Abstract
Objective
The present study investigated the role of miR-181a as a small non-coding RNA molecule in acute myeloid leukemia (AML) pathogenesis and reflected on the effects of Sulforaphane (SFN) on AML progression.
Materials and Methods
This experimental study had two parts. In vivo study, the miR-181a levels was measured in patients with symptoms of AML and compared to healthy controls (HCs) to investigate its role in AML pathogenesis. Afterward, an in vitro study was performed to examine the effects of SFN on the growth, apoptosis and proliferation rate of AML cell lines. Finally, the effect of SFN on miR-181a was evaluated as a major miRNA involved in hematopoiesis.
Results
The results of this study showed an increasing trend (2.9-fold, P=0.0019) in miR-181a expression levels in AML patients as compared with HCs. The data associated with MTT assay and flow cytometry (FCM) additionally demonstrated the anti-proliferative effects of SFN against AML cell lines, with a reduction in miR-181a levels. As well, no significant difference was noted between 24 hours and 48 hours treatments by SFN. It was deduced that modulation of miR-181a expression levels could be one of the mechanisms associated with the anti-proliferative effects of SFN against AML.
Conclusion
MiR-181a levels contribute to AML pathogenesis and thus they can be considered as a strategy in controlling AML progression in patients. Accordingly, SFN can arrest cell proliferation and induce apoptosis in AML cell lines through retardation expression of miR-181a and affecting miR-181a pathway, which already clarified its role in the differentiation of hematopoietic stem cells and indicates another mode of anti-cancer action of sulforaphane.
Keywords: Acute Myeloid Leukemia, miR-181a, Sulforaphane
Introduction
Acute myeloid leukemia (AML) is known as an invasive malignancy of the precursor cells of the blood (1-3). It is also the most common type of cancer resulting from genetic mutations, which can disturb differentiation and apoptosis (4, 5). Disturbance in molecular pathways as well as regulation by some biomarkers such as microRNAs (miRNAs), is thus one cause of this disease (6). These sections or transcripts are referred to as non-coding RNAs, with a length of 19-25 nucleotides, playing several roles in the cells produced by RNA polymerase II or III (7).
MiRNAs show different physiological behaviors and pathological conditions. In addition to tumor suppressor genes, oncogenes, and chromosomal changes, considered to be the main causes of cancer, miRNAs can also play a role the in cancer process (2, 8). Evidence suggests that miRNAs can be diagnostic and therapeutic biomarkers due to their role in pathogens. Studies have further demonstrated the key role of miR-181a in the maintenance of cells and their pathological effects in hematopoietic neoplasms (9). Moreover, miR-181a can directly inhibit the differentiation of granulocytes and quasi-macrophages by protein kinase C delta (PRKCD), calcium/calmodulin-dependent protein kinase kinase 1 (CAMKK1), and CTD small phosphatase-like (CTDSPL) mRNAs. PRKCD is also required for phorbol myristate acetate (PMA) or all-trans retinoic acid (ATRA) induction for the differentiation of myeloid leukemia, and it is a regulator of p38 upstream, which can lead to the phosphorylation of p38 (10).
Today, with the growing use of natural antioxidant compounds, their beneficial effects are being examined by many clinical studies. In addition to the importance of auxiliary compounds (including antioxidants) in reducing chemotherapy side effects, they may be also important in cellular pathways and have an effective new role in cancer treatment strategies (11). Sulforaphane (SFN), as a natural antioxidant, is an alternative product derived from the metabolism of cabbage, and its photochemical activity is high (12). The anti-cancer effects of isothiocyanate in cabbage are attributed to the N=C=S group. These vegetables are rich in SFN, and are respected as anti-cancer drugs (13).
SFN can reduce existing leukemia cells by arresting the G/M phase in the cell cycle and induction of apoptosis. through escalating reactive oxygen species (ROS) and calcium ions (Ca2+) as well as activating some caspase proteins (14). In addition, in previous studies, SFN has been reported to moderate the development and severity of AML by controlling some miRNAs by altering their expressions (2).
Some studies have further shown that SFN has various protective functions against cell damage and tumor processes (15). Overall, SFN has an inhibitory effect on the growth and development of tumors. In addition, SFN can affect the epigenetic control of some key cancer genes resulting in reducing the progression and recurrence of cancer and thus improve patient survival (16). Although many studies have investigated the anti-cancer activities of this antioxidant (17, 18), few investigations have so far examined the effects of SFN on leukemia cells and miRNA expression levels (2). The main objective of this study was to evaluate the effects of SFN on the expression levels of miR-181a in AML cell lines, and AML patients and healthy individuals. The results of the present study may illustrate a new function for the anti-cancer potential of SFN.
Materials and Methods
Human specimens
The experimental part of this research (in vivo study) was to evaluate the pathologically changes in level of miR-181a in AML patients. For this purpose, the blood samples of the cases infected with AML were gained from the Hematology and Oncology Department of Shahid Mohammadi Hospital based in the city of Bandar Abbas. The study was confirmed by the Ethics and Human Rights Committee affiliated with Hormozgan University of Medical Sciences (HUMS), Bandar Abbas, Iran (HUMS.REC.1394.84). The informed consent form was distributed and signed by all the subjects before their participation.
Cell-lines
Based on numerous previous studies and upon determining the features of each cell line (16), four well-known AML cell lines were selected for in vitro part of our study. The selected sell lines are mostly promyelocytes and poorly differentiated cells. The selected AML cell lines were U937 (AML-M4/ M5 monoblasts) (NCBI code, C130), HL-60 (AML-M2 myeloblasts) (NCBI code, C217), NB-4 (AML-M3 promyelocytes) (NCBI code, C 515), and KG-1 (myeloblast or promyelocyte stage of maturation) (NCBI code, C119), obtained from Pasteur Institute of Iran (http://pasteur.ac.ir). Less than 12 hours after being obtained, each of the four cell lines was passaged in the Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen, Germany), consisting of 10% fetal bovine serum (FBS, Gibco, Germany) and 1% penicillin-streptomycin (pen-strep) solution (Invitrogen, Germany), maintained at 95% humidity, a temperature of 37˚C, and 5% carbon dioxide (CO2). The growth rate was calculated in base of the No. of viable cells [((The No. of viable cells each time of measuring *100)/The No. of viable cells in previous measuring)-100] and stated in percentage.
Cell proliferation assay
The effects of D, L-SFN (S4441) on the proliferation of the AML cell lines were evaluated by MTT (3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, USA) assay.
To perform the MTT assay, the number of U-937, NB-4, HL-60, as well as KG-1 cells were counted by a hemocytometer, and then they were cultured in a 96- well plate (U-937: 8000; NB-4: 20000; HL-60: 15000; KG-1: 10000 cells in base of IC50), and incubated for 2 hours under standard conditions. According to previous studies (2, 19), 15, 30, 45, and 60 µM concentrations of SFN (Sigma-Aldrich, USA) were selected . However, the given doses were evaluated independently and their toxicity was assessed according to the half-maximal inhibitory concentration (IC50) measurement. Then, the cells were treated with the selected SFN concentration for 24 and 48 hours. After incubation for 24 hours, 20 μl of MTT solution was added at the final concentration of 0.5 mg/ml to each well and incubated at 37˚C for 4 hours (2). They were subsequently centrifuged for 10 minutes at 4000 g and the crystals formed were dissolved in 150 μl dimethyl sulfoxide (DMSO). Finally, absorption of each well was measured at 495 nm using a micro-plate reader (Anthos 2020, England).
RNA extraction
RNA was extracted from the blood samples with ZR Whole Blood RNA MiniPrep™ kit (ZYMO RESEARCH, Irvine, CA), and then exposed to miScript II RT Kit (Qiagen, Germany) to synthesize cDNA followed by a real-time polymerase chain reaction (RT-PCR) (miScript SYBR Green PCR kit from Qiagen, Germany). To determine the total RNA of the cell lines after an interval (24 hours and 48 hours), TriPure Isolation Reagent (Roche, Germany) kit was applied as per the manufacturer’s instructions.
RNA quantity and quality analysis
A NanoDrop® ND-1000 Spectrophotometer (Thermo Scientific, Germany) was used to determine the purity and the quantity of RNA and cDNA. It should be noted that pure RNA has a photoconductivity ratio of 280/260, 1.9 to 2 times, which could refer to suitable purity of extracted total RNAs. To evaluate quality, the extracted RNAs were run on 1% agarose gel. The quality of the extracted RNA is considered acceptable when the gel had three bands of 28, 18, and 5S, and the intensity of 28S band was two times higher than that of the 18 s band. All of the extracted samples had good quality in this research.
Real time-polymerase chain reaction
To detect the miR-181a expression levels in AML cell lines, the entire extracted RNA of the cell lines and the patients were exposed to miScript II RT Kit (Qiagen, Germany) for cDNA synthesis. Then, 1 ng of this extracted RNA was used to prepare the reverse transcription reaction mixer with a volume of 20 μl. The cDNA samples were subsequently stored at -20˚C until use. The miR-181a and the RNU6 (as housekeeping) primers were also utilized from Qiagen (Cat.No. MS00008827 for miR-181a and MS00033740 for RNU6). RT-PCR was further used and the miScript SYBR® Green PCR Kit (Qiagen, Germany) was applied in accordance with the company’s directions. Briefly, the RT-PCR program was as follows: the primary shock was at 95˚C for 5 minutes accompanied by 40 cycles at 94˚C for 15 s (denaturation), at 55˚C for 30 s (annealing), and finally 70˚C for 30 seconds (extension) (2). After amplification, the relationship between miR-181a expression levels was obtained by dividing the miR-181a mRNA values by the U-6 gene mRNA values in each sample. Each experiment was repeated at least two times and done on three repeats for each time.
Apoptosis analysis
The fluorescein isothiocyanate (FITC) Annexin-V kit (Cat No. 640922, BioLegend, UK) was utilized to measure the effects of SFN on cell apoptosis. The two AML cell lines, KG-1 and HL-60, were evaluated after being treated with SFN.
The doses of SFN used in this study (15, 30, 45, and 60 μM) were evaluated over two time intervals (24 hours and 48 hours). The cells were collected and suspended in 2 ml phosphate-buffered saline (PBS, Cat No. 10010049, Gibco, Thermo Fisher Scientific, Inc.). After that according to manufactures’ instructions, FITC (5 µL), Annexin V (5 µL), and 7-AAD (10 µL) were added to cell suspension (900 μl) in each tube, and incubated in the dark condition for 15 min at room temperature (25˚C). Then, 300 μl of the binding buffer was added to each tube, and analysis was performed by flow cytometry (FACS, Becton Dickinson, Franklin Lakes, NJ, USA). The Flow Jo software was used to analyze the data.
Statistical analysis
The IBM SPSS Statistics software (version 21, IMB, USA), Microsoft Excel software and GraphPad Prism-5 were used for statistical analyses and P≤0.05 were considered to be statistically significant. Kolmogorov-Smirnov test was applied to check data distribution and normality. Expression of miR-181a was evaluated by 2-∆∆Ct. Accordingly, Wilcoxon two-sample test, Mann-Whitney U test, and finally Student’s t test were utilized for detecting significant differences between expression levels.
Results
Demographic results
The patients and the controls were matched in terms of gender, age, and ethnicity as much as possible (2) (Table 1).
Table 1.
Demographic characteristics of AML patients and HC groups
|
| |||
|---|---|---|---|
| Variables | AML patient (n=25) | Healthy control (n=25) | P value |
|
| |||
| Age (Min-Max) | 52.5 (± 22.51) (3-79) | 52.4 (± 22.40) (4-77) | 0.981 |
| ≤50 | 12 (48) | 12 (48) | |
| >50 | 13(52) | 13(52) | |
| Gender | 0.642 | ||
| Female | 10 (40) | 10 (40) | |
| Male | 15 (60) | 15 (60) | |
| % blast cells (Min-Max) | 71.2% (± 20.8) (28-94) | 0 | |
| % Blast ≤70 | 9 (36.0) | 0 | |
| % Blast >70 | 16 (64.0) | 0 | |
| AML stage | |||
| M1 | 4 (16.0) | 0 | |
| M2 | 12 (48.0) | 0 | |
| M4 | 5 (20.0) | 0 | |
| M5 | 5 (20.0) | 0 | |
|
| |||
Data are presented as mean ± SD or n (%). AML; Acute myeloid leukemia and HC; Healthy control.
Cytotoxic effects of SFN on various cell types
The effects of SFN on the growth rate were evaluated by incubation of AML cell lines with various its concentrations for 24 hours and 48 hours. Results were shown as changes in percentages as compared with non-treated cells (SFN concentration was zero), that were randomly assigned as 100% viability.
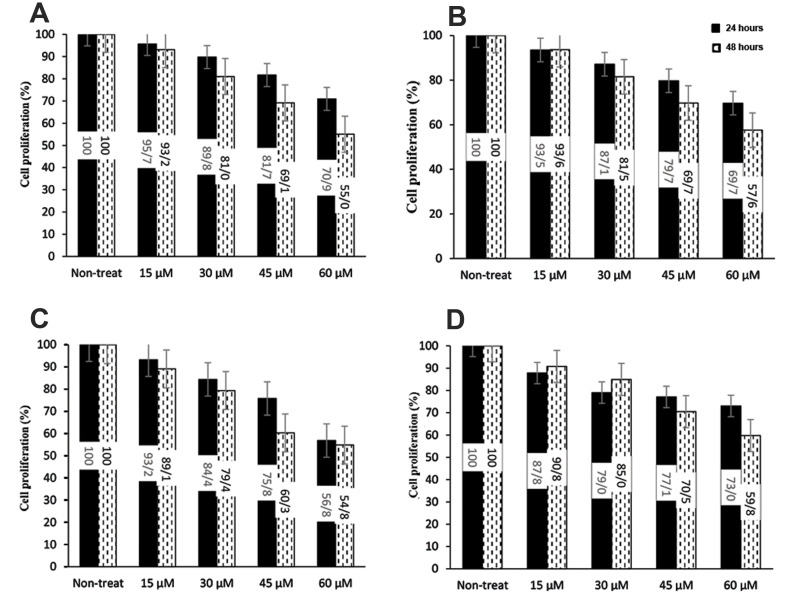
Quantification of the number of cells in all cell lines treated with SFN revealed that the cell viability decreased at higher concentrations of SFN in both tested time points (24 and 48 hours).The growth rate of the cell lines also declined in plates treated with SFN, indicating an inverse relationship between SFN concentration and the growth rate of the cells. The highest and the lowest number of living cells were observed at 15 and 60 μM SFN; respectively. The number of dead cells correspondingly increased as SFN concentration multiplied (Fig .1).
Fig.1.
Effects of concentration and exposure duration of SFN on growth rates of AML cell lines. A. HL60, B. KG-1, C. U-937, and D. NB-4 were evaluated. Each datum was expressed as changes in the percentages of treated cells as compared with non-treated ones. The data were obtained from the number of viable cells based on the MTT assay. The Y-axis shows cell proliferation (changes in percentages of HC group). SFN; Sulforaphane, AML; Acute myeloid leukemia, and HC; Healthy controls.
Apoptosis assays of different SFN concentrations for KG-1 and HL-60 cell-lines
To evaluate the effects of SFN on inducing apoptosis, HL-60 and KG-1 cell lines (representing aggressive and mild AML cell lines, respectively) were treated with different SFN concentrations (15, 30, 45, and 60 μM) for 48 hours. The HC groups (non-treated cells) and cell lines treated with doxorubicin (DOX) (as an anti-cancerous apoptotic agent) were also considered as the negative and positive controls; respectively. The results showed that treatment of HL-60 and KG-1 cells with 60 μM of SFN could induce the highest apoptosis rate (17.9% and 46.6%, respectively), and the lowest apoptosis rate had similarly occurred when HL-60 (4.5%) and KG-1 cells (6.7%) were treated by 15 μM SFN.
Respectively, 12.3 and 5.6 % of cells were engaged in early and late apoptosis at the presence of 60 μM SFN. The lowest initial apoptosis rate (3%) was observed in the treatment with 15 and 30μM SFN (Fig .2) in the KG-1 cell-line. The apoptosis percentage at different doses of SFN demonstrated that the highest apoptosis rate appeared at a dose of 60 μM (46.6%), followed by 45, 30, and 15 μM, showing the highest apoptosis rate with ratios of 20.7%, 10.58%, and 6.59%; respectively. The highest early (26.3%) and late (20.3%) apoptosis rates also happened at 60 μM SFN, and the lowest early (4.7%) and late apoptosis (1.9%) rates were arisen at 15 μM SFN, respectively, for KG-1 cell-lines. The necrosis rate at the mentioned doses was roughly low. Increasing early apoptosis converged with rising doses of SFN in both HL-60 and KG-1 cell lines indicated the anti-proliferative effects of SFN.
Fig.2.
Effects of different concentrations of sulforaphane to induce apoptosis in cell lines after 48 hours treatment. The obtained data of flow-cytometry in cell lines A. HL60 and B. KG1 were shown.
Disruption of MiR-181a expression level in human samples
Comparison of miR-181a expression levels between patients and HC samples showed that miR-181a expression levels were considerably enhanced (Fig .3A) in the patients (P=0.0019). In the M1 stage of the disease, miR-181a expression level in the patient group were increased 2.21- fold and in the M2 stage, 2.9-fold that of the HC group, whereas, no significant changes were observed (M4=0.94 and M5=1.03) in higher stages in comparison with HCs. In other words, gene expression levels were more greatly increased in the first stages (i.e., M1 and M2) than in the higher stages (namely, M3 and M4). This difference of changes in miR-181a expression levels between lower stages (M1 and M2) in comparison with higher stages (M3 and M4) was significant (P=0.0318, Fig .3B).
Fig.3.
The level of miR-181a in blood of patients suffered from AML compared to HC. A. Comparison with AML patients and HCs and B. Comaprison between lower sub-types (AML and M1+M2) and higher sub-types (AML and M3+M4). AML; Acute myeloid leukemia, HC; Healthy control, and M1-M4; Subtypes of acute myeloid leukemia 1 to 4.
Reduction of MiR-181a expression levels in different cell lines by increasing concentration and duration of treatment with SFN
Our data demonstrated that miR-181a expression levels significantly reduced in all treated cell lines compared with the untreated ones (Fig .4). The decline in miR-181a expression levels was also observed following a rise in SFN concentrations in the treated cells. Moreover, miR-181a expression levels gradually diminished during the 24 and 48 hours intervals, though the decline was much more pronounced at the end of the 24 hours period than the 48 hours. At the end of 24 hours, miR-181a expression levels decreased by 0.96-, 0.64-, and 0.41-folds in the HL-60 cell-lines treated with 15, 30, and 45 µM of SFN; respectively.
Fig.4.
The level of miR-181a under 24 and 48 hours treatments with various SFN doses compared in all four studied AML cell lines A. HL60, B. KG-1, C. U-937 and D. NB-4. Y-axis represents relative expression (2-ΔΔCt). NT0; Zero concentration of SFN, SFN; Sulforaphane, and AML; Acute myeloid leukemia.
This pattern was 0.49-, 0.26-, and 0.06-folds for the KG-1 cell line; 0.66-, 0.46-, and 0.11-folds for the U-937 cell line; and 0.26-, 0.16-, and 0.12-folds for the NB4 cell line (P<0.001); respectively. The same descending trend in miR-181a expression level was further observed after 48h treatment with different concentrations of SFN, but no significant difference was notable between 24 and 48 hours treatments (Fig .4). At the end of the 48 hours treatment with 15, 30 and, 45 µM of SFN, the miR-181a expression levels decreased by 0.87-, 0.38-, and 0.11-folds for HL-60. As well the given levels reduced by 0.53-, 0.22-, and 0.03-folds for the KG1 cell line; 0.51-, 0.22-, and 0.09-folds for the U-937 cell line; and 0.31-, 0.21-, and 0.15-folds for the NB-4 cell-line; respectively (P<0.001, Fig .4).
Discussion
AML is caused by the accumulation of myeloid precursors in the bone marrow for a variety of reasons. In addition to recent routine treatments, some complementary therapies have been thus far suggested. Some of the advocated compounds are antioxidant-based supplements that may eliminate several side effects of chemical treatments. One of these compounds is the antioxidant SFN, whose effects have been studied in cancers including apoptosis, angiogenesis, and metastasis, chromatin structure and DNA stability, cell cycle and checkpoints, inflammation and oxidative stress, and cell signaling (20). Mechanisms associated with AML are still being debated.
MiRNAs can significantly contribute to AML and consequently control the differentiation of hematopoietic and tumor cells. On the other hand, any disturbance in their expression levels can cause hematopoietic malignancies, including AML (21-23). In this respect, Liu et al. (24) have revealed that miR-181a expression levels increased in AML patients, accompanied by a rise in proliferation in AML cell lines as well as regulation of transition from stage S to G1 by this miRNA. It has been further shown that miR-181a leads to inhibition of macrophage-like and granulocyte differentiation in HL-60 cells (10). What has been reported in a previous study was the relationship between high expression levels of miR-181a and higher French-American-British (FAB) system percentage of AML M1/M2, higher blood blasts, and better treatment responses (25).
In the present research, miR-181a expression levels were first assessed in vivo and AML patients and HC samples. Our findings suggested that SFN has anti-cancer effects. In addition, a few investigations had been conducted on the effects of SFN on the expression levels. Therefore, the present study aimed to reflect on the effects of SFN on miR-181a expression levels in AML cell lines. According to this study, expression levels in patients with AML samples were significantly higher than those in HC.
The results of the present study were consistent with some previous studies (9, 26). Debernardi et al. (26) have highlighted an association between miR-181a and AML subtypes. Moreover, miR-181a expression levels were higher in AML-M1 or AML-M2 compared with samples with the above morphology, AML-M4 or AML-M5. The miR-181a expression levels were further high in AML subtypes. Nonetheless, overexpression of miR-181a in AML cells could lead to inhibition of cell growth and metabolic activity (9).
Here we showed that the antioxidant SFN increased mortality in AML cells and even apoptosis. Where the growth rate and the number of AML cells diminished as concentration and exposure time of SFN increased. Koolivand et al. (2) have previously reported that SFN use could reduce miRNA expression levels. They found that miR-155 expression levels could be dependent on SFN doses. In another study, Suppipat et al. (27) demonstrated that SFN in leukemic cells could initiate apoptosis and arrest of the cell cycle in G2/M by activating caspases (3, 8, and 9) and inactivating poly ADP-ribose polymerase (PARP). SFN has been also indicated to induce toxicity to HL-60 cells (28). Other studies also reported that SFN act as a potent compound to arrest cell-cycle and induce apoptosis in cancer cells and suggested its contributing factor to combination cancer therapy (29, 30).
In this study, significant pathological changes of miR-181a were observed in AML patients that is likely involved in AML progression. Increasing apoptosis of AML cell lines was also accompanied by decreasing miR-181a expression levels. Higher miR-181a expression levels were interestingly observed at the presence of higher concentration of SFN and longer exposure time to it.
It can be deduced that control of miR-181a expression levels is probably one of the goals associated with SFN use for its anti-proliferative effects against AML. Analyzing the anti-leukemia activity of miR-181a in AML, Huang et al. (31), have also found that suppressing miR-181a expression levels by anti-miR-181a could activate certain pathways such as Kirsten rat sarcoma 2 viral oncogene homolog) gene (KRAS), NRAS proto-oncogene, GTPase (NRAS), and mitogen-activated protein kinase 1 (MAPK1). This property indicated that miR-181a had tumor-suppressing and oncogenic functions.
To conclude, our results show that miR-181a can be affected by SFN. It seems that SFN may have inhibitory effects on AML progression by altering miR-181a expression levels. However, further studies are needed in the future to estimate the contribution of the changes in miR-181a expression levels with relative improvements in AML patients’ conditions.
Conclusion
MiR-181a can be suggested as a candidate prognostic marker for the evaluation of AML progression as well as the effectiveness of treatments. In this study, the anti-proliferative effects of SFN against AML cells were also observed, which converged with decreasing levels of miR-181a. Also, it seems that SFN induced its anti-cancer effects against AML cell lines through the miR-181a pathway involved in the differentiation of hematopoietic stem cells.
Acknowledgements
The authors express their appreciation to Hormozgan University of Medical Sciences (HUMS) for providing Grant No. 94-07-0480. This manuscript was based on the results of a Master’s thesis fulfilled by Mohsen Koolivand. The authors hereby declared no conflict of interest.
Authors’ Contributions
M.K.; Investigation, sample collection, Lab. work and experimental work, writing-original draft, and editing. M.A.; Sample collection, Lab. work and experimental work. S.M.; Supervision, data curation, and writing-original draft. M.A.; Data curation and writing-original draft. K.M.; Conceptualization, data curation and analyzing, investigation, project administration, supervision, writing-original draft, writing review and editing. All authors read and approved the final manuscript.
References
- 1.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Koolivand M, Ansari M, Piroozian F, Moein S, MalekZadeh K. Alleviating the progression of acute myeloid leukemia (AML) by sulforaphane through controlling miR-155 levels. Mol Biol Rep. 2018;45(6):2491–2499. doi: 10.1007/s11033-018-4416-0. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Xu X, Liu Y, Gong A, Wang D, Liao X, et al. Advances in acute myeloid leukemia stem cells.Advances in hematologic malignancies. IntechOpen; 2019. [Google Scholar]
- 4.Koolivand M, Moein S, MalekZadeh K. The relationship of miR- 181a expression level and AML: a systematic review protocol. Int J Surg Protoc. 2019;13:1–4. doi: 10.1016/j.isjp.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Pediatr Clin North Am. 2008;55(1):21-51, ix. doi: 10.1016/j.pcl.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Hickey CJ, Schwind S, Radomska HS, Dorrance AM, Santhanam R, Mishra A, et al. Lenalidomide-mediated enhanced translation of C/EBPα-p30 protein up-regulates expression of the antileukemic microRNA-181a in acute myeloid leukemia. Blood. 2013;121(1):159–169. doi: 10.1182/blood-2012-05-428573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berindan‐Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. Micro- RNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64(5):311–336. doi: 10.3322/caac.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Dahlhaus M, Schult C, Lange S, Freund M, Junghanss C. Micro- RNA 181a influences the expression of HMGB1 and CD4 in acute Leukemias. Anticancer Res. 2013;33(2):445–452. [PubMed] [Google Scholar]
- 10.Su R, Lin HS, Zhang XH, Yin XL, Ning HM, Liu B, et al. MiR-181 family: regulators of myeloid differentiation and acute myeloid leukemia as well as potential therapeutic targets. Oncogene. 2015;34(25):3226–3239. doi: 10.1038/onc.2014.274. [DOI] [PubMed] [Google Scholar]
- 11.Mut-Salud N, Álvarez PJ, Garrido JM, Carrasco E, Aránega A, Rodríguez- Serrano F. Antioxidant intake and antitumor therapy: toward nutritional recommendations for optimal results. Oxid Med Cell Longev. 2016;2016:6719534–6719534. doi: 10.1155/2016/6719534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amjad AI, Parikh RA, Appleman LJ, Hahm ER, Singh K, Singh SV. Broccoli-derived sulforaphane and chemoprevention of prostate cancer: from bench to bedside. Curr Pharmacol Rep. 2015;1(6):382–390. doi: 10.1007/s40495-015-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33(10):1833–1842. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang HS, Shih YL, Lee CH, Hsueh SC, Liu JY, Liao NC, et al. Sulforaphane‐induced apoptosis in human leukemia HL‐60 cells through extrinsic and intrinsic signal pathways and altering associated genes expression assayed by c DNA microarray. Environ Toxicol. 2017;32(1):311–328. doi: 10.1002/tox.22237. [DOI] [PubMed] [Google Scholar]
- 15.Pham N-A, Jacobberger JW, Schimmer AD, Cao P, Gronda M, Hedley DW. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol Cancer Ther. 2004;3(10):1239–1248. [PubMed] [Google Scholar]
- 16.Jiang X, Liu Y, Ma L, Ji R, Qu Y, Xin Y, et al. Chemopreventive activity of sulforaphane. Drug Des Devel Ther. 2018;12:2905–2913. doi: 10.2147/DDDT.S100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnett JP, Lim G, Li Y, Shah RB, Lim R, Paholak HJ, et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017;394:52–64. doi: 10.1016/j.canlet.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mielczarek L, Krug P, Mazur M, Milczarek M, Chilmonczyk Z, Wiktorska K. In the triple-negative breast cancer MDA-MB-231 cell line, sulforaphane enhances the intracellular accumulation and anticancer action of doxorubicin encapsulated in liposomes. Int J Pharm. 2019;558:311–318. doi: 10.1016/j.ijpharm.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Zhou Y, Yang G, Tian H, Geng Y, Hu Y, et al. Sulforaphanecysteine induces apoptosis by sustained activation of ERK1/2 and caspase 3 in human glioblastoma U373MG and U87MG cells. Oncol Rep. 2017;37(5):2829–2838. doi: 10.3892/or.2017.5562. [DOI] [PubMed] [Google Scholar]
- 20.Jabbarzadeh Kaboli P, Afzalipour Khoshkbejari M, Mohammadi M, Abiri A, Mokhtarian R, Vazifemand R, et al. Targets and mechanisms of sulforaphane derivatives obtained from cruciferous plants with special focus on breast cancer-contradictory effects and future perspectives. Biomed Pharmacother. 2020;121:109635–109635. doi: 10.1016/j.biopha.2019.109635. [DOI] [PubMed] [Google Scholar]
- 21.Liao Q, Wang B, Li X, Jiang G. miRNAs in acute myeloid leukemia. Oncotarget. 2017;8(2):3666–3682. doi: 10.18632/oncotarget.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaur V, Chaudhary S, Tyagi A, Agarwal S, Sharawat SK, Sarkar S, et al. Dysregulation of miRNA expression and their prognostic significance in paediatric cytogenetically normal acute myeloid leukaemia. Br J Haematol. 2020;188(6):e90–e94. doi: 10.1111/bjh.16375. [DOI] [PubMed] [Google Scholar]
- 23.Mafra ACP, Calin GA. MicroRNAs in haematological diseases.In Peplow PV, Martinez B, Calin GA, Esquela-Kerscher A, editors.MicroRNAs in diseases and disorders: emerging therapeutic targets. UK: Royal Society of Chemistry; 2019. pp. 293–322. [Google Scholar]
- 24.Liu X, Liao W, Peng H, Luo X, Luo Z, Jiang H, et al. miR-181a promotes G1/S transition and cell proliferation in pediatric acute myeloid leukemia by targeting ATM. J Cancer Res Clin Oncol. 2016;142(1):77–87. doi: 10.1007/s00432-015-1995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwind S, Marcucci G, Maharry K, Radmacher MD, Whitman SP, Paschka P, et al. MicroRNA 181a (miR-181a) expression as a prognosticator in cytogenetically normal acute myeloid leukemia (CN AML). J Clin Oncol. 2009;27(15 suppl):7001–7001. [Google Scholar]
- 26.Debernardi S, Skoulakis S, Molloy G, Chaplin T, Dixon-McIver A, Young BD. MicroRNA miR-181a correlates with morphological sub-class of acute myeloid leukaemia and the expression of its target genes in global genome-wide analysis. Leukemia. 2007;21(5):912–916. doi: 10.1038/sj.leu.2404605. [DOI] [PubMed] [Google Scholar]
- 27.Suppipat K, Park CS, Shen Y, Zhu X, Lacorazza HD. Sulforaphane induces cell cycle arrest and apoptosis in acute lymphoblastic leukemia cells.PLoS One. PLoS One; 2012. pp. e51251–e51251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehner K, Feliciano O, O’Donnell RW. Using carboxyfluorescein succinimidyl ester (CFSE) to measure the effects of sulforaphane on cell division in a human leukemia cell line. FASEB J. 2013;27(Suppl 1):1028–1028. [Google Scholar]
- 29.Chen H, Landen CN, Li Y, Alvarez RD, Tollefsbol TO. Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp Cell Res. 2013;319(5):697–706. doi: 10.1016/j.yexcr.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YH. ROS-mediated activation of AMPK plays a critical role in sulforaphane-induced apoptosis and mitotic arrest in AGS human gastric cancer cells. Gen Physiol Biophys. 2018;37(2):129–140. doi: 10.4149/gpb_2017026. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, Schwind S, Santhanam R, Eisfeld AK, Chiang CL, Lankenau M, et al. Targeting the RAS/MAPK pathway with miR-181a in acute myeloid leukemia. Oncotarget. 2016;7(37):59273–59286. doi: 10.18632/oncotarget.11150. [DOI] [PMC free article] [PubMed] [Google Scholar]