Abstract
Rationale:
Emerging evidence suggests that exposure to environmental tobacco smoke (ETS) may be linked with behavior problems in childhood, but previous research has relied primarily on parent report of exposure, and results are inconclusive.
Objectives:
To investigate the relationship between exposure to ETS and child behavior problems among children with asthma.
Methods:
The sample included 220 children who were enrolled in an asthma intervention trial and regularly exposed to ETS at home. Serum cotinine was used to measure exposure to tobacco smoke, and behavior problems were assessed by parent report on the Behavior Assessment System for Children. Covariates in adjusted analyses included: sex, age, race, asthma severity, asthma medication, maternal education, prenatal tobacco exposure, maternal depression, and Home Observation for Measurement of the Environment score.
Results:
Child behavior problems increased with increasing exposure to ETS. A stratified analysis of boys and girls separately indicated higher exposure among girls, but behavior problems were statistically significantly associated with exposure only in boys. Increasing behavior problems included externalizing behavior problems (β = 2.23, p = .02) such as hyperactivity and aggression, internalizing behavior problems (β = 2.19, p = .01) such as depression, and behavior symptoms (β = 2.55, p = .01).
Conclusions:
Among children with asthma, exposure to ETS is related to increased child behavior problems among boys.
Keywords: environmental tobacco smoke, behavior, children, exposure
There is considerable evidence implicating tobacco smoke exposure, both prenatally and postnatally, with numerous consequences to child health including prematurity,1-3 low birth weight,1,4-7 sudden infant death syndrome,8-11 and asthma severity.12-14 There have been several reports linking prenatal and postnatal tobacco smoke exposure with cognitive deficits in childhood.15-20 However, the research linking environmental tobacco smoke (ETS) exposure with behavioral problems is not as widely recognized.4,12,21-23 Research on the association between tobacco smoke and behavioral problems has yielded inconsistent results, but suggests that tobacco smoke is an exposure which may entail detrimental consequences for child behavior.24 With the increasing prevalence of child behavior problems,25 understanding yet another possible contributor to the rise is critical.
Exposure to tobacco smoke during gestation has been linked with behavior problems in childhood including increased prevalence of attention-deficit hyperactivity disorder (ADHD),26-29 conduct disorder,30,31 disruptive behaviors,30 and externalizing behaviors.32,33 However, these results are inconclusive due to limitations of previous research that failed to properly control for confounders such as postnatal ETS exposure and other known covariates of child behavior.34,35 After conducting a thorough review of case-control and cohort studies of the relationship between prenatal tobacco smoke exposure and child behavior problems, Linnet et al35 concluded that “there may be an association with exposure to tobacco smoke in utero (and ADHD),” but that studies effectively measuring the proper potential covariates of this relationship were “few and of poor quality.”
Although some questions remain about the relative contribution of prenatal tobacco smoke exposure to behavior problems in childhood, we have even more limited information about the impact of postnatal ETS exposure on child behavior. Few studies have specifically investigated the association between postnatal ETS exposure and child behavior, and these have reported inconsistent results. Fergusson et al30 found no relation between childhood ETS exposure and attention, conduct, or disruptive behavior problems at ages 8 to 12 when controlling for maternal smoking during pregnancy. In contrast, Weitzman et al36 reported a dose-response relationship for both internalizing and externalizing behavior problems among children whose mothers smoked at least a pack of cigarettes per day, even after controlling for a variety of potential covariates including maternal smoking during pregnancy. The combination of prenatal and postnatal exposure appeared to have the greatest negative influence on child behavior. Williams et al33 found a similar relationship in which maternal smoking both early in pregnancy and concurrent with the assessment of the child’s behavior at age 5 had the strongest relationship with child externalizing behavior problems. More recent work by Braun et al suggests that ETS may be associated with increased risks for conduct disorder36a but not ADHD.37
One of the most significant limitations of previous studies on ETS exposure and child behavior is that they have relied primarily on parent report to estimate children’s exposure to tobacco. National data indicate that while 25% of parents report child exposure to cigarettes at home, over 50% of children are exposed to tobacco smoke according to biological markers.38 This suggests that parent report may not be a sensitive or accurate way to measure children’s total exposure to ETS. Until researchers systematically measure child exposure to ETS as well as the potential covariates, the contribution of ETS exposure to behavior problems will be uncertain.
Asthma is often associated with an increased prevalence of behavior problems in children. Reports occasionally include elevated externalizing behaviors such as ADHD,39 and oppositional or negative behaviors40,41 but more often indicate more frequent internalizing behaviors such as anxiety and depressive symptoms in children with asthma.39-45 Behavioral symptoms in children with asthma increase in conjunction with increasing asthma severity,39,46 and Alati et al47 reports that internalizing behaviors detected at age 5 remain problematic into adolescence. To our knowledge, the specific association between ETS exposure and increases in behavior problems among children with asthma has not been carefully examined.
The objective of this study was to investigate the relationship between ETS exposure and child behavior problems among a group of children with asthma. We used a biomarker of tobacco exposure, serum cotinine, to objectively quantify exposure from all sources. We hypothesized that children with asthma who are exposed to higher levels of ETS exhibit more behavior problems, as reported by parents, than children with lower levels of exposure to ETS.
METHODS
This study utilized the Cincinnati Asthma Prevention (CAP) Study, a double-blind, placebo controlled intervention trial designed to test the efficacy of reducing environmental tobacco smoke (ETS) exposure among children with asthma using high-efficient particulate air cleaners fitted with carbon-permanganate-zeolite filters. Outcome measures of the CAP study were focused on asthma symptoms, health care utilization, and pulmonary function. For the current study, baseline measures of exposure were used in addition to concurrent measures to assess child behavior and potential covariates.
Children with asthma between 6 and 12 years of age were initially identified for participation in the CAP Study (n = 2240). Letters were mailed to parents introducing the study and providing a postcard that could be returned if they were not interested in being contacted for participation. Among those who did not return the postcard and were able to be reached by telephone, a screening survey with the child’s primary caregiver determined eligibility and interest in study participation (n = 1678). Eligibility criteria for the 6- to 12-year-old children were doctor diagnosed asthma that was treated within the previous 12 months in a pediatric hospital or participating private practice clinic, exposure to ETS from at least 5 cigarettes per day at home, and no plans to move within the 12-month period of the planned intervention trial. Children were excluded if they had other respiratory diseases (n = 646), heart disease (n = 34), were mentally retarded (n = 23), or had other serious conditions barring participation in the study (n = 31). Since the intervention for the parent study required use of an electrical device, children were also excluded if the family did not have working electricity at the time of the screening survey (n = 14). Of the 348 eligible participants, 232 enrolled and completed the study (67% participation rate).
Twelve participants were later excluded from the current analysis plan due to: ineligibility according to initial enrollment criteria and inadvertent enrollment (n = 7), missing baseline cotinine sample (n = 3), target child was an active smoker by survey (n = 1), and cotinine level (112 ng/mL) indicative of active smoking (n = 1). The final sample for this analysis included 220 children. Protocols were approved by the institutional review board.
ETS exposure was measured through analysis of serum cotinine samples that were obtained at the baseline home visit for all 220 children included in this study. Cotinine, a metabolite of nicotine, is currently considered the most reliable biomarker of exposure to tobacco smoke.48 Serum levels provide a short-term view of exposure over the previous 48 to 72 hours. Cotinine detection in serum was performed by the Centers for Disease Control using published methods involving high-performance liquid chromatography linked to atmospheric-pressure chemical ionization tandem mass spectrometry.49
The Behavior Assessment System for Children (BASC)50 was used to measure child behavior problems within the past 2 weeks as reported by the primary caregiver at the baseline home visit. The BASC yields age and sex standardized T-scores for 4 composite clinical scales (externalizing problems, internalizing problems, behavioral symptoms index, adaptive skills) as well as scores on 12 clinical subscales (anxiety, attention problems, atypicality, conduct problems, depression, hyperactivity, somatization, withdrawal, adaptability, leadership, social skills). This instrument is used in clinical and research settings to provide a broad description of child behaviors that may be problematic or adaptive. Internal consistency reliabilities of composite scores are in the middle .80s to low .90s, and test-retest correlations are in the low .90s for the age group of interest. Correlations with the Child Behavior Checklist,51 a conceptually similar measure, range from .65 to .84 for the Internalizing and Externalizing composites. Ostrander et al52 determined that the BASC was a more parsimonious and accurate measure for distinguishing students with and without attention-deficit hyperactivity disorder (ADHD) than the Child Behavior Checklist. To maintain uniformity, this measure was administered as an interview in which caregiver responses were coded by a trained research assistant.
Asthma severity, as perceived by the parent, was reported as mild, moderate, severe or very severe. Parent report of asthma symptoms is an effective means for characterizing child asthma and is not enhanced by asthma diaries or pulmonary function testing.53 Parents additionally reported asthma medication use by the child in the form of short-acting bronchodilators, long-acting inhaled steroids, and oral steroids used for acute exacerbations. To account for the well-established association between maternal depression and child behavior,54,55 we used the Beck Depression Inventory, Second Edition56 to measure maternal depression during the baseline home visit. To account for quality of the home environment, a known contributor to child behavioral outcomes,54,57,58 the Home Observation for Measurement of the Environment (HOME) instrument for elementary school aged children59 was administered at the 12-month follow-up visit. The HOME is primarily an observational tool that assesses the quality of the home environment, including physical characteristics, variety of stimulation, and nurturing behavior from the parent.
A log base 2 transformation was used for analysis of serum cotinine because of the skewed distribution of the raw data. This not only allowed use of parametric methods but provided for a simpler interpretation of the beta coefficients from the regression analyses. Use of the log base 2 transformation means that for each doubling of the cotinine level, there is an increase in the behavior outcome scale equal to the beta coefficient for log (cotinine). Univariate analysis involved estimation of frequencies and means with the associated standard deviation, serum cotinine is reported as a geometric mean and 95% confidence interval, and household income is reported as median and 25th and 75th percentiles. The initial step involved simple regression analysis of log serum cotinine on the behavior outcome scales. Full models were explored including all the potential covariates and confounders: age, race, gender and asthma severity of the child, education and depression (Beck Depression Inventory-II) of the mother, HOME score and prenatal tobacco exposure. The most parsimonious model was created for each of the behavioral outcomes, and the final models included covariates and confounders that were appropriate for any of the outcomes based on p < .05 and change in the independent variable of interest by 10%. This was done so that interpretation of the effect of ETS exposure would be based on the same measures of adjustment for each outcome. Further analysis of the relationship between serum cotinine and child behavior was done using tertiles of serum cotinine to provide another illustration of the relationship. The same approach was used for modeling as described above. SAS® version 9.160 was used for all analyses.
RESULTS
The mean age of the subjects at the baseline visit was 9.4 years. Sixty one percent of the children were boys and 56% were reported to be African-American. The majority of parents reported a high school education or less (65%), and 42% were single and never married with a median household income of $25,000 per year. The majority of children (77%) had moderate to severe asthma (Table 1).
Table 1.
Sample Characteristics at Study Enrollment (N = 220)
| Characteristic | |
|---|---|
| Age (yr) | |
| Mean | 9.40 |
| SD | 1.83 |
| Male | 135 (61.4%) |
| Race | |
| African-American | 123 (55.9%) |
| Caucasian | 92 (41.8%) |
| Other | 5 (2.3%) |
| Parent education | |
| High school graduate or less | 143 (65.0%) |
| Some college | 51 (23.2%) |
| College graduate | 26 (11.8%) |
| Parent marital status | |
| Married/living with someone | 91 (41.4%) |
| Divorced/separated | 34 (15.5%) |
| Single—never married | 92 (41.8%) |
| Widowed | 3 (1.4%) |
| Household incomea | $25,000 ($15,000, $45,000) |
| Asthma severity | |
| Mild | 51 (23.2%) |
| Moderate | 106 (48.2%) |
| Severe/very severe | 63 (28.6%) |
| Maternal smoking during pregnancy | |
| None | 74 (33.6%) |
| Until pregnancy recognition | 32 (14.6%) |
| Throughout pregnancy | 114 (51.8%) |
| Cigarettes smoked in the home daily (at screening)a | 20 (14,40) |
| Cigarettes smoked in the home daily (at baseline home visit)a | 13 (9.5, 20) |
| Serum cotinine (ng/mL)b | 1.18 (0.10, 13.37) |
| HOME scores | |
| Mean | 46.74 |
| SD | 8.08 |
| Maternal depression (BDI) | |
| Mean | 11.12 |
| SD | 9.66 |
| BASC (within clinical range ≥70) | |
| Externalizing | 41 (18.8%) |
| Internalizing | 58 (26.6%) |
| Behavior symptoms | 46 (21.1%) |
Data presented as N (%), mean ± standard deviation. HOME, Home Observation for Measurement of the Environment.
Median (interquartile range).
Geometric mean (95% confidence interval).
By report, 66% of mothers smoked sometime during pregnancy with the index child resulting in both prenatal and postnatal tobacco smoke exposure. Children in the sample were exposed to a median of 13 cigarettes each day at baseline as reported by parents. There was no difference in median reported cigarette exposure for girls and boys. The geometric mean serum cotinine level for the full sample was 1.18 ng/mL, a value indicative of passive exposure. Girls had significantly higher serum cotinine levels than boys (girls = 1.50 ng/mL; boys = 1.01 ng/mL, p = .02). The correlation between serum cotinine and reported exposure (number of cigarettes/day) was 0.39 (p < .0001) for the full sample.
The incidence of behavior problems, as reported by parents on the Behavior Assessment System for Children (BASC), was relatively high among children in this sample. Children scored in a range considered to be clinically relevant (standard T score ≥70) at rates of 19% for externalizing problems, 27% for internalizing problems, and 21% for behavior symptoms. Bivariate analyses of the associations between the log of serum cotinine and child behaviors was significant for all 4 BASC composite scales. Externalizing problems (β = 2.71, p < .0001), Internalizing problems (β = 1.75, p = .002), and Behavior symptoms (β = 2.55, p < .0001) scores increased with increasing cotinine levels, and Adaptive skills (β = −1.27, p = .003) decreased with increasing cotinine levels.
In multivariable analyses including potential contributors to child behaviors, we found that higher levels of environmental tobacco smoke (ETS) exposure were significantly associated with higher scores on the BASC Internalizing Problems and Behavior Symptoms composites. The relationship approached significance for the Externalizing Problems composite, but was not significant for the Adaptive Skills composite (Table 2).
Table 2.
ETS Measured by Log Serum Cotinine and Child Behavior (Adjusteda, N = 220)
| Behavior Subscale | Full Sample |
Males |
Females |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE β | p | β | SE β | p | β | SE β | p | |
| Externalizing | 1.16 | 0.69 | .09 | 2.23 | 0.92 | .02 | −0.91 | 1.27 | .48 |
| Hyperactivity | 1.54 | 0.63 | .02 | 2.14 | 0.88 | .02 | 0.74 | 1.09 | .50 |
| Aggression | 1.18 | 0.68 | .09 | 1.99 | 0.87 | .02 | −0.39 | 1.38 | .78 |
| Conduct | 0.60 | 0.64 | .35 | 1.59 | 0.82 | .06 | −1.51 | 1.24 | .23 |
| Internalizing | 1.31 | 0.61 | .03 | 2.19 | 0.87 | .01 | −0.03 | 1.02 | .98 |
| Somatization | 1.13 | 0.61 | .07 | 1.53 | 0.82 | .06 | −0.07 | 1.17 | .95 |
| Anxiety | 0.73 | 0.53 | .17 | 1.39 | 0.77 | .07 | 0.22 | 0.87 | .80 |
| Depression | 1.21 | 0.61 | .05 | 2.19 | 0.88 | .01 | −0.16 | 0.98 | .87 |
| Behavior Symptoms | 1.39 | 0.66 | .04 | 2.55 | 0.94 | .01 | −0.05 | 1.08 | .96 |
| Adaptive Skills | −0.40 | 0.47 | .40 | −0.96 | 0.62 | .13 | −0.07 | 0.84 | .94 |
Covariates in all models include: sex, age, race, asthma severity, asthma medication, maternal education, prenatal tobacco exposure, maternal depression, HOME score.
To better describe the relationships between ETS exposure and child behavior, we examined the relationship of serum cotinine to the BASC subscales that make up the externalizing and internalizing composites while controlling for the covariates identified previously (Table 2). For the externalizing composite, log serum cotinine was significantly related to higher scores on the hyperactivity subscale. For the BASC internalizing composite, log serum cotinine was significantly related to higher scores on the depression subscale and approached significance for the somatization subscale.
Significant relationships between our measured covariates and child behaviors were found as follows: poorer home quality (Home Observation for Measurement of the Environment [HOME] Score) was related to higher externalizing composite, hyperactivity, aggression, and conduct problems; increasing child age was related to lower hyperactivity and aggression scores; and maternal depression was related to higher internalizing and behavioral symptoms index composites, anxiety, and depression. Greater asthma severity was related to higher internalizing composite and somatization scores, and asthma medication use (short-acting inhaled bronchodilators and oral steroids) was related to higher externalizing composite including 3 subscales, higher internalizing composite and depression, higher behavior symptoms, reduced adaptive skills including social skills and leadership, and higher attention and somatization scores.
Although no clear difference by sex was evident in the effect of serum cotinine on behavior in the main models, and a statistically significant sex interaction was not found (sex × log cotinine interaction p values ranging from .22 to .96), the significantly higher exposure that girls had in relation to boys prompted us to examine the association between child behavior and ETS exposure for girls and boys separately. (Table 2) For boys, significant relationships were found between serum cotinine and higher scores on externalizing behaviors, including subscales of hyperactivity, and aggression; internalizing behaviors including depression; and behavior symptoms. Significant covariates of behavior among boys were asthma severity (internalizing, behavior symptoms, somatization), asthma medications (internalizing, behavior symptoms, adaptive skills, depression, attention problems, social skills, leadership), maternal depression (anxiety, depression), and black race (depression). For girls, no statistically significant relationships were found between serum cotinine and behavior. Significant covariates of behavior were maternal education (externalizing, behavior symptoms, adaptive skills, aggression, conduct), HOME score (externalizing, adaptive skills, aggression, conduct), maternal depression (externalizing, internalizing, adaptive skills, hyperactivity, anxiety, depression), asthma severity (anxiety, somatization), and asthma medications (depression, social skills).
We then divided the sample into tertiles based on serum cotinine levels; low (n = 73); middle (n = 74); high (n = 73). In unadjusted and adjusted analyses, a potential dose-response relationship was found in which more children in the middle and high cotinine tertiles had higher behavior problem scores (BASC) when compared with children in the lowest tertile (Table 3 and Fig. 1).
Table 3.
Unadjusted and Adjusted T Scores on BASC by Serum Cotinine Tertiles (Mean [Standard Deviation], N = 220)
| Low (0.05–0796 ng/mL) |
Middle (0.819–2.16 ng/mL) |
High (2.23–19.6 ng/mL) |
||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Externalizing | 51.7 (13.1) | 53.8 (16.6) | 60.2 (16.8) | 60.6 (17.6) | 62.5 (20.3) | 58.3 (17.4) |
| Hyperactivity | 52.0 (12.9) | 53.8 (15.3) | 58.6 (14.6) | 59.3 (16.0) | 62.5 (18.2) | 58.6 (15.5) |
| Aggression | 51.5 (13.0) | 53.5 (16.3) | 59.4 (17.0) | 60.5 (17.1) | 60.8 (18.4) | 57.0 (17.2) |
| Conduct | 50.8 (11.6) | 53.5 (15.6) | 58.3 (17.4) | 57.9 (16.2) | 59.8 (19.8) | 55.3 (16.1) |
| Internalizing | 57.3 (11.8) | 58.1 (15.3) | 62.0 (14.9) | 63.6 (15.4) | 63.9 (16.1) | 62.7 (15.4) |
| Somatization | 61.3 (12.9) | 61.7 (14.7) | 64.2 (13.4) | 65.4 (15.5) | 66.5 (17.8) | 65.5 (15.4) |
| Anxiety | 53.3 (11.5) | 53.3 (12.8) | 54.8 (12.5) | 55.9 (13.4) | 55.8 (12.6) | 56.4 (13.4) |
| Depression | 52.1 (11.7) | 53.6 (14.6) | 58.5 (15.8) | 60.0 (15.3) | 59.8 (16.1) | 57.4 (15.2) |
| Behavior symptoms | 53.6 (13.2) | 55.5 (15.9) | 61.3 (16.1) | 62.6 (16.6) | 63.0 (17.4) | 59.8 (16.6) |
| Adaptive skills | 46.6 (10.7) | 45.1 (11.1) | 41.5 (11.2) | 40.5 (11.6) | 42.8 (11.1) | 44.9 (11.5) |
Covariates in all models include: sex, age, race, asthma severity, asthma medication, maternal education, prenatal tobacco exposure, maternal depression, HOME score.
Figure 1.
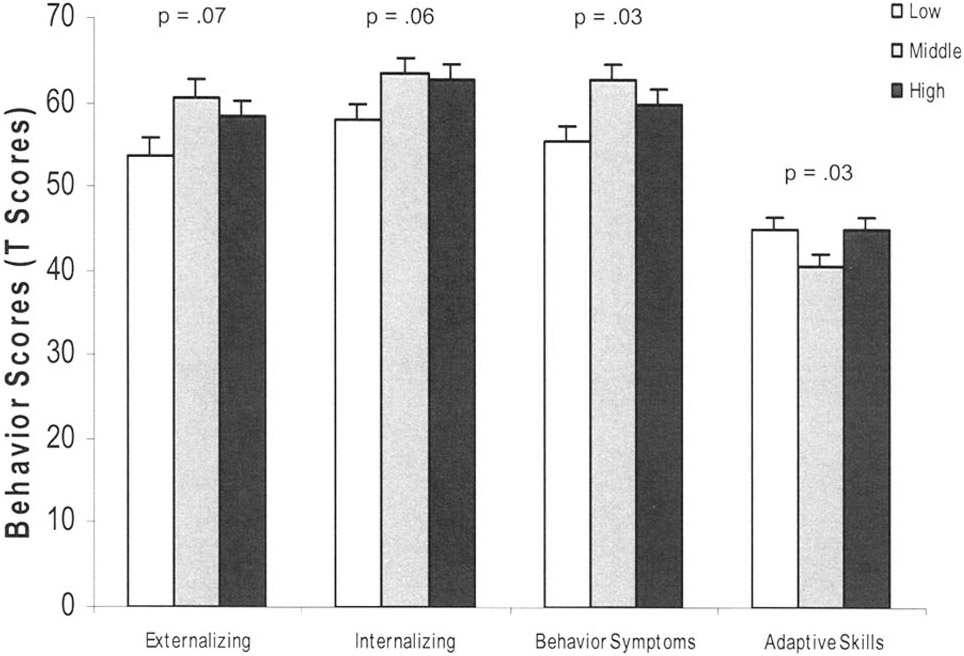
Adjusted (covariates in all models: gender, age, race, asthma severity, asthma medication, maternal education, prenatal tobacco exposure, Home Observation for Measurement of the Environment (HOME) Score, and maternal depression) effect of serum cotinine on child behavior among 6 to 12 year olds with asthma.
* Covariates in all models: gender, age, race, asthma severity, asthma medication, maternal education, prenatal tobacco exposure, HOME Score, and maternal depression
Results for the secondary analysis of behavior problems by serum cotinine tertile for the full sample and divided by sex are shown (Table 4). The lowest tertile of ETS exposure was used as the reference in all analyses, so betas and associated standard errors are given for tertiles 2 and 3 only. The results mirror those found in the previous analysis using the log serum cotinine as a continuous variable. However, a potential leveling in the effect at or near the second tertile is indicated as evidenced by a plateau in the beta values. This may also be seen by examination of the means by tertile presented in Table 3 and Figure 1. The same covariates were used for these adjusted analyses as were used for the previous adjusted analyses (Table 3).
Table 4.
ETS Measured by Log Serum Cotinine Split into Tertilesa and Related to Child Behavior for the Entire Sample and by Sex (Adjustedb, N = 220)
| Behavior Subscale |
Overall | Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 2 |
Tertile 3 |
Tertile 2 |
Tertile 3 |
Tertile 2 |
Tertile 3 |
|||||||
| β (SD) | p | β (SD) | p | β (SD) | p | β (SD) | p | β (SD) | p | β (SD) | p | |
| Externalizing | 6.50 (2.78) | .02 | 3.61 (2.96) | .22 | 8.82 (3.26) | .01 | 8.63 (3.79) | .02 | −2.03 (5.49) | .71 | −6.10 (5.49) | .27 |
| Hyperactivity | 5.51 (2.26) | .03 | 4.84 (2.72) | .08 | 6.30 (3.18) | .05 | 7.63 (3.71) | .04 | 2.27 (4.77) | .64 | 1.78 (4.78) | .71 |
| Aggression | 7.01 (2.74) | .01 | 3.59 (2.92) | .22 | 9.20 (3.06) | .01 | 8.16 (3.56) | .02 | −0.51 (5.84) | .93 | −5.39 (5.93) | .37 |
| Conduct | 4.42 (2.60) | .09 | 1.83 (2.76) | .51 | 7.28 (2.92) | .01 | 6.47 (3.41) | .06 | −5.46 (5.39) | .32 | −7.94 (5.40) | .15 |
| Internalizing | 5.53 (2.46) | .03 | 4.60 (2.62) | .08 | 5.67 (3.14) | .07 | 8.83 (3.66) | .02 | 3.39 (4.39) | .44 | −1.40 (4.40) | .75 |
| Somatization | 3.67 (2.47) | .14 | 3.82 (2.62) | .15 | 5.52 (2.91) | .06 | 7.04 (3.39) | .04 | −2.17 (5.08) | .67 | −2.29 (5.08) | .65 |
| Anxiety | 2.59 (2.15) | .23 | 3.05 (2.29) | .18 | 1.08 (2.78) | .70 | 5.78 (3.24) | .08 | 5.41 (3.72) | .15 | 0.98 (3.72) | .79 |
| Depression | 6.43 (2.44) | .01 | 3.84 (2.60) | .14 | 6.51 (3.16) | .04 | 7.77 (3.68) | .04 | 4.28 (4.17) | .31 | −2.00 (4.17) | .63 |
| Behavior | ||||||||||||
| symptoms | 7.12 (2.66) | .01 | 4.28 (2.83) | .13 | 8.24 (3.36) | .02 | 9.81 (3.91) | .01 | 2.72 (4.64) | .56 | −3.10 (4.64) | .51 |
| Adaptive skills | −4.52 (1.86) | .02 | −0.17 (1.98) | .93 | −3.97 (2.22) | .08 | −2.72 (2.58) | .29 | −6.31 (3.33) | .06 | 0.79 (3.34) | .81 |
The lowest tertile of ETS exposure was used as the reference in all analyses, so betas and associated standard errors are given for tertiles 2 and 3 only.
Covariates in all models: gender, age, race, asthma severity, asthma medication, maternal education, prenatal tobacco exposure, HOME Score, maternal depression.
DISCUSSION
Consistent with previous research studies,33,36 we found that children’s exposure to environmental tobacco smoke (ETS), as measured by serum cotinine, is associated with increased behavior problems of both externalizing and internalizing types among boys. Our findings of increased externalizing behavior problems, in particular hyperactivity and aggression, are consistent with previous reports linking pre and postnatal tobacco smoke exposure with externalizing behavior problems.30,33,36 We also found relationships between ETS exposure and the specific internalizing type behaviors of somatization and depression. Reports of internalizing behaviors in relation to ETS exposure are less common, but Weitzman et al36 reported increased behavior problems related to socialization, anxiety and depression, and immaturity among children who were exposed to higher levels of tobacco smoke.
We additionally found differences between boys and girls in the relationships between ETS exposure and behaviors as well as the influence of covariates. For boys, ETS exposure was associated with increased externalizing behaviors, including hyperactivity, aggression, and conduct problems; internalizing behaviors including depression; and overall behavior symptoms. Although girls were exposed to a greater number of cigarettes by parent report, had higher serum cotinine levels, and were more likely to be in the higher tertiles of serum cotinine, there was no evidence of a relationship between this exposure and behavior problems. Although sex differences in smoking habits, nicotine metabolism, and nicotine effects have been reported among adult smokers, and animal studies reveal sex differences in behavioral and physiological responses to nicotine,61 to our knowledge, sex differences in metabolism and effects of ETS on children have not been thoroughly studied. In contrast to our findings, Braun et al37 suggested that girls may actually be at greater risk of attention-deficit hyperactivity disorder (ADHD) when exposed to tobacco smoke prenatally although there was no statistical significance in the sex differences discussed. A potential sex difference in metabolism and susceptibility to effects of tobacco smoke should be further explored to help explain differences between boys and girls in the relationship between exposure and behaviors.
Covariates that significantly affected the relationships between ETS exposure and behavior also varied by sex. Statistically significant covariates of behavior among boys included asthma severity (internalizing, behavior symptoms, somatization), maternal depression (anxiety, depression), and black race (depression). For girls, significant covariates of behavior included maternal education (externalizing, behavior symptoms, adaptive skills, aggression, conduct), Home Observation for Measurement of the Environment (HOME) score (externalizing, adaptive skills, aggression, conduct), maternal depression (externalizing, internalizing, adaptive skills, hyperactivity, anxiety, depression), and asthma severity (anxiety, somatization).
Maternal depression was an important covariate for both boys and girls but was related primarily to internalizing symptoms in boys and both internalizing and externalizing symptoms in girls. In general, childhood depression is more common among children of depressed parents.62 In addition, girls are generally recognized as having higher rates of depression than boys.62-64 Maternal depression is associated with increased disruptive behaviors, anxiety, and depression in children at rates as high as 2 to 3 times children with nondepressed mothers.55,65
The relationship between asthma severity and child behaviors is not surprising. Previous research has consistently shown a relationship between asthma and increased level of anxious behaviors in children but not depression.43,46,66-68 In addition, disruptive behaviors have been reported rarely and at a lower prevalence among children with asthma.66 In fact, Katon et al68 speculated that up to one third of children with asthma may experience anxiety, and Bussing et al reported that children with severe asthma had nearly 3 times the odds of having severe behavior problems.69
This study is not without limitations. No information was gathered about maternal drug or alcohol use during pregnancy, so we are unable to adjust for these potentially important contributors to later child behavior. We additionally had no birth outcome information such as birth weight or gestational age which can be related to child behavior. All enrolled children had asthma so the results may not be generalizable to populations of children without asthma. Still, these results may be reflective of risks from ETS exposure for the 7 to 10% of children in the US today who have been diagnosed with asthma.70 It is important to reiterate that research on children with asthma generally reveals a tendency toward increased internalizing behavior problems43,46,66-68 whereas prior research on prenatal and postnatal tobacco smoke exposure has primarily yielded associations with externalizing behavior problems.26-33,36 Among children with asthma, reported behavior problems tend to be more frequent than among the general population and may include both internalizing46,47,71 and to a lesser extent externalizing behaviors.72-78 Children with asthma are often more sensitive to the respiratory effects of ETS,14,79 but we are unaware of published studies exploring their susceptibility to the potential behavioral consequences of ETS exposure.
All children in this study were exposed to ETS so we may not be able to generalize our findings to unexposed children. There was, however, a reasonable amount of variability in ETS exposure and asthma severity among the sample allowing for exploration of a potential dose-response relationship between ETS exposure and child behaviors. Additionally, National data suggest that that the majority of children are exposed to ETS at some level.38 Furthermore, our findings of increased behavior problems among those children most heavily exposed suggest a dose-response relationship. In addition, this study suggests that even among families with smokers, variations in child behaviors are observed, diminishing the concern that families of smokers may interpret children’s behaviors differently.
CONCLUSION
Among children with asthma, especially boys, exposure to environmental tobacco smoke (ETS) is related to increased behavior problems, including externalizing problems such as hyperactivity, aggression, and conduct problems, internalizing problems such as depression, and behavior symptoms. The results of this study provide further evidence that even low levels of environmental tobacco smoke may contribute to behavior problems in children and should encourage us to make stronger efforts to prevent childhood exposure to tobacco smoke especially among higher risk populations such as children with asthma.
ACKNOWLEDGMENTS
We thank the staff and participating families of the Cincinnati Asthma Prevention Study for their contribution to this research.
This project was supported by Grants from the National Institutes of Health: NIEHS R21 ES12952-01 and NHLBI R01 HL65731.
REFERENCES
- 1.Bada HS, Das A, Bauer CR, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J. Perinatol 2005;25:631–637. [DOI] [PubMed] [Google Scholar]
- 2.Dejmek J, Solansky I, Podrazilova K, Sram RJ. The exposure of nonsmoking and smoking mothers to environmental tobacco smoke during different gestational phases and fetal growth. Environ Health Perspect. 2002;110:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaakkola JJ, Jaakkola N, Zahlsen K. Fetal growth and length of gestation in relation to prenatal exposure to environmental tobacco smoke assessed by hair nicotine concentration. Environ Health Perspect. 2001;109:557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook DG, Strachan DP. Health effects of passive smoking-10: summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.England LJ, Kendrick JS, Gargiullo PM, Zahniser SC, Hannon WH. Measures of maternal tobacco exposure and infant birth weight at term. Am J Epidemiol. 2001;153:954–960. [DOI] [PubMed] [Google Scholar]
- 6.Steyn K, de Wet T, Saloojee Y, Nel H, Yach D. The influence of maternal cigarette smoking, snuff use and passive smoking on pregnancy outcomes: the Birth to ten study. Paediatr Perinat Epidemiol. 2006;20:90–99. [DOI] [PubMed] [Google Scholar]
- 7.Ward C, Lewis S, Coleman T. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birth weight: retrospective study using Millennium Cohort. BMC Public Health. 2007;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMartin KI, Platt MS, Hackman R, et al. Lung tissue concentrations of nicotine in sudden infant death syndrome (SIDS). J Pediatr. 2002;140:205–209. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell EA, Ford RP, Stewart AW, et al. Smoking and the sudden infant death syndrome. Pediatrics. 1993;91:893–896. [PubMed] [Google Scholar]
- 10.Schoendorf KC, Kiely JL. Relationship of sudden infant death syndrome to maternal smoking during and after pregnancy. Pediatrics. 1992;90:905–908. [PubMed] [Google Scholar]
- 11.Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. A prospective study of smoking during pregnancy and SIDS. Arch Dis Child. 2000;83:203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998;101:E8, 1–6. [DOI] [PubMed] [Google Scholar]
- 13.Gilliland FD, Berhane K, Islam T, et al. Environmental tobacco smoke and absenteeism related to respiratory illness in schoolchildren. Am J Epidemiol 2003;157:861–869. [DOI] [PubMed] [Google Scholar]
- 14.Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409–415. [DOI] [PubMed] [Google Scholar]
- 15.Bauman KE, Flewelling RL, LaPrelle J. Parental cigarette smoking and cognitive performance of children. Health Psychol. 1991;10:282–288. [DOI] [PubMed] [Google Scholar]
- 16.Byrd RS, Weitzman ML. Predictors of early grade retention among children in the United States. Pediatrics. 1994;93:481–487. [PubMed] [Google Scholar]
- 17.Eskenazi B, Bergmann JJ. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. I. Effects on physical growth at age 5 years. Am J Epidemiol. 1995;142(9 Suppl):S10–S18. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DL, Swank PR, Baldwin CD, McCormick D. Adult smoking in the home environment and children’s IQ. Psychol Rep. 1999;84:149–154. [DOI] [PubMed] [Google Scholar]
- 19.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect. 2005;113:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998;20:293–306. [DOI] [PubMed] [Google Scholar]
- 21.Mannino D, Moorman J, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States. Arch Pediatr Adolesc Med. 2001;155:36–41. [DOI] [PubMed] [Google Scholar]
- 22.Martinez FD, Antognoni G, Macri F, et al. Parental smoking enhances bronchial responsiveness in nine-year-old children. Am Rev Respir Dis. 1988;138:518–523. [DOI] [PubMed] [Google Scholar]
- 23.Rylander E, Pershagen G, Eriksson M, Bermann G. Parental smoking, urinary cotinine, and wheezing bronchitis in children. Epidemiology. 1995;6:289–293. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics. Insurance coverage of mental health and substance abuse services for children and adolescents: a consensus statement. American Academy of Pediatrics. Pediatrics. 2000;106:860–862. [DOI] [PubMed] [Google Scholar]
- 25.Kelleher KJ, McInerny TK, Gardner WP, Childs GE, Wasserman RC. Increasing identification of psychosocial problems: 1979-1996. Pediatrics. 2000;105:1313–1321. [DOI] [PubMed] [Google Scholar]
- 26.Linnet KM, Wisborg K, Obel C, et al. Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics. 2005;116:462–467. [DOI] [PubMed] [Google Scholar]
- 27.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry. 1996;153:1138–1142. [DOI] [PubMed] [Google Scholar]
- 28.Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol. 1998;27:352–358. [DOI] [PubMed] [Google Scholar]
- 29.Thapar A, Fowler T, Rice F, et al. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry. 2003;160:1985–1989. [DOI] [PubMed] [Google Scholar]
- 30.Fergusson DM, Horwood LJ, Lynskey MT. Maternal smoking before and after pregnancy: effects on behavioral outcomes in middle childhood. Pediatrics. 1993;92:815–822. [PubMed] [Google Scholar]
- 31.Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry. 1997;54:670–676. [DOI] [PubMed] [Google Scholar]
- 32.Orlebeke JF, Knol DL, Verhulst FC. Child behavior problems increased by maternal smoking during pregnancy. Arch Environ Health. 1999;54:15–19. [DOI] [PubMed] [Google Scholar]
- 33.Williams GM, O’Callaghan M, Najman JM, et al. Maternal cigarette smoking and child psychiatric morbidity: a longitudinal study. Pediatrics. 1998;102:e11. [DOI] [PubMed] [Google Scholar]
- 34.Lambe M, Hultman C, Torrang A, Maccabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–530. [DOI] [PubMed] [Google Scholar]
- 35.Linnet KM, Dalsgaard S, Obel C, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028–1040. [DOI] [PubMed] [Google Scholar]
- 36.Weitzman M, Gortmaker S, Sobol A. Maternal smoking and behavior problems of children. Pediatrics. 1992;90:342–349. [PubMed] [Google Scholar]
- 36a.Braun JM, Froehlich TE, Daniels JL, et al. Association of environmental toxicants and conduct disorder in U.S. children: NHANES 2001–2004. Environ Health Perspect. 2008;116:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Third National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, Division of Laboratory Sciences; 2005. NCEH Publication No. 05-0570. [Google Scholar]
- 39.Blackman JA, Gurka MJ. Developmental and behavioral comorbidities of asthma in children. J Dev Behav Pediatr. 2007;28:92–99. [DOI] [PubMed] [Google Scholar]
- 40.Mrazak DA, Schuman WB, Klinnert M. Early asthma onset: risk of emotional and behavioral difficulties. J Child Psychol Psychiatry. 1998;39:247–254. [PubMed] [Google Scholar]
- 41.Mrazek D, Anderson I, Strunk R. Disturbed emotional development of severely asthmatic preschool children. In: Stevenson JE, ed. Recent Research in Developmental Psychopathology. New York: Pergamon Press; 1985:81–94. [Google Scholar]
- 42.Klinnert MD, McQuaid EL, McCormick D, Adinoff AD, Bryant NE. A multimethod assessment of behavioral and emotional adjustment in children with asthma. J Pediatr Psychol. 2000;25:35–46. [DOI] [PubMed] [Google Scholar]
- 43.Vila G, Nollet-Clemencon C, de Blic J, Mouren-Simeoni MC, Scheinmann P. Prevalence of DSM IV anxiety and affective disorders in a pediatric population of asthmatic children and adolescents. J Affect Disord. 2000;58:223–231. [DOI] [PubMed] [Google Scholar]
- 44.Goodwin RD, Messineo K, Bregante A, Hoven CW, Kairam R. Prevalence of probable mental disorders among pediatric asthma patients in an inner-city clinic. J Asthma. 2005;42:643–647. [DOI] [PubMed] [Google Scholar]
- 45.Halterman JS, Conn KM, Forbes-Jones E, Fagnano M, Hightower AD, Szilagyi PG. Behavior problems among inner-city children with asthma: findings from a community-based sample. Pediatrics. 2006;117:e192–e199. [DOI] [PubMed] [Google Scholar]
- 46.Halterman J, Conn K, Forbes-Jones E, Fagnano M, Hightower D, Szilagyi P. Behavior problems among inner-city children with asthma: findings from a community-based sample. Pediatrics. 2006;117:e192–e198. [DOI] [PubMed] [Google Scholar]
- 47.Alati R, O’Callaghan M, Najman JM, Williams GM, Bor W, Lawlor DA. Asthma and internalizing behavior problems in adolescence: a longitudinal study. Psychosom Med. 2005;67:462–470. [DOI] [PubMed] [Google Scholar]
- 48.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. [DOI] [PubMed] [Google Scholar]
- 49.Bernert JT Jr, Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43:2281–2291. [PubMed] [Google Scholar]
- 50.Reynolds CR, Kamphaus RW. Behavioral Assessment System for Children. Circle Pines, MN: American Guidance Service, Inc.; 1992. [Google Scholar]
- 51.Achenbach T. Child Behavior Checklist. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- 52.Ostrander R, Weinfurt KP, Yarnold PR, August GJ. Diagnosing attention deficit disorders with the Behavioral Assessment System for Children and the Child Behavior Checklist: test and construct validity analyses using optimal discriminant classification trees. J Consult Clin Psychol. 1998;66:660–672. [DOI] [PubMed] [Google Scholar]
- 53.Sharek PJ, Mayer ML, Loewy L, et al. Agreement among measures of asthma status: a prospective study of low-income children with moderate to severe asthma. Pediatrics. 2002;110:797–804. [DOI] [PubMed] [Google Scholar]
- 54.Leve LD, Kim HK, Pears KC. Childhood temperament and family environment as predictors of internalizing and externalizing trajectories from ages 5 to 17. J Abnorm Child Psychol. 2005;33:505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pilowsky DJ, Wickramaratne PJ, Rush AJ, et al. Children of currently depressed mothers: a STAR*D ancillary study. J Clin Psychiatry. 2006;67:126–136. [DOI] [PubMed] [Google Scholar]
- 56.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 57.Bradley RH, Convyn RF, Burchinal M, McAdoo HP, Coll CG. The home environments of children in the United States part II: relations with behavioral development through age thirteen. Child Dev. 2001;72:1868–1886. [DOI] [PubMed] [Google Scholar]
- 58.Pachter LM, Auinger P, Palmer R, Weitzman M. Do parenting and the home environment, maternal depression, neighborhood, and chronic poverty affect child behavioral problems differently in different racial-ethnic groups? Pediatrics. 2006;117:1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caldwell B, Bradley R. Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas at Little Rock; 1984. [Google Scholar]
- 60.SAS [computer program]. Version 9.1. Cary, NC: SAS Institute; 2002. [Google Scholar]
- 61.Pauly JR. Gender differences in tobacco smoking dynamics and the neuropharmacological actions of nicotine. Front Biosci. 2008;13:505–516. [DOI] [PubMed] [Google Scholar]
- 62.Weissman MM, Gammon GD, John K, et al. Children of depressed parents. Increased psychopathology and early onset of major depression. Arch Gen Psychiatry. 1987;44:847–853. [DOI] [PubMed] [Google Scholar]
- 63.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. [DOI] [PubMed] [Google Scholar]
- 64.Wade TJ, Cairney J, Pevalin DJ. Emergence of gender differences in depression during adolescence: national panel results from three countries. J Am Acad Child Adolesc Psychiatry. 2002;41:190–198. [DOI] [PubMed] [Google Scholar]
- 65.Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295:1389–1398. [DOI] [PubMed] [Google Scholar]
- 66.McQuaid EL, Kopel SJ, Nassau JH. Behavioral adjustment in children with asthma: a meta-analysis. J Dev Behav Pediatr. 2001;22:430–439. [DOI] [PubMed] [Google Scholar]
- 67.Ortega AN, Huertas SE, Canino G, Ramirez R, Rubio-Stipec M. Childhood asthma, chronic illness, and psychiatric disorders. J Nerv Ment Dis. 2002;190:275–281. [DOI] [PubMed] [Google Scholar]
- 68.Katon WJ, Richardson L, Lozano P, McCauley E. The relationship of asthma and anxiety disorders. Psychosom Med. 2004;66:349–355. [DOI] [PubMed] [Google Scholar]
- 69.Bussing R, Halfon N, Benjamin B, Wells KB. Prevalence of behavior problems in US children with asthma. Arch Pediatr Adolesc Med. 1995;149:565–572. [DOI] [PubMed] [Google Scholar]
- 70.Moorman JE, Rudd RA, Johnson CA, et al. National surveillance for asthma; United States, 1980–2004. MMWR. 2007;56:18–54. [PubMed] [Google Scholar]
- 71.Gupta S, Mitchell I, Giuffre RM, Crawford S. Covert fears and anxiety in asthma and congenital heart disease. Child Care Health Dev. 2001;27:335–348. [DOI] [PubMed] [Google Scholar]
- 72.Butz AM, Malveaux FJ, Eggleston P, et al. Social factors associated with behavioral problems in children with asthma. Clin Pediatr (Phila). 1995;34:581–590. [DOI] [PubMed] [Google Scholar]
- 73.Calam R, Gregg L, Simpson B, Morris J, Woodcock A, Custovic A. Childhood asthma, behavior problems, and family functioning. J Allergy Clin Immunol. 2003;112:499–504. [DOI] [PubMed] [Google Scholar]
- 74.MacLean WE Jr, Perrin JM, Gortmaker S, Pierre CB. Psychological adjustment of children with asthma: effects of illness severity and recent stressful life events. J Pediatr Psychol. 1992;17:159–171. [DOI] [PubMed] [Google Scholar]
- 75.Stevenson J Relationship between behavior and asthma in children with atopic dermatitis. Psychosom Med. 2003;65:971–975. [DOI] [PubMed] [Google Scholar]
- 76.Vila G, Nollet-Clemencon C, de Blic J, Falissard B, Mouren-Simeoni MC, Scheinmann P. Assessment of anxiety disorders in asthmatic children. Psychosomatics. 1999;40:404–413. [DOI] [PubMed] [Google Scholar]
- 77.Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics. 1999;104:1274–1280. [DOI] [PubMed] [Google Scholar]
- 78.Reichenberg K, Broberg AG. Emotional and behavioural problems in Swedish 7- to 9-year olds with asthma. Chron Respir Dis. 2004;1:183–189. [DOI] [PubMed] [Google Scholar]
- 79.Thomson NC. The role of environmental tobacco smoke in the origins and progression of asthma. Curr Allergy Asthma Rep. 2007;7:303–309. [DOI] [PubMed] [Google Scholar]


