Abstract
Across species, the medial prefrontal cortex guides actions in time. This process can be studied using behavioral paradigms such as simple reaction-time and interval-timing tasks. Temporal control of action can be influenced by prefrontal neurotransmitters such as dopamine and acetylcholine and is highly relevant to human diseases such as Parkinson’s disease, schizophrenia, and attention-deficit hyperactivity disorder (ADHD). We review evidence that across species, medial prefrontal lesions impair the temporal control of action. We then consider neurophysiological correlates in humans, primates, and rodents that might encode temporal processing and relate to cognitive-control mechanisms. These data have informed brain-stimulation studies in rodents and humans that can compensate for timing deficits. This line of work illuminates basic mechanisms of temporal control of action in the medial prefrontal cortex, which underlies a range of high-level cognitive processing and could contribute to new biomarkers and therapies for human brain diseases.
Deciding When to Act
Animal behavior – or the complex symphony of coordinated action in the world – is precisely sequenced in time (Finnerty et al., 2015). Although perceptual events can indicate potential rewards or threats, temporal control of actions over several seconds is required to maximize positive and minimize negative outcomes (Tallot and Doyère, 2020). For mammals, this is critical to foraging and successfully avoiding predators (Bateson, 2003). For humans, temporal control of action is critical for routine activities such as cooking, driving, and crossing the street, and complex activities such as math, language, poetry, and art. Perceptual decision making has been the focus of many neuroscientific studies (Gold and Shadlen, 2007). However, deciding when to act is as important as selecting appropriate actions; indeed, the right action at the wrong time can lead to disastrous consequences. Thus, understanding the underlying neural circuitry involved in temporal control of action has far-reaching importance (Fuster, 2001).
The timing of movements is critical to higher-level cognition. Indeed, temporal control of action underlies many higher-level executive functions such as attention, flexible behavior, reasoning, and working memory. Dual-task studies in which participants perform executive functions concurrently with timing operations reveal marked bidirectional interference; i.e., performing a time production and randomization task concurrently degrades performance of either task (Brown, 2006). Estimating a temporal interval can impair concurrent working-memory performance (Bi et al., 2013). These data imply that temporal control of action and executive functions recruit shared cognitive resources and neural circuits (Parker et al., 2013b).
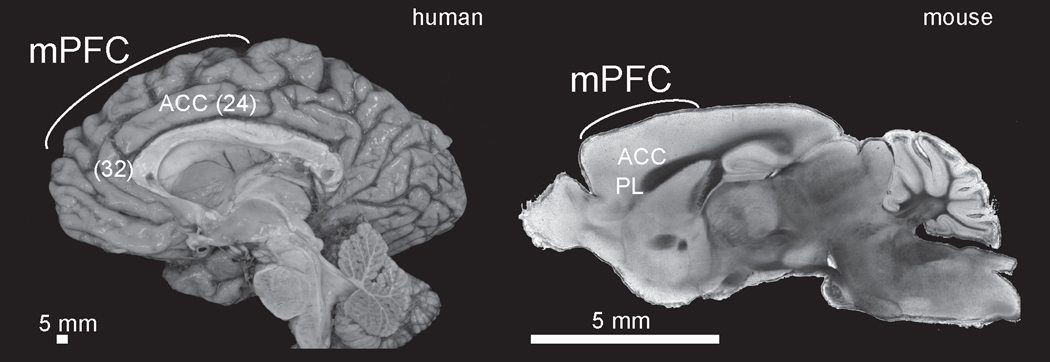
We argue that the medial prefrontal cortex critically controls movements in time. We focus on medial regions of prefrontal cortex that are conserved across mammalian species. Medial prefrontal regions of frontal cortex have been defined by projections from thalamic nuclei and a lack of a prominent cortical layer IV (i.e., ‘agranular’; Rose and Woolsey, 1949; Akert, 1964). Lateral prefrontal human specializations in dorsal and inferior frontal regions have been implicated in specialized functions such as working-memory and language (Fuster, 2008). While these lateral regions have unclear mammalian homologies in rodents, primate medial prefrontal regions can have anatomical and functional homologies with rodents (Preuss, 1995; Uylings et al., 2003; Laubach et al., 2018). Although there are differences between primate and rodent nomenclature because the granular lateral prefrontal cortex does not exist in rodents, the human/primate agranular anterior cingulate cortex covering Brodmann areas 24 and 32 has anatomical homologies to rodent medial prefrontal regions including the anterior cingulate cortex and the prelimbic cortex (Fig 1) (Laubach et al., 2018). For instance, both the human and rodent medial prefrontal cortex have a similar laminar architecture, receive similar neurotransmitter input, and have highly conserved connectivity with thalamic, striatal, hypothalamic and brainstem nuclei (Gabbott et al., 2003, 2005). Although medial prefrontal regions are vastly expanded in primates and humans, there are important differences between cingulate, prelimbic, and supplementary motor regions (Amiez and Procyk, 2019), in this chapter, we argue that across species, medial prefrontal regions have a significant functional homology: the temporal control of action. We start by discussing paradigms to study temporal control of action and then discuss medial prefrontal neurotransmitter systems involved in temporal control of action. We then review diseases with impairments in temporal control of action and lesion studies that disrupt the medial prefrontal cortex and impair timing of movements. Finally, we discuss both neuronal and local field potential correlates of how movements are controlled in time and then relate these signals to protocols that deliver brain stimulation that improves temporal control of action.
Figure 1: Medial prefrontal cortex in humans and rodents:
Sagittal brain section of the medial prefrontal cortex from humans and brightfield microscopy from the mouse. mPFC –medial prefrontal cortex; ACC –anterior cingulate; PL –prelimbic. Brodmann areas in parentheses. Human gross anatomy courtesy of Marco Hefti and the Iowa Brain Bank.
Paradigms for studying the temporal control of action
Time suffuses all paradigms and tasks in behavioral neuroscience, which generally require a specific action in a narrow temporal window. In human studies, the timing of movement is critical to inferences about cognitive processing. For instance, reaction-time linearly increases with the number of working-memory items (Sternberg, 1969; Narayanan et al., 2005) and can be harnessed in perceptual decision-making to capture trade-offs between speed and accuracy (Herz et al., 2017) or countermanding (Verbruggen et al., 2019).
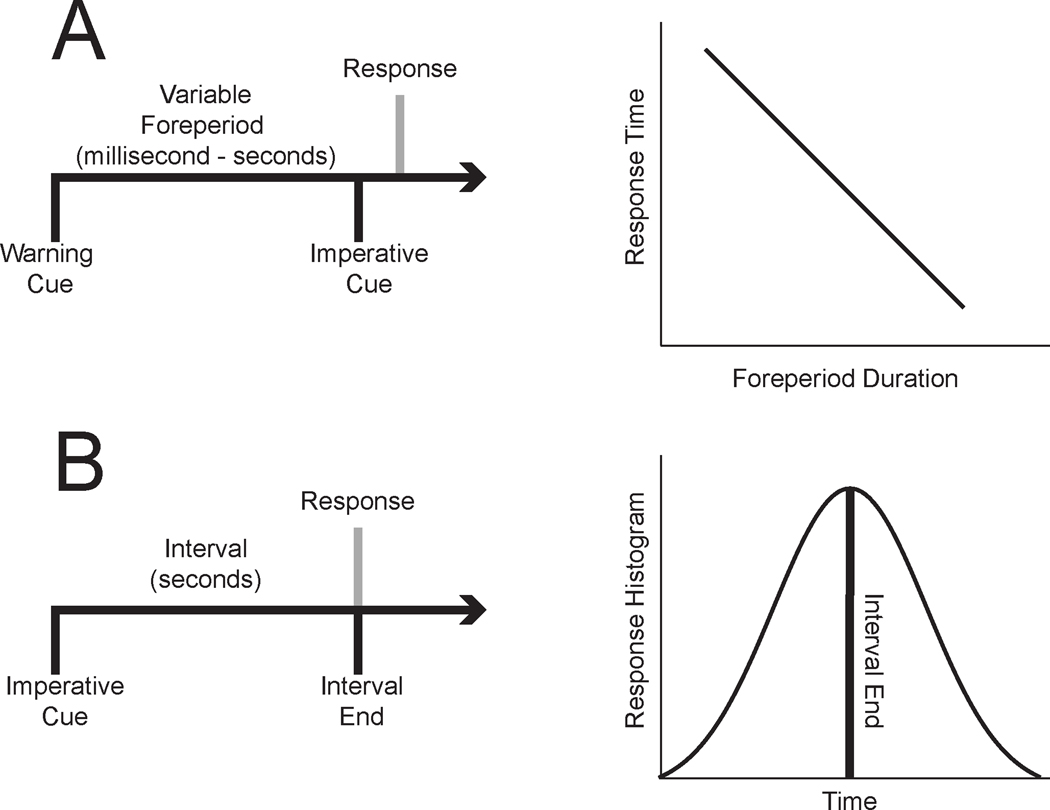
However, the most elementary response in simple-reaction time tasks can be profoundly affected by temporal processing. To ‘control’ for time in responding to a stimulus, many reaction-time tasks included a variable delay or ‘foreperiod’ between a warning cue and the imperative stimulus (Luce, 1991). Studies with the variable delay revealed that human –and non-human participants –were more prepared to respond the longer they waited, leading to delay-dependent speeding (or ‘foreperiod-effect’; Fig 2A) (Naatanen, 1971, 1972; Naatanen et al., 1974; Narayanan et al., 2006). Furthermore, response distributions could be influenced by the temporal probabilities of the task; that is, if a short delay was more likely, response times would decrease (Naatanen et al., 1974). These data suggest that actions are profoundly influenced by the temporal probabilities as specified by the hazard function (Luce, 1991).
Figure 2: Paradigms to study temporal control of action:
Temporal control of action can be studied using two paradigms. A) The simple reaction-time task requires participants to respond as fast as possible to an imperative cue. A warning cue is typically delivered at a variable interval prior to the imperative cue; the time between the warning and imperative cue is called ‘foreperiod’ or ‘delay’. Participants’ reaction-times are higher for short foreperiods compared to long foreperiods, when they are fully prepared to respond. This phenomenon is called the ‘foreperiod-effect’ or ‘delay-dependent speeding’. Another paradigm is B) interval-timing, which requires participants to estimate an interval of several seconds by making a motor response. In this task, the imperative cue indicates the start of the interval. The timing of responses is typically distributed around the end of the interval.
Another paradigm to explicitly study temporal control of action is interval timing (Fig 2B) (Merchant and de Lafuente, 2014). This family of tasks requires participants to estimate an interval of several seconds with a motor response. Although interval timing was designed to capture how time is ‘perceived’, these tasks require a motor report of temporal estimates and thus are inherently tied to action (outside of bisection tasks (Stubbs, 1976)). A beautiful aspect of these tasks is that they are conserved across many species, including mammals and birds (Buhusi and Meck, 2005) and can be performed in a broad range of conditions, from rodent operant behavior to human patients with severe brain diseases or with interoperative human neurophysiology (Carroll et al., 2009; Craig et al., 2014; Kelley et al., 2018). Temporal control of action may influence more complex motor sequences or language (Averbeck et al., 2006; Long et al., 2016); however, we will focus on reaction-time and interval-timing tasks as they are 1) well-suited to isolate temporal control of action from other processes, 2) they are simple enough to study in rodents and humans with cognitive impairments, and 3) complementary evidence across species facilitates convergent inferences on medial prefrontal function.
Neurotransmitter Systems
Human and rodent prefrontal regions receive dense innervation from ascending neurotransmitter systems such as dopamine, acetylcholine, norepinephrine, and serotonin (Narayanan et al., 2013b). Human brain diseases and their treatments involve perturbations of these neurotransmitter systems (Arnsten et al., 2012). Prefrontal norepinephrine and serotonin can have specific effects on goal-directed behaviors, but specific evidence for their role in temporal control of action is lacking. Conversely, dopamine and acetylcholine reliably regulate the timing of movements.
Primate prefrontal dopamine-depletion impairs behaviors across a delay (Brozoski et al., 1979; Goldman-Rakic, 1998). Our group has found specific effects during timing tasks. During simple reaction-time tasks, we found that rodent prefrontal dopamine-depletion and focal infusions of drugs blocking the D1-type dopamine receptors (D1DRs) blunted delay-dependent speeding with faster responses at short intervals. However, we did not observe increased premature responding (Parker et al., 2013a). During interval-timing tasks, we found that disrupting prefrontal dopamine by specific RNAi against tyrosine hydroxylase or dopamine depletion flattened time-response histograms (Narayanan et al., 2012; Parker et al., 2015a; Kim et al., 2017). Focal pharmacology has implicated prefrontal D1DRs but not D2DRs in temporal control of action (Narayanan et al., 2012; Parker et al., 2014, 2015a). Critically, transgenic mouse lines facilitate cell-type-specific experiments, and we have shown that optogenetic inactivation of prefrontal D1DR+ neurons in the mouse medial prefrontal cortex decreased responding at the end of a fixed-interval (Narayanan et al., 2012). These data provide convergent and consistent evidence that prefrontal D1DRs are required for the temporal control of action.
Cholinergic systems also play a clear role in timing tasks. For instance, systemic disruptions of acetylcholine with scopolamine (a muscarinic antagonist) or mecamylamine (a nicotinic antagonist) reliably impair reaction-time tasks (Jones and Higgins, 1995). Cholinergic signaling can affect prefrontal firing (Gill et al., 2000; Parikh and Sarter, 2008). These agents also impair interval timing; however, we found that scopolamine impairs stimulus-processing but not temporal processing by prefrontal neurons (Zhang et al., 2019). These data may be consistent with a view that cholinergic systems are important for attention rather than the temporal control of action (Sarter and Bruno, 1997; Klinkenberg and Blokland, 2010). Indeed, all timing tasks require attention to the passage of time as well as imperative cues. Future work with specific manipulations of cholinergic circuits and localized manipulations in the prefrontal cortex might clarify how acetylcholine modulates prefrontal circuits.
Significance for human disease
The timing of movement provides a unique window into human brain diseases that affect the prefrontal cortex. Because it can be rapidly and objectively assessed, assays built on studying the timing of movement have the potential to contribute to novel biomarkers, diagnostics tools, and intraoperative assays.
Patients with Parkinson’s disease have slow movements. This ‘bradykinesia’ slows all reaction times (Evarts et al., 1981); nonetheless, Parkinson’s disease patients have intact delay-dependent speeding (Jurkowski et al., 2005; Wearden et al., 2008). By contrast, Parkinson’s disease patients have impaired interval timing as a function of levodopa (Malapani et al., 1998, 2002; Jones and Jahanshahi, 2014). When multiple intervals are presented, patients with Parkinson’s disease are not simply slow; rather, they have increased variability by overestimating short intervals and underestimating long intervals. These deficits may reflect, in part, deficits in executive functions in Parkinson’s disease patients (Narayanan et al., 2013b; Parker et al., 2013b p.201).
Another disease with profound prefrontal abnormalities is schizophrenia (Andreasen et al., 1997; Okubo et al., 1997; Abi-Dargham et al., 2002). Like Parkinson’s disease, schizophrenia does not consistently involve delay-dependent speeding but does involve interval-timing impairments (Ward et al., 2011). Indeed, patients with schizophrenia are more variable and less efficient in their temporal estimates but do not reliably under- or overestimate an interval compared to healthy controls (Carroll et al., 2009, 2009; Parker et al., 2017; Thoenes and Oberfeld, 2017). Interestingly, this deficit extends to first-degree relatives (Penney et al., 2005). To date, most studies include patients undergoing therapeutic interventions and little work has been done to explore the role of specific medications in patients, meaning that these interval timing deficits may be more significant than reported. This is particularly important as first-line interventions are thought to target the medial prefrontal dopamine system. While there are inconsistencies in the literature and medication status may play a role; taken together, these data suggest that medial prefrontal dysfunction drives timing deficits in schizophrenia.
By contrast, attention-deficit hyperactivity disorder (ADHD) patients have uniform deficits in timing (Barkley et al., 1997). For instance, patients with ADHD have delay-dependent deficits during reaction-time tasks (Leth-Steensen et al., 2000) as well interval timing deficits (Smith et al., 2002). This interval timing deficit includes both consistent increases in timing variability and the overestimation of perceived time (Rommelse et al., 2008; Hove et al., 2017). Unlike schizophrenia-related timing deficits, several studies have examined the impact of first-line interventions for ADHD. For example, medications that primarily act on the medial prefrontal dopamine system, such as methylphenidate, have been shown to normalize both timing behavior and prefrontal function in ADHD (Hart et al., 2012; Luman et al., 2015). Furthermore, the medial as well as lateral prefrontal regions are dysfunctional in ADHD (Bush, 2011). These data suggest that timing dysfunction may be linked to medial prefrontal dysfunction (Valera et al., 2010; Hart et al., 2012; Noreika et al., 2013; Bluschke et al., 2018).
A broad range of other neurological and psychiatric illness can degrade prefrontal circuits, including Alzheimer’s disease and Huntington’s disease (Hinton et al., 2007; Caselli et al., 2009). More work needs to be done to clarify the role of medial prefrontal networks in these diseases. Nonetheless, this line of work in Parkinson’s disease, schizophrenia and ADHD firmly establishes the clinical relevance of understanding how medial prefrontal regions guide actions in time. Thus, a detailed understanding of these circuits will not only have far-reaching basic-science significance but could lead to new biomarkers, diagnostic tools and neuromodulation therapies for human brain diseases.
Lesion studies
The critical nature of the prefrontal cortex in acting over a delay was identified by Carlyle Jacobsen, who trained macaque monkeys on delayed response and delayed-alternation tasks (Jacobsen, 1935; Jacobsen and Nissen, 1937). Animals with extensive bilateral prefrontal lesions had permanent deficits at a variety of delays, but animals with unilateral lesions or with lesions of premotor or frontal motor areas had no deficits. Although not formally measured, Jacobsen insightfully concluded that frontal lesions in humans and primates affected the “temporal patterning of movement”. This finding was replicated by subsequent primate lesion studies that mapped lesions to lateral and medial regions of the baboon prefrontal cortex (Pribram, 1950; Pribram et al., 1952) and then extended to working memory (Mishkin and Pribram, 1955; Pribram and Mishkin, 1956). Although we are unaware of medial frontal inactivation or lesions during reaction-time tasks, primate medial frontal inactivation increases timing variability during a time-production task (Wang et al., 2018).
Human lesion studies from the laboratory of Donald Stuss definitively implicate human medial prefrontal regions in the temporal control of action. These authors found that superior medial prefrontal lesions spanning Brodmann Areas 24 and 32 slowed reaction-times. By contrast, other prefrontal regions did not produce consistent effects on overall reaction-time, but right lateral prefrontal lesions blunted delay-dependent speeding and prevented speeding of responses at longer intervals (Stuss et al., 2005). Subsequent studies revealed that human prefrontal lesions consistently increased the variability of responses during time-reproduction (Picton et al., 2006a, 2006b). Taken together, these unique human data connect human medial prefrontal areas to the temporal control of action.
In rodents, medial prefrontal regions are reliably involved in guiding movements in time. Excitotoxic prelimbic/infralimbic lesions that spared cingulate regions increased premature responding during simple-reaction time performance in rats; fascinatingly, this premature responding recovered in several days (Risterucci et al., 2003). Work by Narayanan and Laubach that reversibly inactivated dorsal prelimbic / ventral anterior cingulate regions replicated the increased premature responding and decreased delay-dependent speeding and found that animals had faster responses at short intervals (Narayanan and Laubach, 2006; Narayanan et al., 2006). Strikingly, prefrontal inactivation also impaired the ability to optimize responses after errors and improve performance (Narayanan and Laubach, 2008).
During interval-timing, rodent medial prefrontal disruptions impaired selection of a long temporal interval during a maze-running temporal bisection task (Kim et al., 2009). Prefrontal cooling of superficial areas slowed temporal estimates in a time-reproduction task (Namboodiri and Hussain Shuler, 2014; Xu et al., 2014). Our group has reliably shown that reversible prefrontal inactivation impairs fixed-interval timing by ‘flattening’ peaks of time-response histograms, resulting in responses that are more random in time (Narayanan et al., 2012; Parker et al., 2014; Emmons et al., 2017). During peak-interval timing, which allows estimates of both the start and the stop of temporal estimates, medial prefrontal inactivation increased the variability in a dose-dependent fashion (Buhusi et al., 2018). These data provide support for the idea that medial prefrontal lesions reliably increase the variability of how movements are controlled in time. In reaction-time tasks where response distributions are highly skewed, one form of this increased variability might include increased premature or slow responses.
Neuronal Correlates
Recording from single neurons and networks can provide insights into how movements are guided in time (Paton and Buonomano, 2018). Although these patterns can be diverse, and representation of impending movements can be ubiquitous in frontal cortex, recordings from our group and others have identified a common theme in temporal control of action.
This pattern is characterized by ‘ramping’ – or monotonic changes in firing rate over a temporal interval - and is a key form of temporal processing (Narayanan, 2016). In seminal recording studies by Niki and Watanabe, anticipatory patterns of neuronal activity (i.e. ramping activity) were the most common pattern (~25%) during timing and delayed-response tasks (Niki and Watanabe, 1976,1979). These neurons were observed in the cingulate cortex and more common than sustained activity (~20%). While sustained activity may be related to working-memory (Niki and Watanabe, 1976; Goldman-Rakic, 1996), this pattern of activity may also represent ‘time estimation’.
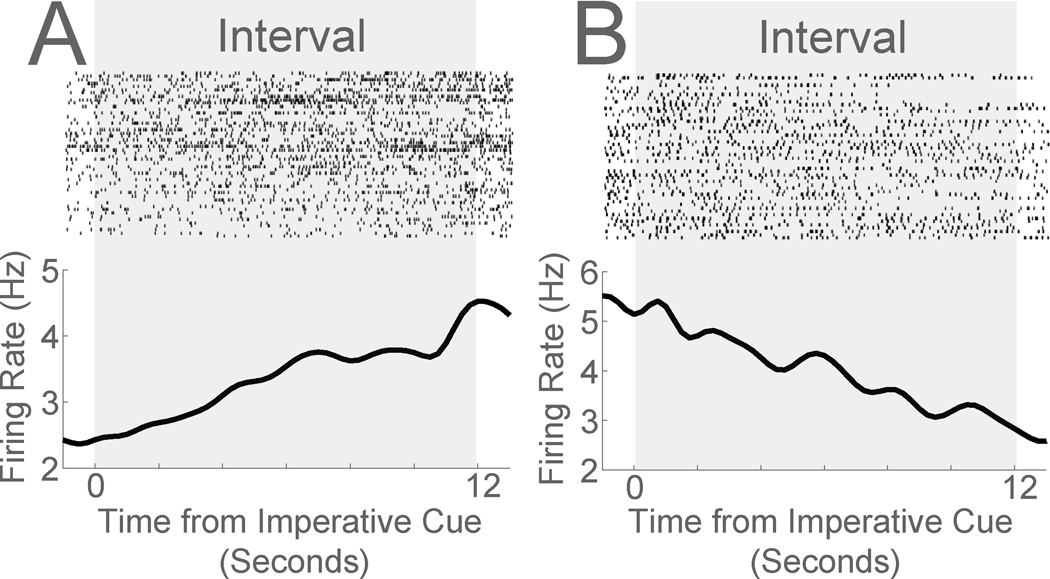
We have found that during timing tasks, ramping activity is the most common pattern of activity in the medial prefrontal cortex (Fig 3A–B) (Narayanan, 2016). This can be quantified by data-driven methods such as principal component analysis or by linear regression (Narayanan and Laubach, 2006, 2009). Linear patterns are a major player in higher-order representation such as neuronal manifolds (Wang et al., 2018). During interval-timing tasks, this pattern is also commonly observed (Matell et al., 2003; Xu et al., 2014), and our work has found that neurons anticipating the interval end are the most common pattern of activity in mice and rats, explaining ~30% of prefrontal variance (range-15–51%) (Narayanan and Laubach, 2009; Parker et al., 2014, 2015c; Emmons et al., 2017; Kim et al., 2017; Emmons et al., 2019). In humans, scalp electrical potentials can steadily depolarize until a voluntary action and represent temporal signals (Shibasaki and Hallett, 2006; Kononowicz and van Rijn, 2014). Notably, human single-unit recordings also reveal ramping patterns of activity (Sheth et al., 2012; Kamiński et al., 2017; Fu et al., 2019).
Figure 3: Ramping neurons in medial prefrontal cortex during a 12-second fixed-interval timing task.
Peri-event rasters of mouse medial prefrontal single neurons during a 12-second fixed-interval timing task. Ramping neurons can exhibit A) monotonic increases or B) monotonic decreases in firing over the fixed interval. Data from recordings described in Zhang et al., 2019.
Ramping activity is notable for several reasons. First, the temporal code is readily evident in that the firing at any given moment represents elapsed time and lends itself to quantitative models based on drift-diffusion dynamics (Simen et al., 2011; Luzardo et al., 2013). These models predict that ramping activity ‘scales’ among prefrontal ensembles to encode short or long intervals and correlates with responses without predicting specific movements (Emmons et al., 2017; Wang et al., 2018). Second, linear models can explain high amounts of variance in ramping activity, although one study reported a logarithmic component to ramping activity during a maze-running task (Kim et al., 2013). While higher order manifolds or multivariate methods may capture additional population encoding (Paton and Buonomano, 2018; Remington et al., 2018; Wang et al., 2018), linear features of ramping activity are important because they are predicted by cellular and behavioral models (Durstewitz, 2003; Simen et al., 2011) and might readily be represented by downstream brain areas. In addition, the degree to which the system is linear might facilitate powerful mathematical tools to understand complex neuronal dynamics. Third, pharmacological manipulations such as dopamine-depletion and D1DR-receptor manipulations that impair temporal control of action also impair ramping patterns of activity without affecting other prefrontal patterns of activity (Parker et al., 2014, 2015b, 2015c). Furthermore, inactivating medial prefrontal cortex disrupts ramping activity in other areas, such as the dorsomedial striatum (Emmons et al., 2017). Fourth, time can be robustly decoded from ramping signals, although non-ramping signals can also contribute (Emmons et al., 2017). Taken together, these data make the compelling case that ramping activity is closely linked to the temporal control of action. Notably, other patterns of neuronal activity can encode temporal features (Matell and Meck, 2004; Karmarkar and Buonomano, 2007; Laje and Buonomano, 2013), but in our hands, ramping activity explains the majority of variance and correlates with behavioral manipulations.
In our work, we argue that medial prefrontal ramping activity is related to guiding upcoming movements in time rather than controlling specific movements. Our premise is supported by three lines of evidence: 1) ramping is observed when animals are holding a lever above a force threshold and not moving (Narayanan and Laubach, 2006, 2009), 2) ramping patterns of neuronal activity are prominent prior to the initial response during interval timing tasks (Emmons et al., 2017), 3) ramping activity persists even when movement-related activity is explicitly accounted for in regression models (Emmons et al., 2017, 2019). Combined with microstimulation studies showing that medial prefrontal stimulation does not reliably evoke movements, and anatomical studies suggesting that medial prefrontal cortex does not have strong links to cortical or subcortical motor nuclei (Neafsey et al., 1986; Gabbott et al., 2005), together these data suggest that medial prefrontal cortex plays a key role in planning movements in time.
At the macro-level, we have consistently observed bursts of midfrontal 4 Hz activity in ‘delta’ or ‘theta’ bands during timing tasks (Narayanan et al., 2013a; Parker et al., 2014, 2015b; Kim et al., 2017). These bursts originate from the human medial prefrontal cortex (Kelley et al., 2018) and are remarkable because they are highly conserved in humans and rodents and depend on dopamine in both species (Narayanan et al., 2013a; Parker et al., 2015b; Kim et al., 2017). Midfrontal 4 Hz activity is a mechanism of cognitive control and is phase-locked to salient events such as novelty, errors, feedback, and to imperative stimuli during timing tasks (Cavanagh and Frank, 2014). Timing-related midfrontal 4 Hz oscillations can be dysfunctional in diseases with impaired temporal control of action such as Parkinson’s disease and schizophrenia (Parker et al., 2015b, 2017; Kim et al., 2017). Because these oscillations can be specifically coherent with distant brain areas and with prefrontal neurons involved in temporal and error-related processing (Narayanan et al., 2013a; Parker et al., 2014, 2015b; Kim and Narayanan, 2018), our working model is that these oscillations engage prefrontal ramping neurons instantiating temporal control of action. Accordingly, midfrontal 4 Hz power is a cognitive control signal that represents the ‘starting gun’ that initiates temporal processing in timing models (Church, 2003).
Stimulation
Preceding lines of evidence from lesion, pharmacology and neurophysiology suggest that there might be opportunities to modulate the temporal control of action, particularly when it is dysfunctional. In addition to the basic-science significance of this manipulation, brain stimulation has the possibility to deliver therapies for human brain diseases (Kim et al., 2015; Zhang et al., 2019). Indeed, for motor symptoms of Parkinson’s disease, detailed circuit mapping and neurophysiology led to breakthroughs for clinical deep-brain stimulation, which vastly improve motor symptoms of Parkinson’s disease (Limousin et al., 1998; Deuschl et al., 2006). Because temporal control of action is intimately linked to a range of cognitive functions in diseases such as Parkinson’s disease, ADHD and schizophrenia, improving temporal control of action may have real-world benefits.
We found that low-frequency brain stimulation at 2 Hz — in the range of midfrontal cognitive control signals — improved interval timing in dopamine-depleted animals (Kim et al., 2017). This intervention was highly specific, targeting prefrontal D1DR+ neurons in the medial prefrontal cortex of mice. Amazingly, 2 Hz brain stimulation also boosted medial prefrontal ramping activity and temporal decoding. Subsequent work from our group found that D1DR+ neurons had specific coherence with ramping neurons and that stimulation early in the interval was sufficient to improve interval timing. Non-specific stimulation of all prefrontal neurons or stimulation in non-dopamine-depleted animals had no reliable effect (Kim and Narayanan, 2018). Similarly, we found that stimulation of cerebellothalamic afferents at 2 Hz could compensate for timing deficits caused by disrupting medial prefrontal D1DRs (Parker et al., 2017). Finally, we found that stimulating corticostriatal projections at 20 Hz could compensate for medial prefrontal inactivation during interval timing (Emmons et al., 2019). These data suggest that specific prefrontal stimulation has the potential to compensate for dysfunctional prefrontal circuits.
These studies also raise the possibility that brain stimulation can improve temporal control of action in human diseases. Fortuitously, clinical deep-brain stimulation targeting the subthalamic nucleus can modulate medial prefrontal networks, possibly via backfiring monosynaptic ‘hyperdirect’ projections (Chen et al., 2020; Narayanan et al., 2020). Subthalamic and prefrontal neurons can have coherence at 4 Hz (Herz et al., 2016; Zavala et al., 2016). Low-frequency clinical deep-brain stimulation of the subthalamic nucleus is able to modulate interval-timing (Wojtecki et al., 2011). We found – to our surprise – that 4 Hz subthalamic brain stimulation increased midfrontal cognitive control signals and improved temporal control of action in Parkinson’s disease patients (Kelley et al., 2018). This finding was supported by data showing that 5 Hz subthalamic nucleus stimulation could also improve conflict tasks (Scangos et al., 2018). This line of work is exciting because it suggests that targeted prefrontal brain stimulation has the potential to improve temporal control of action in human brain diseases, which may point the way for future neuromodulation strategies targeted at cognition.
Conclusion
In summary, we have reviewed the medial prefrontal neurophysiology in the temporal control of action. Much of this data comes from two paradigms: simple-reaction time tasks and interval timing, which can be markedly abnormal in human diseases such as Parkinson’s disease, schizophrenia, and ADHD. Lesion evidence in humans and rodents implicates medial prefrontal regions as well as dopaminergic and cholinergic neurotransmission in temporal control of action. Medial prefrontal recordings consistently report ‘ramping’ patterns of activity in anticipation of temporally controlled movements and ~4 Hz activity in response to imperative stimuli. Low-frequency stimulation can improve temporal control in rodent models of human disease as well as in Parkinson’s disease patients. Together, these studies illuminate how medial prefrontal regions orchestrate behavior in time, which could inspire novel neuromodulation therapies for human diseases.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang D-R, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M (2002) Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22:3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akert K (1964) The frontal granular cortex and behavior. New York: McGraw-Hill. [Google Scholar]
- Amiez C, Procyk E (2019) Chapter 4 - Midcingulate somatomotor and autonomic functions. In: Handbook of Clinical Neurology (Vogt BA, ed), pp 53–71 Cingulate Cortex. Elsevier. Available at: http://www.sciencedirect.com/science/article/pii/B9780444641960000042 [Accessed July 1, 2020]. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD (1997) Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naïve patients. Lancet 349:1730–1734. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Wang MJ, Paspalas CD (2012) Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76:223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Latham PE, Pouget A (2006) Neural correlations, population coding and computation. Nat Rev Neurosci 7:358–366. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Koplowitz S, Anderson T, McMurray MB (1997) Sense of time in children with ADHD: effects of duration, distraction, and stimulant medication. J Int Neuropsychol Soc 3:359–369. [PubMed] [Google Scholar]
- Bateson M (2003) Interval timing and optimal foraging. In: Functional and neural mechanisms of interval timing, pp 113–141. Boca Raton, FL, US: CRC Press. [Google Scholar]
- Bi C, Yuan X, Huang X (2013) The impact of object working memory on timing. Journal of Cognitive Psychology 25:390–399. [Google Scholar]
- Bluschke A, Schuster J, Roessner V, Beste C (2018) Neurophysiological mechanisms of interval timing dissociate inattentive and combined ADHD subtypes. Scientific Reports 8:2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SW (2006) Timing and executive function: Bidirectional interference between concurrent temporal production and randomization tasks. Memory & Cognition 34:1464–1471. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS (1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205:929–932. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH (2005) What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6:755–765. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Reyes MB, Gathers C-A, Oprisan SA, Buhusi M (2018) Inactivation of the Medial-Prefrontal Cortex Impairs Interval Timing Precision, but Not Timing Accuracy or Scalar Timing in a Peak-Interval Procedure in Rats. Front Integr Neurosci 12 Available at: 10.3389/fnint.2018.00020/full [Accessed June 28, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G (2011) Cingulate, Frontal, and Parietal Cortical Dysfunction in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry 69:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP (2009) Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain and cognition 70:181–190. [DOI] [PubMed] [Google Scholar]
- Caselli L, Iaboli L, Nichelli P (2009) Time estimation in mild Alzheimer’s disease patients. Behav Brain Funct 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ (2014) Frontal theta as a mechanism for cognitive control. Trends Cogn Sci (Regul Ed) 18:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Lou S, Huang Z-H, Wang Z, Shan Q-H, Wang Y, Yang Y, Li X, Gong H, Jin Y, Zhang Z, Zhou J-N (2020) Prefrontal Cortex Corticotropin-Releasing Factor Neurons Control Behavioral Style Selection under Challenging Situations. Neuron 0 Available at: https://www.cell.com/neuron/abstract/S0896-6273(20)30060-X [Accessed April 5, 2020]. [DOI] [PubMed] [Google Scholar]
- Church R (2003) A Concise Introduction to Scalar Timing Theory. In: Functional and Neural Mechanisms of Interval Timing (Meck W, ed). CRC Press. Available at: 10.1201/9780203009574.sec1 [Accessed July 23, 2013]. [DOI] [Google Scholar]
- Craig DPA, Varnon CA, Sokolowski MBC, Wells H, Abramson CI (2014) An Assessment of Fixed Interval Timing in Free-Flying Honey Bees (Apis mellifera ligustica): An Analysis of Individual Performance. PLOS ONE 9:e101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G et al. (2006) A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355:896–908. [DOI] [PubMed] [Google Scholar]
- Durstewitz D (2003) Self-Organizing Neural Integrator Predicts Interval Times through Climbing Activity. J Neurosci 23:5342–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons EB, Corte BJD, Kim Y, Parker KL, Matell MS, Narayanan NS (2017) Rodent Medial Frontal Control of Temporal Processing in the Dorsomedial Striatum. J Neurosci 37:8718–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons EB, Kennedy M, Kim Y, Narayanan NS (2019) Corticostriatal stimulation compensates for medial frontal inactivation during interval timing. Scientific Reports 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV, Teräväinen H, Calne DB (1981) Reaction time in Parkinson’s disease. Brain 104:167–186. [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Shadlen MN, Jazayeri M, Nobre AC, Buonomano DV (2015) Time in Cortical Circuits. J Neurosci 35:13912–13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Wu D-AJ, Ross I, Chung JM, Mamelak AN, Adolphs R, Rutishauser U (2019) Single-Neuron Correlates of Error Monitoring and Post-Error Adjustments in Human Medial Frontal Cortex. Neuron 101:165–177.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J (2008) The Prefrontal Cortex, 4th Edition. New York, NY: Academic Press. [Google Scholar]
- Fuster JM (2001) The prefrontal cortex--an update: time is of the essence. Neuron 30:319–333. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Bacon SJ (2003) Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res 993:59–71. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ (2005) Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177. [DOI] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B (2000) Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci 20:4745–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN (2007) The neural basis of decision making. Annu Rev Neurosci 30:535–574. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1996) Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A 93:13473–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1998) The cortical dopamine system: role in memory and cognition. Adv Pharmacol 42:707–711. [DOI] [PubMed] [Google Scholar]
- Hart H, Radua J, Mataix-Cols D, Rubia K (2012) Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD). Neurosci Biobehav Rev 36:2248–2256. [DOI] [PubMed] [Google Scholar]
- Herz DM, Tan H, Brittain J-S, Fischer P, Cheeran B, Green AL, FitzGerald J, Aziz TZ, Ashkan K, Little S, Foltynie T, Limousin P, Zrinzo L, Bogacz R, Brown P (2017) Distinct mechanisms mediate speed-accuracy adjustments in cortico-subthalamic networks. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz DM, Zavala BA, Bogacz R, Brown P (2016) Neural Correlates of Decision Thresholds in the Human Subthalamic Nucleus. Curr Biol 26:916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton SC, Paulsen JS, Hoffmann RG, Reynolds NC, Zimbelman JL, Rao SM (2007) Motor timing variability increases in preclinical Huntington’s disease patients as estimated onset of motor symptoms approaches. Journal of the International Neuropsychological Society 13:539–543. [DOI] [PubMed] [Google Scholar]
- Hove MJ, Gravel N, Spencer RMC, Valera EM (2017) Finger tapping and pre-attentive sensorimotor timing in adults with ADHD. Exp Brain Res 235:3663–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen CF (1935) Functions of frontal association area in primates. Archives of Neurology & Psychiatry 33:558–569. [Google Scholar]
- Jacobsen CF, Nissen HW (1937) Studies of cerebral function in primates. IV. The effects of frontal lobe lesions on the delayed alternation habit in monkeys. Journal of Comparative Psychology 23:101. [Google Scholar]
- Jones CRG, Jahanshahi M (2014) Motor and perceptual timing in Parkinson’s disease. Adv Exp Med Biol 829:265–290. [DOI] [PubMed] [Google Scholar]
- Jones DNC, Higgins GA (1995) Effect of scopolamine on visual attention in rats. Psychopharmacology 120:142–149. [DOI] [PubMed] [Google Scholar]
- Jurkowski AJ, Stepp E, Hackley SA (2005) Variable foreperiod deficits in Parkinson’s disease: Dissociation across reflexive and voluntary behaviors. Brain and Cognition 58:49–61. [DOI] [PubMed] [Google Scholar]
- Kamiński J, Sullivan S, Chung JM, Ross IB, Mamelak AN, Rutishauser U (2017) Persistently active neurons in human medial frontal and medial temporal lobe support working memory. Nat Neurosci 20:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV (2007) Timing in the Absence of Clocks: Encoding Time in Neural Network States. Neuron 53:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R, Flouty O, Emmons EB, Kim Y, Kingyon J, Wessel JR, Oya H, Greenlee JD, Narayanan NS (2018) A human prefrontal-subthalamic circuit for cognitive control. Brain 141:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ghim J-W, Lee JH, Jung MW (2013) Neural correlates of interval timing in rodent prefrontal cortex. J Neurosci 33:13834–13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung AH, Byun J, Jo S, Jung MW (2009) Inactivation of medial prefrontal cortex impairs time interval discrimination in rats. Front Behav Neurosci 3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Alberico S, Emmons E, Narayanan N (2015) New therapeutic strategies targeting D1-type dopamine receptors for neuropsychiatric disease. Frontiers in Biology:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Han S-W, Alberico SL, Ruggiero RN, De Corte B, Chen K-H, Narayanan NS (2017) Optogenetic Stimulation of Frontal D1 Neurons Compensates for Impaired Temporal Control of Action in Dopamine-Depleted Mice. Curr Biol 27:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Narayanan NS (2018) Prefrontal D1 Dopamine-Receptor Neurons and Delta Resonance in Interval Timing. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A (2010) The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev 34:1307–1350. [DOI] [PubMed] [Google Scholar]
- Kononowicz TW, van Rijn H (2014) Decoupling interval timing and climbing neural activity: a dissociation between CNV and N1P2 amplitudes. J Neurosci 34:2931–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laje R, Buonomano DV (2013) Robust timing and motor patterns by taming chaos in recurrent neural networks. Nat Neurosci 16:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach M, Amarante LM, Swanson K, White SR (2018) What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth-Steensen C, King Elbaz Z, Douglas VI (2000) Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica 104:167–190. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL (1998) Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 339:1105–1111. [DOI] [PubMed] [Google Scholar]
- Long MA, Katlowitz KA, Svirsky MA, Clary RC, Byun TM, Majaj N, Oya H, Howard MA, Greenlee JDW (2016) Functional Segregation of Cortical Regions Underlying Speech Timing and Articulation. Neuron 89:1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce RD (1991) Response Times: Their Role in Inferring Elementary Mental Organization. Oxford University Press. [Google Scholar]
- Luman M, Papanikolau A, Oosterlaan J (2015) The Unique and Combined Effects of Reinforcement and Methylphenidate on Temporal Information Processing in Attention-Deficit/Hyperactivity Disorder. J Clin Psychopharmacol 35:414–421. [DOI] [PubMed] [Google Scholar]
- Luzardo A, Ludvig EA, Rivest F (2013) An adaptive drift-diffusion model of interval timing dynamics. Behav Processes 95:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malapani C, Deweer B, Gibbon J (2002) Separating storage from retrieval dysfunction of temporal memory in Parkinson’s disease. J Cogn Neurosci 14:311–322. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J (1998) Coupled temporal memories in Parkinson’s disease: a dopamine-related dysfunction. J Cogn Neurosci 10:316–331. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH (2004) Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res 21:139–170. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH, Nicolelis MAL (2003) Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behavioral Neuroscience 117:760–773. [DOI] [PubMed] [Google Scholar]
- Merchant H, de Lafuente V (2014) Introduction to the neurobiology of interval timing. Adv Exp Med Biol 829:1–13. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Pribram KH (1955) Analysis of the effects of frontal lesions in monkey. I. Variations of delayed alternation. J Comp Physiol Psychol 48:492–495. [DOI] [PubMed] [Google Scholar]
- Naatanen R (1971) Non-aging fore-periods and simple reaction time. Acta Psychologia 35:316–327. [Google Scholar]
- Naatanen R (1972) Time uncertainty and occurrence uncertainty of the stimulus in a simple reaction time task. Acta Psychologia 36:492–503. [Google Scholar]
- Naatanen R, Muranen V, Merisalo A (1974) Timing of expectance peak in simple reaction time situation. Acta Psychol (Amst) 38:461–470. [DOI] [PubMed] [Google Scholar]
- Namboodiri VMK, Hussain Shuler MG (2014) Report of interval timing or action? Proc Natl Acad Sci USA 111:E2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS (2016) Ramping activity is a cortical mechanism of temporal control of action. Curr Opin Behav Sci 8:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, Laubach M (2013a) Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci 16:1888–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M (2006) Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 139:865–876. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ (2012) Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci USA 109:20726–20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M (2006) Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52:921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M (2008) Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J Neurophysiol 100:520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M (2009) Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophysiol 101:2859–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JDE (2005) The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related FMRI analysis. Neuropsychology 19:223–232. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Rodnitzky RL, Uc EY (2013b) Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev Neurosci 24:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Wessel JR, Greenlee JDW (2020) The Fastest Way to Stop: Inhibitory Control and IFG-STN Hyperdirect Connectivity. Neuron 106:549–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR (1986) The organization of the rat motor cortex: a microstimulation mapping study. Brain Res 396:77–96. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M (1976) Prefrontal unit activity and delayed response: relation to cue location versus direction of response. Brain Res 105:79–88. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M (1979) Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res 171:213–224. [DOI] [PubMed] [Google Scholar]
- Noreika V, Falter CM, Rubia K (2013) Timing deficits in attention-deficit/hyperactivity disorder (ADHD): evidence from neurocognitive and neuroimaging studies. Neuropsychologia 51:235–266. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M (1997) Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature 385:634–636. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M (2008) Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci 1129:225–235. [DOI] [PubMed] [Google Scholar]
- Parker KL, Alberico SL, Miller AD, Narayanan NS (2013a) Prefrontal D1 dopamine signaling is necessary for temporal expectation during reaction time performance. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Narayanan NS (2014) D1-Dependent 4 Hz Oscillations and Ramping Activity in Rodent Medial Frontal Cortex during Interval Timing. J Neurosci 34:16774–16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Narayanan NS (2015a) Medial frontal 4-Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. Journal of Neurophysiology 114:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Naryanan NS (2015b) Medial frontal ~4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol:jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Kim YC, Kelley RM, Nessler AJ, Chen K-H, Muller-Ewald VA, Andreasen NC, Narayanan NS (2017) Delta-frequency stimulation of cerebellar projections can compensate for schizophrenia-related medial frontal dysfunction. Mol Psychiatry 22:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Lamichhane D, Caetano MS, Narayanan NS (2013b) Executive dysfunction in Parkinson’s disease and timing deficits. Front Integr Neurosci 7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Ruggiero RN, Narayanan NS (2015c) Infusion of D1 Dopamine Receptor Agonist into Medial Frontal Cortex Disrupts Neural Correlates of Interval Timing. Front Behav Neurosci 9:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Buonomano DV (2018) The Neural Basis of Timing: Distributed Mechanisms for Diverse Functions. Neuron 98:687–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L (2005) Interval-timing deficits in individuals at high risk for schizophrenia. Brain and cognition 58:109–118. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S (2006a) Effects of Focal Frontal Lesions on Response Inhibition. Cereb Cortex Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16699079. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S (2006b) Keeping time: effects of focal frontal lesions. Neuropsychologia 44:1195–1209. [DOI] [PubMed] [Google Scholar]
- Preuss T (1995) Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. Journal of Cognitive Neuroscience 7:1–24. [DOI] [PubMed] [Google Scholar]
- Pribram KH (1950) Some physical and pharmacological factors affecting delayed response performance of baboons following frontal lobotomy. J Neurophysiol 13:373–382. [DOI] [PubMed] [Google Scholar]
- Pribram KH, Mishkin M (1956) Analysis of the effects of frontal lesions in monkey. III. Object alternation. J Comp Physiol Psychol 49:41–45. [DOI] [PubMed] [Google Scholar]
- Pribram KH, Mishkin M, Rosvold HE, Kaplan SJ (1952) Effects on delayed-response performance of lesions of dorsolateral and ventromedial frontal cortex of baboons. J Comp Physiol Psychol 45:565–575. [DOI] [PubMed] [Google Scholar]
- Remington ED, Egger SW, Narain D, Wang J, Jazayeri M (2018) A Dynamical Systems Perspective on Flexible Motor Timing. Trends in Cognitive Sciences 22:938–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M (2003) Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci 17:1498–1508. [DOI] [PubMed] [Google Scholar]
- Rommelse NNJ, Altink ME, Oosterlaan J, Beem L, Buschgens CJM, Buitelaar J, Sergeant JA (2008) Speed, variability, and timing of motor output in ADHD: which measures are useful for endophenotypic research? Behav Genet 38:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Woolsey CN (1949) Organization of the mammalian thalamus and its relationships to the cerebral cortex. Electroencephalogr Clin Neurophysiol 1:391–403; discussion 403–4. [PubMed] [Google Scholar]
- Sarter M, Bruno JP (1997) Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Brain Res Rev 23:28–46. [DOI] [PubMed] [Google Scholar]
- Scangos KW, Carter CS, Gurkoff G, Zhang L, Shahlaie K (2018) A pilot study of subthalamic theta frequency deep brain stimulation for cognitive dysfunction in Parkinson’s disease. Brain Stimul 11:456–458. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN (2012) Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H, Hallett M (2006) What is the Bereitschaftspotential? Clin Neurophysiol 117:2341–2356. [DOI] [PubMed] [Google Scholar]
- Simen P, Balci F, de Souza L, Cohen JD, Holmes P (2011) A model of interval timing by neural integration. J Neurosci 31:9238–9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Taylor E, Rogers JW, Newman S, Rubia K (2002) Evidence for a pure time perception deficit in children with ADHD. Journal of Child Psychology and Psychiatry 43:529–542. [DOI] [PubMed] [Google Scholar]
- Sternberg S (1969) Memory-scanning: mental processes revealed by reaction-time experiments. Am Sci 57:421–457. [PubMed] [Google Scholar]
- Stubbs DA (1976) Scaling of Stimulus Duration by Pigeons1. Journal of the Experimental Analysis of Behavior 26:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI (2005) Multiple frontal systems controlling response speed. Neuropsychologia 43:396–417. [DOI] [PubMed] [Google Scholar]
- Tallot L, Doyère V (2020) Neural encoding of time in the animal brain. Neurosci Biobehav Rev 115:146–163. [DOI] [PubMed] [Google Scholar]
- Thoenes S, Oberfeld D (2017) Meta-analysis of time perception and temporal processing in schizophrenia: Differential effects on precision and accuracy. Clinical Psychology Review 54:44–64. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B (2003) Do rats have a prefrontal cortex? Behav Brain Res 146:3–17. [DOI] [PubMed] [Google Scholar]
- Valera EM, Spencer RMC, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ (2010) Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 68:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F et al. (2019) A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Narain D, Hosseini EA, Jazayeri M (2018) Flexible timing by temporal scaling of cortical responses. Nature Neuroscience 21:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Kellendonk C, Kandel ER, Balsam PD (2011) Timing as a window on cognition in schizophrenia. Neuropharmacology Available at: http://www.ncbi.nlm.nih.gov/pubmed/21530549 [Accessed September 8, 2011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearden JH, Smith-Spark JH, Cousins R, Edelstyn NMJ, Cody FWJ, O’Boyle DJ (2008) Stimulus timing by people with Parkinson’s disease. Brain Cogn 67:264–279. [DOI] [PubMed] [Google Scholar]
- Wojtecki L, Elben S, Timmermann L, Reck C, Maarouf M, Jorgens S, Ploner M, Sudmeyer M, Groiss SJ, Sturm V, Niedeggen M, Schnitzler A (2011) Modulation of Human Time Processing by Subthalamic Deep Brain Stimulation. PLoS One 6 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3171456/ [Accessed June 5, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Zhang S, Dan Y, Poo M (2014) Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proc Natl Acad Sci USA 111:480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala B, Tan H, Ashkan K, Foltynie T, Limousin P, Zrinzo L, Zaghloul K, Brown P (2016) Human subthalamic nucleus-medial frontal cortex theta phase coherence is involved in conflict and error related cortical monitoring. Neuroimage 137:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Jung D, Larson T, Kim Y, Narayanan NS (2019) Scopolamine and Medial Frontal Stimulus-Processing during Interval Timing. Neuroscience 414:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]