-based risk-sharing arrangements-good practices for design, implementation, and evaluation: report of the ISPOR Good Practices for Performance-Based Risk-Sharing Arrangements Task Force. Value Health. 2013;16(5):703-719. doi: 10.1016/j.jval.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 50.Eichler HG, Baird LG, Barker R, et al. From adaptive licensing to adaptive pathways: delivering a flexible life-span approach to bring new drugs to patients. Clin Pharmacol Ther. 2015;97(3):234-246. doi: 10.1002/cpt.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Cohort Creation and Study Design
eMethods. Detailed Methods
eReferences
eFigure 2. Incremental Net Benefit