Abstract
Benzophenanthridines belong to the benzylisoquinolic alkaloids, representing one of the main groups of this class. These alkaloids include over 120 different compounds, mostly in plants from the Fumariaceae, Papaveraceae, and Rutaceae families, which confer chemical protection against pathogens and herbivores. Industrial uses of BZD include the production of environmentally friendly agrochemicals and livestock food supplements. However, although mainly considered toxic compounds, plants bearing them have been used in traditional medicine and their medical applications as antimicrobials, antiprotozoals, and cytotoxic agents have been envisioned. The biosynthetic pathways for some BZD have been established in different species, allowing for the isolation of the genes and enzymes involved. This knowledge has resulted in a better understanding of the process controlling their synthesis and an opening of the gates towards their exploitation by applying modern biotechnological approaches, such as synthetic biology. This review presents the new advances on BDZ biosynthesis and physiological roles. Industrial applications, mainly with pharmacological approaches, are also revised.
Keywords: alkaloids, benzylisoquinoline, benzophenanthridines, natural products, specialized metabolism
1. Introduction
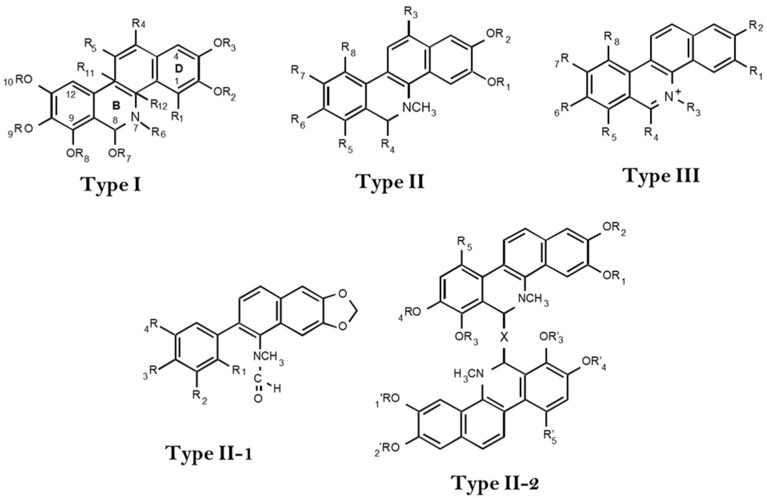
Benzophenanthridines (BZD) represent one of the 11 classes of benzylisoquinoline alkaloids (BIA). BZD include over 120 alkaloids mainly spread in plants from the Fumariaceae, Papaveraceae, and Rutaceae families, within the Ranunculales and Sapindales orders [1]. Structurally, BZD are tetracyclic compounds, which include a non-aromatic heterocyclic system (B ring). Depending on the ring organization, BZD could be classified in three different groups: Type I (hexahydrobenzo[c]phenanthridines) includes two aromatic (A and D) and aliphatic (B and C) rings, in which the N atom in B is usually methylated. In BZD type II (dihydrobenzo[c]phenanthridines), the N atom is also methylated; however, ring B could be absent when the C7-C8 bond is open (type II-1) or modified with a complex substituent at C8 (type II-2). Regardless of ring B being absent, rings A, C, and D are aromatic systems. Finally, BDZ type III results from the N protonation of BDZ type I or II, leading to the corresponding ammonium quaternary salts (Figure 1) [2,3]. Depending on the chemical modifications within these basic structures, up to seven groups can be distinguished: hexahydrobenzophenanthridines (type I), seco-benzophenanthridines (type II-1), dimeric dihydrobenzophenanthridines (type II-2), N-demethylbenzophenanthridines, dihydrobenzophenanthridines, benzophenanthridones, and quaternary benzophenanthridine alkaloids (type III; Figure 1) [2,3].
Figure 1.
Chemical structure of the different benzophenathridines (BZD).
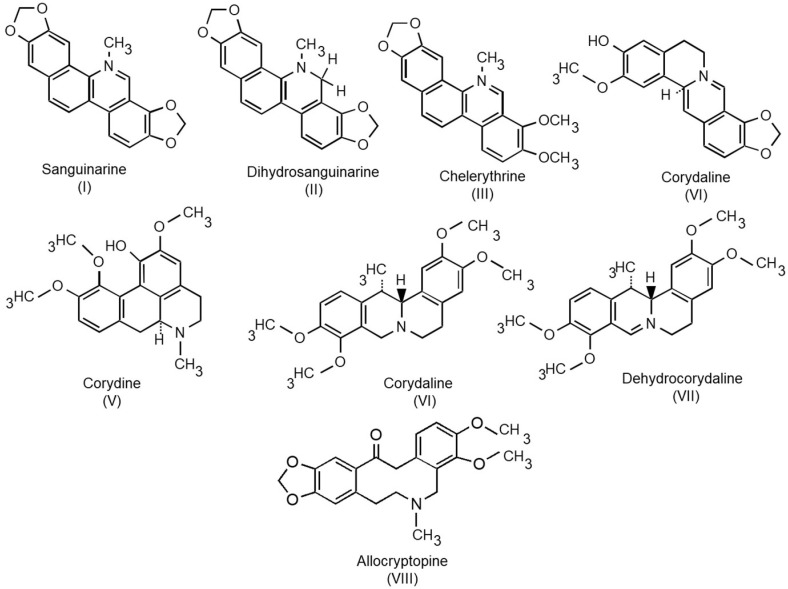
Plants bearing BDZ have had important roles in human traditional medicine in both the old and new world cultures for centuries. For example, the great celandine (Chelidonium majus L.; Papaveraceae) was used by ancient Greeks to treat eye cataracts, whereas the fern leafed corydalis (Corydalis cheilanthifolia Helms; Fumarioideae/Papaveraceae) is mentioned in antique Chinese treatises for irregular menses. In the Americas, prior to Europeans’ arrival, the prickly poppy (Argemone mexicana L., Papaveraceae) was recommended to remove genital warts and for other skin infections [4]. Some of the alkaloids found in these plants are shown in Figure 2 and include sanguinarine (I) and its reduced dihydro form, dihydrosanguinarine (II), chelerythrine (III), cheilanthifoline (IV), corydine (V), corydaline (VI), and dehydrocorydaline (VII), among others. Table 1 lists some plant species that accumulate in BZD alkaloids as well as their different uses for medicinal purposes. There is an increasing interest in sanguinarine and other BZD alkaloids due to their diverse pharmacological effects and possible functions in plant–environment interactions. Here, a review of the new advances on the BDZ biosynthetic process and physiological roles is presented. Industrial applications, mainly with pharmacological approaches, are also revised.
Figure 2.
Chemical structure of the BZD analyzed in this review.
Table 1.
A few plants bearing BZD alkaloids and their traditional medicinal uses.
| Plant Species | Applications | References |
|---|---|---|
| Argemone mexicana L. (Papaveraceae) | Antiprotozoals: to dissolve eye cataracts, to remove warts, and to treat skin infections | [4] |
| Chelidonium majus L. (Papaveraceae) | Skin, liver, and eye diseases; antiparasitic | [5] |
| Corydalis yanhusuo Chou (Papaveraceae) | Analgesic for chest pain, post-partum blood stasis, and spleen and stomach stasis | [6,7] |
|
Macleaya cordata Willd (Papaveraceae) |
Anti-inflammatory and antimicrobial activities | [8] |
|
Eschscholzia californica Cham (Papaveraceae) |
Sedative, anxiolytic, analgesic, soporific, spasmolytic, diuretic, and diaphoretic | [9,10] |
|
Sanguinaria canadensis L. (Papaveraceae) |
To treat cold and congestion, sore throats, emetic, abdominal cramps, lumps, wound infections, and rheumatism | [11] |
2. The Biosynthesis of Sanguinarine and Related BZD: Physiological Roles and Applications
The following sections present a view on the most relevant advancements on the topic.
2.1. Synthesis and Regulation of Sanguinarine and Related Alkaloids
2.1.1. The Biosynthetic Pathway
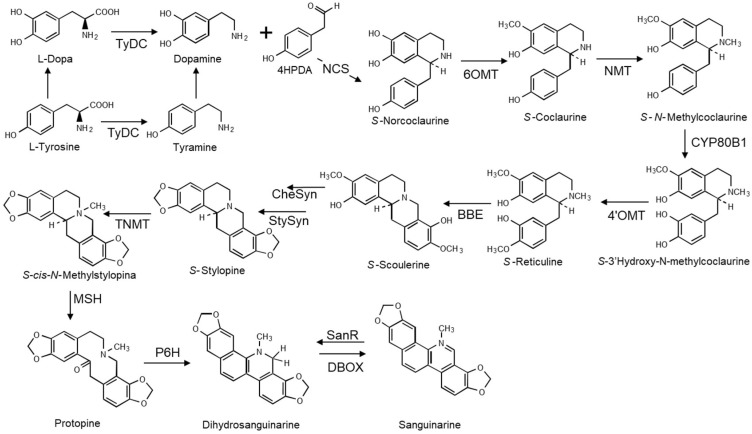
The synthesis of BZD shares initial reactions with other BIA (Figure 3), starting with two units of l-tyrosine. One of them is decarboxylated, forming tyramine, which is then hydroxylated into dopamine. Alternatively, 3,4-dihydroxyphenylalanine (l-dopa) could be directly decarboxylated. Regardless of how dopamine is formed, it is later condensed with 4-hydroxyphenylacetaldehyde (4HPDA), which is the result of tyramine deamination, producing s-norcoclaurine, the central trihydroxylated intermediary from which all the wide BIA diversity will rise (Figure 3). Enzymes involved in these reactions are shown in Table 2. Among those, norcoclaurine synthase (NCS) has been studied in different species, displaying a positive substrate-binding typical of limiting enzymes [12]. Norcoclaurine is transformed in s-reticuline after an ordered series of reactions involving O-6 methylation, N methylation, C3′ hydroxylation, and O-4′ methylation (Figure 3 and Table 2). Although the purified enzymes involved in these reactions could accept structurally related substrates in in vitro assays, all of them display a marked preference for BIA intermediaries [13]. Besides, alternative products could be formed in some species, which results in nearly 300 different alkaloids (Figure 3). This catalytic flexibility, associated to a recent evolutive divergence, is one of the reasons for BIA diversity and the restricted occurrence of different BIA types to a few related species [14].
Figure 3.
Biosynthetic pathways leading to sanguinarine. TyDC, l-tyrosine decarboxylase; NCS, s-norcoclaurine synthase; 6OMT, s-norcoclaurine-6-O-methyltransferase; BBE, reticuline oxidase: berberine bridge enzyme; CheSyn, cheilanthifoline synthase; StySyn, stylopine synthase; TNMT, s-tetrahydroprotoberberine N-methyltransferase; MSH, methyltetrahydroprotoberberine 14-monooxygenase; P6H, protopine 6-hydroxylase; DBOX, dihydrobenzophenanthridine oxidase; and SanR, sanguinarine reductase.
Table 2.
Enzymes involved in sanguinarine biosynthesis, describing reaction catalyzed and subcellular localization. Data were collected from UniProt Beta (https://beta.uniprot.org/ (accessed on 12 December 2021)).
| Enzyme and EC Number | Function | Subcellular Localization | Organisms amd Acc. Number |
|---|---|---|---|
| l-Tyrosine decarboxylase (TyDC) EC 4.1.1.25 | Decarboxylates of l-tyrosine to produce tyramine | Cytosol |
P. somniferum (P54768) Thalictrum flavum (Q9AXN7) A. mexicana (D2SMM8) |
|
s-Norcoclaurine synthase (NCS) EC 4.2.1.78 |
Condenses dopamine and 4-HPDA, producing s-norcoclaurine | Endoplasmic reticulum lumen and vacuole |
P. somniferum (Q4QTJ2) T. flavum (Q67A25) A. mexicana (EU881891) |
|
s-Norcoclaurine-6-O-methyltransferase (6OMT) EC 2.1.1.128 |
Transfers a methyl group from SAM to s-norcoclaurine, forming coclaurine, and to r,s-norcoclaurine, formimg r-norprotosinomenine, s-norprotosinomenine, and (r,s)-isoorientaline | Membrane integral protein |
P. somniferum (Q6WUC1) Coptis japonica (Q9LEL6) |
| Reticuline oxidase: berberine bridge enzyme (BBE) EC:1.21.3.3 |
Converts s-reticuline in s-scoulerine by forming of a carbon–carbon bond (C8) between the N-methyl group and the phenolic ring | Cytoplasmic vesicles |
P. somniferum (P93479) E. californica (P30986) A. mexicana (D2SMM9) |
| Cheilanthifoline synthase (CheSyn) EC:1.14.19.65 | Converts s-scoulerine into r,s-cheilanthifoline by forming a methylenedioxy brigde | Endoplasmic reticulum membrane |
E. californica (B5UAQ8) A. mexicana (CYP719A14) |
| Stylopine synthase (StySyn) EC:1.14.19.73 |
Forms a methylenedioxy bridge on ring A (2,3 position), transforming s-cheilanthifoline to s-stylopine, s-scoulerine to s-nandinine, and s-tetrahydrocolumbamine to s-canadine | Smooth endoplasmic reticulum membrane |
E. californica (Q50LH4) A. mexicana (B1NF19) |
| s-Tetrahydroprotoberberine N-methyltransferase TNMT EC:2.1.1.122 | Converts stylopine, canadine, and tetrahydropalmatine to their corresponding N-methylated products | Cytosol |
P. somniferum (Q108P1) E. californica C3SBS8 |
| Methyltetrahydroprotoberberine 14-monooxygenase (MSH) EC:1.14.14.97 | Transforms, by oxidation, N-methylstylopine and N-methylcanadine into protopine and allocryptopine, respectively | Membranal protein | P. somniferum (L7X3S1) |
| Protopine 6-hydroxylase (P6H) EC:1.14.14.98 | Converts protopine and allocryptopine to dihydrosanguinarine and dihydrochelerythrine by hydroxylation at position 6 | Endoplasmic reticulum membrane |
E. californica (F2Z9C1) P. somniferum (L7X0L7) |
| Dihydrobenzophenanthridine oxidase (DBOX) EC 1.5.3.12 |
Catalyzes a two-electron oxidation of dihydrosanguinarine, forming sanguinarine | Endoplasmic reticulum | P. somniferum (AAC61839) |
| Sanguinarine reductase (SanR) EC:1.3.1.107 | Catalyzes reduction of benzophenanthridines, preferentially sanguinarine, to the dihydroalkaloids; involved in detoxifying the phytoalexins produced by plant itself | Vacoule | E. californica (D5JWB3) |
Reticuline represents another important branching point, leading, on one hand, to the formation of morphinan and protomorphinan alkaloids, and to the groups of protoberberines, secoberberines, protopines, and BZD [14]. The first reaction in this set is the formation of the methylene bridge at C8, producing s-scoulerine, catalyzed in a stereo-specific manner by the berberine bridge enzyme (BBE; Figure 2), which is rapidly induced in response to pathogen and chemical elicitors, and thus related to plant defense in different species [15]. Oxidation of methoxy groups on rings A and D form the corresponding methylendioxy bridges rendering s-chelantifoline (III) and s-stylopine, respectively (Figure 3) [16,17]. N-methylation of stylopine to s-N-methylstylopine and its further oxidation produces protopine, which is then 6-hydroxylated, driving it to the spontaneous opening of the C ring as well as to the rearrangement and dehydration yielding dihydrosanguinarine (II), the first BDZ alkaloid, which is then oxidized to sanguinarine (I) [13] (Figure 3 and Table 2). This biosynthetic route, proposed earlier by the trace of radiolabeled precursors [18], has been confirmed by biochemical and genetic studies, including high throughput approaches in different species such as Argemone mexicana [17], Corydalis yanhuso [6], Eschscholzia californica [19], Macleaya cordata [8], and Papaver somniferum [13], among others. However, species-associated particularities have been noticed in some cases. For example, in A. mexicana, CYP719A13 encodes a cytochrome P450 with activity of both stylopine and canadine synthase since it can accept cheilanthifoline (IV) as well as tetrahydrocolumbamine as substrates, transforming them into stylopine and canadine, respectively, and hence participating in sanguinarine (I) and berberine synthesis [17]. Moreover, enzymes involved in the formation of other BZD, such as chelerythrine (III), have also been identified by combining transcriptomic and metabolomic approaches [8]. Figure 3 depicts the reactions involved in the synthesis of selected BZD alkaloids, showing the acronyms for the enzymes involved in each reaction. Table 2 lists details of the identified enzymes.
2.1.2. Tissue Distribution and Regulation
Commonly, sanguinarine (I) and other BZD alkaloids are synthetized in underground tissues in species of Argemone, Papaver, and Sanguinaria, where enzymes are distributed between the smooth endoplasmic reticulum (SER), both inside in the lumen and membrane-bound, and the cytosol [16,20]. After their synthesis, alkaloids are accumulated in vacuoles with the participation of a vesicle-mediated transport system [21]. In fact, this vesicle-mediated transport system allows for the mobilization of intermediaries, as they are being modified by the membrane-bound enzymes, in the track from SER to vacuole. Moreover, conditions promoting sanguinarine (I) synthesis also concomitantly induce major SER ultrastructural modifications [22].
However, both synthesis and accumulation could also take place in aerial tissues in Bocconia, Chelidonium, and Macleaya [8,23]. Mobilization of sanguinarine (I) through ABCB-type transporters has been determined in A. mexicana and E. californica. EcABCB1 was preferentially expressed in the roots of E. californica, which is the major site of accumulation for this alkaloid [24]. Interestingly, AmABCB1 from A. mexicana was reported mainly to be expressed in seeds with an important expression also observed in roots and in very low levels in leaves [25]. The specific role that sanguinarine could have in seeds of A. mexicana is unknown but it is suggested that it might act as a defense against herbivores due to its high toxicity.
Genes involved in the biosynthesis of sanguinarine (I) and other BIA have been reported under transcriptional regulation of different elements, such as basic Helix-Loop-Helix (bHLH) and WRKY proteins. In E. californica, a number of sanguinarine biosynthetic genes were under the control of two bHLH proteins, named EcbHLH1-1 and -2, whereas tetrahydroprotoberberine cis-N-methyltransferase (TNMT) was regulated by EcbHLH1-1 and EcbHLH1-2-controlled 4′OMT. Interestingly, 6′OMT and stylopine synthase CYP719A3 (Figure 2) were regulated by both proteins [26]. The heterologous expression of a Coptis japonica WRKY protein (CjWRKY) in E. californica-cultured cells increased the production of sanguinarine and 14 other components, and this was related to an increased expression of biosynthetic genes [27].
2.2. Physiological Roles of Benzophenanthridines
Specialized metabolites play critical roles in plant fitness to their surroundings. While some of them can act to attract pollen and seed dispersers, others exert toxic effects to vertebrate and invertebrate animals, fungi, and bacteria. As other plant alkaloids, BZD display toxic effects against herbivores [28] and different soil bacteria and fungi [29]. Sanguinarine has been the most studied among BZD alkaloids, displaying both herbivore deterrent activity as well as antimicrobial effects. Structural features conferring these physiological effects are related to BZD planar configuration, which allows them to intercalate in nucleic acids, affecting both DNA and RNA synthesis. Besides, as heteroaromatic iminium cations, BZD could bind to negatively charged membrane surfaces and proteins, and react with SH-compounds, interfering, in such a way, with the function of several cytosolic and membranal proteins, such as collagenase, tubulin assembly, and Na+/K+ ATPases, among others [30].
Sanguinarine (I), as other alkaloids, modifies its chemical behavior depending on the external pH. It acts as a polar, hydrophilic iminium cation or as a lipophilic, uncharged alkaloamine at pH values lower or higher than six, respectively [2]. In this way, both forms could coexist at a physiological pH. Interestingly, although bioactivity is associated with the iminium form, the low polar alkanolamine exhibits membrane permeability, which allows the alkaloid free movement across organelle membranes. Once inside the compartment’s lumen, interaction with an acidic milieu, such as the presence of a nucleic acid, produces an equilibrium shift towards the iminium active form [2,30]. Alternating these chemical forms ensures movement of the inactive, low toxic forms, which would turn into active ones once the target is reached, without participation of protein receptors.
2.2.1. Herbivore Deterrence of Benzophenanthridines
Sanguinarine (I) interferes with the nerve impulse transmission in arthropods and vertebrates due to its notable inhibitory effects on choline acetyltransferase activity (IC50 284 nM). It also hinders nicotinergic, muscarinergic, and serotonin-2 receptors, impairing, in this way, nerve transmission [10,31,32]. In addition to these effects, sanguinarine also reduces the feeding activity of Lymantria dispar (Lepidoptera) larvae on C. majus plants (LD50 of 4.963 μg/larva). Overall, these responses, which eventually lead to larvae mortality, were associated to the inhibition of digestive enzymes, such as α-amylase, lipase, and serine protease at the transcriptional level [33].
Interestingly, herbivory induces an increase in the contents of sanguinarine in plants, such as E. californica [34], Chelidonium majus [33], M. cordata [29], and Sanguinaria canadensis [35]. Other BZD, such as allocryptopine (VIII), cheilanthifoline (IV), and chelerythrine (III) with similar insecticidal effects, although to a lesser extent, were also augmented in some of these species, suggesting a common effect of BZD on predators’ nerve impulses and herbivore deterrence.
2.2.2. Antimicrobial Activity of Benzophenanthridines
Most studies on BZD antimicrobial activity are related to their potential as pharmaceuticals with relatively few published works on their direct role in plant chemical defense. Different fungi-caused diseases, such as powdery mildew, blight, and root rot, have been observed in plants producing BZD alkaloids, including Eschscholzia [36], Macleaya [37,38], and Papaver [35,39], among others. Fusarium oxysporum and different species of Erysiphe, Dendryphion, and Pleospora have been identified as the etiological agents. Interestingly, even when sanguinarine (I), chelerythrine, and other BZD show antifungal activities [40,41], the direct effects of these fungi infections on alkaloid biosynthesis have not been analyzed in planta, despite abundant studies in in vitro cultures [42]. For instance, cell cultures of A. mexicana respond to the addition of F. oxysporum homogenates by increasing sanguinarine formation after activation of the octadecanoic signaling pathway [43,44]. Similar responses have also been noted in Eschscholzia [21] and Papaver [1], among others. Moreover, complex signaling pathways leading to the activation of alkaloid synthesis, which involve heterotrimeric G proteins, phospholipase A2, and cytoplasmic pH changes, have been established in cell cultures, suggesting an important role of these alkaloids in chemical defense [45,46].
Sanguinarine (I), along with chelerythrine (III) and corydaline (VI) to a lesser extent, displayed fungicidal activity against eight phytopathogenic fungi, including Botrytis cinerea, Fusarium graminearum, F. oxysporum, and Magnaporthe oryzae. These BZD-induced morphological abnormalities in mycelia resulted from deformed hyphae that eventually collapsed due to the disruption of membranes’ integrity. Moreover, an upsurge of reactive oxygen species (ROS) was observed in fungi exposed to BZD, mainly to sanguinarine, and it was associated to alterations in the redox potential of mitochondrial membranes and modifications in the nuclear morphology [47]. These hyphal deformations in M. oryzae resulted in a lower appressorium tissue penetration in barley and disturbed the proper germ tubes’ formation from spores. Such effects are derived from the interference of sanguinarine (I) with the fungus cAMP-mediated signaling pathways [48] and could explain the antifungal effects.
Sanguinarine (I) and chelerythrine (III) also showed antimicrobial effects against soil-borne pathogenic bacteria, such as Agrobacterium tumefaciens, Pseudomonas lachrymans, and Xanthomonas vesicatoria [49]. These effects are mainly due to alkaloid interference in the assembly of the FtsZ protein, a tubulin homologous, into filaments that conform the cytokinesis contracting belt, thus hampering bacterial fission. A direct binding of alkaloids’ dimethoxy and isoquinoline groups to the protein domains involved in polymerization has been observed [50].
Interestingly, even when different pathogenic viruses have been isolated from Papaver species, which accumulates sanguinarine [51,52], no studies on the effect of such infections on alkaloid synthesis have been reported.
2.3. Medical and Industrial Applications of Benzophenanthridines
Perhaps the main industrial use of BDZ in commercial products is the manufacturing of livestock feed additives from M. cordata (Sangrovit®; Phytobiotics Futterzusatzstoffe GmbH; Eltville am Rhein, Germany). The product is sold as pellets or pearls made from dried plant material that contain sanguinarine, protopine, and chelerythrine in no less than 60% on a dry weight basis and it is claimed to improve food digestibility in broilers, swine, cattle, sheep, and farmed fisheries. These effects have been related mainly to alkaloids, helping to control intestinal infections [53]. Interestingly, sanguinarine (I) inhibits the formation of biofilms by different enterobacteria, such as carbapenem-resistant Serratia marcescens and Providencia rettgeri [54,55]. Additionally, sanguinarine could avoid inflammation and retard amino acid transit by inhibiting amino acid decarboxylases’ action [56]. Until recently, antiplaque mouth washes and toothpaste containing extracts from S. canadensis were commercialized (Viadent®; Colgate-Palmolive Inc, New York City, NY, USA), but they are no longer available [11]. Extracts of A. mexicana are mixed with other plants in the elaboration of ecofriendly insecticides (BioDie; Promotora Técnica Industrial SA, Jiutepec México) [4]. Noteworthy is that, although mainly considered toxic compounds [57], BZD alkaloids also display similar pharmaceutical effects, although with different efficacy, due to the same structural features exposed above. BZD exhibit hypotensive, antimicrobial, antioxidant, and anti-inflammatory properties [56]. Moreover, cytotoxic and cytostatic effects on different human cancer cells have also been reported [30]. No cell receptors for these alkaloids have been described; hence, their physiological activity depends on their diffusion into cells and chemical reactivity with membranes, nucleic acids, and proteins, and their consequent interference in different biochemical processes. For example, the disruption of membrane electrochemical gradients, caused by inhibition of Na+/K+ ATPases, also hampers different signal transduction pathways, including those mediated by mitogen-activating protein kinases (MAPK), reactive oxygen species (ROS), and intracellular calcium, which, in turn, could be involved in cell death and apoptosis pathways [58]. It has been observed that chelerythrine, chelidonine (stylophorin), cordatine, and nitidine induce ROS formation in tumoral cell lines from diverse human tissues, including bladder, breast, glioma, lung, kidney, pancreas, prostate, skin, stomach, and uvea, among others [30,57,58,59]. ROS accumulation induced by BZD activates different components of the apoptotic transduction pathways, including both caspase-dependent and independent routes [58]. Such an increase in ROS cell levels is therapeutically valued since recent anticancer approaches are aimed at agents that trigger a controlled upsurge, leading to the activation of the antioxidant cell response, which, in turn, would attenuate tumor development [30,59,60]. Besides these pleiotropic effects and nucleic acid intercalation, direct interaction with enzymes, such as DNA pol, topoisomerases, etc., and membrane lipid peroxidation also account for bioactivity. Increased ROS levels, induced by BZD, also have additional beneficial medical implications for type 2 diabetes (T2D) treatment. Extracts from Fumaria parviflora have shown hypoglycemic effects in rats, which were associated to sanguinarine contents, and bioinformatic prospections pulled out sanguinarine (I) in the top 20 out 6100 candidates with alleged antidiabetic properties [61]. Recently, sanguinarine has been shown to modulate ROS accumulation in kidney tissues of streptozotocine-induced diabetic rats. This not only reduced the expression of tumoral markers but also resulted in protection against diabetic nephropathy [62]. These activities rely on their structural features. Structural requirements for activity include planar configuration, N methyl substitutions, hydroxylation patterns, and the fusion of the B/C ring [2,3,57].
Sanguinarine (I), chelerythrine (III), and their direct dihydro derivatives are the most studied alkaloids among BZD. However, other minor compounds also display similar physiological activities, although with wide variations in their effective doses [2,3]. Table 3 shows some of the main BZD alkaloids with their ascribed pharmacological activity and the biochemical mechanisms involved.
Table 3.
Pharmacological effects of sanguinarine and related alkaloids.
| Alkaloid | Effects | Mechanism | References |
|---|---|---|---|
| Sanguinarine (I) | Antimicrobial | Halts formation of contracting belt by binding to the FtsZ protein | [50,63] |
| Interferes with carbohydrate metabolisms by inhibiting glucose transport and the 2-ketogluconate pathway | |||
| Increases sensitivity to β-lactam antibiotics | |||
| Antiretroviral | Inhibits transcriptase reverse | [64] | |
| Anticancer | Cytotoxic Intercalates DNA and RNA, affecting topoisomerase action and cell division Arrests cell cycle at S and G1 phases by interfering with cyclins and CDK Activates and modulates ROS depending on apoptotic pathways through effects on p53, Bcl-2, caspases, IAP, and autophagy affecting MAPK and ERK Tumor development and metastasis Restrains neovascularization by downregulating expression of the endothelial growth factor Reinforces cell-tight junction Chemosensitization Potentiates cytotoxicity of different agents |
[30,59,65,66,67,68,69,70,71,72] | |
| Anti-inflammatory | Reduces the release of proinflammatory cytokines TNF-α; IL-1β; and IL-6 | [71,72] | |
| Chelerythrine (III) | Adjuvant in COVID-19 treatment Anti-inflammatory |
Prevents hyper-inflammatory immune response regulating signaling pathways mediated by Nrf2, NF-κB, and p38 MAPK | [73,74] |
| Reduces protein kinase C-α/-β inhibitory activity, preventing cerebral vasospasm, eryptosis, and pulmonary inflammation and fibrosis | |||
| Antiviral | Viral RNA-intercalation | [73] | |
| Anticancer | Reduces phosphorylation of ERK and Akt, downplaying the activation of p53, B-cell Bcl-2, caspases, and PARP | [74] | |
| Cheilanthifoline (IV) | Anti-inflammatory | Reduces the release of proinflammatory cytokines and anti-AChE | [75] |
| Antimicrobial | Hinders expression of MRSA genes and disrupts membrane integrity | [76] |
3. Concluding Remarks
In plants, BDZ alkaloids are part of an elaborated chemical defensive system sensitive to potentially harmful environmental stimuli, such as microbial infections and insect foraging. Interaction with these biological agents sets up a chain of biochemical events leading to the transcriptional activation of genes involved in their synthesis and mobilization [21,44,45,46,47]. The defensive role of secondary metabolites, such as alkaloids, in plants has turned into a valuable biotechnological tool, which is frequently used in in vitro cell cultures to increase their accumulation. Cell cultures submitted to conditions mimicking microbial infection or environmental hazards respond by increasing transcriptional activity, leading to the synthesis of these defensive compounds (the process is known as elicitation) [77]. Cell cultures from different species from the Papaveraceae family [42], including A. mexicana [78], E. californica [21], M. cordata [8], and P. somniferum [79], show this response when challenged by exposition to different stimuli. Besides its potential for commercial exploitation, elicitation has also allowed for the discovery of new enzymes [42,80] and has led to the design of ingenious approaches for chemical semisynthetic processes. For example, recently, an interest on BZD dihydro forms, mainly dihydrosanguinarine (II), has surged due to its minor cytotoxicity [81]. In plants, sanguinarine reductase (SanR; Figure 3) leads to the NADH-dependent reduction of sanguinarine and other BZD to the lesser toxic dihydro derivatives. This is part of a mechanism to avoid cell damages caused by an increase in the alkaloid accumulation in response to pathogen infection [21]. This response has instigated the development of a biomimetic approach for the reduction of the N7=C8 double-bond located in the B ring by incubating sanguinarine (I) with NADH under 455 nm blue radiation. This reaction produced a dihydrosanguinarine (II) dimer but also can be applied for the semi-synthesis of different natural substituted dihydroBZD [81]. In fact, an efficient total synthesis of selected BZD, such as chelerythrine (III) and sanguinarine (I), has been recently reported using affordable materials, such as 7-azabenzonorbornadiene and based on enzyme mechanisms [82]. However, although well-established at the cellular level, details on the operation of these mechanisms in integral tissues or the whole plant are yet to be discovered. Moreover, the complete biosynthetic pathway required for sanguinarine (I) and other BZD alkaloids has been isolated from different species [6,8,16,19], and some regulatory genes, and their corresponding cis-elements are also available [26,27]. This has allowed for dissecting which components of the pathway are responsive to specific biochemical mediators [45] and the mechanisms involved in this response. On the other hand, although still under observation due to undesirable side effects, the medical applications of sanguinarine (I) and related alkaloids is an area of intensive research (Table 3). Interestingly, BZD have been considered both cancerogenic and anticancer agents [83]. Epidemiological evidence links the use of mouthwashes added with sanguinarine to maxillary vestibule oral leukoplakia as well as its involuntary consumption in contaminated mustard oil to gall bladder [11,83]. However, different genotoxicity assays and animal tests for cancer genesis often deliver non-conclusive results. Therefore, neither sanguinarine (I) nor other BZD are currently listed as proved cancer-producing agents [83]. The BZD planar structure is similar to other polyaromatic hydrocarbon carcinogens that allow for intercalation in the DNA [2,3]. Besides, BZD induce cell oxidative burst with ROS formation. Paradoxically, although ROS accumulation triggered by BZD could cause DNA damage, it also could be the basis of their therapeutical application against cancer. This may be explained by the fact that a controlled increase in ROS could induce the activation of internal cell mechanisms directed to the mitigation of oxidative harm [58,59]. Moreover, sanguinarine and other BZD inhibited the P-glycoprotein/ABCB1 and related ABCB5 in drug resistance in multidrug-resistant tumor lines, increasing their sensitivity to cytotoxic drugs, which might allow for better treatments [84]. In this way, knowledge generated on the synthesis and regulation of these alkaloids could now be directed towards the generation of tools for their commercial exploitation by modern biotechnological methods, including cell culture technology and synthetic biology approaches. Scaling up for massive culture of elicited cell suspension has been reported [79] and the introduction of the complete sanguinarine (I) pathway to yeast cells has been recently achieved [85]. Moreover, the availability of the complete set of genes involved in sanguinarine (I) synthesis, as well as some of the regulatory genes and those involved in its mobilization, would allow for improving not only the enzymatic catalysis by gene edition but also the internal cell traffic of intermediaries, resulting in more efficient processes for the formation of these valuable alkaloids.
Acknowledgments
The authors thank M.L. Miranda-Ham for her critical review of this article.
Author Contributions
Conceptualization, original draft preparation, review and editing, and project administration, F.V.-F.; review on biosynthesis and enzymes involved, J.I.L.-H.; review on tissue distribution and regulation, L.L.-M.; and review on uses and applications, J.A.M.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was funded by the National Council for Science and Technology (CONACyT; México), grant number CB-2016; 0285887. JIL-H and LL-M are recipients of the CONACYT scholarship for doctoral studies (815650 and 514907/289293, respectively).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh A., Menéndez-Perdomo I.M., Facchini P.J. Benzylisoquinoline alkaloid biosynthesis in opium poppy: An update. Phytochem. Rev. 2019;18:1457–1482. doi: 10.1007/s11101-019-09644-w. [DOI] [Google Scholar]
- 2.Han N., Yang Z., Liu Z., Liu H., Yin J. Research progress on natural benzophenanthridine alkaloids and their pharmacological functions: A review. Nat. Prod. Commun. 2016;11:1181–1188. doi: 10.1177/1934578X1601100838. [DOI] [PubMed] [Google Scholar]
- 3.Bisai V., Saina Shaheeda M.K., Gupta A., Bisai A. Biosynthetic relationships and total syntheses of naturally occurring benzo [c] phenanthridine alkaloids. Asian J. Org. Chem. 2019;8:946–969. doi: 10.1002/ajoc.201900244. [DOI] [Google Scholar]
- 4.Rubio-Piña J., Vazquez-Flota F. Pharmaceutical applications of the benzylisoquinoline alkaloids from Argemone mexicana L. Curr. Top. Med. Chem. 2013;13:2200–2207. doi: 10.2174/15680266113139990152. [DOI] [PubMed] [Google Scholar]
- 5.Zielińska S., Jezierska-Domaradzka A., Wójciak-Kosior M., Sowa I., Junka A., Matkowski A.M. Greater celandine’s ups and downs—21 centuries of medicinal uses of Chelidonium majus from the viewpoint of today’s pharmacology. Front. Pharmacol. 2018;9:299. doi: 10.3389/fphar.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao D., Wang P., Jia C., Sun P., Qi J., Zhou L., Li X. Identification and developmental expression profiling of putative alkaloid biosynthetic genes in Corydalis yanhusuo bulbs. Sci. Rep. 2016;6:1–14. doi: 10.1038/srep19460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J., He S., Wang J., Wang C., Wu J., Wang W., Li F., Li S., Zhao C., Li F. A Review of the traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology of Corydalis yanhusuo. Nat. Prod. Commun. 2020;15:1934578X20957752. [Google Scholar]
- 8.Liu X., Liu Y., Huang P., Ma Y., Qing Z., Tang Q., Cao H., Cheng P., Zheng Y., Yuan Z., et al. The genome of medicinal plant Macleaya cordata provides new insights into benzylisoquinoline alkaloids metabolism. Mol. Plant. 2017;5:975–989. doi: 10.1016/j.molp.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Rolland A., Fleurentin J., Lanhers M.C., Younos C., Misslin R., Mortier F., Pelt J.M. Behavioural effects of the American traditional plant Eschscholzia californica: Sedative and anxiolytic properties. Planta Med. 1991;57:212–216. doi: 10.1055/s-2006-960076. [DOI] [PubMed] [Google Scholar]
- 10.Cahlíková L., Opletal L., Kurfürst M., Macáková K., Kulhánková A., Hošt’álková A. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Chelidonium majus (Papaveraceae) Nat. Prod. Commun. 2010;5:1934578X1000501110. doi: 10.1177/1934578X1000501110. [DOI] [PubMed] [Google Scholar]
- 11.Croaker A., King G.J., Pyne J.H., Anoopkumar-Dukie S., Liu L. Sanguinaria canadensis: Traditional medicine, phytochemical composition, biological activities and current uses. Int. J. Mol. Sci. 2016;17:1414. doi: 10.3390/ijms17091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samanani N., Facchini P.J. Isolation and partial characterization of norcoclaurine synthase, the first committed step in benzylisoquinoline alkaloid biosynthesis, from opium poppy. Planta. 2001;213:898–906. doi: 10.1007/s004250100581. [DOI] [PubMed] [Google Scholar]
- 13.Hagel J.M., Facchini P.J. Benzylisoquinoine alkaloid metabolism: A century of discovery and a brave new world. Plant Cell Physiol. 2013;54:647–672. doi: 10.1093/pcp/pct020. [DOI] [PubMed] [Google Scholar]
- 14.Liscombe D.K., MacLeod B.P., Loukanina N., Nandi O.I., Facchini P.J. Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms. Phytochemistry. 2005;66:2501–2520. doi: 10.1016/j.phytochem.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Bastian D., Konrad B., Toplak M., Lahham M., Messenlehner J., Winkler J., Macheroux P. The family of berberine bridge enzyme-like enzymes: A treasure-trove of oxidative reactions. Arch. Biochem. Biophys. 2017;632:88–103. doi: 10.1016/j.abb.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Beaudoin G.A.W., Facchini P.J. Benzylisoquinoline alkaloid biosynthesis in opium poppy. Planta. 2014;240:19–32. doi: 10.1007/s00425-014-2056-8. [DOI] [PubMed] [Google Scholar]
- 17.Díaz-Chávez M.L., Rolf M., Gesell A., Kutchan T.M. Characterization of two methylenedioxy bridge-forming cytochrome P450-dependent enzymes of alkaloid formation in the Mexican prickly poppy Argemone mexicana. Arch. Biochem. Biophys. 2011;507:186–193. doi: 10.1016/j.abb.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Reed J.W., Hudlicky T. The Quest for a practical synthesis of morphine alkaloids and their derivatives by chemoenzymatic methods. Acc. Chem. Res. 2015;48:674–687. doi: 10.1021/ar500427k. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y., Hirakawa H., Hori K., Minakuchi Y., Toyoda A., Shitan N., Sato F. Comparative analysis using the draft genome sequence of California poppy (Eschscholzia californica) for exploring the candidate genes involved in benzylisoquinoline alkaloid biosynthesis. Biosci. Biotechnol. Biochem. 2021;85:851–859. doi: 10.1093/bbb/zbaa091. [DOI] [PubMed] [Google Scholar]
- 20.Loza-Muller L., Laines-Hidalgo J., Monforte-Gonzalez M., Vazquez-Flota F. Alkaloid distribution in seeds of Argemone mexicana L. (Papaveraceae) J. Mex. Chem. Soc. 2021;65:4. doi: 10.29356/jmcs.v65i4.1574. [DOI] [Google Scholar]
- 21.Weiss D., Baumert A., Vogel M., Roos W. Sanguinarine reductase, a key enzyme of benzophenanthridine detoxification. Plant Cell Environ. 2006;29:291–302. doi: 10.1111/j.1365-3040.2005.01421.x. [DOI] [PubMed] [Google Scholar]
- 22.Alcantara J., Bird D.A., Franceschi V.R., Facchini P.J. Sanguinarine biosynthesis is associated with the endoplasmic reticulum in cultured opium poppy cells after elicitor treatment. Plant Physiol. 2005;138:173–183. doi: 10.1104/pp.105.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X., Gao X., Zhu Z., Cao Y., Zhang Q., Tu P., Chai X. Alkaloids from the tribe Bocconieae (Papaveraceae): A chemical and biological review. Molecules. 2014;19:13042–13060. doi: 10.3390/molecules190913042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nwanyichukwu P. Master’s Thesis. University of Calgary; Calgary, AB, Canada: 2014. Identification and Characterization of an Adenosine Triphosphate Binding Cassette (ABC) Transporter Ecabcb1 Involved in the Transport of Alkaloids in Eschscholzia californica. [Google Scholar]
- 25.Loza-Muller L., Shitan N., Yamada Y., Vázquez-Flota F. AmABCB1, an alkaloid transporter from seeds of Argemone mexicana L. (Papaveraceae) Planta. 2021;254:6. doi: 10.1007/s00425-021-03780-4. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y., Motomura Y., Sato F. CjbHLH1 homologs regulate sanguinarine biosynthesis in Eschscholzia californica cells. Plant Cell Physiol. 2015;56:1019–1030. doi: 10.1093/pcp/pcv027. [DOI] [PubMed] [Google Scholar]
- 27.Yamada Y., Shimada T., Motomura Y., Sato F. Modulation of benzylisoquinoline alkaloid biosynthesis by heterologous expression of CjWRKY in Eschscholzia californica cells. PLoS ONE. 2017;12:e0186963. doi: 10.1371/journal.pone.0186953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salmore A.K., Hunter M.D. Elevational trends in defense chemistry, vegetation, and reproduction in Sanguinaria canadensis. J. Chem. Ecol. 2001;27:1713–1727. doi: 10.1023/A:1010411122739. [DOI] [PubMed] [Google Scholar]
- 29.Liu H., Wang J., Zhao J., Lu S., Wang J., Jiang W., Ma Z., Zhou L. Isoquinoline alkaloids from Macleaya cordata active against plant microbial pathogens. Nat. Prod. Commun. 2009;4:1934578X0900401120. doi: 10.1177/1934578X0900401120. [DOI] [PubMed] [Google Scholar]
- 30.Singh N., Sharma B. Toxicological effects of berberine and sanguinarine. Front. Mol. Biosci. 2018;5:21. doi: 10.3389/fmolb.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmeller T., Latz-Brüning B., Wink M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defense against microorganisms and herbivores. Phytochemistry. 1997;44:257–266. doi: 10.1016/S0031-9422(96)00545-6. [DOI] [PubMed] [Google Scholar]
- 32.Wink M., Schmeller T., Latz-Bruning B. Modes of action of allelochemical alkaloids: Interaction with neuroreceptors, DNA, and other molecular targets. J. Chem. Ecol. 1998;24:1881–1937. doi: 10.1023/A:1022315802264. [DOI] [Google Scholar]
- 33.Zou C., Wang Y., Zou H., Ding N., Geng N., Cao C., Zhang G. Sanguinarine in Chelidonium majus induced antifeeding and larval lethality by suppressing food intake and digestive enzymes in Lymantria dispar. Pest. Biochem. Physiol. 2019;153:9–16. doi: 10.1016/j.pestbp.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Leger E.A., Forister M.L. Increased resistance to generalist herbivores in invasive populations of the California poppy (Eschscholzia californica) Divers. Distrib. 2005;11:311–317. doi: 10.1111/j.1366-9516.2005.00165.x. [DOI] [Google Scholar]
- 35.Watts S.M., Dodson C.D., Reichman O.J. The roots of defense: Plant resistance and tolerance to belowground herbivory. PLoS ONE. 2011;6:e18463. doi: 10.1371/journal.pone.0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camacho-Tapia M., Sánchez-Soto V., Cámara Correia K., Pastirčáková K., Tovar-Pedraza J.M. Powdery mildew of California poppy caused by Erysiphe eschscholziae in Mexico. Can. J. Plant. Pathol. 2018;40:461–466. doi: 10.1080/07060661.2018.1498807. [DOI] [Google Scholar]
- 37.Park M.J., Cho S.E., Piątek M., Shin H.D. First report of powdery mildew caused by Erysiphe macleayae on Macleaya microcarpa in Poland. Plant Dis. 2012;96:1376. doi: 10.1094/PDIS-03-12-0244-PDN. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L., Huang P., Yu L., Zeng J. First report of root rot caused by Fusarium oxysporum on Macleaya cordata in China. J. Plant Pathol. 2020;102:191–192. doi: 10.1007/s42161-019-00382-8. [DOI] [Google Scholar]
- 39.O’Neill N.R., Jennings J.C., Bailey B.A., Farr D.F. Dendryphion penicillatum and Pleospora papaveracea, destructive seedborne pathogens and potential mycoherbicides for Papaver somniferum. Phytopathology. 2000;90:691–698. doi: 10.1094/PHYTO.2000.90.7.691. [DOI] [PubMed] [Google Scholar]
- 40.Yang X.J., Miao F., Yao Y., Cao F.J., Yang R., Ma Y.N., Qin B.-F., Zhou L. In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules. 2012;17:13026–13035. doi: 10.3390/molecules171113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang R., Gao Z.F., Zhao J.Y., Li W.B., Zhou L., Miao F. New class of 2-Aryl-6-chloro-3, 4-dihydroisoquinolinium salts as potential antifungal agents for plant protection: Synthesis, bioactivity and structure–activity relationships. J. Agric. Food Chem. 2015;63:1906–1914. doi: 10.1021/jf505609z. [DOI] [PubMed] [Google Scholar]
- 42.Hagel J.M., Morris J.S., Lee E.J., Desgagné-Penix I., Bross C.D., Chang L., Chen X., Farrow S.C., Zhang Y., Soh J., et al. Transcriptome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants. BMC Plant Biol. 2015;15:227. doi: 10.1186/s12870-015-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trujillo-Villanueva K., Rubio-Piña J., Monforte-González M., Vázquez-Flota F. Fusarium oxysporum homogenates and jasmonate induce limited sanguinarine accumulation in Argemone mexicana cell cultures. Biotechnol. Lett. 2010;32:1005–1009. doi: 10.1007/s10529-010-0252-6. [DOI] [PubMed] [Google Scholar]
- 44.Guízar-González C., Monforte-González M., Vázquez-Flota F. Yeast extract induction of sanguinarine biosynthesis is partially dependent on the octadecanoic acid pathway in cell cultures of Argemone mexicana L., the Mexican poppy. Biotechnol. Lett. 2016;38:1237–1242. doi: 10.1007/s10529-016-2095-2. [DOI] [PubMed] [Google Scholar]
- 45.Färber K., Schumann B., Miersch O., Roos W. Selective desensitization of jasmonate-and pH-dependent signaling in the induction of benzophenanthridine biosynthesis in cells of Eschscholzia californica. Phytochemistry. 2003;6:491–500. doi: 10.1016/S0031-9422(02)00562-9. [DOI] [PubMed] [Google Scholar]
- 46.Roos W., Viehweger K., Dordschbal B., Schumann B., Evers S., Steighardt J., Schwartze W. Intracellular pH signals in the induction of secondary pathways—The case of Eschscholzia californica. J. Plant Physiol. 2006;163:369–381. doi: 10.1016/j.jplph.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Z.M., Shang X.F., Lawoe R.K., Liu Y.Q., Zhou R., Sun Y.F., Li J., Yang G.Z., Yang C.J. Anti-phytopathogenic activity and the possible mechanisms of action of isoquinoline alkaloid sanguinarine. Pest. Biochem. Physiol. 2019;159:51–58. doi: 10.1016/j.pestbp.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Anjago W.M., Zeng W., Chen Y., Wang Y., Biregeya J., Li Y., Zhang T., Peng M., Yan C., Mingyue S., et al. The molecular mechanism underlying pathogenicity inhibition by sanguinarine in Magnaporthe oryzae. Pest. Manag. Sci. 2021;77:4669–4679. doi: 10.1002/ps.6508. [DOI] [PubMed] [Google Scholar]
- 49.Beuria T.K., Santra M.K., Panda D. Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling. Biochemistry. 2005;44:6584–16593. doi: 10.1021/bi050767+. [DOI] [PubMed] [Google Scholar]
- 50.Mingorance J., Rivas G., Vélez M., Gómez-Puertas P., Vicente M. Strong FtsZ is with the force: Mechanisms to constrict bacteria. Trends Microbiol. 2008;18:348–356. doi: 10.1016/j.tim.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Tang J., Lebas B., Liefting L., Veerakone S., Wei T., Ward L. Opium poppy mosaic virus, a new umbravirus isolated from Papaver somniferum in New Zealand. Arch. Virol. 2016;161:197–201. doi: 10.1007/s00705-015-2651-4. [DOI] [PubMed] [Google Scholar]
- 52.Glasa M., Šoltys K., Predajňa L., Sihelská N., Nováková S., Šubr Z., Kraic J., Mihálik D. Molecular and biological characterization of turnip mosaic virus isolates infecting poppy (Papaver somniferum and P. rhoeas) in Slovakia. Viruses. 2018;10:430. doi: 10.3390/v10080430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong Z., Liu M., Zhong X., Ou X., Yun X., Wang M., Ren S., Quing Z., Zeng J. Identification of the impurities in Bopu Powder® and Sangrovit® by LC-MS combined with a screening method. Molecules. 2021;26:3851. doi: 10.3390/molecules26133851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu Y., Liu W., Liu M., Zhang J., Yang M., Wang T., Qian W. In vitro anti-biofilm efficacy of sanguinarine against carbapenem-resistant Serratia marcescens. Biofouling. 2021;37:341–351. doi: 10.1080/08927014.2021.1919649. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q., Lyu Y., Huang J., Zhang X., Yu N., Wen Z., Chen S. Antibacterial activity and mechanism of sanguinarine against Providencia rettgeri in vitro. PeerJ. 2020;8:e9543. doi: 10.7717/peerj.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu C., Guan G., Wang H. The anticancer effect of sanguinarine: A review. Curr. Pharm. Des. 2018;24:2760–2764. doi: 10.2174/1381612824666180829100601. [DOI] [PubMed] [Google Scholar]
- 57.Galadari S., Rahman A., Pallichankandy S., Thayyullathil F. Molecular targets and anticancer potential of sanguinarine—A benzophenanthridine alkaloid. Phytomedicine. 2017;34:143–153. doi: 10.1016/j.phymed.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Khan A.Q., Rashid K., AlAmodi A.A., Agha M.V., Akhtar S., Hakeem I., Raza S.S., Uddin S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharmacother. 2021;143:112142. doi: 10.1016/j.biopha.2021.112142. [DOI] [PubMed] [Google Scholar]
- 59.Akhtar S., Achkar I.W., Siveen K.S., Kuttikrishnan S., Prabhu K.S., Khan A.Q., Eiman I.A., Fairooz S., Jerobin J., Raza A., et al. Sanguinarine induces apoptosis pathway in multiple myeloma cell lines via inhibition of the JaK2/STAT3 signaling. Front. Oncol. 2019;9:285. doi: 10.3389/fonc.2019.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perillo B., di Donato M., Pezone A., di Zazzo E., Giovannelli P., Galasso G., Castoira G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Q., Zhao Z., Shang J., Xia W. Targets and candidate agents for type 2 diabetes treatment with computational bioinformatics approach. J. Diabetes Res. 2014:763936. doi: 10.1155/2014/763936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong J. Sanguinarine ameliorates diabetic nephropathy in rats through nuclear factor-Kappa B and nuclear-factor erythroid 2-related factor 2/hemeoxygenase-1 pathways. Curr. Top. Nutraceutical Res. 2020;19:398–404. [Google Scholar]
- 63.Falchi F.A., Borlotti G., Ferretti F., Pellegrino G., Raneri M., Schiavoni M., Caselli A., Briani F. Sanguinarine inhibits the 2-ketoguconate pathway of glucose utilization in Pseudomonas aeruginosa. Front. Microbiol. 2021;12:744458. doi: 10.3389/fmicb.2021.744458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang Y.C., Chang F.R., Khalil A.T., Hsieh P.W., Wu Y.C. Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Z. Für Nat. C. 2003;58:521–526. doi: 10.1515/znc-2003-7-813. [DOI] [PubMed] [Google Scholar]
- 65.Hazra S., Kumar G.S. Structural and thermodynamic studies on the interaction of iminium and alkanolamine forms of sanguinarine with hemoglobin. J. Phys. Chem. B. 2014;118:3771–3784. doi: 10.1021/jp409764z. [DOI] [PubMed] [Google Scholar]
- 66.Basu P., Kumar G.S. Sanguinarine and its role in Chronic diseases. Adv. Exp. Med. Biol. 2016;928:155–172. doi: 10.1007/978-3-319-41334-1_7. [DOI] [PubMed] [Google Scholar]
- 67.Zhang F., Mao K., Gu Q., Wu W. The antiangiogenic effect of sanguinarine chloride on experimental chloroidal neovascularization in mice via inhibiting vascular endothelial growth factor. Front. Pharmacol. 2021;15:638215. doi: 10.3389/fphar.2021.638215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi Y.H., Choi W.Y., Hong S.H., Kim S.O., Kim G.Y., Lee W.H., Yoo Y.H. Anti-invasive activity of sanguinarine through modulation of tight junctions and matrix metalloproteinase activities in MDA-MB-231 human breast carcinoma cells. Chem. Biol. Interact. 2009;179:185–191. doi: 10.1016/j.cbi.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Choi W.Y., Jin C.Y., Han M.H., Kim G.Y., Kim N.D., Lee W.H., Kim S.K., Choi Y.H. Sanguinarine sensitizes human gastric adenocarcinoma AGS cells to TRAIL-mediated apoptosis via down-regulation of AKT and activation of caspase-3. Anticancer Res. 2009;29:4457–4465. [PubMed] [Google Scholar]
- 70.Achkar I.W., Mraiche F., Mohammed R.M., Uddin S. anticancer potential of sanguinarine for various human malignancies. Future Med. Chem. 2017;9:933–950. doi: 10.4155/fmc-2017-0041. [DOI] [PubMed] [Google Scholar]
- 71.Niu X., Fan T., Li W., Xing W., Huang H. The anti-inflammatory effects of sanguinarine and its modulation of inflammatory mediators from peritoneal macrophages. Eur. J. Pharmacol. 2012;689:262–269. doi: 10.1016/j.ejphar.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 72.Mackraj I., Govender T., Gathiram P. Sanguinarine. Cardiovasc. Drugs Rev. 2008;26:75–83. doi: 10.1111/j.1527-3466.2007.00037.x. [DOI] [PubMed] [Google Scholar]
- 73.Valipour M., Zarghi A., Ebrahimzadeh M.A., Irannejad H. Therapeutic potential of chelerythrine as a multi-purpose adjuvant for the treatment of COVID-19. Cell Cycle. 2021;20:2321–2336. doi: 10.1080/15384101.2021.1982509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X., Zhang M., Fan P., Qin Y., Zhao H. Chelerythrine chloride induces apoptosis in renal cancer HEK-293 and SW-839 cell lines. Oncol. Lett. 2016;11:3917–3924. doi: 10.3892/ol.2016.4520. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Wangchuk P., Sastraruji T., Taweechotipatr M., Keller P., Pyne S. Anti-inflammatory, Anti-bacterial and anti-acetylcholinesterase activities of two isoquinoline alkaloids-scoulerine and cheilanthifoline. Nat. Prod. Commun. 2016;11:1801–1804. doi: 10.1177/1934578X1601101207. [DOI] [PubMed] [Google Scholar]
- 76.He N., Wang P., Wang P., Ma C., Kang W. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. BMC Complement. Altern. Med. 2018;18:261. doi: 10.1186/s12906-018-2317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vázquez-Flota F.A., Loyola-Vargas V.M. In vitro plant cell culture as the basis for the development of a Research Institute in México: Centro de Investigación Científica de Yucatán. In Vitro Cell. Dev. Biol. Plant. 2003;39:250–258. doi: 10.1079/IVP2002398. [DOI] [Google Scholar]
- 78.Xool-Tamayo J., Tamayo-Ordoñez Y., Monforte-González M., Muñoz-Sánchez J.A., Vázquez-Flota F. Alkaloid biosynthesis in the early stages of the germination of Argemone mexicana L. (Papaveraceae) Plants. 2021;10:2226. doi: 10.3390/plants10102226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verma P., Khan S.A., Mathur A.K., Ghosh S., Shanker K., Kalra A. Improved sanguinarine production via biotic and abiotic elicitations and precursor feeding in cell suspensions of latex-less variety of Papaver somniferum with their gene expression studies and upscaling in bioreactor. Protoplasma. 2014;251:1359–1371. doi: 10.1007/s00709-014-0638-8. [DOI] [PubMed] [Google Scholar]
- 80.Takemura T., Ikezawa N., Iwasa K., Sato F. Molecular cloning and characterization of a cytochrome P450 in sanguinarine biosynthesis from Eschscholzia californica cells. Phytochemistry. 2013;91:100–108. doi: 10.1016/j.phytochem.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Wang L., Wang X., Wang W., Liu W., Liu Y., Xie H., Reiser O., Zeng J., Cheng P. Visible-light-promoted biomimetic reductive functionalization of quaternary benzophenanthridine alkaloids. J. Nat. Prod. 2021;84:2390–2397. doi: 10.1021/acs.jnatprod.1c00512. [DOI] [PubMed] [Google Scholar]
- 82.Aravindan N., Jeganmohan M. A short total synthesis of benzophenanthridine alkaloids via a rhodium (III)-catalyzed C−H ring-opening reaction. J. Org. Chem. 2021;86:14826–14843. doi: 10.1021/acs.joc.1c01612. [DOI] [PubMed] [Google Scholar]
- 83.Croaker A., Kinga G.J., Pyned J.H., Anoopkumar-Dukiec S., Simanekf V., Liua L. Carcinogenic potential of sanguinarine. Mutat. Res. Rev. Mutat. Res. 2017;774:45–56. doi: 10.1016/j.mrrev.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Saeed M.E., Mahmoud N., Sugimoto Y., Efferth T., Abdel-Aziz H. Molecular determinants of sensitivity or resistance of cancer cells toward sanguinarine. Front. Pharmacol. 2018;9:136. doi: 10.3389/fphar.2018.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fossati E., Ekins A., Narcross L., Zhu Y., Falgueyret J.P., Beaudoin G.A., Facchini P.J., Martin V.J. Reconstitution of a 10-gene pathway for synthesis of the plant alkaloid dihydrosanguinarine in Saccharomyces cerevisiae. Nat. Commun. 2014;5:1–11. doi: 10.1038/ncomms4283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.