Abstract
Patent foramen ovale (PFO) has a high prevalence in general population and can be implicated in cryptogenic stroke among young people. Recent trials have shown that transcatheter PFO closure is superior to medical treatment in the secondary prevention of ischaemic stroke. The benefit in the reduction of stroke recurrence is particularly evident in patients who have documentation of a PFO with high-risk characteristics. Therefore, after the assessment of a clear causal relationship with the event, a thoughtful documentation of anatomic (height, length, presence of an aneurysmatic or a floppy atrial septum, a prominent Eustachian valve or Chiari’s network, an acute angle with the inferior vena cava) and functional high-risk characteristics is mandatory.
Keywords: Patent foramen ovale (PFO), Transcatheter PFO closure, Ischaemic stroke
Epidemiology
Foramen ovale is a physiological inter-atrial communication that can pathologically persist after foetal life in ∼25% of adult general population; in this case, the condition is defined as patent foramen ovale (PFO). Due to increased thrombogenic diathesis and possible right to left embolization, PFO is thought to be implicated in ischaemic left circulation embolism.
Each year, around 350 000 patients aged from 18 to 60 years with a PFO experience an embolic stroke of otherwise undetermined cause.1 Cryptogenic ischaemic left circulation embolisms—including cryptogenic stroke (CS)—are defined as any definite ischaemia in the arterial bed that lacks of a known cause despite investigation.2 Embolic stroke of undetermined source is a subcategory of ischaemic CS, denoting non-lacunar strokes without an identifiable aetiology.
When a CS coexists with a PFO, it can be re-classified as PFO-related.
Pathophysiology
Paradoxical embolism is the major suggested mechanism linking PFO to stroke, as it may occur when a thrombus shunts from the venous circulation to the arterial circulation. Alternative pathophysiological processes include thrombus forming within the PFO, left atrial dysfunction, and atrial arrhythmias.
However, demonstrating a certain association between PFO and stroke in a given patient is challenging1; actually, given the high prevalence of PFO in the general population, PFO might be also an incidental finding. A thoughtful assessment of PFO characteristics associated with a high risk for stroke is therefore mandatory to identify the patients who are most likely to benefit from an aggressive therapeutic approach.
High-risk PFO
The diagnostic work-up of CS should be modulated with an age-related algorithm, being events occurring in the early decades more likely related to congenital conditions—namely PFO—, while acquired pathological conditions become more prevalent causes of CS after the fifth decade.
Overall, CS are usually related to silent paroxysmal atrial fibrillation (AF); therefore, AF screening, also by prolonged ECG monitoring, represents the first step of the diagnostic work-up in patients ≥55 years old.2 Other major causes of stroke, like aortic arch and intracranial/extracranial atherosclerotic disease, atrial cardiomyopathy, non-PFO-related sources of paradoxical embolism (arteriovenous malformations, atrial or ventricular septal defects, patent ductus arteriosus, and tetralogy of Fallot), malignancy, bacterial and non-bacterial endocarditis, and inherited thrombophilias should also be considered. Once AF and other major causes of stroke are excluded, investigation of PFO presence and successive identification of ‘high-risk’ characteristics are mandatory.
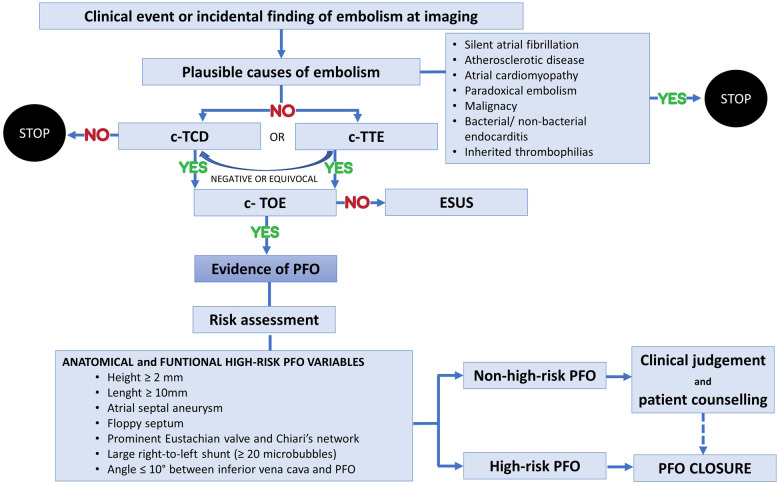
The assessment of the probability that the PFO has a relevant role in the observed clinical scenario and of the likelihood that the event will recur has to be considered to guide the treatment2 (Figure 1).
Figure 1.
Flowchart for PFO-related stroke diagnosis and ‘high-risk PFO’ identification. c-TCD, contrast-enhanced transcranial Doppler; c-TOE, contrast transoesophageal echocardiography; c-TTE, contrast-enhanced transthoracic echocardiography; ESUS, embolic stroke of undetermined source; PFO, patent foramen ovale.
The risk criteria for stroke in patients with a PFO can be categorized into clinical, anatomical, functional, and circumstantial criteria. The Risk of Paradoxical Embolism (RoPE)3 score was based on data from 12 databases and combines some of these criteria to identify stroke-related vs. incidental PFO in CS (Table 1).
Table 1.
RoPe score (modified from Kent et al.3)
| Patient characteristics | Points |
|---|---|
| No history of hypertension | +1 |
| No history of diabetes | +1 |
| No history of stroke or TIA | +1 |
| Non-smoker | +1 |
| Cortical infarct on imaging | +1 |
| Age (years) | |
| 18–29 | +5 |
| 30–39 | +4 |
| 40–49 | +3 |
| 50–59 | +2 |
| 60–69 | +1 |
| ≥70 | 0 |
Although helpful, simple, and practical, the RoPE score should always be used in conjunction with other parameters, as it does not account for high-risk morphological features of the PFO.1 Other clinical clues, conditions that strongly suggest paradoxical embolism in the presence of a PFO, include the simultaneous or previous occurrence of pulmonary emboli or the documentation of a venous source of embolism around the time of stroke, immobilization, recent major surgery, an extended car or airplane journey with possible venous clot development. Activity at the time of the stroke is also relevant—straining manoeuvres, obstructive sleep apnoea with stroke-on-waking should be enquired.
Nakayama et al.4 identified the anatomical and functional high-risk characteristics of PFO by transoesophageal echocardiography (TEE). A ‘large’ PFO is defined for a height ≥ 2 mm, as measured by the maximum separation between the septum primum and septum secundum in the end-systolic frame. A ‘long’ PFO tunnel is defined for a length ≥ 10 mm, as measured by the maximum overlap between the septum primum and septum secundum. Not only the presence of an atrial septal aneurysm (ASA)—a ≥ 10 mm septal excursion from the midline into the right or left atrium or ≥ 15 mm total excursion between the right and left atrium—but also an hypermobile interatrial septum—a floppy septum with excursion ≥ 5 mm in every heartbeat—are characteristics of high-risk PFO. Other anatomic determinants of increased CS risk are the presence of either a prominent Eustachian valve or a Chiari’s network ≥ 10 mm in the right atrium. Finally, also a sharp (≤10°) angle between inferior vena cava and PFO is associated with a high-risk PFO.
The echocardiographic assessment should be completed with a functional evaluation, as a large right-to-left (RL) shunt is defined by the presence of ≥20 microbubbles in the left atrium at rest and during Valsalva manoeuvre, although there is no consensus on the association of a large RL shunt and a higher risk PFO-related stroke. The subgroup analysis of the Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment (RESPECT) trial,5 documented that large shunts were associated with a higher risk of embolic event recurrences, while in the RoPE database also a small shunt was a significant predictor of stroke recurrence among patients who had a higher probability that their PFO was stroke-related rather than an incidental finding.
Nakayama et al.,4 using the above-mentioned anatomical and functional characteristics of PFO by TEE, developed a simple scoring system for the identification of high-risk PFO (Table 2). By scoring each of the factors related to CS as 1 point, a total score ≥ 2 points was strongly associated with CS. A score ≥2 points showed a 91% sensitivity and 80% specificity for the association with CS.
Table 2.
High-risk PFO score calculator (modified from Nakayama et al.4)
| Variables | Point |
|---|---|
| Long-tunnel PFO ≥ 10 mm | 1 |
| Hypermobile interatrial septum | 1 |
| Eustachian valve or Chiari’s network | 1 |
| Large RL shunt during Valsalva maneuver | 1 |
| Low-angle PFO ≤ 10° | 1 |
Among clinical criteria, older age, coagulation disorders, D–dimer >1000 ng/mL at admission, and acetylsalicylic acid use vs. oral anticoagulants expose the patient to a higher PFO-related stroke recurrence rate.
Efficacy of PFO closure
In early 2010s, the availability of percutaneous closure devices was welcomed as the strategy to potentially eradicate the risk of PFO-related stroke recurrence. However, in the early randomized clinical trials (CLOSURE I trial,6 PC trial,7 and RESPECT trial8), PFO closure failed to demonstrate a significant reduction of stroke recurrence as compared with antithrombotic therapy. Lack of high-risk PFO features, clear CS confirmation, and a too short follow-up for a low annual risk of stroke recurrence in the enrolled study populations might have been the major determinants for trials’ failure.
In 2017, other trials including higher risk-PFO patients and with a longer follow-up (CLOSE trial,9 DEFENSE-PFO trial,10 REDUCE trial,11 subgroup analysis of long-term RESPECT study5) demonstrated a significant reduction in ischaemic stroke recurrence with PFO closure as compared with antithrombotic treatment (antiplatelet or anticoagulant therapy).
The most striking benefits favouring the PFO closure strategy were obtained in the DEFENSE-PFO trial,10 where only high-risk PFO patients—with ASA or a PFO size ≥2 mm—were enrolled and adverse events occurred exclusively in the medication-only group during the two-year follow-up.
CLOSE trial9 deemed eligible only 16–60 years old patients with ASA or large shunt PFO characteristics and demonstrated a lower rate of stroke recurrence in the PFO closure plus antiplatelet therapy than in the antiplatelet therapy-only group. A sub-analysis of the RESPECT trial5 confirmed a four-fold reduction in the recurrence of ischaemic events with high-risk PFO closure. Nevertheless, PFO closure was associated with a significant increase in post-procedural AF.5,9–11
A recent meta-analysis from Giacoppo et al.12 documented that the annual incidence of stroke was 0.48/100 person-years after PFO closure and 1.26/100 person-years after medical therapy; the estimated number needed to treat with PFO closure to prevent one stroke over five years was 24 in unselected patients and 13 in patients with high-risk PFO. A larger stroke risk reduction after PFO closure was documented in patients <45 years old, males, with substantial shunts, without no interaction between subgroups.
In conclusion, in patients with the highest probability that PFO has a relevant role in the observed clinical scenario and of recurrence risk, the closure of the PFO should be advised; for patients with intermediate probabilities, clinical judgement is required to allow good decision making in according to the patient. For patients with the lowest probability, medical therapy can be considered. Unclear benefits of PFO closure procedure should be wisely weighted against a non-negligible risk of procedural complications.5
Antithrombotic therapy
Single antiplatelet therapy (SAPT) is the cornerstone of secondary prevention in patients who experienced an ischaemic stroke event, including PFO-related stroke, regardless a PFO-closure procedure has been performed.13 An anticoagulation regimen can be alternatively chosen in the presence of other coexisting indications—such as AF, deep vein thrombosis, pulmonary embolism, or mechanical valve prosthesis.
In patients undergoing percutaneous PFO closure, dual antiplatelet therapy (DAPT) is usually required to prevent an additional 1–2% increase in the risk of thromboembolic events related to device implantation (Figure 2). DAPT with aspirin and clopidogrel is recommended for 1–6 months after device implantation. Caution can be paid to possible delayed device endothelialization and SAPT can also be maintained for at least 5 years to prevent possible late thrombotic events. Anyway, long-term antithrombotic therapy should be considered in patients at high risk for recurrent stroke (e.g. significant residual shunt). Conversely, in high bleeding risk patients, antithrombotic treatment discontinuation after 1 year may be considered, especially if stroke recurrence risk is negligible (young age, successful PFO closure).13
Figure 2.
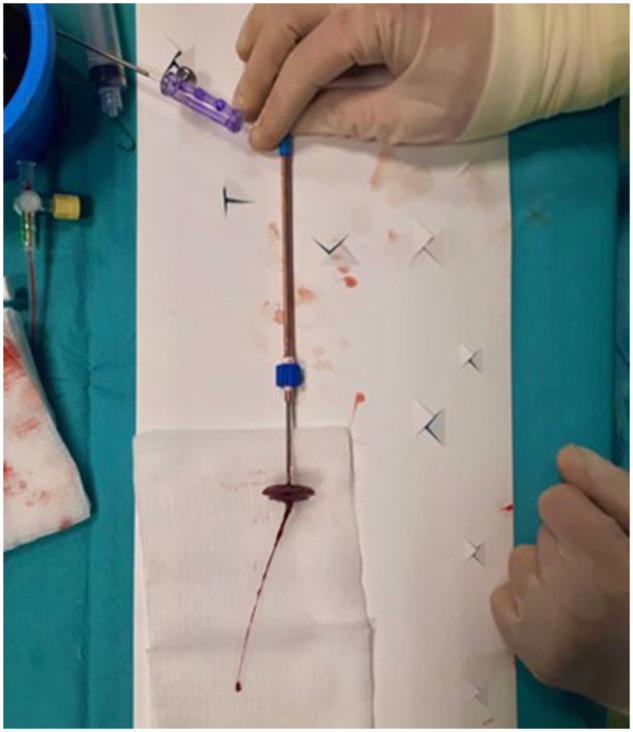
Intra-procedural PFO occluder thrombosis
Long-term implications after PFO closure
After percutaneous PFO closure, rare long-term complications may occur in about 2.6% of cases.2 Residual shunt, atrial arrhythmias, embolization, endocarditis, aortic root erosion, and perforation with pericardial effusion should be systematically searched, especially in the presence of overt symptoms.
Conclusion
In patients with a clear documentation of PFO-related stroke, the risk of annual recurrence rate on medical therapy is low, generally < 2%. Currently available evidence clearly documents an association of percutaneous PFO closure with a stroke recurrence reduction and such benefit seems to be more evident, although not exclusive, in the high-risk PFO group. A cautious approach with a multi-parametric evaluation and a risk-benefit evaluation for both in-hospital and long-term events is anyway recommended, due to the low but not negligible risks related to percutaneous PFO closure.
Conflict of interest: none declared.
References
- 1.Abdelghani M, El-Shedoudy SAO, Nassif M, Bouma BJ, de Winter RJ.. Management of patients with patent foramen ovale and cryptogenic stroke: an update. Cardiology 2019;143:62–72. [DOI] [PubMed] [Google Scholar]
- 2.Pristipino C, Sievert H, D’Ascenzo F, Mas J-L, Meier B, Scacciatella P, Hildick-Smith D, Gaita F, Toni D, Kyrle P, Thomson J, Derumeaux G, Onorato E, Sibbing D, Germonpré P, Berti S, Chessa M, Bedogni F, Dudek D, Hornung M, Zamorano J; European Haematological Society (EHA). European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eurointervention 2019;14:1389–1402. [DOI] [PubMed] [Google Scholar]
- 3.Kent DM, Ruthazer R, Weimar C, Mas J-L, Serena J, Homma S, Di Angelantonio E, Di Tullio MR, Lutz JS, Elkind MSV, Griffith J, Jaigobin C, Mattle HP, Michel P, Mono M-L, Nedeltchev K, Papetti F, Thaler DE.. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013;81:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama R, Takaya Y, Akagi T, Watanabe N, Ikeda M, Nakagawa K, Toh N, Ito H.. Identification of high risk patent foramen ovale associated with cryptogenic stroke: development of a scoring system. J Am Soc Echocardiogr 2019;32:811–816. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med 2017;377:1022–1032. [DOI] [PubMed] [Google Scholar]
- 6.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366:991–999. [DOI] [PubMed] [Google Scholar]
- 7.Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Jüni P; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368:1083–1091. [DOI] [PubMed] [Google Scholar]
- 8.Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368:1092–1100. [DOI] [PubMed] [Google Scholar]
- 9.Mas J-L, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O, Guidoux C, Canaple S, Vaduva C, Dequatre-Ponchelle N, Sibon I, Garnier P, Ferrier A, Timsit S, Robinet-Borgomano E, Sablot D, Lacour J-C, Zuber M, Favrole P, Pinel J-F, Apoil M, Reiner P, Lefebvre C, Guérin P, Piot C, Rossi R, Dubois-Randé J-L, Eicher J-C, Meneveau N, Lusson J-R, Bertrand B, Schleich J-M, Godart F, Thambo J-B, Leborgne L, Michel P, Pierard L, Turc G, Barthelet M, Charles-Nelson A, Weimar C, Moulin T, Juliard J-M, Chatellier G;. CLOSE Investigators. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med 2017;377:1011–1021. [DOI] [PubMed] [Google Scholar]
- 10.Lee PH, Song J-K, Kim JS, Heo R, Lee S, Kim D-H, Song J-M, Kang D-H, Kwon SU, Kang D-W, Lee D, Kwon HS, Yun S-C, Sun BJ, Park J-H, Lee J-H, Jeong HS, Song H-J, Kim J, Park S-J.. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO trial. J Am Coll Cardiol 2018;71:2335–2342. [DOI] [PubMed] [Google Scholar]
- 11.Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, Spence JD, Thomassen L; Gore REDUCE Clinical Study Investigators. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 2017;377:1033–1042. [DOI] [PubMed] [Google Scholar]
- 12.Giacoppo D, Caronna N, Frangieh AH, Michel J, Andò G, Tarantini G, Kasel AM, Capodanno D, Byrne RA.. Long-term effectiveness and safety of transcatheter closure of patent foramen ovale compared with antithrombotic therapy alone: a meta-analysis of six randomised clinical trials and 3,560 patients with reconstructed time-to-event data. Eurointervention 2018;14:857–867. [DOI] [PubMed] [Google Scholar]
- 13.Calabrò P, Gragnano F, Niccoli G, et al. Antithrombotic therapy in patients undergoing transcatheter interventions for structural heart disease. Circulation 2021. doi:CIRCULATIONAHA/2021/054305R1. [DOI] [PubMed] [Google Scholar]