Abstract
Background: In 1991, a mass immunization campaign against the hepatitis B virus (HBV) for children and teenagers was introduced in Italy. This study evaluated the impact of the immunization campaign on the incidence and modes of HBV transmission. Method: Acute HBV cases of viral hepatitis were reported to the National Surveillance System (SEIEVA). Hepatitis A cases reported to the same system were used as controls to calculate the adjusted odds ratios and the population attributable risk for potential risk factors. Results: The incidence of acute HBV declined from 5.0 in 1990 to 0.4 in 2019 per 100,000 population. The fall was almost total in people targeted by the campaign: in 2019, zero cases (100% reduction) in the age-group 0–14 years and 0.1 cases per 100,000 population (99.4% reduction) in the age-group 15–24 years were reported. In the decade 2010–2019, nearly one-fifth (19.3%) of cases occurred in foreigners. Intravenous drug use is no longer a risk factor (OR = 0.7; 95% CI = 0.5–1.02). Beauty treatments, risky sexual exposure, and household contact with an HBsAg carrier were found to be independent predictors of acute hepatitis B. Conclusions: The HB vaccination campaign proved effective in minimising acute HBV in Italy. Control of the infection is close to being reached for the first time in Europe.
Keywords: HBV, Italy, epidemiology, risk factors, intravenous drug users
1. Introduction
In 1991, Italy was the first European country to introduce mandatory vaccination against the hepatitis B virus (HBV) for 3-month-old infants and for 12-year-old children (limited to the first 12 years of the campaign for the latter category). In 1992, the program became fully operative. In 2004, the vaccination of 12-year-old adolescents ended, whereas the program for infants was maintained. As consequence, all subjects <40 years of age are currently vaccinated against HBV. Immunization of teenagers was an important target because it afforded protection before the onset of risk-taking behaviors such as sexual activity and drug use, which are more likely to start in the teenage years.
The vaccination program also included the mandatory screening of pregnant women for the hepatitis B surface antigen (HBsAg), in order to administer active-passive immunization to newborns of positive mothers. In addition, it recommended the vaccine for groups at high risk of infection (including households of chronic HBsAg carriers and intravenous drug users).
This immunization campaign has reached millions of children, with an immunization rate of 95% [1]. In this context, HBV vaccine not only prevents the spread of the virus, but generally reduces the global burden of HB-associated disease. For these reasons, a pivotal role in the combat of HBV infection is held by the HBV vaccine, as further suggested by a recent report [2].
Data from the national surveillance system of acute viral hepatitis (SEIEVA) have previously shown the effectiveness of the vaccination policy in reducing the incidence of acute hepatitis B in Italy in the period 1991–2005, mostly in the age groups targeted by the campaign (a 50-fold decrease in the subjects aged 0–14 years and a 20-fold decrease in the those aged 15–24 years) [3,4].
We report herein the impact of the HB vaccination campaign on the incidence rate of acute HBV after three decades, and on changes, if any, in transmission pattern.
2. Materials and Methods
2.1. Study Population and Data Collection
SEIEVA is an enhanced surveillance system coordinated by the Italian National Institute of Health and established in 1985 [5]. The number of Italian health units that voluntarily participate in the system has progressively increased, reaching 82.4% of the Italian population under surveillance in 2019.
Case definition and data collection procedures used within the SEIEVA surveillance system are described elsewhere [5]. During the study period, the surveillance methods and notification system were unchanged.
2.2. Incidence Rates Estimate
In order to estimate the incidence rates for each type of hepatitis and for each year, the number of the new reported cases was used as the numerator, and the denominator was the population of the local health units participating in the surveillance system.
2.3. Case Control Study
To estimate the association linking acute HBV cases with characteristics of subjects and potential sources of exposure, and the population attributable risk (PAR) to various exposures, cases of hepatitis A reported during the same time period to the SEIEVA were used as controls.
2.4. Statistical Analysis
To evaluate the statistical significance of the differences observed between groups, chi-squared test or chi-squared for trend was used for proportion, and Mann–Whitney or Kruskal–Wallis test was used for continuous variables. A p-value < 0.05 was considered to be significant. A multiple logistic regression model was used to evaluate the independent effect of the considered risk factors on HBV infection; socio-demographic characteristics were considered as adjustment variables.
The population attributable risk (PAR) was calculated according to Levine’s formula [6]: P × (r − 1)/P × (r − 1) +1, where P is the proportion of the general population (in this case acute HAV cases) with the characteristics or exposure, and r is the odds ratio.
3. Results
3.1. Incidence Data
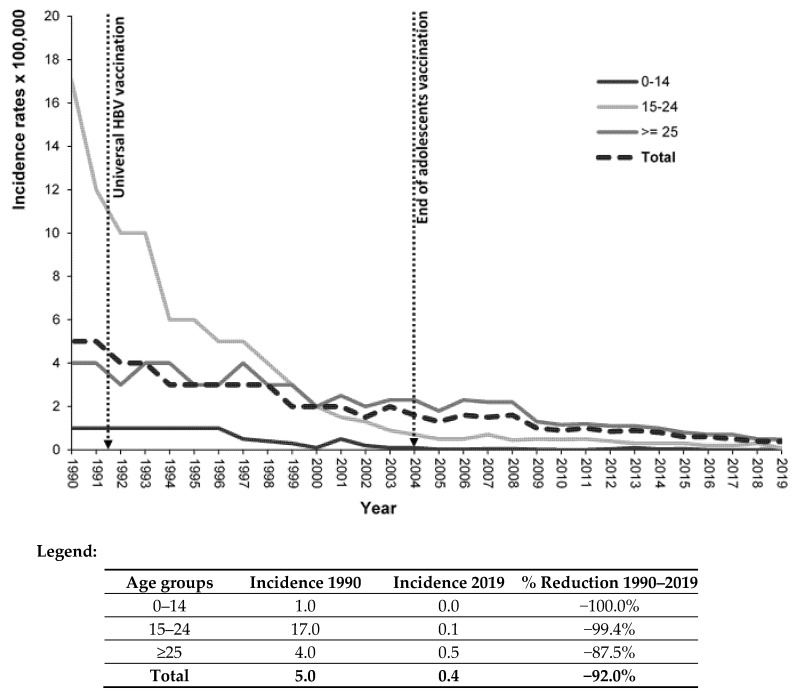
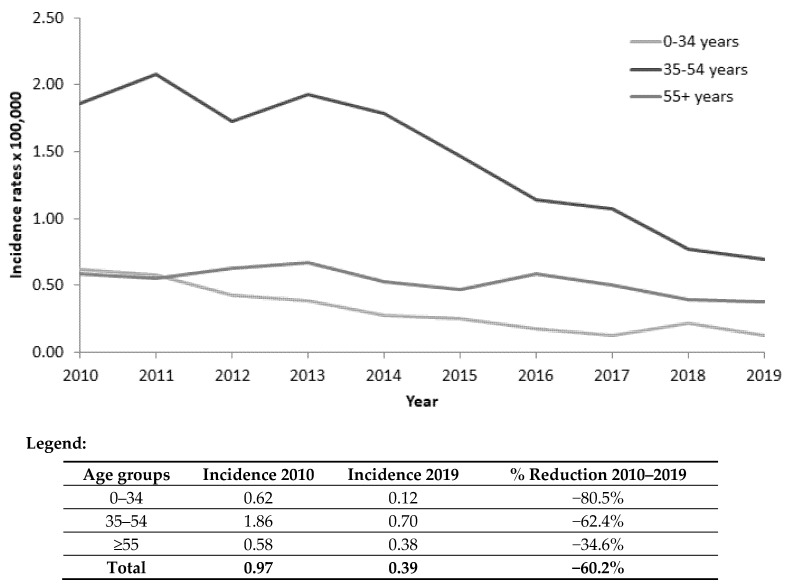
The overall incidence rate of acute hepatitis B cases per 100,000 inhabitants decreased from 5.0 in 1990 to 0.4 in 2019 (92.0% reduction). The decrease was nearly total in the age group targeted by the vaccination campaign: 0 cases in the age group 0–14 (100% reduction) and 0.1 cases per 100,000 population (99.4% reduction) in subjects 15–24 years old (Figure 1). In the last decade, the decrease was evident even in older age groups (Figure 2).
Figure 1.
Incidence rates (number of cases × 100,000 inhabitants) of acute hepatitis B in Italy (total and by age group). SEIEVA 1990–2019.
Figure 2.
Age-specific incidence rates (number of cases per 100,000 inhabitants) of acute hepatitis B in Italy. SEIEVA 2010–2019.
3.2. Main Characteristics
Table 1 reports the features of acute HBV cases by decade. The median age of cases rose from 29 years in the period 1991–1999 to 45 years in 2010–2019 (p < 0.01). The proportion of cases having higher years of education, diagnosis in northern/central areas, and escaping mandatory vaccination significantly increased over time (p < 0.01). Nearly one-fifth of cases in the decade 2010–2019 occurred in people born abroad. The median number of days spent in hospital decreased from 16 to 10 days (p < 0.01); the case-fatality rate increased from 0.5% to 1.1% (p < 0.001).
Table 1.
Main features of acute hepatitis B in Italy by decade. SEIEVA 1991–2019.
| Characteristics | 1991–1999 N = 6443 n (%) |
2000–2009 N = 5526 n (%) |
2010–2019 N = 3148 n (%) |
p-Value for Trend |
|---|---|---|---|---|
| Sex ratio (M/F) | 2.8 | 3.1 | 3.0 | 0.044 |
| Age distribution: | ||||
| ≤25 | 2354 (36.7) | 596 (10.9) | 174 (5.5) | <0.001 |
| 26–40 | 2483 (38.7) | 2743 (50.3) | 909 (28.9) | |
| 41–60 | 1063 (16.6) | 1616 (29.6) | 1617 (51.5) | |
| ≥61 | 516 (8.0) | 502 (9.2) | 443 (14.1) | |
| Median age (range) | 29 (1–98) | 37 (0–100) | 45 (0–98) | <0.001 |
| Years of education: | ||||
| ≤8 | 3489 (71.2) | 1977 (53.9) | 737 (48.4) | <0.001 |
| ≥9 | 1414 (28.8) | 1691 (46.1) | 784 (51.6) | |
| Area of diagnosis: | ||||
| North/central | 5295 (82.2) | 4915 (88.9) | 2918 (92.7) | <0.001 |
| South/islands | 1148 (17.8) | 611 (11.1) | 230 (7.3) | |
| Area of birth *: | ||||
| Italy | 2541 (80.7) | - | ||
| Abroad | 607 (19.3) | |||
| Escaped mandatory vaccination | 55 (0.9) | 279 (5.2) | 280 (9.2) | <0.001 |
| Icterus | 5170 (83.5) | 4588 (85.4) | 2530 (82.7) | 0.869 |
| Hospitalization rate | 5893 (93.1) | 5084 (93.7) | 2853 (91.7) | 0.050 |
| Median days spent in hospital (range) | 16 (1–98) | 13 (1–118) | 10 (1–84) | <0.001 |
| Death | 30 (0.5) | 25 (0.4) | 33 (1.1) | 0.002 |
* Information available since 2004.
The frequency (%) of non-mutually exclusive risk factors reported in HBV cases by decade (Table 2) highlights that beauty treatments and risky sexual behavior were the most frequent sources of exposure. Intravenous drug use went down from 20.8% in the decade 1991–1999 to 3.3% in 2010–2019 (p < 0.01). Blood transfusion without surgical intervention was stable under 1%.
Table 2.
Frequency (%) of non-mutually exclusive risk factors reported by acute hepatitis B cases according to decade. SEIEVA 1991–2019. (Information was missing in some cases).
| Risk Factors | 1991–1999 N = 6443 n (%) |
2000–2009 N = 5526 n (%) |
2010–2019 N = 3148 n (%) |
p-Value for Trend |
|---|---|---|---|---|
| Blood transfusion without surgical intervention | 43 (0.7) | 22 (0.4) | 16 (0.6) | |
| Surgical intervention without blood transfusion | 623 (10.4) | 622 (12.0) | 291 (10.0) | 0.013 |
| Surgical intervention and blood transfusion | 107 (1.8) | 86 (1.7) | 26 (0.9) | |
| Intravenous drug use | 1267 (20.8) | 378 (7.2) | 96 (3.3) | <0.001 |
| Unvaccinated among IDU * | 1137 (95.7) | 301 (89.9) | 72 (91.1) | <0.001 |
| Beauty treatments ** | 1827 (29.9) | 1662 (31.5) | 949 (31.8) | 0.044 |
| Sexual exposure *** | 1601 (30.4) | 1642 (32.5) | 818 (28.3) | 0.174 |
| Household contact of an HBsAg+ carrier | 598 (12.9) | 421 (10.4) | 201 (8.7) | <0.001 |
| Aware of the positivity of the household | 181 (47.8) | 160 (43.7) | 77 (41.8) | 0.153 |
* Information missing in 79 cases in 1991–1999, 43 cases in 2000–2009, and 17 cases in 2010–2019. ** Piercing, tattooing, acupuncture, manicurist/chiropodist attendance, and barbershop shaving. *** Two or more sexual partners or lack of condom use in cases of casual sexual intercourse.
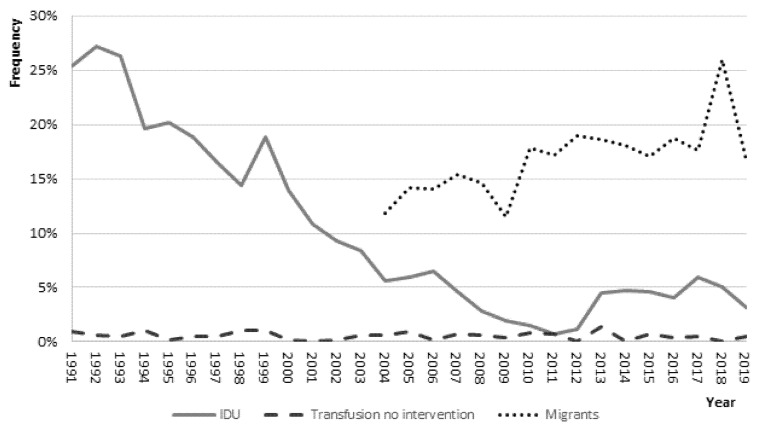
Figure 3 reports the yearly trends of some characteristics of the subjects: the proportion of people reporting intravenous drug use shows a dramatic decline over time from 25.4% in 1991 to 3.2% in 2019; conversely, acute HBV among foreigners increased over time, peaking at 26% in 2018.
Figure 3.
Frequency (%) of some non-mutually exclusive risk factors reported in acute hepatitis B cases by year. SEIEVA 1991–2019.
In the last decade (2010–2019), acute hepatitis B in subjects born abroad, as compared to Italian cases, was more likely in subjects who were of female sex (sex ratio 2.0 vs. 3.4; p < 0.001), younger (median age 32 years vs. 47 years; p < 0.01), and part of households with an HBsAg positive carrier (15.5% vs. 7.4%; p < 0.01), but less likely to report risky sexual behavior (22.9% vs. 29.5 p = 0.003) (Table 3).
Table 3.
Comparison of acute hepatitis B cases in Italy by area of birth. SEIEVA 2010–2019.
| Characteristics | Italians N = 2541 n (%) |
Foreigners N = 607 n (%) |
p-Value |
|---|---|---|---|
| Sex ratio (M/F): | 3.4 | 2.0 | <0.001 |
| Age: | |||
| ≤40 | 631 (24.9) | 452 (74.7) | <0.001 |
| 41–60 | 1474 (58.1) | 143 (23.6) | |
| ≥61 | 433 (17.0) | 10 (1.6) | |
| Median age (range) | 47 (0–98) | 32 (2–80) | <0.001 |
| Area of diagnosis: | |||
| North/Central | 2343 (92.2) | 575 (94.7) | 0.032 |
| South/Islands | 198 (7.8) | 32 (5.3) | |
| I.V. Drug use | 64 (2.7) | 32 (6.0) | <0.001 |
| Household contact of an HBsAg+ carrier | 144 (7.4) | 57 (15.5) | <0.001 |
| Sexual exposure * | 698 (29.5) | 120 (22.9) | 0.003 |
* Two or more sexual partners or lack of condom use in cases of casual sexual intercourse.
3.3. Risk Factor Analysis
Using acute hepatitis A cases reported by the same surveillance system as a control group, the adjusted OR for the association of acute HBV cases with the characteristics of subjects and reported risk factors in the decade 2010–2019 were evaluated by multiple logistic regression analysis (Table 4). All the considered characteristics, except IDU (OR = 0.7; CI 95% = 0.5–1.02) proved to be independent predictors of acute HBV. Blood transfusion was confirmed to be an efficient mode of transmission (OR = 5.08; 95% CI = 1.8–14.3); however, only 16 HBV cases reported such exposure (Table 4).
Table 4.
Frequency (%) of characteristics reported by acute hepatitis B and acute hepatitis A (control group) cases and adjusted * odds ratio (OR) for the association of acute hepatitis B with characteristics of subjects. SEIEVA 2010–2019.
| Characteristics | HBV N = 3148 n (%) |
HAV N = 7517 n (%) |
OR (95% CI) |
|---|---|---|---|
| Sex: | |||
| Females | 780 (24.8) | 2240 (29.9) | 1 |
| Males | 2360 (75.2) | 5258 (70.1) | 1.42 (1.24–1.63) |
| Age: | |||
| ≤40 | 1083 (34.5) | 4917 (65.5) | 1 |
| ≥41 | 2060 (65.5) | 2591 (34.5) | 5.33 (4.70–6.05) |
| Area of diagnosis: | |||
| South/islands | 230 (7.3) | 903 (12.0) | 1 |
| North/central | 2918 (92.7) | 6614 (88.0) | 1.59 (1.28–1.92) |
| Area of birth: | |||
| Italy | 2541 (80.7) | 6416 (85.4) | 1 |
| Abroad | 607 (19.3) | 1101 (14.6) | 3.02 (2.54–3.60) |
| Blood transfusion without surgical intervention | 16 (0.6) | 7 (0.1) | 5.08(1.81–14.3) |
| Surgical intervention without blood transfusion | 291 (10.0) | 300 (4.5) | 2.25 (1.83–2.76) |
| Surgical intervention and blood transfusion | 26 (0.9) | 10 (0.1) | 4.76 (2.01–11.3) |
| Intravenous drug use | 96 (3.3) | 230 (3.5) | 0.72 (0.51–1.02) |
| Beauty treatments ** | 949 (31.8) | 1581 (23.4) | 1.62 (1.43–1.83) |
| Sexual exposure *** | 818 (28.3) | 983 (15.4) | 2.38 (2.06–2.74) |
| Household contact of an HBsAg+ carrier | 201 (8.7) | 71 (1.2) | 10.8 (7.88–14.9) |
* Adjusted by logistic regression analysis for the confounding effect of all listed variables. ** Piercing, tattooing, acupuncture, manicurist/chiropodist attendance, and barbershop shaving. *** Two or more sexual partners or lack of condom use in cases of casual sexual intercourse.
Population attributable risk (PAR) estimates indicate that 10.2%, 9.1%, and 7.6% of acute HBV cases may be explained by beauty treatments, risky sexual behavior, and household contact with an HBsAg positive case, respectively. Only 1.1% of cases are due to blood transfusion (Table 5).
Table 5.
Population attributable risk (PAR *) for risk factors in acute hepatitis B cases in Italy. SEIEVA 2010–2019.
| Characteristics | PAR |
|---|---|
| Blood transfusion without surgical intervention | 1.1% |
| Surgical intervention without blood transfusion | 5.2% |
| Surgical intervention and blood transfusion | 1.8% |
| Intravenous drug use | - |
| Beauty treatments ** | 10.2% |
| Sexual exposure *** | 9.1% |
| Household contact of an HBsAg+ carrier | 7.6% |
* Calculated by Levine’s formula. ** Piercing, tattooing, acupuncture, manicurist/chiropodist attendance, and barbershop shaving. *** Two or more sexual partners or lack of condom use in cases of casual sexual intercourse.
4. Discussion
The present findings highlight the effectiveness of the HB vaccination campaign on the incidence of acute hepatitis B in Italy. The overall decrease in the infection over time was 92%; most important, acute symptomatic HBV infection results were nearly eliminated in the age groups targeted by the campaign: zero cases in subjects 0–14 years old and 0.1 per 100,000 in those 15–24 years old. In the last decade, the favorable impact of the vaccination is evident even in the older age groups, reflecting the reduced spread of the virus.
The decreasing incidence figures are in line with the results of surveys showing the effectiveness of HB vaccination in preventing chronic liver disease (CLD) and HBsAg carriage among the young Italian generation. The mean age of CLD HBsAg-positive cases has shifted from 30.8 years in the decade 1980–1989 [7] to 57.3 years in 2019 [8]. In 2010, no subject below 30 years of age tested anti-HBc-positive in a study performed in a small southern Italian town [9]. In another study during the years 2008–2009 focusing on 13,008 pregnant women, the HBsAg prevalence rate was 0.4%, but no woman younger than 30 years of age (i.e., those targeted by vaccination) tested HBsAg-positive [10].
Taken together, all these findings indicate a current minimal spread of HBV infection in Italy, where control of the virus is close to being reached for the first time in a European country.
The key explanation for this favorable condition may be the peculiar model of the HBV vaccination policy adopted in Italy. The combined immunization of 3-month-old infants and 12-year-old subjects (limited to the first 12 years of the campaign for the latter category) generated an early immune cohort of youths, who are at high risk of acquiring HBV infection.
Other countries where comprehensive HB vaccination programs were adopted also reported successful results. In Taiwan, children born before and after the introduction of universal HBV vaccination showed a dramatic decrease in the HBsAg carrier rate from 10% in 1984 to 0.9% in 2012 [11]. Moreover, a dramatic declining trend in liver-related mortality was observed after the introduction of the national vaccination program: 68% decline in mortality from fulminant hepatitis in infants and a 75% decrease in the incidence of HCC in children 6–8 years old [12]. Universal newborn vaccination has eliminated HCC and symptomatic HBV infection among Alaskan native children [13]. In Gambia, infant vaccination has achieved substantial protection against chronic HBsAg carriage in early adulthood [14].
A changing pattern is observed in the main modes of HBV transmission. Intravenous drug use, the strongest independent predictor of acute HBV in the period 1995–1997 (adj. OR = 33.1%; 95% CI = 20.4–53.6) [3], is no longer associated with acute infection in the last decade (OR = 0.72; CI 95% = 0.51–1.02). Moreover, a dramatic downtrend in cases reporting this source of exposure from 20.8% in 1991–1999 to 3.3% in 2010–2019 is observed. This finding is different from the corresponding figures in other developed countries; in the USA, despite an overall decline in acute HBV cases attributable to successful vaccination programs, a rise in cases related to drug use has been reported in some states [15]. Further insight into the role of intravenous drug use as an important source of HBV infection in the USA has been recently provided [16]. The prevalence of the hepatitis B core antibody (anti-HBc) in serum (the marker for any previous exposure to HBV) among USA-born individuals has remained flat (around 2.5%) since 2007, coincident with a nearly doubled prevalence from 35.3% in 2001–2006 to 58.4% among people reporting previous intravenous drug use [16].
Taken together, all these findings reflect the reported decline in vaccine uptake among USA-born children and adolescents aged 6–18 years from 1999 to 2016 [17], leaving open the opportunity for new infections in young people.
Our findings might reflect the avoidance of sharing drug use equipment and/or the low proportion of IVDU still susceptible to HBV infection due to already being exposed or previously vaccinated. This latter point seems to be the most likely explanation. Among the 96 acute HBV cases reporting IDU in the decade 2010–2019, as many as 91.1% were unvaccinated subjects. Higher vaccine coverage may further decrease the rate of infection in this risk group, already targeted by the vaccination campaign.
Despite the fact that blood transfusion without surgical intervention continued to be an efficient mode of HBV transmission (OR = 5.1; 95% CI = 1.8–14.3) even in the decade 2010–2019, it accounts for only 1.1% of all HBV cases according to the PAR estimates, as only 16 subjects reported this source of exposure. This favorable finding reflects the introduction in 2008 of nucleic acid testing (NAT) for HBV in blood donor screening, capable of detecting infections in the window phase as well as the presence of occult infections that could potentially be transmitted. A recent survey [18] showed that the overall residual risk of HBV amounts to only 1 in 2,566,854 donations. Moreover, the risk of acquiring HBV infection could also be related to the hospital setting more than to the transfused blood [19].
Cohabitation with a chronic HBAg+ carrier results in the most efficient mode of transmission (OR = 10.8; 95% CI = 7.8–14.9). Alarmingly, rejection of the opportunity for immunization is present in this group, as more than 40% of these subjects, aware of the carrier condition of their cohabitant, were not vaccinated despite the strong recommendation of immunization.
Risky sexual behavior and beauty treatments continue to be efficient modes of HBV transmission. The use of HAV cases as a control group has likely generated an underestimate of the association of sexual behaviors with HBV infection (OR = 2.38; 95% CI = 2.06–2.74), because of an HAV epidemic among homosexual people that occurred in Italy, as well as in several other European countries, during 2016–2017 [20].
According to the PAR estimates, those two risk factors may explain nearly one-fifth of all HBV cases occurring in Italy. Once again, the importance of condom use in cases of casual sexual intercourse and proper sterilization of instruments in beauty treatment settings need to be strongly recommended.
HBV infection in migrant populations represents a new challenge for Italy and the rest of Europe; in fact, migrants may face a higher risk of contracting communicable diseases because they are often exposed to various risk factors before, during, and after migration. Moreover, instability and disruption of national healthcare services in their countries of origin may lead to suboptimal vaccine coverage at arrival [21]. As a consequence, most of them are still susceptible to infection, and, once they have acquired HBV, they may maintain the spread of the infection. Currently, they represent nearly one-fifth of all HBV cases occurring in Italy and are three-fold (95% CI = 2.5–3.6) more likely to acquire HBV than Italian natives. Testing and active proposal of HB vaccine to migrants entering Italy represents a useful and advisable preventive measure [22].
A potential limit of the present study is that incidence rates of acute HBV herein reported underestimate the true incidence of the infection, due to the voluntary nature of reporting cases and occurrence of subclinical infections. However, the incidence trend of the infection should not be affected by this limit, as the system was unchanged during the study period. The use of hepatitis A cases as a control group to estimate the odds ratios and the PAR might raise concern, because of differences between HAV and HBV cases by age, sex, and geographical area. The use of multiple logistic regression analysis likely removed the potential confounding effect of these socio-demographic variables. Moreover, comparability between cases and the control is the crucial point in a case-control study, and this is ensured by the fact that, in this study, hepatitis A and B cases were both identified by the same surveillance system, and thus exposed to the same selective factors, if any. Thus, reliable comparison between cases and controls was obtained in this survey. Despite the fact that using HAV cases cannot be considered as absolutely the best control, it may represent a feasible choice in this setting.
Finally, the high proportion of the Italian population covered by SEIEVA, with a nationwide reach, provides representativeness to the observed findings of acute HBV in Italy.
In conclusion, the current spread of HBV infection in Italy is minimal, reflecting the effectiveness of the vaccination campaign. Vaccination coverage of the households of chronic HBsAg carriers and of migrants may accelerate the road towards control of the virus for the first time in a European country.
These features, even if generated in Italy, may also be of interest for other developed countries.
Acknowledgments
SEIEVA collaborating group: ISS: Valeria Alfonsi, Franca D’Angelo; Piemonte: Carla Zotti, Erika Rainero, Noemi Marengo, Silvana Malaspina, Angela Gallone, Annalisa Castella, Maria Teresa Galati, Annamaria Scala, Paolo Castagna, Virginia Silano, Sebastiano D’Agosta, Maria Grazia Tacca, Silvia Iodice, Vilma Corvi, Maurizio Oddone, Daniela Rivetti, Paola Ravaschietto, Franco Giovanetti, Teresa Cappello, Alessandro Smaniotto; Valle D’Aosta: Mario Ruffier, Marina Verardo; Lombardia: Maria Gramegna, Sabrina Senatore, Danilo Cereda, Annalisa Donadini, Carla Nespoli, Livia Trezzi, Giorgio Gennati, Angelo Monteverdi, Liana Boldori, Franco Paolo Tortorella, Paola Elvira Merlini, Davide Di Caterina, Marino Faccini, Anna Lamberti, Eva Rossetti, Rita Brugnoli, Giulia Mainardi, Alessandra Vezzoli, Daniela Caso, Milena Testa, Alice Erba, Paola Senegaglia, Annamaria Spagna, Silvia Lodola, Marcello Tirani, Enza Giompapa, Luigi Guido Roveri, Gabriele Guardigli, Vanna Scalvinoni; Provincia autonoma di Bolzano: Silvia Spertini, Sabine Gamper, Andrea Grünfelder, Barbara Ploner, Verena Runggatscher; Provincia autonoma di Trento: Silvia Molinaro, Maria Grazia Zuccali, Silvia Franchini; Veneto: Francesca Russo, Francesca Zanella, Donatella Rizzato, Graziella Carpene, Nadia Lamonato, Emanuela Destefani, Alessandra Dal Zotto, Rita Dal Zotto, Paola Casagrande, Felice Foglia, Gemma Zorzi, Ester Chermaz, Liana Gava, Luigi Nicolardi, Lorena Pavanetto, Cecilia Battiston, Francesco De Grandi, Laura Rizzato, Michele Tonon, Elisabetta Cannizzo, Maurizio Foroni, Laura Colucci, Monica Barbieri, Rosanna Ledri; Friuli Venezia Giulia: Tolinda Gallo, Giulio Rocco, Rossana Stacul, Ariella Breda, Andrea Iob, Simonetta Micossi, Oriana Feltrin, Giuseppina Caernelos; Liguria: Marco Mela, Virna Frumento, Anna Opisso, Alessandro Cuccu, Giorgio Zoppi, Patrizia Torracca, Armanda Capellini, Francesco Maddalo; Emilia Romagna: Giovanna Mattei, Claudio Gualanduzzi, Erika Massimiliani, Anna Rita Sacchi, Laura Gardenghi, Anna Rosa Gianninoni, Elena Dalle Donne, Roberto Rangoni, Annalisa Califano, Chiara Reali, Emilia Biguzzi, Barbara Bondi, Anna Pecci, Bianca Maria Borrini; Toscana: Lucia Pecori, Emanuela Balocchini, Costanza Pierozzi, Rosa Luzzoli, Paola Marchini, Lara Lucchesi, Alberto Tomasi, Elisabetta Raso, Nadia Olimpi, Cristiana Berti, Francesca Matarazzo, Nicoletta Galletti, Laura Puppa, Marinella Frasca, Alessandro Barbieri, Chiara Cinughi, Maria Bandini, Iorio Lezzi, Fabrizia Verdelli, Renzo Paradisi, Rita Bindi, Cinzia Monica Sansone, Maria Rosati, Federica Zacchini, Simonetta Baretti, Rossella Cecconi, Chiara Staderini, Poalo Filidei, Elisabetta Alfaroli, Franco Barghini, Marina Cadoni; Umbria: Anna Tosti, Giovannini G, Giovanni Piattellini, Alessandra Buscosi, Anna Pasquale, Carla Ciani, Maria Claudia Paoloni, Franco Santocchia, Maria Laura Proietti; Marche: Daniel Fiacchini, Dzenana Hazurovic, Maria Sole Giamprini, Katia Gatti, Jacqueline Van Will, Alfredo Vaccaro, Maria Margherita Sbarbati, Alessandra Amelio, Daniela Cimini, Vania Moroni, Daniela Francoletti, Federica Scaccia, Elisabetta Branchesi, Selena Saracino, Catia Mezzanotte, Susanna Cimica, Vita Vitale, Franca Laici, Irene Petrelli, Barbara Airini, Gabriella Passarini, Lucia Ruffini, Anna Maria Lambertucci, Annarita Mogetta, Francesca Cioccoloni, Giuseppe Ciarrocchi, Marina Pistolesi, Erika Fratello, Francesca Picciotti, Claudio Angelini; Lazio: Paola Scognamiglio, Francesco Vairo, Andreina Ercole, Antonio Salvatore Maglietta, Fabrizio Magrelli, Fabrizio Perrelli, Carlo Cerocchi, Paolo Grillo, Cristina Vazzoler, Maria Rosaria Loffredo, Alessio Pendenza, Maria Rosaria Nappi, Paola Bueti, Luigi Santucci, Franca Mangiagli, Donatella Varrenti, Silvia Aquilani, Pietro Dionette, Daniela Corpolongo, Giuseppe Di Luzio; Abruzzo: Manuela Di Giacomo, Enrico Giansanti, Cristiana Mancini, Claudio Turchi, Carla Granchelli, Graziella Soldato, Felesina D’Eugenio, Ida Albanesi; Campania: Maria Antonietta Ferrara, Annarita Citarella, Elena Fossi, Rosa Alfieri, Milena Scotto, Anna Luisa Caiazzo; Puglia: Rosa Prato, Maria Chironna, Domenico Martinelli, Francesca Fortunato, Maria Giovanna Cappelli, Daniela Loconsole, Anna Morea, Giulia Del Matto, Raffaele Angelillis, Marcello Antonazzo, Valerio Aprile, Grazia Maria Avella, Roberta Cambria, Giovanni Caputi, Rosati Cipriani, Carlo De Santis, Francesco Desiante, Marisa Ferraro, Vera Laforgia, Antonino Madaro, Maria Giuseppina Maluccio, Anna Maria Matera, Stefania Menolascina, Giuseppina Moffa, Maria Nesta, Rita Olivieri, Onofrio Pagone, Pasquale Pedote, Rosella Squicciarini, Stefano Termite, Viviana Vitale; Basilicata: Francesco Negrone, Manuela Maldini, Giovanni Laugello, Teresa Russo; Calabria: Anna Domenica Mignuoli, Giuseppe Afflitto, Benedetto Caroleo, Maria Montesanti, Vincenzo De Giorgio, Antonio Maradei, Rocco Cataldo Romeo, Francesca Scrivano, Elisa Lazzarino, Vittoria Surace, Antonella Giordano, Alessandro Bisbano, Anita Arcuri, Ida Valentini; Sicilia: Rossana Mangione, Valentina Meli, Mario Cuccia, Elena Longhitano; Sardegna: Fiorenzo Delogu, Donatella Fracasso, Maria Vittoria Marceddu, Antonina Puggioni, Maria Valentina Eugenia Marras, Rita Serpi, Simonetta Santus, Valentina Marras.
Author Contributions
Conceptualization, methodology, and supervision, T.S., M.E.T. and L.F.; validation and investigation, T.S., M.E.T. and L.F.; formal analysis, M.E.T. and L.F.; visualization and writing—original draft preparation, T.S.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data used to support the findings in this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mastrodomenico M., Muselli M., Provvidenti L., Scatigna M., Bianchi S., Fabiani L. Long-term immune protection against HBV: Associated factors and determinants. Hum. Vaccines Immunother. 2021;17:2268–2272. doi: 10.1080/21645515.2020.1852869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers R.C., Hermann G.E. Hindbrain astrocytes and glucose counter-regulation. Physiol. Behav. 2019;204:140–150. doi: 10.1016/j.physbeh.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroffolini T., Mele A., Tosti M.E., Gallo G., Balocchini E., Ragni P., Santonastasi F., Marzolini A., Ciccozzi M., Moiraghi A. The impact of the hepatitis B mass immunisation campaign on the incidence and risk factors of acute hepatitis B in Italy. J. Hepatol. 2000;33:980–985. doi: 10.1016/S0168-8278(00)80132-4. [DOI] [PubMed] [Google Scholar]
- 4.Mele A., Tosti M.E., Mariano A., Pizzuti R., Ferro A., Borrini B., Zotti C.M., Lopalco P.L., Curtale F., Balocchini E., et al. Acute Hepatitis B 14 Years after the Implementation of Universal Vaccination in Italy: Areas of Improvement and Emerging Challenges. Clin. Infect. Dis. 2008;46:868–875. doi: 10.1086/528687. [DOI] [PubMed] [Google Scholar]
- 5.Tosti M.E., Longhi S., de Waure C., Mele A., Franco E., Ricciardi W., Filia A. Assessment of timeliness, representativeness and quality of data reported to Italy's national integrated surveillance system for acute viral hepatitis (SEIEVA) Public Health. 2015;129:561–568. doi: 10.1016/j.puhe.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Walter S.D. Calculation of Attributable Risks from Epidemiological Data. Int. J. Epidemiol. 1978;7:175–182. doi: 10.1093/ije/7.2.175. [DOI] [PubMed] [Google Scholar]
- 7.Giusti G., Ruggiero G., Galanti B., Piccinino F., Sagnelli E. Chronic active hepatitis in Italy: A multicentric study. Hepatogastroenterology. 1994;41:397–400. [Google Scholar]
- 8.Stroffolini T., Ciancio A., Furlan C., Vinci M., Niro G.A., Russello M., Colloredo G., Morisco F., Coppola N., Babudieri S., et al. Chronic hepatitis B virus infection in Italy during the twenty-first century: An updated survey in 2019. Eur. J. Clin. Microbiol. Infect. Dis. 2020;40:607–614. doi: 10.1007/s10096-020-04065-6. [DOI] [PubMed] [Google Scholar]
- 9.Stroffolini T., Guadagnino V., Rapicetta M., Ippolito F.M., Caroleo B., De Sarro G., Focà A., Liberto M.C., Giancotti A., Barreca G.S., et al. The impact of a vaccination campaign against hepatitis B on the further decrease of hepatitis B virus infection in a southern Italian town over 14 years. Eur. J. Intern. Med. 2012;23:e190–e192. doi: 10.1016/j.ejim.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Spada E., Tosti M.E., Zuccaro O., Stroffolini T., Mele A. Evaluation of the compliance with the protocol for preventing perinatal hepatitis B infection in Italy. J. Infect. 2011;62:165–171. doi: 10.1016/j.jinf.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Ni Y.-H., Chang M.-H., Wu J.-F., Hsu H.-Y., Chen H.-L., Chen D.-S. Minimization of hepatitis B infection by a 25-year universal vaccination program. J. Hepatol. 2012;57:730–735. doi: 10.1016/j.jhep.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Chien Y.-C., Jan C.-F., Kuo H.-S., Chen C.-J. Nationwide Hepatitis B Vaccination Program in Taiwan: Effectiveness in the 20 Years After It Was Launched. Epidemiologic Rev. 2006;28:126–135. doi: 10.1093/epirev/mxj010. [DOI] [PubMed] [Google Scholar]
- 13.McMahon B.J., Bulkow L.R., Singleton R.J., Williams J., Snowball M., Homan C., Parkinson A.J. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54:801–807. doi: 10.1002/hep.24442. [DOI] [PubMed] [Google Scholar]
- 14.Peto T.J., Mendy M.E., Lowe Y., Webb E.L., Whittle H.C., Hall A.J. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia Hepatitis Intervention Study (1986–90) and in the nationwide immunisation program. BMC Infect. Dis. 2014;14:7. doi: 10.1186/1471-2334-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iqbal K., Klevens M., Kainer M.A., Baumgartner J., Gerard K., Poissant T., Sweet K., Vonderwahl C., Knickerbocker T., Khudyakov Y., et al. Epidemiology of acute hepatitis B in the United States from population-based sur-veillance, 2006–2011. Clin. Infect. Dis. 2015;61:584–592. doi: 10.1093/cid/civ332. [DOI] [PubMed] [Google Scholar]
- 16.Ly K.N., Xing J., Spradling P.R. Trends in Prevalence and Characteristics of Resolved and Current Hepatitis B Among US-Born Persons: National Health and Nutrition Examination Survey, 2001–2018. J. Infect. Dis. 2021;224:804–812. doi: 10.1093/infdis/jiab224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le M.H., Yeo Y.H., So S., Gane E., Cheung R.C., Nguyen M.H. Prevalence of Hepatitis B Vaccination Coverage and Serologic Evidence of Immunity Among US-Born Children and Adolescents From 1999 to 2016. JAMA Netw. Open. 2020;3:e2022388. doi: 10.1001/jamanetworkopen.2020.22388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velati C., Romanò L., Prati E., Marano G., Piccinini V., Catalano L., Pupella S., Vaglio S., Veropalumbo E., Masiello F., et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis B virus in-fection in Italy from 2009 to 2018. Blood Transfus. 2019;17:409–417. doi: 10.2450/2019.0245-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smellie M.K.R., Carman W.F., Elder S., Walker D., Lobidel D., Hardie R., Downie G., McMenamin J., Cameron S., Morrison D., et al. Hospital transmission of hepatitis B virus in the absence of exposure prone procedures. Epidemiology Infect. 2005;134:259–263. doi: 10.1017/S0950268805004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndumbi P., Freidl G.S., Williams C.J., Mårdh O., Varela C., Avellón A., Friesema I., Vennema H., Beebeejaun K., Ngui S.L., et al. Hepatitis A outbreak disproportionately affecting men who have sex with men (MSM) in the European Union and European Economic Area, June 2016 to May 2017. Euro Surveill. 2018;33:1700641. doi: 10.2807/1560-7917.ES.2018.23.33.1700641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Regional Office for Europe (WHO-EURO) Health of Refugees and Migrants Regional Situation Analysis, Practices, Experiences, Lessons Learned and Ways Forward, WHO European Region (2018) [(accessed on 15 December 2021)]. Available online: https://www.euro.who.int/en/publications/abstracts/report-on-the-health-of-refugees-and-migrants-in-the-who-european-region-no-public-health-without-refugee-and-migrant-health-2018.
- 22.Tosti M.E., Marceca M., Eugeni E., D’Angelo F., Geraci S., Declich S., Della Seta M., Ferrigno L., Marrone R., Pajno C., et al. Health assessment for migrants and asylum seekers upon arrival and while hosted in reception centres: Italian guidelines. Health Policy. 2020;125:393–405. doi: 10.1016/j.healthpol.2020.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings in this study are available from the corresponding authors upon request.