Abstract
Cordyceps militaris (C. militaris) is a medicinal mushroom possessing a variety of biofunctionalities. It has several biologically important components such as polysaccharides and others. The diverse pharmacological potential of C. militaris has generated interest in reviewing the current scientific literature, with a particular focus on prevention and associated molecular mechanisms in inflammatory diseases. Due to rising global demand, research on C. militaris has continued to increase in recent years. C. militaris has shown the potential for inhibiting inflammation-related events, both in in vivo and in vitro experiments. Inflammation is a multifaceted biological process that contributes to the development and severity of diseases, including cancer, colitis, and allergies. These functions make C. militaris a suitable functional food for inhibiting inflammatory responses such as the regulation of proinflammatory cytokines. Therefore, on the basis of existing information, the current study provides insights towards the understanding of anti-inflammatory activity-related mechanisms. This article presents a foundation for clinical use, and analyzes the roadmap for future studies concerning the medical use of C. militaris and its constituents in the next generation of anti-inflammatory drugs.
Keywords: Cordyceps militaris, inflammation, polysaccharides, pharmacokinetics, COX-2, matrix metalloproteinases
1. Introduction
Inflammation is a complex process that occurs as a result of various chemical or physical substances in the body, including pathogens, trauma, toxic substances and others [1]. Type of inflammatory stimulus, and the effectiveness of the response, determine the nature of inflammation as acute or chronic. Augmented synthesis of inflammatory mediators has been associated with chronic ailments such as cancer, arthritis, asthma, viral diseases, atherosclerosis, and others [2]. As a result, slowing down inflammatory events has become crucial, and non-steroid anti-inflammatory medicines (NSAIDs) are commonly utilized for this purpose. NSAIDs have a number of side effects, including renal failure, bronchospasm, gastrointestinal problems, water retention, and hypersensitivity responses [3].
As a result, substantial attention has recently been given to the development of natural anti-inflammatory products with greater safety and minimal side effects. Natural products have been in continuous use since prehistoric times for the alleviation of diseases and overall wellbeing. The modern system of medicine was initiated through the isolation of pharmacologically active morphine, which served as the basis for the construction of a therapeutic empire composed of compounds isolated or derived from natural sources [4]. Previously the shifting of paradigm from isolation from natural sources towards synthesis or combinatorial chemistry urged scientists to focus on the large-scale synthesis of drug substances. Due to considerable efforts and reduced productivity in the synthetic process, recent decades have witnessed the regaining of involvement of natural products in drug development in conjunction with new technological approaches such as high-throughput selection [4,5]. Traditional acceptance, cost effectiveness and unique chemical diversity of metabolites have led to exploitation of natural resources for the identification of various pharmacological leads and scaffolds, serving the benefit of humanity [6].
Medicinal mushrooms have remained an important component of human culture. The genus Cordyceps is one of the largest genera in the family Clavicipitaceae, containing over 750 species, and being extremely diverse in terms of the number of species, their morphology and acclimatization on varied hosts [7,8]. These diverse species are mostly distributed in Asian countries (such as Korea, Japan, Nepal, China), and other parts of the world in humid temperate and tropical habitats. Occurrence of a variety of species in different environmental conditions throughout the world indicates its global distribution [9,10]. Specialized and coordinated mechanisms are involved in the association between the Cordyceps species and related hosts. They maintain their life cycles according to the characteristics of the hosts for the growth and survival, after evading their immune systems and subsequent production of defensive secondary metabolites by the hosts, which can be considered as promising sources of new drugs [9]. Based upon this property, these species have gained profound importance as a source of natural products having diverse biological activities [10]. Recently, Cordyceps species growing in the wild have been surpassed by artificially cultured specimens due to the scarcity and high price associated with collection and processing [11]. C. militaris is an ethnomedicinal fungi having importance in traditional Chinese medicines and extensively used as a crude drug and a functional food in Asia [12]. C. militaris is the second most popular, investigated species in its genus. Several pharmacological activities of this species have been documented, including blood glucose control, hypolipidemic, anti-tumor, anti-microbial, anti-viral, antiprotozoal, anti-inflammatory, neuroprotective, anti-oxidant and immuno-protective activities [10,12]. Therefore, the C. militaris may be considered an important candidate for the treatment of different ailments. Mycological data of C. militaris is given below [12]. Natural and cultured C. militaris are shown in Figure 1.
Figure 1.
Natural (A) and cultivated (B) C. militaris.
In today’s world, the concept of “prevention is better than cure” is prevalent, resulting in the establishment of safety and therapeutic profiles of food items. Functional foods can be categorized as foods marketed under the labels of healthy food and food sources having physiological properties besides their nutritional uses. Additionally, natural materials that can be used daily for regulating or affecting the body system upon intake can also be called functional foods [13,14]. According to the European Consensus, the most accurate definition is that “a food can be considered as functional beyond adequate nutritional effects, if it reasonably improves target functions in the body in a way that contributes to improving health and minimizing disease risk”. Functional foods are natural substances; however, different constituents can be incorporated or removed from them through biotechnological procedures [15] to improve health, or help avert diseases in individual or defined groups based upon gender and age [16,17]. The existence or deliberate addition of certain factors such as fibers, flavonoids, polyphenols, anthocyanins, minerals, fatty acids and carotenoids, among others, enhances the nutritional value of the dietary components. Vitamins, minerals and other nutritional components were formerly incorporated in the manufacturing of functional foods. Prebiotics and probiotics, which have a tendency to target metabolic syndrome and other ailments [18,19], along with high blood pressure, cellular damage, oxidation [18,20], are well documented functional foods. These have unique immune-modulatory and immune-boasting potential, and they can enhance immunity through symbiotic association with humans [12]. Apart from treatment functionalities, C. militaris might be a good candidate for consideration as functional food due to the presence of metabolites of therapeutic or protective capacities.
An extensive number of bioactive constituents have been identified from this species including cordycepin, ergosterol, carotenoids, mannitol, proteins, essential amino acids, volatile oils, carotenoids, minerals, vitamins, nucleosides, and sterols, as well as several types of carbohydrates such as mono, oligo, and polysaccharides, which shows its pharmacological and palliative significance [12]. The industrial sector in China is involved in large-scale fermentation and commercial cultivation of its stromata, which is attributed to a decline in the wild population, and satisfies the increasing demand for medicinal, edible and nutritional purpose [21]. Further, the bioactive compounds produced in fermented culture is a major reason for its industrial production [21,22]. Currently, at least 36 health foods prepared from this medicinal fungus have been approved in China. Moreover, health food products of C. militaris mycelia in form of powder (Z20030034) and capsules (Z20030035) have gained the status of commercially approved moieties with claims of having beneficial effects on kidneys and lungs, and being effective against cough, asthma, phlegm, cold limbs, fatigue, dizziness, tinnitus, and other ailments. Furthermore, since the Chinese Ministry of Health recognized it as a Novel Food in 2009, it has been widely consumed as a regular food item [23].
The objective of the present study is to provide a comprehensive update on the pharmacological effects of C. militaris in terms of inflammation prevention, as well as the mechanisms of action at the molecular levels that may contribute to its anti-inflammatory properties.
1.1. Chemical Constituents
1.1.1. Proteins and Peptides
Fresh raw material is preferred over processed or dried parts for the isolation of the maximum content of bioactive peptides and proteins from medicinal mushrooms [24]. An important peptide, cordymin, isolated from C. militaris is about 10.9 kDa in size and its N-terminal sequence makes it unique among other peptides. Cordymin was observed to inhibit many fungal species, such as Rhizoctonia solani, Mycosphaerella arachidicola and others. It is also reported to decrease the proliferation of MCF-7 breast cancer cells and levels of HIV-1 reverse transcriptase [25]. Additionally, it has preventive effects against osteopenia by increasing bone mineral content and density in diabetic rats [26]. Another lectin, named CML, has been isolated from C. militaris by gel filtration chromatography, and is approximately a 31 kDa peptide. It is distinctive from the lectins identified from several mushroom species including Polyporus squamosus, Laetiporus sulphureus, and others in terms of differences in secondary structure, containing 29% β-turns, 12% β-sheets, 27% α-helix, and 32% random-coil structures. CML has been observed to exert hemagglutinating activity in experimental animals by specifically combining sialoglycoproteins. This compound has also shown to have mitogenic activity on mouse splenocytes [27]. Similarly, another report discussed the isolation of C. militaris protein (CMP). It has also exhibited antiproliferative effects on human breast cancer MCF-7 cells (IC50 9.3 μM) and bladder cancer 5637 cells (IC50 8.1 μM). It also possesses antifungal activity against Fusarium oxysporum at a minimum concentration of 1.6 μM [28]. Three protein-polysaccharide polymer complexes (CPSP-F1, CPSP-F2, and CPSP-F3) have been isolated form by C. militaris using an ultrasound-extraction and column chromatography technique. Among these compounds, CPSP-F1 and CPSP-F2 exhibited inhibitory IC50 as 32.2 ± 0.2 mg/mL and 5.3 ± 0.0 mg/mL in an acetylcholinesterase inhibitory assay, suggesting its ability to be used against Alzheimer’s disease [29]. A fibrinolytic enzyme has also been produced by C. militaris using a submerged fermentation technique. The specific enzyme was purified from culture supernatant by hydrophobic interaction, ion exchange and gel filtration chromatographic strategies. It prevented clot formation by degrading the α, β and γ chains of fibrinogen, and also activated plasminogen into plasmin, thus proving to be a good anticoagulant agent [30]. Another isolated novel protein is “C. militaris immune-regulatory protein” (CMIP), which showed anti-metastatic activity on a 4T1 breast cancer lung metastasis, numbers of tumor nodules in the lung, and improved survival rate in experimental animals [31].
1.1.2. Polysaccharides
Polysaccharides in fungal species are among the most prevalent and significant biologically active constituents extracted from fruiting bodies, mycelium and fermentation broth. These substances show diverse physicochemical properties and have been the target of developing quality control of mushrooms, especially C. militaris, containing functional foods [32]. In this species, polysaccharides have been divided into two types i.e., intracellular polysaccharides and extracellular polysaccharides, based on their formation or origin in fungal cells. The polysaccharides of C. militaris are reported to exhibit a range of biofunctions, such as immunomodulatory, antioxidant, antitumor and anti-inflammatory effects. A novel polysaccharide (PCLM) obtained from the culture broth of C. militaris enhanced the immune-stimulatory activity of RAW264.7 macrophages by modulating the release of toxic molecules (nitric oxide (NO) and super oxide dismutase (SOD)) and cytokine tumor necrosis factor (TNF)-α, as well as inducing phagocytosis [33]. Another report suggested that Cordyceps polysaccharides have the tendency to overcome CY-induced immunosuppression, and significantly enhanced the function of spleen lymphocytes and macrophages [34]. A previous study reported CMP-W1 and CMP-S1 (two polysaccharides) showed significant enhancement of proliferation of spleen cells from an animal model [35]. In another finding, the functional polysaccharides CMP40 and CMP50 enhanced lymphocyte proliferation, interleukin (IL)-4 concentrations and antibody titers in the serum [32]. The polysaccharides obtained from C. militaris also possess strong antioxidant activities. Three polysaccharides extracted from C. militaris exhibited strong anti-radical activity. The W-CBP50II polysaccharide was observed to have more activity against hydroxyl, superoxide and 1,1-diphenyl-2-picrylhyrazyl (DPPH)-radical scavenging activity [36]. Other novel polysaccharides such as CBP-1, P70-1 extracted and purified from the fruiting body of cultured C. militaris, exhibited strong hydroxyl radical scavenging activity with an IC50 value of 0.638 mg/mL and 0.548 mg/mL, respectively [32,37]. The exopolysaccharides of mutant C. militaris SU5-08 enhanced adaptive immune responses. Further, these polysaccharides have shown anticancer activity against a variety of cell lines. The polysaccharide CMP-I significantly inhibited the proliferation of HepG2, HeLa, K562 and HT29 cells [38]. Another report showed the dose-dependent inhibitory potential of C. militaris derived polysaccharides against BGC-823, MCF-7, and SMMC-7721 cells [39]. Another study reported the antiproliferative potential of a polysaccharide from C. militarism against adenocarcinomic human alveolar basal epithelial cells (A549) cells, with an IC50 of 39.08 μg/mL [38]. Additionally, the report confirm the inhibitory effects of C. militaris polysaccharides on colon 205, NCI-H460, PC-3 cell lines [40,41]. These components also inhibited tumor in animal model [42].
Yuko et al., reported a C. militaris-derived acidic polysaccharide (APS) containing arabinose (Ara), galacturonic acid, D-Gal, Rha, and Xyl with an MW of 5.76 × 105 Da. It showed beneficial health improving properties on influenza A virus infection via modulation of the macrophages related to immune functions [43]. Another study investigated the beneficial effects of a C. militaris-derived polysaccharide on maturation of dendritic cells (DC), suggesting that it might be used in cancer immunotherapy [44]. The protective effects of a C. militaris-derived polysaccharide on hydrogen peroxide-stimulated apoptosis in HL-7702 cells was investigated previously. The tested polysaccharide significantly reduced hydrogen peroxide-stimulated apoptosis, mitochondrial dysfunction, production of reactive oxygen species (ROS), decreased intracellular adenosine triphosphate levels, and augmented secretion of cytochrome C. [45]. The polysaccharides from cultured C. militaris have demonstrated anti-aging potential by preventing mitochondrial injury. It was observed that the tested sample inhibited Fe2(+)-L Cysteine activated swelling and mitochondrial damage in a dose-dependent manner [46]. In other studies, polysaccharides of C. militaris were found to boost immunological effectiveness against Newcastle disease vaccination in animals. The study suggested that the polysaccharides might be contenders for a novel form of immunological adjuvant [47]. Various studies on the isolation of polysaccharides from this species have been documented, such as CPS-1 polysaccharide from C. militaris composed of d-glucose (D-Glc), xylose (Xyl), rhamnose (Rha), d-mannose (D-Man), and d-galactose (D-Gal), with a molecular weight of 2.3 × 104 Da, which was extracted and reported as an immune-modulating agent [48]. Other polysaccharides such as cordlan [44], cysinocan consisting of D-Gal, D-Man, D-Glc, with an MW of 8.2 × 104 Da [49], two hetero-polysaccharides (CMP-W1; 3.66 × 105 Da and CMP-S1; 4.6 × 105 Da) consisting of D-Glc, D-Gal and D-Man, have been reported as immunomodulatory agents [35]. The polysaccharide CMPB90-1 with an MW of 5.8 × 103 Da was studied as a tumor eradication agent through immune regulatory activity [50]. Polysaccharide structure, including monosaccharide content and glycosidic linkages, is intimately associated with their immunomodulatory action. TLR2 is most likely to identify polysaccharides containing galactose and glucose, which regulate immunomodulatory functions [51,52]. Polysaccharides with α-d- and β-d-glucosidic bonds have been found to have a more positive impact on NO generation in macrophages. Therefore, it can be inferred that immunomodulatory potential of the CMPB90-1 polysaccharide may be attributed toward its monosaccharide like galactose and glucose. A previous study demonstrated polysaccharide mediated induction of M1 polarization, along with TLR2 as the membrane receptor of MC-2 on macrophages. These polysaccharides were structurally identical to CMPB90-1 [51,53]. The structure of CMPB90-1 regulates its activity on the phenotype of macrophages
1.1.3. Nucleosides
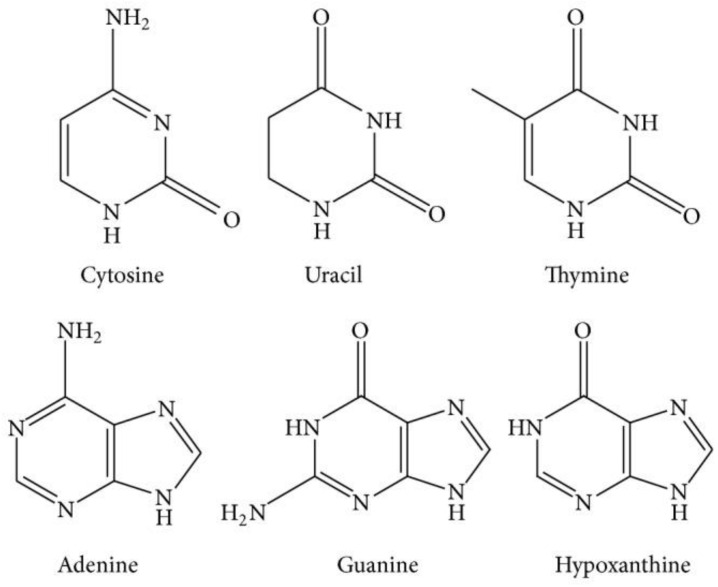
C. militaris contains nucleosides and nitrogenous bases (adenosine, guanosine, cytidine, uridine, adenine and uracil) as its main components, among which, adenosine significantly suppressed the release of neurotransmitters in the central nervous system and could be used to treat chronic heart failure, and also tonically inhibits the release of excitatory neurotransmitters [54]. This nucleoside is reported to be a neuronal modulator and to inhibit neuronal action, including regulation of the sleep cycle, regulation of seizure susceptibility, and locomotor effects. Pharmacologically it can be used as an analgesic agent, and also mediates the effects of ethanol and chronic drug use. Additionally, it is important as a neuroprotection agent [55]. Adenosine receptor subtypes in blood arteries have been identified and characterized, and when triggered have a considerable influence on peripheral circulation [56]. Another nucleoside, cytidine, has shown antidepressant-like effects in a forced swim test with rat models [57]. Moreover, intraperitoneal and oral administration of guanosine can inhibit α-dendrotoxin- and quinolinic acid-induced seizures in experimental animals [58]. The chemical structures of various nucleosides are presented in Figure 2.
Figure 2.
Chemical structure of six nucleosides.
Cordycepin, also known as 3′-deoxyadenosine, is a bioactive metabolite that was initially found in the fermented broth of C. militaris [59]. It is a nucleoside analogue that has been proved to possess an array of biological activities including antibacterial, antifungal, antitumor, antileukemia, and antiviral activities, as well as an immunoregulatory effect [60,61]. Different mechanisms of action have been identified, including inhibition of purine synthesis, RNA chain termination and interference in mTOR signal transduction [62]. Cordycepin has various pharmacological functions, one being antidiabetic activity. This compound has proved to be effective in reducing the blood glucose levels as well as blood glucose tolerance levels of alloxan-induced diabetic mice [63]. The mechanism of its antidiabetic activity is not yet fully understood. However, different reports investigated that cordycepin can suppress the production of proinflammatory cytokines (such as IL-1β, IL-6, TNF-α and NO) in LPS stimulated macrophages, leading to downregulation of type 2 diabetes-regulating genes (11β-hydroxysteroid dehydrogenase type 1 and peroxisome proliferated activated receptor-λ) [64]. It inactivates nuclear factor kappa B (NF-κB)-regulated inflammatory responses and corresponding reduced expression of diabetes-regulating genes [65,66]. Cordycepin has also been observed to exert anti-hyperlipidemic activity due to its structural resemblance to adenosine (activating adenosine mono phosphate kinase-AMPK), which can lead to inhibition of Acetyl CoA carboxylase and result in reduction of fatty acid synthesis [67]. It can also reduce the levels and accumulation rate of low-density lipoprotein cholesterol, triglycerides and total cholesterol in an effective manner [68]. Pertaining to its lipid lowering activities, this compound can be recognized as a prominent bioactive agent for cardiovascular illnesses treatment. It also enhances the cytokine release of resting peripheral blood mononuclear cells (PBMCs), and proliferation and transcription factors in PBMCs are amplified in a leukemia cells (THP-1), suggesting an immunomodulatory function [69]. Pure bioactive constituents isolated from C. militaris have demonstrated immunomodulatory effects, lowering the generation of anti-ds-DNA and boosting the survival rate of lupus mice [20]. An antiosteoporotic effect of cordycepin has been observed in osteopenic rat models, in which loss of bone was retrieved in the test animals. The reduced activity of alkaline phosphatase enzymes and tartrate-resistant acid phosphatase was also found in a variety of experiments models. Cordycepin consumption can induce the levels of osteocalcin (prominent marker of bone development), reduce oxidative stress and decrease the C-terminal cross-linked telopeptide of type I collagen content (bone resorption marker) in ovariectomized experimental animals [70]. The effects of cordycepin against malarial parasites in mice have been detected, in which the nucleic acids and protein synthesis of the parasites ceased [71]. Cordycepin is also a good anti-inflammatory, antihyperuricemic, and antioxidant agent, and considered to have the ability to treat respiratory disorders, kidney malfunctioning, arthritis, cancer and fertility issues [20]. In addition, the main component, cordycepin itself, has demonstrated antitumor, anticancer, insecticidal and antimicrobial activities [8]. The structure of cordycepin is shown in Figure 3.
Figure 3.
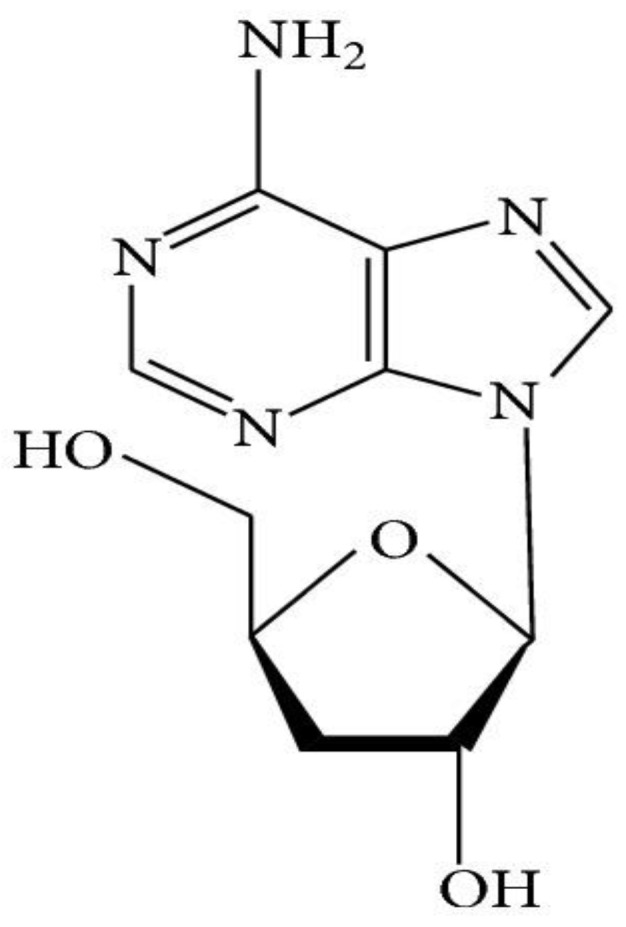
Chemical structure of cordycepin.
1.1.4. Phenolic Compounds
Medicinal mushrooms have a significant number of phenolic compounds, specifically polyphenols and flavonoids. These compounds have vital roles as antioxidants with various mechanisms including metal chelation, free radical scavenging, inhibition of LDL oxidation and enzyme modulation activities [72]. Yu et al., detected the presence of polyphenolic (60.2 μg/mL) and flavonoid (0.598 μg/mL) content in cultured C. militaris extracts [73]. Similarly, according to another report, phenols, flavonoids, lycopene, beta carotene and ascorbic acid were detected in methanolic extracts of C. militaris by UV visible spectroscopy [74].
1.1.5. Others
Along with the aforementioned constituents, C. militaris contains other metabolites that elevates its therapeutic significance. Ergosterol analogues of C. militaris are reported to exert their actions as antiviral and antiarrhythmic substances [75]. They have the tendency to suppress activated human mesangial cells as well as alleviate immunoglobulin A nephropathy (Berger’s disease) [76]. In addition, mannitol (sugar alcohol) and trehalose free sugars have been found in C. militaris. Mannitol and trehalose free sugars have also been found in C. militaris. Mannitol has certain bioactivities such as antitussive, diuretic, free radical inhibition and other bio-functionalities [77]. Certain novel carotenoids have been separated from its fruit bodies and identified as xanthophylls [78]. Pigmented compounds, such as carotene, can be incorporated, and functional foods produced in the food industry acting as improved anticancer functional foods in addition to traditional carotenoids. Moreover, a new cerebroside (glycosphingolipid) cordycerebroside A, along with soyacerebroside I and glucocerebroside, have been isolated from C. militaris. These compounds reduce the augmented pro-inflammatory iNOS and suppress cyclooxygenase (COX)-2 in LPS-stimulated monocyte/macrophage-like (RAW264.7) cells [79].
2. Pharmacological Actions of Cordyceps militaris
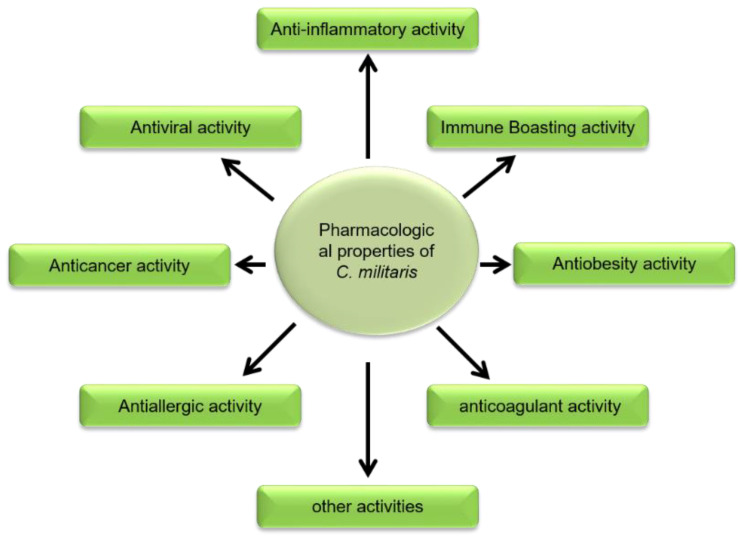
The medicinal mushroom C. militaris has been widely consumed in China for medication purpose since ancient times (3000 years). It is used for therapeutic treatment of lung and kidney malfunction, hyperglycemia and hyperlipidemia, respiratory disorders, fatigue, treatment of night sweating, fertility issues, cardiac arrhythmias, and other heart diseases. On a broader scale, C. militaris has an array of pharmacological properties, including as inflammation inhibition, and antioxidant, antitumor, antimetastatic, immunomodulatory, hypoglycemic, and steroidogenic activities [12]. Various pharmacological activities are presented in Figure 4 and explained bellow.
Figure 4.
Pharmacological potential of Cordyceps militaris and its constituents.
2.1. Immune Boosting Activity
Several reports have suggested the immune regulation activities of extracts of this medicinal mushroom. The oral administration of aqueous extracts from the C. militaris fruiting body at a concentration of 20 mg/kg resulted in induced interferon (IFN) secretion by macrophages via IL-18 [80]. Both fresh and dried C. militaris extracts have been observed to have equivalent immunomodulatory effects in clophosphamide (cy)-activated immunosuppressed experimental animals. Quantitative investigation of phytochemicals revealed that levels of cordycepin and adenosine in fresh and dried C. militaris were similar, whereas fresh extracts contained more polysaccharides, total polyphenol, and total flavonoids compared to dried ones. Both types of extracts reversed the inhibition of the thymus and spleen index in a dose-dependent manner in a diseased mice model. Additionally, these extracts were able to increase the levels of IL-2 and IFN-γ secretion levels in test animals [81]. An ethyl alcohol extract of C. militaris administered to healthy Korean male volunteers enhanced cell-mediated immunity at a dose of 1.5 g/day via an oral route. Researchers observed no side effects after a treatment duration of 4 weeks along, with significant enhancement in the levels of IL-2 and the IFN-γ compared to the placebo group. Further, due to enhanced T cell proliferation and improved natural killer cell activity, this fungus was promoted as a safe immunomodulator to boost cell-mediated immunity [82]. In another study, C. militaris fruiting body extracts displayed immune modulation potential and antioxidative activity in healthy Kunming mice. Oral administration of different extracts concentrations i.e., 50, 100, or 200 mg/kg on a daily basis caused a significant increase in thymic and splenic indices. Total white blood cell count, as well as monocytes and lymphocytes, were enhanced, neutrophils were decreased, but eosinophils and basophils did not undergo any changes. Augmented IL and TNF-α levels were reported in the spleen and increased total antioxidant capacity, glutathione peroxidase, and SOD were observed in different organs such as the heart, kidney, and liver. These results suggest an immune boosting response in heathy organisms [46] and indicate the positive impact of C. militaris as an immunomodulatory agent.
2.2. Antiviral Potential
C. militaris extract has been used to determine its protective effects in influenza A/NWS/33 (H1N1) virus-infected mouse. The virus preventive effects of C. militaris extracts were investigated by administering different doses at 30, 100, or 300 mg/kg per day for seven days to H1N1-infected animals. The results inferred that the protective effect of the extracts could be attributed to suppressed TNF-α level along with increased IL-12, natural killer (NK) cells in experimental mice models [83]. Viral hepatitis occurs commonly worldwide, and when untreated causes hepatocellular carcinomas and cirrhosis. C. militaris showed moderate anti-HCV potential in conjunction with standard antivirals (IFN-α or ribavirin) in a cell-based HCV RNA replication assay system used to investigate antiviral activity [84].
2.3. Anticoagulant Activity
Fibrin is formed by fibrinogen by the activity of thrombin. The accumulation of fibrin in blood vessels can lead to clot formation resulting in thrombosis and cardiovascular diseases. A number of reports have confirmed the presence of fibrinolytic enzymes in C. militaris with anti-coagulant or thrombolytic activities. Cui et al., successfully purified a novel fibrinolytic enzyme from culture broth of the species and named it C. militaris fibrinolytic enzyme (CMase) [85]. Similarly, Liu et al. purified a fibrinoyltic enzyme, which had the ability to hydrolyze fibrin or fibrinogen by cleaving the α-chains more efficiently than β- and γ-chains, revealing its plasmin like nature. It was able to degrade thrombin, indicating its benefits as an anticoagulant and antithrombotic protein [86]. The same authors reported the biochemical characterization of another fibrinolytic protease from C. militaris [30]. Hence, C. militaris can be suggested as good source of novel thrombolytic agents, although work related to the anticoagulant activities of crude extracts is scarce.
2.4. Anticancer Activity
C. militaris is one of the important medicinal species and well documented for its anticancer effects. C. militaris ethanolic extract was orally administered to a xenograft in mice bearing murine T cell lymphoma (RMA) cell-derived cancers, which resulted in significant anticancer activity by the suppressing the size and mass of cancer. Furthermore, reduced proliferation of RMA cells and C6 glioma cells, downregulation of phosphorylation of AKT, p85 and augmented cleaved caspase-3, phosphoglycogen synthase kinase 3β (p-GSK3β) were reported. The extract significantly increased the proapoptotic cell population and reduced viability compared to control cells. The finding indicates the anticancerous activity of C. militaris occurred by regulating of p85/AKT- or GSK3β-related caspase 3-dependent apoptosis [87]. Similarly, methanolic extracts showed good cytotoxic activity via the MTT assay against Hep-2 cancer cell lines with an IC50 value of 20 μg/mL [74]. In another study, the effect of fluoride was monitored in the culture medium of C. militaris, and positive effects were observed on the synthesis of secondary bioactive metabolites and growth of fruiting bodies, which eventually caused reduced proliferation and apoptosis in a human osteosarcoma (U2OS) cell line [88]. Another study discussed the decreased apoptotic activity of aqueous extract of C. militaris (AECM) on MDA-MB-231 cells. It showed significant induction of mitochondrial dysfunction and loss of mitochondrial membrane permeability by modulating Bcl2/Bax proteins, and also caspase activation [89]. Another report showed the tumor inhibitory effects of an ethanolic extract of C. militaris in xenograft Balb/c nude mice transfected with human colorectal carcinoma RKO cells. The oral administration of test extracts led to delayed growth of RKO cell-derived tumors. It also stimulated cell cycle arrest in G2/M phase (66.33% at 300 μg/mL) and enhanced early apoptosis (18.07% at 300 μg/mL). Western blot analysis indicated an increase in the expression levels of p53, cleaved caspase 9, cleaved caspase-3, cleaved PARP, and Bim, Bak, and Bad proteins [90]. A mechanistic based study conducted by Chou et al., revealed that anticancerous effects of C. militaris on leukemia cell lines might be attributed to activation of AKT and p38 mitogen activated protein kinase (MAPK), during the course of apoptosis induction, suggesting the possible use of its extracts against leukemia by activating the p38 MAPK pathway [91]. Another mechanism of the apoptosis of lung carcinoma by C. militaris extracts is related to downregulation of TCTN3 expression, which affected the hedgehog signaling cascade and contributed to the serial activation of caspases. Additionally, the extract negatively modulated GLI1 transcriptional activity by inhibiting SMO/PTCH1 molecules, subsequently regulating the intrinsic apoptotic signaling cascade [92]. All these findings support the possible use of C. militaris extracts as anticancer agents in future studies.
2.5. Anti-Obesity Activity
C. militaris extracts possess lipid lowering activities. A novel extract of mulberry leaves fermented with C. militaris was exploited to detect its effect on lipid metabolism. Administration of an extract in a high fat diet fed to (HFD)-activated obese C57BL/6 mice for 12 weeks showed significantly decreased concentrations of triglyceride, glucose, total cholesterol and low-density lipoprotein, and induced production of levels of high-density lipoproteins were observed. The amount of abdominal fat and the size of adipocytes were reduced compared to control groups. Moreover, the sample reduced the Fas cell surface death receptor for lipogenesis and inhibited adipocyte protein 2 and peroxisome proliferator-activated receptor-γ mRNA expression [93]. Recently, strawberry extracts fermented with C. militaris showed enhanced levels of secondary metabolites as well as different extents of inhibition of adipogenesis in a 3T3-L1 cell line [94,95]. The extract also showed dose-dependent suppressed differentiation of 3T3-L1 preadipocytes into mature adipocytes and did not show any toxic effects on cells. An associated reduction in lipid accumulation, increased levels of adipocyte markers including peroxisome proliferator-activated receptor-γ, adiponectin, and CCAAT/enhancer binding protein-α, as well as continuous expression of monocyte chemoattractant protein (pre adipocytes marker) were observed [96].
2.6. Anti-Allergic Activity
Allergic responses are associated with disorders related to the immune system, where intense immune reactions occur in response to various triggers such as foods, chemicals, pollens and particulate matter. Primarily, production of CD4+ specific allergen cells i.e., type 2 helper, Th2 are accompanied by the generation of interleukins (IL-4, IL-5, IL-9, and IL-13) by the effector Th2 cells, which subsequently results in the generation of IgE i.e., allergen related immunoglobins from B cells. IgE reactions with allergen cytokines generate the allergic reaction. Therefore, suppression of both allergen cytokines and IgE are effects of therapeutic agents for allergies. Aqueous extracts of C. militaris have demonstrated asthma-preventing potential in ovalbumin (OVA)-activated experimental animals at a dose of 4 g/kg/day. Results revealed a decreased concentration of serum immunoglobulin E (IgE) as well as fewer infiltrating cells in the airways of mice treated with test extracts, although efficiency was less compared to montelukast and steroids, which are standard drugs for the treatment of asthma [97]. In another study, C. militaris (ethyl acetate extract) inhibited allergic reactions in a concentration-dependent manner in basophilic leukemia (RBL-2H3) cells. Extracts inhibited antigen-activated degranulation in RBL-2H3 cells with an IC50 value of 28.5 μg/mL. The extract prevented antigen-induced passive cutaneous anaphylaxis in experimental animals in a concentration-dependent manner [98]. In another study, an extract of C. militaris cultured on germinated soybean extract showed inhibitory potential in 2,4-dinitro-1-fluorobenzene (DNFB)-activated contact dermatitis mice models at a concentration of 300 mg/kg. It not only led to reduced ear swelling but also reduced infiltration of T, CD4 and CD8 cells in the ear tissues of the mice [99]. A study was performed to determine the molecular mechanisms of allergy prevention. An ethyl alcohol extract prepared from silkworm pupa-cultivated C. militaris fruiting bodies in immunogen triggered RBL-2H3 mast cells inhibited the release of β-hexosaminidase (a degranulation marker) and mRNA levels of TNF-α, as well as IL-4. Western blotting results revealed the inhibition of the Syk/phosphatidylinositol 3-kinases (PI3K)/MEKK4/JNK/c-Jun signaling cascade associated with the expression of various allergic cytokines in stimulated RBL-2H3 cells. Additionally, inhibited PLCγ evocation, and Erk activation were involved in stimulating the synthesis of lipid mediators and Ca2+ mobilization, which favor degranulation in activated RBL-2H3 cells [100].
2.7. Other
Several studies have documented the antihyperglycemic potential of C. militaris. Oral administration of aqueous and ethanolic extracts of C. militaris to diabetic Sprague-Dawley rats caused significant reduction in blood glucose levels. These results were due to increased glucose metabolism and suppression of total cholesterol and triglyceride concentrations [101]. The diabetic preventive potential of different fractions of C. militaris in streptozotocin-induced diabetic animals was determined in another study, resulting reduced blood glucose levels in which C. militaris extract acted as an insulin sensitizer (enhanced insulin secretion and insulin resistance in type II diabetic rats) [102]. This medicinal fungus has also proved its importance as a fertility enhancer, antimicrobial and antiaging species [103].
3. Inflammation
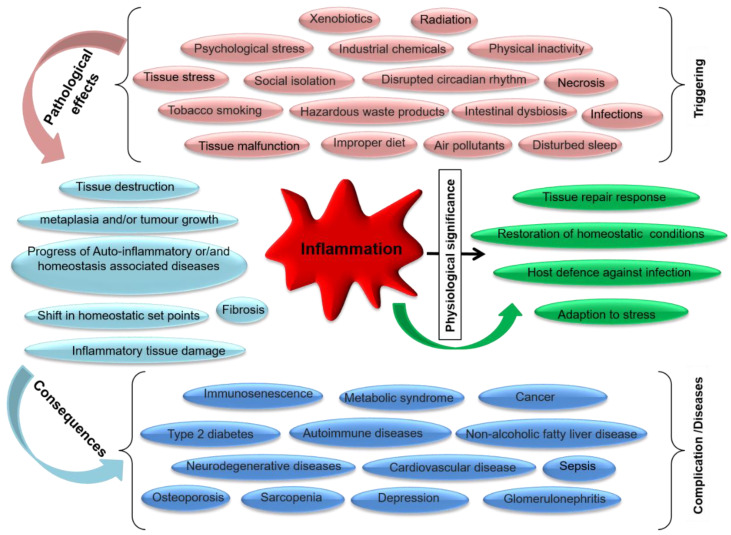
Inflammation is a key function in biological processes initiated by various stimuli and noxious factors such as irradiation by ultraviolet light, irritants, infections and cell injury. The main features of inflammation are redness, elevated temperature, pain and alteration in physiological functions at infected sites [104,105]. Generally, inflammation is considered to be protective mechanisms against pathogen-induced tissue damage, and it may be acute or chronic. It involves neutrophils, natural killer cells, mast cells, and T and B cells supporting the undesired immune reaction [104]. Prolonged chronic inflammation increases the risk of various inflammation associated disorders such as arthritis, asthma, cancer, and atherosclerosis. A variety of regulatory enzymes such as phospholipase A2 (PLA2), lipoxygenases (LOX), COX, phosphatidylinositol kinase, and tyrosine kinases have a substantial role in inflammation and immune responses. Injured or physiologically altered tissues generate stress, which acts as either an endogenous or exogenous inducer of the inflammatory response [106]. These inducers elicit both mast cells and macrophages residing at inflamed tissues that subsequently promote cellular inflammatory mediators [107]. Elicitation of the inflammatory cascade can proceed through three different paths linked with a variety of pathological events as depicted in Figure 5. Among them, the immune response occurs in infection allied inflammation. Inflammatory mediators such as lipid mediators, chemokines, proteolytic enzymes, complement component fragments, cytokines, vasoactive amines and peptides are characterized on the basis of their biochemistry [108], while IL and TNF-α alter the function of effectors such as cells and tissues during inflammatory responses [109]. Overall, these inflammatory mediators have divergent effects in different cell and tissue types, with a wide range of functions in the inflammatory response, regulating homeostasis and adoption [110].
Figure 5.
Triggering, pathological consequences, and physiological significance of inflammation.
Stimulators and bacterial endotoxin-triggered immune cells such as leukocytes generate pro-inflammatory cytokines, which participate in elicitation, activation and regulation of cellular adhesion molecules (CAMs). CAMs comprise different groups such as integrins, selectins, and various glycoproteins (immunoglobulins). Tethering, rolling, transmigration to inflammation related areas, and binding of white blood cells and endothelium are all crucial functions. Different studies have demonstrated mechanisms such as migration and mobility white blood cell during inflammation [104]. Inflammatory cells are characterized by augmented inflammation-related chemicals including TNF-α, IL-1, IL-6 and IL-1β, which contribute to inflammation related response such as regulation of chemokines, cytokines and CAMs production. Furthermore, reduced inflammation related reactions have been found against TNF-α and IL-1β specific antibodies [111], showing their involvement in inflammation regulation.
Arachidonic acid (A.A) has a vital role in inflammation by regulating inflammatory metabolites, and inflammation regulatory enzymes such as LOX, and COX. A.A is one of the prominent contributing factors and biomarkers of inflammation, and its inhibition has been recognized as a molecular target to protect against inflammatory ailments [104].
Exogenous factors including microbes and tumor promoters promote inflammatory cells such as macrophages eosinophils, and neutrophils, which are connected with oxidative stress. Various reactive nitrogen species (RNS) and ROS act as fuel for inflammatory processes by prompting the production of proinflammatory cytokines and adhesion related components via NF-κB pathway activation. Augmented inflammatory responses, including activated NF-κB, and induced expression of adhesion molecule have been investigated in oxidative stress activated neutrophils [112].
NF-κB is a prominent molecular component that activate and regulates gene transcription [113] of pro-inflammatory chemicals, the A.A cascade, cytokines, chemokines, and inflammatory mediator-induced phosphorylation of I-κB via I-κB kinase and damage to the I-κB complex. It initiates the activation of NF-κB and its related reactions associated with inflammation [114]. Variations in NF-κB pathway activation have been linked with chronic inflammation related ailments such as cancer, inflammatory bowel illness, asthma, atherosclerosis, and rheumatoid arthritis, [115], as well as maintaining different inducible transcription factors in inflammation. Inflammation involves several enzymatic proteins that are related to immune regulation and removal or destruction of various substance through by blood cells [116]. The role of these proteins as inflammatory mediators has long been known. These proteins attach to pathogens after being activated by a recognition protein such as C-reactive protein (CRP), natural IgM, or mannan-binding lectin.
Inflammatory response results in the production of enzymes involved in degradation of the cellular matrix, such as matrix metalloproteinases (MMPs) [117]. Various biomolecules occur on the surface of leucocytes, such as selectins, which support their entry into tissue spaces by rolling on the endothelial surfaces [104].
Currently, steroidal and nonsteroidal inflammation preventive medications are used to treat acute inflammation and related diseases [118]. However, these medicines are not fully effective against chronic inflammation-related conditions and may have adverse impacts on human health. As a result, it is critical to investigate materials with low adverse effects for the management and treatment of inflammation [119]. Functional foods have attractions in developing novel therapeutics because of their nutritional and pharmacological potential. Furthermore, a lot of attention has been diverted towards natural sources including mushrooms that contain medicinally important biofunctional components that can reduce the severity of inflammatory ailments via different mechanisms such as regulating oxidative stress in the physiological range and controlling pro-inflammatory cytokines. C. militaris and its bioactive components are being investigated for biomedical applications, due to their reduced side effects, rich nutritional and bioactive constituents and suppression of inflammation. C. militaris and its active constituents have both in vitro and in vivo inflammation preventing potential in a variety of experimental models [120,121,122].
4. Cordyceps militaris and Inflammation
Chronic inflammation causes diseases that are characterized by devastating effects. Many different factors induce inflammation, such as chemicals, physical injury, infectious agents, immunological responses, and metabolic disorders. Medicinal fungi such as C. militaris, or their constituents such as cordycepin, are currently being investigated as therapeutic agents against inflammation as they prevent acute and chronic inflammatory responses. The actual mechanisms involved in anti-inflammatory responses include antioxidant activity, transcription factors, matrix metalloproteinases, complement cascade properties, and adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), selectin, and vascular cell adhesion molecule-1 (VCAM-1). Moreover, C. militaris, and its constituents also regulate pro-inflammatory enzyme actions and gene expression of inflammatory genes. Recent reports revealed that Cordycepin, one of the prominent components of C. militaris, inhibited inflammation-associated gene expressions of COX-2 and iNOS [123]. Furthermore, ethanol extract of silkworm pupa-cultivated C. militaris fruiting bodies inhibit the secretion of histamine, protein kinases [124], and regulate gene transcription associated with inflammation or inflammation associated diseases [125]. Many studies have demonstrated the potential of C. militaris in prevention of inflammation. Hence, this study comprehensively reviews the available data on the inhibition potential of C. militaris against inflammation along with related molecular mechanisms. The anti-inflammatory mode of action and molecular events linked with suppression of inflammation associated with C. militaris in various experimental models are presented in Table 1 and briefly explained below.
Table 1.
Biofunctional components, concentration, experimental/disease model and anti-inflammatory actions of Cordyceps militaris.
| Bioactive Component | Dose/Disease Model | Study Type/Experimental Model | Results/Mechanism | References |
|---|---|---|---|---|
| Cordycepin | 2.5–10 mg per kg of rat/Parkinson’s disease | In vivo/ Male Sprague-Dawley rats |
Reduced neuro-inflammation, dynamin-related protein 1 (Drp1), IL-1β, IL-18 and tyrosine hydroxylase. Amplified NLRP3 inflammasome activation, ATP production, AMP-activated protein kinase and mitochondrial functions | [164] |
| Cordycepin | 0.0005–0.008 nM/L | In vitro/ PC12 rat pheochromocytoma cell line |
Improved mitochondrial functioning by increased ATP content, maintaining membrane potential, inhibiting fission protein 1(Fis1) and mitochondrial ROS levels. | [164] |
| Cordycepin | 0–40 µg per mL/ TNF-α-induced inhibition of osteogenic differentiation in ADMSCs |
In vitro/ ADMSCs |
Restoration of cell proliferation and osteogenic differentiation by regulating Runx2 and Osx mRNA expressions, and NF-κB signaling via inhibition of IκBα phosphorylation. | [165] |
| Cordycepin | 0–40 µg per mL/LPS-stimulated RAW264.7 cells | In vitro/ RAW264.7 cells |
Reduced proinflammatory chemicals such as IL-1β, IL-6, TNF-α, iNOS, COX-2 and NO synthesis | [64] |
| C. militaris extract (WIB801C) | 20, 50, 100 mg per kg of rat/Focal cerebral ischemia | In vivo/ Male Sprague-Dawley rat |
Neuroprotection, inhibited MCP-1-induced microglial migration, oedema and the infiltration of ED-1-and MPO-positive inflammatory cells. | [158] |
| Asterina pectinifera fermented C. militaris extract (FACM) | 0–40 µg per mL/LPS-induced RAW264.7 macrophages | In vitro/ RAW264.7 macrophages |
Amelioration of LPS-stimulated phosphorylation levels of MAPKs (p38, JNK1/2, and ERK1/2), NO synthase expression, IL-6 and TNF-α. | [140] |
| Cordycepin | 10, 20, 400 mg per kg of rat/ Acute lung injury, asthma. |
In vivo/ Male BALB/c mice |
Inhibited OVA-specific immunoglobulin (Ig) E, mucus hypersecretion, eotaxin, IL-4, -5, -13 and ICAM-1, NF-kB activation and p38-MAPK signaling cascades, recruitment of inflammatory cells in an experimental model. | [143] |
| Militarin Derivatives | 0–100 µM/ LPS-treated RAW264.7 |
In vitro/ RAW264.7 cells, peritoneal macrophages |
Inhibited NO production and PGE2 by downregulating p38/AP-1, IKKe/IRF-3, and Syk/NF-kB pathways | [122] |
| Militarin Derivatives | 5–20 mg per kg in DSS-induced colitis, 5–30 mg per kg in gastritis model and ear oedema model |
In vivo/ male C57BL/6 and ICR mice |
Anti-inflammatory effects by reducing gastric damage (gastritis), inhibited colon size and up-regulated phospho-p38 (colitis), and inhibited ear oedema. | [122] |
| Cordycepin and adenosine |
0, 1, 10 and 100 µg per mL/ LPS induced inflammatory response |
In vitro/ Murine macrophage |
Inhibition of inflammation by reducing expression of M1 chemokines (CX3CR1, RANTES) and cytokines (IL-1β, TNF-α). | [166] |
| Extract of C. militaris grown on soybean | 5–20 mg per kg of mice/ DSS-induced colitis |
In vivo/ C57BL/6 mice |
Inhibited TNF-𝛼, iNOS, MMP-3, MMP-9 mRNA Expressions in colonic tissue of a colitis model. | [167] |
| Extract of C. militaris grown on soybean | 10 and 100 µg per mL/ LPS-induce RAW264.7 Cells. |
In vitro/ RAW264.7 cells |
Suppressed TNF-𝛼 and iNOS in a cell model | [167] |
| Extract (Mulberry leaves fermented with C. Militaris) | High fat diet-induce -obese mice | In vivo/ C57BL/6N male mice |
Inhibited mast cell infiltration, COX-2, iNOS, IL-6, -1β, TNF-α, NF-κB. Anti-inflammatory response via the PI3K/AKT/mTOR signaling pathway. | [148] |
| Cordycepin | 0, 10, 50 or 100 µM/ Nucleus pulposus cell and intervertebral disc organ culture inflammatory models |
In vitro/ rats |
Increased type-II collagen, aggrecan synthesis. Inhibited PGE2, NO, and matrix damaging enzymes (MMP-3, -13; ADAMTS-4, and -5). | [146] |
| C. militaris extract, fractions, ergosterol | 0.1, 1, 10 and 100 µg per mL/ LPS-stimulated BV2 microglia cells | In vitro/ BV2 microglia cells |
Significantly reduction in LPS induced nitric oxide. | [168] |
| C. militaris-fermented product extract | 0.603–1.809 g per kg per day/ liver fibrosis BALB/c mice |
In vivo/ Male BALB/c mice |
Suppressed proinflammatory cytokines, such as TNF-α, IL-6, and NF-κB. | [151] |
| Cordycepin, C. militaris butanol extract | 0–30 µg of Cordycepin per mL/ or 0–75 µg of extract per mL/ LPS-triggered RAW264.7 cells |
In vitro/ RAW264.7 cells |
Anti-inflammatory effect by inhibiting NO synthesis, NF-κB activation, iNOS, COX-2 expressions and phosphorylation of p38 and Akt. | [136] |
| Ergosterol palmitate; palmitic acid; ergosterol; ergosterol peroxide; 3,4-O-isopropylidene-d-mannitol; Cordycepin; d-mannitol; d-glucose | LPS/IFN-α stimulated murine peritoneal macrophage cells | In vitro/ macrophage cells |
Suppressed synthesis of cytokines including IL-12 and TNF-α and NO production | [40] |
| Soya-cerebroside, C. militaris extract |
0, 1, 5, and 10 µM/ IL-1β-induced monocytes |
In vitro/ Monocyte |
Reduced monocytes migration and MCP-1 expressions. Downregulated SP1 expression by activating miR-432 and inducing phosphorylation of AKT and AMPK. | [159] |
| Soya-cerebroside, C. militaris extract |
3 and 10 mg per kg per day/ IL-1β-induced inflammatory rat model |
In vivo/ Severe combined immunodeficiency |
Inhibited edema and cartilage damage. Induction in CD68 and MCP-1 (a marker for monocyte/macrophages) positive cells, | [159] |
| Soya-cerebroside | 0, 1, 5, and 10 µM/ Osteoarthritis synovial fibroblasts (OASFs) |
In vitro/ humans |
Decreased monocyte migration, activated AKT and AMPK signaling pathways, MCP-1 and microRNA (miR)-432 expression in OASFs. | [159] |
| C. militaris extract | 1, 10, 100 and 1000 µg per mL/ In LPS-stimulated RAW264.7 and antigen-induced RBL-2H3 cells |
In vitro/ RAW264.7 and RBL-2H3 cells |
Inhibited nitrite production, iNOS, and TNF-α. | [169] |
| C. militaris extract | 500 mg per kg of animal per day/ DSS induced acute colitis |
In vivo/ BALB/c mice |
Alleviated the severity of the disease in a colitis mouse model by decreasing mRNA expression of TNF- α and iNOS. | [169] |
| GRC, GRC-ON89A |
250, 500 µg per mL/ LPS-induced Macrophages |
In vitro/ RAW264.7 cells |
Reduced NO production, iNOS, COX-2, and TNF-α mRNA expression, and that of MAPKs (ERK, JNK, and P38), NF-κB. | [120] |
| GRC, GRC-ON89A |
25 mg per kg of animal/ DNFB induced allergic contact dermatitis |
In vivo/ BALB/c, C57BL/6N mice models |
Decreased inflammatory response such as ear swelling in an experimental model | [120] |
| Cordycepin | 12.5, 25, 50, 100 µg per mL/ Cholecystokinin-stimulated pancreatic acinar cancer cell |
In vitro/ pancreatic acinar cancer cell |
Anti-inflammatory effect by down regulating NLRP3 inflammasome activation and NF-κB via AMPK. | [152] |
| Cordycepin | 100 mg per kg of animal/ Caerulein induced acute pancreatitis |
In vivo/ Male ICR mice |
Augmented neutrophil infiltration and reduced edema, acinar cell vacuolization, serum amylase, lipase levels. Inhibited TNF-α, IL-1β, IL-6 by suppressing the activation of NLRP3 inflammasome and NF-κB. | [152] |
| C. militaris aqueous extract | 1 and 2 g per kg of animal/ Cationic bovine serum albumin-induced membranous glomerulonephritis rat model |
In vivo/ Wistar male rats |
Amplification of total protein, serum albumin, MDA, SOD, and glutathione peroxidase. Attenuated IL-1, TNF-α, 6-keto-PGF1α, NF-κB p65. Reduced serum levels of VCAM-1, ICAM-1, and MCP-1 and urine protein serum creatinine, triglyceride, blood urea nitrogen and total cholesterol. | [144] |
| Extract of fruiting bodies C. militaris | 500 µg per mL/ LPS-induced inflammatory response in macrophages |
In vitro/ RAW264.7 Macrophages |
Reduced Synthesis of IL-6, NO, and TNF-α. | [170] |
| Cordycepin | 50, 100, and 200 g per kg / LPS-induced acute lung injury mice model |
In vivo/ Male BALB/c mice |
Inhibition of Nrf2 and HO-1 expressions, MDA content, IL-1β, TNF-α and NF-κB activation. |
[133] |
| C. militaris and Rumex crispus Mixture | 50 and 100 µg per mL/ LPS-induced splenocytes |
Ex vivo/ splenocytes |
Suppressed COX-2, iNOS, IL-1β, IL-6, TNF-α, IFN-γ) and NO synthesis | [135] |
| C. militaris-based nanoemulsion | 25 and 50 µg per mL/ LPS-induced Macrophages |
In vitro/ RAW264.7 Macrophages |
Reduced expression of proinflammatory cytokines (TNF-α, IL-1β, IKKa, iNOS, IL-6, NF-kß) and NO production. | [171] |
| Mulberry leaves fermented with C. militaris |
100, 200 and 400 μg per mL/ LPS-induced Macrophages |
In vitro/ RAW264.7 Macrophages |
Anti-inflammatory activity by iNOS-mediated COX-2, expression of inflammatory cytokines (IL-1β, IL-6 and TNF-α), and MAPK signaling pathway | [148] |
| Cordycepin | 10, 50 and 100 μM/ IL-1β-stimulated human osteoarthritic chondrocytes |
Ex vivo/ osteoarthritic chondrocytes |
Suppressed IL-1β, PGE2, MMP-13, IL-6, iNOS, COX-2 and NO synthesis. | [145] |
| Cordycepin | PBMCs (Kawasaki disease patients), LPS-induced Macrophages | In vitro and Ex-Vivo/ PBMCs, macrophages | Inhibition of LPS-stimulated TNFα production in mouse macrophages and in PBMCs | [172] |
| Cordycepin | 1, 5, 10 and 20 mg per kg/ Traumatic brain injury |
In vivo/ Sprague-Dawley rats |
Increased arginase 1 and IL-10. Inhibition of IL-1β, iNOS, MPO and MMP-9, and NADPH oxidase expression. | [173] |
| C. militaris fruiting bodies extract | 4 g per kg/ OVA sensitized airway inflammatory mice model |
In vivo/ BALB/c mice |
Inhibited asthmatic airway inflammation and blocked bronchoconstriction mediators-leukotrienes | [97] |
| C. militaris, C. militaris fermented Haliotis discus hannai (HFCM-5) | 50, 100 and 200 µg per mL/ LPS-induced Macrophages |
In vitro/ RAW264.7 Macrophages |
Decreased proinflammatory cytokines, TNF-α and IL-6 in a concentration-dependent manner. In addition, showed nitric oxide inhibitory activity. | [174] |
| Cordycepin | 50 and 100 μM/ Palmitic acid and oleic acid in inflammation in Hepatocytes |
In vitro/ hepatocytes |
Attenuated the increased expression of inflammatory genes (TNF-α, IL-1β, Cxcl10, Ccl2 and Ccl5) | [121] |
| Cordycepin | 100 and 200 mg per kg/ Lipotoxic model), nonalcoholic steatohepatitis |
In vivo/ Mice |
Suppressed inflammatory genes (IL-1β, Cxcl2, Cxcl10, Ccl2, and Ccl5), activation of NF-κB signaling, and inflammatory cell infiltration. Anti-inflammatory effects through AMPK pathways | [121] |
| Spent mushroom (C. militaris) | 0.5, 1 and 1.5 g per kg/ | In vitro/ pigs |
Improved health conditions. Inhibition of IL-1β and TNF-α. | [175] |
| Fermented cultured C. militaris (GRC-SC11) | 0–300 µg per mL/ allergic model (RBL-2H3 cells) |
In vitro/ RBL-2H3 |
IL-4 and TNF-α inhibition | [176] |
| Cordycepin | 2 g per liter in drinking water/ LPS stimulated animals |
In vivo/ Male broilers (Ross 308) |
Inhibition of COX-2 and iNOS | [123] |
| C. militaris powder | 3, 1.5, and 0.5 g powder per individual/ | In vivo/ Humans |
Suppressed inflammatory cytokines including EGF, eotaxin, fractalkine, IP-10, IL-1α, -6, -8, IFN-α2, -γ, MIP-1α, -1β, GRO, G-CSF, GM-CSF, MCP-1, sCD40L, TGF-α, VEGF | [137] |
Abbreviations: 1-fluoro-2,4-dinitrofluorobenzene: DNFB; Adipose-derived mesenchymal stem cells: ADMSCs; Cordyceps militaris grown on germinated Rhynchosia nulubilis: GRC; Cyclooxygenase 2: COX-2; Dextran sodium sulfate-induced: DSS; Epidermal growth factor: EGF; Fibroblast growth factor-2: FGF-2; Granulocyte-colony stimulating factor: G-CSF; Granulocyte-macrophage colony-stimulating factor: GM-CSF; GRC: fermented with Pediococcus pentosaceus ON89A isolated from onion hexane extract: GRC-ON89A; Growth regulated oncogene: GRO; Interferon-α2 (IFN-α2), IFN-γ; Heme oxygenase-1: HO-1; IFN-γ inducible protein 10: IP-10; Inducible NO synthase: iNOS; Intercellular adhesion molecule 1: ICAM-1; Interleukin-1α: (IL)-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17; Lipopolysaccharide: LPS; Macrophage inflammatory protein-1α: (MIP)-1α, MIP-1β; Macrophage-derived chemokine: MDC; Malondialdehvde: MDA; Monocyte chemoattractant protein-1: (MCP)-1, MCP-3; Monocyte chemoattractant protein-1: MCP-1; Nuclear factor erythroid 2–related factor 2: Nrf2; Nuclear factor-κB: NF-κB; Mitogen -activated protein kinases: MAPKs; Ovalbumin: OVA; Peripheral Blood Mononuclear Cells: PBMCs; Transforming growth factor-α: TGF-α; Tumor necrosis factor alpha: TNF-α; Tumor necrosis factor-α: (TNF)-α, TNF-β; Vascular endothelial growth factor: VEGF; Vascular adhesion molecule 1: VCAM-1.
4.1. Antioxidant Potential
Oxidative stress arises mainly because of the antioxidant system being unable to remove systematic production of ROS or restore damage produced by the ROS in living systems [112]. RNS are also associated with oxidative stress. Vital biological molecules are susceptible to ROS, and RNS. Their interaction can result in damage of cellular components, causing modifications and altered functioning of bio-molecules such as proteins, DNA and others [112,126]. Changes in these processes result in the activation of inflammatory pathways mediated by inflammatory mediators, which cause damage and altered functioning. Inflammatory cells, such as macrophages and neutrophils, contribute to maintaining oxidative stress. Neutrophils play a vital role in the regulation of inflammatory processes by the generation of superoxides via triggering NADPH oxidase, that leads to a worsening effect in cells [127]. Numerous studies have demonstrated the antioxidant potential of C. militaris. Zhang et al. documented the substantial radical scavenging activity of the polysaccharide-iron (III) on DPPH, hydroxyl ABTS, and superoxide species [128]. In another study, the antioxidant capacity of neutral polysaccharide was assessed with assays of reducing power, ABTS radical scavenging activity, oxygen radical absorbance capacity (ORAC-fluorescein), and hydroxyl radical scavenging activity [129]. An ethanol extract of C. militaris showed in vitro antioxidant activity against DPPH, superoxide, and hydroxyl radicals and low-density lipoproteins [130]. Extracellular polysaccharide from C. militaris effectively regulated key proteins such as HO-1, Nrf2, Kelch-like ECH-associated protein-1 (Keap1), and quinone oxidoreductase 1 (NQO1) in the Nrf2 signaling pathway [131]. He et al. also supported the anti-inflammatory property of an ethanol extract of C. militaris via the suppression of H2O2-stimulated cell injury related to ROS overproduction and downregulated mitogen-activated protein kinases in C6 glial cells [132]. The stimulation of redox-sensitive transcription factor Nrf2 averts oxidative stress damage by activating HO-1 [126]. C. militaris polysaccharides prominently increased catalase, SOD, glutathione peroxidase levels and total antioxidant capacity, and reduced malondialdehyde (MDA) in experimental animals [34]. Additionally, use of C. militaris was a potent therapy for inflammation associated damaging effects in neurological illnesses [132]. Cordycepin suppressed LPS-induced MDA content and inflammatory cytokines (IL-1β, TNF-α) production. It also inhibited LPS-stimulated NF-κB activation, Nrf2 and HO-1 expression [133].
4.2. Effects on Proinflammatory Enzymes
Various inflammatory mediators including prostaglandins (PGs) i.e., PGE2, 6-keto prostaglandin F1α (6-keto-PGF1α) and other active lipids are found in all human cells. Cyclooxygenases and prostaglandin synthases catalyze a series of events that create PGs from A.A at the site of inflammation [134]. The increased production of iNOS takes place mostly at inflammatory sites, resulting in the augmented synthesis of NO, which ultimately induces synthesis of PGs [119,126]. PLA2 and LOX enzymes are also linked in the regulation of the A.A pathway. Likewise, LOX participates in the derivation of leukotrienes (LT) by the A.A route, which has been connected to inflammatory diseases such as cancer, inflammatory bowel disease, asthma, allergy and other ailments. C. militaris ameliorated IL-1β-stimulated COX-2 expression in splenocytes [135]. LPS-induced LPS-stimulated RAW264.7 cells exposed to Cordycepin showed concentration-dependent reduced synthesis of NO and proinflammatory cytokines including IL-6, IL-1β, TNF-α, COX-2 and iNOS, [64]. Another study demonstrated the anti-inflammatory effect of cordycepin attributed to inhibiting NO synthesis, suppressing COX-2 and NF-κB activation, Akt and p38 phosphorylation and iNOS expression [136]. C. militaris powder at a dose of 0–3 g per individual suppressed inflammatory cytokines such as EGF, eotaxin, fractalkine, GM-CSF, GRO, G-CSF, IFN-α2, IFN-γ, IL-1α, IL-6, IL-8, IP-10, MCP-1, MIP-1β, MCP-1, MIP-1α, TGF-α, sCD40L, VEGF that were reported in the blood samples from volunteers of both sexes, i.e., male and female [137]. In comparison to the LPS-treated control cells, GRC-ON89A decreased the release of NO. It also suppressed the production of COX-2, iNOS, and TNF-mRNA in LPS-triggered macrophages. In addition, pretreatment with GRC-ON89A suppressed LPS-induced activation of NF-κB and MAPKs (ERK, JNK, and P38) [120].
Leukotrienes are crucial molecules related to inflammatory processes in inflammatory bowel disease cancer, asthma, and rheumatoid arthritis. These molecules are derivatives of the LOX-mediated arachidonic acid pathway [104,126,138,139]. NO is a significant important secondary messenger in the signaling pathway regulating pathophysiological conditions. The advantageous roles of NO in neurological and defensive systems have been widely discussed for many years, and NO has evolved as a mediator of several bio-activities. In addition to these effects, there is a link between increased NO production and inflammation-related disorders. The interaction of NO with metal causes changes in the activity of enzymes such as catalase, which results in H2O2 accumulation and harmful consequences. Additionally, peroxynitrite is an effective oxidant associated with apoptosis and DNA damage. Oxidation of low-density lipoproteins and suppressing of mitochondrial respiration occurs as an outcome of interaction between superoxide anions and NO [126]. NO tends to promote the synthesis of pro-inflammatory cytokines such as TNF-α and others [104]. Asterina pectinifera fermented C. militaris extract decreases LPS-triggered expression of inducible NO synthase and inhibited pro-inflammatory cytokines such as TNF-α and IL-6. Furthermore, it ameliorates the LPS-triggered phosphorylation levels of JNK1/2, ERK1/2, and p38 MAPKs in RAW264.7 macrophages [140].
4.3. Effects on Inflammation-Associated Gene Expression
C. militaris and its constituents regulate numerous molecular mechanisms, including PI3K, MAPK pathways, activator of transcription (JAK/STAT) pathways, Janus kinase-Signal Transducer, protein kinase C (PKC), and others. These mechanisms contribute to inflammation and related diseases via regulating inflammatory mediators and are comprehensively discussed in this study. Inflammatory conditions are linked to kinases that affect the production and regulation of transcription factors such as activator protein-1 (AP-1) [141].
The MAPK family includes important serine/threonine protein kinases such as c-jun N-terminal kinase (JNK), p38, and extracellular signal-regulated kinase1/2 (Erk1/2) [142]. Inflammatory stimuli initiate these pathways that ultimately control the expression of targeted genes such as TNF-α, IL-1, and COX-2. Cordycepin inhibited LPS-stimulated NF-κB activation and suppressed LPS induced lung wet/dry ratio, MDA content, and inflammatory cytokine (IL-1β, TNF-α) production [133]. Asterina pectinifera fermented C. militaris extract decreased LPS-induced expression of iNOS and inhibits proinflammatory cytokines such as TNF-α and IL-6. Additionally, it ameliorates the LPS-induced phosphorylation levels of ERK1/2, JNK1/2, and p38 MAPKs [140]. Cordycepin attenuated airway hyper responsiveness, mucus hypersecretion, and OVA-specific immunoglobulin (Ig) E in an experimental model. It blocked severe OVA-induced inflammatory cell recruitment to the lungs such as eosinophils, neutrophils, macrophages, and lymphocytes. It decreased the upregulation of eotaxin, ICAM-1, IL-4, IL-5, and IL-13 in mice. Furthermore, it inhibited p38-MAPK and NF-κB signaling pathway activation in OVA-driven asthmatic mice [143]. An aqueous extract of C. militaris significantly reduced the serum levels of MCP-1, ICAM-1, VCAM-1, and NF-κB p65 compared with disease model rats, and total cholesterol, serum creatinine, triglyceride, blood urea nitrogen and urine protein in a cationic bovine serum albumin-induced membranous glomerulonephritis rat model. It also attenuated altered levels of inflammatory factors such as IL, TNF-α and 6-keto-PGF1α, and NF-κB p65. In addition, increased the levels of serum albumin, total protein, MDA, and induced the production of SOD levels and glutathione peroxidase were observed [144]. Cordycepin inhibited IL-1β, PGE2, NO synthesis, along with production of MMP-13, IL-6, iNOS and COX-2 in IL-1β-induced chondrocytes [145]. In another study, cordycepin reduced the production MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5. It also inhibited oxidative stress-associated factors (NO and PGE2) and increased the synthesis of aggrecan and collagen-2 in LPS-activated models [146]. A militarin derivative inhibited the synthesis of NO and PGE2 at the transcriptional level through the inhibition of multiple targets including Syk/NF-kB, IKKe/IRF-3, and p38/AP-1 pathways in LPS-activated RAW264.7 cells and peritoneal macrophages [122]. PI3K has a critical role as a mediator of inflammatory signaling pathways [67]. Stimulation of PI3K leads to AKT activation via a cascade of events that subsequently causes cytokine synthesis [147]. C. militaris fermented mulberry (Morus alba) leaves exerted anti-inflammatory response through the PI3K/AKT/mTOR signaling pathway in high fat diet-induced obese mice. It reduced mast cell infiltration, inflammatory mediator expression (iNOS and COX-2), and proinflammatory cytokine synthesis (NF-κB, IL-1, -6, and TNF-α) [148]. The anomalous activity of the JAK/STAT pathway in inflammation has been detected in response to various stimuli, resulting in the stimulation of gene transcription related to inflammatory conditions. Various cytokines enhance STAT phosphorylation, which controls gene transcription [104,149]. Similarly, JAK-phosphorylated STATs are implicated in the control of target gene transcription, such as TARC chemokine [150].
4.4. Effects on Transcription Factors
There are numerous stimuli, such as LPS, RNS, ROS and proinflammatory mediators activating I-κB phosphorylation through I-κB kinase, that break down the I-κB complex and cause NF-κB activation. NF-κB then translocates into the nucleus where it induces the transcription of genes such as IL-1, iNOS, TNF-α, myeloperoxidase lipoxygenases, adhesion molecules, COX-2, and pro-inflammatory cytokines [104,114]. Furthermore, NF-κB activation is linked to a number of degenerative inflammatory diseases such as inflammatory bowel disease, asthma, atherosclerosis, and rheumatoid arthritis [115].
Nonsteroidal anti-inflammatory therapeutic agents, including aspirin and sulindac have been shown to inhibit the NF-κB response. The pharmaceutical substance that adverts these pathways can reduce inflammatory processes in living beings. Various reports have shown that C. militaris reduces NF-κB-regulated inflammation and related responses in a variety of experiments [40,120,136,148,151]. Various inflammatory genes, such as COX-2 IL-8, IL-6, and TNF-α, are activated by the NF-κB transcription factor, which is susceptible to oxidative stress. Cordycepin attenuates caerulein-stimulated degenerative histological features of pancreatic injury, such as augmented inflammatory cell (neutrophil) infiltration, and reduced edema, acinar cell vacuolization, and serum amylase and lipase levels. It inhibited inflammatory chemicals (IL-6, TNF-α and IL-1β,) by suppressing the activation of NF-κB and NLRP3 inflammasomes in an experimental model [152]. Additionally, C. militaris serves as an effective inhibitor of pro-inflammatory cytokines, demonstrating its anti-inflammatory tendency via NF-κB inhibition, and down regulating the iNOS, COX-2, MAPKs and AKT cascades in LPS-mediated inflammations in RAW264.7 macrophages [120]. Repression of RANKL is also linked with events that may be important targets for controlling inflammation-associated condition such as cancer and colitis. C. militaris extract demonstrated concentration-dependent suppression of receptor activator of NF-κB ligand (RANKL)-triggered osteoclast differentiation. In addition, cordycepin considerably suppressed RANKL-activated NF-κB and p38 phosphorylation [153].
4.5. Effects on Adhesion Molecules
Endothelial adhesion molecules such as vascular cell adhesion molecule (VCAM)-1, P-selectins (platelets), monocyte chemotactic protein (MCP)-1, L-selectin (leukocytes), intracellular adhesion molecule (ICAM)-1, and integrin, play a critical role in the interactions of endothelial and leukocytes during inflammatory processes [104]. These molecules have a variety of functions such as infiltration, adhesion, tethering, and rolling. These are only a few of the critical functions of leukocyte-mediated inflammation. Augmented immunoglobin-G adhesion components, including ICAM-1, VCAM-1 participate in migration, activation of T cells, and leucocyte recruitment [104,154]. In vitro experiments exhibited that diverse inflammatory related molecules, such as IL-1β and TNF-α, cause the synthesis of E-selectin [155], P-selectin [156], and leukocyte adhesion molecules [157] in endothelial cells. This illustrates the implication of proinflammatory cytokines in the recruitment of white blood cells at the site of inflammation. Aqueous extract of C. militaris prominently decreased the serum levels of NF-κB p65, VCAM-1, ICAM-1, MCP-1, compared with disease model rats, as well as blood urea nitrogen, serum creatinine, total cholesterol, triglyceride, and urine protein. It also attenuated altered inflammatory chemicals such as IL, TNF-α and 6-keto-PGF1α, and NF-κB p65. Furthermore, increased levels of serum albumin, total protein, MDA, SOD levels, and glutathione peroxidase were observed [144]. C. militaris extract showed neuroprotection against focal and permanent ischemic brain damage through anti-inflammatory activities. It suppressed oedema and the infiltration of ED-1-and MPO-positive inflammatory cells into ischemic lesions in an experiment model. It also inhibited chemoattractant (MCP-1)-induced microglial migration [158]. A soya-cerebroside of C. militaris attenuates IL-1β-activated monocyte migration and MCP-1 expression and inhibits SP1 levels via upregulation of miR-432 and the phosphorylation of AMPK and AKT [159]. Suppressed inflammatory cytokines including eotaxin, EGF, fractalkine, GRO, GM-CSF, G-CSF, IFN-α2, IFN-γ, IP-10, IL-8, IL-6, IL-1α, MCP-1, MIP-1β, MIP-1α, sCD40L, VEGF, TGF-α were reported in the blood samples of both male and female volunteers [137]. Extravasion of neutrophil was effectively inhibited in the peritoneal inflammatory model. Increased selectin receptors are linked to inflammatory diseases including pancreatitis. Various noninfectious inflammatory ailments such as acute pancreatitis are characterized by high mortality and morbidity, and accompanied by tissue necrosis and severe inflammation. Cordycepin inhibited a variety of proinflammatory cytokines, including TNF-α, IL-6, and IL-1β via downregulating NLRP3 inflammasomes and NF-κB inhibition in experimental model [152].
4.6. Effects on Matrix Metalloproteinase
The enzyme inhibitory potential of C. militaris against a variety of enzymes including COX, matrix metalloproteases (MMPs) and others has been well reported. Inhibition of these enzymes confines tissue injuries in various diseases, reduces inflammation and mitigates metastasis. MMPs are a group of zinc-containing calcium-dependent proteases (endopeptidases) involved in the breakdown and remodeling of extracellular matrix components [160]. Hormones, growth factors, and cytokines all have a role in regulating MMP expression.
Tissue inhibitors of metalloproteinases (TIMPs) control MMPs in the physiological range. Increased or decreased TIMP or MMPs can trigger different diseases or increase their severity including inflammation-related ailments [161].
Proinflammatory cytokines such as IL-1, IL-17, TNF-α and others induced the production of MMPs in bovine primary chondrocytes, chondrosarcomal cells (SW1353), and human primary chondrocytes [104,162]. Cordycepin inhibited IL-1β-stimulated MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts in a concentration-dependent manner. It also suppressed IL-1β-stimulated p38/JNK and AP-1 activation [163]. C. militaris-derived soya-cerebroside inhibits IL-1β-induced MMP-1 synthesis by regulating MEK, ERK, FAK, and AP-1 signaling cascades [159].
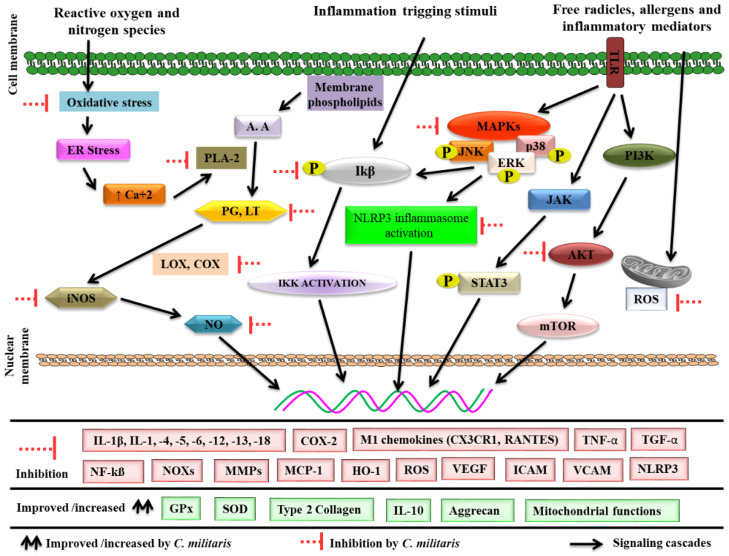
C. militaris associated anti-inflammatory molecular mechanisms are depicted in Figure 6.
Figure 6.
C. militaris and its constituents associated anti-inflammatory molecular mechanisms. Arachidonic acid: A.A; Cyclooxygenase 2: COX-2; Heme oxygenase-1: HO-1; Interleukin-1β: (IL)-1β, -4, -5, -6, -10, -12, -13, -18; Intracellular adhesion molecule: ICAM; Inducible nitric oxide synthase: iNOS; Leukotrienes LT; Lipoxygenases: LOX; Monocyte chemotactic protein-1: MCP-1; Matrix metalloproteinases: MMPs; Nuclear factor-κB: NF-κB; phospholipase A2: PLA-2; Glutathione peroxidase: GPx; Prostaglandin: PG; Superoxide dismutase: SOD; Transforming growth factor-α: TGF-α; Tumor necrosis factor alpha: TNF-α; Vascular endothelial growth factor: VEGF; Vascular cell adhesion molecule: VCAM. Upward double arrow shows the improved/increased content or functionality. Single black arrow indicates signaling cascades, while the red symbol specifies the inhibition of inflammation associated signals and biomolecules.
5. Functional Resemblance of Cordyceps militaris and Nonsteroidal Inflammation Preventing Drugs
The anti-inflammatory potential of C. militaris shows it has similar molecular targets to those of steroidal and nonsteroidal drugs. Nonsteroidal inflammation preventing drugs and C. militaris both reduce inflammation related mechanistic cascades including
-
➢
Production of ROS
-
➢
Triggering and augmented production of pro-inflammatory cytokines
-
➢
Inflammation-associated markers, pro-inflammatory cytokine mediated regulation of CAMs
-
➢
NF-κB activation
-
➢
Enhancing the production of arachidonic acid metabolites
Various contributing factors, such as autoimmune injury, infections, trauma, toxins, postischemia, and microbes elicit inflammatory events, Usually these responses are a part of healing or eradicating the infectious agent. Prolonged or chronic inflammation can cause persistent tissue damage through collagen and leukocytes. Inflammation for prolonged periods leads to the chronic disorders, while inhibition or downregulation of the above-indicated inflammatory responses can avert disease development or lessen its severity. Therefore, C. militaris may be noteworthy as an anti-inflammatory substance due to its effects on NF-κB and other inflammation-linked events for treating inflammation and chronic ailments. Furthermore, improved knowledge of bioavailability and proper consumption, such as daily recommended intake, would improve its efficacy as a therapeutic food for inflammation control.
6. Limitations and Future Prospects
Naturally occurring C. militaris is exceptionally expensive because of its rarity, host specificity, and habitat. Furthermore, due to practical constraints, such as rigorous growing conditions, cordycepin production from this medicinal fungus is unlikely to reach commercial levels. It is important to highlight that the production of cordycepin through chemical procedures is a time-consuming procedure that results in decreased productivity, as well as the discharge of a considerable volume of organic solvents that are disadvantageous for our environment. Moreover, natural production of significantly important constituents from C. militaris such as cordycepin on a commercial level is carried out by culturing this fungus. Although cultivation on a large scale has some concerns, several studies have reported the similarity in quantity and the nature of chemical constituents in cultivated and natural C. militaris.
C. militaris as a therapeutic agent has a number of limitations including negligence, lack of suitable systematic information, inadequate in-depth research and awareness. Formerly, it was solely used by an elite class of people but with the passage of time has become available universally. Research on C. militaris in terms of its therapeutic effectiveness, adverse effects, biosafety, biosecurity, and appropriate standardization should be prioritized not only by individual producers, but also by different industries including functional foods, pharmaceutical, and others.
The raw sources for natural medicines come from nature. The majority of medications derive from botanical origins. People select these medications for a variety of reasons, including the fact that chemically created pharmaceuticals may cause patients to become sicker, whereas natural measures such as medicinal mushrooms may combat life-threatening diseases with lower side effects on human health. The pharmaceutical industry requires innovation providing low-cost materials with appropriate health security in order to maintain healthy growth. The combination of chemistry and biotechnology with bio-originated starting materials, such as secondary metabolites, has the potential to revolutionize edible mushroom-based pharmaceuticals. As a result, C. militaris derived metabolites, including cordycepin, peptides, polysaccharides, and other active constituents, will be a major driving force in the field of green pharmacognosy and pharmacology.
7. Concluding Remarks
Medicinal fungi are classical examples of natural riches pharmacological importance. Our review encompasses the therapeutic effects of C. militaris in the context of ameliorating inflammation-related conditions and disorders. This species has gained importance as a potent bio-functional food source due to its diverse biological activities against inflammation-mediated disorders such as diabetes, allergies, obesity, infectious diseases and cancer. Different bio-functional components from the species are able to regulate inflammation at the molecular level. The inhibition of inflammation at the molecular level proceeds through various mechanisms including inhibition of prostaglandins, cytokines and chemokines, MMPs, oxidative stress, and suppressed inflammation associated transcription factors. In the pharmaceutical sector, C. militaris-associated bioactive ingredients may result in the development of a viable base for pharmaceutical industries for treatment and management of emerging diseases. In this regard, the species needs to be investigated by contemporary scientific methods including gene sequencing, precise analysis of bioactive chemicals and their screening, as well as pharmacological research and clinical trials. The food industry is already generating formulations with C. militaris constituents but two important parameters that require extensive work are the development of a complete safety profile, bioavailability studies and standardization of the pharmacodynamics parameters, as well as consideration of regulatory aspects.
Acknowledgments
We greatly acknowledge the Government of Republic of Korea for supporting this project through National Research Foundation (MSIT, NO. NRF-2021R1F1A1048156).
Author Contributions
All authors made substantial contributions to the study and approved the final draft of manuscript for submission. All authors have read and agreed to the published version of the manuscript.
Funding
National Research Foundation of Korea (MSIT, NO. NRF-2021R1F1A1048156).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors state that they have no competing interests in the publication of this work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ashley N.T., Weil Z.M., Nelson R.J. Inflammation: Mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. Syst. 2012;43:385–406. doi: 10.1146/annurev-ecolsys-040212-092530. [DOI] [Google Scholar]
- 2.Rock K.L., Kono H. The inflammatory response to cell death. Annu. Rev. Pathol. Mech. Dis. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambriz-Pérez D.L., Leyva-López N., Gutierrez-Grijalva E.P., Heredia J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016;2:1131412. [Google Scholar]
- 4.Ngo L.T., Okogun J.I., Folk W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013;30:584–592. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu F., Ma X.H., Qin C., Tao L., Liu X., Shi Z., Zhang C.L., Tan C.Y., Chen Y.Z., Jiang Y.Y. Drug discovery prospect from untapped species: Indications from approved natural product drugs. PLoS ONE. 2012;7:e39782. doi: 10.1371/journal.pone.0039782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galm U., Shen B. Natural product drug discovery: The times have never been better. Chem. Biol. 2007;14:1098–1104. doi: 10.1016/j.chembiol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Kim H.G., Song H., Yoon D.H., Song B.W., Park S.M., Sung G.H., Cho J.Y., Park H.I., Choi S., Song W.O., et al. Cordyceps pruinosa extracts induce apoptosis of HeLa cells by a caspase dependent pathway. J. Ethnopharmacol. 2010;128:342–351. doi: 10.1016/j.jep.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Sung G.H., Shrestha B., Han S.K., Sung J.M. Cultural Characteristics of Ophiocordyceps heteropoda Collected from Korea. Mycobiology. 2011;39:1–6. doi: 10.4489/MYCO.2011.39.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajek A., St. Leger R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994;39:293–322. [Google Scholar]
- 10.Olatunji O.J., Tang J., Tola A., Auberon F., Oluwaniyi O., Ouyang Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia. 2018;129:293–316. doi: 10.1016/j.fitote.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Cleaver P.D., Loomis-Powers M., Patel D. Analysis of quality and techniques for hybridization of medicinal fungus Cordyceps sinensis (Berk.) Sacc. (Ascomycetes) Int. J. Med. Mushrooms. 2004;6:151–164. [Google Scholar]
- 12.Das S.K., Masuda M., Sakurai A., Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia. 2010;81:961–968. doi: 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Rivera G., Bocanegra-García V., Monge A. Traditional plants as source of functional foods: A review Plantas tradicionales como fuente de alimentos funcionales: Una revisión. CyTA J. Food. 2010;8:159–167. doi: 10.1080/19476330903322978. [DOI] [Google Scholar]
- 14.Maroyi A. Utilization of Bridelia mollis as herbal medicine, nutraceutical and functional food in southern Africa: A review. Trop. J. Pharm. Res. 2019;18:203–209. doi: 10.4314/tjpr.v18i1.30. [DOI] [Google Scholar]
- 15.Ukeyima M.T., Enujiugha V.N., Sanni T.A. Current applications of probiotic foods in Africa. Afr. J. Biotechnol. 2010;9:394–401. [Google Scholar]
- 16.Arvanitoyannis I.S., Van Houwelingen-Koukaliaroglou M. Functional foods: A survey of health claims, pros and cons, and current legislation. Crit. Rev. Food Sci. Nutr. 2005;45:385–404. doi: 10.1080/10408390590967667. [DOI] [PubMed] [Google Scholar]
- 17.Silva M.A., Albuquerque T.G., Alves R.C., Oliveira M.B.P., Costa H.S. Melon (Cucumis melo L.) by-products: Potential food ingredients for novel functional foods? Trends Food Sci. Technol. 2020;98:181–189. doi: 10.1016/j.tifs.2018.07.005. [DOI] [Google Scholar]
- 18.Green M., Arora K., Prakash S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020;21:2890. doi: 10.3390/ijms21082890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziemer C.J., Gibson G.R. An overview of probiotics, prebiotics and synbiotics in the functional food concept: Perspectives and future strategies. Int. Dairy J. 1998;8:473–479. doi: 10.1016/S0958-6946(98)00071-5. [DOI] [Google Scholar]
- 20.Ashraf S.A., Elkhalifa A.E.O., Siddiqui A.J., Patel M., Awadelkareem A.M., Snoussi M., Ashraf M.S., Adnan M., Hadi S. Cordycepin for Health and Wellbeing: A Potent Bioactive Metabolite of an Entomopathogenic Cordyceps Medicinal Fungus and Its Nutraceutical and Therapeutic Potential. Molecules. 2020;25:2735. doi: 10.3390/molecules25122735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holliday J., Cleaver M.P. Medicinal value of the caterpillar fungi species of the genus Cordyceps (Fr.) Link (Ascomycetes). A review. Int. J. Med. Mushrooms. 2008;10:219–234. doi: 10.1615/IntJMedMushr.v10.i3.30. [DOI] [Google Scholar]
- 22.Shrestha B., Zhang W., Zhang Y., Liu X. The medicinal fungus Cordyceps militaris: Research and development. Mycol. Prog. 2012;11:599–614. doi: 10.1007/s11557-012-0825-y. [DOI] [Google Scholar]
- 23.Dong C., Guo S., Wang W., Liu X. Cordyceps industry in China. Mycology. 2015;6:121–129. doi: 10.1080/21501203.2015.1043967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong K.L., Wong R.N., Zhang L., Liu W.K., Ng T.B., Shaw P.C., Kwok P.C., Lai Y.M., Zhang Z.J., Zhang Y., et al. Bioactive proteins and peptides isolated from Chinese medicines with pharmaceutical potential. Chin. Med. 2014;9:19. doi: 10.1186/1749-8546-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong J.H., Ng T.B., Wang H., Sze S.C., Zhang K.Y., Li Q., Lu X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine. 2011;18:387–392. doi: 10.1016/j.phymed.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Qi W., Zhang Y., Yan Y.B., Lei W., Wu Z.X., Liu N., Liu S., Shi L., Fan Y. The Protective Effect of Cordymin, a Peptide Purified from the Medicinal Mushroom Cordyceps sinensis, on Diabetic Osteopenia in Alloxan-Induced Diabetic Rats. Evid. Based Complement. Altern. Med. 2013;2013:985636. doi: 10.1155/2013/985636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung E.C., Kim K.D., Bae C.H., Kim J.C., Kim D.K., Kim H.H. A mushroom lectin from ascomycete Cordyceps militaris. Biochim. Biophys. Acta. 2007;1770:833–838. doi: 10.1016/j.bbagen.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Park B.T., Na K.H., Jung E.C., Park J.W., Kim H.H. Antifungal and Anticancer Activities of a Protein from the Mushroom Cordyceps militaris. Korean J. Physiol. Pharm. 2009;13:49–54. doi: 10.4196/kjpp.2009.13.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai C.H., Yen Y.H., Yang J.P. Finding of polysaccharide-peptide complexes in Cordyceps militaris and evaluation of its acetylcholinesterase inhibition activity. J. Food Drug Anal. 2015;23:63–70. doi: 10.1016/j.jfda.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X., Kopparapu N.K., Li Y., Deng Y., Zheng X. Biochemical characterization of a novel fibrinolytic enzyme from Cordyceps militaris. Int. J. Biol. Macromol. 2017;94 Pt B:793–801. doi: 10.1016/j.ijbiomac.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 31.Yang Q., Yin Y., Yu G., Jin Y., Ye X., Shrestha A., Liu W., Yu W., Sun H. A novel protein with anti-metastasis activity on 4T1 carcinoma from medicinal fungus Cordyceps militaris. Int. J. Biol. Macromol. 2015;80:385–391. doi: 10.1016/j.ijbiomac.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Wen C., Duan Y., Zhang H., Ma H. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019;132:906–914. doi: 10.1016/j.ijbiomac.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.S., Kwon D.S., Lee K.R., Park J.M., Ha S.J., Hong E.K. Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth. Carbohydr. Polym. 2015;120:29–37. doi: 10.1016/j.carbpol.2014.11.059. [DOI] [PubMed] [Google Scholar]
- 34.Wang M., Meng X.Y., Yang R.L., Qin T., Wang X.Y., Zhang K.Y., Fei C.Z., Li Y., Hu Y., Xue F.Q. Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydr. Polym. 2012;89:461–466. doi: 10.1016/j.carbpol.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Luo X., Duan Y., Yang W., Zhang H., Li C., Zhang J. Structural elucidation and immunostimulatory activity of polysaccharide isolated by subcritical water extraction from Cordyceps militaris. Carbohydr. Polym. 2017;157:794–802. doi: 10.1016/j.carbpol.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 36.Chen X., Wu G., Huang Z. Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. Int. J. Biol. Macromol. 2013;58:18–22. doi: 10.1016/j.ijbiomac.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 37.Yu R., Wang L., Zhang H., Zhou C., Zhao Y. Isolation, purification and identification of polysaccharides from cultured Cordyceps militaris. Fitoterapia. 2004;75:662–666. doi: 10.1016/j.fitote.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Jing Y., Cui X., Chen Z., Huang L., Song L., Liu T., Lv W., Yu R. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr. Polym. 2014;102:288–296. doi: 10.1016/j.carbpol.2013.11.061. [DOI] [PubMed] [Google Scholar]
- 39.Chen C., Wang M.-L., Jin C., Chen H.-J., Li S.-H., Li S.-Y., Dou X.-F., Jia J.-Q., Gui Z.-Z. Cordyceps militaris polysaccharide triggers apoptosis and G0/G1 cell arrest in cancer cells. J. Asia-Pac. Entomol. 2015;18:433–438. doi: 10.1016/j.aspen.2015.04.015. [DOI] [Google Scholar]
- 40.Rao Y.K., Fang S.H., Wu W.S., Tzeng Y.M. Constituents isolated from Cordyceps militaris suppress enhanced inflammatory mediator’s production and human cancer cell proliferation. J. Ethnopharmacol. 2010;131:363–367. doi: 10.1016/j.jep.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Park S.E., Kim J., Lee Y.W., Yoo H.S., Cho C.K. Antitumor activity of water extracts from Cordyceps militaris in NCI-H460 cell xenografted nude mice. J. Acupunct. Meridian Stud. 2009;2:294–300. doi: 10.1016/S2005-2901(09)60071-6. [DOI] [PubMed] [Google Scholar]
- 42.Lee J.S., Hong E.K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharmacol. 2011;11:1226–1233. doi: 10.1016/j.intimp.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Ohta Y., Lee J.B., Hayashi K., Fujita A., Park D.K., Hayashi T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007;55:10194–10199. doi: 10.1021/jf0721287. [DOI] [PubMed] [Google Scholar]
- 44.Kim H.S., Kim J.Y., Kang J.S., Kim H.M., Kim Y.O., Hong I.P., Lee M.K., Hong J.T., Kim Y., Han S.B. Cordlan polysaccharide isolated from mushroom Cordyceps militaris induces dendritic cell maturation through toll-like receptor 4 signalings. Food Chem. Toxicol. 2010;48:1926–1933. doi: 10.1016/j.fct.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 45.Lin R., Liu H., Wu S., Pang L., Jia M., Fan K., Jia S., Jia L. Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int. J. Biol. Macromol. 2012;51:153–157. doi: 10.1016/j.ijbiomac.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y., E Q., Zuo J., Tao Y., Liu W. Protective effect of Cordyceps polysaccharide on hydrogen peroxide-induced mitochondrial dysfunction in HL-7702 cells. Mol. Med. Rep. 2013;7:747–754. doi: 10.3892/mmr.2012.1248. [DOI] [PubMed] [Google Scholar]
- 47.Wang M., Meng X., Yang R., Qin T., Li Y., Zhang L., Fei C., Zhen W., Zhang K., Wang X., et al. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Biol. Macromol. 2013;59:178–183. doi: 10.1016/j.ijbiomac.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Yu R., Song L., Zhao Y., Bin W., Wang L., Zhang H., Wu Y., Ye W., Yao X. Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris. Fitoterapia. 2004;75:465–472. doi: 10.1016/j.fitote.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Cheung J.K., Li J., Cheung A.W., Zhu Y., Zheng K.Y., Bi C.W., Duan R., Choi R.C., Lau D.T., Dong T.T., et al. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: Signaling cascade and induction of cytokines. J. Ethnopharmacol. 2009;124:61–68. doi: 10.1016/j.jep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Bi S., Huang W., Chen S., Huang C., Li C., Guo Z., Yang J., Zhu J., Song L., Yu R. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. Int. J. Biol. Macromol. 2020;150:261–280. doi: 10.1016/j.ijbiomac.2020.02.050. [DOI] [PubMed] [Google Scholar]
- 51.Bi S., Jing Y., Zhou Q., Hu X., Zhu J., Guo Z., Song L., Yu R. Structural elucidation and immunostimulatory activity of a new polysaccharide from Cordyceps militaris. Food Funct. 2018;9:279–293. doi: 10.1039/C7FO01147D. [DOI] [PubMed] [Google Scholar]
- 52.Figueiredo R.T., Bittencourt V.C.B., Lopes L.C.L., Sassaki G., Barreto-Bergter E. Toll-like receptors (TLR2 and TLR4) recognize polysaccharides of Pseudallescheria boydii cell wall. Carbohydr. Res. 2012;356:260–264. doi: 10.1016/j.carres.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 53.Wu D.-T., Xie J., Wang L.-Y., Ju Y.-J., Lv G.-P., Leong F., Zhao J., Li S.-P. Characterization of bioactive polysaccharides from Cordyceps militaris produced in China using saccharide mapping. J. Funct. Foods. 2014;9:315–323. doi: 10.1016/j.jff.2014.05.005. [DOI] [Google Scholar]
- 54.Ribeiro J.A. Purinergic inhibition of neurotransmitter release in the central nervous system. Pharm. Toxicol. 1995;77:299–305. doi: 10.1111/j.1600-0773.1995.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 55.Dunwiddie T.V., Masino S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 56.Tabrizchi R., Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharm. Ther. 2001;91:133–147. doi: 10.1016/S0163-7258(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 57.Carlezon W.A., Jr., Mague S.D., Parow A.M., Stoll A.L., Cohen B.M., Renshaw P.F. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol. Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 58.Vinadé E.R., Schmidt A.P., Frizzo M.E., Izquierdo I., Elisabetsky E., Souza D.O. Chronically administered guanosine is anticonvulsant, amnesic and anxiolytic in mice. Brain Res. 2003;977:97–102. doi: 10.1016/S0006-8993(03)02769-0. [DOI] [PubMed] [Google Scholar]
- 59.Cunningham K.G., Manson W., Spring F.S., Hutchinson S.A. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature. 1950;166:949. doi: 10.1038/166949a0. [DOI] [PubMed] [Google Scholar]
- 60.Ahn Y.J., Park S.J., Lee S.G., Shin S.C., Choi D.H. Cordycepin: Selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostridium spp. J. Agric. Food Chem. 2000;48:2744–2748. doi: 10.1021/jf990862n. [DOI] [PubMed] [Google Scholar]
- 61.Zhou X., Meyer C.U., Schmidtke P., Zepp F. Effect of cordycepin on interleukin-10 production of human peripheral blood mononuclear cells. Eur. J. Pharm. 2002;453:309–317. doi: 10.1016/S0014-2999(02)02359-2. [DOI] [PubMed] [Google Scholar]
- 62.Tuli H.S., Sandhu S.S., Sharma A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech. 2014;4:1–12. doi: 10.1007/s13205-013-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma L., Zhang S., Du M. Cordycepin from Cordyceps militaris prevents hyperglycemia in alloxan-induced diabetic mice. Nutr. Res. 2015;35:431–439. doi: 10.1016/j.nutres.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Shin S., Lee S., Kwon J., Moon S., Lee S., Lee C.K., Cho K., Ha N.J., Kim K. Cordycepin Suppresses Expression of Diabetes Regulating Genes by Inhibition of Lipopolysaccharide-induced Inflammation in Macrophages. Immune Netw. 2009;9:98–105. doi: 10.4110/in.2009.9.3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji D.B., Ye J., Li C.L., Wang Y.H., Zhao J., Cai S.Q. Antiaging effect of Cordyceps sinensis extract. Phytother. Res. 2009;23:116–122. doi: 10.1002/ptr.2576. [DOI] [PubMed] [Google Scholar]
- 66.Patel K., Ingalhalli R.S. Cordyceps militaris (L.: Fr.) Link-An Important Medicinal Mushroom. J. Pharmacogn. Phytochem. 2013;2:315–319. [Google Scholar]
- 67.Thomson D.M., Winder W.W. AMP-activated protein kinase control of fat metabolism in skeletal muscle. Acta Physiol. (Oxf.) 2009;196:147–154. doi: 10.1111/j.1748-1716.2009.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atkinson L.L., Kozak R., Kelly S.E., Onay Besikci A., Russell J.C., Lopaschuk G.D. Potential mechanisms and consequences of cardiac triacylglycerol accumulation in insulin-resistant rats. Am. J. Physiol. Endocrinol. Metab. 2003;284:E923–E930. doi: 10.1152/ajpendo.00360.2002. [DOI] [PubMed] [Google Scholar]
- 69.Zhou X., Luo L., Dressel W., Shadier G., Krumbiegel D., Schmidtke P., Zepp F., Meyer C.U. Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis. Am. J. Chin. Med. 2008;36:967–980. doi: 10.1142/S0192415X08006387. [DOI] [PubMed] [Google Scholar]
- 70.Zhang D.-W., Wang Z.-L., Qi W., Lei W., Zhao G.-Y. Cordycepin (3′-deoxyadenosine) down-regulates the proinflammatory cytokines in inflammation-induced osteoporosis model. Inflammation. 2014;37:1044–1049. doi: 10.1007/s10753-014-9827-z. [DOI] [PubMed] [Google Scholar]
- 71.Trigg P.I., Gutteridge W.E., Williamson J. The effects of cordycepin on malaria parasites. Trans. R. Soc. Trop. Med. Hyg. 1971;65:514–520. doi: 10.1016/0035-9203(71)90162-3. [DOI] [PubMed] [Google Scholar]
- 72.Rodrigo R., Bosco C. Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp. Biochem. Physiol. C Toxicol. Pharm. 2006;142:317–327. doi: 10.1016/j.cbpc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 73.Yu H.M., Wang B.S., Huang S.C., Duh P.D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2006;54:3132–3138. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- 74.Joshi M., Sagar A., Kanwar S.S., Singh S. Anticancer, antibacterial and antioxidant activities of Cordyceps militaris. Indian J. Exp. Biol. 2019;57:15–20. [Google Scholar]
- 75.Cui J.D. Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit. Rev. Biotechnol. 2015;35:475–484. doi: 10.3109/07388551.2014.900604. [DOI] [PubMed] [Google Scholar]
- 76.Li-Tong C., Hong-Feng C., Wen-Fang H.J. Chemical composition, pharmic effect and application of Cordyceps militaris. Guangzhou Food Sci. Technol. 2005;21:192–197. [Google Scholar]
- 77.Reis F.S., Barros L., Calhelha R.C., Cirić A., van Griensven L.J., Soković M., Ferreira I.C. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013;62:91–98. doi: 10.1016/j.fct.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 78.Dong J.Z., Wang S.H., Ai X.R., Yao L., Sun Z.W., Lei C., Wang Y., Wang Q.J. Composition and characterization of cordyxanthins from Cordyceps militaris fruit bodies. J. Funct. Foods. 2013;5:1450–1455. doi: 10.1016/j.jff.2013.06.002. [DOI] [Google Scholar]
- 79.Chiu C.P., Liu S.C., Tang C.H., Chan Y., El-Shazly M., Lee C.L., Du Y.C., Wu T.Y., Chang F.R., Wu Y.C. Anti-inflammatory Cerebrosides from Cultivated Cordyceps militaris. J. Agric. Food Chem. 2016;64:1540–1548. doi: 10.1021/acs.jafc.5b05931. [DOI] [PubMed] [Google Scholar]
- 80.Kim C.S., Lee S.Y., Cho S.H., Ko Y.M., Kim B.H., Kim H.J., Park J.C., Kim D.K., Ahn H., Kim B.O., et al. Cordyceps militaris induces the IL-18 expression via its promoter activation for IFN-gamma production. J. Ethnopharmacol. 2008;120:366–371. doi: 10.1016/j.jep.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Zhu S.J., Pan J., Zhao B., Liang J., Ze-Yu W., Yang J.J. Comparisons on enhancing the immunity of fresh and dry Cordyceps militaris in vivo and in vitro. J. Ethnopharmacol. 2013;149:713–719. doi: 10.1016/j.jep.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 82.Kang H.J., Baik H.W., Kim S.J., Lee S.G., Ahn H.Y., Park J.S., Park S.J., Jang E.J., Park S.W., Choi J.Y., et al. Cordyceps militaris Enhances Cell-Mediated Immunity in Healthy Korean Men. J. Med. Food. 2015;18:1164–1172. doi: 10.1089/jmf.2014.3350. [DOI] [PubMed] [Google Scholar]
- 83.Lee H.H., Park H., Sung G.H., Lee K., Lee T., Lee I., Park M.S., Jung Y.W., Shin Y.S., Kang H., et al. Anti-influenza effect of Cordyceps militaris through immunomodulation in a DBA/2 mouse model. J. Microbiol. 2014;52:696–701. doi: 10.1007/s12275-014-4300-0. [DOI] [PubMed] [Google Scholar]
- 84.Ueda Y., Mori K., Satoh S., Dansako H., Ikeda M., Kato N. Anti-HCV activity of the Chinese medicinal fungus Cordyceps militaris. Biochem. Biophys. Res. Commun. 2014;447:341–345. doi: 10.1016/j.bbrc.2014.03.150. [DOI] [PubMed] [Google Scholar]
- 85.Cui L., Dong M.S., Chen X.H., Jiang M., Lv X., Yan G. A novel fibrinolytic enzyme from Cordyceps militaris, a Chinese traditional medicinal mushroom. World J. Microbiol. Biotechnol. 2008;24:483–489. doi: 10.1007/s11274-007-9497-1. [DOI] [Google Scholar]
- 86.Liu X., Kopparapu N.K., Shi X., Deng Y., Zheng X., Wu J. Purification and biochemical characterization of a novel fibrinolytic enzyme from culture supernatant of Cordyceps militaris. J. Agric. Food Chem. 2015;63:2215–2224. doi: 10.1021/jf505717e. [DOI] [PubMed] [Google Scholar]
- 87.Park J.G., Son Y.J., Lee T.H., Baek N.J., Yoon D.H., Kim T.W., Aravinthan A., Hong S., Kim J.H., Sung G.H., et al. Anticancer Efficacy of Cordyceps militaris Ethanol Extract in a Xenografted Leukemia Model. Evid. Based Complement. Altern. Med. 2017;2017:8474703. doi: 10.1155/2017/8474703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X., Wang J., Zhang H., Xiao L., Lei Z., Kaul S.C., Wadhwa R., Zhang Z. Low Dose of Fluoride in the Culture Medium of Cordyceps militaris Promotes Its Growth and Enhances Bioactives with Antioxidant and Anticancer Properties. J. Fungi. 2021;7:342. doi: 10.3390/jof7050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin C.Y., Kim G.Y., Choi Y.H. Induction of apoptosis by aqueous extract of Cordyceps militaris through activation of caspases and inactivation of Akt in human breast cancer MDA-MB-231 Cells. J. Microbiol. Biotechnol. 2008;18:1997–2003. [PubMed] [Google Scholar]
- 90.Lee H.H., Lee S., Lee K., Shin Y.S., Kang H., Cho H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. Daru. 2015;23:35. doi: 10.1186/s40199-015-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chou S.M., Lai W.J., Hong T., Tsai S.H., Chen Y.H., Kao C.H., Chu R., Shen T.L., Li T.K. Involvement of p38 MAPK in the Anticancer Activity of Cultivated Cordyceps militaris. Am. J. Chin. Med. 2015;43:1043–1057. doi: 10.1142/S0192415X15500603. [DOI] [PubMed] [Google Scholar]
- 92.Jo E., Jang H.J., Shen L., Yang K.E., Jang M.S., Huh Y.H., Yoo H.S., Park J., Jang I.S., Park S.J. Cordyceps militaris Exerts Anticancer Effect on Non-Small Cell Lung Cancer by Inhibiting Hedgehog Signaling via Suppression of TCTN3. Integr. Cancer. 2020;19:1534735420923756. doi: 10.1177/1534735420923756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee M.R., Kim J.E., Choi J.Y., Park J.J., Kim H.R., Song B.R., Choi Y.W., Kim K.M., Song H., Hwang D.Y. Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: Study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp. Ther. Med. 2019;17:2185–2193. doi: 10.3892/etm.2019.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Q., Hong I.P., Ahn M.J., Yoo H.S., Han S.B., Hwang B.Y., Lee M.K. Anti-adipogenic activity of Cordyceps militaris in 3T3-L1 cells. Nat. Prod. Commun. 2011;6:1839–1841. doi: 10.1177/1934578X1100601213. [DOI] [PubMed] [Google Scholar]
- 95.Guo L., Li K., Kang J.S., Kang N.J., Son B.G., Choi Y.W. Strawberry fermentation with Cordyceps militaris has anti-adipogenesis activity. Food Biosci. 2020;35:100576. doi: 10.1016/j.fbio.2020.100576. [DOI] [Google Scholar]
- 96.Shimada T., Hiramatsu N., Kasai A., Mukai M., Okamura M., Yao J., Huang T., Tamai M., Takahashi S., Nakamura T., et al. Suppression of adipocyte differentiation by Cordyceps militaris through activation of the aryl hydrocarbon receptor. Am. J. Physiol. Endocrinol. Metab. 2008;295:E859–E867. doi: 10.1152/ajpendo.90373.2008. [DOI] [PubMed] [Google Scholar]
- 97.Hsu C.H., Sun H.L., Sheu J.N., Ku M.S., Hu C.M., Chan Y., Lue K.H. Effects of the immunomodulatory agent Cordyceps militaris on airway inflammation in a mouse asthma model. Pediatr. Neonatol. 2008;49:171–178. doi: 10.1016/S1875-9572(09)60004-8. [DOI] [PubMed] [Google Scholar]
- 98.Oh J.Y., Choi W.S., Lee C.H., Park H.J. The ethyl acetate extract of Cordyceps militaris inhibits IgE-mediated allergic responses in mast cells and passive cutaneous anaphylaxis reaction in mice. J. Ethnopharmacol. 2011;135:422–429. doi: 10.1016/j.jep.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 99.Park D.K., Choi W.S., Park H.J. Antiallergic activity of novel isoflavone methyl-glycosides from Cordyceps militaris grown on germinated soybeans in antigen-stimulated mast cells. J. Agric. Food Chem. 2012;60:2309–2315. doi: 10.1021/jf205199j. [DOI] [PubMed] [Google Scholar]
- 100.Wu T.F., Chan Y.Y., Shi W.Y., Jhong M.T. Uncovering the Molecular Mechanism of Anti-Allergic Activity of Silkworm Pupa-Grown Cordyceps militaris Fruit Body. Am. J. Chin. Med. 2017;45:497–513. doi: 10.1142/S0192415X17500306. [DOI] [PubMed] [Google Scholar]
- 101.Dong Y., Jing T., Meng Q., Liu C., Hu S., Ma Y., Liu Y., Lu J., Cheng Y., Wang D., et al. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley rats. BioMed Res. Int. 2014;2014:160980. doi: 10.1155/2014/160980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Silva D.D., Rapior S., Hyde K.D., Bahkali A.H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012;56:1–29. doi: 10.1007/s13225-012-0187-4. [DOI] [Google Scholar]
- 103.Mehra A., Zaidi K.U., Mani A., Thawani V.J.K. The health benefits of Cordyceps militaris—A review. Kavaka. 2017;48:27–32. [Google Scholar]
- 104.Phull A.R., Kim S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods. 2017;38:415–426. doi: 10.1016/j.jff.2017.09.051. [DOI] [Google Scholar]
- 105.Segal J.P., Tresidder K.A., Bhatt C., Gilron I., Ghasemlou N.J. Circadian control of pain and neuroinflammation. J. Neurosci. Res. 2018;96:1002–1020. doi: 10.1002/jnr.24150. [DOI] [PubMed] [Google Scholar]
- 106.Montero-Vega M.T. The inflammatory process underlying atherosclerosis. Crit. Rev. Immunol. 2012;32:373–462. doi: 10.1615/CritRevImmunol.v32.i5.10. [DOI] [PubMed] [Google Scholar]
- 107.Kornstädt L., Pierre S., Weigert A., Ebersberger S., Schäufele T.J., Kolbinger A., Schmid T., Cohnen J., Thomas D., Ferreirós N. Bacterial and Fungal Toll-Like Receptor Activation Elicits Type I IFN Responses in Mast Cells. Front. Immunol. 2021;11:3872. doi: 10.3389/fimmu.2020.607048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galvão I., Sugimoto M.A., Vago J.P., Machado M.G., Sousa L.P. Immunopharmacology and Inflammation. Springer; Berlin/Heidelberg, Germany: 2018. Mediators of inflammation; pp. 3–32. [Google Scholar]
- 109.Chen Y., Yang M., Huang W., Chen W., Zhao Y., Schulte M.L., Volberding P., Gerbec Z., Zimmermann M.T., Zeighami A. Mitochondrial metabolic reprogramming by CD36 signaling drives macrophage inflammatory responses. Circ. Res. 2019;125:1087–1102. doi: 10.1161/CIRCRESAHA.119.315833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chovatiya R., Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol. Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sommer C., Lindenlaub T., Teuteberg P., Schäfers M., Hartung T., Toyka K.V. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001;913:86–89. doi: 10.1016/S0006-8993(01)02743-3. [DOI] [PubMed] [Google Scholar]
- 112.Phull A.R., Nasir B., Haq I.U., Kim S.J. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem. Biol. Interact. 2018;281:121–136. doi: 10.1016/j.cbi.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 113.Baldwin A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Investig. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baldwin A.S., Jr. The NF-κB and IκB proteins: New discoveries and insights. Annu. Rev. Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 115.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tissot B., Montdargent B., Chevolot L., Varenne A., Descroix S., Gareil P., Daniel R. Interaction of fucoidan with the proteins of the complement classical pathway. Biochim. Biophys. Acta. 2003;1651:5–16. doi: 10.1016/S1570-9639(03)00230-9. [DOI] [PubMed] [Google Scholar]
- 117.Maity N., Nema N.K., Sarkar B.K., Mukherjee P.K. Standardized Clitoria ternatea leaf extract as hyaluronidase, elastase and matrix-metalloproteinase-1 inhibitor. Indian J. Pharm. 2012;44:584–587. doi: 10.4103/0253-7613.100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Terzi M., Altun G., Şen S., Kocaman A., Kaplan A.A., Yurt K.K., Kaplan S. The use of non-steroidal anti-inflammatory drugs in neurological diseases. J. Chem. Neuroanat. 2018;87:12–24. doi: 10.1016/j.jchemneu.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 119.Phull A.-R., Majid M., Haq I.-U., Khan M.R., Kim S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017;97:468–480. doi: 10.1016/j.ijbiomac.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 120.Kwon H.K., Song M.J., Lee H.J., Park T.S., Kim M.I., Park H.J. Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis. Int. J. Mol. Sci. 2018;19:3504. doi: 10.3390/ijms19113504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lan T., Yu Y., Zhang J., Li H., Weng Q., Jiang S., Tian S., Xu T., Hu S., Yang G., et al. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology. 2021;74:686–703. doi: 10.1002/hep.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu T., Shim J., Yang Y., Byeon S.E., Kim J.H., Rho H.S., Park H., Sung G.H., Kim T.W., Rhee M.H., et al. 3-(4-(tert-Octyl)phenoxy)propane-1,2-diol suppresses inflammatory responses via inhibition of multiple kinases. Biochem. Pharm. 2012;83:1540–1551. doi: 10.1016/j.bcp.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 123.Cheng Y.H., Hsieh Y.C., Yu Y.H. Effect of Cordyceps militaris Hot Water Extract on Immunomodulation-associated Gene Expression in Broilers, Gallus gallus. J. Poult. Sci. 2019;56:128–139. doi: 10.2141/jpsa.0180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu T.F., Shi W.Y., Chiu Y.C., Chan Y.Y. Investigation of the molecular mechanism underlying the inhibitory activities of ethanol extract of Bombyx mori pupa-incubated Cordyceps militaris fruiting bodies toward allergic rhinitis. Biomed. Pharm. 2021;135:111248. doi: 10.1016/j.biopha.2021.111248. [DOI] [PubMed] [Google Scholar]
- 125.Kim H.J., Joe H.I., Zhang Z., Woo Lee S., Lee K.Y., Kook Y.B., An H.J. Anti-inflammatory effect of Acalypha australis L. via suppression of NF-κB signaling in LPS-stimulated RAW264.7 macrophages and LPS-induced septic mice. Mol. Immunol. 2020;119:123–131. doi: 10.1016/j.molimm.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 126.Devi K.P., Malar D.S., Nabavi S.F., Sureda A., Xiao J., Nabavi S.M., Daglia M. Kaempferol and inflammation: From chemistry to medicine. Pharm. Res. 2015;99:1–10. doi: 10.1016/j.phrs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 127.Winterbourn C.C., Kettle A.J., Hampton M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016;85:765–792. doi: 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 128.Zhang X., Zhang X., Gu S., Pan L., Sun H., Gong E., Zhu Z., Wen T., Daba G.M., Elkhateeb W.A. Structure analysis and antioxidant activity of polysaccharide-iron (III) from Cordyceps militaris mycelia. Int. J. Biol Macromol. 2021;178:170–179. doi: 10.1016/j.ijbiomac.2021.02.163. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y., Zeng Y., Cui Y., Liu H., Dong C., Sun Y. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohydr. Polym. 2020;235:115969. doi: 10.1016/j.carbpol.2020.115969. [DOI] [PubMed] [Google Scholar]
- 130.Wu H.C., Chen S.T., Chang J.C., Hsu T.Y., Cheng C.C., Chang H.S., Liu C.S., Wu Y.C., Liang Z.C. Radical Scavenging and Antiproliferative Effects of Cordycepin-Rich Ethanol Extract from Brown Rice-Cultivated Cordyceps militaris (Ascomycetes) Mycelium on Breast Cancer Cell Lines. Int. J. Med. Mushrooms. 2019;21:657–669. doi: 10.1615/IntJMedMushrooms.2019031138. [DOI] [PubMed] [Google Scholar]
- 131.Song Q., Zhu Z. Using Cordyceps militaris extracellular polysaccharides to prevent Pb(2+)-induced liver and kidney toxicity by activating Nrf2 signals and modulating gut microbiota. Food Funct. 2020;11:9226–9239. doi: 10.1039/D0FO01608J. [DOI] [PubMed] [Google Scholar]
- 132.He M.T., Lee A.Y., Park C.H., Cho E.J. Protective effect of Cordyceps militaris against hydrogen peroxide-induced oxidative stress in vitro. Nutr. Res. Pract. 2019;13:279–285. doi: 10.4162/nrp.2019.13.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lei J., Wei Y., Song P., Li Y., Zhang T., Feng Q., Xu G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharm. 2018;818:110–114. doi: 10.1016/j.ejphar.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 134.Nilsson A., Wilhelms D.B., Mirrasekhian E., Jaarola M., Blomqvist A., Engblom D. Inflammation-induced anorexia and fever are elicited by distinct prostaglandin dependent mechanisms, whereas conditioned taste aversion is prostaglandin independent. Brain Behav. Immun. 2017;61:236–243. doi: 10.1016/j.bbi.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park E.S., Song G.H., Kim S.H., Lee S.M., Kim Y.G., Lim Y.L., Kang S.A., Park K.Y. Rumex crispus and Cordyceps militaris Mixture Ameliorates Production of Pro-Inflammatory Cytokines Induced by Lipopolysaccharide in C57BL/6 Mice Splenocytes. Prev. Nutr. Food Sci. 2018;23:374–381. doi: 10.3746/pnf.2018.23.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim H.G., Shrestha B., Lim S.Y., Yoon D.H., Chang W.C., Shin D.J., Han S.K., Park S.M., Park J.H., Park H.I., et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW264.7 macrophage cells. Eur. J. Pharm. 2006;545:192–199. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 137.Sun Y., Shao Y., Zhang Z., Wang L., Mariga A.M., Pang G., Geng C., Ho C.T., Hu Q., Zhao L. Regulation of human cytokines by Cordyceps militaris. J. Food Drug Anal. 2014;22:463–467. doi: 10.1016/j.jfda.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Phull A.R., Kim S.J. Fucoidan from Undaria pinnatifida regulates type II collagen and COX-2 expression via MAPK and PI3K pathways in rabbit articular chondrocytes. Biologia. 2017;72:1362–1369. doi: 10.1515/biolog-2017-0158. [DOI] [Google Scholar]
- 139.Sharma J., Mohammed L.A. The role of leukotrienes in the pathophysiology of inflammatory disorders: Is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology. 2006;14:10–16. doi: 10.1007/s10787-006-1496-6. [DOI] [PubMed] [Google Scholar]
- 140.Kim Y.S., Shin W.B., Dong X., Kim E.K., Nawarathna W., Kim H., Park P.J. Anti-inflammatory effect of the extract from fermented Asterina pectinifera with Cordyceps militaris mycelia in LPS-induced RAW264.7 macrophages. Food Sci. Biotechnol. 2017;26:1633–1640. doi: 10.1007/s10068-017-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sheng W.Y., Chen Y.R., Wang T.C. A major role of PKC theta and NFkappaB in the regulation of hTERT in human T lymphocytes. FEBS Lett. 2006;580:6819–6824. doi: 10.1016/j.febslet.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 142.Phull A., Eo S., Kim S.J.C., Biology M. Oleanolic acid (OA) regulates inflammation and cellular dedifferentiation of chondrocytes via MAPK signaling pathways. Cell. Mol. Biol. (Noisy-le-Grand) 2017;63:12–17. doi: 10.14715/cmb/2017.63.3.3. [DOI] [PubMed] [Google Scholar]
- 143.Yang X., Li Y., He Y., Li T., Wang W., Zhang J., Wei J., Deng Y., Lin R. Cordycepin alleviates airway hyperreactivity in a murine model of asthma by attenuating the inflammatory process. Int. Immunopharmacol. 2015;26:401–408. doi: 10.1016/j.intimp.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 144.Song J., Wang Y., Liu C., Huang Y., He L., Cai X., Lu J., Liu Y., Wang D. Cordyceps militaris fruit body extract ameliorates membranous glomerulonephritis by attenuating oxidative stress and renal inflammation via the NF-κB pathway. Food Funct. 2016;7:2006–2015. doi: 10.1039/C5FO01017A. [DOI] [PubMed] [Google Scholar]
- 145.Ying X., Peng L., Chen H., Shen Y., Yu K., Cheng S. Cordycepin prevented IL-β-induced expression of inflammatory mediators in human osteoarthritis chondrocytes. Int. Orthop. 2014;38:1519–1526. doi: 10.1007/s00264-013-2219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Y., Li K., Mao L., Han X., Zhang K., Zhao C., Zhao J. Cordycepin inhibits LPS-induced inflammatory and matrix degradation in the intervertebral disc. PeerJ. 2016;4:e1992. doi: 10.7717/peerj.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kong D., Yamori T. Phosphatidylinositol 3-kinase inhibitors: Promising drug candidates for cancer therapy. Cancer Sci. 2008;99:1734–1740. doi: 10.1111/j.1349-7006.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee M.R., Kim J.E., Park J.J., Choi J.Y., Song B.R., Choi Y.W., Kim D.S., Kim K.M., Song H.K., Hwang D.Y. Protective role of fermented mulberry leave extract in LPS-induced inflammation and autophagy of RAW264.7 macrophage cells. Mol. Med. Rep. 2020;22:4685–4695. doi: 10.3892/mmr.2020.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kisseleva T., Bhattacharya S., Braunstein J., Schindler C.W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/S0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 150.Ju S.M., Song H.Y., Lee S.J., Seo W.Y., Sin D.H., Goh A.R., Kang Y.-H., Kang I.-J., Won M.-H., Yi J.-S., et al. Suppression of thymus-and activation-regulated chemokine (TARC/CCL17) production by 1, 2, 3, 4, 6-penta-O-galloyl-β-d-glucose via blockade of NF-κB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 2009;387:115–120. doi: 10.1016/j.bbrc.2009.06.137. [DOI] [PubMed] [Google Scholar]
- 151.Hung Y.P., Lee C.L. Higher Anti-Liver Fibrosis Effect of Cordyceps militaris-Fermented Product Cultured with Deep Ocean Water via Inhibiting Proinflammatory Factors and Fibrosis-Related Factors Expressions. Mar. Drugs. 2017;15:168. doi: 10.3390/md15060168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang J., Zhou Y., Shi J. Cordycepin protects against acute pancreatitis by modulating NF-κB and NLRP3 inflammasome activation via AMPK. Life Sci. 2020;251:117645. doi: 10.1016/j.lfs.2020.117645. [DOI] [PubMed] [Google Scholar]
- 153.Kim J., Lee H., Kang K.S., Chun K.H., Hwang G.S. Cordyceps militaris mushroom and cordycepin inhibit RANKL-induced osteoclast differentiation. J. Med. Food. 2015;18:446–452. doi: 10.1089/jmf.2014.3215. [DOI] [PubMed] [Google Scholar]
- 154.Wulfert F.M., van Meurs M., Kurniati N.F., Jongman R.M., Houwertjes M.C., Heeringa P., Struys M.M., Zijlstra J.G., Molema G. Age-dependent role of microvascular endothelial and polymorphonuclear cells in lipopolysaccharide-induced acute kidney injury. J. Am. Soc. Anesthesiol. 2012;117:126–136. doi: 10.1097/ALN.0b013e31825b57c9. [DOI] [PubMed] [Google Scholar]
- 155.Etter H., Althaus R., Eugster H.P., Santamaria-Babi L.F., Weber L., Moser R. IL-4 and IL-13 downregulate rolling adhesion of leukocytes to IL-1 or TNF-alpha-activated endothelial cells by limiting the interval of E-selectin expression. Cytokine. 1998;10:395–403. doi: 10.1006/cyto.1997.0308. [DOI] [PubMed] [Google Scholar]
- 156.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc. Res. 1996;32:733–742. doi: 10.1016/S0008-6363(96)00066-1. [DOI] [PubMed] [Google Scholar]
- 157.Friedrichs B., Müller C., Brigelius-Flohé R. Inhibition of tumor necrosis factor-alpha- and interleukin-1-induced endothelial E-selectin expression by thiol-modifying agents. Arter. Thromb. Vasc. Biol. 1998;18:1829–1837. doi: 10.1161/01.ATV.18.12.1829. [DOI] [PubMed] [Google Scholar]
- 158.Hwang S., Cho G.S., Ryu S., Kim H.J., Song H.Y., Yune T.Y., Ju C., Kim W.K. Post-ischemic treatment of WIB801C, standardized Cordyceps extract, reduces cerebral ischemic injury via inhibition of inflammatory cell migration. J. Ethnopharmacol. 2016;186:169–180. doi: 10.1016/j.jep.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 159.Liu S.C., Chiu C.P., Tsai C.H., Hung C.Y., Li T.M., Wu Y.C., Tang C.H. Soya-cerebroside, an extract of Cordyceps militaris, suppresses monocyte migration and prevents cartilage degradation in inflammatory animal models. Sci. Rep. 2017;7:43205. doi: 10.1038/srep43205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X., Khalil R.A. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv. Pharmacol. 2018;81:241–330. doi: 10.1016/bs.apha.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alameddine H.S., Morgan J.E. Matrix Metalloproteinases and Tissue Inhibitor of Metalloproteinases in Inflammation and Fibrosis of Skeletal Muscles. J. Neuromuscul. Dis. 2016;3:455–473. doi: 10.3233/JND-160183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Choi M.-C., Choi W.H. Mithramycin A alleviates osteoarthritic cartilage destruction by inhibiting HIF-2α expression. Int. J. Mol. Sci. 2018;19:1411. doi: 10.3390/ijms19051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Noh E.M., Kim J.S., Hur H., Park B.H., Song E.K., Han M.K., Kwon K.B., Yoo W.H., Shim I.K., Lee S.J., et al. Cordycepin inhibits IL-1beta-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxf.) 2009;48:45–48. doi: 10.1093/rheumatology/ken417. [DOI] [PubMed] [Google Scholar]
- 164.Zhang X.L., Huang W.M., Tang P.C., Sun Y., Zhang X., Qiu L., Yu B.C., Zhang X.Y., Hong Y.X., He Y., et al. Anti-inflammatory and neuroprotective effects of natural cordycepin in rotenone-induced PD models through inhibiting Drp1-mediated mitochondrial fission. Neurotoxicology. 2021;84:1–13. doi: 10.1016/j.neuro.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 165.Yang J., Cao Y., Lv Z., Jiang T., Wang L., Li Z. Cordycepin protected against the TNF-α-induced inhibition of osteogenic differentiation of human adipose-derived mesenchymal stem cells. Int. J. Immunopathol. Pharm. 2015;28:296–307. doi: 10.1177/0394632015592160. [DOI] [PubMed] [Google Scholar]
- 166.Shin S., Moon S., Park Y., Kwon J., Lee S., Lee C.K., Cho K., Ha N.J., Kim K. Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines. Immune Netw. 2009;9:255–264. doi: 10.4110/in.2009.9.6.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park D.K., Park H.J. Ethanol extract of Cordyceps militaris grown on germinated soybeans attenuates dextran-sodium-sulfate-(DSS-) induced colitis by suppressing the expression of matrix metalloproteinases and inflammatory mediators. Biomed. Res. Int. 2013;2013:102918. doi: 10.1155/2013/102918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nallathamby N., Guan-Serm L., Vidyadaran S., Abd Malek S.N., Raman J., Sabaratnam V. Ergosterol of Cordyceps militaris Attenuates LPS Induced Inflammation in BV2 Microglia Cells. Nat. Prod. Commun. 2015;10:885–886. doi: 10.1177/1934578X1501000623. [DOI] [PubMed] [Google Scholar]
- 169.Han E.S., Oh J.Y., Park H.J. Cordyceps militaris extract suppresses dextran sodium sulfate-induced acute colitis in mice and production of inflammatory mediators from macrophages and mast cells. J. Ethnopharmacol. 2011;134:703–710. doi: 10.1016/j.jep.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 170.Chien R.C., Lin L.M., Chang Y.H., Lin Y.C., Wu P.H., Asatiani M.D., Wasser S.G., Krakhmalnyi M., Agbarya A., Wasser S.P., et al. Anti-Inflammation Properties of Fruiting Bodies and Submerged Cultured Mycelia of Culinary-Medicinal Higher Basidiomycetes Mushrooms. Int. J. Med. Mushrooms. 2016;18:999–1009. doi: 10.1615/IntJMedMushrooms.v18.i11.50. [DOI] [PubMed] [Google Scholar]
- 171.Rupa E.J., Li J.F., Arif M.H., Yaxi H., Puja A.M., Chan A.J., Hoang V.A., Kaliraj L., Yang D.C., Kang S.C. Cordyceps militaris Fungus Extracts-Mediated Nanoemulsion for Improvement Antioxidant, Antimicrobial, and Anti-Inflammatory Activities. Molecules. 2020;25:5733. doi: 10.3390/molecules25235733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang J.L., Xu Y., Shen J. Cordycepin inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production via activating amp-activated protein kinase (AMPK) signaling. Int. J. Mol. Sci. 2014;15:12119–12134. doi: 10.3390/ijms150712119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yuan J., Wang A., He Y., Si Z., Xu S., Zhang S., Wang K., Wang D., Liu Y. Cordycepin attenuates traumatic brain injury-induced impairments of blood-brain barrier integrity in rats. Brain Res. Bull. 2016;127:171–176. doi: 10.1016/j.brainresbull.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 174.Joung H.J., Kim Y.S., Hwang J.W., Han Y.K., Jeong J.H., Lee J.S., Moon S.H., Jeon B.T., Park P.J. Anti-inflammatory effects of extract from Haliotis discus hannai fermented with Cordyceps militaris mycelia in RAW264.7 macrophages through TRIF-dependent signaling pathway. Fish. Shellfish Immunol. 2014;38:184–189. doi: 10.1016/j.fsi.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 175.Boontiam W., Wachirapakorn C., Phaengphairee P., Wattanachai S. Effect of Spent Mushroom (Cordyceps militaris) on Growth Performance, Immunity, and Intestinal Microflora in Weaning Pigs. Animals. 2020;10:2360. doi: 10.3390/ani10122360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Phull A.-R., Dhong K.-R., Park H.-J. Lactic Acid Bacteria Fermented Cordyceps militaris (GRC-SC11) Suppresses IgE Mediated Mast Cell Activation and Type I Hypersensitive Allergic Murine Model. Nutrients. 2021;13:3849. doi: 10.3390/nu13113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.