Abstract

Stimuli-responsive coordination cages allow reversible control over guest binding and release, relevant for adaptive receptors, carriers, catalysts, and complex systems. Light serves as an advantageous stimulus, as it can be applied with precise spatial and temporal resolution without producing chemical waste products. We report the first Pd-mediated coordination cage based on ligands embedding a diazocine photoswitch. While the thermodynamically more stable cis-photoisomer sloppily assembles to a mixture of species with general formula [Pdncis-L2n], the less stable trans-isomer yields a defined [Pd2trans-L4] cage that reversibly converts back to the cis-system by irradiation at 530 nm or thermal relaxation. The [Pdncis-L2n] species do not bind a given guest; however, [Pd2trans-L4] is able to encapsulate a bis-sulfonate as long as it is kept assembled, requiring continuous irradiation at 385 nm. In the absence of UV light, thermal relaxation results in back-switching and guest release. Assembly and properties of the system were characterized by a combination of NMR, ion mobility ESI-MS, single-crystal X-ray diffraction, and UV–vis absorption studies.
Introduction
The coordination-driven assembly of functional building blocks into supramolecular structures allows creating advanced nanosystems such as allosteric receptors, regulated catalysts, molecular machines, and stimuli-responsive materials. In many cases, the function of such systems requires nanosized cavities able to host guest molecules.1−4 Response to external stimuli can then be employed to trigger structural rearrangements, affecting the host–guest binding affinity, thus establishing control over guest uptake and release.5−12 Among various stimuli (e.g., pH,13−15 temperature,16 solvent,17 electrochemical,18,19 or chemical input20,21), light is a highly advantageous reagent, as it can be precisely administered with temporal and spatial resolution, is available from cheap LED sources, and is essentially waste-free. The light-triggered modification of structure and function of (supra)molecular systems can be achieved using dedicated photoswitches such as azobenzenes,22,23 dithienylethenes,24,25 other hindered alkenes,26,27 indigoids,28 and spiropiranes,29,30 just to name a few. At the same time, light can be used as a fuel to drive systems away from thermal equilibrium, generating dissipative self-assembled systems.31,32
Although the commonly known photoswitches have been studied intensively before, reports on their implementation into discrete metallosupramolecular self-assemblies, especially as an integral part of the ligand backbone, remain rather scarce. For example, Wezenberg and Feringa incorporated their overcrowded alkene motor to trigger structural reorganization in Pd2L4 cages,33 Liu and co-workers reported a Pd2L4 species that undergoes disassembly/reassembly upon isomerization of azobenzene moieties,34 while the group of Hardie exploited the same photochromic unit to report a structural switch in an Ir3L2 assembly.35 Besides these examples, the so far most intensely studied photoswitchable unit in ligand backbones is the dithienylethene (DTE) moiety. Our group previously reported a series of systems based on PdII cations and DTE ligands to control structural rearrangements and to tune guest selectivity, uptake, and release in a fully reversible fashion in homo- and heteroleptic cages.8,10,36,37 Similar systems have been applied to control the topology of supramolecular gels38 and to tune the emission properties of Eu2L4 helicates39 or have been implemented in Pt-based metallacycles to trigger structural rearrangements or photochromic properties.25,40
Recently a new class of azo group-based photoswitches, namely, diazocines, has emerged as promising candidates for creating molecular switches and stimuli-responsive materials.41,42 At first glance, the bridged diazocines seem similar to the archetypical azobenzenes; however, they are thermodynamically most stable in their cis-isomeric form. The metastable trans-isomer is formed upon irradiation at 385 nm, possessing a thermal lifetime of a few hours at room temperature. As compared to azobenzenes, diazocines are more rigid with a smaller number of conformational degrees of freedom and therefore allow a more controlled photochemically induced movement.43 Herein, we report the implementation of a diazocine moiety into the backbone of a banana-shaped bis-pyridyl ligand under full preservation of its photoswitching properties. Self-assembly with PdII cations leads to the formation of PdnL2n supramolecular assemblies. Interestingly, when the diazocine is used in the stable cis-isomeric form, structural strain seems to hamper formation of a defined structure as exclusive product. The diazocine in the metastable trans-isomeric form, however, neatly assembles to lantern-shaped cage [Pd2(trans-L)4] as a single species. Formation of this species by metallosupramolecular assembly seems energetically favored compared to the cis-analogue, but it exists only as a transient assembly, whose population has to be kept up by continuous irradiation with UV light. Interestingly, the [Pd2(trans-L)4] species is able to host a bis-sulfonate guest, while the structural change to the cis-analogue leads to guest release. Hence, the system resides in a state dominated by the prevalence of the [G@Pd2trans-L4] host–guest complex via a dissipative self-assembly process, only as long as it is powered with light of 385 nm wavelength. Removal of the stimulus leads to thermal relaxation of the backbone, formation of [Pdncis-L2n], and guest release.
Results and Discussion
Photoswitchable bis-pyridyl ligand (Z)-3,8-bis(pyridin-3-ylethynyl)-11,12-dihydrodibenzo[c,g]-[1,2]diazocine (L) was synthesized by Sonogashira coupling between (Z)-3,8-diiodo-11,12-dihydrodibenzo[c,g][1,2]diazocine42 and 3-ethynylpyridine (Supporting Information). The ligand forms as a thermodynamically stable cis-isomer (cis-L). Irradiation at 385 nm rapidly generates the trans-isomer (trans-L), while irradiation at 530 nm or thermal relaxation reverses the process (Figure 1a). The photochemical properties were investigated by 1H NMR and UV–vis absorption spectroscopies (Figure 1b–d). Upon irradiation of a dimethylformamide (DMF) or acetonitrile solution of cis-L, a new set of signals appeared in the NMR spectrum (Figure 1bi,ii), assigned to formation of trans-L. The conversion is not quantitative, and the photostationary state (PSS) was determined as a 62% trans/cis-form ratio by NMR signal integration. The coexistence of the two isomers is confirmed also by DOSY measurements, where the two species have clearly distinguishable diffusion coefficients measured as Dcis-L = 4.35 × 10–10 and Dtrans-L = 6.22 × 10–10 (Figure 1biii). Thermal relaxation from trans-L to cis-L was monitored by 1H NMR, resulting in a half-lifetime of 7.2 h at 298 K (Figure 1d). The thermal relaxation process (or irradiation with 530 nm) results also in a color change, from red to pale yellow in solution. In the UV–vis spectrum, cis-L is characterized by an absorption maximum at 399 nm (Figure 1c). Upon irradiation with a 385 nm LED, a new absorption band at 483 nm appears, characteristic for trans-L (Figure 1c). Upon irradiation with a 530 nm LED, the cis-isomer is fully restored within 5 min. Thermal back-switching of metastable trans-L was investigated also with absorption spectroscopy (Figure 1c, SI), and rate constants at different temperatures were used to calculate the activation parameters for the process (Figure S33).
Figure 1.

(a) Photoswitching between the two L isomers; (b) 1H NMR (500 MHz, DMF-d7, 298 K) of (i) cis-L and (ii) the mixture of cis-L and trans-L after irradiation at 385 nm for 10 min, (iii) DOSY of the two ligand isomers; (c) absorption spectra showing the thermal relaxation from trans-L (red line) to cis-L (beige line) obtained every 30 min (DMF, 1.12 mM, 298 K); (d) kinetics of trans-L isomerization, monitored by 1H NMR.
Next, we investigated the formation of Pd-based assemblies starting with the stable isomer cis-L. Reaction of the ligand with [Pd(CH3CN)4](BF4)2 in a 2:1 stoichiometry in DMF as a solvent was investigated by NMR and ESI-MS. Upon addition of PdII, the 1H NMR signals of cis-L are significantly broadened, complicating the assignment, while clearly downfield shifted, as typically observed for pyridyl ligands upon Pd coordination (Figure 2bi,ii). From geometric considerations, the expected species would be a Pd2L4 cage; however, the NMR spectra suggest a more complex scenario, with the presence of multiple species obeying the formula [Pdncis-L2n] (hereafter named “cis-Cage”). High-resolution ESI-MS analysis supports this hypothesis, showing the formation of species with stoichiometry Pd2L4 at m/z = 647.47 ([Pd2cis-L4+BF4]3+) and Pd3L6 at 748.15 ([Pd3cis-L6+2BF4]4+), while the peaks at 463.10 and 1013.21 consist of an overlap of Pd2L4 and Pd3L6 species (Figure 2c). Trapped ion mobility spectra (tims) for the peaks corresponding to the empty cages show two collisional cross section (CCS) values, clearly indicating the difference in size between the two species (Figure S38). Formation of a mixture of species with different nuclearity is not uncommon in metallosupramolecular assembly;44−48 however, the broad NMR spectra suggest the additional formation of larger oligomers, probably undergoing rapid ligand exchange, that are not detectable by ESI-MS. As an explanation for observing such a mixture of ill-defined assembly products, we assumed the accumulation of strain in the assemblies, resulting from a nonideal geometry of the cis-isomeric ligand that hampers formation of stable cage- or ring-like three-dimensional structures. In an attempt to release strain from the system, we combined cis-L with the metal precursor [PdCl2(CH3CN)2] in a 1:1 ratio, indeed resulting in the clean formation of a [Pd2Cl4cis-L2] (cis-Ring) metallocycle (Figure 2a). Single crystals of the compound were obtained from a DMF/CH2Cl2 mixture and subjected to single-crystal X-ray diffraction, delivering a structure in the P1̅ space group with half a molecule in the asymmetric unit (Figure 3b). In this structure, the two bis-pyridyl ligands, and consequently the coordinated Cl anions, were found to adopt a trans-arrangement, hence leaving more space and conformational freedom for the diazocine-based ligands than in the corresponding [Pd2cis-L4] assembly, which is conformationally more constrained owing to its tricyclic topology.
Figure 2.

(a) Assembly of cis-Ring, cis-Cage (+ higher assemblies), and trans-Cage and structural interconversion via light or thermal relaxation; (b) 1H NMR spectra (500 MHz, DMF-d7, 298 K) of (i) cis-L + [Pd(CH3CN)4](BF4)2 (2:1 mol ratio), (ii) a mixture of cis-L and trans-L after 385 nm UV irradiation, (iii) formation of trans-Cage, (iv) DOSY spectra for trans-Cage; (c) ESI mass spectra of cis-Cage (+ higher assemblies; top) and trans-Cage (bottom).
Figure 3.

Crystal structures and molecular dimensions of (a) cis-Ring, (b) trans-Cage, and (c) DFT-optimized model for [Pd2cis-L4]. Short distances on the left side measured between diazocine Cipso atoms; distances on the right, between opposite ethylene bridge carbon atoms.
On the other hand, self-assembly of PdII ions with the meta stable trans-L in a 1:2 ratio unambiguously results in formation of [Pd2trans-L4] (trans-Cage), as shown by NMR, ESI-MS, and X-ray analysis. 1H NMR spectra show rather sharp signals compared to the ones obtained for the cis-system. By addition of an internal standard (1,3,5-tris-tert-butylbenzene), we estimated the conversion into trans-Cage to be about 65% (Figure S14) with respect to the entire ligand content by 1H NMR integration. In line with the values determined for the PSS-governed equilibrium of ligand isomers, this speaks for a quantitative conversion of trans-L into trans-Cage. Species of the cis-Cage mixture are visible as very broad signals below the new set assigned to the trans-Cage. Overlap with these broad signals prevented a precise determination of the PSS of the assembly mixture. In trans-Cage, the diagnostic protons Ha and Hb from the pyridine donor groups are downfield shifted from 8.86 to 9.88 ppm and from 8.66 to 9.66 ppm, respectively, confirming coordination to PdII (Figure 2biii). DOSY analysis corroborates the presence of a single component with rH = 12.4 Å (Figure 2bvi), a value matching with the formation of a [Pd2L4] topology. The [Pd2L4] stoichiometry was further supported by ESI-MS analysis. A comparison with the data obtained for the assembly products of cis-L with PdII cations clearly showed exclusive formation of [Pd2trans-L4] and no species with higher nuclearity (Figure 2c). Ultimate confirmation for the formation of a dinuclear cage was provided by single-crystal X-ray crystallography. Single crystals of [Pd2trans-L4] were obtained by vapor diffusion of diethyl ether into a DMF solution of the cage at 4 °C in the absence of light (Figure 3a). Low temperature and dark conditions were essential for successful crystal growth and measurement in order to isolate the compound with all diazocine photoswitches residing in the metastable trans-isomeric state. [Pd2trans-L4] crystallizes in a triclinic P1̅ space group, revealing a C2-symmetry with respect to the relative conformation of the eight-membered rings. The Pd···Pd distance was measured as 17.0 Å, significantly longer than that found in the cis-Ring (10.9 Å), the latter showing a more folded conformation. To the best of our knowledge, only two examples of crystal structures for a trans-diazocine have been reported so far,49,50 none of them carrying any substituents.
As no X-ray structure could be obtained for the [Pd2cis-L4] species, a gas-phase structure for this cage photoisomer was determined by density functional theory (DFT) geometry optimization (for details see the Supporting Information). When comparing the calculated [Pd2cis-L4] cis-Cage structure with the X-ray result of cis-Ring, it caught our attention that the cis-diazocine moiety is significantly stretched in the former structure (Cipso–Cipso distance = 6.4 Å; Figure 3c) as compared to the latter (5.6 Å; corresponding distance in the DFT geometry-optimized ring: 5.7 Å; X-ray of free cis-diazocine: 5.9 Å41), and a similar observation was made when comparing the Pd–Pd distances (Figure 3b and c). Apparently, the assembly of cis-L to [Pd2cis-L4] would force the diazocines to stretch to an unfavorable extent. This energetic disadvantage drives the system towards the formation of an ill-defined mixture of species with different nuclearities showing highly dynamic ligand exchange.
Furthermore, a plausible explanation for the clean formation of a [Pd2trans-L4] species, in contrast to the cis-Cage situation, could be obtained by comparing energies derived from DFT calculations. Therefore, the strain energies of the structures of [Pd2cis-L4], trans-Cage, and cis-Ring were compared by computing energies for ligand and coordination site fragments in their assembly-derived and relaxed conformations, respectively. Results of the calculations show that formation of the tentative [Pd2cis-L4] (Figure 3c) species suffers from significant strain compared to the sterically less congested and conformationally more flexible cis-Ring, as well as compared to the trans-Cage (see the Supporting Information for details). These results support that formation of the experimentally observed trans-Cage is favored, even if it persists only in a transient state under continuous irradiation with UV light to prevent thermal back-transformation.
Interestingly, the photoswitching properties of the ligand were maintained in the cage structure. Upon irradiation of the cis-Cage mixture with a 385 nm LED for 10 min, the sample converts into the trans-Cage. Again, the process is reversible, and irradiation at λ = 530 nm or thermal relaxation restore the cis-Cage mixture, as clearly shown by 1H NMR analysis (Figures 2b, S13). UV–vis analysis results of the cage species are similar to those of the ligands, with an absorption band centered at 395 nm for cis-Cage and an increasing band at 483 nm for trans-Cage upon irradiation at λ = 385 nm (Figure 4a). The photoswitching is reversible and shows no significant fatigue over numerous cycles (Figure 4b). The thermal relaxation follows a first-order kinetics, and rate constant k and half-lifetime t1/2 of the trans-Cage were determined (Supporting Information). The half-life for the supramolecular cage (t1/2 = 6.1 h) at 298 K is comparable, while slightly shorter, to that of trans-L (t1/2 = 7.2 h) at the same temperature. Temperature-dependent kinetic studies were carried out for both cage and ligand to determine the activation parameters using the Eyring–Polanyi equation, as reported in Table 1. The results clearly show how the ligand’s photoswitching properties are maintained upon formation of the supramolecular cage.
Figure 4.

(a) Cage solution color change after irradiation (pale yellow: cis-Cage (+ higher assemblies), red: trans-Cage); (b) fatigue experiment of structural interconversion upon irradiation with 385 and 530 nm in an alternating sequence; absorbance values measured at λ = 483 nm; (c) time-dependent UV–vis spectra showing thermal relaxation from the trans-Cage to cis-Cage mixture; (d) UV–vis monitoring of the system reaching the PSS and maintaining the out-of-equilibrium state (trans-Cage) with constant irradiation at 385 nm; turning off the light leads to thermal decay.
Table 1. Activation Energies for Trans to Cis Isomerization.
| ΔS⧧ (J/mol K) | ΔH⧧ (kJ/mol) | ΔG⧧ (kJ/mol) at 298 K | |
|---|---|---|---|
| trans-C to cis-C | 30.7 | 97.3 | 88.1 |
| trans-L to cis-L | 24.2 | 96.2 | 89.0 |
As detailed above, trans-Cage is accessible only when energy, in the form of light, is provided to the system. To prove this, we monitored the UV–vis spectrum of the system under constant irradiation at a fixed temperature, using a home-built setup. Irradiation of the cis-Cage at λ = 385 nm was performed with a 90° incidence angle with respect to the direction of the source and detector of the spectrophotometer, equipped with a thermostat to keep the sample at 288 K. The diagnostic absorption bands at 395 and 483 nm reveal that upon irradiation, the cis-Cage system rapidly converts into trans-Cage, and upon reaching the PSS, the ratio of cis- and trans-isomers is kept constant while the light stimulus is continuously applied. Upon removal of the light source, the transient state of the system dominated by the trans-Cage immediately starts transforming into the cis-Cage system by thermal relaxation (Figure 4c,d).
Finally, we investigated the host–guest properties of both cis- and trans-systems with bis-anionic guest 2,6-naphthalene bissulfonate (G) (Figure 5a). NMR titration of the cis-Cage mixture with G resulted in even further broadening of the signals, accompanied by decreasing intensity and onset of precipitation after the addition of 0.3 equiv of guest, indicative of an outside association mode, creating a salt of low solubility (Figure S35).51,52 On the contrary, trans-Cage is clearly able to bind the guest molecule in its cavity, in accordance with previously reported hosts for G showing a comparable cavity size.52 According to 1H NMR spectroscopy, stepwise addition of G into the cage solution results in a gradual downfield shift of the inward-pointing protons He and Ha, evidencing encapsulation (Figure 5b). Unfortunately, it was not possible to determine the association constant due to an onset of precipitation after the addition of 1 guest equivalent. The host–guest behavior was investigated also by trapped ion mobility spectrometry coupled to ESI-TOF-MS (timsTOF). Here, it was possible to detect signals indicative of an interaction of G with both cis- and trans-Cage (Figures 5c, S31). In the case of the cis-isomer, however, the main species are the “empty” assembly [Pd2cis-L4]4+ at m/z = 463.10, as well as compounds with stoichiometry [Pd3cis-L5G]4+ at m/z = 664.36, [Pd4cis-L6G2]4+ at m/z = 865.11, and [Pd4cis-L7G2]4+ at m/z = 967.90, where presumably the guest replaces one or more ligands (or ligand arms) in coordinated positions in order to reduce strain. Species [Pd2cis-L4+G]2+, corresponding to a typical 1:1 host:guest complex stoichiometry at m/z = 1070.19, was only detected as a minor component (Figure S36). On the contrary, the mass spectrum of the trans-isomeric host in the presence of G features the signal at m/z = 1070.19 assigned to [G@Pd2trans-L4]2+ as the predominant species (Figure 5c). In order to further investigate whether the guest binds inside or interacts with the outside of the cages, we performed an ion mobility analysis focusing on the 2+ peaks assigned to the [Pd2L4+2BF4]2+ and [Pd2L4+G]2+ species for both the cis- and trans-isomers. Addition of G to the trans-Cage shows two CCSs, corresponding to larger objects (494.1 and 504.4 Å2) as compared to the “empty” host (487.4 Å2) and assigned to inside and outside guest binding modes, respectively (Figure 5d). Analysis of the small MS peak at m/z = 1070.19 for [Pd2cis-L4+G]2+ shows only one CCS value of 501.7 Å2, comparable to the outside guest binding mode observed for trans-Cage, hence further supporting that the guest cannot enter the cavity of [Pd2cis-L4] but rather associates nonspecifically to the cage’s exterior (Figure 5d).
Figure 5.
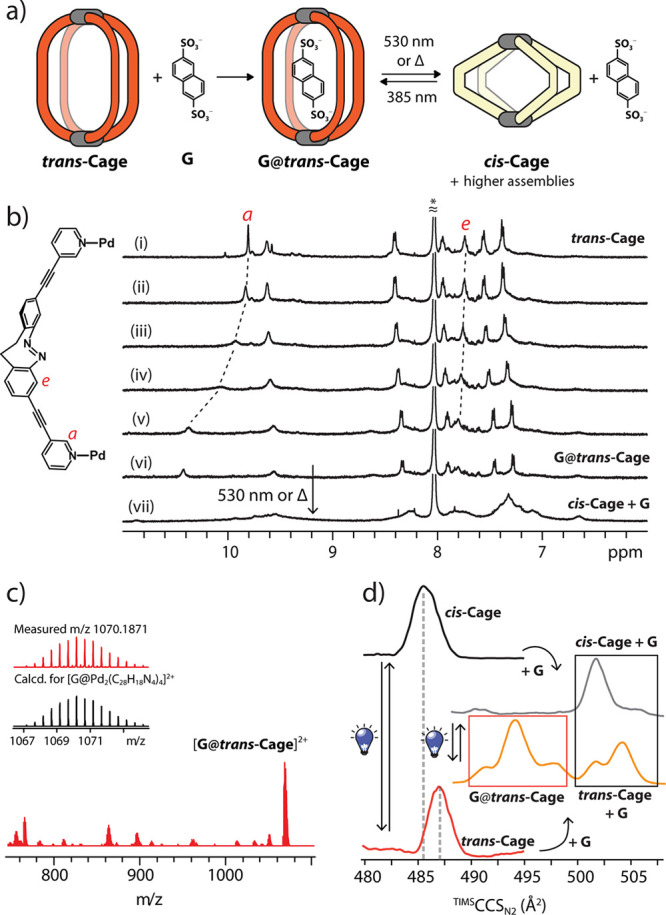
(a) Guest uptake of trans-Cage and release by isomerization to cis-Cage; (b) 1H NMR (500 MHz, DMF-d7, 298 K) stepwise addition of guest G to trans-Cage (mole ratio of host:guest = (i) 1:0.2, (ii) 1:0.4, (iii) 1:0.6, (iv) 1:0.8, and (v) 1:1.0) followed by photoswitching (vii); (c) ESI-MS of G@trans-Cage; (d) ion mobility spectra for cis-Cage, cis-Cage+G, trans-Cage, and G@trans-Cage and light-induced reversibility of the process. The red box highlights signals for inside binding; the black box, signals for outside binding.
Furthermore, guest binding and release was found to be reversible upon application of the light stimulus. Irradiation (λ = 385 nm) of the system to the metastable trans-Cage leads to guest binding, while reversing the photoswitching (λ = 530 nm or Δ) leads to guest release, as determined by stepwise irradiation combined with ion mobility experiments (Figure 5d, Figure S40). Noteworthy, formation of [G@Pd2trans-L4]2+ represents an example of dissipative self-assembly, since this state is existing only under continuous irradiation. Removal of the light stimulus fueling maintenance of this complex leads to thermal relaxation of the diazocine moiety to the cis-form, disassembly of the host, and consequently guest release.
Conclusions
In conclusion, we reported the first coordination-driven cage embedding a diazocine photoswitch.53 The photophysical properties of the diazocine moiety are maintained in the supramolecular assembly, and the system can reversibly switch between a cis- and trans-Cage system. The thermodynamically more stable ligand isomer cis-L leads to an ill-defined mixture of [Pdncis-L2n]n (n = 2, 3, ...) compounds as a consequence of an unfavorable ligand conformation. Strain in this system can be released when assembling a ring topology, as shown by the crystal structure of a [Pd2Cl4cis-L2] ring and DFT calculations. Metastable trans-L self-assembles with PdII to a [Pd2L4] cage as exclusive product, as confirmed by NMR, MS, and single-crystal X-ray results. However, [Pd2trans-L4] is maintained only under continuous irradiation with UV light, and removal of the stimulus results in thermal relaxation to re-form the cis-Cage system. Only the metastable trans-Cage is able to bind a guest molecule. The host–guest system is thus formed via dissipative self-assembly and exists only under constant input of energy in the form of light. Upon reversal of ligand isomerization, by irradiation at 530 nm or thermal back-switching, trans-Cage disassembles and releases the guest. The herein introduced building blocks and principles offer potential for controlling functions in molecular machinery, generating stimuli-responsive complex systems and models for autopoietic processes.
Acknowledgments
This work was supported by the European Research Council (ERC Consolidator grant 683083, RAMSES). H.L. thanks the National Research Foundation of Korea (grant NRF- 2021R1C1C1013037). R.H. and D.L. are grateful for support by the DFG via SFB 677.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c12011.
Synthetic procedures, NMR, MS, UV–vis spectroscopic data, SCXRD results, and DFT calculation data (PDF)
Accession Codes
CCDC 2117884–2117885 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Saha S.; Regeni I.; Clever G. H. Structure Relationships between Bis-Monodentate Ligands and Coordination Driven Self-Assemblies. Coord. Chem. Rev. 2018, 374, 1–14. 10.1016/j.ccr.2018.06.010. [DOI] [Google Scholar]
- Pullen S.; Tessarolo J.; Clever G. H. Increasing Structural and Functional Complexity in Self-Assembled Coordination Cages. Chem. Sci. 2021, 12, 7269–72393. 10.1039/D1SC01226F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty R.; Mukherjee P. S.; Stang P. J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. 10.1021/cr200077m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. D.; Hunter C. A.; Williams N. H. Coordination Cages Based on Bis(Pyrazolylpyridine) Ligands: Structures, Dynamic Behavior, Guest Binding, and Catalysis. Acc. Chem. Res. 2018, 51, 2073–2082. 10.1021/acs.accounts.8b00261. [DOI] [PubMed] [Google Scholar]
- Davis A. V.; Fiedler D.; Seeber G.; Zahl A.; van Eldik R.; Raymond K. N. Guest Exchange Dynamics in an M4L6 Tetrahedral Host. J. Am. Chem. Soc. 2006, 128, 1324–1333. 10.1021/ja056556+. [DOI] [PubMed] [Google Scholar]
- Löffler S.; Wuttke A.; Zhang B.; Holstein J. J.; Mata R. A.; Clever G. H. Influence of Size, Shape, Heteroatom Content and Dispersive Contributions on Guest Binding in a Coordination Cage. Chem. Commun. 2017, 53, 11933–11936. 10.1039/C7CC04855F. [DOI] [PubMed] [Google Scholar]
- August D. P.; Nichol G. S.; Lusby P. J. Maximizing Coordination Capsule–Guest Polar Interactions in Apolar Solvents Reveals Significant Binding. Angew. Chem., Int. Ed. 2016, 55, 15022–15026. 10.1002/anie.201608229. [DOI] [PubMed] [Google Scholar]
- Li R.-J.; Holstein J. J.; Hiller W. G.; Andréasson J.; Clever G. H. Mechanistic Interplay between Light Switching and Guest Binding in Photochromic [Pd2Dithienylethene4] Coordination Cages. J. Am. Chem. Soc. 2019, 141, 2097–2103. 10.1021/jacs.8b11872. [DOI] [PubMed] [Google Scholar]
- Castilla A. M.; Ronson T. K.; Nitschke J. R. Sequence-Dependent Guest Release Triggered by Orthogonal Chemical Signals. J. Am. Chem. Soc. 2016, 138, 2342–2351. 10.1021/jacs.5b13016. [DOI] [PubMed] [Google Scholar]
- Li R.-J.; Tessarolo J.; Lee H.; Clever G. H. Multi-Stimuli Control over Assembly and Guest Binding in Metallo-Supramolecular Hosts Based on Dithienylethene Photoswitches. J. Am. Chem. Soc. 2021, 143, 3865–3873. 10.1021/jacs.0c12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto F. J.; von Krbek L. K. S.; Nitschke J. R. Strategies for Binding Multiple Guests in Metal–Organic Cages. Nat. Rev. Chem. 2019, 3, 204–222. 10.1038/s41570-019-0085-3. [DOI] [Google Scholar]
- Kim T. Y.; Vasdev R. A. S.; Preston D.; Crowley J. D. Strategies for Reversible Guest Uptake and Release from Metallosupramolecular Architectures. Chem.—Eur. J. 2018, 24, 14878–14890. 10.1002/chem.201802081. [DOI] [PubMed] [Google Scholar]
- Jansze S. M.; Cecot G.; Severin K. Reversible Disassembly of Metallasupramolecular Structures Mediated by a Metastable-State Photoacid. Chem. Sci. 2018, 9, 4253–4257. 10.1039/C8SC01108G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Zhang D.; Ronson T. K.; Nitschke J. R. Improved Acid Resistance of a Metal–Organic Cage Enables Cargo Release and Exchange between Hosts. Angew. Chem., Int. Ed. 2020, 59, 7435–7438. 10.1002/anie.202001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansze S. M.; Severin K. Palladium-Based Metal–Ligand Assemblies: The Contrasting Behavior upon Addition of Pyridine or Acid. J. Am. Chem. Soc. 2019, 141, 815–819. 10.1021/jacs.8b12738. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Ronson T. K.; Güryel S.; Thoburn J. D.; Wales D. J.; Nitschke J. R. Temperature Controls Guest Uptake and Release from Zn 4 L 4 Tetrahedra. J. Am. Chem. Soc. 2019, 141, 14534–14538. 10.1021/jacs.9b07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L.-X.; Yan D.-N.; Cheng P.-M.; Xuan J.-J.; Li S.-C.; Zhou L.-P.; Tian C.-B.; Sun Q.-F. Controlled Self-Assembly and Multistimuli-Responsive Interconversions of Three Conjoined Twin-Cages. J. Am. Chem. Soc. 2021, 143, 2016–2024. 10.1021/jacs.0c12064. [DOI] [PubMed] [Google Scholar]
- Szalóki G.; Croué V.; Carré V.; Aubriet F.; Alévêque O.; Levillain E.; Allain M.; Aragó J.; Ortí E.; Goeb S.; Sallé M. Controlling the Host–Guest Interaction Mode through a Redox Stimulus. Angew. Chem., Int. Ed. 2017, 56, 16272–16276. 10.1002/anie.201709483. [DOI] [PubMed] [Google Scholar]
- Croué V.; Goeb S.; Szalóki G.; Allain M.; Sallé M. Reversible Guest Uptake/Release by Redox-Controlled Assembly/Disassembly of a Coordination Cage. Angew. Chem., Int. Ed. 2016, 55, 1746–1750. 10.1002/anie.201509265. [DOI] [PubMed] [Google Scholar]
- Preston D.; Fox-Charles A.; Lo W. K. C.; Crowley J. D. Chloride Triggered Reversible Switching from a Metallosupramolecular [Pd2L4]4+ Cage to a [Pd2L2Cl4] Metallo-Macrocycle with Release of Endo - and Exo -Hedrally Bound Guests. Chem. Commun. 2015, 51, 9042–9045. 10.1039/C5CC02226F. [DOI] [PubMed] [Google Scholar]
- Zhu R.; Lübben J.; Dittrich B.; Clever G. H. Stepwise Halide-Triggered Double and Triple Catenation of Self-Assembled Coordination Cages. Angew. Chem., Int. Ed. 2015, 54, 2796–2800. 10.1002/anie.201408068. [DOI] [PubMed] [Google Scholar]
- Bandara H. M. D.; Burdette S. C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. 10.1039/C1CS15179G. [DOI] [PubMed] [Google Scholar]
- Oshchepkov A. S.; Namashivaya S. S. R.; Khrustalev V. N.; Hampel F.; Laikov D. N.; Kataev E. A. Control of Photoisomerization of an Azoazacryptand by Anion Binding and Cucurbit[8]Uril Encapsula-tion in an Aqueous Solution. J. Org. Chem. 2020, 85, 9255–9263. 10.1021/acs.joc.0c01260. [DOI] [PubMed] [Google Scholar]
- Tian H.; Yang S. Recent Progresses on Diarylethene Based Photochromic Switches. Chem. Soc. Rev. 2004, 33, 85–97. 10.1039/b302356g. [DOI] [PubMed] [Google Scholar]
- Qin Y.; Wang Y.-T.; Yang H.-B.; Zhu W.. Recent Advances on the Construction of Diarylethene-Based Supramolecular Metallacycles and Metallacages via Coordination-Driven Self-Assembly. Chem. Synth. 2021, 1:2. [Google Scholar]
- Koumura N.; Zijlstra R. W. J.; van Delden R. A.; Harada N.; Feringa B. L. Light-Driven Monodirectional Molecular Rotor. Nature 1999, 401, 152–155. 10.1038/43646. [DOI] [PubMed] [Google Scholar]
- Ruangsupapichat N.; Pollard M. M.; Harutyunyan S. R.; Feringa B. L. Reversing the Direction in a Light-Driven Rotary Molecular Motor. Nat. Chem. 2011, 3, 53–60. 10.1038/nchem.872. [DOI] [PubMed] [Google Scholar]
- Petermayer C.; Dube H. Indigoid Photoswitches: Visible Light Responsive Molecular Tools. Acc. Chem. Res. 2018, 51, 1153–1163. 10.1021/acs.accounts.7b00638. [DOI] [PubMed] [Google Scholar]
- Berton C.; Busiello D. M.; Zamuner S.; Scopelliti R.; Fadaei-Tirani F.; Severin K.; Pezzato C. Light-Switchable Buffers. Angew. Chem., Int. Ed. 2021, 60, 21737–21740. 10.1002/anie.202109250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton C.; Busiello D. M.; Zamuner S.; Solari E.; Scopelliti R.; Fadaei-Tirani F.; Severin K.; Pezzato C. Thermodynamics and Kinetics of Protonated Merocyanine Photoacids in Water. Chem. Sci. 2020, 11, 8457–8468. 10.1039/D0SC03152F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzon G.; Prins L. J. Energy Consumption in Chemical Fuel-Driven Self-Assembly. Nat. Nanotechnol. 2018, 13, 882–889. 10.1038/s41565-018-0250-8. [DOI] [PubMed] [Google Scholar]
- Ragazzon G.; Baroncini M.; Silvi S.; Venturi M.; Credi A. Light-Powered Autonomous and Directional Molecular Motion of a Dissipative Self-Assembling System. Nat. Nanotechnol. 2015, 10, 70–75. 10.1038/nnano.2014.260. [DOI] [PubMed] [Google Scholar]
- Stuckhardt C.; Roke D.; Danowski W.; Otten E.; Wezenberg S. J.; Feringa B. L. A Chiral Self-Sorting Photoresponsive Coordination Cage Based on Overcrowded Alkenes. Beilstein J. Org. Chem. 2019, 15, 2767–2773. 10.3762/bjoc.15.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S.; Luo Q.; Zang M.; Tian J.; Zhang Z.; Zeng M.; Ji Y.; Xu J.; Liu J. Light-Triggered Reversible Disassembly of Stimuli-Responsive Coordination Metallosupramolecular Pd2L4 Cages Mediated by Azobenzene-Containing Ligands. Mater. Chem. Front. 2019, 3, 1238–1243. 10.1039/C9QM00160C. [DOI] [Google Scholar]
- Oldknow S.; Martir D. R.; Pritchard V. E.; Blitz M. A.; Fishwick C. W. G.; Zysman-Colman E.; Hardie M. J. Structure-Switching M3L2Ir(III) Coordination Cages with Photo-Isomerising Azo-Aromatic Linkers. Chem. Sci. 2018, 9, 8150–8159. 10.1039/C8SC03499K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.; Luo Y.; Damaschke B.; Gómez L.; Ribas X.; Jose A.; Peretzki P.; Seibt M.; Clever G. H. Light-Controlled Interconversion between a Self-Assembled Triangle and a Rhombicuboctahedral Sphere. Angew. Chem., Int. Ed. 2016, 55, 445–449. 10.1002/anie.201508307. [DOI] [PubMed] [Google Scholar]
- Li R.; Han M.; Tessarolo J.; Holstein J. J.; Lübben J.; Dittrich B.; Volkmann C.; Finze M.; Jenne C.; Clever G. H. Successive Photoswitching and Derivatization Effects in Photochromic Dithienylethene-Based Coordination Cages. Chemphotochem 2019, 3, 378–383. 10.1002/cptc.201900038. [DOI] [Google Scholar]
- Gu Y.; Alt E. A.; Wang H.; Li X.; Willard A. P.; Johnson J. A. Photoswitching Topology in Polymer Networks with Metal–Organic Cages as Crosslinks. Nature 2018, 560, 65–69. 10.1038/s41586-018-0339-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhou Y.; Gao T.; Yan P.; Li H. Metal-Directed Synthesis of Quadruple-Stranded Helical Eu(III) Molecular Switch: A Significant Improvement in Photocyclization Quantum Yield. Chem. Commun. 2020, 56, 13213–13216. 10.1039/D0CC05698G. [DOI] [PubMed] [Google Scholar]
- Li M.; Chen L.-J.; Zhang Z.; Luo Q.; Yang H.-B.; Tian H.; Zhu W.-H. Conformer-Dependent Self-Assembled Metallacycles with Photo-Reversible Response. Chem. Sci. 2019, 10, 4896–4904. 10.1039/C9SC00757A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewertsen R.; Neumann H.; Buchheim-Stehn B.; Herges R.; Näther C.; Renth F.; Temps F. Highly Efficient Reversible Z–E Photoisomerization of a Bridged Azobenzene with Visible Light through Resolved S1(Nπ*) Absorption Bands. J. Am. Chem. Soc. 2009, 131, 15594–15595. 10.1021/ja906547d. [DOI] [PubMed] [Google Scholar]
- Moormann W.; Langbehn D.; Herges R. Synthesis of Functionalized Diazocines for Application as Building Blocks in Photo- and Mechanoresponsive Materials. Beilstein J. Org. Chem. 2019, 15, 727–732. 10.3762/bjoc.15.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moormann W.; Tellkamp T.; Stadler E.; Röhricht F.; Näther C.; Puttreddy R.; Rissanen K.; Gescheidt G.; Herges R. Efficient Conversion of Light to Chemical Energy: Directional, Chiral Photoswitches with Very High Quantum Yields. Angew. Chem., Int. Ed. 2020, 59, 15081–15086. 10.1002/anie.202005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarolo J.; Lee H.; Sakuda E.; Umakoshi K.; Clever G. H. Integrative Assembly of Heteroleptic Tetrahedra Controlled by Backbone Steric Bulk. J. Am. Chem. Soc. 2021, 143, 6339–6344. 10.1021/jacs.1c01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Zhou L.-P.; Guo X.-Q.; Cai L.-X.; Sun Q.-F. Adaptive Self-Assembly and Induced-Fit Transformations of Anion-Binding Metal-Organic Macrocycles. Nat. Commun. 2017, 8, 15898. 10.1038/ncomms15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand D. K.; Biradha K.; Kawano M.; Sakamoto S.; Yamaguchi K.; Fujita M. Dynamic Self-Assembly of an M3L6Molecular Triangle and an M4L8 Tetrahedron from Naked PdII Ions and Bis(3-pyridyl)-Substituted Arenes. Chem.—Asian J. 2006, 1, 82–90. 10.1002/asia.200600029. [DOI] [PubMed] [Google Scholar]
- Rancan M.; Tessarolo J.; Zanonato P. L.; Seraglia R.; Quici S.; Armelao L. Self-Assembly of a Constitutional Dynamic Library of Cu(II) Coordination Polygons and Reversible Sorting by Crystallization. Dalton Trans. 2013, 42, 7534–7538. 10.1039/c3dt50827g. [DOI] [PubMed] [Google Scholar]
- Hasenknopf B.; Lehn J.-M.; Boumediene N.; Dupont-Gervais A.; Van Dorsselaer A.; Kneisel B.; Fenske D. Self-Assembly of Tetra- and Hexanuclear Circular Helicates. J. Am. Chem. Soc. 1997, 119, 10956–10962. 10.1021/ja971204r. [DOI] [Google Scholar]
- Jun M.; Joshi D. K.; Yalagala R. S.; Vanloon J.; Simionescu R.; Lough A. J.; Gordon H. L.; Yan H. Confirmation of the Structure of Trans-Cyclic Azobenzene by X-Ray Crystallography and Spectroscopic Characterization of Cyclic Azobenzene Analogs. Chem. 2018, 3, 2697–2701. 10.1002/slct.201703126. [DOI] [Google Scholar]
- Krämer R.; Nöthling N.; Lehmann C. W.; Mohr F.; Tausch M. W. E-Diazocine in Chemical Education: Synthesis, Structure, Photochromism and Thermal Stability. Chemphotochem 2018, 2, 6–11. 10.1002/cptc.201700124. [DOI] [Google Scholar]
- Clever G. H.; Tashiro S.; Shionoya M. Light-Triggered Crystallization of a Molecular Host–Guest Complex. J. Am. Chem. Soc. 2010, 132, 9973–9975. 10.1021/ja103620z. [DOI] [PubMed] [Google Scholar]
- Clever G. H.; Kawamura W.; Shionoya M. Encapsulation versus Aggregation of Metal–Organic Cages Controlled by Guest Size Variation. Inorg. Chem. 2011, 50, 4689–4691. 10.1021/ic200517r. [DOI] [PubMed] [Google Scholar]
- For a recent example of cages based on ligands with an azobenzene backbone see:Cecot P.; Walczak A.; Markiewicz G.; Stefankiewicz A. R. Gating the photoactivity of azobenzene-type ligands trapped within a dynamic system of an M4L6 tetrahedral cage, an M2L2 metallocycle and mononuclear MLn complexes. Inorg. Chem. Front. 2021, 8, 5195–5200. 10.1039/D1QI01063H. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


