Abstract
Patients receiving maintenance dialysis (MD) are vulnerable to COVID-19-related morbidity and mortality. Currently, data on SARS-CoV-2-specific cellular and humoral immunity post-vaccination in this population are scarce.
We conducted a prospective single-center study exploring the specific cellular (interferon-γ and interleukin-2 ELISpot assays) and humoral immune responses (dot plot array and chemiluminescent microparticle immunoassay [CMIA]) at 4 weeks and 6 weeks following a single dose or a complete homologous dual dose SARS-CoV-2 vaccine regimen in 60 MD patients (six with a history of COVID-19).
Our results show that MD patients exhibit a high seroconversion rate (91.7%) but the anti-spike IgG antibodies (CMIA) tend to wane rapidly after full immunization. Only 51.7% of the patients developed T cell immune response. High anti-spike IgG antibodies may predict a better cellular immunity. While patients with prior COVID-19 showed the best response after one, SARS-CoV-2-naïve patients may benefit from a third vaccine injection.
Keywords: Cellular immune response, Hemodialysis, Immunoglobulins, Peritoneal dialysis, T cells
1. Introduction
Compared with the general population, patients receiving maintenance dialysis (MD) are at increased risk for morbidity and mortality associated with coronavirus disease 2019 (COVID-19) [1]. The poor outcomes have been attributed to their higher comorbidity burden as well as their increased age, immunologically deficient state from kidney failure, or immunosuppressive medications [[2], [3], [4]]. Compounding these factors, patients undergoing in-center dialysis cannot self-isolate, as they are required to attend their dialysis treatments thrice weekly at a dialysis center, to which they are often transported by a ride-sharing vehicle.
Currently, the European Medicines Agency (EMA) has approved two mRNA-based COVID-19 vaccines (mRNA-1273 [Moderna Biotech] and BNT162b2 [Pfizer–BioNTech]) and two vector-based vaccines (ChAdOx1 nCoV-19 [Oxford–AstraZeneca]; Ad26.COV2·S [Johnson & Johnson–Janssen]) [5]. These vaccines induce robust humoral and cellular immune responses against the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) spike protein in healthy individuals, which, protect from the risk of subsequent infection [6,7]. However, whether the results are generalizable to patients with kidney failure is unknown, as the effectiveness of the vaccines has not been explicitly tested in this population due to their common exclusion from SARS-CoV-2 vaccination trials [8,9]. So far, the limited data available suggest a diminished vaccine response in MD patients [10,11]. Therefore, there is an urgent need for more data to allow adaptation of the vaccination protocols to achieve adequate antiviral protection for this vulnerable population, if needed.
The main objective of the present study was to examine the humoral and cellular immunogenicity and reactogenicity of a homologous mRNA-based and vector-based SARS-CoV-2 vaccine regimen in patients receiving MD.
2. Materials and methods
2.1. Study design and participants
This prospective, single-center cohort study included patients aged ≥18 years receiving thrice-weekly in-center dialysis (hemodialysis or peritoneal dialysis) at the Patienten-Heimversorgung outpatient dialysis center at University Hospital Giessen and Marburg, Giessen, Germany. At the time of enrollment, the dialysis center served 84 hemodialysis patients and 5 peritoneal dialysis patients. Patients were enrolled if they had: i) received a homologous mRNA-based or a single-dose or homologous dual dose vector-based vaccine regimen (with or without history of COVID-19), and ii) no laboratory evidence of current SARS-CoV-2 infection. The interval between the first and second injections was determined as per EMA guidelines [5]: 3–4 weeks for homologous mRNA-based vaccines and 4–12 weeks for the vector-based single dose (Ad26.COV2·S) or homologous vector-based ChAdOx1 nCoV-19 vaccine. All blood samples were obtained prior to dialysis treatment at 4 weeks (T1) and 6 weeks (T2) after complete vaccination, with a tolerance range of ±2 days. Local and systemic adverse events after the first and second dose were self-reported using a standardized questionnaire.
The study was approved by the local human research ethics committee (AZ 126/21) and complied with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrollment in the study.
2.2. Procedures and measurements
2.2.1. Stimulation assays
Vaccine-induced SARS-CoV-2-specific T cells were quantified from citrated whole blood using a multicolor FluoroSpot Immune assay kit (CoV-iSpot, FluoroSpot Assay, AID/GenID, Straßberg, Germany) that detects functional T cells and an interferon (IFN)-γ and interleukin (IL)-2 reaction specifically against SARS-CoV-2. The results were evaluated by calculating the ratio of the antigen specific reaction and negative control (NC) (stimulation index, SI). Any antigen-specific FluoroSpot test with an SI of ≤2 (NC > 2) or ≤ 5 (NC < 2), depending on the background stimulation, was considered negative when assessed quantitatively. For NC <2 a stimulation index of >5 – < 7 was considered borderline and a SI ≥ 7 was considered positive, whereas for NC ≥2 a stimulation index of >2 – ≤ 3 was considered borderline and a SI > 3 was considered positive. A reactive response was considered when at least one timepoint showed a reactive pattern and the other timepoint was reactive or borderline or negative or invalid. A borderline response was considered when one timepoint was borderline and the other timepoint was borderline or negative or invalid. A negative response was considered when one timepoint was negative and the other timepoint was negative or invalid. Only when both timepoints were invalid the response was considered as invalid. GenID performed all FluoroSpot tests, blinded to the clinical data. The Supplementary Material provides a detailed description of the ELISpot method.
2.2.2. Analysis of SARS-CoV-2-specific antibodies
SARS-CoV-2-specific antibodies were quantified using plasma from citrated whole blood samples using an immunoglobulin G (IgG) assay coated with a recombinant receptor-binding domain of the SARS-CoV-2 spike protein antigen using an in-house dot plot array by GenID, blinded to the clinical data. Antibody levels are expressed in % intensity of gray scale, ranging from 0 to 100% black, with an intensity of >16% considered positive and ≤ 16% considered negative, respectively. In addition, SARS-CoV-2-specific antibodies from serum samples against the spike protein and nucleocapsid protein were performed by the Institute of Medical Virology (Giessen, Germany) using antibody chemiluminescent microparticle immunoassay (CMIA; Anti-S AdviseDx SARS-CoV-2 IgG II and Anti-N Abbott Architect SARS-CoV-2 IgG, Abbott, Chicago, IL, USA). The anti-N array (measured in S/CO) was used to detect a previous infection. Anti-S levels after infection or vaccination were expressed as AU (arbitrary unit)/mL with levels >50 AU/mL defined as positive and ≤ 50 AU/mL as negative.
2.2.3. Other laboratory methods
All other blood samples were analyzed at the local Institute of Laboratory Medicine at University Hospital Giessen and Marburg, Giessen, where they were processed within 30 min of collection and centrifuged for 10 min at 3000 ×g. Serum creatinine was measured by a photometric-enzymatic method on an ADVIA Chemistry XPT analyzer (enzymatic creatinine; Siemens Healthineers, Erlangen, Germany), with calibration to reference measurements for isotope dilution mass spectrometry. Ferritin was measured by chemiluminescent immunoassay (CLIA) on a Centaur XPT analyzer (Siemens Healthineers). Soluble IL-2 receptor and IL-6 were determined on a Siemens Immulite 1000 system with Siemens reagents.
2.2.4. Other measures
The other variables included kidney failure etiology, dialysis vintage and dose (Kt/V for thrice-weekly hemodialysis [12] and weekly Kt/V for peritoneal dialysis [13]), previous SARS-CoV-2 infection, and use of immunosuppressive therapy.
2.3. Statistical analysis
Descriptive statistics are expressed as the median [interquartile range] for numeric variables and as n (%) for categorical variables. Differences between two independent groups were tested with the Mann-Whitney test or independent t-test according to the variables' distribution normality. Categorical variables were tested with the chi-square test or Fisher's exact test. Paired ordinal data were compared using the Wilcoxon signed-rank test; paired nominal data were compared using the McNemar test. Univariate and multivariate logistic regression analyses with backwards elimination were conducted with IL-2 or IFN-γ ELISpot test reactivity at either T1 or T2 as a dependent variable. Independent variables with significant associations in the univariate analysis (p < 0.1) were further included in the multivariate regression analysis. The statistical analysis was performed with SPSS Statistics 26 (IBM, Ehningen, Germany). P-values <0.05 were considered significant.
3. Results
3.1. Participants
A total of 60 patients were enrolled in this study. All patients had either received a single dose or a dual-dose homologous vaccine regimen. Fifty-one patients had received mRNA-based vaccines (BNT162b2, n = 49; mRNA-1273, n = 2); nine patients had received vector-based vaccines (ChAdOx1 nCoV-19, n = 6; Ad26.COV2·S, n = 3). Six patients had received a booster vaccination 6 months after SARS-CoV-2 infection. Table 1 shows the characteristics of the study population; the median age of the population was 65.4 years; 63.3% were male; 35% had diabetes mellitus; 90% had hypertension, and 50% had coronary heart disease. The main causes of kidney failure were glomerulonephritis (21.7%), diabetes (20%), and nephrosclerosis (16.7%). No demographic differences in the demographic data were observed between patients receiving mRNA-based vaccines vs. vector-based vaccines. The median length of time on dialysis was 33 [15–49] months. Hemodialysis was the dialysis modality adopted by 88.3% of the study population. No difference was observed in key laboratory parameters between T1 and T2 (Supplementary Material Table S1). A total of 50 and 58 patient samples were analyzed at T1 and T2, respectively.
Table 1.
Demographic and clinical characteristics of the cohort.
| Total (n = 60) | mRNA-based vaccines (mRNA-1273/BNT162b2) (n = 51) | Vector-based vaccines (ChAdOx1 nCoV-19Ad26.COV2-S) (n = 9) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 65.4 [54–75] | 66 [51–75] | 64 [58–73] | 0.99 |
| Male sex, n (%) | 38 [63.3%] | 33 [64.7%] | 5 [55.6%] | 0.71 |
| Dry weight, kg | 76 [64–89.5] | 75 [64–89] | 84 [54–92] | 0.81 |
| Body mass index, kg/m2 | 26.1 [22–29] | 26.0 [22–28] | 27.6 [20−1] | 0.88 |
| Comorbidities | ||||
| Hypertension, n (%) | 54 (90%) | 46 (90.2%) | 8 (88.9%) | 1.0 |
| Diabetes mellitus, n (%) | 21 (35%) | 18 (35.3%) | 3 (33.3%) | 1.0 |
| Coronary artery disease, n (%) | 30 (50%) | 25 (49%) | 5 (55.6%) | 1.0 |
| History of stroke, n (%) | 7 (11.7%) | 5 (9.8%) | 2 (22.2%) | 0.28 |
| Immunosuppressive therapy, n (%) | 7 (12.5%) | 5 (9.8%) | 2 (22.2%) | 0.28 |
| Cause of kidney failure, n (%) | 0.91 | |||
| Nephrosclerosis | 10 (16.7%) | 9 (17.6%) | 1 (11.1%) | |
| Diabetic nephropathy/nephrosclerosis | 12 (20%) | 10 (19.6%) | 2 (22.2%) | |
| Cardiorenal syndrome | 10 (16.7%) | 9 (17.6%) | 1 (11.1%) | |
| Glomerulonephritis | 13 (21.7%) | 10 (19.6%) | 3 (33.3%) | |
| Interstitial nephritis | 3 (5%) | 3 (5.9%) | – | |
| ADPKD | 4 (6.7%) | 4 (7.8%) | – | |
| Cancer | 5 (8.3%) | 4 (7.8%) | 1 (11.1%) | |
| Unknown | 3 (5%) | 2 (3.9%) | 1 (11.1%) | |
| Dialysis data | ||||
| Dialysis modality, n (%) | ||||
| Hemodialysis | 53 (88.3%) | 44 (86.3%) | 9 (100%) | 0.58 |
| Peritoneal dialysis | 7 (11.7%) | 7 (13.7%) | – | N/A |
| Dialysis vintage, months | 32.5 [15–49] | 35.0 [15.0–50.0] | 26.0 [6.5–34.0] | 0.16 |
| Kt/V | 1.6 [1.4–1.7] | 1.6 [1.4–1.7] | 1.4 [1.3–1.6] | 0.11 |
| Hemodialysis | 1.6 [1.4–1.7] | 1.6 [1.4–1.7] | 1.4 [1.3–1.6] | |
| Peritoneal dialysis | 2.4 [1.6–3.5] | 2.4 [1.7–2.9] | – | |
| Baseline clinical data | ||||
| Leucocyte count, g/L | 6.8 [4.8–8.3] | 7.0 [5.2–8.4] | 5.0 [4.4–7.3] | 0.34 |
| Differential count, g/L | ||||
| Total neutrophils | 4.4 [2.9–6.3] | 4.7 [2.8–6.5] | 3.6 [2.9–5.2] | 0.46 |
| Total lymphocytes | 1.2 [0.8–1.5] | 1.2 [0.9–1.5] | 1.1 [0.7–1.4] | 0.62 |
| Total basophils | 0.03 [0.02–0.04] | 0.02 [0.01–0.04] | 0.03 [0.02–0.03] | 0.87 |
| Total monocytes | 0.6 [0.5–0.8] | 0.6 [0.5–0.8] | 0.6 [0.4–0.7] | 0.70 |
| Total eosinophils | 0.2 [0.1–0.3] | 0.2 [0.1–0.3] | 0.3 [0.1–0.4] | 0.48 |
| Hemoglobin, g/dL | 10.8 [10.3–11.4] | 10.9 [10.4–11.4] | 10.5 [9.8–11.8] | 0.44 |
| Serum creatinine, mg/dLa | 7.0 [5.2–8.7] | 6.9 [5.5–8.9] | 7.6 [4.4–8.6] | 1.0 |
| Urea, mg/dLb | 120 [97–135] | 120 [110–136] | 85 [79–134] | 0.32 |
| Phosphate, mmol/L | 1.7 [1.4–1.9] | 1.8 [1.4–1.9] | 1.4 [1.3–1.9] | 0.44 |
| Parathyroid hormone, pg/mL | 299 [207–400] | 315 [214–413] | 280 [80–314] | 0.19 |
| Albumin, g/dL | 40.4 [38.2–42.5] | 40.1 [38.0–42.2] | 42.3 [40.9–42.7] | 0.12 |
| C-reactive protein, mg/L | 9.8 [2.2–20.2] | 10.2 [2.3–20.0] | 2.7 [0.8–25.0] | 0.74 |
| Total IgG, g/L | 10.8 [7.3–14.0] | 10.5 [7.3–14.0] | 10.9 [6.8–14.0] | 0.94 |
| IL-6, μg/L | 10.0 [10.0–11.5] | 10.0 [10.0–11.5] | 10.0 [10.0–13.6] | 1.0 |
| Soluble IL-2 receptor, U/mL | 1284 [873–1864] | 1284 [873–1927] | 1571 [1001–1844] | 0.71 |
| Ferritin, μg/L | 228 [82–542] | 214 [76–479] | 523 [108–916] | 0.17 |
Values are the median [interquartile range], or n (%).
ADPKD, autosomal dominant polycystic kidney disease; IgG, immunoglobulin G; IL-2, interleukin-2; IL-6, interleukin-6.
To convert the values for serum creatinine to μmol/L, multiply by 88.4.
To convert the values for urea to blood urea nitrogen, multiply by 0.467.
3.2. IL-2 and IFN-γ stimulation assays
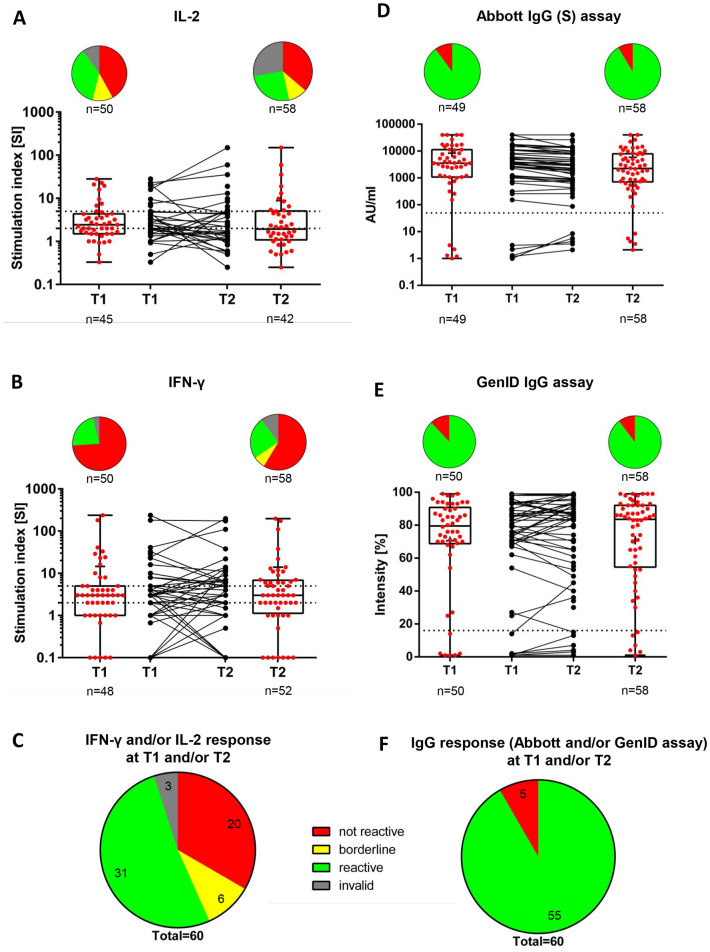
We first analyzed all patient samples irrespective of the vaccine type and history of COVID-19. The majority of patients showed no reactivity in the IL-2 and IFN-γ ELISpot assays at T1 (42.0% and 74.0%, respectively) and T2 (36.2% and 58.6%, respectively), while there was a reactive pattern in the IL-2 and IFN-γ ELISpot assays at T1 in 36.0% and 22.0% of the patients, respectively, and at T2 in 25.9% and 24.1% of the patients, respectively (Fig. 1A and B). An IL-2 and/or IFN-γ reactivity in at least one time point was observed in 51.7% of patients (Fig. 1C), whereas a non-response in all samples was observed in 33.3% of the patients. Borderline reactivities and invalid test results were observed in 10.0% and 5.0% of patients, respectively (Fig. 1A). There was no difference in the median IL-2 and IFN-γ reactivities between both time points (p = 0.93 and p = 0.85, respectively; Table 2 ).
Fig. 1.
Vaccine-induced SARS-CoV-2-specific IL-2 (A) and IFN-γ (B) and IL-2 and/or IFN-γ (C)-producing T cells and SARS-CoV-2 anti-spike IgG antibodies as determined using the Abbott assay (D), GenID assay (E), and both (F) at T1 and T2.
A) T1 (n = 50): Not reactive, 21 (42.0%); borderline, 6 (12.0%); reactive, 18 (36.0%); invalid, 5 (10.0%). T2 (n = 58): Not reactive, 21 (36.2%); borderline, 6 (10.3%); reactive, 15 (25.9%); invalid, 16 (27.6%). T1 vs. T2 (p = 0.73).
B) T1 (n = 50): Not reactive, 37 (74.0%); borderline, 0 (0.0%); reactive, 11 (22.0%); invalid, 2 (4.0%). T2 (n = 58): Not reactive, 34 (58.6%); borderline, 4 (6.9%); reactive, 14 (24.1%); invalid, 6 (10.3%). T1 vs. T2 (p = 0.12).
C) T1 and/or T2 (n = 60): Not reactive, 20 (33.3%); borderline, 6 (10.0%); reactive, 31 (51.7%); invalid, 3 (5.0%).
D) T1 (n = 50): Positive, 44 (88.0%)a; negative, 6 (12.0%)b. T2 (n = 58): Positive, 52 (89.7%)a; negative, 6 (10.3%)b. T1 vs. T2 (p = 1.0)e.
E) T1 (n = 49): Positive, 44 (89.8%)c; negative, 5 (10.2%)d. T2 (n = 58): Positive, 53 (91.4%)c; negative, 5 (8.6%)d. T1 vs. T2 (p = NS)e.
F) T1 and/or T2 (n = 60): Positive, 55 (91.7%); negative, 5 (8.3%).
The dashed horizontal lines indicate the cut-off for positivity (reactive); the area between the horizontal lines indicates the borderline zone used in each GenID assay.
aPositive refers to antibody levels >16%.
bNegative refers to antibody levels ≤16%.
cPositive refers to antibody concentration > 50 AU/mL.
dNegative refers to antibody concentration ≤ 50 AU/mL.
eMcNemar's test for paired nominal data was used.
IFN-γ, interferon-γ; IL-2, interleukin-2; SARS-CoV-2, NS, not significant; severe acute respiratory syndrome-coronavirus type-2; T1, timepoint 1; T2, timepoint 2.
Table 2.
Cellular and humoral immune responses following SARS-CoV-2 vaccination at T1 and T2.
| Vaccines (mRNA- and vector-based) |
p-value | ||
|---|---|---|---|
| T1 | T2 | ||
| IL-2, SI | 2.4 [1.5–4.5] (n = 33) |
1.6 [1.0–4.7] (n = 33) |
0.93 |
| IFN-γ, SI | 3 [1–5] (n = 43) |
3 [1–7] (n = 43) |
0.85 |
| SARS-CoV-2 anti-spike IgG, AU/mL (Abbott array) | 3517 [1070–11,164] (n = 48) |
2240 [756–7687] (n = 48) |
<0.001 |
| SARS-CoV-2 anti-spike IgG, % (GenID assay) | 79.5 [68.5–92.2] (n = 48) |
84.5 [58–92.7] (n = 48) |
0.37 |
Values are the median [interquartile range]. Bolded p-values denote statistical significance at the p < 0.05 level. We conducted analyses of patients who had valid measurements at both time points.
IFN-γ, interferon-γ; IgG, immunoglobulin G; IL-2, interleukin-2; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; T1, timepoint 1; T2, timepoint 2.
3.3. Analysis of SARS-CoV-2-specific antibodies
In the Abbott array, 89.8% of patients had SARS-CoV-2 anti-spike IgG antibodies above the detection limit (IgG >50 AU/mL at T1 and remained positive at T2, whereas 8.6% of patients were classified as negative (IgG ≤ 50 AU/mL) at both time points (Fig. 1E). However, the median SARS-CoV-2 anti-spike IgG antibody levels were significantly lower at T2 compared to T1 (2240 [756–7687] AU/mL vs. 3517 [1070–11,164] AU/mL; p < 0.001; Table 2). In contrast, when using the GenID assay, the median percentage of median SARS-CoV-2 anti-spike IgG antibodies were not significantly different between T2 and T1 (84.5% [58%–92.7%] vs. 79.5% [68.5%–92.2%]; p = 0.371; Table 2). The GenID IgG assay showed that 55 of 60 patients (91.7%) were positive (IgG intensity >16%) in at least one timepoint, while five patients (8.3%) were negative at both T1 and T2 (IgG intensity ≤16%) (Fig. 1E).
Except for one case, both the Abbott and GenID assays yielded the same results when classifying the humoral response as either positive or negative (Supplementary Material Fig. S1). A positive test result in at least one assay at either T1 or T2 was observed in 91.7% of patients, whereas 8.3% were consistently negative in both assays (Fig. 1D).
Overall, five patients (8.3%) did not generate anti-spike IgG antibodies. Four of these patients showed no cellular immune response and one patient showed a positive cellular immune response at time T1 but a negative one at time T2 (Table S2 in the Supplementary Material). Four patients were on immunosuppressive therapy (two patients received rituximab and prednisolone due to MPO-ANCA (myeloperoxidase–anti-neutrophil cytoplasmic antibody)-positive vasculitis, one patient was a pancreatic islet transplant recipient, one patient received anti-myeloma chemotherapy), while the fifth patient had a history of Hodgkin lymphoma. Notably, the remaining 2 patients who were on immunosuppressive therapy did develop anti-spike IgG antibodies (one patient received full vaccination approximately 1 month prior to the initiation of immunosuppressive therapy due to first diagnosis of MPO-ANCA positive vasculitis with renal involvement, one patient had tacrolimus monotherapy after kidney allograft failure).
3.4. Association between humoral and cellular immune response following vaccination
Patients showing IL-2 producing T cells had higher levels of SARS-CoV-2 anti-spike IgG at both timepoints in both the Abbott array (T1, p = 0.024; T2, p = 0.001) and the GenID assay (T1, p = 0.024; T2, p = 0.007) compared to those who did not show any IL-2 reactivity. We excluded patients with borderline results from the comparison. Likewise, we found higher levels of SARS-CoV-2 anti-spike IgG at both timepoints in both the Abbott array (T1, p = 0.002; T2, p > 0.001) and GenID assay (T1, p = 0.019; T2, p = 0.01) in patients with IFN-γ ELISpot positive reactivity compared to the non-reactive patients.
Multivariate logistic regression analysis showed that among the variables included, the ELISpot assay reactivity regarding IL-2 (T1: odds ratio [OR] = 2.40 (95% confidence interval [CI]: 1.10–5.40), p = 0.028; T2: OR = 6.85 [95% CI: 1.49–31.50], p = 0.014) and IFN-γ (T1: OR = 4.18 [95% CI: 1.62–10.80], p = 0.003; T2: OR = 9.45 [95% CI: 2.05–43.60], p = 0.004) was independently associated with SARS-CoV-2 IgG levels as measured with the Abbott array at both timepoints. We did not find other confounders that had an effect on ELISpot reactivity (Table 3 ).
Table 3.
Association of demographic and clinical characteristics with positive SARS-CoV-2-specific IL-2 reactivity (A) and IFN-γ reactivity (B) at T1 and T2.
| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | T1a |
T2b |
||||||||||
| Univariate association |
Multivariate association |
Univariate association |
Multivariate association |
|||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | 0.982 | (0.944–1.02) | 0.353 | 0.967 | (0.927–1.008) | 0.114 | ||||||
| Diabetes mellitus (yes)c | 1.25 | (0.320–4.8) | 0.748 | 0.733 | (0.192–2.806) | 0.651 | ||||||
| Dialysis modality (PD)c | 0.353 | (0.033–3.7) | 0.387 | 0 | (0–0) | 0.999 | ||||||
| Dialysis duration | 1 | (0.988–1.001) | 0.880 | 1.001 | (0.990–1.013) | 0.853 | ||||||
| Previous COVID-19 infection (recovered)c | 7.69 | (0.805–73.5) | 0.077d | 5 | (0.466–53.6) | 0.184 | ||||||
| Type of vaccine (vector vaccine)c | 0.353 | (0.33–3.7) | 0.387 | 0.429 | (0.040–4.57) | 0.483 | ||||||
| SARS-CoV-2 anti-spike antibodies (in 10,000) | 2.44 | (1.1–5.4) | 0.028d | 2.401 | (1.102–5.402) | 0.028 | 6.848 | (1.487–31.5) | 0.014d | 6.848 | (1.487–31.50) | 0.014 |
| Immunosuppressive drugs (yes)c | 0.750 | (0.111–5.074) | 0.768 | 0 | (0–0) | 0.999 | ||||||
| CRP | 1.053 | (0.983–1.128) | 0.140 | 0.957 | (0.890–1030) | 0.241 | ||||||
| IL-6 | 0.931 | (0.825–1.050) | 0.243 | 0.905 | (0.775–1057) | 0.209 | ||||||
| Ferritin | 1 | (0.999–1.002) | 0.807 | 1 | (0.998–1001) | 0.615 | ||||||
|
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (B) | ||||||||||||
| Variable | T1a |
T2a |
||||||||||
| Univariate association |
Multivariate association |
Univariate association |
Multivariate association |
|||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | 0.989 | (0.949–1.032) | 0.622 | 0.975 | (0.939–1.013) | 0.201 | ||||||
| Diabetes mellitus (yes)b | 1.190 | (0.291–4.867) | 0.808 | 0.794 | (0.219–2.880) | 0.725 | ||||||
| Dialysis modality (PD)b | 0.640 | (0.067–6.142) | 0.699 | 0.446 | (0.047–4.210) | 0.481 | ||||||
| Dialysis duration | 1.004 | (0.989–1.018) | 0.618 | 1.004 | (0.990–1.018) | 0.599 | ||||||
| Previous COVID-19 infection (recovered)b | 1.195e+10 | (0–0) | 0.999 | 5.493e+9 | (0–0) | 0.999 | ||||||
| Type of vaccine (vector vaccines)b | 0.640 | (0.067–6.142) | 0.699 | 0.577 | (0.059–5.674) | 0.637 | ||||||
| SARS-CoV-2 anti-spike antibodies (in 10,000) | 4.18 | (1.6–10.8) | 0.003c | 4.18 | (1.62–10.80) | 0.003 | 9.447 | (2.047–43.60) | 0.004c | 9.447 | (2.047–43.60) | 0.004 |
| Immunosuppressive drugs (yes)b | 0.640 | (0.067–6.14) | 0.699 | 2.667 | (0.337–21.11) | 0.353 | ||||||
| CRP | 0.997 | (0.978–1.016) | 0.730 | 0.973 | (0.931–1.016) | 0.216 | ||||||
| IL-6 | 0.952 | (0.847–1.069) | 0.402 | 0.948 | (0.864–1.040) | 0.261 | ||||||
| Ferritin | 1 | (0.999–1.001) | 0.570 | 1.001 | (1–1.003) | 0.090c | ||||||
Bolded p-values denote statistical significance at the p < 0.05 level.
aThirty-nine cases were included in the logistic regression analysis due to missing cases.
bThirty-six cases were included in the logistic regression analysis due to missing cases.
cRefers to indicator.
dRefers to variables included in the multivariate logistic regression analysis with backward elimination.
95% CI, 95% confidence interval; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; IL-6, interleukin-6; OR, odds ratio; PD, peritoneal dialysis; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; T1, timepoint 1; T2, timepoint 2.
Bolded p-values denote statistical significance at the p < 0.05 level.
aForty-eight cases were included in the logistic regression analysis due to missing cases.
bRefers to indicator.
cRefers to variables included in the multivariate logistic regression analysis with backward elimination.
95% CI, 95% confidence interval; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; IL-6, interleukin-6; OR, odds ratio; PD, peritoneal dialysis; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; T1, timepoint 1; T2, timepoint 2.
3.5. Comparison of cellular and humoral responses between SARS-CoV-2-naïve patients and those with previous COVID-19 and between vaccine regimens
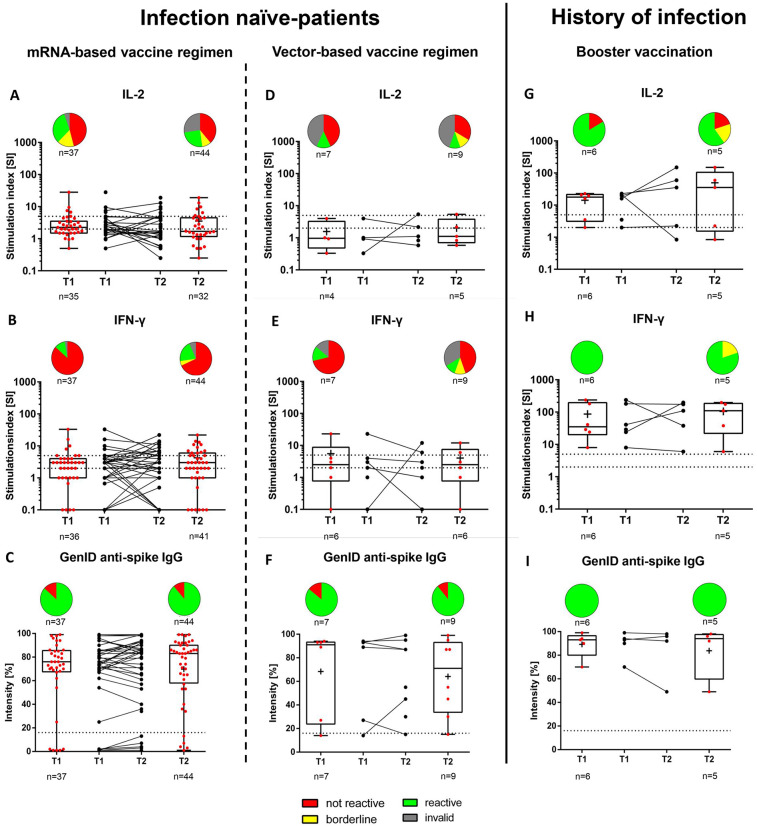
The Abbott assay showed that patients with previous SARS-CoV-2 infection who had received a booster vaccination exhibited higher SARS-CoV-2 anti-spike IgG concentrations at both timepoints when compared to SARS-CoV-2-naïve patients with full vaccination (T1 and T2, p < 0.001; Table 4 and Fig. 2 ). Also, patients with previous SARS-CoV-2 infection showed a higher SI in the IL-2 ELISpot at T1 (p = 0.004) but not at T2 (p = 0.07). Such patients also had a higher SI in the IFN-γ ELISpot at both timepoints (T1 and T2, p < 0.001). Patients with history of COVID had significantly higher levels of response in the GenID assay (91.5% vs. 76%, p = 0.04) at time T1, but not at time T2 (92% vs. 83%, p = 0.15) (Table 4). The vaccination regimen in the infection-naïve patients did not affect the vaccination response compared to the group with history of COVID-19 (Table S3 and S4 in the Supplementary Material). Also, the dialysis regime (hemodialysis vs. peritoneal dialysis) had no effect on the immunity response (data not shown). However, it should be noted that there was only a small number of patients receiving a vector-based vaccination regimen and peritoneal dialysis and, thus, the groups may be underpowered.
Table 4.
Comparison of cellular and humoral responses between SARS-CoV-2-naïve and SARS-CoV-2-infected patients with full vaccination.
| Vaccinated with no history of COVID-19 |
Vaccinated with history of COVID-19 |
p-value | |||
|---|---|---|---|---|---|
| Median [IQR] | n | Median [IQR] | n | ||
| SARS-CoV-2 anti-spike IgG antibodies at T1, AU/mL (Abbott) | 3180 [1008–6029] | 43 | 40,000 [17,230–40,000] | 6 | <0.001 |
| SARS-CoV-2 anti-spike IgG antibodies at T2, AU/mL (Abbott) | 1894 [583–5268] | 53 | 14,653 [10,584–40,000] | 5 | <0.001 |
| SARS-CoV-2 anti-spike IgG antibodies at T1, % (GenID) | 76 [67.2–88.2] | 44 | 91.5 [82.7–95.2] | 6 | 0.042 |
| SARS-CoV-2 anti-spike IgG antibodies at T2, % (GenID) | 83 [54–91] | 53 | 92 [68.5–97] | 5 | 0.15 |
| IL-2 at T1, SI | 2 [1.5–3.5] | 39 | 17.7 [3.1–21.4] | 6 | 0.004 |
| IL-2 at T2, SI | 1.67 [1–4.4] | 37 | 35.3 [1.5–105] | 5 | 0.07 |
| IFN-γ at T1, SI | 3 [1–4] | 42 | 35 [20–195] | 6 | <0.001 |
| IFN-γ at T2, SI | 3 [1–6] | 47 | 110 [22–186] | 5 | <0.001 |
Bolded p-values denote statistical significance at the p < 0.05 level.
COVID-19, coronavirus disease 2019; IFN-γ, interferon-γ; IL-2, interleukin-2; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus type-2; T1, timepoint 1; T2, timepoint 2.
Fig. 2.
Vaccine-induced SARS-CoV-2-specific IL-2- and IFN-γ-producing T cells and SARS-CoV-2 anti-spike IgG antibodies as determined using the GenID assay at T1 and T2, stratified by mRNA vaccine regimen (A–C), vector-based vaccine regimen (D—F), for infection naïve patients, or booster vaccination in patients with a history of SARS-CoV-2 infection (G–I).
A) IL-2: T1 (n = 37): Not reactive, 17 (45.9%); borderline, 6 (16.2%); reactive, 12 (32.4%); invalid, 2 (5.4%). T2 (n = 44): Not reactive, 17 (38.6%); borderline, 4 (9.1%); reactive, 11 (25.0%); invalid, 12 (27.3%).
B) IFN-γ: T1 (n = 37): Not reactive, 32 (86.5%); borderline, 0 (0%); reactive, 4 (10.8%); invalid, 1 (2.7%). T2 (n = 44): Not reactive, 30 (68.1%); borderline, 2 (4.5%); reactive, 9 (20.5%); invalid, 3 (6.8%).
C) IgG: T1 (n = 37): Positive, 32 (86.5%)a; negative, 5 (13.5%)b. T2 (n = 44): Positive, 39 (88.6%)a; negative, 5 (11.4%)b.
D) IL-2: T1 (n = 7): Not reactive, 3 (42.9%); borderline, 0 (0%); reactive, 1 (14.3%); invalid, 3 (42.9%). T2 (n = 9): Not reactive, 3 (33.3%); borderline, 1 (11.1%); reactive, 1 (11.1%); invalid, 4 (44.4%).
E) IFN-γ: T1 (n = 7): Not reactive, 5 (71.4%); borderline, 0 (0%); reactive, 1 (14.3%); invalid, 1 (14.3%). T2 (n = 9): Not reactive, 4 (44.4%); borderline, 1 (11.1%); reactive, 1 (11.1%); invalid, 3 (44.4%).
F) IgG: T1 (n = 7): Positive, 6 (85.7%)a; negative, 1 (14.3%)b. T2 (n = 9): Positive, 8 (88.9%)a; negative, 1 (11.1%)b.
G) IL-2: T1 (n = 6): Not reactive, 1 (16.7%); borderline, 0 (0%); reactive, 5 (83.3%); invalid, 0 (0%). T2 (n = 5): Not reactive, 1 (20.0%); borderline, 1 (20.0%); reactive, 3 (60.0%); invalid, 0 (0%).
H) IFN-γ: T1 (n = 6): Not reactive, 0 (0%); borderline, 0 (0%); reactive, 6 (100%); invalid, 0 (0%). T2 (n = 5): Not reactive, 0 (0%); borderline, 1 (20.0%); reactive, 4 (80.0%); invalid, 0 (0%).
I) IgG: T1 (n = 6): Positive, 6 (100%)a; negative, 0 (0%)b. T2 (n = 5): Positive, 5 (100%)a; negative 0 (0%)b.
All individuals with a history of SARS-CoV-2 infection were vaccinated with one dose of mRNA- BNT162b2 (n = 6).
The dashed horizontal lines indicate the cut-off for positivity (reactive); the area between the horizontal lines indicates the borderline zone used in each GenID assay.
aPositive refers to antibody levels >16%.
bNegative refers to antibody levels ≤16%.
IgG, immunoglobulin G; IFN-γ, interferon-γ; IL-2, interleukin-2.
3.6. Reactogenicity of SARS-CoV-2 vaccine regimen
Adverse events were reported by 28 (46.7%) and 22 (43.1%) patients after the first and second dose, respectively (Supplementary Material Table S5). The onset of adverse events was mostly reported within 3 days after vaccination (after the first dose, 89.3% of patients; after the second dose, 100% of patients). The main reported adverse events were pain and swelling or redness at the injection site (after the first dose, 31.6%; after the second dose, 27.4%) and tiredness/fatigue (after the first dose, 16.7%; after the second dose, 23.5%). No association was observed between the reported reactogenicity and the immune response (data not shown).
4. Discussion
4.1. Key findings
We prospectively quantified the humoral and cellular immune response at both 4 weeks and 6 weeks after full vaccination in patients receiving MD. Most patients (91.7%) had detectable SARS-CoV-2 anti-spike IgG antibodies in at least one time point. However, the median SARS-CoV-2 anti-spike IgG antibody levels measured with the Abbott assay were significantly lower at 6 weeks post-vaccination compared to 4 weeks, indicating that although a full vaccination regimen was sufficient for inducing SARS-CoV-2-specific antibodies, the antibody levels tended to decrease rapidly. In addition, although only 51.7% of patients were reactive in the SARS-CoV-2-specific T cell activation ELISpot assays, there were no detectable differences in the IL-2 or IFN-γ reactivities between both time points, suggesting the short-term stability of cellular immunity compared to humoral immunity after full vaccination. Finally, patients receiving a booster vaccination after SARS-CoV-2 infection showed both better humoral and cellular immunity compared to SARS-CoV-2-naïve patients with full vaccination, whereas patients with immunosuppressive therapy developed almost no detectable humoral or cellular immunity.
4.2. Comparison with previous studies
The observed high humoral immune response to the SARS-CoV-2 spike protein is similar to a recent vaccine trial involving healthy adults [14]. Some studies on the humoral response in hemodialysis patients have reported similar (>90%) seroconversion results [[15], [16], [17], [18]], which are comparatively higher to that of other studies, where 77%–82% of dialysis patients had positive anti-spike IgG antibody levels [11,[19], [20], [21]]. The observed differences in the seroconversion rate among the selected studies may be in part due to the large variation in the number of dialysis patients on immunosuppressive therapy (0%–12.0%) or with a history of cancer (0%–35.2%), as both are recognized risk factors for a diminished immune response following vaccination [11,19,20].
Comparable longitudinal data analyzing the humoral response dynamics at 4 weeks and 6 weeks post-vaccination in MD patients are not available. However, a study group recently indicated waning humoral response 6 months after SARS-CoV-2 vaccination in patients receiving MD [22]. Whether the antibody loss is caused by the impaired immune system in dialysis patients or by the vaccine platforms, or both, remains unclear. Nevertheless, strategies for prolonging and/or boosting host immunity should be evaluated to protect vulnerable populations against SARS-CoV-2 and its variants. Therefore, a third booster dose after 6 months, which is common recommendation in many countries including Germany should be considered to sustain protective humoral immunity in patients with kidney failure [23].
Emerging evidence suggests the requirement of both antibody-mediated and T cell-mediated immunity for effective protection against SARS-CoV-2 [24]. However, our results indicate that a significant proportion of patients may not develop cellular immunity, although almost all patients developed antibodies. Our results are in line with previous studies involving MD patients describing an impaired cellular immune response after SARS-CoV-2 vaccination, with T cell activation of 31%–78% [11,16,18], which is lower compared to that of the general population (88.2%) [25] but higher compared to that of kidney transplant recipients (5.1%–35.0%) [26,27]. The inadequate cellular response after SARS-CoV-2 vaccination in MD patients is consistent with the impaired response reported after hepatitis B virus vaccination in hemodialysis patients [28]. Notably, however, the findings regarding cellular immune response from such studies must be interpreted carefully, given the different vaccine platforms used.
MD patients receiving a booster vaccination after SARS-CoV-2 infection showed better humoral and cellular immunity compared to SARS-CoV-2-naïve patients with full vaccination. Other studies also suggest that prior seropositivity seems to be protective against SARS-CoV-2 infection in MD patients [19,29]. Forbes et al. reported that hemodialysis patients have a robust and sustained antibody response after confirmed COVID-19 infection, with 71% of the cohort having a positive response, indicating increasing antibody positivity during the 6-month follow-up period [30]. The reason for the stronger humoral and cellular immune response in MD patients after natural COVID-19 infection is unknown, but similar findings were reported for immunocompetent individuals and transplant recipients [11,29,30]. It might be intuitive that the high inflammation level observed in MD patients during COVID-19 may contribute to a stronger antigenic challenge and lymphocyte recruitment, generating stronger cellular and humoral immune responses as compared to vaccine-mediated prime-boost immune response.
Yet, it is unclear whether the higher antibody level correlates with better protection against SARS-CoV-2 infection [31,32]. However, our data show that the higher estimates of SARS-CoV-2 anti-spike IgG at both timepoints are associated with a higher rate of cellular immunity. Therefore, we speculate that these patients also have higher protection against SARS-COV-2 infection.
Vaccination side effects did occur in (up to 46.7%) of cases, but were usually mild (local pain and swelling), and were mostly restricted to the first 3 days after vaccination (in 89.3%), in line with previous reports [16,33].
4.3. Study strengths and limitations
A major strength of our study is that we compared humoral and cellular immunity, which have received little attention in the literature. We also measured cellular immunity including both IL-2 and IFN-γ, which allows assessment of both the early and late cellular immune response [34].
The important limitations of our study include the observational, non-randomized study character, small sample size, and limited number of patients receiving a vector-based vaccine regimen and peritoneal dialysis. Moreover, we did not include healthy controls; therefore, we were unable to compare the humoral and cellular immune response against SARS-CoV-2 vaccines between MD patients and healthy controls.
5. Conclusions
The majority of patients receiving MD develop SARS-CoV-2 anti-spike IgG antibodies after a single dose or homologous dual dose vaccine regimen, but these antibodies tend to wane rapidly by 6 weeks after full immunization. Only approximately 50% of patients develop T cell immunity. High anti-spike IgG antibodies may predict a better cellular immunity. The level of vaccine response was significantly higher in patients who have had a history of COVID-19. Therefore, while patients with prior COVID-19 showed the best response after one dose, SARS-CoV-2-naïve patients may benefit from a third vaccine injection to optimize immunogenicity and sustain protection.
Compliance with ethical standards
Approval by the local ethics committee (Ethikkommission des Fachbereich Medizin, Justus-Liebig-Universität Giessen) was granted before initiating enrolment (AZ 126/21). Written informed consent was obtained from the patients by a member of the research team.
Availability of data and material
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Competing interests
KS is employee of AID/GenID, the manufacturer of the ELISpot assay. None of the other authors declare any competing interests.
Funding
There was no funding source for this study. The dot plot arrays were generously supplied and analyzed by AID/GenID GmbH, Straßberg, Germany.
Author contributions
Study concept and design: HK, CN, KS, HS, CGS, MS, H-WB, and FH-S. FH-S is the senior author of the paper.
Literature research and clinical advice: KS, JC, MA, HS, CGS, IE, MW, DT, SJ, AA, CR, WS, and RW.
Acquisition, analysis, or interpretation of data: HK, CN, KS, JC, MA, HS, CGS, IE, MW, DT, SJ, AA, CR, WS, RW, MS, H-WB, and FH-S.
Drafting of the manuscript: HK, CN, KS, MA, and FH-S.
Critical revision of the manuscript for important intellectual content: HK, CN, KS, JC, MA, HS, CGS, IE, MW, DT, SJ, AA, CR, WS, RW, MS, H-WB, and FH-S.
Preparation of figures: KS.
Statistical analysis: MA.
Study supervision: HK, MS, H-WB, and FH-S.
The authors shared study design, data collection, data analyses, and data interpretation, as well as preparation, review, and approval of the manuscript. The authors declare that the results presented in this paper have not been published previously in whole or part, except in abstract format. The corresponding authors had full access to all study data and had final responsibility for the decision to submit for publication.
Acknowledgements
The authors thank the nursing staff of the Patienten-Heimversorgung outpatient dialysis center at the University Hospital Giessen for their efforts and commitment to the patient well-being. Without their support, this work would not have been possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2022.108961.
Appendix A. Supplementary data
Supplementary data to "Immunogenicity and reactogenicity of homologous mRNA-based and vector-based SARS-CoV-2 vaccine regimen in patients receiving maintenance dialysis"
References
- 1.Ng J.H., Hirsch J.S., Wanchoo R., Sachdeva M., Sakhiya V., Hong S., Jhaveri K.D., Fishbane S., Northwell C.-R.C., C.-R.C. the Northwell Nephrology Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Himmelfarb J., Vanholder R., Mehrotra R., Tonelli M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020;16:573–585. doi: 10.1038/s41581-020-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen D.E., Sibbel S., Marlowe G., Bludorn K., Miller D., Kelley T., Connaire J., Young A., Tentori F., Brunelli S.M. Antibody status, disease history, and incidence of SARS-CoV-2 infection among patients on chronic dialysis. J. Am. Soc. Nephrol. 2021;32:1880–1886. doi: 10.1681/ASN.2021030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato S., Chmielewski M., Honda H., Pecoits-Filho R., Matsuo S., Yuzawa Y., Tranaeus A., Stenvinkel P., Lindholm B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Medicines Agency COVID-19 Vaccines. 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines Accessed July 25.
- 6.Barrett J.R., Belij-Rammerstorfer S., Dold C., Ewer K.J., Folegatti P.M., Gilbride C., Halkerston R., Hill J., Jenkin D., Stockdale L., Verheul M.K., Aley P.K., Angus B., Bellamy D., Berrie E., Bibi S., Bittaye M., Carroll M.W., Cavell B., Clutterbuck E.A., Edwards N., Flaxman A., Fuskova M., Gorringe A., Hallis B., Kerridge S., Lawrie A.M., Linder A., Liu X., Madhavan M., Makinson R., Mellors J., Minassian A., Moore M., Mujadidi Y., Plested E., Poulton I., Ramasamy M.N., Robinson H., Rollier C.S., Song R., Snape M.D., Tarrant R., Taylor S., Thomas K.M., Voysey M., Watson M.E.E., Wright D., Douglas A.D., Green C.M., Hill A.V.S., Lambe T., Gilbert S., Pollard A.J., Oxford C.V.T.G. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 7.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Tureci O., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Sahin U., Gruber W.C. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. New Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glenn D.A., Hegde A., Kotzen E., Walter E.B., Kshirsagar A.V., Falk R., Mottl A. Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease. Kidney Int. Rep. 2021:1407–1410. doi: 10.1016/j.ekir.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr E.J., Kronbichler A., Graham-Brown M., Abra G., Argyropoulos C., Harper L., Lerma E.V., Suri R.S., Topf J., Willicombe M., Hiremath S. Systematic review of early immune response to SARS-CoV-2 vaccination among patients with chronic kidney disease. Kidney Int. Rep. 2021;6:2292–2304. doi: 10.1016/j.ekir.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yau K., Abe K.T., Naimark D., Oliver M.J., Perl J., Leis J.A., Bolotin S., Tran V., Mullin S.I., Shadowitz E., Gonzalez A., Sukovic T., Garnham-Takaoka J., de Launay K.Q., Takaoka A., Straus S.E., McGeer A.J., Chan C.T., Colwill K., Gingras A.C., Hladunewich M.A. Evaluation of the SARS-CoV-2 antibody response to the BNT162b2 vaccine in patients undergoing hemodialysis. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espi M., Charmetant X., Barba T., Koppe L., Pelletier C., Kalbacher E., Chalencon E., Mathias V., Ovize A., Cart-Tanneur E., Bouz C., Pellegrina L., Morelon E., Fouque D., Juillard L., Thaunat O. The ROMANOV study found impaired humoral and cellular immune responses to SARS-CoV-2 mRNA vaccine in virus-unexposed patients receiving maintenance hemodialysis. Kidney Int. 2021;100:928–936. doi: 10.1016/j.kint.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.F. National Kidney KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am. J. Kidney Dis. 2015;66:884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Blake P.G., Bargman J.M., Brimble K.S., Davison S.N., Hirsch D., McCormick B.B., Suri R.S., Taylor P., Zalunardo N., Tonelli M., D. Canadian Society of Nephrology Work Group on Adequacy of Peritoneal Clinical practice guidelines and recommendations on peritoneal dialysis adequacy 2011. Perit. Dial. Int. 2011;31:218–239. doi: 10.3747/pdi.2011.00026. [DOI] [PubMed] [Google Scholar]
- 14.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O'Dell S., Schmidt S.D., Swanson P.A., 2nd, Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H., R.N.A.S.G. m An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos-Araujo C., Veiga P.M., Santos M.J., Santos L., Romaozinho C., Silva M., Lucas C., Duarte M.L., Haarhaus M., Haase M., Macario F. Time-dependent evolution of IgG antibody levels after first and second dose of mRNA-based SARS-CoV-2 vaccination in hemodialysis patients: a multicenter study. Nephrol. Dial. Transplant. 2021 doi: 10.1093/ndt/gfab293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stumpf J., Siepmann T., Lindner T., Karger C., Schwobel J., Anders L., Faulhaber-Walter R., Schewe J., Martin H., Schirutschke H., Barnett K., Huther J., Muller P., Langer T., Pluntke T., Anding-Rost K., Meistring F., Stehr T., Pietzonka A., Escher K., Cerny S., Rothe H., Pistrosch F., Seidel H., Paliege A., Beige J., Bast I., Steglich A., Gembardt F., Kessel F., Kroger H., Arndt P., Sradnick J., Frank K., Klimova A., Mauer R., Grahlert X., Anft M., Blazquez-Navarro A., Westhoff T.H., Stervbo U., Tonn T., Babel N., Hugo C. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021;9:100178. doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanay N.B., Freiman S., Shapira M., Wishahi S., Hamze M., Elhaj M., Zaher M., Armaly Z. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99:1496–1498. doi: 10.1016/j.kint.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broseta J.J., Rodriguez-Espinosa D., Rodriguez N., Mosquera M.D.M., Marcos M.A., Egri N., Pascal M., Soruco E., Bedini J.L., Bayes B., Maduell F. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am. J. Kidney Dis. 2021;78:571–581. doi: 10.1053/j.ajkd.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giot M., Fourie T., Lano G., Villarroel P.M.S., de Lamballeri X., Gully M., Samson L., Farault J., Bouchouareb D., Jehel O., Brunet P., Jourde-Chiche N., Ninove L., Robert T. Spike and neutralizing antibodies response to COVID-19 vaccination in haemodialysis patients. Clin. Kidney J. 2021;14:2239–2245. doi: 10.1093/ckj/sfab128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speer C., Goth D., Benning L., Buylaert M., Schaier M., Grenz J., Nusshag C., Kalble F., Kreysing M., Reichel P., Tollner M., Hidmark A., Ponath G., Schnitzler P., Zeier M., Susal C., Morath C., Klein K. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin. J. Am. Soc. Nephrol. 2021;16:1073–1082. doi: 10.2215/CJN.03700321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand S., Montez-Rath M.E., Han J., Garcia P., Cadden L., Hunsader P., Kerschmann R., Beyer P., Dittrich M., Block G.A., Boyd S.D., Parsonnet J., Chertow G.M. Antibody response to COVID-19 vaccination in patients receiving dialysis. J. Am. Soc. Nephrol. 2021;32:2435–2438. doi: 10.1681/ASN.2021050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidovic T., Schimpf J., Abbassi-Nik A., Stockinger R., Sprenger-Mahr H., Lhotta K., Zitt E. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: time for a boost. Kidney Int. 2021 doi: 10.1016/j.kint.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert Koch Institut Epidemiologisches Bulletin. 2021. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/39/Art_01.html Accessed November 2.
- 24.Sariol A., Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity. 2020;53:248–263. doi: 10.1016/j.immuni.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., Pascal K.E., Maurus D., Brachtendorf S., Lorks V., Sikorski J., Koch P., Hilker R., Becker D., Eller A.K., Grutzner J., Tonigold M., Boesler C., Rosenbaum C., Heesen L., Kuhnle M.C., Poran A., Dong J.Z., Luxemburger U., Kemmer-Bruck A., Langer D., Bexon M., Bolte S., Palanche T., Schultz A., Baumann S., Mahiny A.J., Boros G., Reinholz J., Szabo G.T., Kariko K., Shi P.Y., Fontes-Garfias C., Perez J.L., Cutler M., Cooper D., Kyratsous C.A., Dormitzer P.R., Jansen K.U., Tureci O. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 26.Cucchiari D., Egri N., Bodro M., Herrera S., Del Risco-Zevallos J., Casals-Urquiza J., Cofan F., Moreno A., Rovira J., Banon-Maneus E., Ramirez-Bajo M.J., Ventura-Aguiar P., Perez-Olmos A., Garcia-Pascual M., Pascal M., Vilella A., Trilla A., Rios J., Palou E., Juan M., Bayes B., Diekmann F. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sattler A., Schrezenmeier E., Weber U.A., Potekhin A., Bachmann F., Straub-Hohenbleicher H., Budde K., Storz E., Pross V., Bergmann Y., Thole L.M., Tizian C., Holsken O., Diefenbach A., Schrezenmeier H., Jahrsdorfer B., Zemojtel T., Jechow K., Conrad C., Lukassen S., Stauch D., Lachmann N., Choi M., Halleck F., Kotsch K. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Invest. 2021;131 doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litjens N.H., Huisman M., van den Dorpel M., Betjes M.G. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J. Am. Soc. Nephrol. 2008;19:1483–1490. doi: 10.1681/ASN.2007090971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikizler T.A., Coates P.T., Rovin B.H., Ronco P. Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int. 2021;99:1275–1279. doi: 10.1016/j.kint.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes S., Davari M., Gnanasampanthan S., Roth N., Young G., Rajakariar R., Cove-Smith A., Yaqoob M.M., Cutino-Moguel T., Mahalingasivam V., McCafferty K. Persistence of antibody response to SARS-CoV-2 in a cohort of haemodialysis patients with COVID-19. Nephrol. Dial. Transplant. 2021 doi: 10.1093/ndt/gfab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterhoff D., Gluck V., Vogel M., Schuster P., Schutz A., Neubert P., Albert V., Frisch S., Kiessling M., Pervan P., Werner M., Ritter N., Babl L., Deichner M., Hanses F., Lubnow M., Muller T., Lunz D., Hitzenbichler F., Audebert F., Hahnel V., Offner R., Muller M., Schmid S., Burkhardt R., Gluck T., Koller M., Niller H.H., Graf B., Salzberger B., Wenzel J.J., Jantsch J., Gessner A., Schmidt B., Wagner R. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection. 2021;49:75–82. doi: 10.1007/s15010-020-01503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Criscuolo E., Diotti R.A., Strollo M., Rolla S., Ambrosi A., Locatelli M., Burioni R., Mancini N., Clementi M., Clementi N. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J. Med. Virol. 2021;93:2160–2167. doi: 10.1002/jmv.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon B., Rubey H., Treipl A., Gromann M., Hemedi B., Zehetmayer S., Kirsch B. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol. Dial. Transplant. 2021;36:1709–1716. doi: 10.1093/ndt/gfab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantaleo G., Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to "Immunogenicity and reactogenicity of homologous mRNA-based and vector-based SARS-CoV-2 vaccine regimen in patients receiving maintenance dialysis"
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.