Microporous crystalline materials, such as zeolites and metal–organic frameworks (MOFs), possess well-defined cavities and channels of molecular dimensions. While these micropores offer unique molecular sieving properties, their small size (usually <1.5 nm) constrains molecular diffusion within crystals, thereby limiting their efficiency in separation and catalytic applications. Introducing mesopores (2–50 nm) or macropores (>50 nm) in microporous crystals would facilitate intracrystalline molecular diffusion, but there is a lack of effective methods to achieve this goal for MOF crystals. In this issue of ACS Central Science, Gu and coauthors report a novel metal–organic framework solid solution (MOSS) strategy that enables the homogeneous mixing of microporous MOF NU-901 and mesoporous MOF NU-1000, with tunable ratios, in single nanocrystals.1 When used as gas chromatography stationary phases for separation of various isomers, the MOSS nanocrystals exhibit unique elution sequences and significantly enhanced separation resolution compared to single-component MOF nanocrystals.1
The preparation of hierarchical zeolites to combine the advantages of micropores (size and shape selectivity) and mesopores/macropores (fast molecular diffusion) in one material has long been an important area of research. Among various synthetic methods, the most commonly used is the “templating” method, in which organic macromolecules (e.g., surfactants and polymers) are embedded in zeolite crystals during synthesis and subsequently removed by calcination to produce mesoporosity.2,3
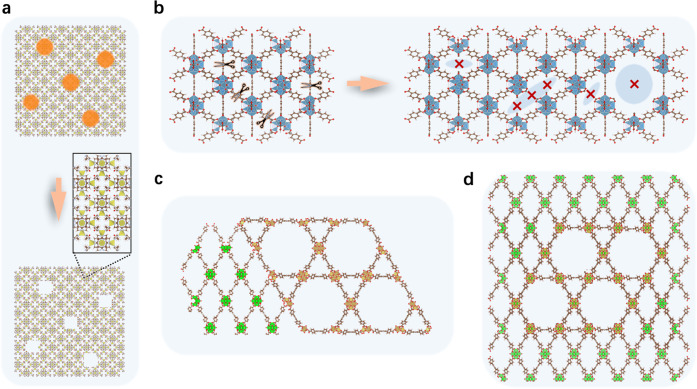
In recent years, efforts have also been made to prepare hierarchical MOFs by creating additional secondary porosity in MOF crystals. Unlike inorganic zeolites, MOFs are organic–inorganic hybrid materials whose structures cannot withstand the calcination required to remove organic templates. Therefore, new synthetic strategies have been developed to generate secondary porosity in MOFs.4−9 For example, macropores were created in ZIF-8 single crystals by using a modified “templating” method, in which polystyrene microspheres acted as templates that could be removed by solvent dissolution rather than calcination (Figure 1a). The obtained hierarchically structured ZIF-8 exhibited superior catalytic activity compared to the conventional ZIF-8.5 Secondary porosity can also be generated by the partial removal of organic linkers or metal clusters from the framework (Figure 1b). It has been demonstrated that the use of excess modulators during synthesis can lead to the formation of missing-linker and missing-cluster defects, locally producing larger pores;6 alternatively, postsynthesis treatments (e.g., hydrolysis, thermolysis, ozonolysis, and laser photolysis) have also been used to generate mesopores in MOFs by partially disrupting their structures.7−9
Figure 1.
Schematic illustrations of various methods for generating secondary porosity in MOFs: (a) the “templating” method, (b) the creation of structural defects, (c) the construction of a heterostructure, and (d) the metal–organic framework solid solution (MOSS) strategy.
Although these methods can efficiently generate secondary porosity in MOFs, they cannot control the size, density, and distribution of secondary pores with molecular precision. In most cases, the secondary pores produced are irregular and disordered. Constructing heterostructures of microporous and mesoporous MOFs by epitaxial growth is another strategy to integrate different types of pores in one material (Figure 1c).10 However, in this configuration, the two types of pores are separated rather than mixed, and thus, molecular diffusion in the microporous MOF remains constrained.
Here, Gu and coauthors prepared a series of MOSS materials by homogeneously mixing NU-901 (channel diameter: ∼1.2 nm) and NU-1000 (primary channel diameter: ∼2.8 nm) in single nanocrystals. In comparison with previous hierarchical MOFs (Figure 1a–c), these MOSS materials are characterized by uniformly distributed, well-defined mesopores arising from a crystalline MOF structure (Figure 1d). The presence of mesoporous NU-1000 divides the microporous NU-901 crystal into small domains, thereby reducing diffusion lengths and improving molecular transport. This unique two-in-one MOF structure is obtained by using mixed ligands of [1,1′,3′,1″-terphenyl]-4,4″-dicarboxylic acid (LB) and 1,3,6,8-tetrakis (p-benzoic acid) pyrene (H4TBApy), in which the ratio of mesopores to micropores can be modulated by tuning the ratio of LB to H4TBApy.
The synthesized MOSS materials were carefully characterized using powder X-ray diffraction, N2 adsorption, and electron microscopy techniques. The results collectively demonstrated the coexistence of NU-901 and NU-1000 structures with tunable ratios. Impressively, the intergrowth of the two MOF structures and their distributions in single nanocrystals were imaged by scanning transmission electron microscopy at a nearly atomic resolution, which provided the most direct evidence for the proposed model.1
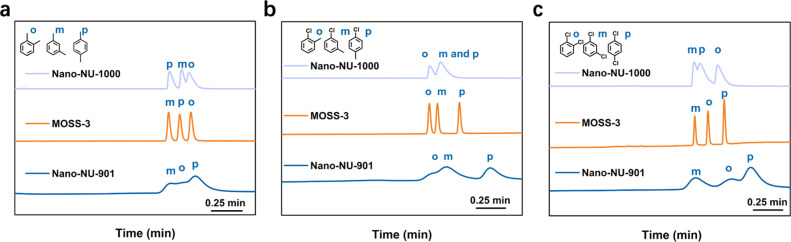
In the test of separating disubstituted benzene and alkane isomers, the MOSS materials exhibited significantly higher separation resolution than either NU-901 or NU-1000 alone, and MOSS-3 with a 1:1 volume ratio of mesopore to micropore provided the best performance. Interestingly, in addition to higher separation resolution, MOSS-3 also showed different elution sequences compared to NU-901 and NU-1000 (Figure 2). Combining experimental observations and Grand Canonical Monte Carlo and molecular dynamics simulations, the authors attribute the excellent separation performance of MOSS-3 to its optimal mesopore-to-micropore ratio that balances the kinetic diffusion barrier and thermodynamic interactions.
Figure 2.
Gas chromatograms of (a) xylene, (b) chlorotoluene, and (c) dichlorobenzene isomers using MOSS-3-, nano-NU-901-, and nano-NU-1000-coated GC columns. Reproduced from ref (1). Copyright 2022 The Authors. Published by American Chemical Society.
Further research can be carried out on the basis of this interesting work. Given the availability of different mesoporous MOFs, it will be interesting to explore whether the MOSS strategy can be generally applied to MOF combinations other than NU-901/NU-1000. Moreover, efforts are needed to determine more precisely the domain sizes and distribution of the two MOF structures in MOSS. Additionally, atomic-resolution imaging to clearly identify metal clusters and organic linkers should be employed to fully comprehend the interfacial structure between the two MOFs. The insights gained will guide the design of other hierarchical MOF structures.
References
- Xu M.; Meng S.-S.; Cai P.; Gu Y.-H.; Yan T.-A.; Yan T.-H.; Gu L.; Liu D.-H.; Zhou H.-C.; Gu Z.-Y.. Homogeneously Mixing Different Metal-Organic Framework Structures in Single Nanocrystals through Forming Solid Solutions. ACS Central Science 2022, in press. 10.1021/acscentsci.1c01344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.; Na K.; Kim J.; Sakamoto Y.; Terasaki O.; Ryoo R. Stable Single-Unit-Cell Nanosheets of Zeolite MFI as Active and Long-Lived Catalysts. Nature 2009, 461 (7261), 246–249. 10.1038/nature08288. [DOI] [PubMed] [Google Scholar]
- Tian Q.; Liu Z.; Zhu Y.; Dong X.; Saih Y.; Basset J.-M.; Sun M.; Xu W.; Zhu L.; Zhang D.; Huang J.; Meng X.; Xiao F.-S.; Han Y. Beyond Creation of Mesoporosity: The Advantages of Polymer-Based Dual-Function Templates for Fabricating Hierarchical Zeolites. Adv. Funct. Mater. 2016, 26 (12), 1881–1891. 10.1002/adfm.201504888. [DOI] [Google Scholar]
- Qiu L.-G.; Xu T.; Li Z.-Q.; Wang W.; Wu Y.; Jiang X.; Tian X.-Y.; Zhang L.-D. Hierarchically Micro- and Mesoporous Metal-Organic Frameworks with Tunable Porosity. Angew. Chem., Int. Ed. 2008, 47 (49), 9487–9491. 10.1002/anie.200803640. [DOI] [PubMed] [Google Scholar]
- Shen K.; Zhang L.; Chen X.; Liu L.; Zhang D.; Han Y.; Chen J.; Long J.; Luque R.; Li Y.; Chen B. Ordered Macro-Microporous Metal-Organic Framework Single Crystals. Science 2018, 359 (6372), 206–210. 10.1126/science.aao3403. [DOI] [PubMed] [Google Scholar]
- Liu L.; Chen Z.; Wang J.; Zhang D.; Zhu Y.; Ling S.; Huang K.-W.; Belmabkhout Y.; Adil K.; Zhang Y.; Slater B.; Eddaoudi M.; Han Y. Imaging Defects and Their Evolution in a Metal–Organic Framework at Sub-Unit-Cell Resolution. Nat. Chem. 2019, 11 (7), 622–628. 10.1038/s41557-019-0263-4. [DOI] [PubMed] [Google Scholar]
- Feng L.; Day G. S.; Wang K.-Y.; Yuan S.; Zhou H.-C. Strategies for Pore Engineering in Zirconium Metal-Organic Frameworks. Chem. 2020, 6 (11), 2902–2923. 10.1016/j.chempr.2020.09.010. [DOI] [Google Scholar]
- Zhang L.; Yuan S.; Feng L.; Guo B.; Qin J.; Xu B.; Lollar C.; Sun D.; Zhou H. Pore-Environment Engineering with Multiple Metal Sites in Rare-Earth Porphyrinic Metal–Organic Frameworks. Angew. Chem., Int. Ed. 2018, 57 (18), 5095–5099. 10.1002/anie.201802661. [DOI] [PubMed] [Google Scholar]
- Wang K.; Feng L.; Yan T.; Wu S.; Joseph E. A.; Zhou H. Rapid Generation of Hierarchically Porous Metal–Organic Frameworks through Laser Photolysis. Angew. Chem., Int. Ed. 2020, 59 (28), 11349–11354. 10.1002/anie.202003636. [DOI] [PubMed] [Google Scholar]
- Zhao M.; Chen J.; Chen B.; Zhang X.; Shi Z.; Liu Z.; Ma Q.; Peng Y.; Tan C.; Wu X.-J.; Zhang H. Selective Epitaxial Growth of Oriented Hierarchical Metal–Organic Framework Heterostructures. J. Am. Chem. Soc. 2020, 142 (19), 8953–8961. 10.1021/jacs.0c02489. [DOI] [PubMed] [Google Scholar]