Abstract
Protein tyrosine kinase 7 (PTK7), a catalytically defective receptor protein tyrosine kinase, is upregulated in tumor tissues and cell lines of esophageal squamous cell carcinoma (ESCC). We showed that PTK7 plays an oncogenic role in various ESCC cell lines. However, its role as an oncogene has not been demonstrated in vivo. Here, we examined the influence of PTK7 on the tumorigenic potential of ESCC KYSE-30 cells, which are known to establish xenograft tumors. Overexpression of PTK7 enhanced the proliferation, adhesion, wound healing, and migration of KYSE-30 cells, and these effects were reversed by the knockdown of PTK7. PTK7 overexpression and knockdown, respectively, increased and decreased the tyrosine phosphorylation of cellular proteins and the phosphorylation of ERK, AKT, and FAK, which are important for cell proliferation, survival, adhesion, and migration. Additionally, PTK7 overexpression and silencing, respectively, increased and decreased the weight, volume, and number of Ki-67-positive proliferating cells in xenograft tumors of KYSE-30 cells. Therefore, we propose that PTK7 plays an important role in the tumorigenesis of ESCC cells in vivo and is a potential therapeutic target for ESCC.
Keywords: esophageal squamous cell carcinoma, KYSE-30 cells, oncogene, PTK7, receptor protein tyrosine kinase
1. Introduction
Protein tyrosine kinase 7 (PTK7) (also known as colon carcinoma kinase-4, CCK-4) is a catalytically defective receptor protein tyrosine kinase (RPTK) molecule that contains an extracellular domain with seven immunoglobulin-like loops, a transmembrane domain, and a tyrosine kinase domain lacking catalytic activity [1].
Homozygous PTK7 knockout mice are perinatally lethal with severe developmental defects, including defective neural tube closure [2]. The PTK7 knockout mice were phenotypically similar to mice and Xenopus with mutations in the planar cell polarity (PCP) genes. PTK7 is also genetically linked to the PCP gene Vangl2. In addition, PTK7 interacts with canonical Wnt signaling pathway proteins, including β-catenin, and activates genes involved in Xenopus development, such as the formation of Spemann’s organizer [3]. Moreover, PTK7 functions in non-canonical Wnt signaling by switching off canonical Wnt signaling [4]. PTK7 interacts with Wnt5A, a non-canonical Wnt ligand, and induces morphogenetic cell movements in Xenopus [5]. These findings suggest that PTK7 regulates the PCP and canonical and noncanonical Wnt signaling pathways during development.
PTK7 is an important modulator of tumorigenesis in adult tissues and is upregulated in esophageal squamous cell carcinoma (ESCC) [6,7], colorectal cancer [8,9], and other cancers [10,11,12]. PTK7 specifically promotes proliferation, survival, migration, invasion, and wound healing, and decreases apoptosis in many cell types [6,10,13,14,15,16]. However, PTK7 also acts as a tumor suppressor in some cell types, such as epithelial ovarian carcinoma and lung squamous cell carcinoma cells [17,18].
Esophageal cancer (EC) is the seventh most common cancer worldwide and the sixth leading cause of malignancy-related deaths [19]. EC is divided into two major subtypes, namely, esophageal adenocarcinoma (EAC) and ESCC. EAC is the most common subtype in Western countries, and the main risk factors include gastroesophageal reflux disease and obesity [20]. Globally, ESCC is the most common EC, with the highest incidence in East Asia and parts of Africa. Cigarette smoking and alcohol consumption are the main risk factors for ESCC [21,22].
PTK7 is upregulated in ESCC [6,7] and plays a role in ESCC cell proliferation, survival, migration, and invasion [15,16]. Fibroblast growth factor receptor 1 (FGFR1) is frequently upregulated in ESCC tumor tissues and cell lines [23]. PTK7 binds and activates FGFR1 independent of FGF, thereby increasing the tumorigenicity of PTK7- and FGFR1-positive cells [23].
We have shown that PTK7 plays a role in enhancing oncogenic properties in TE-6, 9, 10, and 11 ESCC cells harboring TP53 mutations [24]. However, TE-10 cells, which showed a distinct PTK7-dependent tumorigenic response [6,15], did not induce xenograft tumors in nude mice (unpublished data). KYSE-30 ESCC cells harboring epidermal growth factor receptor (EGFR) overexpression and TP53 mutations [25,26,27] can generate tumors in xenografts of orthotopic and subcutaneous implantation [28,29]. In this study, we analyzed the role of PTK7 in oncogenic phenotypes and the activation of signaling proteins in KYSE-30 cells using PTK7 knockdown and overexpression. We then analyzed the role of PTK7 in tumorigenesis in subcutaneous xenograft mice with KYSE-30 cells carrying knocked-down and overexpressed PTK7.
2. Results
2.1. PTK7 Overexpression Increases Proliferation, Adhesion, and Migration in ESCC KYSE-30 Cells
The effect of PTK7 overexpression on the oncogenic properties of ESCC KYSE-30 cells was first examined. Infecting KYSE-30 cells with a PTK7-FLAG lentivirus increased PTK7 expression compared to that by the overexpression vector control (Figure 1A). PTK7 overexpression increased cell growth to 204.5 ± 11.8%, 342.6 ± 8.3%, and 665.5 ± 22.6% at 1, 2, and 3 days of culture, respectively, compared to that by the overexpression vector control (144.0 ± 3.9%, 256.3 ± 11.1%, and 447.0 ± 19.0% at 1, 2, and 3 days of culture, respectively) (Figure 1B). Moreover, PTK7 overexpression increased adhesion by 131.5 ± 2.5% (Figure 1C), wound healing by 130.1 ± 2.1% (Figure 1D), and migration by 141.6 ± 6.1% (Figure 1E), compared to that by the overexpression vector control. The differences in the values observed in the overexpression vector control and those in the mock control were not statistically significant.
Figure 1.
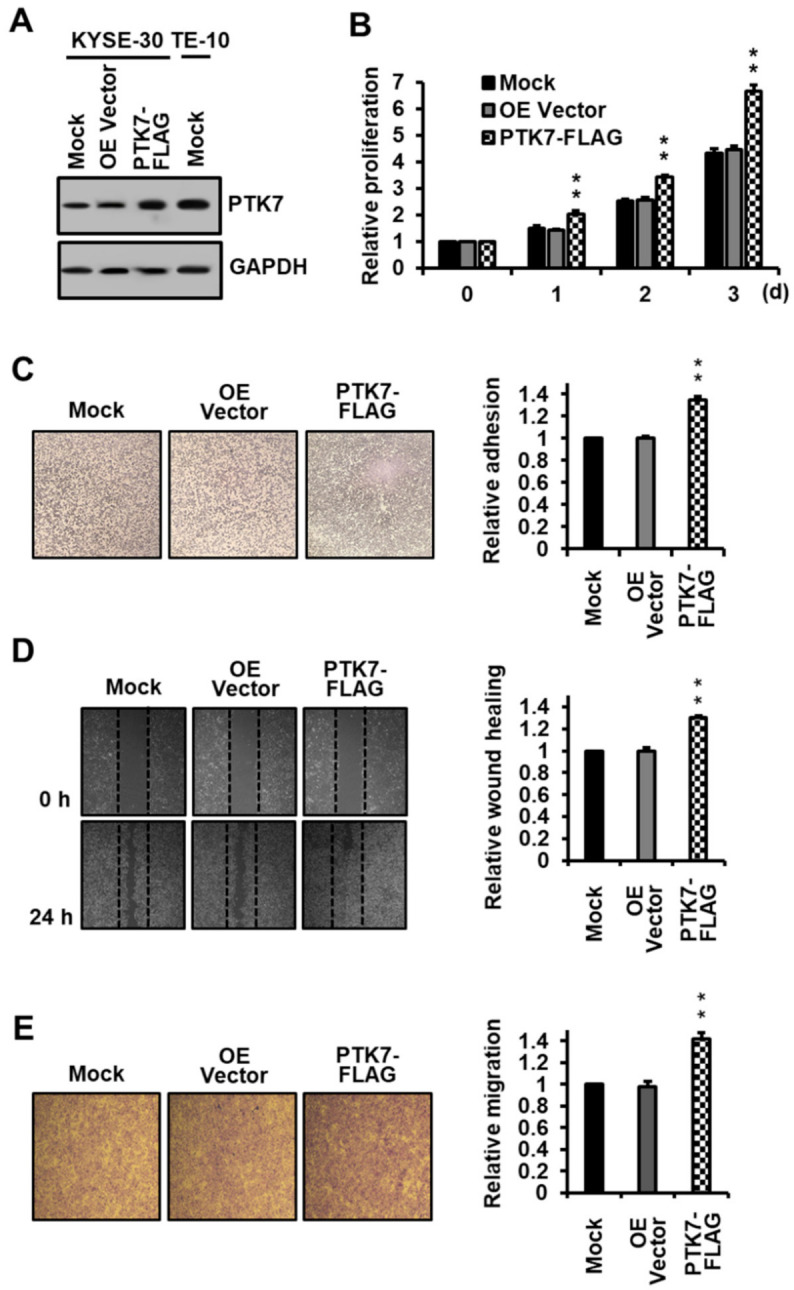
Effect of PTK7 overexpression on the oncogenic properties of ESCC KYSE-30 cells. (A) PTK7 expression in ESCC KYSE-30 cells mock-infected or infected with lentiviruses for vector control (OE Vector) and for expression of PTK7-FLAG and in ESCC TE-10 cells mock-infected was analyzed by Western blotting with PTK7 and GAPDH antibodies. (B) Proliferation of the mock, vector control, and PTK7-overexpressing KYSE-30 cells was analyzed for 3 days. (C) Adhesion of the mock, vector control, and PTK7-overexpressing KYSE-30 cells was analyzed 1 h after plating on dishes coated with type I collagen (1 μg/well). (D) Wound healing of the mock, vector control, and PTK7-overexpressing KYSE-30 cells was analyzed 24 h after the wounding in the monolayer of cells. (E) Chemotactic migration of the mock, vector control, and PTK7-overexpressing KYSE-30 cells was analyzed 24 h after loading of the cells in the upper transwell chamber. The representative images for (C–E) are shown. Each bar represents the mean ± SD from three independent experiments. ** p < 0.01 vs. 0 day (B) or vector control (C–E).
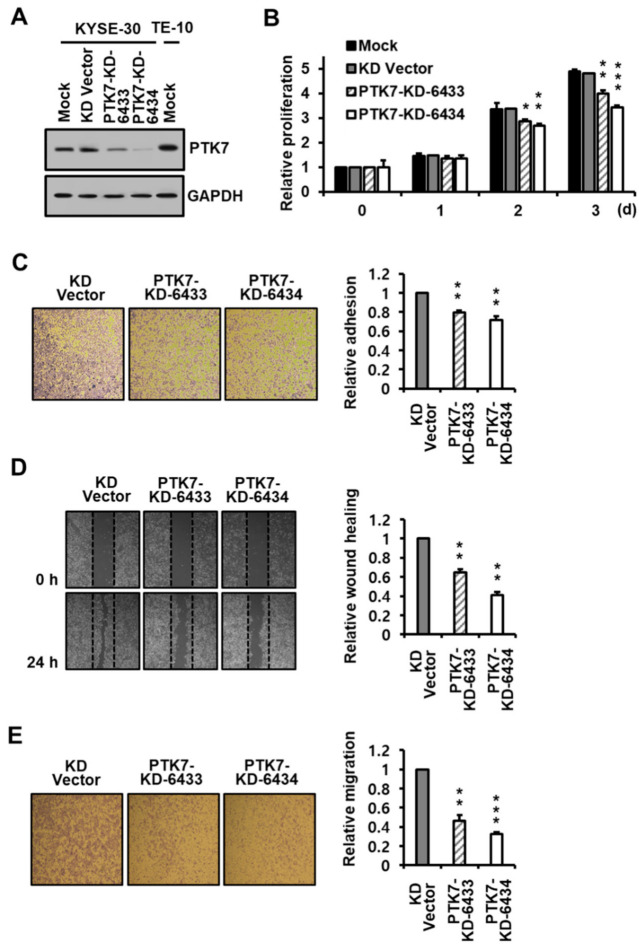
2.2. PTK7 Knockdown Reduces Proliferation, Adhesion, and Migration in ESCC KYSE-30 Cells
ESCC KYSE-30 cells were infected with lentiviruses encoding PTK7 shRNAs (PTK7-KD-6433 and -6434) to silence PTK7 expression and examine its effect on the oncogenic properties of these cells. PTK7 knockdown reduced PTK7 expression in KYSE-30 cells (Figure 2A), and PTK7 knockdown using PTK7-KD-6433 and -6434 decreased cell proliferation to 398.1 ± 14.4% and 341.9 ± 9.4%, respectively, compared to that by the vector control at 482.1 ± 14.2%, after 3 days of culturing (Figure 2B). PTK7 knockdown using PTK7-KD-6433 and -6434 also reduced adhesion to 80.9 ± 2.3% and 69.5 ± 1.6% (Figure 2C), wound healing to 65.0 ± 3.1% and 41.2 ± 3.2% (Figure 2D), and migration to 46.4 ± 5.7% and 32.5 ± 2.0% (Figure 2E), respectively, in comparison to that by the knockdown vector control. PTK7 overexpression and knockdown exhibited an opposing effect on the tumorigenic effect, indicating that PTK7 acts as an oncogene in KYSE-30 cells.
Figure 2.
Effect of PTK7 knockdown on the oncogenic properties of ESCC KYSE-30 cells. (A) PTK7 expression in ESCC KYSE-30 cells mock-infected or infected with lentiviruses for vector control (KD Vector) and for PTK7 knockdown shRNA (PTK7-KD-6433 and PTK7-KD-6434) and in ESCC TE-10 cells mock-infected was analyzed by Western blotting with PTK7 and GAPDH antibodies. Proliferation (B), adhesion (C), wound healing (D), and chemotactic migration (E) of the mock, vector control, and PTK7 knockdown shRNA (PTK7-KD-6433 and PTK7-KD-6434) KYSE-30 cells were analyzed using the same methods as in the overexpressing cells. The representative images for (C–E) are shown. Each bar represents the mean ± SD from three independent experiments. * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. 0 day (B) or vector control (C–E).
2.3. PTK7 Increases the Activation of Oncogenic Signaling Proteins
Cell proliferation, adhesion, and migration involve the activation of various signaling pathways, including the MAPK, PI3-kinase/AKT, and FAK pathways [30,31,32]. We investigated the phosphorylation of these signaling proteins in PTK7-overexpressing or knockdown KYSE-30 cells. The tyrosine phosphorylation of cellular proteins and the phosphorylation of ERK, AKT, and FAK were increased by PTK7 overexpression and decreased by PTK7 knockdown (Figure 3). Thus, PTK7 activates the signaling pathways involved in cell proliferation, adhesion, and migration, resulting in increased tumorigenesis of ESCC KYSE-30 cells.
Figure 3.
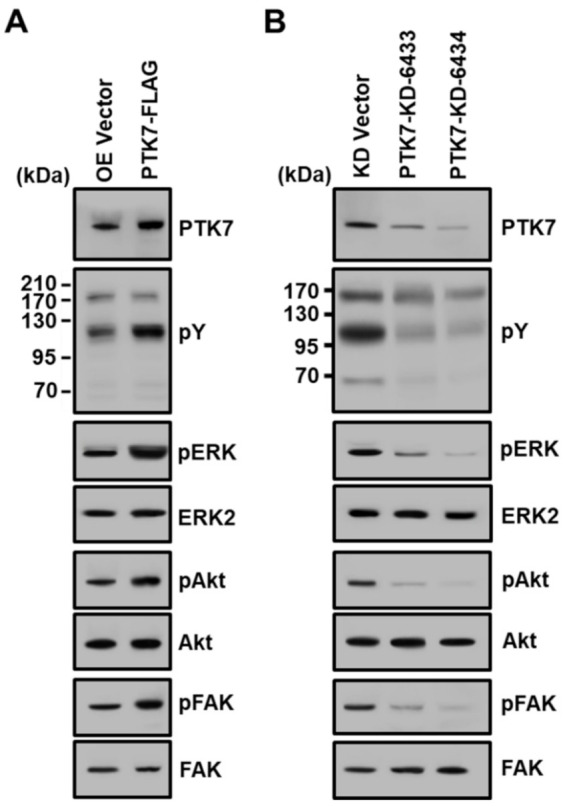
Effect of PTK7 overexpression and knockdown on activation of signaling molecules in ESCC KYSE-30 cells. Tyrosine phosphorylation of cellular proteins, as well as phosphorylation of ERK, AKT, and FAK, was examined by Western blotting in KYSE-30 cells infected with lentiviruses for vector control (OE Vector) and PTK7 overexpression (PTK7-FLAG) (A), as well as the vector control (KD Vector) and PTK7 knockdown shRNA (PTK7-KD-6433 and -6434) (B).
2.4. PTK7 Expression Correlates with the Tumorigenic Effect of ESCC KYSE-30 Cells In Vivo
To further elucidate the effect of PTK7 expression on tumorigenesis in vivo, we transplanted mock, PTK7-FLAG, PTK7-KD-6433, or PTK7-KD-6434 KYSE-30 cells into immune-deficient athymic nude (BALB/c nu/nu) mice. Xenografted mice bearing PTK7-overexpressing KYSE-30 cells showed accelerated tumor growth compared to mock-control mice (Figure 4A), while mice harboring PTK7-knockdown KYSE-30 cells exhibited a delay in tumor growth compared to control mice (Figure 5A). Mice were sacrificed 6 weeks post injection. The tumor weight increased to 190.5 ± 17.2% in PTK7-overexpressing tumors with PTK7-FLAG (Figure 4B), compared to the tumor weight (0.72 ± 0.11 g) of the mock control tumors. The tumor weight decreased to 73.8 ± 5.2% and 31.7 ± 8.6% in PTK7-knockdown tumors with PTK7-KD-6433 and PTK7-KD-6434, respectively (Figure 5B), compared to the tumor weight (0.73 ± 0.08 g) of the mock control tumors.
Figure 4.
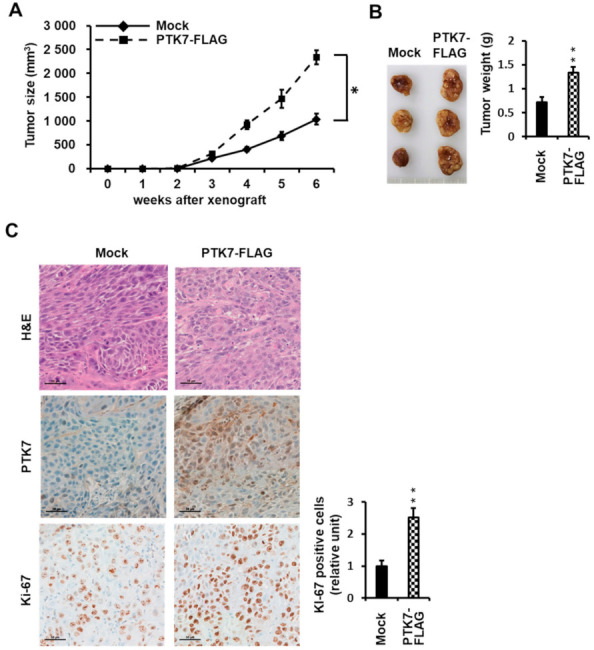
Effect of PTK7 overexpression on tumor growth in xenograft mice. KYSE-30 cells with mock-transfected or overexpressing PTK7-FLAG were injected subcutaneously into the dorsal regions of mice. (A) Tumor volumes were monitored once per week for 6 weeks. (B) Images of tumors from mice at 6 weeks after injection (left) and the corresponding weights (right) are shown. Each bar represents the mean ± SD of the three mice. * p < 0.05, ** p < 0.01 vs. mock-transfected cells. (C) Representative images of histologic and immunohistochemical (PTK7 and Ki-67) analysis of tumors are shown in the indicated groups of mice. Scale bar represents 50 μm.
Figure 5.
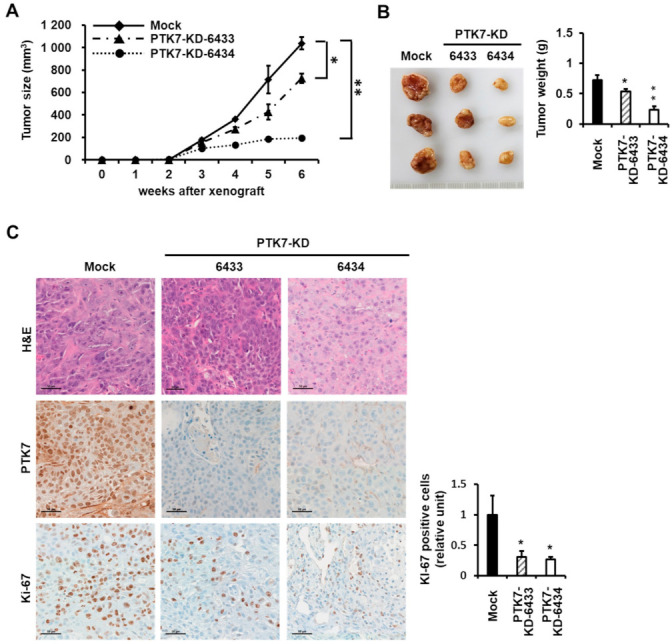
Effect of PTK7 knockdown on tumor growth in xenograft mice. KYSE-30 cells with mock-transfected or PTK7 knockdown (PTK7-KD-6433 and -6434) were subcutaneously xenografted into the dorsal regions of mice. (A) Tumor volumes were recorded for 6 weeks. (B) Images of tumors from mice at 6 weeks after injection (left) and the corresponding weights (right) are shown. Each bar represents the mean ± SD of the three mice. * p < 0.05, ** p < 0.01 vs. mock-transfected cells. (C) Representative images of histologic and immunohistochemical (PTK7 and Ki-67) analysis of tumors are shown in the indicated groups of mice. Scale bar represents 50 μm.
Hematoxylin and eosin staining and immunohistochemical staining for PTK7 and Ki-67 were performed on the tumor sections to assess changes in cell morphology and proliferation. PTK7 expression was elevated in PTK7-FLAG tumors and decreased in PTK7-KD-6433 and -6434 tumors compared to that in the control tumors (Figure 4C and Figure 5C). Expression of Ki-67, a proliferation marker in solid tumors, was stronger (252.1 ± 29.2%) in PTK7-overexpressing tumors and weaker (30.6 ± 10.1% in PTK7-KD-6433 and 26.8 ± 4.0% in PTK7-KD-6434) in PTK7-knockdown tumors, compared to that in control tumors (Figure 4C and Figure 5C). This confirmed that PTK7 promotes tumor progression in ESCC.
3. Discussion
Analysis of the biological pathways deregulated in ESCC showed that the RPTK-MAPK-PI3K pathway, cell cycle, and epigenetic regulatory mechanisms are frequently dysregulated by multiple molecular abnormalities [33,34,35]. In ESCC tumor tissues, EGFR is frequently overexpressed or often amplified, with activation of the PI3K/AKT signaling pathway due to mutations in the PI3KCA gene and loss of PTEN expression [36,37,38]. TP53 and CDKN2A mutations and CCND1 amplification are additional changes observed in the genes regulating the cell cycle [39,40]. Mutations are often found in genes involved in epigenetic regulation, such as histone H3 lysine-4 mono-methyltransferases (KMT2D/MLL2 and KMT2C/MLL3) and histone acetyltransferases (CREBBP and EP300) [39].
EGFR overexpression and TP53 mutations are common in precancerous ESCC lesions [36,41]. TP53 mutations were also correlated with EGFR overexpression. These findings suggest that patients with ESCC may benefit from EGFR-targeted therapy. Nevertheless, the EGFR-neutralizing antibody cetuximab only reacted to ESCC patient-derived xenografts with high EGFR expression and/or amplification [42]. In addition, gefitinib, a low molecular weight EGFR inhibitor, did not improve overall survival in unselected patients with EC, including ESCC, but provided a palliative effect in a subgroup of difficult-to-treat patients with short life expectancy [43]. Another low molecular weight EGFR inhibitor, icotinib, was effective in 17.6% of patients with high EGFR-expressing tumors, but not in patients with moderate EGFR-expressing tumors [44]. EGFR inhibitors are effective for epithelial-like ESCC cells but are ineffective for mesenchymal-like ESCC cells, as EGFR signaling cannot be blocked [45]. EGFR inhibitors alone are, therefore, considered less effective in treating ESCC.
PTK7 is upregulated in various cancer types, including ESCC [6,7]. PTK7 reduces apoptosis and promotes proliferation, survival, migration, invasion, and wound healing in ESCC cells [6,16]. PTK7 knockdown reduced the phosphorylation of Akt, Erk, and FAK [6]. PTK7 upregulates MMP-9 through activation of AP-1 and NF-κB, thus increasing the invasive properties of ESCC cells [15]. These results suggest that PTK7 has potential as a prognostic marker for ESCC and could be a candidate for targeted therapy.
In our analysis, the PTK7 levels in ESCC TE-10 and TE-11 cells were higher than those in TE-5, TE-9, and TE-14 cells [6]. TE-10 cells exhibited a PTK7-dependent tumorigenic response, including cell proliferation, survival, wound healing, and invasion [6,15]. TE-10 cells harbor a TP53 mutation [24] and co-amplified INT-2/FGF3 and HST-1/FGF4. The INT-2/FGF3 and HST-1/FGF4 polypeptides are members of the FGF family, which have mitogenic activity in various cell types [46]. Additionally, PTK7 knockdown reduced not only ligand-free and FGF-induced FGFR1 phosphorylation, but also the interaction of signaling adaptor proteins with FGFR1 and activation of downstream signaling proteins in TE-10 cells [23]. Collectively, these results suggest that the FGF/FGFR signaling pathway, in cooperation with PTK7, plays an important role in the pathogenesis of EC in an autocrine or paracrine manner.
We also wanted to use a mouse xenograft model of ESCC cells to demonstrate the role of PTK7 in tumorigenesis. However, it was unclear whether TE-10 cells could establish xenograft tumors in nude mice. Nishihara et al. reported that xenograft tumors could not be generated [46], while Yang et al. recently reported that xenograft tumors were produced using TE-10 cells [47]. However, we were unable to generate tumors after performing xenografts in nude mice with TE-10 cells (unpublished data).
KYSE-30 cells were established in nude mice tumors generated through transplantation of well-differentiated invasive ESCC tissue from a 64-year-old Japanese man [26]. KYSE-30 cells have a highly rearranged hypertriploid karyotype with 8% polyploidy and harbor TP53 mutations and amplification of EGFR, MYC, and CCND1. Additionally, KYSE-30 cells produce xenograft tumors in nude mice [48,49].
In this study, we showed that KYSE-30 cells express PTK7 at lower levels relative to TE-10 cells. Consistent with our previous results using other ESCC cells [6,15,50], PTK7 overexpression increased proliferation, adhesion, wound healing, and migration as well as tyrosine phosphorylation of cellular proteins and phosphorylation of ERK, AKT, and FAK in KYSE-30 cells. When PTK7 was silenced in KYSE-30 cells, these effects were reversed. ERK inhibition by PD98059 (MEK inhibitor) and Akt inhibition by LY294002 (PI3K inhibitor) in ESCC TE-10 cells, in which PTK7 expression increases invasion via MMP-9 secretion, decreased MMP-9 secretion [15]. Similarly, ERK inhibition by PD98059 and Akt inhibition by LY294002 significantly suppress migration and invasion in ESCC TE-8 and TE-9 cells [51]. Furthermore, knockdown or inhibition (by defactinib) of FAK decreases proliferation, migration, and invasion in various ESCC cells, including KYSE-30 cells [52,53]. Therefore, PTK7-dependent activation of ERK, Akt and FAK appears to be directly related to the oncogenic phenotypes.
Various studies have shown that PTK7 and FGFR1 are significantly upregulated in ESCC tumor tissues and cell lines [6,23,54]. We also demonstrated that PTK7 plays an important role in FGFR1 activation in various ESCC cells [23]. Therefore, regulation of FGFR1 activity, based on PTK7 expression, could possibly modulate oncogenic phenotypes and signaling pathways. However, considering the high EGFR levels in KYSE-30 cells, it was interesting how PTK7 knockdown could reduce oncogenic processes. It was recently reported that PTK7 is involved in the activation of EGFR and Akt signaling in triple-negative breast cancer cells [55]. These results suggest that counteracting PTK7 can efficiently reduce the tumorigenesis of esophageal squamous cells in ESCC cells by blocking FGFR and EGFR signaling.
Importantly, we could demonstrate that xenograft tumors of KYSE-30 cells with PTK7 overexpression and silencing respectively increased and decreased the weight, volume, and number of Ki-67-positive proliferating cells. This proved that PTK7 expression is positively correlated with the tumorigenic process of ESCC in vivo. In addition, this xenograft tumor model of KYSE-30 cells can be used to analyze anti-cancer agents targeting PTK7 and the role of PTK7 in vivo.
4. Materials and Methods
4.1. Cell Culture
Human ESCC KYSE-30 cells and human embryonic kidney 293 (HEK293) cells expressing SV40 T antigen (HEK293T) were provided by Dr. Sang Kil Lee (Yonsei University College of Medicine, Seoul, Korea) and Dr. Jong Bae Park (National Cancer Center, Goyang, Gyeonggi, Korea), respectively. KYSE-30 cells were grown in Dulbecco’s modified Eagle medium/nutrient mixture F-12 medium (DMEM/F12; Gibco of Thermo Fisher Scientific, Grand Island, NY, USA), supplemented with 2.5 mM sodium glutamine and 2% fetal bovine serum (FBS). HEK293T cells were grown in DMEM supplemented with 10% bovine serum (BS). All media were supplemented with 100 U/ml penicillin and 100 μg/mL streptomycin, and all cells were maintained at 37 °C in 5% CO2.
4.2. Generation of PTK7 Overexpression and Knockdown Lentiviruses and Infection of KYSE-30 Cells
To construct the pHRST-PTK7-FLAG-IRES-eGFP lentiviral transfer vector for PTK7 expression, a DNA fragment including the PTK7-FLAG-coding sequence was amplified by polymerase chain reaction (PCR), using the pcDNA3-hPTK7-FLAG vector [56] as a template, and PrimeSTAR GXL DNA polymerase. The upstream primer (5′-GATCTCGACGCGGCCGCTACGACTCACTATAGGGAGACCCAAGCT-3′) contained 17 nucleotides of pHRST-IRES-eGFP (nt. 2839-2855), including a NotI site (underlined) and 28 nucleotides of pcDNA3 (nt. 866-893; italicized). The downstream primer (5′-GGGCGGAATTGGATCCGAAGGCACAGTCGAGGCTGATCAG-3′) contained 16 nucleotides of pHRST-IRES-eGFP (nt. 2871-2856), including a BamHI site (underlined) and 24 nucleotides of pcDNA3 (nt. 1028-1051; italicized). The PCR product was ligated into the NotI and BamHI sites of the pHRST-IRES-eGFP vector using the In-fusion HD cloning kit (TaKaRa, Kusatsu, Japan). The resulting construct, pHRST-PTK7-FLAG-IRES-eGFP, was bi-directionally sequenced to avoid PCR errors. The pLKO.1-shRNA-PTK7-6433 and -6434 constructs for human PTK7 knockdown and the pLKO.1-control were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lentiviruses were produced in HEK293T cells, as previously described [6], with the exception of using 13 μg of pHRST-PTK7-FLAG-IRES-eGFP for PTK7 overexpression. KYSE-30 cells infected with PTK7 knockdown lentiviruses were incubated with 1 μg/ml puromycin for 14 days, and puromycin-resistant PTK7-knockdown colonies were pooled in a mixed culture. Cells infected with PTK7-overexpression lentiviruses were used without further selection.
4.3. Antibodies
The following antibodies were used: anti-phospho-ERK (sc-7383) and anti-FAK (sc-557), obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-phospho-Akt (Ser473) (4060s) and anti-Akt (9272s), obtained from Cell Signaling Technology (Danvers, MA, USA); anti-phospho-tyrosine (clone 4G10) (05-321) and anti-phospho-FAK (Tyr397) (abt135), obtained from Merck Millipore (Burlington, MA, USA); anti-ERK2 (bms-52068R), obtained from Bioss (Boston, MA, USA); anti-FLAG-M2 antibody (F1804), obtained from Sigma-Aldrich; anti-Ki-67 (ab15580), obtained from Abcam (Cambridge, UK); anti-GAPDH (abc2003), obtained from AbClone (Seoul, Korea); and horseradish peroxidase-conjugated goat anti-mouse IgG (K0211589) and rabbit IgG (K0211708), obtained from KOMA Biotech (Seoul, Korea). The generation of anti-PTK7 anti-serum was described previously [57].
4.4. Western Blot Analysis
For Western blot analysis, cells were lysed with RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) for 10 min at 4 °C. Lysates were subjected to SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. Blots were incubated with the indicated antibodies, and the immunoreactive bands were visualized using West-Q PICO Dura ECL solution (GenDepot, Barker, TX, USA), Immobilon Western Chemiluminescent HRP Substrate (Merck Millipore), and an LAS-3000 imaging system (Fujifilm, Tokyo, Japan).
4.5. Cell Proliferation Assay
Cell proliferation assays were performed as previously described [58]. Briefly, cells (0.1 ml, 1.5 × 104 cells/ml) were plated in a 96-well flat-bottomed cell culture plate (SPL, Pocheon, Korea) in DMEM/F12 medium supplemented with 2% FBS and 2.5 mM sodium glutamine and incubated for 1–3 days. To measure the number of live cells, cells were washed with phosphate-buffered saline (PBS; 8.06 mM Na2HPO4, 1.47 mM KH2PO4, 137 mM NaCl, 2.6 mM KCl; pH 7.4) containing 1 mM CaCl2 and 0.5 mM MgCl2 and incubated with 0.1 ml DMEM/F12 medium containing 0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide for 4 h. After removal of the medium, cells were washed with PBS and solubilized with 0.1 ml DMSO. Absorbance was measured at 565 nm using a SpectraMax M3 microplate reader (Molecular Devices, San Jose, CA, USA).
4.6. Cell Adhesion Assay
The cell adhesion assay was performed as previously described [59]. Briefly, cells were incubated in serum-free DMEM/F12 medium for 24 h. Detached cell suspensions (3 × 104 cells/0.1 mL for overexpression analysis or 5 104 cells/0.1 mL for knockdown analysis) were loaded onto 96-well plates, which were precoated with rat-tail type I collagen (1 μg/well) overnight and were incubated in DMEM/F12 medium with 1% FBS for 1 h. Cells were fixed with 3.7% paraformaldehyde in PBS and stained with 0.005% crystal violet. The stained cells were lysed with 1% SDS, and the absorbance was measured at 600 nm.
4.7. Wound Healing and Chemotactic Migration Assay
A wound was introduced by scraping the monolayer with a micropipette tip. Cells were incubated for 24 h in DMEM/F12 medium containing 2% FBS and evaluated by light microscopy [59]. The chemotactic migration assay was performed as previously described [50]. Briefly, detached cell suspensions (7 × 104 cells/0.1 mL serum-free DMEM/F12 medium) were loaded into the upper compartment of transwell chambers (Corning, Tewksbury, MA, USA). The bottom surface of each transwell was coated with 10 μL of 0.1% gelatin. The lower compartment of each well was filled with 0.65 mL DMEM/F12 medium, with 10% FBS as a chemoattractant. The chamber was then incubated at 37 °C for 24 h. After incubation, the remaining cells in the upper compartment were removed using a cotton swab. Cells that migrated to the bottom surface of the filter were fixed with 3.7% paraformaldehyde in PBS and stained with 0.005% crystal violet. The stained cells were solubilized with 1% SDS, and the absorbance was measured at 600 nm.
4.8. Xenograft Mouse Model
Four to five-week-old male immune-deficient athymic nude (BALB/c nu/nu) mice were purchased from Orient Bio Inc. (Gyeonggi, Korea). To investigate the effect of PTK7 on tumorigenesis in a xenograft mouse model, 1 × 106 KYSE-30 cells expressing either PTK7-FLAG or PTK7 shRNA (PTK7-KD-6433 and 6434) were resuspended in growth-factor-reduced Matrigel (Corning) and subcutaneously injected into the backs of mice. Tumor growth in each group was evaluated by measuring the tumor size twice per week using calipers (length × width × depth/2). Mice were sacrificed 6 weeks after cell injection. The xenograft tumors were recovered to measure tumor weight, fixed in formalin, and were paraffin embedded for histological and immunohistochemical analyses [60].
4.9. Ethics Statement
All animal experiments were approved and performed in accordance with the Institutional Animal Care and Use Committee (IACUC) review board of the National Cancer Center, which is an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) accredited facility that abides by the Institute of Laboratory Animal Resources guide (NCC-20-561).
4.10. Statistical Analysis
Statistical analyses were performed using Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Statistical significance was analyzed using the Student’s t test. For all tests, p values less than 0.05 were considered statistically significant.
5. Conclusions
We previously showed that PTK7 expression correlates with the promotion of oncogenic properties in several ESCC cells. In this study, we analyzed the effect of PTK7 on tumorigenicity at the cellular level and in vivo using KYSE-30 cells, which are known to produce xenograft tumors in nude mice. KYSE-30 cells expressed PTK7 at a relatively lower level than TE-10 cells. Overexpression of PTK7 increased proliferation, adhesion, wound healing, and migration, as well as tyrosine phosphorylation of cellular proteins and phosphorylation of ERK, AKT, and FAK in KYSE-30 cells. PTK7 silencing in KYSE-30 cells was reversed by PTK7 overexpression. PTK7 overexpression and silencing increased and decreased, respectively, the weight, volume, and number of Ki-67-positive proliferating cells in xenograft tumors of KYSE-30 cells. The results showed that the expression of PTK7 in vivo correlates positively with the oncogenic process of ESCC. Furthermore, a xenograft tumor model using KYSE-30 cells can be used to analyze PTK7-targeted anti-cancer drugs and the role of PTK7 in vivo.
Acknowledgments
W.-S.S. is a recipient of the Yonsei Frontier Laboratory Young Researcher Supporting Program of the Yonsei University Research Fund. J.H.K. and S.W.O. were fellowship awardees of the BK21 FOUR program.
Abbreviations
| DMEM | Dulbecco’s modified Eagle medium |
| EAC | esophageal adenocarcinoma |
| EC | esophageal cancer |
| EGFR | epidermal growth factor receptor |
| ESCC | esophageal squamous cell carcinoma |
| FBS | fetal bovine serum |
| FGFR | fibroblast growth factor receptor |
| HEK293 | human embryonic kidney 293 |
| PBS | phosphate-buffered saline |
| PCP | planar cell polarity |
| PCR | polymerase chain reaction |
| PTK7 | protein tyrosine kinase 7 |
| RPTK | receptor protein tyrosine kinase |
Author Contributions
W.-S.S., M.-K.P., H.L., and S.-T.L. designed the project, analyzed the data, and wrote the manuscript. W.-S.S., M.-K.P., J.H.K., S.W.O., and J.-Y.J. performed the experiments. H.L. and S.-T.L. conceptualized and supervised the project. All the authors critically reviewed and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Cancer Center of Korea (NCC-2010271 and 2110150 to H.L.), the National Research Foundation of Korea (2019M3A9A8065054 to S.-T.L.), and the Korea Drug Development Fund funded by the Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare of Korea (HN21C1214000021 to S.-T.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Park S.-K., Lee H.-S., Lee S.-T. Characterization of the human full-length PTK7 cDNA encoding a receptor protein tyrosine kinase-like molecule closely related to chick KLG. J. Biochem. 1996;119:235–239. doi: 10.1093/oxfordjournals.jbchem.a021228. [DOI] [PubMed] [Google Scholar]
- 2.Lu X., Borchers A.G., Jolicoeur C., Rayburn H., Baker J.C., Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 3.Puppo F., Thome V., Lhoumeau A.C., Cibois M., Gangar A., Lembo F., Belotti E., Marchetto S., Lecine P., Prebet T., et al. Protein tyrosine kinase 7 has a conserved role in Wnt/beta-catenin canonical signalling. EMBO Rep. 2011;12:43–49. doi: 10.1038/embor.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peradziryi H., Kaplan N.A., Podleschny M., Liu X., Wehner P., Borchers A., Tolwinski N.S. PTK7/Otk interacts with Wnts and inhibits canonical Wnt signalling. EMBO J. 2011;30:3729–3740. doi: 10.1038/emboj.2011.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez S., Scerbo P., Giordano M., Daulat A.M., Lhoumeau A.-C., Thomé V., Kodjabachian L., Borg J.-P. The PTK7 and ROR2 protein receptors interact in the vertebrate WNT/planar cell polarity (PCP) pathway. J. Biol. Chem. 2015;290:30562–30572. doi: 10.1074/jbc.M115.697615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin W.-S., Kwon J., Lee H.W., Kang M.C., Na H.-W., Lee S.-T., Park J.H. Oncogenic role of protein tyrosine kinase 7 in esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1120–1126. doi: 10.1111/cas.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashyap M.K., Abdel-Rahman O. Expression, regulation and targeting of receptor tyrosine kinases in esophageal squamous cell carcinoma. Mol. Cancer. 2018;17:1–11. doi: 10.1186/s12943-018-0790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mossie K., Jallal B., Alves F., Sures I., Plowman G.D., Ullrich A. Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene. 1995;11:2179–2184. [PubMed] [Google Scholar]
- 9.Lhoumeau A.-C., Martinez S., Boher J.-M., Monges G., Castellano R., Goubard A., Doremus M., Poizat F., Lelong B., De Chaisemartin C., et al. Overexpression of the promigratory and prometastatic PTK7 receptor is associated with an adverse clinical outcome in colorectal cancer. PLoS ONE. 2015;10:e0123768. doi: 10.1371/journal.pone.0123768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin X., Huang T., Ma C., Duan J., Li R., Zhang W., Tian W. Protein tyrosine kinase 7-knockdown inhibits oral squamous cell carcinoma cell viability, proliferation, migration and invasion via downregulating dishevelled segment polarity protein 3 expression. Exp. Ther. Med. 2021;22:1–9. doi: 10.3892/etm.2021.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan F., Tang J., Kong F.-L., Zou H.-W., Ni B.-L., Yu J.-C. Identification of PTK7 as a promising therapeutic target for thyroid cancer. Eur. Rev. Med. Pharmacol. Sci. 2020;24:6809–6817. doi: 10.26355/eurrev_202006_21670. [DOI] [PubMed] [Google Scholar]
- 12.Zou R.-C., Liang Y., Li L.-L., Tang J.-Z., Yang Y.-P., Geng Y.-C., He J., Luo L.-Y., Li W.X., Sun Z.-W., et al. Bioinformatics analysis identifies protein tyrosine kinase 7 (PTK7) as a potential prognostic and therapeutic biomarker in stages I to IV hepatocellular carcinoma. Med. Sci. Monit. 2019;25:8618–8627. doi: 10.12659/MSM.917142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng L., Sefah K., O’Donoghue M.B., Zhu G., Shangguan D., Noorali A., Chen Y., Zhou L., Tan W. Silencing of PTK7 in colon cancer cells: Caspase-10-dependent apoptosis via mitochondrial pathway. PLoS ONE. 2010;5:e14018. doi: 10.1371/journal.pone.0014018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prebet T., Lhoumeau A.-C., Arnoulet C., Aulas A., Marchetto S., Audebert S., Puppo F., Chabannon C., Sainty D., Santoni M.-J., et al. The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood. 2010;116:2315–2323. doi: 10.1182/blood-2010-01-262352. [DOI] [PubMed] [Google Scholar]
- 15.Shin W.S., Hong Y., Lee H.W., Lee S.T. Catalytically defective receptor protein tyrosine kinase PTK7 enhances invasive phenotype by inducing MMP-9 through activation of AP-1 and NF-kappaB in esophageal squamous cell carcinoma cells. Oncotarget. 2016;7:73242–73256. doi: 10.18632/oncotarget.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu K., Song G., Zhang X., Li Q., Zhao Y., Zhou Y., Xiong R., Hu X., Tang Z., Feng G. PTK7 is a novel oncogenic target for esophageal squamous cell carcinoma. World J. Surg. Oncol. 2017;15:105. doi: 10.1186/s12957-017-1172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H., Li G., Yin Y., Wang J., Wang H., Wei W., Guo Q., Ma H., Shi Q., Zhou X., et al. PTK7 protein is decreased in epithelial ovarian carcinomas with poor prognosis. Int. J. Clin. Exp. Pathol. 2014;7:7881–7889. [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J.-H., Kwon J., Lee H.W., Kang M.C., Yoon H.-J., Lee S.-T., Park J.H. Protein tyrosine kinase 7 plays a tumor suppressor role by inhibiting ERK and AKT phosphorylation in lung cancer. Oncol. Rep. 2014;31:2708–2712. doi: 10.3892/or.2014.3164. [DOI] [PubMed] [Google Scholar]
- 19.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. Erratum in CA Cancer J. Clin. 2020, 70, 313. [DOI] [PubMed] [Google Scholar]
- 20.Lagergren J., Bergström R., Lindgren A., Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 21.Kamangar F., Chow W.-H., Abnet C.C., Dawsey S.M. Environmental causes of esophageal cancer. Gastroenterol. Clin. N. Am. 2009;38:27–57. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islami F., Fedirko V., Tramacere I., Bagnardi V., Jenab M., Scotti L., Rota M., Corrao G., Garavello W., Schüz J., et al. Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: A systematic review and meta-analysis. Int. J. Cancer. 2010;129:2473–2484. doi: 10.1002/ijc.25885. [DOI] [PubMed] [Google Scholar]
- 23.Shin W., Lee H.W., Lee S. Catalytically inactive receptor tyrosine kinase PTK7 activates FGFR1 independent of FGF. FASEB J. 2019;33:12960–12971. doi: 10.1096/fj.201900932R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnas C., Martel-Planche G., Furukawa Y., Hollstein M., Montesano R., Hainaut P. Inactivation of the p53 protein in cell lines derived from human esophageal cancers. Int. J. Cancer. 1997;71:79–87. doi: 10.1002/(SICI)1097-0215(19970328)71:1<79::AID-IJC14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L., He L.-R., Xi M., Cai M.-Y., Shen J.-X., Li Q.-Q., Liao Y.-J., Qian D., Feng Z.-Z., Zeng Y.-X., et al. Nimotuzumab promotes radiosensitivity of EGFR-overexpression esophageal squamous cell carcinoma cells by upregulating IGFBP-3. J. Transl. Med. 2012;10:249. doi: 10.1186/1479-5876-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada Y., Imamura M., Wagata T., Yamaguchi N., Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69:277–284. doi: 10.1002/1097-0142(19920115)69:2<277::AID-CNCR2820690202>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H., Shibagaki I., Shimada Y., Wagata T., Imamura M., Ishizaki K. Characterization of p53 gene mutations in esophageal squamous cell carcinoma cell lines: Increased frequency and different spectrum of mutations from primary tumors. Int. J. Cancer. 1996;65:372–376. doi: 10.1002/(SICI)1097-0215(19960126)65:3<372::AID-IJC16>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Song S., Chang D., Cui Y., Hu J., Gong M., Ma K., Ding F., Liu Z.-H., Wang T.-Y. New orthotopic implantation model of human esophageal squamous cell carcinoma in athymic nude mice. Thorac. Cancer. 2014;5:417–424. doi: 10.1111/1759-7714.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo L.-L., Zhao L., Xi M., He L.-R., Shen J.-X., Li Q.-Q., Liu S.-L., Zhang P., Xie D., Liu M.-Z. Association of insulin-like growth factor-binding protein-3 with radiotherapy response and prognosis of esophageal squamous cell carcinoma. Chin. J. Cancer. 2015;34:514–521. doi: 10.1186/s40880-015-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y.J., Pan W.W., Liu S.B., Shen Z.F., Xu Y., Hu L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J., Nie J., Ma X., Wei Y., Peng Y., Wei X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer. 2019;18:1–28. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanteti R., Batra S.K., Lennon F.E., Salgia R. FAK and paxillin, two potential targets in pancreatic cancer. Oncotarget. 2016;7:31586–31601. doi: 10.18632/oncotarget.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin D.-C., Hao J.-J., Nagata Y., Xu L., Shang L., Meng X., Sato Y., Okuno Y., Varela A.M., Ding L.-W., et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat. Genet. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Testa U., Castelli G., Pelosi E. Esophageal cancer: Genomic and molecular characterization, stem cell compartment and clonal evolution. Medicines. 2017;4:67. doi: 10.3390/medicines4030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R., Li P., Xing W., Qiu H. Heterogeneous genomic aberrations in esophageal squamous cell carcinoma: A review. Am. J. Transl. Res. 2020;12:1553–1568. [PMC free article] [PubMed] [Google Scholar]
- 36.Hanawa M., Suzuki S., Dobashi Y., Yamane T., Kono K., Enomoto N., Ooi A. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int. J. Cancer. 2006;118:1173–1180. doi: 10.1002/ijc.21454. [DOI] [PubMed] [Google Scholar]
- 37.Shigaki H., Baba Y., Watanabe M., Murata A., Ishimoto T., Iwatsuki M., Iwagami S., Nosho K., Baba H. PIK3CA mutation is associated with a favorable prognosis among patients with curatively resected esophageal squamous cell carcinoma. Clin. Cancer Res. 2013;19:2451–2459. doi: 10.1158/1078-0432.CCR-12-3559. [DOI] [PubMed] [Google Scholar]
- 38.Chang D., Wang T.-Y., Li H.-C., Wei J.-C., Song J.-X. Prognostic significance of PTEN expression in esophageal squamous cell carcinoma from Linzhou City, a high incidence area of northern China. Dis. Esophagus. 2007;20:491–496. doi: 10.1111/j.1442-2050.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 39.Gao Y.-B., Chen Z.-L., Li J.-G., Hu X.-D., Shi X.-J., Sun Z.-M., Zhang F., Zhao Z.-R., Li Z.-T., Liu Z.-Y., et al. Genetic landscape of esophageal squamous cell carcinoma. Nat. Genet. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 40.Sawada G., Niida A., Uchi R., Hirata H., Shimamura T., Suzuki Y., Shiraishi Y., Chiba K., Imoto S., Takahashi Y., et al. Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology. 2016;150:1171–1182. doi: 10.1053/j.gastro.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 41.Abedi-Ardekani B., Dar N.A., Mir M.M., Zargar S.A., Lone M.M., Martel-Planche G., Villar S., Mounawar M., Saidi F., Malekzadeh R., et al. Epidermal growth factor receptor (EGFR) mutations and expression in squamous cell carcinoma of the esophagus in central Asia. BMC Cancer. 2012;12:602. doi: 10.1186/1471-2407-12-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu H., Wang C., Wang J., Chen D., Deng J., Deng J., Fan J., Badakhshi H., Huang X., Zhang L., et al. A subset of esophageal squamous cell carcinoma patient-derived xenografts respond to cetuximab, which is predicted by high EGFR expression and amplification. J. Thorac. Dis. 2018;10:5328–5338. doi: 10.21037/jtd.2018.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutton S.J., Ferry D.R., Blazeby J., Abbas H., Dahle-Smith A., Mansoor W., Thompson J., Harrison M., Chatterjee A., Falk S., et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): A phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15:894–904. doi: 10.1016/S1470-2045(14)70024-5. [DOI] [PubMed] [Google Scholar]
- 44.Wang X., Niu H., Fan Q., Lu P., Ma C., Liu W., Liu Y., Li W., Hu S., Ling Y., et al. Predictive value of EGFR overexpression and gene amplification on icotinib efficacy in patients with advanced esophageal squamous cell car-cinoma. Oncotarget. 2016;7:24744–24751. doi: 10.18632/oncotarget.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshioka M., Ohashi S., Ida T., Nakai Y., Kikuchi O., Amanuma Y., Matsubara J., Yamada A., Miyamoto S., Natsuizaka M., et al. Distinct effects of EGFR inhibitors on epithelial- and mesenchymal-like esophageal squamous cell carcinoma cells. J. Exp. Clin. Cancer Res. 2017;36:1–13. doi: 10.1186/s13046-017-0572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishihira T., Hashimoto Y., Katayama M., Mori S., Kuroki T. Molecular and cellular features of esophageal cancer cells. J. Cancer Res. Clin. Oncol. 1993;119:441–449. doi: 10.1007/BF01215923. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y., Li W., Wei B., Wu K., Liu D., Zhu D., Zhang C., Wen F., Fan Y., Zhao S. MicroRNA let-7i inhibits histone lysine demethylase KDM5B to halt esophageal cancer progression. Mol. Ther. Nucleic Acids. 2020;22:846–861. doi: 10.1016/j.omtn.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M., Hu Y., Zhang M.-F., Luo K.-J., Xie X.-Y., Wen J., Fu J.-H., Yang H. MMP1 promotes tumor growth and metastasis in esophageal squamous cell carcinoma. Cancer Lett. 2016;377:97–104. doi: 10.1016/j.canlet.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 49.Lee N.P., Chan C.M., Tung L.N., Wang H.K., Law S. Tumor xenograft animal models for esophageal squamous cell car-cinoma. J. Biomed. Sci. 2018;25:66. doi: 10.1186/s12929-018-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin W.-S., Gim J., Won S., Lee S.-T. Biphasic regulation of tumorigenesis by PTK7 expression level in esophageal squamous cell carcinoma. Sci. Rep. 2018;8:8519. doi: 10.1038/s41598-018-26957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kodama T., Koma Y.-I., Arai N., Kido A., Urakawa N., Nishio M., Shigeoka M., Yokozaki H. CCL3–CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab. Investig. 2020;100:1140–1157. doi: 10.1038/s41374-020-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang F., Zhang Y., Da J., Jia Z., Wu H., Gu K. Downregulation of SPARC expression decreases cell migration and invasion involving epithelial-mesenchymal transition through the p-FAK/p-ERK pathway in esophageal squamous cell carcinoma. J. Cancer. 2020;11:414–420. doi: 10.7150/jca.31427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L., Zhao D., Wang Y., Zhang W., Zhang J., Fan J., Zhan Q., Chen J. Focal adhesion kinase (FAK) inhibitor-defactinib suppresses the malignant progression of human esophageal squamous cell carcinoma (ESCC) cells via effective blockade of PI3K/AKT axis and downstream molecular network. Mol. Carcinog. 2021;60:113–124. doi: 10.1002/mc.23273. [DOI] [PubMed] [Google Scholar]
- 54.Luo H., Quan J., Xiao H., Luo J., Zhang Q., Pi G., Ye Y., He R., Liu Y., Su X., et al. FGFR inhibitor AZD4547 can enhance sensitivity of esophageal squamous cell carcinoma cells with epithelial-mesenchymal transition to gefitinib. Oncol. Rep. 2018;39:2270–2278. doi: 10.3892/or.2018.6304. [DOI] [PubMed] [Google Scholar]
- 55.Cui N.-P., Qiao S., Jiang S., Hu J.-L., Wang T.-T., Liu W.-W., Qin Y., Wang Y.-N., Zheng L.-S., Zhang J.-C., et al. Protein tyrosine kinase 7 regulates EGFR/Akt signaling pathway and correlates with malignant progression in triple-negative breast cancer. Front. Oncol. 2021;11:699889. doi: 10.3389/fonc.2021.699889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Na H.W., Shin W.S., Ludwig A., Lee S.T. The cytosolic domain of PTK7, generated from sequential cleavage by ADAM17 and gamma-secretase, enhances cell proliferation and migration in colon cancer cells. J. Biol. Chem. 2012;287:25001–25009. doi: 10.1074/jbc.M112.348904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin W.-S., Maeng Y.-S., Jung J.-W., Min J.-K., Kwon Y.-G., Lee S.-T. Soluble PTK7 inhibits tube formation, migration, and invasion of endothelial cells and angiogenesis. Biochem. Biophys. Res. Commun. 2008;371:793–798. doi: 10.1016/j.bbrc.2008.04.168. [DOI] [PubMed] [Google Scholar]
- 58.Lee Y.H., Seo E.K., Lee S.-T. Skullcapflavone II inhibits degradation of type I collagen by suppressing MMP-1 transcription in human skin fibroblasts. Int. J. Mol. Sci. 2019;20:2734. doi: 10.3390/ijms20112734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin W.-S., Shim H.J., Lee Y.H., Pyo M., Park J.S., Ahn S.Y., Lee S.-T. PTK6 localized at the plasma membrane promotes cell proliferation and migration through phosphorylation of Eps8. J. Cell. Biochem. 2017;118:2887–2895. doi: 10.1002/jcb.25939. [DOI] [PubMed] [Google Scholar]
- 60.Shin W., Park M.K., Lee Y.H., Kim K.W., Lee H., Lee S. The catalytically defective receptor protein tyrosine kinase EphA10 promotes tumorigenesis in pancreatic cancer cells. Cancer Sci. 2020;111:3292–3302. doi: 10.1111/cas.14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.