Abstract
The members of the Nesterenkonia genus have been isolated from various habitats, like saline soil, salt lake, sponge-associated and the human gut, some of which are even located in polar areas. To identify their stress resistance mechanisms and draw a genomic profile across this genus, we isolated four Nesterenkonia strains from the lakes in the Tibetan Plateau, referred to as the third pole, and compared them with all other 30 high-quality Nesterenkonia genomes that are deposited in NCBI. The Heaps’ law model estimated that the pan-genome of this genus is open and the number of core, shell, cloud, and singleton genes were 993 (6.61%), 2782 (18.52%), 4117 (27.40%), and 7132 (47.47%), respectively. Phylogenomic and ANI/AAI analysis indicated that all genomes can be divided into three main clades, named NES-1, NES-2, and NES-3. The strains isolated from lakes in the Tibetan Plateau were clustered with four strains from different sources in the Antarctic and formed a subclade within NES-2, described as NES-AT. Genome features of this subclade, including GC (guanine + cytosine) content, tRNA number, carbon/nitrogen atoms per residue side chain (C/N-ARSC), and amino acid composition, in NES-AT individuals were significantly different from other strains, indicating genomic adaptation to cold, nutrient-limited, osmotic, and ultraviolet conditions in polar areas. Functional analysis revealed the enrichment of specific genes involved in bacteriorhodopsin synthesis, biofilm formation, and more diverse nutrient substance metabolism genes in the NES-AT clade, suggesting potential adaptation strategies for energy metabolism in polar environments. This study provides a comprehensive profile of the genomic features of the Nesterenkonia genus and reveals the possible mechanism for the survival of Nesterenkonia isolates in polar areas.
Keywords: microbial adaptation, comparative genome, polar environments, Nesterenkonia
1. Introduction
The Qinghai-Tibet Plateau (QTP) is referred to as the third pole of the world because it shares many characteristics with the Arctic and Antarctic regions. The common harsh conditions include low temperature, limited nutrient availability, and strong ultraviolet radiation [1]. In addition, lakes in these regions also suffer from a wide range of salinity and pH, which together contribute to them becoming research hotspots for studies of extremophiles adaption [2,3,4]. Microbial community structures in the Antarctic, Arctic, and Tibetan lakes have been investigated alone and it has been found that the main impact factors include light, temperature, and physicochemical conditions, including the availability of organic carbon and nutrients [5,6,7]. The bacterial diversity and community composition in lakes from the three polar regions were found to also share some common microbial taxa [8]. However, this traditional amplicon sequencing method cannot capture the specific genomic and functional differentiations below the species level. The higher-precision method should be applied to clarify mechanisms by which the bacteria have evolved to survive in these harsh conditions.
Bacterial strains isolated from the polar region have shown many molecular mechanisms for adaptation to extreme conditions. For instance, Marisediminicola antarctica ZS314T, isolated from intertidal sediments in East Antarctica, has reddish-orange pigments synthesis capacity at low temperatures [9]. This carotenoid product may contribute to the regulation of membrane fluidity and can also protect cells against UV radiation [10]. Antarctic Streptomyces and Kribbella strains harbor biosynthetic gene clusters that encodes lipopeptide biosurfactant molecules, the primary ecological role of which is accelerating nutrient flow [11]. The psychrophilic Arthrobacter isolate contains more copies of nucleic acid-binding cold-shock proteins (CSPs) and, also, the homologs of the CspA-like cold acclimation protein. These proteins can show different expression patterns to a sudden temperature transition and can help to stabilize DNA and RNA secondary structures during growth under low conditions [12].
Nesterenkonia, is a genus within the Micrococcaceae family, consisting of many mesophilic moderate haloalkaliphiles from various sources. Members of this group possess high genomic G + C content (64%–72%) and are generally aerobic, gram-positive, and chemo-organotrophic. They have been found in diverse environmental habitats, like hypersaline and saline lakes [13,14,15], cotton pulp mills [16], and the human gut [17,18]. Members belonging to this genus are also isolated from the Antarctic and Arctic [19,20], suggesting that Nesterenkonia spp. might have specific adaptation mechanisms to the polar environment. Previous research about the strain Act20 from the high-altitude-Andean-lake in Argentina found that is has multi-resistance, especially towards UV radiation, drought, and copper [21]. Transcriptional analysis of the Antarctic strain AN1 showed that genes related to antioxidants-coding, cold stress, and the glyoxylate cycle were significantly upregulated during cold growth [20]. However, the systematic analysis of genomic features across all genomes within this genus was absent, which can be resolved by pan-genome analyses. This method can encompass the entire gene pool of target species and offer a framework for genomic diversity estimation [22]. By dividing the full gene repertoire into a few parts (core, shell, cloud, and singleton gene), many important scientific projects can be studied, such as environmental adaptation [23,24], speciation mechanisms [25], and pathogenic drug resistance [26].
In this study, we isolated and sequenced four Nesterenkonia strains from different Tibetan lakes and attempted to draw the genomic profile to extreme niche adaptation using the comparative genomic method with all readily published and available genomes of Nesterenkonia species in the public National Center for Biotechnology Information (NCBI) database. Among the four strains and other strains from the Arctic and Antarctic regions, we observed some genomic adaptation to cold, nutrient-limited, osmotic, and ultraviolet conditions, as well as energy metabolism adaptation to polar environments.
2. Materials and Methods
2.1. Sampling, Isolation, and Physiological Measurement
Large-scaled samplings from Tibetan lakes and bacterial isolation were conducted in August of 2015 and 2016. The samples from freshwater and saline lakes were cultivated in Luria–Bertani (LB, 4% NaCl) and CM medium (0.5% NaCl) [27], respectively. Water samples were gradient diluted and plated directly, while the sediment samples were dissolved in the NaCl solution first. The NaCl concentration of the solution was adjusted according to the salinity of its source lake. The taxonomy of the selected colony was confirmed by PCR reaction, which amplified the 16s rRNA with primer pair 27F-1492R. The obtained sequence was blast searching against the EZBioCloud database to find the closest related taxa [28]. Finally, four isolates, which have 99.86% identity with Nesterenkonia aurantiaca, were selected to perform further analysis. The strain LB17, AY15, YGD6, and DZ6 are from samples in Lubu Cuo water, Ayong Cuo sediment, Yagedong Cuo water, and Daze Cuo sediment, respectively. The conductivity, pH, water temperature (Temp), and dissolved oxygen (DO) concentration measurements were conducted in the field using a multi-parameter water quality sonde (YSI 6600, Yellow Springs, Greene, OH, USA). Other environment parameters, including total phosphorus, total nitrogen, ammonium, nitrate, nitrite, and phosphate, were analyzed using the standard methods [29].
2.2. Whole Genome Sequencing, Assembly, and Reference Genomes Collection
Bacterial genomic DNA was extracted using the SDS method [30]. After evaluating the DNA quality and quantity using NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA), the sequencing libraries were generated using the NEBNext® Ultra™ DNA Library Prep Kit for Illumina (New England Biolabs, MA, USA). Finally, the sequencing process was conducted using Illumina NovaSeq PE150 at the Beijing Novogene Bioinformatics Technology Co., Ltd. The adapter sequence of raw reads was detected using BBmap v37.0 [31] and then removed using Trimmomatic v0.33 [32]. The resulting high-quality reads were assembled to the scaffolds using SPAdes v3.9 [33]. The genome data obtained in this study have been deposited at NCBI under the BioProject number PRJNA786453. The genomes of all Nesterenkonia isolates in the Genbank and Neomicrococcus aestuarii strain B18 were downloaded using the ncbi-genome-download script (https://github.com/kblin/ncbi-genome-download, accessed on 7 Octobor 2021). In all genomes, only the scaffold with longer than 1000-bp length was kept for downstream analysis.
2.3. Genome Quality Estimation, Gene Annotation, and Phylogenetic Analysis
The completeness and contamination of each genome were evaluated using CheckM v1.1.3 [34]. Only the genome with >95% completeness and <5% contamination was selected for downstream analysis. Other genomic statistic parameters were calculated by seqkit v0.16.1 [35]. Gene calling for each genome was performed using PROKKA v1.14.5 [36]. The tRNAscan was used to predict the tRNA [37]. All protein-coding genes were annotated by searching the KEGG KOfam [38] and COGs (Clusters of Orthologous Groups) databases [39] using HMMER v3.3.2 [40]. The 16s rRNA sequences of all genomes were exacted and aligned using mafft v7.474 [41] with the “-auto” command. The maximum likelihood phylogenomic tree was constructed using IQ-TREE v1.6.12 [42] by default parameters. For species-tree building, protein-coding genes were first clustered into orthologous groups (OG) using OrthoFinder v2.2.1 [43]. The sequences in each shared single-copy OG were aligned and trimmed using mafft v7.474 and trimAl v1.2 [44], respectively. The no-gap alignments were concatenated and used as the input to construct a maximum likelihood phylogenomic tree using IQ-TREE v1.6.12.
2.4. Comparative Genomic Analysis
The pairwise average nucleotide identity (ANI) analysis of all genomes was performed using FastANI [45]. Amino acid identity (AAI) between each proteome pair was estimated by the online tool AAI-Matrix [46]. The amino acid (AA) composition and average nitrogen and carbon content of each proteome were calculated by a homemade script. All descriptive statistical analysis and difference significance tests were conducted in R (R Core Team, 2020) [47]. Pan-genome analysis and visualization were conducted on the anvi’o platform [48]. Briefly, the pangenome of all 34 Nesterenkonia genomes was computed using ‘anvi-pan-genome’ with default –mcl-inflation 2, which uses the Markov Cluster algorithm [49] to cluster the annotating genes into groups. Pan-genome openness was estimated using the ‘micropan’ R package [50] with 100 permutations. The map for pan-genome, core, and accessory gene distribution was performed by the PanGP program with a distance guide (DG) subsampling algorithm [51]. Combined with the KO and COG annotation, clade-specific functions were identified by the ‘anvi-compute-functional-enrichment-in-pan’ command. Only genes with a <0.05 ‘adjusted q-value’, which represents the false-discovery rate adjusted p-value corrected for multiple testing, were considered.
3. Results and Discussion
3.1. Tibetan Isolates Show High Similarities with Antarctic Isolates
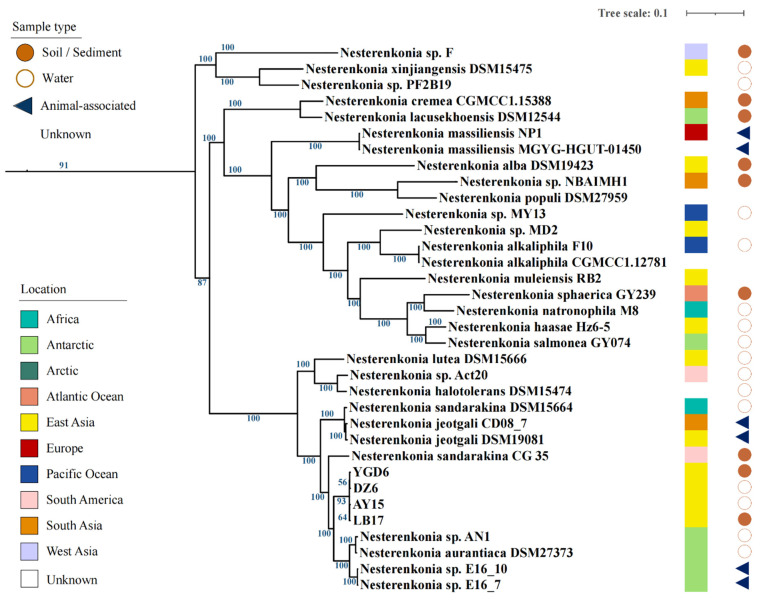
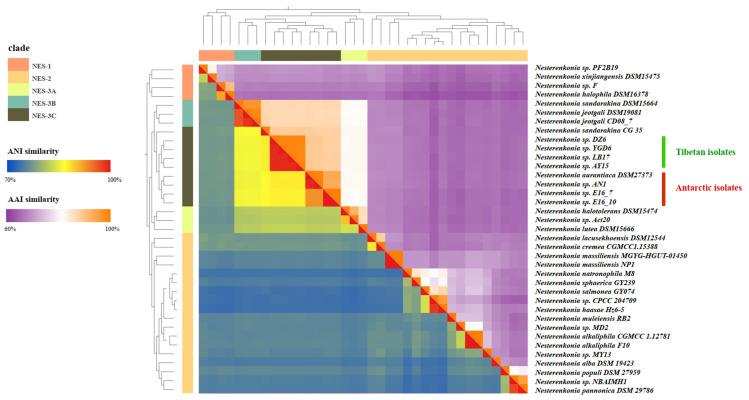
A total of 6041 orthologous groups common to all Nesterenkonia isolates were identified. Phylogenetic analysis based on 304 single-copy OG showed that the whole genus contains three major clades, named herein NES-1, NES-2, and NES-3 (Figure 1, Table 1). The four Tibetan isolates and four Antarctic isolates were found to form a separate subclade (NES-AT) within the major clade NES-2, which may indicate a close evolutionary relationship among these isolates. Phylogenetic analysis based on 16S rRNA gene sequences also indicated the grouping of the eight isolates from the Tibetan and Antarctic regions (Figure S1). The isolates within NES-AT subclade shared 1923 single-copy OGs, which were extracted to build the evolutionary tree. According to the tree topology, Tibetan and Antarctic isolates generate their respective cluster, together with the internal subcluster division, indicating potential local adaptions. For Tibetan clusters, environmental salinity seems to be more important than habitat type for microbial divergence. This is because the strains from saline and freshwater lake samples were clustered separately, whether they were isolated from water or sediment. For the Antarctic cluster, the sponge-associated strain E16_7 and E16_10 as well as the soil-derived strain AN1 and DSM 2737 formed two subclusters respectively, which is consistent with the previous results [52]. ANI and AAI analysis also showed similar results (Figure 2). ANI analysis based on whole-genome comparison showed that NES-AT clade strains shared 93.5% of their identity between them, and below 80% of their identity with all Nesterenkonia genomes. These results suggest the evolution of bacteria affiliated with NES-AT clade towards the polar environment. The annual mean temperature in the Tibetan Plateau is lower than 10 °C because of the high elevation (>4000 m) [53], while even in the warmest January, the average air temperature in the Antarctic region was only 2.5 ± 0.49 °C [54]. The cold weather and serious UV radiation in both regions limit the growth of organisms and lead to low productivity [55]. On the other hand, this high similarity can also provide some supports for the hypothesis about the geological history of the two regions. More specifically, Tibet originated from the Gondwanaland plate including India and these eight isolates may come from the common ancestor [56].
Figure 1.
Phylogenetic tree of 34 high-quality Nesterenkonia strains. Colored bars and circles/tringles indicate isolation location and habitat type, respectively. The species Neomicrococcus aestuarii was used as the outgroup reference genome.
Table 1.
Genomes information of the strains within the genus Nesterenkonia.
| Strain Name | Accession Number | Scaffold Number | Geneome Size (M) | Gene Number | GC% | rRNA Number | tRNA Number | Completeness | Contamination |
|---|---|---|---|---|---|---|---|---|---|
| Nesterenkonia sp. AY15 | JAJOYV000000000 | 20 | 2,801,805 | 2574 | 67.24 | 5 | 234 | 99.01 | 0.46 |
| Nesterenkonia sp. DZ6 | JAJOYW000000000 | 9 | 2,869,920 | 2614 | 67.2 | 5 | 360 | 99.01 | 0.57 |
| Nesterenkonia sp. F | GCA_000220985.2 | 134 | 2,809,541 | 2514 | 71.49 | 3 | 93 | 98.59 | 0.52 |
| Nesterenkonia alba DSM 19423 | GCA_000421745.1 | 36 | 2,591,866 | 2384 | 63.75 | 6 | 140 | 98.38 | 0 |
| Nesterenkonia massiliensis NP1 | GCA_000455245.1 | 19 | 2,672,431 | 2550 | 62.16 | 3 | 261 | 97.73 | 0.46 |
| Nesterenkonia sp. AN1 | GCA_000582475.1 | 42 | 3,040,130 | 2932 | 67.42 | 3 | 167 | 97.15 | 0.69 |
| Nesterenkonia jeotgali strain CD08_7 | GCA_001483765.1 | 8 | 2,925,195 | 2715 | 67.63 | 3 | 364 | 98.51 | 0.98 |
| Nesterenkonia sp. PF2B19 | GCA_001758425.2 | 134 | 3,696,919 | 3701 | 69.49 | 5 | 91 | 96.95 | 0.07 |
| Nesterenkonia sandarakina strain CG 35 | GCA_003003175.1 | 56 | 3,224,976 | 3001 | 67.46 | 9 | 124 | 99.39 | 0.46 |
| Nesterenkonia natronophila strain M8 | GCA_003595215.1 | 5 | 2,520,774 | 2363 | 61.82 | 3 | 402 | 98.34 | 0.07 |
| Nesterenkonia muleiensis strain RB2 | GCA_003600155.1 | 57 | 3,676,111 | 3493 | 63.55 | 2 | 95 | 97.79 | 0.86 |
| Nesterenkonia salmonea strain GY074 | GCA_005771525.1 | 110 | 3,267,177 | 3175 | 61.13 | 3 | 114 | 99.16 | 0.34 |
| Nesterenkonia sphaerica strain GY239 | GCA_005771565.1 | 52 | 2,770,794 | 2633 | 64.28 | 4 | 143 | 99.03 | 1.41 |
| Nesterenkonia sp. NBAIMH1 | GCA_007922635.1 | 1 | 2,691,978 | 2605 | 66.41 | 6 | 1128 | 97.94 | 0.18 |
| Nesterenkonia populi strain DSM 27959 | GCA_007994735.1 | 2 | 2,551,278 | 2414 | 66.85 | 6 | 1177 | 98.17 | 0.88 |
| Nesterenkonia sp. MD2 | GCA_008711175.1 | 41 | 3,733,063 | 3593 | 63.15 | 5 | 142 | 98.41 | 1.41 |
| Nesterenkonia alkaliphila strain F10 | GCA_009758175.1 | 103 | 3,318,774 | 3105 | 64.83 | 2 | 66 | 98.85 | 1.43 |
| Nesterenkonia haasae strain Hz 6-5 | GCA_010119385.1 | 29 | 3,422,101 | 3258 | 60.8 | 7 | 193 | 99.16 | 1.21 |
| Nesterenkonia sp. MY13 | GCA_012641515.1 | 41 | 3,101,056 | 2965 | 63.07 | 3 | 144 | 98.8 | 1.68 |
| Nesterenkonia sandarakina strain DSM 15664 | GCA_013410215.1 | 2 | 3,017,448 | 2780 | 67.51 | 6 | 1128 | 98.58 | 1.05 |
| Nesterenkonia xinjiangensis strain DSM 15475 | GCA_013410745.1 | 1 | 3,569,370 | 3182 | 68.81 | 6 | 1225 | 99.77 | 0.61 |
| Nesterenkonia jeotgali strain DSM 19081 | GCA_014138825.1 | 1 | 3,002,985 | 2767 | 67.44 | 6 | 1275 | 98.51 | 3.28 |
| Nesterenkonia alkaliphila CGMCC 1 | GCA_014639295.1 | 81 | 3,386,621 | 3181 | 64.79 | 4 | 103 | 98.85 | 1.43 |
| Nesterenkonia cremea CGMCC 1 | GCA_014642675.1 | 37 | 3,082,200 | 2850 | 66.86 | 5 | 167 | 99.56 | 0.88 |
| Nesterenkonia lutea strain DSM 15666 | GCA_014873955.1 | 2 | 2,958,123 | 2702 | 66.73 | 6 | 1128 | 99.54 | 0.07 |
| Nesterenkonia halotolerans strain DSM 15474 | GCA_014874065.1 | 3 | 2,966,101 | 2742 | 66.24 | 6 | 648 | 99.16 | 1.28 |
| Nesterenkonia sp. E16_7 | GCA_017347075.1 | 82 | 3,294,162 | 3074 | 67.28 | 3 | 111 | 98.88 | 1.44 |
| Nesterenkonia sp. E16_10 | GCA_017347085.1 | 49 | 3,295,232 | 3071 | 67.28 | 3 | 122 | 98.42 | 1.44 |
| Nesterenkonia lacusekhoensis strain DSM 12544 | GCA_017876395.1 | 2 | 2,742,649 | 2662 | 66.68 | 6 | 1486 | 100 | 0.99 |
| Nesterenkonia sp. Act20 | GCA_019173455.1 | 2 | 2,930,097 | 2732 | 65.93 | 7 | 697 | 99.58 | 0.75 |
| Nesterenkonia massiliensis MGYG-HGUT-01450 | GCA_902375145.1 | 19 | 2,672,431 | 2550 | 62.16 | 3 | 261 | 97.73 | 0.46 |
| Nesterenkonia sp. LB17 | JAJOYX000000000 | 10 | 2,819,602 | 2569 | 67.19 | 4 | 297 | 99.01 | 0 |
| Nesterenkonia aurantiaca strain DSM 27373 | GCA_004364585.1 | 25 | 2,948,026 | 2704 | 67.58 | 4 | 186 | 99.11 | 0 |
| Nesterenkonia sp. YGD6 | JAJOYY000000000 | 11 | 2,853,887 | 2592 | 67.12 | 3 | 250 | 99.01 | 0 |
Figure 2.
Average nucleotide (ANI, lower left triangle) and amino acid identity (AAI, upper right triangle) analysis of Nesterenkonia genus isolates. Genomes were clustered according to the phylogenetic tree.
3.2. Comparative Genomic Analysis
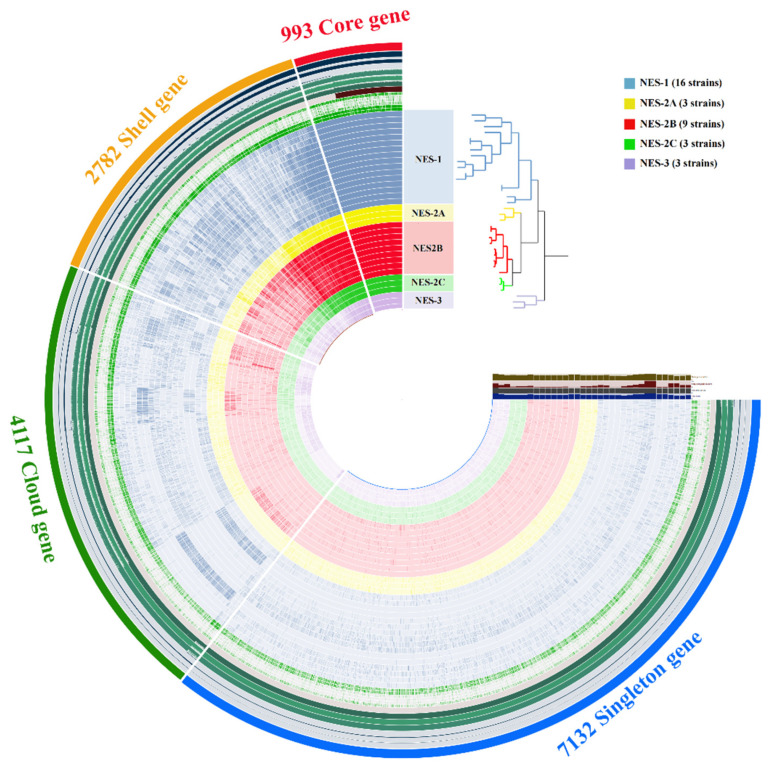
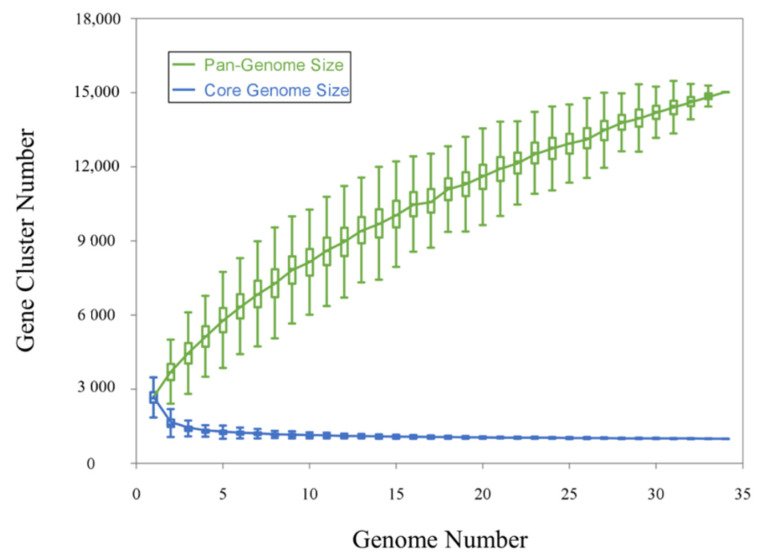
The pan-genome analysis found a total of 15,024 gene clusters across the 34 high-quality Nesterenkonia genomes (Figure 3), which include 30 reference genomes from NCBI and also the strains that we isolated. Gene clusters were defined as core (present in each genome) and accessory (non-core genes) types. The latter was then classified into “shell” (present in 99%-15% genomes), “cloud” (found in less than 15% single genome), and “singleton” (found in only one genome). The number of core, shell, cloud and singleton genes were 993 (6.61%), 2782 (18.52%), 4117 (27.40%), and 7132 (47.47%), respectively. Heaps law model parameter α estimation was equal to 0.3976, less than the threshold of 1.00 [57]. In addition, the gene accumulation curves of the pan-genome show that the power trend line has not arrived at the platform stage (Figure 4). Both results suggest that this genus has an open pan-genome and the sequenced genomes cannot contain the complete gene repertoire. As more Nesterenkonia strains are sequenced, more novel genes will be found, leading to a larger pan-genome. This openness indicates that isolates within this genus have great potential to integrate exogenous genetic material and broaden genetic diversity by other evolutionary mechanisms, like recombination and mutation. Nesterenkonia strains can thus adapt to diverse habitats by accessory genes acquisition and loss and have a very large and flexible gene pool [58].
Figure 3.
Pan-genome analysis of Nesterenkonia genus using Anvi’o workflow. The genomes are organized in radial layers as core, shell, cloud, and singleton gene clusters.
Figure 4.
Characteristic curves of the pan-genome and core genome using PanGP.
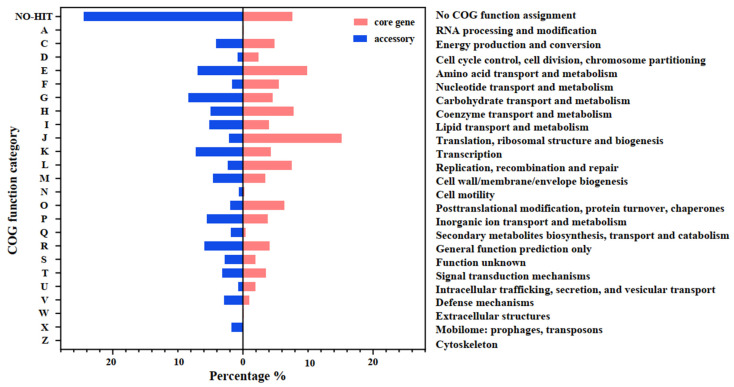
We then compared the COG functional categories distribution between the core and accessory genes (Figure 5). As expected, the COG functional categories of some highly conserved and low evolution rate biological processes, such as COG-J (translation, ribosomal structure, and biogenesis), COG-L (replication, recombination, and repair), COG-O (posttranslational modification, protein turnover, chaperones), COG-U (intracellular trafficking, secretion, and vesicular transport), and COG-F (nucleotide transport and metabolism) concentrated more in the core genome but were low in the accessory genes. Instead, COG-V (defense mechanisms), COG-X (mobilome: prophages, transposons), and COG-K (transcription) enrich more in accessory genes, indicating that strains from different sources possess the distinct capacity of genetic material processing [59] and diverse transcription mechanisms to deal with the changing ecological conditions. COG categories corresponding to amino acid (COG-E) and coenzyme (COG-H) transport and metabolism are dominant in the entire genomes but overrepresented in core genes, while the secondary (COG-Q) and carbohydrate metabolism (COG-G) functions have the opposite distribution. This finding is in line with the high secondary metabolites biosynthetic diversity and polysaccharide degradation ability in the Actinobacteria phylum, which are often present in the flexible genome and are varied below the genus level. Finally, genes within COG-S (unknown function), COG-R (general function prediction only) categories, as well as genes without any COG annotation (NO-HIT), were abundant across the pan-genome and had higher proportions in accessory genes.
Figure 5.
The COG functional categories distribution comparison between core and accessory genes.
3.3. Genomic Feature Comparison between NES-AT Isolates and Other Nesterenkonia Isolates
Since the bacteria that lived in similar niches shared more genomic features, we calculated some genome characteristic parameters and compared them between NES-AT clade and other isolates (Figure 6).
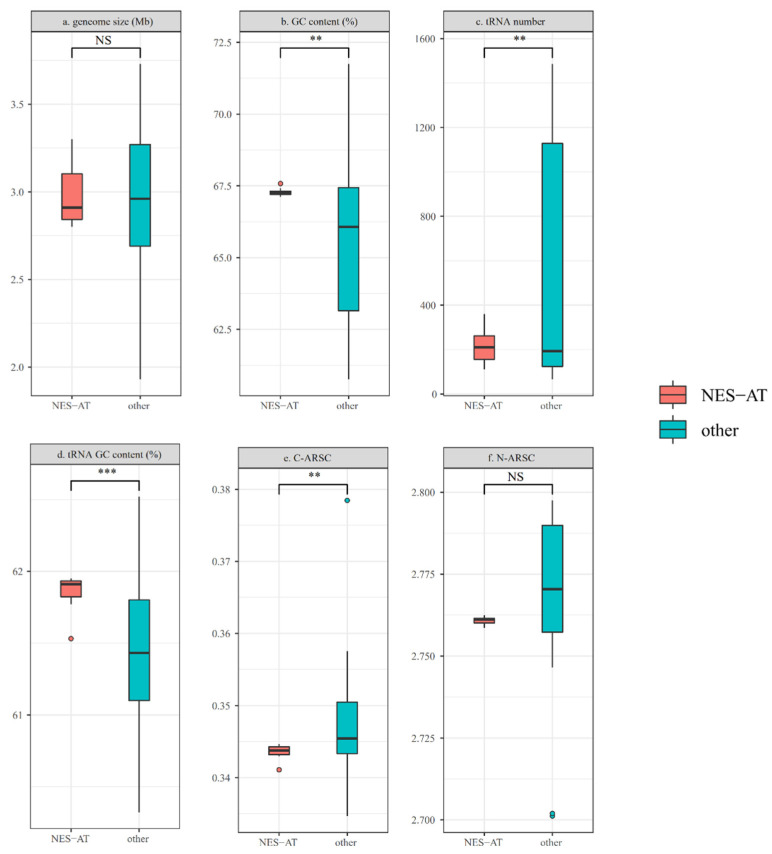
Figure 6.
Genomic feature comparisons between NES-AT clades and other references Nesterenkonia, marked as NES-AT and other, respectively. (a) genome size (Mb), (b) GC content (%), (c) tRNA number, (d) tRNA GC content (%), (e) C-ARSC and (f) N-ARSC C-ARSC and N-ARSC represent the number of carbon and nitrogen atoms per residue side chain, respectively. (NS: not significant; **: p < 0.01; ***: p < 0.001). The dot represents the outliers.
Genome size (Figure 6a) and GC content (Figure 6b): GC (guanine + cytosine) content, as an important indicator of microbial evolution, is often thought to be positive relative to the genome size [60]. Although the difference in genome size is not significant, the GC content in the NES-AT clade is higher than that in other Nesterenkonia strains (t-test, p < 0.01). Previous studies found that increased GC content often accompanies high rates of genetic damage in the manner of a double-strand break [61], which is often caused by severe ultraviolet radiation [62]. This base composition tendency probably implies the adaptation to serious UV exposure, due to the ozone depletion in the Antarctic and high elevation in Tibetan areas [63,64].
tRNA: Transfer RNAs (tRNA), as an adaptor molecule that participates in the peptide chain synthesis [65], also have an important role in gene expression regulation and cell membrane modification [66]. In addition, yeast can change the tRNA gene abundance (Figure 6c) during stressful conditions, which also reflects its essential function in survival [67]. In Nesterenkonia genomes, a higher number of tRNA genes was found in the NES-AT clade (t-test, p < 0.01, p = 0.0067), which showed an opposite tendency in thermophiles [68]. Due to the positive relations between tRNA genes and their relative concentration [69], more tRNA genes may increase the tRNA amount. Higher tRNA numbers can accelerate the transcription/translation speed and make up for the low diffusion rate and metabolic activity in polar environments [70]. Since significant correlations between tRNA abundance and growth rate/optimal growth temperature have been reported in other prokaryotes [68], more tRNA allows organisms to grow fast in the cold. Similar results were also found in other psychrophilic isolates [71]. Mean GC contents of tRNA genes (Figure 6d) for all Nesterenkonia genomes were calculated and NES-AT isolates showed significantly higher (t-test, p < 0.001, p = 0.00043) values than others. Similar high tRNA GC% has only been found in hyperthermophiles, the RNA stability of which is needed in high temperatures [72].
C-ARSC (Figure 6e) and N-ARSC (Figure 6f): We also calculated the nitrogen content of protein-coding sequences (N-ARSC) and the numbers of carbon atoms per residue side chain (C-ARSC) in all genomes. These indexes can reflect the nutrient availabilities in the environment, as the comparison studies between epipelagic and mesopelagic Marinimicrobia genomic modalities [73]. Only a reduced use of carbon in the AA sequences was found in the NES-AT clade genomes, indicating that carbon-limited conditions in polar regions are likely an important factor influencing the evolution of Nesterenkonia [74]. Harsh conditions often limit plant growth, which provides a primary source of organic carbon. Thus, microbes in the polar region usually face the challenge of carbon-poor adaptation [75,76,77].
AA composition: Due to the prevalence of amino acid (AA) preference in microbial cold adaption, AA usage of each proteome was calculated. Since charged polar AA will lead to a stable protein structure by formatting the salt bridge on the protein surface [78], psychrophilic organisms often adjust the AA composition for cold adaption. In NES-AT clade, isolates tend to harbor more nonpolar AA and less polar AA, which includes positively charged histidine, negatively charged aspartate, and glutamine, and uncharged tyrosine and glutamine. The different AA preferences in NES-AT isolates may contribute to the protein flexibility improvement at low temperatures [79]. In addition, the substitution of alanine to glutamine was also found in a psychroactive Antarctic salt-lake archaea Halorubrum lacusprofundi [80], which could explain the higher alanine and lower glutamine proportion in NES-AT isolates. Researches about cold-adapted bacterial lipase and cell surface proteins showed remarkably lower aromatic residues [81,82], which is consistent with less tyrosine and tryptophan in the NES-AT clade. Similar results were also found in other prokaryotes groups, like marine Shewanella spp. [83] and subzero-growing Arctic permafrost bacteria [84]. A striking feature is a significant leucine preference in the NES-AT clade, which is unfavorable for helical structure flexibility. Leucine, one of the widely used nutrient sources [85], its accumulation might enhance survival in oligotrophic conditions. After all, genome AA composition can also be impacted by environmental concentration [86]. Cystine, another preferred AA that is common to many psychrophiles, is shown in low abundance in NES-AT isolates. Cysteine can form disulfide bonds to assist the cell envelope proteins folding and stability [87]. Reduced content is possibly beneficial to loose protein structure. The third contrary result is shown on phenylalanine, which is nonpolar but capable of cation-π interactions formation [88], whose enrichment is likely to relate with other stress, such as UV defense. This is because phenylalanine is the precursor of mycosporines and mycosporines-like amino acids, which can be used as sunscreen compounds to protect against severe UV damage [89]. Some disagreements with previous studies [90] may arise from species specificity, as there is huge divergence between proteobacteria and actinobacteria phylum.
3.4. Functional Genes Related to the “Polar” Environmental Adaption
We first classified all 34 isolates into the “NES-AT” and “Other” clades and compared the COG category difference between them using a student’s t-test. We found that the NES-AT clade has significantly more genes in COG-I (lipid transport and metabolism, p < 0.001), COG-C (energy production and conversion, p < 0.001), COG-B (chromatin structure and dynamics, p < 0.001), COG-T (signal transduction mechanisms, p < 0.001) and COG-D (cell cycle control, cell division, chromosome partitioning, p < 0.01). Lipid is the main component of the cellular membrane and its content and composition can influence membrane fluidity. Bacteria often change the lipid composition of cell walls and membranes for adaptation to cold and oligotrophic conditions. For example, Antarctic Pseudoalteromonas isolates PhTAC125 showed better performance in cold adaptation than the closely related strain PspTB41, which contains fewer COG-I genes [91]. COG-C and COG-T classes have been proved to harbor a high number of cold-adapted proteins, which can be helpful for energy acquisition and maintenance under low-temperature stress [92]. Previous transcriptomic analysis of Nesterenkonia sp. AN1 in cold response showed that COG-B and COG-D genes were significantly upregulated [20]. This might be consistent with the change of growth cycle and speed when the isolates survive in cold conditions [93]. The same trend is also present in the COG-S category (p < 0.01), reflecting the genes with unique and unexplored functions in complex and extreme niches. In contrast, COG-N (Cell motility), COG-p (Inorganic ion transport and metabolism, p < 0.05), COG-E (p < 0.01), and COG-F (p < 0.05) related genes have significantly decreased proportions among the NES-AT clade isolates. Oligotrophic bacteria contained fewer genes involved in COG-N because of their lower demand for the transient microscale nutrient sources in the environment [94]. The decreased gene amount of other nutrients (amino acid, nucleotide, and inorganic ion) transporter and metabolism can also be an indication of the adaptive evolution to nutrient-limiting habitats.
Following this, the more specific function enrichment analysis was performed using KOfam and COG annotation results. Some KOfam and COG functions are found to be overrepresented and only the items with an adjusted q value < 0.01 were shown (Table 2). Bacteriorhodopsin (COG5524), also called the actinorhodopsin in previous researches, was a putative light-activated proton pump [95]. Its appearance provides NES-AT isolates the potential for the phototrophy lifestyle and improves survival during the nutrient starvation situation, which is similar to the rhodopsin in Pelagibacter [96]. Both COG3049 (penicillin V acylase or related amidase from Ntn superfamily) and COG4978 (GyrI-like small molecule binding domain) can act as the transcriptional regulators that control the biofilm formation [97,98], which is a widespread mechanism for bacterial survival under adverse environments. Cellulase/cellobiase (COG5297), most likely the endoglucanase, can give NES-AT isolates the capacity of plant cell wall degradation. Increasing the carbohydrate metabolism diversity can be helpful to energy starvation [99,100]. The glucose they produced can further become the nucleotide sugar precursors (UDP-glucose) and participate in the synthesis of cell surface polysaccharides with the help of O-antigen ligase (COG3307, K16567) [101], which was only enriched in NES-AT isolates. These structures can contribute to diverse biological functions, like nutrient gathering, cold defense, and motility, which protect cells against abiotic and biotic stress [102,103].
Table 2.
KOfam and COG functions enrichment summary.
| Function Class | COG Function | Enrichment Score | Adjusted q Value | Enriched Groups | Accession |
| Energy production | Bacteriorhodopsin | 37 | 0 | AT | COG5524 |
| Transcriptional regulators | Penicillin V acylase or related amidase, Ntn superfamily (YxeI) | 37 | 0 | AT | COG3049 |
| GyrI-like small molecule binding domain (BltR2) | 31.7525 | 0 | AT | COG4978 | |
| HD superfamily phosphodieaserase, includes HD domain of RNase Y (RnaY) | 16.3079 | 0.0061 | AT | COG1418 | |
| Polysaccharides metabolism | Cellulase/cellobiase CelA1 (CelA1) | 27.5549 | 0.0001 | AT | COG5297 |
| O-antigen ligase (RfaL) | 24.1298 | 0.0004 | AT | COG3307 | |
| Phosphoglycerol transferase MdoB/OpgB, AlkP superfamily (MdoB) | 14.9693 | 0.0089 | AT | COG1368 | |
| Glycolysis | Phosphoenolpyruvate synthase/pyruvate phosphate dikinase (PpsA) | 18.8444 | 0.0024 | AT | COG0574 |
| Lysine metabolism | Saccharopine dehydrogenase, NADP-dependent (Lys9) | 18.8444 | 0.0024 | AT | COG1748 |
| Ion transporters | H+/Cl− antiporter ClcA (ClcA) | 18.8444 | 0.0024 | AT | COG0038 |
| Mg2+ and Co2+ transporter CorA (CorA) | 14.9693 | 0.0089 | AT | COG0598 | |
| Electron transfer chain | Flavodoxin (FldA) | 18.8444 | 0.0024 | AT | COG0716 |
| Flavodoxin/ferredoxin-NADP reductase (Fpr) | 18.8444 | 0.0024 | AT | COG1018 | |
| Fe-S cluster carrier ATPase, Mrp/ApbC/NBP35 family (Mrp) | 14.9693 | 0.0089 | AT | COG0489 | |
| Cell motility | Flagellar motor protein MotB (MotB) | 18.8444 | 0.0024 | other | COG1360 |
| Cell surface structure | Sialic acid synthase SpsE, contains C-terminal SAF domain (SpsE) | 14.9693 | 0.0089 | other | COG2089 |
| CDP-glycerol glycerophosphotransferase, TagB/SpsB family | 14.9693 | 0.0089 | other | COG1887 | |
| Murein tripeptide amidase MpaA (MpaA) | 17.1582 | 0.0054 | AT | COG2866 | |
| Thiol:disulfide interchange protein DsbD (DsbD) | 16.7683 | 0.0058 | AT | COG4232 | |
| Unknown | Uncharacterized conserved protein YchJ, contains N- and C-terminal SEC-C domains (YchJ) | 14.9693 | 0.0089 | AT | COG3012 |
| Uncharacterized membrane protein YccF, DUF307 family (YccF) | 14.9693 | 0.0089 | AT | COG3304 | |
| Predicted peptidase | 16.7683 | 0.0058 | AT | COG4099 | |
| Function Class | KOfam | Enrichment Score | Adjusted q Value | Enriched Groups | Accession |
| Polysaccharides metabolism | exopolysaccharide production protein ExoQ | 31.7525 | 0 | AT | K16567 |
| Lysine metabolism | saccharopine dehydrogenase (NAD+, L-lysine forming) | 31.7525 | 0 | AT | K00290 |
| Glycolysis | pyruvate, water dikinase | 21.2667 | 0.0012 | AT | K01007 |
| Solute transporter | solute: Na+ symporter, SSS family | 24.1298 | 0.0006 | other | K03307 |
| ethanolamine permease | 14.9693 | 0.0095 | AT | K16238 | |
| putative amide transporter protein | 18.8444 | 0.0029 | AT | K22112 | |
| Dimethylamine oxidation | dimethylamine monooxygenase subunit B | 21.2667 | 0.0012 | AT | K22343 |
| dimethylamine monooxygenase subunit C | 21.2667 | 0.0012 | AT | K22344 | |
| dimethylamine monooxygenase subunit A | 21.2667 | 0.0012 | AT | K22342 | |
| Cell surface structure | prokaryotic ubiquitin-like protein Pup | 20.925 | 0.0012 | other | K13570 |
| 3-deoxy-manno-octulosonate cytidylyltransferase (CMP-KDO synthetase) | 14.9693 | 0.0095 | other | K00979 | |
| N5-(carboxyethyl)ornithine synthase | 14.9693 | 0.0095 | AT | K00298 | |
| phosphoglycerol transferase | 14.9693 | 0.0095 | AT | K01002 | |
| Stress defense | glyoxylase I family protein | 18.8444 | 0.0029 | AT | K08234 |
| Rhamnose metabolism | rhamnulokinase | 16.7683 | 0.0073 | other | K00848 |
| Methionine biosynthesis | 5-methyltetrahydropteroyltriglutamate-homocysteinmethyltransferase | 16.7683 | 0.0073 | other | K00549 |
| Methanogenesis | formylmethanofuran dehydrogenase subunit E | 16.2576 | 0.0088 | AT | K11261 |
| Antibiotic resistance | fluoroquinolone resistance protein | 14.9693 | 0.0095 | AT | K18555 |
| Transcriptional regulators | MarR family transcriptional regulator, lower aerobic nicotinate degradation pathway regulator | 14.9693 | 0.0095 | AT | K22296 |
| Lipid metabolism | mitochondrial enoyl-[acyl-carrier protein] reductase/trans-2-enoyl-CoA reductase | 14.9693 | 0.0095 | AT | K07512 |
| 4′-phosphopantetheinyl transferase | 14.9693 | 0.0095 | AT | K06133 | |
| sterol 3beta-glucosyltransferase | 14.9693 | 0.0095 | AT | K05841 | |
| Unknown | SEC-C motif domain protein | 14.9693 | 0.0095 | AT | K09858 |
NES-AT isolates additionally contain the genes that encoded the NADP-dependent saccharopine dehydrogenase (COG1748, K00290). It can mediate the biosynthesis alpha-aminoadipate pathway of lysine, whose accumulation is a common strategy to block the negative effects of many stress conditions like high salinity [104]. Members of the NES-AT clade also harbor the phosphoenolpyruvate (PEP) synthase (COG0574, K01007) that is capable of catalyzing the PEP to pyruvate with the dependence of AMP and phosphate. As the essential enzyme in glycolysis of the modified Embden-Meyerhof pathway, its appearance would be helpful for energy flux stabilization in energy and ADP-limited environments [105]. More abundant carbon metabolism capacity in the NES-AT clade also reflected on the enrichment of the dimethylamine (DMA) monooxygenase gene cluster (dmmABC, K22342-K22344). This enzyme is required for bacterial growth using DMA, the oxidation product of trimethylamine oxide [106]. On the contrary, the abundance of the gene that encoded the SSS family solute: Na+ symporter (K03307) in other strains is significantly higher than in the NES-AT clade. The solutes carried include many nutrients, like carbohydrates, osmolytes, and cofactors [106]. The above results suggested that in the way of nutrient acquirement, non-polar isolates prefer to absorb from the environments, whereas NES-AT strains tend to broaden the metabolic capacity of alternative carbon substances.
4. Conclusions
In this study, four Nesterenkonia strains from the lakes on Tibetan Plateau were isolated and sequenced to identify their stress resistance mechanisms in comparison with all other 30 high-quality Nesterenkonia genomes deposited in the NCBI. The results showed that Tibetan isolates have a close evolutionary relationship with four Antarctic strains and form a subclade NES-AT. Genomes within this clade showed similar genomic properties with other psychrophilic bacteria, such as higher GC content and increased number of tRNA. The reduced use of carbon in the amino acid sequence of NES-AT members is consistent with the nutrient-limited conditions in polar regions. Similar patterns are also present in the results of functional genes enrichment. That is, Tibetan and Antarctic genomes contain more genes that are involved in diverse carbohydrate metabolism and biofilm formation, which can be helpful to stress defense. This study improved our knowledge about how Nesterenkonia strains from Tibetan and Antarctic regions changed their genomic properties and gene content towards adaptation of polar extreme conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10020233/s1, Figure S1: Phylogenetic tree based on 16S rRNA gene sequences of genus Nesterenkonia.
Author Contributions
Conceptualization, D.D. and Q.W.; methodology, D.D. and H.L.; software, D.D.; formal analysis, D.D.; investigation, D.D.; resources, Q.W.; data curation, D.D.; writing—original draft preparation, D.D.; writing—review and editing, D.D., P.X. and Q.W.; visualization, D.D.; supervision, Q.W.; project administration, Q.W.; funding acquisition, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31730013) and the Key Research Program of Frontier Science, Chinese Academy of Sciences (QYZDJ-SSW-DQC030).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The genomes of four isolates (sp. AY15, DZ6, LB17 and YGD6) in this study were submitted to the GenBank database under accession numbers JAJOYV000000000, JAJOYW000000000, JAJOYX000000000 and JAJOYY0000000001, respectively.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yao T., Thompson L.G., Mosbrugger V., Zhang F., Ma Y., Luo T., Xu B., Yang X., Joswiak D.R., Wang W., et al. Third Pole Environment (TPE) Environ. Dev. 2012;3:52–64. doi: 10.1016/j.envdev.2012.04.002. [DOI] [Google Scholar]
- 2.Williams T.J., Allen M.A., DeMaere M., Kyrpides N., Tringe S., Woyke T., Cavicchioli R. Microbial ecology of an Antarctic hypersaline lake: Genomic assessment of ecophysiology among dominant haloarchaea. ISME J. 2014;8:1645–1658. doi: 10.1038/ismej.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheridan P.O., Thames Consortium. Raguideau S., Quince C., Holden J., Zhang L., Williams T.A., Gubry-Rangin C. Gene duplication drives genome expansion in a major lineage of Thaumarchaeota. Nat. Commun. 2020;11:5494. doi: 10.1038/s41467-020-19132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen L., Liu Y., Allen M.A., Xu B., Wang N., Williams T.J., Wang F., Zhou Y., Liu Q., Cavicchioli R. Linking genomic and physiological characteristics of psychrophilic Arthrobacter to metagenomic data to explain global environmental distribution. Microbiome. 2021;9:136. doi: 10.1186/s40168-021-01084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teufel A.G., Li W., Kiss A.J., Morgan-Kiss R.M. Impact of nitrogen and phosphorus on phytoplankton production and bacterial community structure in two stratified Antarctic lakes: A bioassay approach. Polar Biol. 2017;40:1007–1022. doi: 10.1007/s00300-016-2025-8. [DOI] [Google Scholar]
- 6.Liu Y., Yao T., Zhu L., Jiao N., Liu X., Zeng Y., Jiang H. Bacterial Diversity of Freshwater Alpine Lake Puma Yumco on the Tibetan Plateau. Geomicrobiol. J. 2009;26:131–145. doi: 10.1080/01490450802660201. [DOI] [Google Scholar]
- 7.Møller A.K., Søborg D.A., Abu Al-Soud W., Sørensen S., Kroer N. Bacterial community structure in High-Arctic snow and freshwater as revealed by pyrosequencing of 16S rRNA genes and cultivation. Polar Res. 2013;32:17390. doi: 10.3402/polar.v32i0.17390. [DOI] [Google Scholar]
- 8.Liu K., Yao T., Pearce D.A., Jiao N., Zeng Y., Guo B., Liu Y. Bacteria in the lakes of the Tibetan Plateau and polar regions. Sci. Total. Environ. 2021;754:142248. doi: 10.1016/j.scitotenv.2020.142248. [DOI] [PubMed] [Google Scholar]
- 9.Liao L., Su S., Zhao B., Fan C., Zhang J., Li H., Chen B. Biosynthetic Potential of a Novel Antarctic Actinobacterium Marisediminicola antarctica ZS314T Revealed by Genomic Data Mining and Pigment Characterization. Mar. Drugs. 2019;17:388. doi: 10.3390/md17070388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva T.R.E., Silva L.C.F., de Queiroz A.C., Moreira M.S.A., Fraga C.A.D.C., de Menezes G.C.A., Rosa L.H., Bicas J., de Oliveira V.M., Duarte A.W.F. Pigments from Antarctic bacteria and their biotechnological applications. Crit. Rev. Biotechnol. 2021;41:809–826. doi: 10.1080/07388551.2021.1888068. [DOI] [PubMed] [Google Scholar]
- 11.Benaud N., Edwards R.J., Amos T.G., D’Agostino P.M., Gutiérrez-Chávez C., Montgomery K., Nicetic I., Ferrari B.C. Antarctic desert soil bacteria exhibit high novel natural product potential, evaluated through long-read genome sequencing and comparative genomics. Environ. Microbiol. 2021;23:3646–3664. doi: 10.1111/1462-2920.15300. [DOI] [PubMed] [Google Scholar]
- 12.Dsouza M., Taylor M.W., Turner S.J., Aislabie J. Genomic and phenotypic insights into the ecology of Arthrobacter from Antarctic soils. BMC Genom. 2015;16:36. doi: 10.1186/s12864-015-1220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W.J., Chen H.H., Zhang Y.Q., Schumann P., Stackebrandt E., Xu L.H., Jiang C.L. Nesterenkonia halotolerans sp. nov.; Nesterenkonia xinjiangensis sp. nov.; actinobacteria from saline soils in the west of China. Int. J. Syst. Evol. Microbiol. 2004;54:837–841. doi: 10.1099/ijs.0.02935-0. [DOI] [PubMed] [Google Scholar]
- 14.Delgado O., Quillaguaman J., Bakhtiar S., Mattiasson B., Gessesse A., Hatti-Kaul R. Nesterenkonia aethiopica sp. nov.; an alkaliphilic, moderate halophile isolated from an Ethiopian soda lake. Int. J. Syst. Evol. Microbiol. 2006;56:1229–1232. doi: 10.1099/ijs.0.63633-0. [DOI] [PubMed] [Google Scholar]
- 15.Finore I., Orlando P., Di Donato P., Leone L., Nicolaus B., Poli A. Nesterenkonia aurantiaca sp. nov.; an alkaliphilic actinobacterium isolated from Antarctica. Int. J. Syst. Evol. Microbiol. 2016;66:1554–1560. doi: 10.1099/ijsem.0.000917. [DOI] [PubMed] [Google Scholar]
- 16.Liu J.M., Tuo L., Habden X., Guo L., Jiang Z.K., Liu X.F., Chen L., Zhang Y.Q., Sun C.H. Nesterenkonia populi sp. nov.; an actinobacterium isolated from Populus euphratica. Pt 5Int. J. Syst. Evol. Microbiol. 2015;65:1474–1479. doi: 10.1099/ijs.0.000123. [DOI] [PubMed] [Google Scholar]
- 17.Chander A., Nair R.G., Kaur G., Kochhar R., Dhawan D.K., Bhadada S.K., Mayilraj S. Genome Insight and Comparative Pathogenomic Analysis of Nesterenkonia jeotgali Strain CD08_7 Isolated from Duodenal Mucosa of Celiac Disease Patient. Front. Microbiol. 2017;8:129. doi: 10.3389/fmicb.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edouard S., Sankar S., Dangui N.P.M., Lagier J.-C., Michelle C., Raoult D., Fournier P.-E. Genome sequence and description of Nesterenkonia massiliensis sp. nov. strain NP1T. Stand. Genom. Sci. 2014;9:866–882. doi: 10.4056/sigs.5631022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh P., Kapse N., Gowdaman V., Tsuji M., Singh S., Dhakephalkar P. Comparative Genomic Analysis of Arctic Permafrost Bacterium Nesterenkonia sp. PF2B19 to Gain Insights into Its Cold Adaptation Tactic and Diverse Biotechnological Potential. Sustainability. 2021;13:4590. doi: 10.3390/su13084590. [DOI] [Google Scholar]
- 20.Aliyu H., De Maayer P., Cowan D. The genome of the Antarctic polyextremophile Nesterenkonia sp. AN1 reveals adaptive strategies for survival under multiple stress conditions. FEMS Microbiol. Ecol. 2016;92:fiw032. doi: 10.1093/femsec/fiw032. [DOI] [PubMed] [Google Scholar]
- 21.Alonso-Reyes D.G., Galván F.S., Portero L.R., Alvarado N.N., Farías M.E., Vazquez M.P., Albarracín V.H. Genomic insights into an andean multiresistant soil actinobacterium of biotechnological interest. World J. Microbiol. Biotechnol. 2021;37:116. doi: 10.1007/s11274-021-03129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vernikos G., Medini D., Riley D.R., Tettelin H. Ten years of pan-genome analyses. Curr. Opin. Microbiol. 2015;23:148–154. doi: 10.1016/j.mib.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Sugawara M., Epstein B., Badgley B.D., Unno T., Xu L., Reese J., Gyaneshwar P., Denny R., Mudge J., Bharti A.K., et al. Comparative genomics of the core and accessory genomes of 48 Sinorhizobium strains comprising five genospecies. Genome Biol. 2013;14:1–20. doi: 10.1186/gb-2013-14-2-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong C., Wang L., Ning K. Pan-genome study of Thermococcales reveals extensive genetic diversity and genetic evidence of thermophilic adaption. Environ. Microbiol. 2021;23:3599–3613. doi: 10.1111/1462-2920.15234. [DOI] [PubMed] [Google Scholar]
- 25.Lefébure T., Stanhope M.J. Evolution of the core and pan-genome of Streptococcus: Positive selection, recombination, and genome composition. Genome Biol. 2007;8:1–17. doi: 10.1186/gb-2007-8-5-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Auria G., Hernández N.J., Peris-Bondia F., Moya A., Latorre A. Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genom. 2010;11:181. doi: 10.1186/1471-2164-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehgal S.N., Gibbons N.E. Effect of Some Metal Ions on the Growth of Halobacterium Cutirubrum. Can. J. Microbiol. 1960;6:165–169. doi: 10.1139/m60-018. [DOI] [PubMed] [Google Scholar]
- 28.Yoon S.-H., Ha S.-M., Kwon S., Lim J., Kim Y., Seo H., Chun J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Federation W.E., Association A. Standard Methods for the Examination of Water and Wastewater. American Public Health Association (APHA); Washington, DC, USA: 2005. [Google Scholar]
- 30.Lim H.J., Lee E., Yoon Y., Chua B., Son A. Portable lysis apparatus for rapid single-step DNA extraction of Bacillus subtilis. J. Appl. Microbiol. 2016;120:379–387. doi: 10.1111/jam.13011. [DOI] [PubMed] [Google Scholar]
- 31.Bushnell B., Rood J., Singer E. BBMerge–Accurate paired shotgun read merging via overlap. PLoS ONE. 2017;12:e0185056. doi: 10.1371/journal.pone.0185056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parks D.H., Imelfort M., Skennerton C.T., Hugenholtz P., Tyson G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen W., Le S., Li Y., Hu F. SeqKit: A Cross-Platform and Ultrafast Toolkit for FASTA/Q File Manipulation. PLoS ONE. 2016;11:e0163962. doi: 10.1371/journal.pone.0163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seemann T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 37.Chan P.P., Lin B.Y., Mak A.J., Lowe T.M. tRNAscan-SE 2.0: Improved detection and functional classification of transfer RNA genes. Nucleic Acids Res. 2021;49:9077–9096. doi: 10.1093/nar/gkab688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galperin M.Y., Wolf Y.I., Makarova K.S., Alvarez R.V., Landsman D., Koonin E.V. COG database update: Focus on microbial diversity, model organisms, and widespread pathogens. Nucleic Acids Res. 2021;49:D274–D281. doi: 10.1093/nar/gkaa1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eddy S.R. Genome Informatics 2009. Imperial College Press and Distributed by World Scientific Publishing Co.; London, UK: 2009. A new generation of homology search tools based on probabilistic inference; pp. 205–211. [PubMed] [Google Scholar]
- 41.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen L.-T., Schmidt H.A., Von Haeseler A., Minh B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emms D.M., Kelly S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015;16:157. doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain C., Rodriguez R.L.M., Phillippy A.M., Konstantinidis K.T., Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018;9:1–8. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez R.L.M., Konstantinidis K.T. The Enveomics Collection: A Toolbox for Specialized Analyses of Microbial Genomes and Metagenomes. PeerJ Preprints; London, UK: 2016. [Google Scholar]
- 47.R Core Team . R: A Language and Environment for Statistical Computing. R Core Team; Vienna, Austria: 2013. [Google Scholar]
- 48.Eren A.M., Kiefl E., Shaiber A., Veseli I., Miller S.E., Schechter M.S., Fink I., Pan J.N., Yousef M., Fogarty E.C. Community-led, integrated, reproducible multi-omics with anvi’o. Nat. Microbiol. 2021;6:3–6. doi: 10.1038/s41564-020-00834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enright A.J., van Dongen S., Ouzounis C.A. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30:1575–1584. doi: 10.1093/nar/30.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snipen L.-G., Liland K.H. Micropan: An R-package for microbial pan-genomics. BMC Bioinform. 2015;16:1–8. doi: 10.1186/s12859-015-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y., Jia X., Yang J., Ling Y., Zhang Z., Yu J., Wu J., Xiao J. PanGP: A tool for quickly analyzing bacterial pan-genome profile. Bioinformatics. 2014;30:1297–1299. doi: 10.1093/bioinformatics/btu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreno-Pino M., Ugalde J.A., Valdés J.H., Rodríguez-Marconi S., Parada-Pozo G., Trefault N. Bacteria Isolated from the Antarctic Sponge Iophon sp. Reveals Mechanisms of Symbiosis in Sporosarcina, Cellulophaga, and Nesterenkonia. Front. Microbiol. 2021;12:660779. doi: 10.3389/fmicb.2021.660779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu S., Tang Y., Du M., Kato T., Li Y., Cui X., Zhao X. Short-term variation of CO2 flux in relation to environmental controls in an alpine meadow on the Qinghai-Tibetan Plateau. J. Geophys. Res. Space Phys. 2003:108. doi: 10.1029/2003jd003584. [DOI] [Google Scholar]
- 54.Schloss I.R., Abele D., Moreau S., Demers S., Bers A., González O., Ferreyra G.A. Response of phytoplankton dynamics to 19-year (1991–2009) climate trends in Potter Cove (Antarctica) J. Mar. Syst. 2012;92:53–66. doi: 10.1016/j.jmarsys.2011.10.006. [DOI] [Google Scholar]
- 55.Lieth H. Primary Productivity of the Biosphere. Springer; Berlin/Heidelberg, Germany: 1975. Modeling the Primary Productivity of the World; pp. 237–263. [Google Scholar]
- 56.Crawford A.R. The Indus Suture Line, the Himalaya, Tibet and Gondwanaland. Geol. Mag. 1974;111:369–383. doi: 10.1017/S0016756800039935. [DOI] [Google Scholar]
- 57.Tettelin H., Riley D., Cattuto C., Medini D. Comparative genomics: The bacterial pan-genome. Curr. Opin. Microbiol. 2008;11:472–477. doi: 10.1016/j.mib.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Medini D., Donati C., Tettelin H., Masignani V., Rappuoli R. The microbial pan-genome. Curr. Opin. Genet. Dev. 2005;15:589–594. doi: 10.1016/j.gde.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Thomas C.M., Nielsen K.M. Mechanisms of, and Barriers to, Horizontal Gene Transfer between Bacteria. Nat. Rev. Microbiol. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 60.Mende D.R., Bryant J.A., Aylward F., Eppley J.M., Nielsen T., Karl D.M., Delong E.F. Environmental drivers of a microbial genomic transition zone in the ocean’s interior. Nat. Microbiol. 2017;2:1367–1373. doi: 10.1038/s41564-017-0008-3. [DOI] [PubMed] [Google Scholar]
- 61.Weissman J.L., Fagan W.F., Johnson P.L.F. Linking high GC content to the repair of double strand breaks in prokaryotic genomes. PLoS Genet. 2019;15:e1008493. doi: 10.1371/journal.pgen.1008493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slieman T.A., Nicholson W.L. Artificial and Solar UV Radiation Induces Strand Breaks and Cyclobutane Pyrimidine Dimers in Bacillus subtilis Spore DNA. Appl. Environ. Microbiol. 2000;66:199–205. doi: 10.1128/AEM.66.1.199-205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith R.C., Prézelin B.B., Baker K.S., Bidigare R.R., Boucher N.P., Coley T., Karentz D., MacIntyre S., Matlick H.A., Menzies D., et al. Ozone Depletion: Ultraviolet Radiation and Phytoplankton Biology in Antarctic Waters. Science. 1992;255:952–959. doi: 10.1126/science.1546292. [DOI] [PubMed] [Google Scholar]
- 64.Liu J., Liu J., Linderholm H.W., Chen D., Yu Q., Wu D., Haginoya S. Observation and calculation of the solar radiation on the Tibetan Plateau. Energy Convers. Manag. 2012;57:23–32. doi: 10.1016/j.enconman.2011.12.007. [DOI] [Google Scholar]
- 65.Su Z., Wilson B., Kumar P., Dutta A. Noncanonical Roles of tRNAs: tRNA Fragments and Beyond. Annu. Rev. Genet. 2020;54:47–69. doi: 10.1146/annurev-genet-022620-101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raina M., Ibba M. tRNAs as regulators of biological processes. Front. Genet. 2014;5:171. doi: 10.3389/fgene.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torrent M., Chalancon G., de Groot N.S., Wuster A., Babu M.M. Cells alter their tRNA abundance to selectively regulate protein synthesis during stress conditions. Sci. Signal. 2018;11:eaat6409. doi: 10.1126/scisignal.aat6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dutta A., Chaudhuri K. Analysis of tRNA composition and folding in psychrophilic, mesophilic and thermophilic genomes: Indications for thermal adaptation. FEMS Microbiol. Lett. 2010;305:100–108. doi: 10.1111/j.1574-6968.2010.01922.x. [DOI] [PubMed] [Google Scholar]
- 69.Kanaya S., Yamada Y., Kudo Y., Ikemura T. Studies of codon usage and tRNA genes of 18 unicellular organisms and quantification of Bacillus subtilis tRNAs: Gene expression level and species-specific diversity of codon usage based on multivariate analysis. Gene. 1999;238:143–155. doi: 10.1016/S0378-1119(99)00225-5. [DOI] [PubMed] [Google Scholar]
- 70.Satapathy S.S., Dutta M., Ray S.K. Higher tRNA diversity in thermophilic bacteria: A possible adaptation to growth at high temperature. Microbiol. Res. 2010;165:609–616. doi: 10.1016/j.micres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Médigue C., Krin E., Pascal G., Barbe V., Bernsel A., Bertin P.N., Cheung F., Cruveiller S., D’Amico S., Duilio A., et al. Coping with cold: The genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res. 2005;15:1325–1335. doi: 10.1101/gr.4126905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saunders N.F., Thomas T., Curmi P.M., Mattick J.S., Kuczek E., Slade R., Davis J., Franzmann P.D., Boone D., Rusterholtz K., et al. Mechanisms of Thermal Adaptation Revealed From the Genomes of the Antarctic Archaea Methanogenium frigidum and Methanococcoides burtonii. Genome Res. 2003;13:1580–1588. doi: 10.1101/gr.1180903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Getz E.W., Tithi S.S., Zhang L., Aylward F.O. Parallel Evolution of Genome Streamlining and Cellular Bioenergetics across the Marine Radiation of a Bacterial Phylum. MBio. 2018;9:e01089-18. doi: 10.1128/mBio.01089-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang X., Yu L., Yi Y., Wang J., Wang S., Meng C., Liu S., Hao Y., Zhang Y., Cao X., et al. Phylogenomic analysis reveals a two-stage process of the evolutionary transition of Shewanella from the upper ocean to the hadal zone. Environ. Microbiol. 2021;23:744–756. doi: 10.1111/1462-2920.15162. [DOI] [PubMed] [Google Scholar]
- 75.Yoshitake S., Uchida M., Koizumi H., Nakatsubo T. Carbon and nitrogen limitation of soil microbial respiration in a High Arctic successional glacier foreland near Ny-Ålesund, Svalbard. Polar Res. 2007;26:22–30. doi: 10.1111/j.1751-8369.2007.00001.x. [DOI] [Google Scholar]
- 76.Vero S., Garmendia G., Martínez-Silveira A., Cavello I., Wisniewski M. The Ecological Role of Micro-Organisms in the Antarctic Environment. Springer; Berlin/Heidelberg, Germany: 2019. Yeast activities involved in carbon and nitrogen cycles in Antarctica; pp. 45–64. [Google Scholar]
- 77.Hu Y., Wang Z., Wang Q., Wang S., Zhang Z., Zhang Z., Zhao Y. Climate change affects soil labile organic carbon fractions in a Tibetan alpine meadow. J. Soils Sediments. 2017;17:326–339. doi: 10.1007/s11368-016-1565-4. [DOI] [Google Scholar]
- 78.Grzymski J.J., Carter B.J., DeLong E.F., Feldman R.A., Ghadiri A., Murray A.E. Comparative Genomics of DNA Fragments from Six Antarctic Marine Planktonic Bacteria. Appl. Environ. Microbiol. 2006;72:1532–1541. doi: 10.1128/AEM.72.2.1532-1541.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ayala-Del-Río H.L., Chain P.S., Grzymski J.J., Ponder M.A., Ivanova N., Bergholz P.W., Di Bartolo G., Hauser L., Land M., Bakermans C., et al. The Genome Sequence of Psychrobacter arcticus 273-4, a Psychroactive Siberian Permafrost Bacterium, Reveals Mechanisms for Adaptation to Low-Temperature Growth. Appl. Environ. Microbiol. 2010;76:2304–2312. doi: 10.1128/AEM.02101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DasSarma S., Capes M.D., Karan R., DasSarma P. Amino Acid Substitutions in Cold-Adapted Proteins from Halorubrum lacusprofundi, an Extremely Halophilic Microbe from Antarctica. PLoS ONE. 2013;8:e58587. doi: 10.1371/journal.pone.0058587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ronholm J., Raymond-Bouchard I., Creskey M., Cyr T., Cloutis E.A., Whyte L.G. Characterizing the surface-exposed proteome of Planococcus halocryophilus during cryophilic growth. Extremophiles. 2015;19:619–629. doi: 10.1007/s00792-015-0743-4. [DOI] [PubMed] [Google Scholar]
- 82.Arpigny J., Lamotte J., Gerday C. Molecular adaptation to cold of an Antarctic bacterial lipase. J. Mol. Catal. B: Enzym. 1997;3:29–35. doi: 10.1016/S1381-1177(96)00041-0. [DOI] [Google Scholar]
- 83.Zhao J.-S., Deng Y., Manno D., Hawari J. Shewanella spp. Genomic Evolution for a Cold Marine Lifestyle and In-Situ Explosive Biodegradation. PLoS ONE. 2010;5:e9109. doi: 10.1371/journal.pone.0009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raymond-Bouchard I., Goordial J., Zolotarov Y., Ronholm J., Stromvik M., Bakermans C., Whyte L.G. Conserved genomic and amino acid traits of cold adaptation in subzero-growing Arctic permafrost bacteria. FEMS Microbiol. Ecol. 2018;94:fiy023. doi: 10.1093/femsec/fiy023. [DOI] [PubMed] [Google Scholar]
- 85.Bölter M. Effects of carbohydrates and leucine on growth of bacteria from Antarctic soils (Casey Station, Wilkes Land) Polar Biol. 1993;13:297–306. doi: 10.1007/BF00238356. [DOI] [Google Scholar]
- 86.Moura A., Savageau M.A., Alves R. Relative Amino Acid Composition Signatures of Organisms and Environments. PLoS ONE. 2013;8:e77319. doi: 10.1371/journal.pone.0077319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dutton R.J., Boyd D., Berkmen M., Beckwith J. Bacterial species exhibit diversity in their mechanisms and capacity for protein disulfide bond formation. Proc. Natl. Acad. Sci. USA. 2008;105:11933–11938. doi: 10.1073/pnas.0804621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gromiha M.M., Suwa M. Structural analysis of residues involving cation-π interactions in different folding types of membrane proteins. Int. J. Biol. Macromol. 2005;35:55–62. doi: 10.1016/j.ijbiomac.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Oren A., Gunde-Cimerman N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007;269:1–10. doi: 10.1111/j.1574-6968.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- 90.Metpally R.P.R., Reddy B.V.B. Comparative proteome analysis of psychrophilic versus mesophilic bacterial species: Insights into the molecular basis of cold adaptation of proteins. BMC Genom. 2009;10:11. doi: 10.1186/1471-2164-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mocali S., Chiellini C., Fabiani A., Decuzzi S., De Pascale D., Parrilli E., Tutino M.L., Perrin E., Bosi E., Fondi M., et al. Ecology of cold environments: New insights of bacterial metabolic adaptation through an integrated genomic-phenomic approach. Sci. Rep. 2017;7:839. doi: 10.1038/s41598-017-00876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goordial J., Raymond-Bouchard I., Zolotarov Y., de Bethencourt L., Ronholm J., Shapiro N., Woyke T., Stromvik M., Greer C.W., Bakermans C., et al. Cold adaptive traits revealed by comparative genomic analysis of the eurypsychrophile Rhodococcus sp. JG3 isolated from high elevation McMurdo Dry Valley permafrost, Antarctica. FEMS Microbiol. Ecol. 2016;92:fiv154. doi: 10.1093/femsec/fiv154. [DOI] [PubMed] [Google Scholar]
- 93.Reyes-Lamothe R., Sherratt D.J. The bacterial cell cycle, chromosome inheritance and cell growth. Nat. Rev. Genet. 2019;17:467–478. doi: 10.1038/s41579-019-0212-7. [DOI] [PubMed] [Google Scholar]
- 94.Lauro F.M., McDougald D., Thomas T., Williams T.J., Egan S., Rice S., DeMaere M., Ting L., Ertan H., Johnson J., et al. The genomic basis of trophic strategy in marine bacteria. Proc. Natl. Acad. Sci. USA. 2009;106:15527–15533. doi: 10.1073/pnas.0903507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma A.K., Zhaxybayeva O., Papke R.T., Doolittle W.F. Actinorhodopsins: Proteorhodopsin-like gene sequences found predominantly in non-marine environments. Environ. Microbiol. 2008;10:1039–1056. doi: 10.1111/j.1462-2920.2007.01525.x. [DOI] [PubMed] [Google Scholar]
- 96.Delong E.F., Béjà O. The Light-Driven Proton Pump Proteorhodopsin Enhances Bacterial Survival during Tough Times. PLoS Biol. 2010;8:e1000359. doi: 10.1371/journal.pbio.1000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin Y.-H., Xu J.-L., Hu J., Wang L.-H., Ong S.L., Leadbetter J., Zhang L.-H. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol. 2003;47:849–860. doi: 10.1046/j.1365-2958.2003.03351.x. [DOI] [PubMed] [Google Scholar]
- 98.Chambers J.R., Liao J., Schurr M.J., Sauer K. BrlR from P seudomonas aeruginosa is ac-di-GMP-responsive transcription factor. Mol. Microbiol. 2014;92:471–487. doi: 10.1111/mmi.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Medie F.M., Davies G., Drancourt M., Henrissat B. Genome analyses highlight the different biological roles of cellulases. Nat. Rev. Genet. 2012;10:227–234. doi: 10.1038/nrmicro2729. [DOI] [PubMed] [Google Scholar]
- 100.Ramasamy K.P., Telatin A., Mozzicafreddo M., Miceli C., Pucciarelli S. Draft Genome Sequence of a New Pseudomonas sp. Strain, ef1, Associated with the Psychrophilic Antarctic Ciliate Euplotes focardii. Microbiol. Resour. Announc. 2019;8:e00867-19. doi: 10.1128/MRA.00867-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel K.B., Toh E., Fernandez X.B., Hanuszkiewicz A., Hardy G.G., Brun Y.V., Bernards M.A., Valvano M.A. Functional Characterization of UDP-Glucose:Undecaprenyl-Phosphate Glucose-1-Phosphate Transferases of Escherichia coli and Caulobacter crescentus. J. Bacteriol. 2012;194:2646–2657. doi: 10.1128/JB.06052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Becker A. Challenges and perspectives in combinatorial assembly of novel exopolysaccharide biosynthesis pathways. Front. Microbiol. 2015;6:687. doi: 10.3389/fmicb.2015.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.John M.S., Nagoth J.A., Ramasamy K.P., Ballarini P., Mozzicafreddo M., Mancini A., Telatin A., Liò P., Giuli G., Natalello A., et al. Horizontal gene transfer and silver nanoparticles production in a new Marinomonas strain isolated from the Antarctic psychrophilic ciliate Euplotes focardii. Sci. Rep. 2020;10:10218. doi: 10.1038/s41598-020-66878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rönsch H., Krämer R., Morbach S. Impact of osmotic stress on volume regulation, cytoplasmic solute composition and lysine production in Corynebacterium glutamicum MH20-22B. J. Biotechnol. 2003;104:87–97. doi: 10.1016/S0168-1656(03)00166-4. [DOI] [PubMed] [Google Scholar]
- 105.Imanaka H., Yamatsu A., Fukui T., Atomi H., Imanaka T. Phosphoenolpyruvate synthase plays an essential role for glycolysis in the modified Embden-Meyerhof pathway in Thermococcus kodakarensis. Mol. Microbiol. 2006;61:898–909. doi: 10.1111/j.1365-2958.2006.05287.x. [DOI] [PubMed] [Google Scholar]
- 106.Lidbury I., Mausz M.A., Scanlan D., Chen Y. Identification of dimethylamine monooxygenase in marine bacteria reveals a metabolic bottleneck in the methylated amine degradation pathway. ISME J. 2017;11:1592–1601. doi: 10.1038/ismej.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genomes of four isolates (sp. AY15, DZ6, LB17 and YGD6) in this study were submitted to the GenBank database under accession numbers JAJOYV000000000, JAJOYW000000000, JAJOYX000000000 and JAJOYY0000000001, respectively.