Abstract
Background
Access to various kidney replacement therapy (KRT) modalities for patients with end-stage kidney disease differs substantially within Europe.
Methods
European adults on KRT filled out an online or paper-based survey about factors influencing and experiences with modality choice (e.g. information provision, decision-making and reasons for choice) between November 2017 and January 2019. We compared countries with low, middle and high gross domestic product (GDP).
Results
In total, 7820 patients [mean age 59 years, 56% male, 63% on centre haemodialysis (CHD)] from 38 countries participated. Twenty-five percent had received no information on the different modalities, and only 23% received information >12 months before KRT initiation. Patients were not informed about home haemodialysis (HHD) (42%) and comprehensive conservative management (33%). Besides nephrologists, nurses more frequently provided information in high-GDP countries, whereas physicians other than nephrologists did so in low-GDP countries. Patients from low-GDP countries reported later information provision, less information about other modalities than CHD and lower satisfaction with information. The majority of modality decisions were made involving both patient and nephrologist. Patients reported subjective (e.g. quality of life and fears) and objective reasons (e.g. costs and availability of treatments) for modality choice. Patients had good experiences with all modalities, but experiences were better for HHD and kidney transplantation and in middle- and high-GDP countries.
Conclusion
Our results suggest European differences in patient-reported factors influencing KRT modality choice, possibly caused by disparities in availability of KRT modalities, different healthcare systems and varying patient preferences. Availability of home dialysis and kidney transplantation should be optimized.
Keywords: chronic haemodialysis, dialysis, ESRD, kidney transplantation, peritoneal dialysis
KEY LEARNING POINTS
What is already known about this subject?
access to the various treatments for patients with end-stage kidney disease (ESKD) differs substantially in Europe, suggesting that patients may not receive the most preferable treatments with respect to clinical outcomes and quality of life;
patients’ opinions on and experiences with kidney replacement therapy (KRT) modality choice are essential to improve access to preferable treatments for individuals with ESKD; and
so far, most studies on patients’ opinions about factors influencing modality choice have been performed in higher income countries. Moreover, patients were rarely questioned about objective reasons [e.g. acute start or (un)availability of treatments] for modality choice.
What this study adds?
according to patients, timing and content of information provision about KRT modalities and comprehensive conservative management differed across Europe and seemed to be less optimal in low-gross domestic product (GDP) countries. Usually, both patient and nephrologist were involved in treatment modality choice;
patients reported objective and subjective reasons for modality choice. Some reasons were more often selected by patients from middle- and high-GDP countries (e.g. work or study, social life) and others by patients from low-GDP countries (e.g. out of pocket costs, limited availability of treatments other than centre haemodialysis); and
most patients were satisfied with information provision, decision-making and the treatment they received, but patients from low-GDP countries tended to be less satisfied.
What impact this may have on practice or policy?
the substantial variation in patient-reported factors influencing modality choice is partially caused by limited availability of home dialysis and kidney transplantation in several countries. The availability of these treatments should be optimized, accompanied by education, policy measures and funding; and
patient preferences for treatment, which may be influenced by information provision and method of decision-making, might differ between countries. Discussing the preferences with individual patients may help nephrologists to personalize the process of KRT modality choice.
INTRODUCTION
Access to the various treatments for patients with end-stage kidney disease (ESKD) differs substantially in Europe [1–3]. Of the available kidney replacement therapy (KRT) modalities, kidney transplantation (KTx) offers the best survival and quality of life against the lowest costs [4–7]. However, since the demand for KTx exceeds supply and not all patients are suitable to receive a KTx, many patients depend on dialysis treatment. Home-based dialysis treatments [home haemodialysis (HHD) and peritoneal dialysis (PD)] may offer a better quality of life and seem to be associated with similar clinical outcomes as centre haemodialysis (CHD) [6, 8–10]. Nevertheless, the majority of European dialysis patients are treated with CHD [1]. It therefore seems that the most preferable KRT modalities may not be accessible for all patients.
Patients’ opinion on and experiences with KRT modality choice are essential to improve access to preferable treatments for individuals with ESKD. Several studies have questioned patients with ESKD on factors influencing modality choice, such as information provision [11–14] and involvement in decision-making [11, 14–16]. In other studies, patients were surveyed on reasons for their modality choice, e.g. personal values [15], treatment features [14, 17, 18] and consequences for daily life [14, 17, 18]. In addition, researchers asked patients about their satisfaction with information provision [11, 12, 16] and decision-making [16] or experience with treatments [12].
However, most of these studies [12–18] were performed in single, higher-income countries (e.g. Australia and the USA), whereas macro-economic factors, such as gross domestic product (GDP), may substantially influence treatment availability [3, 19, 20], which in turn may affect patients’ choice. Moreover, previous studies mainly included subjective reasons (e.g. personal preferences or fears), whereas patients were rarely questioned about objective reasons (e.g. acute start or availability of treatments) influencing modality choice.
We therefore surveyed a large number of European adults with ESKD on dialysis or living with a functioning KTx on factors influencing their KRT modality choice (i.e. information provision, decision-making and general and treatment-specific reasons), as well as on their experience with treatments. We compared opinions and experiences from patients living in lower, middle and higher income countries in Europe.
MATERIALS AND METHODS
Development and translation
We designed the Effect of Differing Kidney Disease Treatment Modalities and Organ Donation and Transplantation Practices on Health Expenditure and Patient Outcomes (EDITH) kidney patient survey in English using existing literature [11–18] and input from a kidney patients’ advocate. Seven patients and two nephrologists in training provided feedback on draft versions, and we modified the survey accordingly. The survey was part of the EDITH project [21].
For each language, two native speakers with a medical background (e.g. medical doctor, nurse or medical student) voluntarily translated the survey into their native language. Translators were asked to translate as literally as possible, but were encouraged to use language-specific and patient-friendly terms. The final survey was available online in LimeSurvey [22] in 29 languages and on paper in 31 languages. The English version of the survey, detailed methods using the CHERRIES (Checklist for Reporting Results of Internet E-Surveys) checklist [23] and an overview of all available languages are available as Supplementary material.
Ethical aspects
The Medical Ethics Committee of the Amsterdam University Medical Centers—location Academic Medical Center judged that a comprehensive evaluation was not required since this study was not subject to the Dutch Medical Research Involving Human Subjects Act (W17 291#17.343). If deemed necessary by local hospitals, nephrologists obtained approval from their local ethics committee. Participation in this survey was voluntary.
Data collection
Adults with ESKD treated by any form of dialysis or KTx and living in Europe were eligible to participate in this survey from November 2017 to January 2019. The survey was distributed and promoted by local and national kidney patient associations, the European Kidney Patients’ Federation (EKPF), the European Renal Association – European Dialysis and Transplantation Association (ERA-EDTA), national societies of nephrology and by individual nephrologists and their colleagues.
Data processing and statistical analysis
In the analyses, we included respondents from European countries, who had started KRT above the age of 18 years and reported to have received at least one KRT modality. Data from paper surveys were entered manually into LimeSurvey and were subject to the same value limits and logic as responses from online surveys. Duplicate responses on paper surveys were detected with IBM SPSS Statistics 26.0 [24] ‘Duplicate’ function using combinations of variables. Potential duplicates were manually checked and removed if necessary.
Results are presented for all respondents together and by countries’ income status. We categorized countries into three income groups by using tertiles based on GDP Power Purchasing Parity (further indicated as GDP) 2016 data from the World Bank [25] (Supplementary data, Table S1). Note that we did not use the World Bank income classification itself [26] as this would result in three groups of very unequal size. We compared sex, age and GDP distribution of our sample with those of prevalent patients in the ERA-EDTA Registry in 2017. Data of individual countries cannot be shared publicly due to the privacy of individuals that participated in the study. We tested for differences between the GDP tertiles using Chi-square tests for categorical variables and Kruskall–Wallis tests for continuous variables. P-values <0.01 were considered statistically significant. Statistical analyses were performed with IBM SPSS Statistics 26.0 [24] and SAS software 9.4 [27].
RESULTS
In total, 7820 of 12 014 respondents from 38 European countries were included in the analysis (Table 1; Supplementary data, Figures S1 and S2, Table S1). Respondents were excluded because of missing information on country (n = 3311), treatment (n = 134), KRT initiation before 18 years (n = 386), duplicates (n = 186) or because respondents were not living in Europe (n = 177). Of the respondents with missing information on country, only 27% completed >5 questions and 10% completed >10 questions, which did not allow comparison of their characteristics with those who responded appropriately.
Table 1.
Demographic and disease-related characteristics of respondents per GDP tertile
| All respondents | Lower GDP tertilea | Middle GDP tertilea | Higher GDP tertilea | P-valueb | |
|---|---|---|---|---|---|
| n = 7820 | n = 3399 | n = 2060 | n = 2361 | ||
| Male sex (%) | 55.7 | 53.8 | 56.7 | 57.7 | 0.009 |
| Age, mean (SD), years | 59.2 (14.0) | 58.7 (14.3) | 58.0 (13.6) | 60.9 (13.8) | <0.001 |
| Marital status, % married | 60.7 | 62.1 | 58.2 | 60.8 | 0.018 |
| Educational level, % with vocational or higher education | 59.2 | 52.7 | 57.2 | 70.0 | <0.001 |
| Employed and working, % of patients ≤65 years | 36.5 | 24.3 | 41.5 | 46.6 | <0.001 |
| Duration of KRT, mean (SD), years | 8.9 (8.2) | 7.4 (6.4) | 10.1 (9.2) | 9.9 (9.2) | <0.001 |
| Current treatment, % | <0.001 | ||||
| CHD | 62.5 | 82.9 | 55.3 | 39.5 | |
| HHD | 2.2 | 0.5 | 0.9 | 5.9 | |
| PD | 6.0 | 2.2 | 7.9 | 9.8 | |
| LTX | 8.7 | 4.6 | 6.5 | 16.4 | |
| DTX | 20.6 | 9.8 | 29.5 | 28.3 | |
| First treatment (%) | <0.001 | ||||
| CHD | 77.6 | 90.5 | 75.0 | 61.7 | |
| HHD | 1.0 | 0.3 | 1.2 | 1.7 | |
| PD | 16.1 | 6.7 | 18.5 | 27.5 | |
| LTX | 3.4 | 1.6 | 2.6 | 6.5 | |
| DTX | 1.9 | 0.9 | 2.7 | 2.7 | |
| On the kidney transplant waitlist, % of current dialysis patients | 22.0 | 13.7 | 34.9 | 27.4 | <0.001 |
| Reason for not being on the kidney transplant waitlist, % | <0.001 | ||||
| Medical reasons | 32.0 | 30.0 | 35.3 | 34.7 | |
| Will receive kidney from living donor | 2.9 | 2.2 | 4.0 | 3.9 | |
| Will be on the waiting list later on | 17.6 | 13.5 | 23.0 | 24.1 | |
| Do not want a kidney transplant | 21.4 | 21.6 | 19.5 | 22.5 | |
| Cannot afford a kidney transplant | 4.9 | 6.8 | 4.0 | 0.5 | |
| My hospital does not offer kidney transplantation | 5.9 | 7.3 | 2.4 | 5.4 | |
| Reason unknown | 15.2 | 18.7 | 11.7 | 8.8 | |
| Self-reported diseases, % | |||||
| Diabetes mellitus | 27.8 | 32.7 | 23.2 | 25.7 | <0.001 |
| Polycystic kidney disease | 26.8 | 29.7 | 26.9 | 23.2 | <0.001 |
| Glomerulonephritis | 25.0 | 37.9 | 21.1 | 11.8 | <0.001 |
| Malignancy | 5.9 | 4.8 | 6.7 | 6.5 | 0.033 |
| Received help with filling out survey, % | 23.8 | 36.5 | 18.7 | 10.0 | <0.001 |
For the categorization of countries per GDP category, see Supplementary data, Figure S2.
P-values calculated with Chi-square tests and Kruskall–Wallis tests to compare GDP tertiles on categorical and continuous outcomes.
LTX, living donor kidney transplantation; DTX, deceased donor kidney transplantation.
Demographic and disease-related characteristics
The mean age of respondents was 59.2 years, and 55.7% were male (Table 1). About 43.5% of respondents lived in a lower GDP tertile country (low-GDP), 26.3% in a middle GDP tertile country (middle-GDP) and 30.2% in a higher GDP tertile country (high-GDP) (Supplementary data, Figure S2). Sex and age distribution matched fairly with those of prevalent patients from the same countries in the ERA-EDTA Registry, but patients from middle-GDP countries were underrepresented (Supplementary data, Table S2).
The majority of respondents were married and higher educated, and approximately one-third of the respondents ≤65 years of age were employed. At the time of survey completion, the mean duration of KRT was 8.9 years and two-thirds of the respondents received CHD, 2.2% HHD and 6.0% PD, whereas 29.3% lived with a KTx. Twenty-two percent of the dialysis patients were on the waiting list for KTx, and medical reasons were the most frequently reported reason for not being on the waiting list (32.0%). One-quarter of respondents reported to suffer from diabetes mellitus (27.8%), whereas only 5.9% declared having a malignancy. Almost one-quarter of respondents received help with filling out the survey.
Respondents from high-GDP countries were slightly older, more often men, higher educated and more often employed compared with respondents from middle- and low-GDP countries (P < 0.01). Most respondents from low-GDP countries received CHD, whereas the proportion of respondents receiving PD or HHD or living with a KTx was higher in middle- and/or high-GDP countries (P < 0.001). The longest mean duration of KRT and highest percentage of dialysis patients on the KTx waiting list were found in middle-GDP countries, followed by high-GDP and low-GDP countries (P < 0.001). Self-reported prevalence of diabetes mellitus was higher in low-GDP countries (P < 0.001). The proportion of respondents receiving help with filling out the survey decreased with increasing GDP (P < 0.001).
Information provision
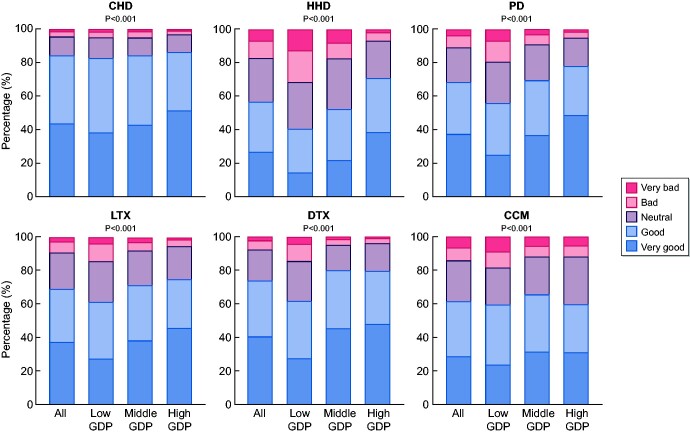
About a quarter of the respondents received information on KRT modalities >12 months before KRT initiation, whereas another quarter reported not to have received any information before KRT initiation (Table 2). Virtually all respondents received information about CHD, but a substantial proportion was not informed about HHD (42.1%) and comprehensive conservative management (33.0%). The most common sources of information were nephrologists (92.1%), nurses (38.1%) and brochures/booklets (26.7%). Just over half of the respondents who reported to have received information were satisfied or very satisfied with the information provided about all modalities. Respondents were most satisfied with the information on CHD and deceased donor KTx and were least satisfied with information on HHD (Figure 1).
Table 2.
Information provision and decision-making per GDP tertile
| All respondents | Lower GDP tertilea | Middle GDP tertilea | Higher GDP tertilea | P-valueb | |
|---|---|---|---|---|---|
| Timing of information before KRT initiation, % | <0.001 | ||||
| No information received | 25.2 | 34.1 | 21.1 | 16.6 | |
| <1 month | 17.8 | 19.2 | 18.4 | 15.3 | |
| 1–3 months | 17.2 | 15.4 | 18.2 | 18.9 | |
| 4–12 months | 16.4 | 11.9 | 16.7 | 22.3 | |
| >12 months | 23.4 | 19.4 | 25.6 | 27.0 | |
| No information received about, % | |||||
| CHD | 7.4 | 10.0 | 4.7 | 6.2 | <0.001 |
| HHD | 42.1 | 51.7 | 48.4 | 26.5 | <0.001 |
| PD | 24.5 | 34.7 | 21.8 | 16.0 | <0.001 |
| LTX | 22.7 | 29.0 | 20.2 | 17.9 | <0.001 |
| DTX | 19.8 | 28.7 | 12.6 | 16.6 | <0.001 |
| CCM | 33.0 | 33.8 | 28.5 | 36.2 | <0.001 |
| Information source, % | |||||
| Nephrologist | 92.1 | 89.7 | 95.2 | 92.9 | <0.001 |
| Doctor other than nephrologist | 19.5 | 21.4 | 19.7 | 16.5 | <0.001 |
| Nurse | 38.1 | 27.2 | 32.3 | 58.8 | <0.001 |
| Other kidney patients | 24.5 | 24.2 | 27.1 | 22.5 | 0.002 |
| Brochure/booklet | 26.7 | 18.7 | 24.2 | 40.4 | <0.001 |
| Website/Internet | 21.2 | 16.7 | 23.8 | 25.1 | <0.001 |
| Kidney patients' federation | 14.9 | 6.4 | 18.8 | 23.6 | <0.001 |
| Persons influencing decision making, % | |||||
| Husband/wife or partner | 39.9 | 29.5 | 39.3 | 55.2 | <0.001 |
| Other family members | 30.2 | 30.0 | 30.4 | 30.3 | 0.940 |
| Friends | 5.3 | 4.7 | 5.4 | 6.1 | 0.074 |
| Other kidney patients | 10.2 | 8.4 | 13.2 | 10.0 | <0.001 |
| My doctor | 81.3 | 82.0 | 78.5 | 82.7 | 0.002 |
| My nurse | 17.2 | 7.0 | 14.7 | 33.9 | <0.001 |
| Employer/supervisor at work | 0.8 | 0.2 | 0.7 | 1.6 | <0.001 |
| Another person | 1.4 | 1.2 | 1.5 | 1.7 | 0.389 |
| Nobody | 0.3 | 0.3 | 0.3 | 0.1 | 0.411 |
For the categorization of countries per GDP category, see Supplementary data, Figure S2.
P-values calculated with Chi-square tests to compare GDP tertiles on categorical outcomes.
LTX, living donor kidney transplantation; DTX, deceased donor kidney transplantation; CCM, comprehensive conservative management.
FIGURE 1.
Satisfaction with information provision about treatments. For the categorization of countries per GDP category, see Supplementary data, Figure S2. P-values calculated with Chi-square tests. LTX, living donor kidney transplantation; DTX, deceased donor kidney transplantation; CCM, comprehensive conservative management.
Time between information provision and KRT initiation was longer in high-GDP countries than in low- and middle-GDP countries (P < 0.001) (Table 2). In low-GDP countries, a higher percentage of respondents stated that they had not received any information about HHD, PD, or living or deceased donor KTx (P < 0.001). We found lower percentages of respondents who reported no information about particular modalities if we restricted the analyses to patients aged ≤60 years at KRT initiation, those without diabetes mellitus and in countries where the treatment was available (HHD) or more prevalent (PD, living or deceased donor KTx) as reported by data from the ERA-EDTA Registry [1] (Supplementary data, Table S3). However, the proportion of uninformed patients in most strata remained significantly larger in low-GDP countries (P < 0.001), compared with the countries with a higher GDP. The vast majority of respondents received their information from nephrologists, but in high-GDP countries, the proportion of respondents who obtained information from nurses and brochures/booklets was larger (P < 0.001). Respondents from middle- and high-GDP countries were slightly more satisfied with the information provision than those from low-GDP countries (P < 0.001) (Figure 1).
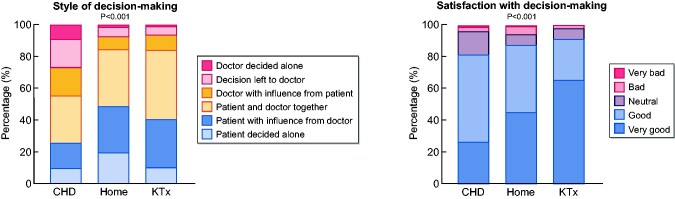
Decision-making
Figure 2 shows the results of method of decision-making and the satisfaction about this by respondents who had received only one form of KRT so far. For each treatment, the majority of decisions were made involving both patient and nephrologist (CHD 63.4%, HHD and PD 73.1%, pre-emptive KTx 83.4%). A smaller proportion of respondents reported that they had left the decision to their doctor (CHD 17.9%, HHD and PD 6.0%, pre-emptive KTx 5.7%) or that the doctor decided alone (CHD 9.1%, HHD and PD 1.5%, pre-emptive KTx 0.9%). Regardless of their treatment, most respondents were satisfied with the way decisions were made (>80% good or very good), but pre-emptive KTx recipients were most satisfied (good or very good: CHD 81.2%; HHD and PD 87.2%; pre-emptive KTx 91.1%). Virtually all respondents mentioned that other people such as their doctors (81.3%), partners (39.9%), other family members (30.2%) or nurses (17.2%) had affected their treatment choice (Table 2).
FIGURE 2.
Decision-making for respondents undergoing only one treatment. ‘Home’ includes respondents on home haemodialysis and peritoneal-dialysis. P-values are calculated with Chi-square tests.
Similar trends in the method of decision-making and the satisfaction about this were observed across the GDP tertiles (Supplementary data, Table S4). In high-GDP countries, a larger proportion of respondents reported that their partners and/or nurses had influenced their choice (P < 0.001) (Table 2).
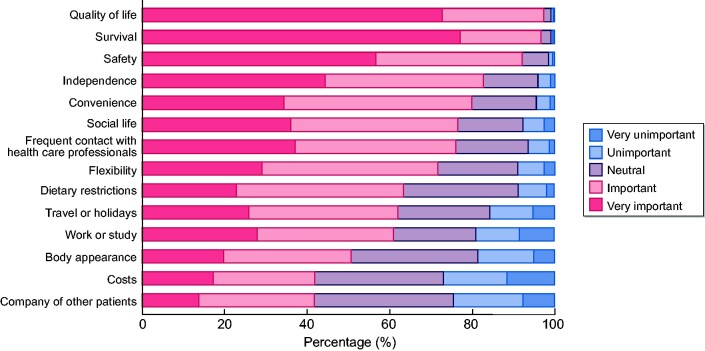
Factors influencing treatment modality choice
According to the respondents, the three most important factors affecting treatment modality choice were ‘quality of life’ (97.3% reported ‘important’ or ‘very important’), ‘survival’ (96.6%) and ‘safety’ (92.0%) (Figure 3). The three least important factors included ‘company of other patients’ (41.8% reported this as ‘important’ or ‘very important’), ‘costs’ (42.0%) and ‘body appearance’ (50.7%). The three most important factors were similar across the GDP tertiles. Social life and work or study were more important for respondents from high-GDP countries, whereas costs were more often reported as important by those from low-GDP countries (Supplementary data, Table S5).
FIGURE 3.
Factors influencing treatment modality choice.
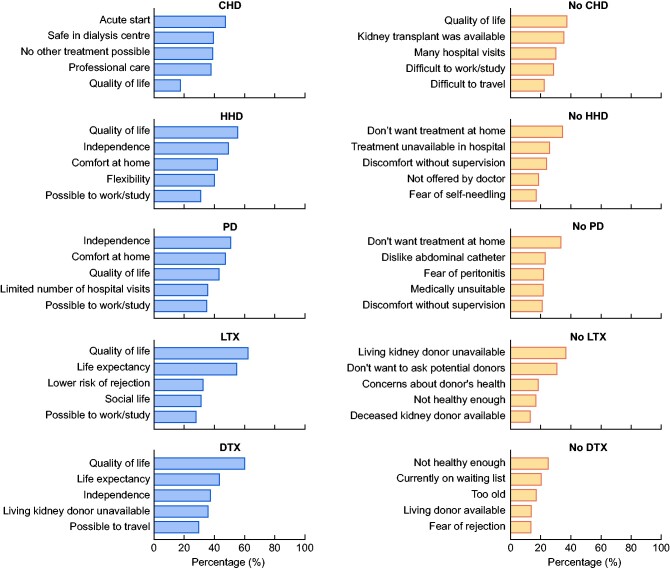
Treatment-specific reasons
Respondents selected a maximum of five main reasons why they did or did not receive a particular KRT modality (Figure 4 and Supplementary data, Table S6). Reasons for receiving HHD or PD or a KTx from a living or deceased donor partly overlapped. Quality of life played an important role in the choice of each of these modalities, whereas limited availability, fears and concerns, age and health status played an important role in not receiving a specific treatment.
FIGURE 4.
Top five treatment-specific reasons. LTX, living donor kidney transplantation; DTX, deceased donor kidney transplantation.
Experience with treatments
More than 80% of respondents had a good or very good experience with the treatment(s) they (had) received (Figure 5). Respondents from middle- and high-GDP countries reported more frequently very good experience with CHD and deceased donor KTx than those from low-GDP countries (P < 0.001).
FIGURE 5.
Experience with treatments. For the categorization of countries per GDP category, see Supplementary data, Figure S2. P-values calculated with Chi-square tests. LTX, living donor kidney transplantation; DTX, deceased donor kidney transplantation.
DISCUSSION
We surveyed a large group of adults with ESKD on KRT from 38 European countries about factors influencing their treatment modality choice. We found that timing and content of information provision differed across Europe and seemed to be less optimal in low-GDP countries. Treatment modality choice was often made involving both patient and nephrologist. When patients reported both objective and subjective reasons for modality choice, some reasons were more often selected by patients in middle- and high-GDP countries (e.g. work or study, social life) and others by patients in low-GDP countries (e.g. out of pocket costs, limited availability of treatments other than CHD). Most patients were satisfied with information provision, decision-making and the treatment they received, but patients in low-GDP countries tended to be less satisfied.
Limited or absent information provision was in particular prevalent in low-GDP countries and could be related to limited availability of specific KRT modalities [1, 3]. Treatment availability on a national level does not always imply availability in all regions due to, for example, long travel distances, limited capacity or financial barriers. Absent information provision about home dialysis or KTx also existed in countries with better availability, which may be at least partly due to contraindications for particular treatments, such as older age or the presence of comorbidities [13, 14, 28]. Objective data on the health status and living circumstances are needed to investigate the influence of these factors on information provision and treatment modality choice.
Our results show that it is most often the nephrologist who gives information about KRT modalities, irrespective of a country’s GDP. Besides, nurses were more often involved in information provision in high-GDP countries, whereas patients from low-GDP countries reported more involvement of other physicians than nephrologists in information provision. Nephrologists, other physicians and nurses may provide information in a different way, with respect to content, comprehensibility and available time, which could influence patients’ choice. However, patients perceived information from their doctor or nurse as equally helpful [11].
Although patients described several shortcomings in information provision and several were not even involved in the decision-making process, their overall satisfaction about the received information and decision-making was high. Please note that patients who were not informed about particular treatments could not rate the quality of the information. Future research might examine patients’ opinions about several aspects of information provision in more detail (e.g. timing, comprehensibility and usefulness) and in specific patient subgroups. We realize that the patients’ opinions may not always correspond to reality and patients may, for example, not remember exactly the timing of information provision or may not recognize or understand the information. Nevertheless, we believe that quality of information prevails on satisfaction and that the findings from our study and other studies [11, 12, 16, 29] suggest the need to improve information provision.
Other studies show substantial variation in patients’ preferences about their own role and the involvement of others (e.g. nephrologist, partner or children) in decision-making [28, 30]. There is also considerable variation in patients’ interest in outcomes (e.g. survival, clinical variables, symptoms, maintaining lifestyle) of a particular KRT modality [31, 32]. Healthcare professionals may explore these preferences beforehand in order to personalize information provision and decision-making.
In our study, quality of life was the most important factor in the choice of KRT modality for patients in all GDP categories, and it was also frequently mentioned as a treatment-specific reason. Since the meaning of quality of life may differ from patient to patient, as it depends on personal circumstances such as age, finances and living situation, it is important that healthcare professionals discuss quality of life and its meaning with individual patients [33].
Many respondents selected fears or concerns as reasons for not receiving a particular treatment. Consistent with literature, they selected fear of self-needling, dislike of abdominal catheter, fear of peritonitis and concerns about no supervision as reasons against home dialysis [14, 17, 18, 34]. Furthermore, several patients reported hesitation to ask potential donors and concerns about donors’ health as reasons for not receiving living donor KTx, whereas fears of transplant surgery or rejection were mentioned by a smaller group [35, 36]. Some reasons patients gave for being against home dialysis or KTx may be based on incorrect information leading to misperceptions (‘patients should not dialyze without supervision’ [34] or ‘a kidney transplant is a risk of life and death’ [37]). Providing adequate education to patients and their families may help to overcome these barriers [38, 39].
Besides subjective reasons, patients frequently mentioned objective reasons why they did or did not receive a treatment. Acute KRT initiation was a main reason for almost half of respondents who (had) received CHD. Half of the patients starting dialysis experience an unplanned or acute start [40–42] that may hinder adequate training and preparation for home dialysis or pre-emptive KTx. However, patients facing an acute start of KRT may still be able to receive home dialysis or KTx—even if only at a later stage—if they receive adequate education and support [43].
Another frequently selected reason for receiving CHD was that ‘no other treatments were possible’. This could be due to the limited availability of home dialysis or KTx in several countries [3], to inadequate information provision about these treatments or to patient-related factors. For example, patients may have contra-indications against treatments other than CHD or their living situation may be unsuitable to perform home dialysis [15, 17, 34, 44].
Half of the respondents from low-GDP countries indicated that costs were important when considering treatment modality. In another survey, one-fifth of the patients with ESKD mentioned that costs hindered them receiving the optimal treatment, but results from patients from lower- and higher-income countries were not described separately [11]. Financial barriers do not necessarily correlate to GDP. For example, patients with ESKD from several higher income countries have significant out-of-pocket costs [45–47]. Moreover, dialysis and/or KTx were excluded from public funding in some countries, patient co-payment for dialysis or KTx was reported in both Western and Eastern European countries, and funding for dialysis or transplant medication was unavailable in several European countries across all GDP categories [3, 48]. The amount of reimbursement of KRT varies largely in Europe, as found in the EDITH project [21] and by others [49], but the amount of out-of-pocket costs and the consequences for patients (such as poverty, non-adherence or declining KTx) should be investigated as well in order to create a complete overview of costs of KRT.
Poor health and older age were two main reasons for not receiving KTx. Although age alone is not considered a contraindication in most kidney transplant guidelines [50, 51], a large variability exists in the percentage of nephrologists who would recommend KTx for patients aged >60 years [52]. However, research has shown that certain older patients may also experience a survival advantage with KTx over dialysis, and the survival after receiving a KTx, including in patients >75 years of age, has increased over time [53, 54]. Increased awareness about these benefits of KTx in older patients may influence attitudes towards KTx of both professionals and patients.
Consistent with studies from Australia and the USA, patients receiving home dialysis and those living with a functioning KTx were more satisfied with their treatment than those receiving CHD [12, 29]. In addition, patients from middle- or high-GDP countries were more satisfied than their counterparts from low-GDP countries. This could be related to poorer information provision or decision-making experiences, since these two features have been reported as risk factors for lower treatment satisfaction [12]. Furthermore, poor treatment experience may be related to financial and social consequences of that treatment, including high out-of-pocket costs, becoming unemployed or long travel time to the hospital.
A main strength is that the EDITH kidney patient survey is one of the largest kidney patient surveys to date, including more than 7800 patients on KRT from 38 European countries, all surveyed in their own language. We pursued inclusion of older and sicker patients by offering paper surveys and assistance when filling out the survey. Furthermore, we are among the first to compare opinions of patients from lower-, middle- and higher-income countries in Europe.
This study also has limitations. Despite careful translation by native speakers with a medical background and patients’ feedback on survey comprehensibility, differences in interpretation of questions and answers may have occurred. Moreover, the respondents represent a fraction of all European patients on KRT, and our results may not be representative for the overall KRT population. To maximize the group of potential respondents and respect the anonymity of respondents in the context of the General Data Protection Regulation, we did not directly contact potential respondents. Therefore, we do not know which persons received the survey and whether they responded or not. Consequently, we were not able to calculate a response rate or compare the characteristics of responders with non-responders. Finally, respondents’ opinions about information provision, decision-making or factors influencing modality choice may have changed over time (recall bias), and their opinion may have been influenced by later experiences with other treatments. However, we tried to limit the influence of later experiences by selecting respondents who received only one form of KRT when investigating method of decision-making and satisfaction.
CONCLUSION
The results of our study including over 7800 European patients with ESKD suggest substantial variation in patient-reported factors influencing the choice of KRT modality. This variation is partially caused by limited availability of home dialysis and KTx in several countries. In addition, disparities in healthcare systems such as medical workforce, funding and health insurance system, may affect patients’ access to treatments for ESKD. Moreover, patient preferences, which may be influenced by information provision and method of decision-making, might differ between countries. The availability of home dialysis and KTx should be optimized, accompanied by education, policy measures and funding, so that all European patients with ESKD can choose the form of KRT that is most suitable for them.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors sincerely thank all patients who filled out the EDITH kidney patient survey. Moreover, we are grateful to all patients who pre-tested the survey, provided advice about the content of the survey or help to distribute the survey in their country or personal network. Amongst others, we are grateful for support from Austria [ARGE Niere (D. Trinkl), Österreichische Transplantationsverband (M. Krimbacher), Verein Niere Vorarlberg (E. Längle)]; Belgium [Fenier-Fabir and Nier Limburg (M. Mues)]; Bosnia and Herzegovina [Udruženje Dializiranih & Transplantiranih Bolesnika Federacije BiH (T. Žuljević)]; Bulgaria [Association of patients with kidney diseases and friends Bulgaria (ABPZ) (H. Nikolova and R. Vaklinova)]; Cyprus [Pancyprian Kidney Organization (E. Groutidou Petridou)]; Denmark [Nyreforeningen (K. Ertmann Krammer and P. Holm Harmsen)]; Finland [Munais-ja maksaliitto (MUMA) (S. Högström)]; France [France REIN, Transforme (E. Lacour)]; Greece [Greek Kidney Patients Federation (T. Papadopoulos and M. Sekadakis), Sports Federation For Dialysis & Transplant Athletes (D. Arka)]; Hungary [Trapilap (J. Berente)], Vesebetegek Egyesületeinek Országos Szövetsége (VORSZ) (A. Ádám); Ireland [Irish Kidney Association (IKA) (M. Murphy and C. White)]; Latvia [Latvijas nieru slimnieku asociācijas (LNSA) (J. Baranovska-Kaša)]; Lithuania (Gyvastis, Lietuvos nefrologiniu ligoniu asociacija); Luxembourg [Dialyse Patienten Lëtzebuerg a.s.b (L. Chrisnach)]; the Netherlands [Leef Nierpatiënten Vereniging Hilversum (P. Floor), NierNieuws, Nierpatiënten Vereniging Nederland (W. Konijn and K. Prantl), Nierpatiënten Vereniging Solidair (H. Zomer), Stichting Doneren en transplanteren (M. van der Gaag), Stichting Sport en Transplantatie (J. Folkers and F. Kuipers), Vereniging voor Nierpatiënten regio Den Haag (G. van der Pol)]; North Macedonia [NEFRON Kidney Patient Association (P. Baliska and D. Gorgiev)]; Norway [Landsforeningen for Nyrepasienter og Transplanterte (LNT) (J. Anker Lisberg Sarpebakken, K. Fosser, M. Gonsholt, I. Østli and L. Skar)]; Poland [Ogólnopolskie Stowarzyszenie Osób Dializowanych (OSOD) (M. Dębski-Korzec and A. Serwadczak)]; Portugal [Associação Portuguesa de Insuficientes Renais (APIR) (M. Campos)]; Romania [Asociatia Transplantatilor din Romania (ATR) G. Tache and A. Teodorescu)]; Slovenia [Sve Osmeh (J. Antic and B. Danilovic)], Zveza Društev Ledvičnih Bolnikov Slovenije (ZDLBS) (L. Hajdinjak and B. Tome); Spain [Federación Nacional de Asociaciones para la Lucha Contra las Enfermedades del Riñón (ALCER) (J. Carlos Julián Mauro and D. Gallego Azurro)]; Sweden [Njürforbundet (H. Heddman, S. Norman and L. Pellborn)]; and Ukraine (Nephro Nadiya); and from the following organizations EKPF/CEAPIR (M. Murphy and C. White), South-east Europe transplant network Seentransplant (M. Osterc), PKD International (T. Harris) and NephCure (M. van Meel).
In addition, we wish to thank many international colleagues for their help. We are grateful to every person who has helped us by translating, promoting or distributing the EDITH kidney patient survey. Amongst others, we received support from Albania (A. Idrizi), Austria (R. Kramar, U. Lang, G. Mayer, R. Oberbauer, R. Reindl-Schwaighofer and S. Tabernig), Belarus (K. Komissarov and K. Kurylovich), Belgium (D. Abramowicz, F. Bonkain, M. Claes, S. Claus, F. Collart, J. De Meester, L. Heemeryck, E. Meersman, L. Moyson, C. Olbrechts, S. Verhofstede, C. Swyns, C. Tielemans, W. Van Biesen and R. Vanholder), Bosnia and Herzegovina (B. Čengić-Roljic and H. Resic), Bulgaria (E. Vazelov), Croatia (I. Bubić, M. Bušić, M. Dragović, D. Lazarin and S. Živčić Ćosić), Cyprus (K. Ioannou and G. Marangou d'Avernas), Czech Republic (V. Borcany and I. Rychlik), Denmark (L. Boesby and J. Goya Heaf), Estonia (Ü. Pechter and M. Rosenberg—Ots), Finland (A. Cornér, P. Finne, R. Ikäheimo and R. Muroma-Karttunen), France [C. Couchoud, B. Hodemon Corne, V. Juventin, M. Laville, Z. Massy and M. Mongé, colleagues from Réseau épidémiologie et Information en Néphrologie (REIN)], Germany (M. Lingemann, A. Rahmel, F. Schaub and S. Venhaus), Greece (N. Afentakis, T. Apostolou and E. Dounousi), Hungary (O. Deme, A. Mezõ and G. Reúsz), Iceland (R. Pálsson and H. Runolfsdottir), Ireland (W. Plant), Italy (R. Benevento, V. Caramia, C. Carella, P. di Caccio, L. Gesualdo, E. Mancini, A. Meterangelis, A. Nanni Costa, M. Nordio, M. Postorino, V. Sparacino and C. Zoccali), Latvia (H. Cernevskis, L. Kucane, A. Petersons and A. Silda), Lithuania (I. Arūnė Bumblytė, V. Tilvikaitė and E. Žiginskienė), Luxembourg (M. Aurich, S. Azorin Contesse and C. Braun), Moldova (A. Tanase), the Netherlands (J. Bijlsma, M. Boonstra, W. Bos, M. de Jong, G. Guggenheim-van der Hout, M. Hemmelder, A. Jaho, S. Kragten—Lathouwers, S. Logtenberg, I. van de Meerakker—Hegge, A. Neradova, M. van Oosten, A. Ozyilmaz, M. Pippias, D. Pitters, V. Sauve and D. Struijk), North Macedonia (G. Selim and O. Stojceva—Taneva), Norway (M. Andersen, A. Asberg, K. Lønning and A. Varberg Reisaeter), Poland (S. Dudzicz, G. Korejwo, M. Nowicki and A. Wieçek), Portugal (R. Aguiar, E. Almeida, S. Fernandes and F. Marcario), Romania (L. Garneata, G. Mircescu, G. Stefan and L. Tuta), Russia (A. Andrusev and E. Zakharova), Serbia (S. Bjelica, N. Maksimovic, R. Naumovic and M. Zaric), Slovakia (R. Roland, J. Rosenberger and V. Spustova), Slovenia (J. Buturovic Ponikvar, D. Kovac and M. Malgaj), Spain (P. Barreda, J. Cannata, M. Collado, M. del Pino y Pino, M. Enma Huarte Loza, A. Ortiz and H. Zambrana), Sweden (O. Bratt, M. Evans, H. Rydell and M. Stendahl), Switzerland (I. Françoise Binet, U. Huynh-Do, F. Immer and M. Weder, staff from Inselspital Universitätsspital Bern), Turkey (M. Arici and N. Seyahi), Ukraine (M. Kolesnyk, N. Stepanova and L. Surzkho), and from Eurotransplant (P. Branger, M. van Meel and U. Samuel).
FUNDING
R.W.d.J., V.S.S. and K.J.J. report grants from European Union (Grant number PP-01-2016) and from ERA-EDTA during the conduct of the study. The funders did not have any role in study design; collection, analysis and interpretation of data, writing the report; and the decision to submit the report for publication. This publication is part of the EDITH project, which has received funding from the European Union.
CONFLICT OF INTEREST STATEMENT
R.W.d.J., V.S.S. and K.J.J. report grants from European Union (Grant number PP-01-2016) and from ERA-EDTA during the conduct of the study. All other authors have nothing to disclose. This work has been presented as oral presentation at the 55th ERA-EDTA congress (Copenhagen, 24–27 May 2018) and as poster at the BENELUX Kidney Meeting (Eindhoven, 11 October 2019) and at the American Society of Nephrology Kidney Week 2019 (Washington, DC, 5–10 November 2019). The content of this publication represents the views of the authors only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission or any other body of the European Union. The European Commission does not accept any responsibility for use that may be made of the information it contains.
AUTHORS’ CONTRIBUTIONS
R.W.d.J., V.S.S., M.M. and K.J.J. were responsible for research idea and study design; R.W.d.J., V.S.S., M.M., Z.A.M. and K.J.J. were responsible for data acquisition; R.W.d.J., V.S.S., A.R., M.M., R.C.V., Z.A.M. and K.J.J. were responsible for data analysis/interpretation; R.W.d.J., V.S.S. and K.J.J. were responsible for statistical analysis; V.S.S., Z.A.M. and K.J.J. were responsible for supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
REFERENCES
- 1. ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2017. Amsterdam, the Netherlands: Amsterdam UMC, location AMC, Department of Medical Informatics, 2019
- 2. Kramer A, Boenink R, Noordzij M. et al. The European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2017: a summary. Clin Kidney J 2020; 13: 693–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bello AK, Levin A, Lunney M. et al. Global Kidney Health Atlas: a report by the International Society of Nephrology on the Global Burden of End-stage Kidney Disease and Capacity for Kidney Replacement Therapy and Conservative Care across World Countries and Regions. Brussels, Belgium: International Society of Nephrology, 2019
- 4. Wolfe RA, Ashby VB, Milford EL. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 5. Tonelli M, Wiebe N, Knoll G. et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011; 11: 2093–2109 [DOI] [PubMed] [Google Scholar]
- 6. Cameron JI, Whiteside C, Katz J, Devins GM.. Differences in quality of life across renal replacement therapies: a meta-analytic comparison. Am J Kidney Dis 2000; 35: 629–637 [DOI] [PubMed] [Google Scholar]
- 7. Jarl J, Desatnik P, Peetz Hansson U. et al. Do kidney transplantations save money? A study using a before–after design and multiple register-based data from Sweden. Clin Kidney J 2018; 11: 283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehrotra R, Chiu YW, Kalantar-Zadeh K. et al. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171: 110–118 [DOI] [PubMed] [Google Scholar]
- 9. van de Luijtgaarden MW, Jager KJ, Segelmark M. et al. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol Dial Transplant 2016; 31: 120–128 [DOI] [PubMed] [Google Scholar]
- 10. Weinhandl ED, Liu J, Gilbertson DT. et al. Survival in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. J Am Soc Nephrol 2012; 23: 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Biesen W, van der Veer SN, Murphey M. et al. Patients’ perceptions of information and education for renal replacement therapy: an independent survey by the European Kidney Patients’ Federation on information and support on renal replacement therapy. PLoS One 2014; 9: e103914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fadem SZ, Walker DR, Abbott G. et al. Satisfaction with renal replacement therapy and education: the American Association of Kidney Patients survey. Clin J Am Soc Nephrol 2011; 6: 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehrotra R, Marsh D, Vonesh E. et al. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 2005; 68: 378–390 [DOI] [PubMed] [Google Scholar]
- 14. Ludlow MJ, Lauder LA, Mathew TH. et al. Australian consumer perspectives on dialysis: first national census. Nephrology (Carlton) 2012; 17: 703–709 [DOI] [PubMed] [Google Scholar]
- 15. Dahlerus C, Quinn M, Messersmith E. et al. Patient perspectives on the choice of dialysis modality: results from the empowering patients on choices for renal replacement therapy (EPOCH-RRT) study. Am J Kidney Dis 2016; 68: 901–910 [DOI] [PubMed] [Google Scholar]
- 16. Zee J, Zhao J, Subramanian L. et al. Perceptions about the dialysis modality decision process among peritoneal dialysis and in-center hemodialysis patients. BMC Nephrol 2018; 19: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jennette C, Derebail V, Baldwin J. et al. Renal replacement therapy and barriers to choice: using a mixed methods approach to explore the patient’s perspective. J Nephrol Soc Work 2009; 32: 15–26 [PMC free article] [PubMed] [Google Scholar]
- 18. Morton RL, Devitt J, Howard K. et al. Patient views about treatment of stage 5 CKD: a qualitative analysis of semistructured interviews. Am J Kidney Dis 2010; 55: 431–440 [DOI] [PubMed] [Google Scholar]
- 19. van de Luijtgaarden MWM, Jager KJ, Stel VS. et al. Global differences in dialysis modality mix: the role of patient characteristics, macroeconomics and renal service indicators. Nephrol Dial Transplant 2013; 28: 1264–1275 [DOI] [PubMed] [Google Scholar]
- 20. Spasovski G, Rroji M, Vazelov E. et al. Nephrology in the Eastern and Central European region: challenges and opportunities. Kidney Int 2019; 96: 287–290 [DOI] [PubMed] [Google Scholar]
- 21. Jager KJ, Stel VS, Branger P. et al. The effect of differing kidney disease treatment modalities and organ donation and transplantation practices on health expenditure and patient outcomes. Nephrol Dial Transplant 2018; 33: 560–562 [DOI] [PubMed] [Google Scholar]
- 22. LimeSurvey GmbH. LimeSurvey: An Open Source survey tool. LimeSurvey GmbH, http://www.limesurvey.org
- 23. Eysenbach G. Improving the quality of web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004; 6: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. IBM Corporation. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp., Released 2017
- 25. World Bank. Indicators, https://data.worldbank.org/indicator (20 August 2019, date last accessed)
- 26. World Bank. World Bank Country and Lending Groups, https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (25 August 2020, date last accessed)
- 27. SAS Institute Inc. SAS Enterprise Miner 9.4. Cary, NC: SAS Institute Inc.
- 28. Durand MA, Bekker HL, Casula A. et al. Can we routinely measure patient involvement in treatment decision-making in chronic kidney care? A service evaluation in 27 renal units in the UK. Clin Kidney J 2016; 9: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fortnum D, Grennan K, Ludlow M.. Dialysis Consumer Perspectives Survey Two: Complete dataset report. South Melbourne, Australia: Kidney Health Australia, 2015
- 30. Orsino A, Cameron JI, Seidl M. et al. Medical decision-making and information needs in end-stage renal disease patients. Gen Hosp Psychiatry 2003; 25: 324–331 [DOI] [PubMed] [Google Scholar]
- 31. Urquhart-Secord R, Craig JC, Hemmelgarn B. et al. Patient and caregiver priorities for outcomes in hemodialysis: an International Nominal Group Technique Study. Am J Kidney Dis 2016; 68: 444–454 [DOI] [PubMed] [Google Scholar]
- 32. Morton RL, Tong A, Webster AC. et al. Characteristics of dialysis important to patients and family caregivers: a mixed methods approach. Nephrol Dial Transplant 2011; 26: 4038–4046 [DOI] [PubMed] [Google Scholar]
- 33. Ramer SJ, McCall NN, Robinson-Cohen C. et al. Health outcome priorities of older adults with advanced CKD and concordance with their nephrology providers’ perceptions. J Am Soc Nephrol 2018; 29: 2870–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLaughlin K, Manns B, Mortis G. et al. Why patients with ESRD do not select self-care dialysis as a treatment option. Am J Kidney Dis 2003; 41: 380–385 [DOI] [PubMed] [Google Scholar]
- 35. Gordon EJ. “They don’t have to suffer for me”: why dialysis patients refuse offers of living donor kidneys. Med Anthropol Q 2001; 15: 245–267 [DOI] [PubMed] [Google Scholar]
- 36. Zimmerman D, Albert S, Llewellyn-Thomas H et al.. The influence of socio-demographic factors, treatment perceptions and attitudes to living donation on willingness to consider living kidney donor among kidney transplant candidates. Nephrol Dial Transplant 2006; 21: 2569–2576 [DOI] [PubMed] [Google Scholar]
- 37. Landreneau K, Ward-Smith P.. Perceptions of adult patients on hemodialysis concerning choice among renal replacement therapies. Nephrol Nurs J 2007; 34: 513–519 [PubMed] [Google Scholar]
- 38. Manns BJ, Taub K, Vanderstraeten C. et al. The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: A randomized trial. Kidney Int 2005; 68: 1777–1783 [DOI] [PubMed] [Google Scholar]
- 39. Ismail SY, Luchtenburg AE, Timman R. et al. Home-based family intervention increases knowledge, communication and living donation rates: a randomized controlled trial. Am J Transplant 2014; 14: 1862–1869 [DOI] [PubMed] [Google Scholar]
- 40. Brown PA, Akbari A, Molnar AO. et al. Factors associated with unplanned dialysis starts in patients followed by nephrologists: a retrospective cohort study. PLoS ONE 2015; 10: e0130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arulkumaran N, Navaratnarajah A, Pillay C. et al. Causes and risk factors for acute dialysis initiation among patients with end-stage kidney disease – a large retrospective observational cohort study. Clin Kidney J 2019; 12: 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hughes SA, Mendelssohn JG, Tobe SW. et al. Factors associated with suboptimal initiation of dialysis despite early nephrologist referral. Nephrol Dial Transplant 2013; 28: 392–397 [DOI] [PubMed] [Google Scholar]
- 43. Rioux JP, Cheema H, Bargman JM. et al. Effect of an in-hospital chronic kidney disease education program among patients with unplanned urgent-start dialysis. Clin J Am Soc Nephrol 2011; 6: 799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morton RL, Tong A, Howard K. et al. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ 2010; 340: c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nickel M, Rideout W, Shah N. et al. Estimating patient-borne water and electricity costs in home hemodialysis: a simulation. CMAJ Open 2017; 5: E61–E65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kidney Foundation of Canada. The Burden of Out-of-Pocket Costs for Canadians with Kidney Failure 2018 REPORT, 2018
- 47. Hirth RA, Greer SL, Albert JM. et al. Out-of-pocket spending and medication adherence among dialysis patients in twelve countries. Health Aff (Millwood) 2008; 27: 89–102 [DOI] [PubMed] [Google Scholar]
- 48. Bello AK, Levin A, Lunney M. et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ 2019; 367: l5873. [DOI] [PubMed] [Google Scholar]
- 49. van der Tol A, Lameire N, Morton RL. et al. An international analysis of dialysis services reimbursement. Clin J Am Soc Nephrol 2019; 14: 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Segall L, Nistor I, Pascual J. et al. Criteria for and appropriateness of renal transplantation in elderly patients with end-stage renal disease: a literature review and position statement on behalf of the European Renal Association-European Dialysis and Transplant Association Descartes Working Group and European Renal Best Practice. Transplantation 2016; 100: e55–e65. [DOI] [PubMed] [Google Scholar]
- 51. Chadban SJ, Ahn C, Axelrod DA. et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation 2020; 104 (4S1 Suppl 1): S11–S103 [DOI] [PubMed] [Google Scholar]
- 52. Tong A, Hanson CS, Chapman JR. et al. Nephrologists’ preferences and perspectives on patient's access to kidney transplantation: a systematic review. Nephrol Dial Transplant 2014; 29: iii561–iii562 [DOI] [PubMed] [Google Scholar]
- 53. McAdams-DeMarco MA, James N, Salter ML. et al. Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc 2014; 62: 2235–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pippias M, Stel VS, Kramer A. et al. Access to kidney transplantation in European adults aged 75-84 years and related outcomes: an analysis of the European Renal Association-European Dialysis and Transplant Association Registry. Transpl Int 2018; 31: 540–553 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.