Abstract
Whether there is an association between dietary quality and sleep disorder in American adults is unclear. We conducted this study to analyze whether dietary quality, using the Healthy Eating Index-2015 (HEI-2015) scores as the measure, was associated with self-reported sleep disorders. Data came from the National Health and Nutrition Examination Survey (2005–2014). Step-weighted logistic regression models were performed to explore the relationships between the HEI-2015 scores and sleep disorder. Weighted quantile sum regression model was used to identify the HEI-2015 components most strongly associated with sleep disorders. According to quartiles, HEI scores were categorized into inadequate (<25%), average (25%–75%), and optimal (>75%). Compared to inadequate HEI status, average HEI status (OR: 0.961, 95%CI: 0.959–0.962) and optimal HEI status (OR: 0.913, 95% CI: 0.912–0.915) were associated with reduced risk of sleep disorder after multivariable adjustments. Greens and beans, added sugars, saturated fats, total vegetables and total protein foods were the top five important components for sleep disorders. Our results suggest that there is a statistically significant association between better dietary quality and reduced risk of sleep disorder among United States adults.
Keywords: sleep disorder, dietary quality, healthy eating index
1. Introduction
Sleep disorders are a group of syndromes characterized by disturbance in the patient’s amount of sleep, quality or timing of sleep, or in behaviors or physiological conditions associated with sleep. Insomnia, sleep apnea, and circadian rhythm sleep–wake disorders are typical sleep disorders. More than one-third of adults experience transient insomnia at some point in their life, and circadian rhythm sleep–wake disorders are common [1]. In the general middle-aged population, moderate-to-severe sleep apnea can be found in about 30–50% of men and 11–23% of women [2,3].
Sleep disorder is an important public health issue that has been confirmed to be associated with many adverse health outcomes, such as obesity [4], hypertension [4], cardiovascular disease [4,5], premature mortality [6,7,8], and impaired quality of life [9].
Many studies have confirmed that nutrients, such as macronutrients, vitamins, etc., are related to sleep. For example, intake of fats was negatively associated with total sleep time [10], vitamin D deficiency was associated with a higher risk of sleep disorders [11], and vitamin C and selenium were found to be related to sleep duration [12]. In addition, high carbohydrate diets were suggested to improve sleep outcomes by the previous review [13]. However, the impact of overall diet quality is inconclusive because the health outcome of diet is often driven by the joint effect of all dietary intakes. For example, no associations between dietary scores and sleep parameters were found in Swedish older men [14]. American adults with higher energy-adjusted dietary inflammatory index were more likely to report sleep disturbances [15]. Moreover, the previous review pointed out that no sufficient evidence can support the conclusion that it is possible to improve sleep quality through interventions on dietary habits or on individual components of the diet [16].
The Healthy Eating Index-2015 (HEI-2015) was designed by the United States Department of Agriculture to measure how well a set of foods aligns with key recommendations of the 2015–2020 Dietary Guidelines for Americans.
We aim to explore the effect of overall diet quality in a more macroscopic way, using HEI-2015 as the measure, to determine whether general American adults with sleep disorders can benefit from adherence to American dietary guidelines.
2. Materials and Methods
2.1. Data Source
The participants who engaged in the 2005–2014 National Health and Nutrition Examination Survey (NHANES) were used in this study. NHANES, a major program of the National Center for Health Statistics (NCHS), is designed to assess the health and nutritional status of adults and children in the United States. The survey combined interviews and physical examinations, having high representation in the US population. More information can be found at https://www.cdc.gov/nchs/nhanes/ (accessed on 24 July 2021).
2.2. Participants
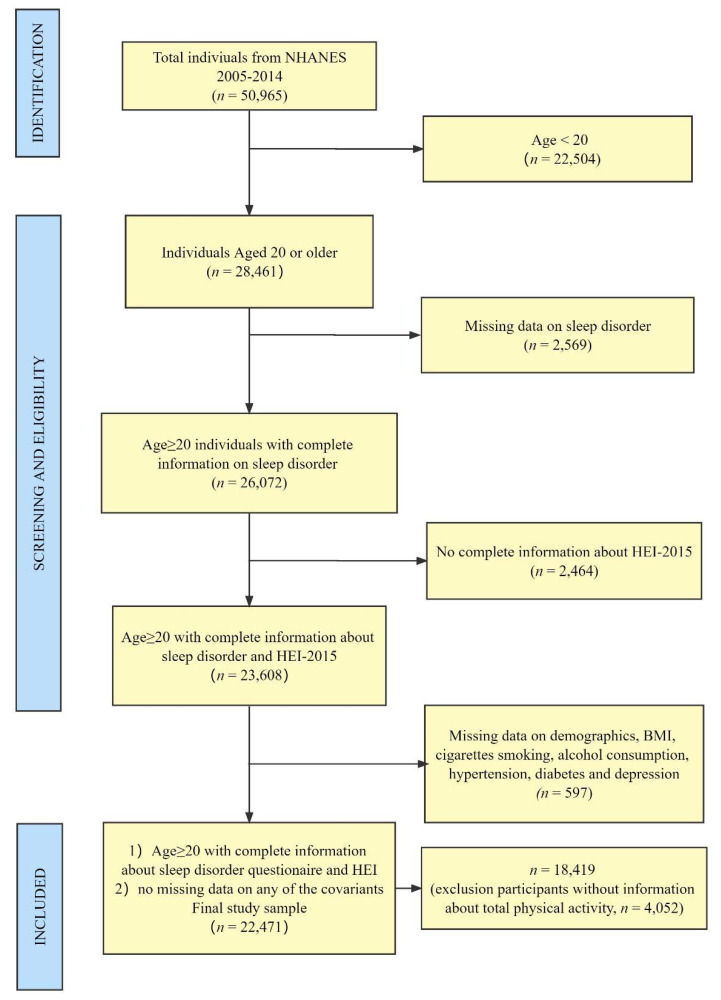
Figure 1 describes the process of the study design, sampling, and exclusion. The study combined data from 2005 to 2014 NHANES cycles (i.e., 2005–06; 2007–08, 2009–10, 2011–12, 2013–14), and 28,494 participants were excluded because of the exclusion of participants aged less than 20 and missing data on any of the measures or covariates. In the NHANES, participants aged less than 20 years old were treated as a youth by education level. The average age of the participants was 47.5, of which 47.2% were men.
Figure 1.
Flowchart of the population included in our final analysis.
Questions about sleep habits and sleep disorders were added to the NHANES questionnaire beginning in 2005. Since 2015, we could not obtain information about sleep disorders, because of the updated questionnaires; thus, we only chose the data from 2005 to 2014 for this study.
2.3. Measures
2.3.1. Sleep Disorder
Sleep disorder: In NHANES, sleep disorder was measured in the “Sleep Disorders Questionnaire”, where respondents are asked “Have you ever been told by a doctor or other health professional that you have a sleep disorder?”. If the respondent answered “Yes”, s/he was classified as “adults with sleep disorder”, or if the respondent answered “No”, s/he was classified as “adults without sleep disorder”.
2.3.2. Healthy Eating Index 2015
Previous studies have assessed the HEI-2015 of construct validity, reliability, criterion validity, and so on, and concluded that it was a useful means by which to measure diet quality [17,18,19]. Moreover, HEI-2015 has been applied to the study of sleep duration [20], cardiovascular disease [21], and cognitive performance [22].
HEI-2015 is composed of nine adequacy components and four moderation components. Adequacy components represent the food groups, subgroups, and dietary elements that are encouraged. For these components, higher scores reflect higher intakes, because higher intakes are desirable. Moderation components represent the food groups and dietary elements for which there are recommended limits to consumption. For moderation components, higher scores reflect lower intakes because lower intakes are more desirable. The adequacy components are total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, and fatty acids (ratio of unsaturated to saturated fatty acids). The moderation components are refined grains, sodium, added sugars, and saturated fats [23].
Participants who had both two 24 h dietary recalls were included, and the dietary recall status was restricted to be reliable or had met the minimum criteria for each day, which can reduce the bias of HEI-2015. The HEI-2015 total and component scores were calculated using the simple HEI scoring algorithm using publicly available SAS macros.
The HEI-2015 is scored out of 100 points, with higher scores indicating better overall diet quality. According to the quartiles, we divided the HEI scores into three groups: <25% (inadequate, reference group), 25–75% (average), and >75% (optimal), as HEI Category.
2.4. Covariates
Covariates of interest consisted of sociodemographic, behavioral, and health characteristics deemed a priori as potential confounders. Sociodemographic characteristics included age group (20–39, 40–59, 60+), sex, race (non-Hispanic White, Mexican American, Non-Hispanic Black, other race/multiracial), highest level of education (<High School, High school /GED equivalent, College/AA degree and College or above), and ratio of household income to the federal poverty level (FPL; 0~130% FPL, >130~350% FPL, >350% FPL). Behavioral characteristics included smoking status (never, former, current) [24], drink level (none, light, moderate and heavy) [25], and caffeine intake categories (<Q1, Q1~Q3 and >Q3). The categories of caffeine intake were defined by quartiles of the mean amounts of two 24 h dietary recalls. Health characteristics included body mass index categories (underweight, normal weight, overweight and obese) [26], hypertension (Yes, No) [27,28], diabetes (Yes, No) [27,29] and depression(Yes, No) [30,31].
2.5. Statistical Analysis
To make nationally representative estimates, a full sample 2-year Mobile Examination Center exam weight was used to account for different sampling probabilities and participation rates across the ten-year period according to the NHANES data analysis tutorials.
Descriptive statistics were used to compare distributions of measures and covariates with survey waves and sleep disorder. Weighted mean was used to calculate the mean HEI scores across years. Rao–Scott Chi-square test was used to compare differences among groups. A series of step-weighted logistic regression models was performed to analyze the association between HEI scores and sleep disorder. No covariates were adopted in the crude model, sociodemographic characteristics were adopted in model I, model II was additionally adjusted for behavioral factors, and model III was additionally adjusted for health characteristics. Weighted quantile sum regression model (WQS Model) was used to identify the individual HEI-2015 components most strongly associated with sleep disorder while adjusting for risk factors. The receiver operating characteristic (ROC) curve was performed to examine the fitness of the WQS model, using the original database. Statistical analyses were performed using the R software (version 4.1.0, R Foundation for Statistical Computing). All statistical tests were two-sided, and significance was considered at p < 0.05.
In the sensitivity analysis, we added the total physical activity in the logistics regression model and the WQS model, as consistent total physical activity assessment in NHANES was conducted starting from 2007. Total physical activity was assessed from 2007–2014, which included work and recreational moderate and vigorous activity and was categorized as 0, 1–149, 150–299, and ≥300 min/week.
3. Results
3.1. Descriptive Analysis
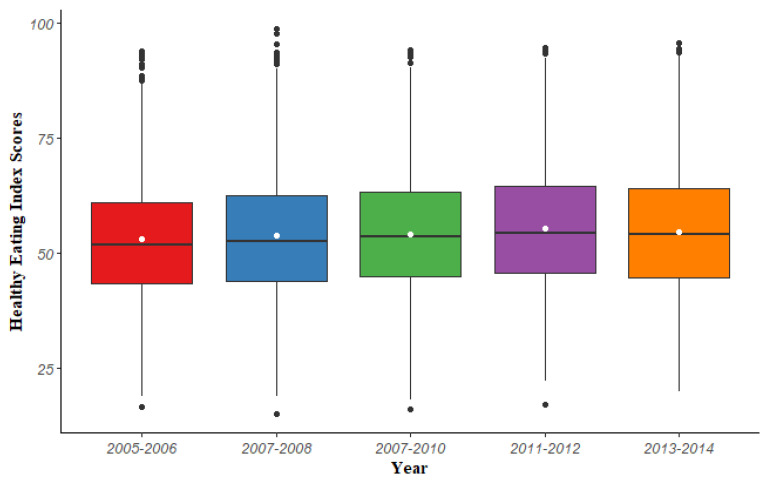
The overall mean and standard error of HEI-2015 scores were 54.05 and 0.21, respectively. This indicated that average diets of American adults do not conform to dietary recommendations. The mean of HEI scores rose from 52.76 in 2005–2006 to 54.45 in 2013–2014 (Figure 2). More accurately, American adults’ diet quality showed a slight improvement over time from 2005–2012 but a small decrease during 2013–2014, and there is room for improvement.
Figure 2.
The trend of HEI-2015 scores in the five cycles from NHANES 2005–2014. The white points in the graph represent the weighted mean of the HEI-2015 scores for each study wave.
Table 1 depicts the prevalence of sleep disorder and the characteristic of HEI across five consecutive NHANES cycles. During this decade, the prevalence of sleep disorders in US adults increased from 7.50% in 2005–2006 to 10.41% in 2013–2014, showing significant growth (F = 5.4848, p < 0.001). Additionally, the proportion of those who have inadequate HEI scores is decreasing, while the proportion of those who have optimal HEI scores are increasing, which indicates that dietary quality has some degree of improvement during this decade. This is consistent with the results in Figure 2.
Table 1.
Characteristics of adults aged 20+ years in NHANES 2005–2014.
| Characteristics | 2005–2006 (n = 4046) |
2007–2008 (n = 4661) |
2009–2010 (n = 5010) |
2011–2012 (n = 4291) |
2013–2014 (n = 4463) |
χ2 | p Value |
|---|---|---|---|---|---|---|---|
| Sleep disorder (No. Weighted%) | 286 (7.50%) | 384 (8.05%) | 395 (7.65%) | 382 (9.24%) | 462 (10.41%) | 5.48 | <0.001 |
| HEI Category (No. Weighted%) | 3.64 | 0.003 | |||||
| Inadequate | 1042 (27.28%) | 1145 (26.29%) | 1179 (23.22%) | 942 (21.76%) | 1093 (24.73%) | ||
| Average | 2129 (51.63%) | 2373 (50.08%) | 2610 (51.38%) | 2158 (50.36%) | 2149 (47.90%) | ||
| Optimal | 875 (21.09%) | 1143 (23.63%) | 1221 (25.40%) | 1191 (27.88%) | 1221 (27.37%) |
HEI, Healthy Eating Index.
Each of the potential confounders was associated with sleep disorders at p < 0.05; see Table 2. Older adults and males are more likely to report sleep disorders than not reporting, whereas younger adults and females were more likely to report no sleep disorders compared to reporting sleep disorders. People who have higher education levels and lower family income, abnormal weight, chronic diseases, and depression were more likely to report sleep disorders than not reporting. Participants who had higher HEI scores and higher intake of caffeine and alcohol were less likely to report sleep disorders than reporting.
Table 2.
Characteristics of adults aged 20+ years by sleep disorder.
| Characteristics | Adults without Sleep Disorder | Adults With Sleep Disorder | χ2 | p Value |
|---|---|---|---|---|
| HEI Category (No. Weighted%) | 4.02 | 0.019 | ||
| Inadequate | 4881 (90.60%) | 520 (9.40%) | ||
| Average | 10467 (91.29%) | 952 (8.71%) | ||
| Optimal | 5214 (92.44%) | 437 (7.56%) | ||
| Age Group (No. Weighted%) | 56.40 | <0.001 | ||
| 20–39 | 6800 (95.10%) | 342 (4.90%) | ||
| 40–59 | 6766 (89.67%) | 785 (10.33%) | ||
| 60+ | 6996 (89.39%) | 782 (10.61%) | ||
| Gender (No. Weighted%) | 5.89 | 0.018 | ||
| Female | 10841 (91.97%) | 938 (8.03%) | ||
| Male | 9721 (90.79%) | 971 (9.21%) | ||
| Race (No. Weighted%) | 19.97 | <0.001 | ||
| Non-Hispanic White | 9504 (90.71%) | 1040 (9.29%) | ||
| Mexican American | 3260 (95.39%) | 175 (4.61%) | ||
| Non-Hispanic Black | 4347 (91.43%) | 417 (8.57%) | ||
| Other | 3451 (93.00%) | 277 (7.00%) | ||
| Education Level (No. Weighted%) | 2.89 | 0.045 | ||
| <High School | 5141 (92.20%) | 428 (7.80%) | ||
| High school /GED | 4711 (90.72%) | 461 (9.28%) | ||
| College/AA degree | 5966 (90.77%) | 615 (9.23%) | ||
| College or above | 4744 (92.20%) | 405 (7.80%) | ||
| Family Income (No. Weighted%) | 3.47 | 0.035 | ||
| 0~130% FPL | 5807 (90.12%) | 636 (9.88%) | ||
| 130%~350% FPL | 7108 (91.54%) | 626 (8.46%) | ||
| 350% FPL | 7647 (91.85%) | 647 (8.15%) | ||
| Smoke Status (No. Weighted%) | 27.61 | <0.001 | ||
| Never Smoker | 11450 (92.91%) | 843 (7.09%) | ||
| Former Smoker | 5031 (89.26%) | 605 (10.74%) | ||
| Current Smoker | 4081 (89.99%) | 461 (10.01%) | ||
| Drink Level (No. Weighted%) | 16.11 | <0.001 | ||
| None | 6377 (89.54%) | 700 (10.46%) | ||
| Light | 5883 (90.47%) | 598 (9.53%) | ||
| Moderate | 6566 (93.26%) | 476 (6.74%) | ||
| Heavy | 1736 (92.43%) | 135 (7.57%) | ||
| Caffeine Category (No. Weighted%) | 4.08 | 0.019 | ||
| <Q1 | 6310 (91.72%) | 524 (8.28%) | ||
| Q1~Q3 | 10556 (91.85%) | 928 (8.15%) | ||
| >Q3 | 3696 (90.19%) | 457 (9.81%) | ||
| BMI Category (No. Weighted%) | 80.31 | <0.001 | ||
| Normal Weight | 5655 (95.60%) | 256 (4.40%) | ||
| Underweight | 337 (94.46%) | 12 (5.54%) | ||
| Overweight | 6981 (93.93%) | 453 (6.07%) | ||
| Obese | 7589 (85.88%) | 1188 (14.12%) | ||
| Hypertension (No. Weighted%) | 196.81 | <0.001 | ||
| No | 13595 (93.88%) | 825 (6.12%) | ||
| Yes | 6967 (86.12%) | 1084 (13.88%) | ||
| Diabetes (No. Weighted%) | 157.57 | <0.001 | ||
| No | 18299 (92.43%) | 1449 (7.57%) | ||
| Yes | 2263 (81.09%) | 460 (18.91%) | ||
| Depression (No. Weighted%) | 183.08 | <0.001 | ||
| No | 17831 (92.71%) | 1341 (7.29%) | ||
| Yes | 2731 (82.41%) | 568 (17.59%) |
HEI, Healthy Eating Index; FPL, family income to poverty; BMI, Body Mass Index.
3.2. Logistic Regression Models to Assess the Association between HEI-2015 Total Scores and Sleep Disorder
The results of step-weighted logistics regression models are illustrated in Table 3. Compared to the crude model, model I, and model II, the effect of the HEI-2015 on sleep disorders was reduced in Model III. However, this association did not alter, suggesting that a higher HEI score or better dietary quality may be associated with decreased risk of sleep disorders.
Table 3.
Relationship between HEI and sleep disorder among US adults aged 20+ years.
| Characteristics | OR (95% CI) | |||
|---|---|---|---|---|
| Crude Model | Model I | Model II | Model III | |
| HEI Category (reference, Inadequate) | ||||
| Average | 0.920 (0.919,0.921) | 0.875 (0.874,0.876) | 0.907 (0.906,0.909) | 0.961 (0.959,0.962) |
| Optimal | 0.789 (0.787,0.790) | 0.720 (0.719,0.721) | 0.771 (0.770,0.772) | 0.913 (0.912,0.915) |
| Age Group (reference, 20–39) | ||||
| 40–59 | 2.365 (2.361,2.368) | 2.255 (2.252,2.258) | 1.706 (1.704,1.709) | |
| 60+ | 2.460 (2.456,2.463) | 2.192 (2.188,2.195) | 1.468 (1.465,1.470) | |
| Sex (reference, Female) | ||||
| Male | 1.185 (1.183,1.186) | 1.227 (1.226,1.229) | 1.344 (1.342,1.346) | |
| Race (reference, Non-Hispanic White) | ||||
| Mexican American | 0.529 (0.528,0.530) | 0.549 (0.548,0.551) | 0.546 (0.545,0.548) | |
| Non-Hispanic Black | 0.915 (0.913,0.917) | 0.903 (0.901,0.904) | 0.728 (0.726,0.729) | |
| Other | 0.827 (0.825,0.828) | 0.812 (0.811,0.814) | 0.809 (0.808,0.811) | |
| Education Level (reference, <High School) | ||||
| High school /GED equivalent | 1.202 (1.199,1.204) | 1.256 (1.254,1.259) | 1.360 (1.358,1.363) | |
| College/AA degree | 1.319 (1.317,1.321) | 1.416 (1.414,1.419) | 1.522 (1.520,1.525) | |
| College or above | 1.143 (1.140,1.145) | 1.331 (1.328,1.333) | 1.682 (1.679,1.686) | |
| Family Income (reference, 0~130% FPL) | ||||
| 130%~350% FPL | 0.715 (0.714,0.716) | 0.744 (0.743,0.745) | 0.841 (0.84,0.842) | |
| 350% FPL | 0.645 (0.644,0.646) | 0.705 (0.704,0.706) | 0.871 (0.87,0.873) | |
| Smoke Status (reference, Never Smoker) | ||||
| Former Smoker | 1.437 (1.435,1.439) | 1.346 (1.345,1.348) | ||
| Current Smoker | 1.439 (1.436,1.441) | 1.509 (1.507,1.511) | ||
| Drink Level (reference, None) | ||||
| Light | 0.955 (0.953,0.956) | 0.991 (0.990,0.993) | ||
| Moderate | 0.617 (0.616,0.618) | 0.752 (0.751,0.754) | ||
| Heavy | 0.754 (0.753,0.756) | 0.411 (0.41,0.412) | ||
| Caffeine Category (reference, <Q1) | ||||
| Q1~Q3 | 0.900 (0.899,0.901) | 0.901 (0.900,0.902) | ||
| >Q3 | 0.886 (0.884,0.887) | 0.865 (0.864,0.867) | ||
| BMI Category (reference, Normal Weight) | ||||
| Underweight | 1.254 (1.248,1.261) | |||
| Overweight | 1.241 (1.239,1.243) | |||
| Obese | 2.733 (2.728,2.737) | |||
| Hypertension (reference, No) | ||||
| Yes | 1.605 (1.603,1.607) | |||
| Diabetes (reference, No) | ||||
| Yes | 1.616 (1.613,1.618) | |||
| Depression (reference, No) | ||||
| Yes | 3.365 (3.360,3.370) | |||
OR, odds ratio; 95% CI, 95% confidence interval; HEI, Healthy Eating Index; BMI, Body Mass Index. Model I: Adjusted for sociodemographic characteristics. Model II: Adjusted for sociodemographic characteristics and behavioral characteristics. Model III: Adjusted for sociodemographic characteristics, behavioral characteristics, and health characteristics.
In the sensitivity analysis, compared to those with inadequate HEI status, people with optimal HEI status were less likely to report sleep disorders (OR: 0.995, 95% CI: 994–0.997), indicating that higher HEI scores were associated with the decreased risk of sleep disorders. Detailed information was presented in the Supplementary Table S1.
3.3. WQS Regression Model to Assess the Association between HEI-2015 Components and Sleep Disorder
In the WQS regression model, component values were quantiled and combined into a unidirectional weighted index, thereby reducing dimensionality and avoiding multi-collinearity. WQS provides a single overall effect estimate of the mixture, and individual components were ranked by their overall contribution to the index, indicating relative importance.
Adjusted for sociodemographic, behavioral and health covariates, the overall effect of 13 components on sleep disorder was protective (OR: 0.977, 95% CI: 0.964–0.990); see Table 4. The results of the WQS model were similar to the results of the logistic model.
Table 4.
The results of the weighted quantile sum regression model.
| Model | OR | 95% CI | p Value |
|---|---|---|---|
| Model I | 0.963 | (0.951,0.975) | <0.001 |
| Model II | 0.974 | (0.961,0.986) | <0.001 |
| Model III | 0.977 | (0.964,0.990) | <0.001 |
OR, odds ratio; 95% CI, 95% confidence interval. Model I: Adjusted for sociodemographic characteristics. Model II: Adjusted for sociodemographic characteristics and behavioral characteristics. Model III: Adjusted for sociodemographic characteristics, behavioral characteristics, and health characteristics.
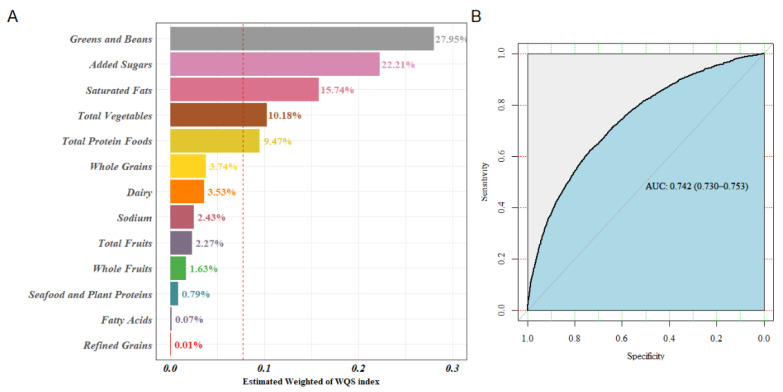
Figure 3A displays the WQS model regression index weights. Greens and beans, added sugars, saturated fats, total vegetables, and total protein foods were the top five important components, which account for more than 85% of the weight. Accordingly, a higher intake of greens and beans, total vegetables, and total protein foods, which were adequacy components, were related to a reduced risk of sleep disorder. Inversely, moderation components, with higher points awarded for lower consumption, such as added sugars and saturated fats, were positively associated with the risk of sleep disorder. Thus, people with sleep disorders should limit their intake of added sugars and saturated fats. The other six adequacy components, whole grains, dairy, total fruits, whole fruits, seafood, and plant proteins, accounted for 12% of the weights; therefore, they were important components that cannot be ignored. Nevertheless, sodium and refined grains moderation components, accounting for 3% of the weights, contributed much less to the overall effect.
Figure 3.
WQS regression index weights for sleep disorders. (A) ROC curve of the WQS model (B).
The area under the curve was 0.742 (95% CI: 0.730–0.753), which indicated that the results of the WQS model were considered acceptable; see Figure 3B.
The results of sensitivity analysis are presented in Supplementary Table S2. When controlling for total physical activity, the association between the overall 13 components and sleep disorders remained inverse (OR: 0.987, 95% CI: 0.975–0.999), indicating the stability of our findings. The top five components associated with sleep disorders did not alter, and the detailed information about the WQS regression index weights of 13 components for sleep disorders is illustrated in Supplementary Figure S1.
4. Discussion
Our study found compelling evidence that dietary quality and dietary components, using HEI-2015 as the measure, were linked to sleep disorders, assessed by the statement, “ever told by a doctor or other health professional that they have sleep disorder”. A higher HEI score or better dietary quality may be correlated to the reduced risk of sleep disorder.
Despite that these were not fully consistent with the measurements of self-reported sleep disturbances, several previous studies showed a similar association between diet and sleep disorders. A case-control study targeted at Iranian adults found that people with greater adherence to the healthy dietary pattern were less likely to have primary insomnia [32]. Meanwhile, diet quality score was found to be associated with adequate sleep duration and reduced odds for short sleep duration in Australian women [33]. In American adults aged 45–84, adherence to the Mediterranean diet was less likely to report insomnia [34], and poor diet quality, characterized by the Alternative Healthy Eating Index-2010, was associated with moderate-to-severe obstructive sleep apnea [35]. Moreover, the energy-adjusted Dietary Inflammatory Index was found to be associated with the risk of short sleep duration and self-reported sleep disturbances in American adults [15]. However, we used different measurements of self-reported sleep disturbances from this work, where sleep disturbances were assessed by the statement, “ever told by a doctor or other health professional that you have trouble sleeping”. Additional meta-analyses showed that diet treatment can reduce the severity of obstructive sleep apnea [36].
In addition, some researchers deemed that individuals with sleep disorders may select an unhealthy diet. A prospective cohort study found that women with poor sleep quality were prone to intake more and had a lower-quality diet [37]. Some reviews pointed out that sleep influenced dietary choices; people who slept less were more likely to prefer fats, eat fewer vegetables, and choose irregular eating patterns [38,39]. The proposed mechanisms by which sleep may stimulate food intake were by the upregulation of the activity of the central nervous hypocretin system and changes in key appetite hormones [40]. However, since the data are cross sectional, the causal relationship between HEI and sleep disorders remains unclear in our study.
Greens and beans, total vegetables, total protein foods adequacy components and added sugars and saturated fats moderate components were the top five dietary components associated with sleep disorder in our study. Previous studies have found that short sleep duration, one kind of sleep disorder, was associated with poor diet quality, such as lower bean, protein, fruits, and whole grain intake [39,41], or low intake of vegetables and fish, and high intakes of confectionary foods [33]. Another cross-sectional study found that industrialized dietary pattern (high in sugar-sweetened beverages, fast foods) yielded higher odds for obstructive sleep apnea, compared with the traditional dietary pattern (high in legumes and tortillas) [42].
Possible mechanisms may explain these relationships. A cross-sectional study based on a Chinese sample found that vegetables and beans or bean products can reduce the risk of depression, which can influence sleep [43]. Foods such as plant species, including roots, leaves, fruits, and seeds, contain melatonin and serotonin. These foods may also contain tryptophan, which is a precursor for serotonin and melatonin [44]. However, the consumption of saturated fatty acids deteriorates sleep wellness, and total sleep time was negatively associated with intake of total fat and saturated fat [45]. Moreover, the systematic review found that the Healthy Eating Index showed better correlation with obesity [46], and dietary weight loss was effective in reducing the severity of obstructive sleep apnea. These factors may explain why a healthy diet is important for sleep.
Caffeine was generally considered to be bad for sleep [47], but in this study, it was contradictory. This may be due to interference from other covariates, such as education level and family income, as copious caffeine intake was a risk factor of sleep disorders before including these covariates. In addition, people with higher caffeine intake also had a higher prevalence of sleep disorders than those with lower caffeine intake. Another possible explanation is that caffeine is anti-inflammatory. If coffee is consumed earlier in the day, it is unlikely to influence sleep and can help lower systemic inflammation. The previous study found that, compared with non-drinkers, people who drank ≥4 cups of total coffee/day had lower concentrations of C-reactive protein and interleukin 6 (IL-6) [48], which are related to sleep disorders [49]. Caffeine may be helpful to sleep through the inflammatory pathway. However, we could not obtain the information about the time of coffee consumption.
The effect of the 13-component mixture in the WQS model was relatively smaller than HEI total effect in the logistic regression. The possible reason is that, in the WQS model, the values for the 13 components can be scored into quantiles by the median, and the odds ratio represents the comparison between higher than the median and lower than the median. However, in the logistic regression model, we divided HEI total scores into three groups and compared the optimal and inadequate scores, making the protective effect of HEI seem to be larger than that of the WQS model.
There are several limitations to this study. First, only adults were investigated in this study, making it difficult to generalize our findings to children and adolescents. Second, whether participants had a sleep disorder or not was measured by asking if they had ever been told by a doctor or health professional that they had a sleep disorder. This self-reported but physician-diagnosed sleep disorder may cause bias because no objective sleep measures were collected. Third, the HEI does not incorporate the timing of eating, which may be an important aspect to consider in future analyses on the effect of sleep disorder on diet quality. Last but not least, the causal relationship of HEI and sleep disorder cannot be inferenced because of the cross-sectional design.
In conclusion, within this sample of nationally representative American adults, our primary finding is that sleep disorder is rapidly growing in prevalence among American adults, which raises demand for public health interventions. More importantly, we discovered that sleep disorder was significantly associated with lower dietary quality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14040873/s1, Table S1: The sensitivity analysis results of the logistics regression model from 2007-2014; Table S2: The sensitivity analysis results of the Weighted Quantile Sum regression model; Figure S1: The sensitivity analysis results of (A) WQS regression index weights for sleep disorders; (B) The ROC curve of the WQS model.
Author Contributions
Z.-J.Z. designed the study and edited the manuscript. M.-G.D. performed the statistical analysis, J.-Q.N. drafted the manuscript. M.-G.D., Y.-Y.L., X.Y. and Z.-J.Z. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Study details are available online at cdc.gov/nchs/nhanes/irba98.htm.
Data Availability Statement
The NHANES dataset is publicly available online, accessible at cdc.gov/nchs/nhanes/index.htm.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pavlova M.K., Latreille V. Sleep Disorders. Am. J. Med. 2019;132:292–299. doi: 10.1016/j.amjmed.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Arnardottir E.S., Bjornsdottir E., Olafsdottir K.A., Benediktsdottir B., Gislason T. Obstructive sleep apnoea in the general population: Highly prevalent but minimal symptoms. Eur. Respir. J. 2016;47:194–202. doi: 10.1183/13993003.01148-2015. [DOI] [PubMed] [Google Scholar]
- 3.Heinzer R., Vat S., Marques-Vidal P., Marti-Soler H., Andries D., Tobback N., Mooser V., Preisig M., Malhotra A., Waeber G., et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccolo R.S., Yang M., Bliwise D.L., Yaggi H.K., Araujo A.B. Racial and socioeconomic disparities in sleep and chronic disease: Results of a longitudinal investigation. Ethn. Dis. 2013;23:499–507. [PMC free article] [PubMed] [Google Scholar]
- 5.Redline S., Foody J. Sleep disturbances: Time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation. 2011;124:2049–2051. doi: 10.1161/CIRCULATIONAHA.111.062190. [DOI] [PubMed] [Google Scholar]
- 6.Grandner M.A., Hale L., Moore M., Patel N.P. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med. Rev. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huyett P., Siegel N., Bhattacharyya N. Prevalence of Sleep Disorders and Association With Mortality: Results From the NHANES 2009–2010. Laryngoscope. 2021;131:686–689. doi: 10.1002/lary.28900. [DOI] [PubMed] [Google Scholar]
- 8.Kripke D.F., Garfinkel L., Wingard D.L., Klauber M.R., Marler M.R. Mortality associated with sleep duration and insomnia. Arch. Gen. Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 9.Silva G.E., An M.W., Goodwin J.L., Shahar E., Redline S., Resnick H., Baldwin C.M., Quan S.F. Longitudinal evaluation of sleep-disordered breathing and sleep symptoms with change in quality of life: The Sleep Heart Health Study (SHHS) Sleep. 2009;32:1049–1057. doi: 10.1093/sleep/32.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandner M.A., Kripke D.F., Naidoo N., Langer R.D. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010;11:180–184. doi: 10.1016/j.sleep.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Q., Kou T., Zhuang B., Ren Y., Dong X., Wang Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients. 2018;10:1395. doi: 10.3390/nu10101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandner M.A., Jackson N., Gerstner J.R., Knutson K.L. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite. 2013;64:71–80. doi: 10.1016/j.appet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binks H., Vincent G.E., Gupta C., Irwin C., Khalesi S. Effects of Diet on Sleep: A Narrative Review. Nutrients. 2020;12:936. doi: 10.3390/nu12040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Egmond L., Tan X., Sjögren P., Cederholm T., Benedict C. Association between Healthy Dietary Patterns and Self-Reported Sleep Disturbances in Older Men: The ULSAM Study. Nutrients. 2019;11:1029. doi: 10.3390/nu11051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kase B.E., Liu J., Wirth M.D., Shivappa N., Hebert J.R. Associations between dietary inflammatory index and sleep problems among adults in the United States, NHANES 2005–2016. Sleep Health. 2021;7:273–280. doi: 10.1016/j.sleh.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Godos J., Grosso G., Castellano S., Galvano F., Caraci F., Ferri R. Association between diet and sleep quality: A systematic review. Sleep Med. Rev. 2021;57:101430. doi: 10.1016/j.smrv.2021.101430. [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick S.I., Reedy J., Krebs-Smith S.M., Pannucci T.E., Subar A.F., Wilson M.M., Lerman J.L., Tooze J.A. Applications of the Healthy Eating Index for Surveillance, Epidemiology, and Intervention Research: Considerations and Caveats. J. Acad. Nutr. Diet. 2018;118:1603–1621. doi: 10.1016/j.jand.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panizza C.E., Shvetsov Y.B., Harmon B.E., Wilkens L.R., Le Marchand L., Haiman C., Reedy J., Boushey C.J. Testing the Predictive Validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: Is the Score Associated with a Reduced Risk of All-Cause and Cause-Specific Mortality? Nutrients. 2018;10:452. doi: 10.3390/nu10040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reedy J., Lerman J.L., Krebs-Smith S.M., Kirkpatrick S.I., Pannucci T.E., Wilson M.M., Subar A.F., Kahle L.L., Tooze J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018;118:1622–1633. doi: 10.1016/j.jand.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen E.C., Prather A., Leung C.W. Associations between sleep duration and dietary quality: Results from a nationally-representative survey of US adults. Appetite. 2020;153:104748. doi: 10.1016/j.appet.2020.104748. [DOI] [PubMed] [Google Scholar]
- 21.Hu E.A., Steffen L.M., Coresh J., Appel L.J., Rebholz C.M. Adherence to the Healthy Eating Index-2015 and Other Dietary Patterns May Reduce Risk of Cardiovascular Disease, Cardiovascular Mortality, and All-Cause Mortality. J. Nutr. 2020;150:312–321. doi: 10.1093/jn/nxz218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y., Zhang Y., Li J., Liu Y., Chang H., Jiang Y., Tuo X., Zhou L., Yu Y. Association between healthy eating index-2015 and various cognitive domains in US adults aged 60 years or older: The National Health and Nutrition Examination Survey (NHANES) 2011–2014. BMC Public Health. 2021;21:1862. doi: 10.1186/s12889-021-11914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs-Smith S.M., Pannucci T.E., Subar A.F., Kirkpatrick S.I., Lerman J.L., Tooze J.A., Wilson M.M., Reedy J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SSY A.L., Natto Z.S., Midle J.B., Gyurko R., O’Neill R., Steffensen B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J. Periodontol. 2019;90:16–25. doi: 10.1002/jper.18-0183. [DOI] [PubMed] [Google Scholar]
- 25.Gay I.C., Tran D.T., Paquette D.W. Alcohol intake and periodontitis in adults aged ≥30 years: NHANES 2009-2012. J. Periodontol. 2018;89:625–634. doi: 10.1002/JPER.17-0276. [DOI] [PubMed] [Google Scholar]
- 26.Soares M.J., Macedo A., Bos S.C., Maia B., Marques M., Pereira A.T., Gomes A.A., Valente J., Nogueira V., Azevedo M.H. Sleep disturbances, body mass index and eating behaviour in undergraduate students. J. Sleep Res. 2011;20:479–486. doi: 10.1111/j.1365-2869.2010.00887.x. [DOI] [PubMed] [Google Scholar]
- 27.Leigh L., Hudson I.L., Byles J.E. Sleep Difficulty and Disease in a Cohort of Very Old Women. J. Aging Health. 2016;28:1090–1104. doi: 10.1177/0898264315624907. [DOI] [PubMed] [Google Scholar]
- 28.Moon C., Hagen E.W., Johnson H.M., Brown R.L., Peppard P.E. Longitudinal sleep characteristics and hypertension status: Results from the Wisconsin Sleep Cohort Study. J. Hypertens. 2021;39:683–691. doi: 10.1097/HJH.0000000000002692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Gao X., Winkelman J.W., Cespedes E.M., Jackson C.L., Walters A.S., Schernhammer E., Redline S., Hu F.B. Association between sleeping difficulty and type 2 diabetes in women. Diabetologia. 2016;59:719–727. doi: 10.1007/s00125-015-3860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang H., Tu S., Sheng J., Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019;23:2324–2332. doi: 10.1111/jcmm.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadat S., Salehi-Sahlabadi A., Pourmasoumi M., Feizi A., Clark C.C.T., Akkasheh G., Ghiasvand R. A healthy dietary pattern may be associated with primary insomnia among Iranian adults: A case-control study. Int. J. Vitam. Nutr. Res. 2021;91:479–490. doi: 10.1024/0300-9831/a000644. [DOI] [PubMed] [Google Scholar]
- 33.Mondin T.C., Stuart A.L., Williams L.J., Jacka F.N., Pasco J.A., Ruusunen A. Diet quality, dietary patterns and short sleep duration: A cross-sectional population-based study. Eur. J. Nutr. 2019;58:641–651. doi: 10.1007/s00394-018-1655-8. [DOI] [PubMed] [Google Scholar]
- 34.Castro-Diehl C., Wood A.C., Redline S., Reid M., Johnson D.A., Maras J.E., Jacobs D.R., Jr., Shea S., Crawford A., St-Onge M.P. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep. 2018;41:zsy158. doi: 10.1093/sleep/zsy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid M., Maras J.E., Shea S., Wood A.C., Castro-Diehl C., Johnson D.A., Huang T., Jacobs D.R., Jr., Crawford A., St-Onge M.P., et al. Association between diet quality and sleep apnea in the Multi-Ethnic Study of Atherosclerosis. Sleep. 2019;42:zsy194. doi: 10.1093/sleep/zsy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards B.A., Bristow C., O’Driscoll D.M., Wong A.M., Ghazi L., Davidson Z.E., Young A., Truby H., Haines T.P., Hamilton G.S. Assessing the impact of diet, exercise and the combination of the two as a treatment for OSA: A systematic review and meta-analysis. Respirology. 2019;24:740–751. doi: 10.1111/resp.13580. [DOI] [PubMed] [Google Scholar]
- 37.Zuraikat F.M., Makarem N., Liao M., St-Onge M.P., Aggarwal B. Measures of Poor Sleep Quality Are Associated With Higher Energy Intake and Poor Diet Quality in a Diverse Sample of Women From the Go Red for Women Strategically Focused Research Network. J. Am. Heart Assoc. 2020;9:e014587. doi: 10.1161/JAHA.119.014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vernia F., Di Ruscio M., Ciccone A., Viscido A., Frieri G., Stefanelli G., Latella G. Sleep disorders related to nutrition and digestive diseases: A neglected clinical condition. Int. J. Med. Sci. 2021;18:593–603. doi: 10.7150/ijms.45512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaput J.P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014;134:86–91. doi: 10.1016/j.physbeh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Schmid S.M., Hallschmid M., Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3:52–62. doi: 10.1016/S2213-8587(14)70012-9. [DOI] [PubMed] [Google Scholar]
- 41.Haghighatdoost F., Karimi G., Esmaillzadeh A., Azadbakht L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition. 2012;28:1146–1150. doi: 10.1016/j.nut.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Gaona-Pineda E.B., Martinez-Tapia B., Rodríguez-Ramírez S., Guerrero-Zúñiga S., Perez-Padilla R., Shamah-Levy T. Dietary patterns and sleep disorders in Mexican adults from a National Health and Nutrition Survey. J. Nutr. Sci. 2021;10:e34. doi: 10.1017/jns.2021.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X., Bi B., Zheng L., Li Z., Yang H., Song H., Sun Y. The prevalence and risk factors for depression symptoms in a rural Chinese sample population. PLoS ONE. 2014;9:e99692. doi: 10.1371/journal.pone.0099692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peuhkuri K., Sihvola N., Korpela R. Diet promotes sleep duration and quality. Nutr. Res. 2012;32:309–319. doi: 10.1016/j.nutres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Zhao M., Tuo H., Wang S., Zhao L. The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediat. Inflamm. 2020;2020:3142874. doi: 10.1155/2020/3142874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asghari G., Mirmiran P., Yuzbashian E., Azizi F. A systematic review of diet quality indices in relation to obesity. Br. J. Nutr. 2017;117:1055–1065. doi: 10.1017/S0007114517000915. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhary N.S., Grandner M.A., Jackson N.J., Chakravorty S. Caffeine consumption, insomnia, and sleep duration: Results from a nationally representative sample. Nutrition. 2016;32:1193–1199. doi: 10.1016/j.nut.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hang D., Kværner A.S., Ma W., Hu Y., Tabung F.K., Nan H., Hu Z., Shen H., Mucci L.A., Chan A.T., et al. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. Am. J. Clin. Nutr. 2019;109:635–647. doi: 10.1093/ajcn/nqy295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irwin M.R., Olmstead R., Carroll J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NHANES dataset is publicly available online, accessible at cdc.gov/nchs/nhanes/index.htm.