Abstract
Tricuspid regurgitation (TR) is common in patients with left-sided valvular heart disease and is independently associated with increased mortality and morbidity because it leads to right-sided heart failure and recurrent hospitalization. The prognostic benefit of isolated TR surgical repair or replacement is unclear and medical treatment of decompensated right heart failure alone does not prevent the progression of the disease. Recently, minimal invasive catheter-based techniques have emerged as a feasible and effective option for TR treatment in selected high-risk patients who would clinically benefit from tricuspid valve repair. We provide an overview of the current state of transcatheter TR treatment using the edge-to-edge technique.
Keywords: Edge-to-edge repair, Tricuspid regurgitation, Percutaneous transcatheter valve repair
Tricuspid valve regurgitation (TR) is most often secondary, i.e. functional, rather than resulting from morpho-pathological changes of the tricuspid valve (TV) complex.1 Frequently, the cause of TR is a disease of left-sided heart valves.1 Functional TV regurgitation is the result of right ventricular (RV) pressure and/or volume overload altering TV annulus size as well as producing abnormalities of RV dimension and function, which thereby alter leaflet coaptation and cause tethering. Pressure overload is most often due to pulmonary hypertension resulting from left-sided heart disease, especially in the setting of mitral valve pathology or, less frequently, pulmonary disease, while RV volume overload is observed in left-to-right shunts or intrinsic RV disease. It is noteworthy that atrial fibrillation, especially if chronic, can be another important aetiologic factor, primarily through its effect on RV function and TV annular dilation.2 Additionally, there is a growing population of patients without significant cardiopulmonary comorbidities who nonetheless have severe TR possibly related to intra-cardiac pacemaker/defibrillator leads.3 Finally, there are patients who, despite no significant comorbidities and overt cardiac causes develop severe isolated TR. This is characterized by dilation of RV base and TV annulus, leading to less tethering but the exhaustion of the reserve of TV annulus coverage. The pathological mechanism of TV annular enlargement may be a degenerative alteration of TV annular fibrous structure.
Tricuspid valve disease has long been ignored, with the belief that TR would improve after surgical correction of left-sided valvular disease. On the contrary, moderate TR may worsen after surgery and is associated with increased morbidity and decreased survival depending on its severity.4,5 Management of patients with severe functional TR after left-sided valve surgery is difficult because they are usually in poor clinical condition compared with when they had the initial intervention. Thus, a large body of evidence now supports the strong negative effect of significant TR on outcomes, and this recognition has led physicians to indicate more frequently combined TV surgery. However, this is associated with the risk of subsequent dysfunction of TV repair or replacement. Indeed, for patients undergoing TV surgery, recurrence of moderate, or severe TR can be as high as 60% at 5 years. Of note, redo TV surgery is often associated with high morbidity and mortality rates. Moreover, a significant number of patients are deemed inoperable or are at high risk for a surgical approach.6 For these reasons, the TV has been referred to as the ‘forgotten valve’ because this valve disease was historically perceived to be less important than left-sided valvular disease and often was not considered for surgical treatment.
Following the introduction of transcatheter techniques for aortic and mitral valve disease, the possibility of lower-risk TV intervention is therefore particularly attractive. However, the development of transcatheter devices for TR treatment faces several anatomical challenges because the TV apparatus is more complex than that of the mitral valve (Table 1). Despite these challenges, our ageing population, the rising prevalence of atrial fibrillation, the increase in iatrogenic TR from transvenous pacemaker/defibrillator leads,3 and the likely higher life expectancy of patients with effectively treated aortic and mitral valves underscore the importance of developing transcatheter solutions. In the last few years, numerous catheter-based devices have been designed and underwent preclinical and clinical testing, but most of them are not available for regular use yet and clinical experience is limited. At present, the most widely applied technique in selected high-risk patients is the edge-to-edge repair that improving the coaptation of the TV leaflets has shown to achieve effective TR reduction resulting in significant clinical improvement.
Table 1.
Anatomical challenges for catheter-based TV repair
| Frequent presence of a large (>40 mm) tricuspid annulus size with a non-planar and elliptical shape. |
| Thin and more delicate valve leaflets, often with deep clefts and folds. |
| High variability (quantity, density and location) of thin and fragile chordae. |
| Lack of calcium for device anchoring. |
| Angulation between the tricuspid annulus and the inferior and superior vena cava. |
| Frequent presence of pacemaker/defibrillator leads. |
| Proximity of structures such as the AV node or right His bundle branch, right coronary artery, coronary sinus, vena cava and right ventricular outflow tract. |
TV, tricuspid valve; AV, atrio-ventricular.
Leaflet coaptation devices
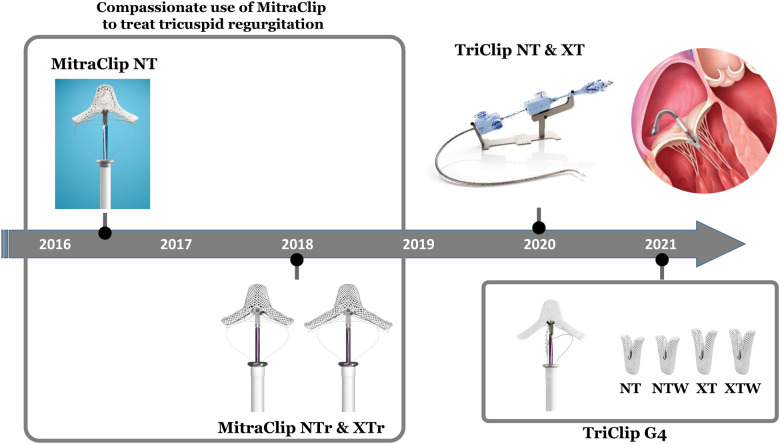
Two different technologies mimicking the edge-to-edge Alfieri surgical repair are currently available. The MitraClip System (Abbott Vascular, Santa Clara, CA, USA) is a transvenous edge-to-edge device grasping the anterior and posterior mitral leaflets within a locking. This device has been established as an effective and durable alternative treatment for both organic and functional severe mitral regurgitation in over 100 000 patients worldwide. After clinical studies with the MitraClip have shown promising results for TR treatment, The TriClip Transcatheter Tricuspid Valve Repair System was developed based on the modification of the MitraClip. The TriClip was initially available in two sizes: NT and XT. The TriClip XT provides a 40% greater coaptation surface area than the TriClip NT. Recently, two additional devices with wider arms and independent leaflet grasping (NTW and XTW) became available (Figure 1).
Figure 1.
Evolution of the MitraClip System and the TriClip transcatheter tricuspid valve repair system. Compassionate treatment of tricuspid regurgitation started in 2016 with the MitraClip NT. Subsequently, MitraClip NTr and XTr were used. MitraClip XTr features 3-mm longer arms compared to NTr. In 2020, the TriClip Transcatheter Tricuspid Valve Repair System, which is based on modifications of the MitraClip System, was introduced for clinical use with two devices (NT and XT) that were joined in 2021 by two new generation devices, NTW and XTW. The new generation features wider arms and independent leaflet grasping.
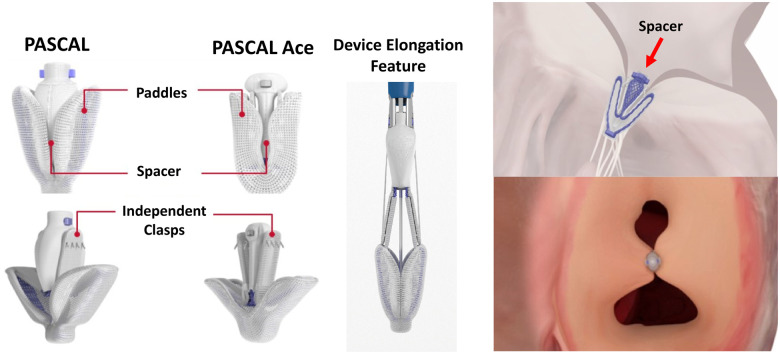
The PASCAL System (Edwards Lifesciences, Irvine, CA, USA) is another percutaneous edge-to-edge repair device similar to the MitraClip and TriClip Systems. The PASCAL features independent leaflet capture, which can facilitate grasping, as well as a central spacer, which may improve TR correction in the most severe cases (Figure 2).
Figure 2.
The PASCAL System for mitral and tricuspid regurgitation treatment. Device key characteristics include: (i) a central spacer designed to fill the coaptation gap and minimize regurgitation; (ii) two paddles designed to minimize stress concentration on valve leaflet; (iii) device elongation featuring a slim profile for repositioning and navigation in dense cordae with reduced risk of entanglement; (iv) independent grasping and a single row of retention elements allowing optimal placement on valve leaflets. The PASCAL Ace System features narrower profile and central spacer to further optimizing patient treatment.
However, two aspects must be taken into consideration when transferring the percutaneous edge-to-edge technique from the mitral to the tricuspid space. First, the difficulty of obtaining high-resolution echocardiographic images of the TV to guide the procedure and second the limited steering of the device in the right atrium using standard techniques because of the close proximity between the inferior vena cava orifice and the atrial septum.
Clinical studies with the MitraClip System and the TriClip transcatheter tricuspid valve repair system
So far, the MitraClip has been the device most widely used to correct severe TR in selected patients and therefore has the largest evidence base. After the publication in 2016 of the first successful case in a patient with congenitally corrected transposition of the great arteries, Hausleiter et al.7 reported a series of 13 symptomatic patients with severe TR treated with this approach. All patients experienced a TR grade reduction (3.2 ± 0.6 vs. 1.5 ± 0.6). At 30 days, New York Heart Association (NYHA) functional class improved in 84% (3.3 ± 0.5 vs. 2.4 ± 0.5) and only one major adverse event (severe biventricular dysfunction and stroke) was reported. One year later, Nikening et al.8 published the results of 64 treated patients, 88% of whom had functional TR. In 34%, TR was treated at the time of transcatheter repair of mitral regurgitation. In 48%, a single clip was used, while 42% received between two and four clips, and in 10% either no clip was deployed or data was missing. Acute procedural success was achieved in 97%, with a reduction in TR severity by at least one grade in 91%. However, severe TR was still present in 13%. While no major procedural complications occurred, there were three in-hospital deaths (one cardiogenic shock, one systemic inflammatory response syndrome, and one hyperkalaemia-mediated arrhythmia). At a mean follow-up of 14 days, the per cent of patients with functional class III/IV was reduced from 93 to 63, and persistent functional class IV symptoms disappeared in all. Moreover, there was a significant improvement in 6-min walk distance (6MWD) (from 177 to 193 m, P = 0.007). However, no significant change in the NTproBNP level was observed, and all but 15 patients still required diuretic therapy. The TriValve registry included 249 patients with severe TR treated with the MitraClip in 14 centres in Europe and North America between June 2015 and June 2018.9 A successful procedure (≤2+ TR grade) was achieved in 77% by the placement of 2 ± 1 clips. Concomitant treatment of severe mitral regurgitation was performed in 52%. Predictors of procedural failure included larger effective regurgitant orifice area (EROA), tricuspid coaptation gap, tricuspid tenting area, and absence of central or anteroseptal TR jet location. Predictors of 1-year mortality were a procedural failure, worsening kidney function, and absence of sinus rhythm. In 2020, Cai et al.10 reported a retrospective observational study of the outcomes of 124 patients assessed for severe TR between 2015 and 2019. Seventy-one patients were ineligible for transcatheter treatment because of anatomy and/or clinical factors and received guideline directed medical therapy (GDMT), while 53 underwent TV intervention with the MitraClip. Procedural success (TR grade ≤ moderate) was 77.3% without periprocedural complications. The most common clip location was between the septal and anterior leaflet, with 75.5% of patients receiving at least one clip at this position. Out of 96 clips deployed in 53 patients, there was only one single leaflet device attachment (SLDA), which was subsequently treated with an additional clip. After the procedure, there was a significant improvement in functional class, with more patients having class I/II symptoms (6.5% vs. 86.8%, P < 0.001) compared to the GDMT group. A significant improvement in 6MWD (240.7 ± 138.5 vs. 334.4 ± 141.5 m, P = 0.01) was also observed at follow-up. However, the total daily dose of post-procedural diuretics and mineralocorticoid receptor antagonists remained similar. At a median follow-up of 17 months, the GDMT group had significantly lower survival (46.9% vs. 75.1%, P = 0.047), with a trend towards reduced freedom from heart failure hospitalization (43.6% vs. 61.7%, P = 0.074), and a significantly decreased freedom from the combined endpoint of heart failure hospitalization and all-cause mortality (33.2% vs. 62.7%, P = 0.027). Besler et al.11 analysed the predictors of procedural outcome in 117 TR patients treated with the MitraClip and found that smaller EROA, tenting area, vena contracta, and coaptation gap together with a central/anteroseptal jet location were predictors of procedural success at univariate analysis, whereas only the last two factors independently predicted repair success. Using the MitraClip XTR, Braun et al.12 treated 31 patients, 16 (52%) of whom with a coaptation gap ≥7 mm. The overall procedural success was 87%, whereas the success rate was 75% in those with larger gaps, and the 30-day residual TR ≤2 was 69% and 43%, respectively. Of note, they observed single leaflet clip detachment in four patients with ≥7-mm coaptation gap. The TRILUMINATE pivotal trial was launched in 2019 to investigate the safety and efficacy of the TriClip System in patients with moderate/severe TR who were randomized to transcatheter TV repair or GMDT.13 Acute procedural success was 90.5% and TR severity was moderate or less in 57% at 30 days and 6 months. At 1 year, TR severity was moderate or less in 64%. A reduction of ≥1 TR grade was observed in 85.5% at 30 days and increased to 87.1% at 6 months. Patients treated with the TriClip experienced significant improvements in their quality of life at 30 days, which was sustained through 6 months post-procedure. A significant clinical improvement [functional class I/II increased from 22% to 80%, Kansas City Cardiomyopathy Questionnaire (16.8 points), and 6MWD (33.1 m)] persisted at 1 year. Of note, a significant reduction in right atrial and RV dimensions, with significant improvement in RV function, suggesting positive remodelling was observed at 6 months. The trial also met the primary safety endpoint. Only 3.7% of patients had a major adverse events at 6 months, significantly less than the pre-specified performance goal of 39% (P < 0.0001).
Clinical studies with the PASCAL System
Between September 2017 and February 2019, 28 consecutive patients were treated for symptomatic severe TR using the PASCAL System at six centres as part of a first-in-human, compassionate-use observational study.14 The aetiology of TR was functional in 23, degenerative in 2, and mixed in 3. All patients had severe right-sided heart failure (functional class III or IV) due to ≥3+ TR despite medical therapy and were deemed at high surgical risk or inoperable. In general, patients with >15-mm coaptation gaps, severe leaflet tethering, and pacemaker lead-induced TR were excluded. In total, 40 PASCAL devices (1.4 ± 0.6 per patient) were implanted, 28 between the anterior and septal leaflet and 12 between the posterior and septal leaflet. Of note, independent grasping for implantation was used in 90% (36 of 40 PASCAL devices). Acute procedural success (implantation of at least one device with post-procedural TR grade ≤2+, with no mortality or conversion to surgery) was achieved in 24 of 28 (86%) patients. In one patient, implantation was not possible because intraprocedural echocardiographic imaging was not of sufficient quality to guide the procedure. In another patient, implantation failed because of a large coaptation gap. This patient died from terminal heart failure 1 week later. Two patients experienced SLDA and were managed conservatively with medical therapy. At 30 days, mortality was 7.1% (2 of 28 patients). One SLDA patient died 29 days after the procedure of a presumed cardiac cause. Another patient was rehospitalized for heart failure despite sustained TR reduction. There was a significant improvement in functional class in 23 of 26 (88%) patients. Moreover, the incidence of patients in functional class ≥III was reduced from 100% (28 of 28 patients) to 12% (3 of 26 patients) (P < 0.001). Consistent with functional class improvement, an increase in 6MWD from 240 to 335 m (P < 0.001) was observed. At 30 days, the presence of ≥3+ TR grade was reduced from 100% (28 of 28 patients) to 15% (4 of 26 patients) (P < 0.001). It is noteworthy that no worsening of RV function (TV annular plane systolic excursion 15.7 ± 3.9 mm at baseline vs. 14.0 ± 3.6 mm at 30 days, P < 0.10) occurred despite effective TR reduction. Furthermore, there was evidence of favourable RV remodelling at 30 days, as TV annular diameter decreased from 47.4 ± 7.3 to 40.3 ± 7.1 mm (P < 0.001).
Future perspective and conclusions
Clinicians are confronted with different types of symptomatic TR affecting patients in varying stages of acute or chronic heart disease. The future direction of transcatheter TR treatment must aim at refining patient selection with a focus on haemodynamic, clinical, and anatomical characteristics. Clinical response and procedural success rates seem significantly affected by the severity of chronic RV failure, which directly influences patient selection. This suggests that TR, when treated at an earlier disease stage, i.e. before the development of overt RV failure, might have higher procedural success and derive larger clinical benefit. The TV apparatus has its own complexity, which must be taken into account when approaching with catheter-based techniques severe or massive TR with large central coaptation defects. Moreover, interventionalists have to overcome the known challenges of echocardiographic visualization of valve leaflets for catheter-based edge-to-edge TV repair. Therefore, refinements in procedural strategy, anatomical and clinical patient selection, imaging and modification of the device for dedicated use in TV disease are underway to further increase procedural success and expand the applicability of this technique. However, given that the results achieved so far are from high-volume centres with extensive knowledge of transcatheter TV intervention, they may not be generalizable to less experienced centres indicating the imperative need of increasing operator expertise and skill. Future studies (Table 2) with long-term follow-up will have to confirm the efficacy and durability of interventional TR repair using the edge-to-edge technique in larger subset of high-risk patients in order to further defining its clinical value.
Table 2.
Ongoing trials of transcatheter treatment of tricuspid regurgitation with dedicated devices using the edge-to-edge technique
| Trial | TriClip System |
|---|---|
| TRILUMINATE Pivotal | The primary objective of this trial is to demonstrate the safety and effectiveness of the TriClip device in improving clinical outcomes in symptomatic patients with severe tricuspid regurgitation, who are at intermediate or greater estimated risk for mortality or morbidity with tricuspid valve surgery. This randomized controlled trial will compare TriClip to medical therapy. |
| bRIGHT | An observational single-arm, multicenter, real-world study evaluating severe tricuspid regurgitation in 200 patients treated with the TriClip System. |
| TRI-FR | Evaluation of tricuspid valve percutaneous repair system compared to conventional pharmacological treatment in the treatment of severe secondary tricuspid disorders in patients unsuitable for surgical isolated correction of tricuspid regurgitation. |
|
| |
| PASCAL System | |
|
| |
| CLASP TR EFS | Early feasibility study of the PASCAL system for tricuspid valve repair in patients with tricuspid insufficiency. Enrollment is currently limited to patients in the United States. |
| CLASP II TR | Prospective, multicenter, randomized, controlled pivotal trial to evaluate the safety and effectiveness of transcatheter tricuspid valve repair with the PASCAL System and OMT compared to OMT alone in patients with tricuspid regurgitation. |
| TriCLASP | European prospective, multicenter post-market study of transcatheter repair of tricuspid regurgitation. |
OMT, optimal medical therapy.
Conflict of interest: none declared.
References
- 1.Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, Mahoney DW, Enriquez-Sarano M.. Burden of tricuspid regurgitation in patients diagnosed in the community setting. J Am Coll Cardiol Imaging 2019;12:433–442. [DOI] [PubMed] [Google Scholar]
- 2.Utsunomiya H, Itabashi Y, Mihara H, Berdejo J, Kobayashi S, Siegel RJ, Shiota T.. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging 2017;10:e004897. [DOI] [PubMed] [Google Scholar]
- 3.Addetia K, Harb SC, Hahn RT, Kapadia S, Lang RM.. Cardiac implantable electronic device lead-induced tricuspid regurgitation. J Am Coll Cardiol Imaging 2019;12:622–636. [DOI] [PubMed] [Google Scholar]
- 4.Nath J, Foster E, Heidenreich PA.. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405–409. [DOI] [PubMed] [Google Scholar]
- 5.Axtell AL, Bhambhani V, Moonsamy P, Healy EW, Picard MH, Sundt TM, Wasfy JH.. Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J Am Coll Cardiol 2019;74:715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zack CJ, Fender EA, Chandrashekar P, Reddy YNV, Bennett CE, Stulak JM, Miller VM, Nishimura RA.. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol 2017;70:2953–2960. [DOI] [PubMed] [Google Scholar]
- 7.Hausleiter J, Braun D, Orban M, Latib A, Lurz P, Boekstegers P, von Bardeleben RS, Kowalski M, Hahn RT, Maisano F, Hagl C, Massberg S, Nabauer M.. Patient selection, echocardiographic screening and treatment strategies for interventional tricuspid repair using the edge-to-edge repair technique. Eurointervention 2018;14:645–653. [DOI] [PubMed] [Google Scholar]
- 8.Nickenig G, Kowalski M, Hausleiter J, Braun D, Schofer J, Yzeiraj E, Rudolph V, Friedrichs K, Maisano F, Taramasso M, Fam N, Bianchi G, Bedogni F, Denti P, Alfieri O, Latib A, Colombo A, Hammerstingl C, Schueler R.. Transcatheter treatment of severe tricuspid regurgitation with the edge-to-edge MitraClip technique. Circulation 2017;135:1802–1814. [DOI] [PubMed] [Google Scholar]
- 9.Mehr M, Taramasso M, Besler C, Ruf T, Connelly KA, Weber M, Yzeiraj E, Schiavi D, Mangieri A, Vaskelyte L, Alessandrini H, Deuschl F, Brugger N, Ahmad H, Biasco L, Orban M, Deseive S, Braun D, Rommel K-P, Pozzoli A, Frerker C, Näbauer M, Massberg S, Pedrazzini G, Tang GHL, Windecker S, Schäfer U, Kuck K-H, Sievert H, Denti P, Latib A, Schofer J, Nickenig G, Fam N, von Bardeleben S, Lurz P, Maisano F, Hausleiter J.. 1-Year outcomes after edge-to-edge valve repair for symptomatic tricuspid regurgitation. Results from the TriValve registry. J Am Coll Cardiol Interv 2019;12:1451–1461. [DOI] [PubMed] [Google Scholar]
- 10.Cai S, Bowers N, Dhoot A, Ho EC, Ong G, Eckstein J, Edwards J, Fam N, Connelly KA.. Natural history of severe tricuspid regurgitation: outcomes after transcatheter tricuspid valve intervention compared to medical therapy. Intern J Cardiol 2020;320:49–54. [DOI] [PubMed] [Google Scholar]
- 11.Besler C, Orban M, Rommel K-P, Braun D, Patel M, Hagl C, Borger M, Nabauer M, Massberg S, Thiele H, Hausleiter J, Lurz P.. Predictors of procedural and clinical outcomes in patients with symptomatic tricuspid regurgitation undergoing transcatheter edge-to-edge repair. J Am Coll Cardiol Cardiovasc Interv 2018;11:1119–1128. [DOI] [PubMed] [Google Scholar]
- 12.Braun D, Rommel K-P, Orban M, Karam N, Brinkmann I, Besler C, Massberg S, Nabauer M, Lurz P, Hausleiter J.. Acute and short-term results of transcatheter edge-to-edge repair for severe tricuspid regurgitation using the mitraclip XTR system. J Am Coll Cardiol Cardiovasc Interv 2019;12:604–605. [DOI] [PubMed] [Google Scholar]
- 13.Lurz P, Stephan von Bardeleben R, Weber M, Sitges M, Sorajja P, Hausleiter J, Denti P, Trochu J-N, Nabauer M, Tang GHL, Biaggi P, Ying S-W, Trusty PM, Dahou A, Hahn RT, Nickenig G.. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol 2021;77:229–239. [DOI] [PubMed] [Google Scholar]
- 14.Fam NP, Braun D, von Bardeleben RS, Nabauer M, Ruf T, Connelly KA, Ho E, Thiele H, Lurz P, Weber M, Nickenig G, Narang A, Davidson CJ, Hausleiter J.. Compassionate use of the PASCAL transcatheter valve repair system for severe tricuspid regurgitation. A multicenter, observational, first-in-human experience. J Am Coll Cardiol Interv 2019;12:2488–2495. [DOI] [PubMed] [Google Scholar]