Abstract
Aims:
Continuous glucose monitoring (CGM) has the potential to promote diabetes self-management at home with a better glycemic control as outcome. Investigation of the effect of CGM has typically been carried out based on randomized controlled trials with prespecified CGM devices on CGM-naïve participants. The aim of this study was to investigate the effect on glycemic control in people using their personal CGM before and during the trial.
Materials and Methods:
Data from the Onset 5 trial of 472 people with type 1 diabetes using either their personal CGM (n = 117) or no CGM (n = 355) and continuous subcutaneous insulin infusion in a 16-week treatment period were extracted. Change from baseline in glycated hemoglobin A1c (HbA1c), number of hypoglycemic episodes, and CGM metrics at the end of treatment were analyzed with analysis of variance repeated-measures models.
Results:
Use of personal CGM compared with no CGM was associated with a reduction in risk of documented symptomatic hypoglycemia (event rate ratio: 0.82; 95% CI: 0.69-0.97) and asymptomatic hypoglycemia (event rate ratio: 0.72; 95% CI: 0.53-0.97), reduced time spent in hypoglycemia (P = .0070), and less glycemic variability (P = .0043) without a statistically significant increase in HbA1c (P = .2028).
Conclusions:
Results indicate that use of personal CGM compared with no CGM in a population of type 1 diabetes is associated with a safer glycemic control without a statistically significantly deteriorated effect on HbA1c, which adds to the evidence about the real-world use of CGM, where device type is not prespecified, and users are not CGM naïve.
Keywords: continuous glucose monitoring, type 1 diabetes, continuous subcutaneous insulin infusion, hypoglycemia, glycemic management
Introduction
Glycemic management of people with type 1 diabetes is a complex interplay between multiple factors, and the patients need to deal with the majority of these at home without close contact to clinical care.1,2 Effective approaches to promote diabetes self-management at home are thus wanted, and use of continuous glucose monitoring (CGM) might be one of the solutions. 3 CGM devices provide an interstitial glucose reading typically every five minutes and are thus an obvious tool for reduction of glycemic excursions. However, inaccuracy caused by physiological differences between the plasma and interstitial glucose hampers these detection capabilities.4,5 Still, CGM has a great potential for improving metabolic control through promotion of self-management, education, and person empowerment. In a meta-analysis of six randomized controlled studies of real-time CGM compared with self-monitoring of blood glucose (SMBG) in people with type 1 diabetes, Pickup et al 6 showed that use of CGM was associated with a significant reduction in glycated hemoglobin A1c (HbA1c) of 0.3% points. Furthermore, the reduction was larger for people with less controlled glycemia at baseline, and the perspective was to target CGM at people with continued poor glycemic control during intensified insulin therapy. In another meta-analysis by Szypowska et al 7 of seven randomized controlled studies of type 1 diabetes, a modest reduction in HbA1c of 0.25% points was reported for CGM versus SMBG. With respect to hypoglycemia, the results of the meta-analyses were unclear.Szypowska et al 7 did not find any significant reduction in time spent in hypoglycemia in CGM users compared to SMBG. Pickup et al 6 reported that baseline area under the curve of hypoglycemia was only weakly related to the effect of CGM compared with SMBG on hypoglycemia outcome. Both meta-analyses included studies conducted more than a decade ago. In newer randomized controlled studies of adults with type 1 diabetes, CGM has been associated with a reduction in HbA1c compared with SMBG of around 0.5% point,8,9 with one trial reporting a reduction of 0.9% points during a three-year follow-up. 10 Furthermore, the studies report reduced time in hypoglycemia for users of CGM, and in a study of people with type 1 diabetes and impaired hypoglycemia awareness or history of severe hypoglycemia, a 72% reduction in incidence of hypoglycemic episodes for users of CGM compared with SMBG was reported. 11 In most of these studies, the CGM product type was prespecified, and previous use of CGM was not an inclusion criterion, and in many cases previous use of CGM was even an exclusion criterion. A real-world situation where people were allowed to use their personal CGM without any restriction on type and previous use could lead to different results.
This aim of this study was to investigate the effect of CGM on glycemic management in a trial of people with type 1 diabetes using their personal CGM.
Methods
Study Data
Study data were obtained from the Onset 5 clinical trial 13 investigating the efficacy and safety of fast-acting insulin aspart compared with insulin aspart in a 16-week parallel-arm study of people with type 1 diabetes using continuous subcutaneous insulin infusion. Up to half of the participants were allowed to use their personal CGM throughout the trial. During the 16-week treatment period, all participants were further monitored thrice with a blinded Dexcom G4 Platinum (Dexcom, San Diego, CA, USA) CGM device (1) at randomization, (2) at week 8, and (3) at the end of trial. Key inclusion criteria were adults with an HbA1c of 53-75 mmol/mol (7%-9%) diagnosed with type 1 diabetes more than one year before informed consent and being pump users (for at least six months before screening). Hypoglycemic episodes were recorded by the participants and investigator during trial conduct according to guidelines by the American Diabetes Association. 12 The definition of the hypoglycemic episodes was 13 (1) severe hypoglycemia: an episode requiring assistance of another person to actively administer carbohydrate, glucagon, or take other corrective actions. Blood glucose concentrations may not be available during an episode, but neurological recovery following the return of blood glucose to normal is considered sufficient evidence that the episode was induced by a low blood glucose concentration. (2) Asymptomatic hypoglycemia: an episode not accompanied by typical symptoms of hypoglycemia, but with a measured blood glucose concentration ≤70 mg/dL (3.9 mmol/L). (3) Documented symptomatic hypoglycemia: an episode during which typical symptoms of hypoglycemia are accompanied by a measured blood glucose concentration ≤70 mg/dL (3.9 mmol/L). (4) Pseudo-hypoglycemia: an episode during which the person with diabetes reports any of the typical symptoms of hypoglycemia with a measured blood glucose concentration >70 mg/dL (3.9 mmol/L) but approaching that level. (5) Probable symptomatic hypoglycemia: an episode during which symptoms of hypoglycemia are not accompanied by a blood glucose determination but that was presumably caused by a blood glucose concentration ≤70 mg/dL (3.9 mmol/L).
Endpoints
This post hoc analysis compared the following endpoints between CGM users and people without CGM: (1) laboratory: change from baseline in HbA1c; (2) self-report: number and rate of severe hypoglycemia, documented symptomatic hypoglycemia, asymptomatic hypoglycemia, probable symptomatic hypoglycemia, and pseudo-hypoglycemia; and (3) CGM: time spent in hyperglycemia (>180 mg/dL [10.0 mmol/L]), time spent in target (70 mg/dL [3.9 mmol/L] <CGM ≤180 mg/dL [10.0 mmol/L]), time spent in hypoglycemia (≤70 mg/dL [3.9 mmol/L]), time spent in hypoglycemia (≤53 mg/dL [3.0 mmol/L]), coefficient of variation (CV), and mean interstitial glucose. Only periods without gaps in CGM data contributed to the calculations. Finally, the endpoint of change from baseline in total daily insulin dose was calculated and compared between people using personal CGM and people without CGM.
Statistical Analyses
Baseline patient characteristics are presented with mean and SDs, percentage, or numbers. Test for difference in each characteristic was performed with t-tests, chi-square tests, or Mann–Whitney U-tests where applicable. The effect of using personal CGM versus no CGM on the endpoints of change from baseline in HbA1c and total daily insulin dose was analyzed with an analysis of variance repeated-measures model adjusted for age, sex, region, smoking status, body mass index (BMI), diabetes duration, and HbA1c at baseline. The effect of using personal CGM versus no CGM on self-reported hypoglycemic episodes was summarized descriptively and is presented according to the American Diabetes Association’s categories 11 as number of people with episodes, percentage of people with episodes, number of episodes, and number of episodes per 100 person years of exposure. Furthermore, adjusted event rate ratios are presented, and they were estimated with negative binomial regression models with the logarithm of the person exposure as offset. The models were adjusted for age, sex, diabetes duration, region, smoking status, and BMI. Finally, the effect of using personal CGM versus no CGM on metrics from the blinded CGM session at the end of treatment was analyzed with analysis of variance repeated-measures models adjusted for age, sex, region, smoking status, BMI, diabetes duration, and the metric at baseline. Missing data were not imputed.
Results
In Table 1, the baseline patient characteristics are shown. People using personal CGM were older, had higher BMI, longer diabetes duration, and more hypoglycemia unawareness compared to people without CGM. Furthermore, they were typically from North America and not current smokers.
Table 1.
Baseline Characteristics of People in This Study.
| Parameter | Personal CGM | No CGM | P value |
|---|---|---|---|
| N | 117 | 355 | |
| Age, mean (SD) | 49 (13) | 42 (15) | <.0001 |
| Sex (%) | |||
| Female | 53 | 58 | .3136 |
| Male | 47 | 42 | |
| Region (%) | |||
| Europe | 44 | 61 | .0016 |
| North America | 56 | 39 | |
| Smoking status (%) | |||
| Current | 4 | 12 | .0342 |
| Previous | 26 | 21 | |
| Never | 70 | 67 | |
| Body mass index (kg/m2), mean (SD) | 27.2 (4.1) | 26.0 (3.9) | .0070 |
| Duration of diabetes (yr), mean (SD) | 28 (12) | 23 (12) | .0004 |
| Total daily insulin dose (U/kg), mean (SD) | 0.60 (0.23) | 0.63 (0.24) | .2221 |
| HbA1c at baseline, mean (SD) | |||
| mmol/mol | 58.2 (5.7) | 58.4 (5.9) | .7174 |
| % | 7.48 (0.52) | 7.50 (0.54) | |
| Hypoglycemia unawareness a , n (%) | 20 (17) | 34 (10) | .0267 |
| Number of days CGM b worn (days), mean (SD) | 42 (7) | 41 (8) | .8173 |
| Percentage of time CGM b is active (%), mean (SD) | 95 (6) | 94 (6) | .3270 |
CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin A1c.
According to the Clarke questionnaire, question 8. 14
Use of blinded CGM during 3 × 2 weeks.
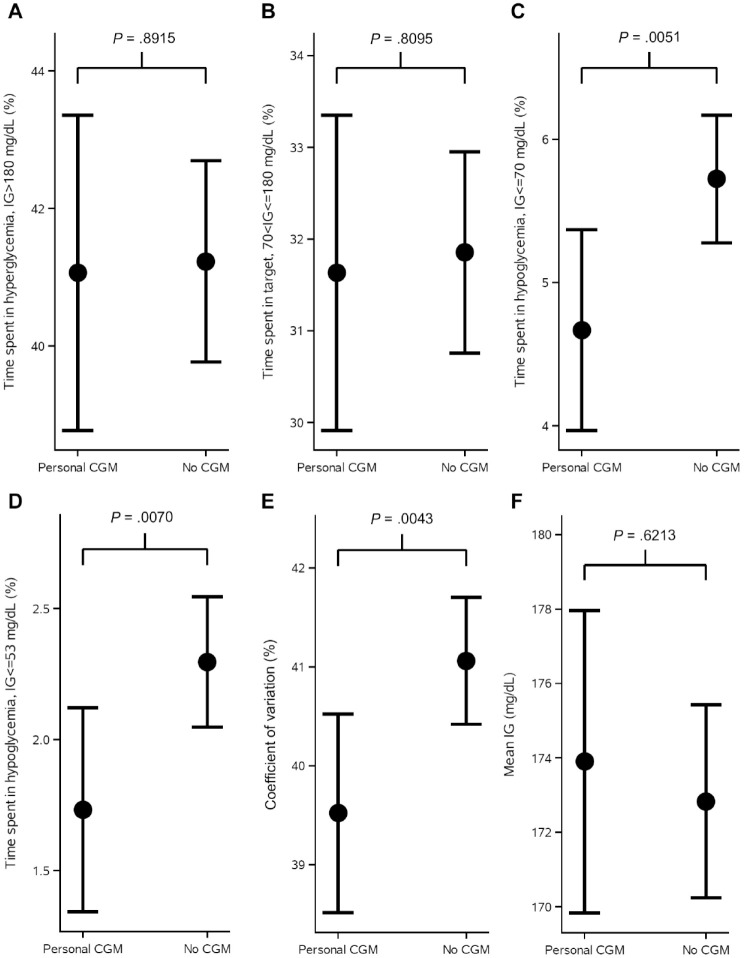
Figure 1 shows the observed mean values and standard errors of change from baseline in HbA1c in the weeks after randomization. Estimated adjusted mean values and 95% confidence limits are shown as stars and associated error bars. People without CGM experienced a larger drop in HbA1c compared to people using personal CGM, but the difference was not statistically significant. Table 2 shows the number of hypoglycemic episodes after 16 weeks of treatment and estimated event rate ratio. Use of personal CGM is associated with a 18% reduction in risk of documented hypoglycemia and a 28% reduction in risk of asymptomatic hypoglycemia compared with no CGM. Furthermore, use of personal CGM is associated with a reduction in risk of severe hypoglycemia and an increase in risk of probably symptomatic hypoglycemia and pseudo-hypoglycemia, but all three estimates were not statistically significant. Figure 2 shows estimated CGM metrics after 16 weeks of treatment. Results from analyses of all endpoints except self-reported hypoglycemia can be seen in Table 3. No statistically significant difference in time spent in hyperglycemia, time spent in target, and mean interstitial glucose was observed in use of personal CGM compared to no CGM. On the other hand, time in hypoglycemia was statistically significantly reduced with 15 and 8 minutes for interstitial glucose ≤70 and ≤53 mg/dL, respectively, for use of personal CGM compared with no CGM. Furthermore, variation in interstitial glucose (CV) was statistically significantly reduced with 1.5%.
Figure 1.
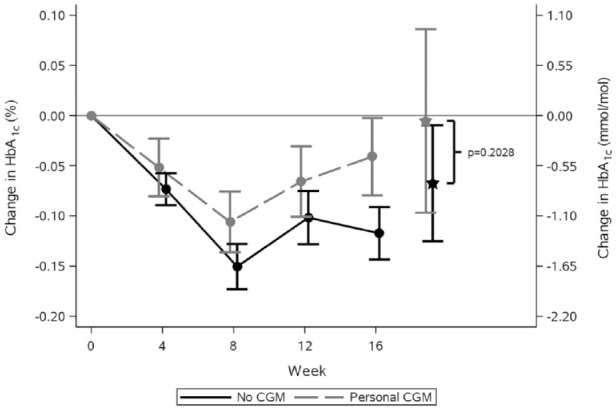
Observed mean values and standard error of the mean of the change from baseline in HbA1c by treatment week. Values marked with stars and associated error bars are estimated mean values and 95% confidence limits at week 16 from an analysis of variance repeated-measures model adjusted for age, sex, region, smoking status, body mass index, diabetes duration, and HbA1c at baseline. The conversion factor from HbA1c in % to mmol/mol is 10.93.
CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin A1c.
Table 2.
Number of Hypoglycemic Episodes After 16 weeks of Treatment.
| Personal CGM |
No CGM |
Estimated event |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | E | R | N | % | E | R | Rate ratio (95% CI) | |
| Severe hypoglycemia | 5 | 4 | 7 | 19 | 11 | 3 | 21 | 19 | 0.79 (0.18-3.44) |
| Documented symptomatic | 113 | 97 | 3462 | 9614 | 346 | 97 | 13 814 | 12 739 | *0.82 (0.69-0.97) |
| Asymptomatic | 99 | 85 | 1094 | 3038 | 295 | 83 | 3709 | 3420 | *0.72 (0.53-0.97) |
| Probable symptomatic | 11 | 9 | 67 | 186 | 24 | 7 | 53 | 49 | 3.59 (0.93-13.82) |
| Pseudo-hypoglycemia | 9 | 8 | 92 | 255 | 26 | 7 | 123 | 113 | 2.90 (0.81-10.40) |
Classification follows American Diabetes Association’s 2013 guidelines. 12
n is the number of people, % percentage of people, E is the number of hypoglycemic episodes, R is the actual event rate calculated as number of hypoglycemic episodes per 100 person-years of exposure within each group.
The estimated event rate ratios (personal CGM/no CGM) are derived from a negative binomial regression model with the logarithm of the person exposure as offset. The models were adjusted for age, sex, diabetes duration, region, smoking status, and body mass index.
CGM, continuous glucose monitoring.
Statistically significant.
Figure 2.
CGM metrics at the end of treatment period shown as estimated mean and 95% confidence intervals as error bars and P-values from analysis of variance repeated-measures models analyzing the effect of CGM use on each of the metrics. Time spent in hyperglycemia, time spent in range, time spent in hypoglycemia, and measures of variation are shown for CGM users versus non-CGM users. The models were adjusted for age, sex, region, smoking status, body mass index, diabetes duration, and the metric at baseline.
CGM, continuous glucose monitoring; IG, interstitial glucose.
Table 3.
Estimated Change From Baseline in HbA1c, Change From Baseline in Total Daily Dose, and CGM Metrics at the End of Treatment.
| Endpoint | Personal CGM | No CGM | Difference (95% CI) | P value |
|---|---|---|---|---|
| Change from baseline in HbA1c | −0.01% –0.06 mmol/mol |
−0.07% –0.74 mmol/mol |
0.06% (−0.03%; 0.16%) 0.68 (−0.37; 1.72) mmol/mol |
.2028 |
| Change from baseline in total daily insulin dose | −0.0033 U/kg | −0.0082 U/kg | 0.0049 (−0.0239; 0.0336) U/kg | .7394 |
| Time spent in hyperglycemia (>180 mg/dL [10.0 mmol/L]) | 41.1% 591.3 min |
41.2% 593.7 min |
−0.17% (−2.56%; 2.22%) −2.4 (−36.8; 32.0) min |
.8915 |
| Time spent in target (70 mg/dL [3.9 mmol/L] <CGM ≤180 mg/dL [10.0 mmol/L]) | 31.6% 455.5 min |
31.9% 458.7 min |
−0.22% (−2.01%; 1.57%) −3.2 (−29.0; 22.6) min |
.8055 |
| Time spent in hypoglycemia (≤70 mg/dL [3.9 mmol/L]) | 4.5% 67.2 min |
5.7% 82.4 min |
−1.05% (−1.79%; −0.32%)*
−15.2 (−28.8; −4.6) min* |
.0051 |
| Time spent in hypoglycemia (≤53 mg/dL [3.0 mmol/L]) | 1.7% 24.9 min |
2.3% 33.1 min |
−0.56% (−0.97%; −0.16%)*
−8.1 (−14.0; −2.2) min* |
.0070 |
| Coefficient of variation | 39.5% | 41.1% | −1.54% (−2.60%; −0.49%) * | .0043 |
| Mean interstitial glucose | 173.9 mg/dL | 172.8 mg/dL | 1.07 (−3.17; 5.30) mg/dL | .6213 |
Estimated means, differences, 95% confidence limits, and P value are from analysis of variance repeated-measures models adjusted for age, sex, region, smoking status, body mass index, diabetes duration, and value at baseline (HbA1c, total daily insulin dose, or CGM metric).
CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin A1c.
Statistically significant.
Discussion
In this post hoc analysis of the Onset 5 trial, use of personal CGM compared with no CGM for people with type 1 diabetes was associated with a reduction in risk of documented symptomatic and asymptomatic hypoglycemia, reduced time spent in hypoglycemia, and less glycemic variability without a statistically significant increase in HbA1c. Furthermore, a reduction in severe hypoglycemia was observed for people using personal CGM, though it was not statistically significant.
Three meta-analyses by Pickup et al, 6 Benkhadra et al, 15 and Szypowska et al 7 examining randomized controlled trials of CGM use in people with type 1 diabetes have reported HbA1c improvements of 0.2%-0.3% points for CGM compared to standard of care, which in most cases included SMBG. Although the effect is modest, current evidence is that CGM results in a larger reduction in HbA1c compared with SMBG. This is especially evident for newer trials, such as the COMISAIR, 10 GOLD, 8 and DIAMOND 9 trial. In our study, CGM did not result in a larger reduction in HbA1c; on the contrary, people without CGM dropped more in HbA1c, but the observation was not statistically significant. The populations of other studies were people with type 1 diabetes treated with either multiple daily injections or continuous subcutaneous insulin infusion. That is, they are comparable to the population of this study. The baseline HbA1c was in most studies much higher (>65 mmol/mol)8–10 compared to this study (58 mmol/mol), which may result in larger HbA1c reductions. Furthermore, people in the randomized controlled studies included were in most cases CGM naïve. We anticipate that the most prominent effect of a technology solution that promotes self-management at home like CGM is in the initial phase. This is confirmed in a meta-analysis by Su et al, 16 where they investigated the effect of telemedicine on treatment outcomes in diabetes and found that the effect on HbA1c was most prominent during the first six months. The same phenomena could be seen for CGM with the result that people in our study exhibited an effect on HbA1c from the CGM use before enrollment in the clinical trial.
From the CGM metrics it can be observed that the reductions in time spent in hypoglycemia do not come at a cost of increased time spent in hyperglycemia, and mean interstitial glucose is only slightly increased for people using personal CGM. The changes in time spent in hypoglycemia are small, but they still reduce the risk of severe (not statistically significant), documented symptomatic and asymptomatic hypoglycemia with 18%-28%.
In this study, 17% of the people using personal CGM had a history with hypoglycemia unawareness compared to 10% of the people without CGM. Especially, people suffering from hypoglycemia unawareness might benefit from using CGM, 17 which might explain the higher prevalence in the CGM group in this study. A recommendation to insulin-treated patients with hypoglycemia unawareness is to raise glycemic targets for several weeks, 17 and the most obvious way would be to reduce daily insulin use. From the analysis of total daily insulin dose during the treatment period, no statistically significant difference was found between people using personal CGM and people without. Therefore, a more protective behavior to insulin administration cannot explain the numerically higher HbA1c level for people using personal CGM in this study.
A limitation in this study is that the choice to use CGM was not random. People using personal CGM in our study might be a self-selected population who are more proactive in their glycemic management and/or have a greater fear of hypoglycemia. One way to reduce this skewness could be to control for related confounders, but these behavioral and socioeconomic factors were not available in data. Furthermore, we have no knowledge about which personal CGM device was used by the patients, which makes the results less specific, and the devices are older versions (the trial was conducted in 2016-2017), and the results might thus not be generalizable to today’s devices. Another limitation is the low number of patients, which makes results less generalizable, and the imbalanced groups in baseline variables, which may have biased the statistical tests. Finally, a limitation is that the study is underpowered to show a statistically significant effect on rate of severe hypoglycemia, and a conclusion on severe hypoglycemia, therefore, cannot be drawn.
Conclusion
The results of this study indicate that use of personal CGM compared with no CGM in a population of type 1 diabetes on continuous subcutaneous insulin infusion is associated with a decreased risk of both symptomatic and asymptomatic hypoglycemia and decreased glycemic variation without a statistically significant increase in HbA1c and mean interstitial glucose. As people in this study were not randomized to use of personal CGM or no CGM, lack of data on possible confounders, such as behavioral and socioeconomic factors, is a limitation. The study provides evidence about the real-world use of CGM where device type is not prespecified, and users are not CGM naïve, which is important in the assessment of the real-world long-term effect of CGM.
Acknowledgments
None
Footnotes
Abbreviations: HbA1c, glycated hemoglobin A1c; CGM, continuous glucose monitoring; SMBG, self-measured blood glucose; CV, coefficient of variation.
Authors’ Contribution Statement: MJ designed the study, did the statistics, and drafted the article. PV, IH, and OH came with valuable input to the study design and the article writing. All authors have read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics: Ethics approval has been obtained according to local regulations for observational studies.
ORCID iD: Morten Hasselstrøm Jensen  https://orcid.org/0000-0002-6649-8644
https://orcid.org/0000-0002-6649-8644
References
- 1. Davidson M, Penney ED, Muller B, Grey M. Stressors and self-care challenges faced by adolescents living with type 1 diabetes. Appl Nurs Res. 2004;17(2):72-80. [DOI] [PubMed] [Google Scholar]
- 2. Ramchandani N, Way N, Melkus GD, Sullivan-Bolyai S. Challenges to diabetes self-management in emerging adults with type 1 diabetes. Diabetes Educ. 2019;45(5):484-497. [DOI] [PubMed] [Google Scholar]
- 3. Kruger D, Marcus AO. Psychological motivation and patient education: a role for continuous glucose monitoring. Diabetes Technol Ther. 2000;2(suppl 1):S93-S97. [DOI] [PubMed] [Google Scholar]
- 4. Jensen MH, Dethlefsen C, Hejlesen O, Vestergaard P. Simple post-processing of continuous glucose monitoring measurements improves endpoints in clinical trials [published online ahead of print May 16, 2019]. J Diabetes Sci Technol. doi: 10.1177/1932296819848721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuroda A, Taniguchi S, Akehi Y, et al. Accuracy and time delay of glucose measurements of continuous glucose monitoring and bedside artificial pancreas during hyperglycemic and euglycemic hyperinsulinemic glucose clamp study. J Diabetes Sci Technol. 2017;11(6):1096-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Szypowska A, Ramotowska A, Dzygało K, Golicki D. Beneficial effect of real-time continuous glucose monitoring system on glycemic control in type 1 diabetic patients: systematic review and meta-analysis of randomized trials. Eur J Endocrinol. 2012;166(4):567-574. [DOI] [PubMed] [Google Scholar]
- 8. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the gold randomized clinical trial. JAMA. 2017;317(4):379-387. [DOI] [PubMed] [Google Scholar]
- 9. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the diamond randomized clinical trial. JAMA. 2017;317(4):371-378. [DOI] [PubMed] [Google Scholar]
- 10. Šoupal J, Petruželková L, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care. 2020;43(1):37-43. [DOI] [PubMed] [Google Scholar]
- 11. Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367-1377. [DOI] [PubMed] [Google Scholar]
- 12. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013;98(5):1845-1859. [DOI] [PubMed] [Google Scholar]
- 13. Novo Nordisk. Protocol: Efficacy and Safety of Continuous Subcutaneous Insulin Infusion of Faster-acting Insulin Aspart compared to NovoRapid® in Adults with Type 1 Diabetes. 2016. https://clinicaltrials.gov/ct2/show/NCT02825251. Accessed date: 9-SEP-2020
- 14. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM: a prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18(4):517-522. [DOI] [PubMed] [Google Scholar]
- 15. Benkhadra K, Alahdab F, Tamhane S, et al. Real-time continuous glucose monitoring in type 1 diabetes: a systematic review and individual patient data meta-analysis. Clin Endocrinol (Oxf). 2017;86(3):354-360. [DOI] [PubMed] [Google Scholar]
- 16. Su D, Zhou J, Kelley MS, et al. Does telemedicine improve treatment outcomes for diabetes? A meta-analysis of results from 55 randomized controlled trials. Diabetes Res Clin Pract. 2016;116:136-148. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association. Standards of medical care in diabetes - 2018. Diabetes Care. 2018;41(suppl 1): S55-S64. [DOI] [PubMed] [Google Scholar]