Supplemental Digital Content is available in the text.
Keywords: hypertension, incidence, Nonalcoholic fatty liver disease, ultrasound
Abstract
Several studies reported an association between nonalcoholic fatty liver disease (NAFLD) and the risk of incident hypertension. The objective of this systematic review and meta-analysis was to obtain a precise and reliable estimate of the nature and magnitude of this association. We systematically searched Ovid-MEDLINE up to March 2021 for observational studies in which NAFLD was diagnosed in adults using blood-based panels, imaging techniques or liver biopsy and with a follow-up ≥1 year. Measures of association from individual studies were meta-analyzed using random-effects models. Of the 1108 titles initially scrutinized, we included 11 cohort studies with data on 390 348 participants (52% male) and a mean follow-up of 5.7 years. In the overall analysis, NAFLD was associated with a moderately increased risk of incident hypertension (hazard ratio 1.66; 95% confidence interval (CI), 1.38-2.01; test for overall effect z = 5.266; P < 0.001). There was significant heterogeneity among the studies (P < 0.001). Sensitivity analyses showed that estimates were not affected by geographical location, duration of follow-up and adjustment for baseline blood pressure values. On the other hand, the magnitude of the association was lower in studies that adjusted for baseline adiposity compared with those that did not, explaining part of the observed heterogeneity. No significant publication bias was detected by funnel plot analysis and Egger’s and Begg’s tests. This large meta-analysis indicates that NAFLD is associated with a ~1.6-fold increased risk of developing hypertension. Further studies are needed to investigate the role of NAFLD severity in terms of inflammation and fibrosis on incident hypertension.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease, affecting ~25% of the adult world population [1]. It is an umbrella term including patients with different degrees of histologic severity spanning from simple steatosis to lobular inflammation and hepatocyte ballooning (nonalcoholic steatohepatitis) to collagen deposition leading to liver fibrosis and possibly cirrhosis [2,3]. It is increasingly recognized as a frequent cause of liver-related morbidity and mortality and its global prevalence is expected to further increase in the foreseeable future, given the widespread rise in obesity rates among adolescents and young adults [4].
Being frequently associated with insulin resistance and ectopic fat deposition, its prevalence is even higher in patients with metabolic disorders, such as type 2 diabetes [5], and in patients displaying signs of the metabolic syndrome, including visceral obesity, dyslipidemia and essential hypertension [6–8].
Accumulating evidence suggests that NAFLD is associated with an increased prevalence and incidence of hypertension [9,10], which still represents by far the most common disease that affects human beings and is considered the top contributor to the burden of disease worldwide [11–13].
To date, two previous meta-analyses examined the association between γ-glutamyl transpeptidase (γ-GT) levels and risk of incident hypertension [14,15], whereas no quantitative summary of the available evidence is present on studies using more accurate measures of liver fat content such as specific blood-based panels, imaging techniques or liver biopsy.
We have therefore undertaken a systematic review and meta-analysis of observational cohort studies of adults from different geographical locations examining the association between NAFLD (diagnosed based on imaging, blood biomarkers or liver biopsy) and incident hypertension. A meta-analytic approach might help resolve inconsistencies among previously published studies and more precisely define the nature and the magnitude of the association.
Methods
The data of the meta-analysis are available from the corresponding author at reasonable request.
Data sources and search strategy
We systematically searched Ovid-MEDLINE to identify articles reporting the results of longitudinal observational studies published up to March 2021 investigating the association between NAFLD and incident hypertension. The search, designed by S.C. and G.P., was performed by S.C. Articles were selected by using the terms “nonalcoholic fatty liver disease” OR “NAFLD” OR “fatty liver” OR “nonalcoholic steatohepatitis” AND “incidence” OR “new-onset” AND “hypertension” (Supplementary Table S1, Supplemental digital content 1, http://links.lww.com/EJGH/A725). We limited our searches to human studies without predefined language restrictions. Reference lists of included manuscripts and review articles were hand searched to identify additional studies not covered by the original database searches. The systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) as outlined in Supplementary Table S2, Supplemental digital content 1, http://links.lww.com/EJGH/A72516. Given the observational nature of the included studies, we followed the reporting items proposed by the Meta-analysis Of Observational Studies in Epidemiology for the meta-analysis of these studies [16].
Study selection
Only studies that met the following inclusion criteria were considered for the present systematic review and meta-analysis: (1) longitudinal design; (2) duration of follow-up ≥1 year; (3) assessment of the relationship between NAFLD and incident hypertension; (4) availability of a measure of association [hazard ratio or odds ratio (OR)] with 95% confidence intervals (CI) for the outcome of interest; (5) a diagnosis of liver steatosis based either on imaging techniques (ultrasonography, computerized tomography or transient elastography), blood/biomarkers [fatty liver index (FLI) [17], hepatic steatosis index [18] or other scores of liver steatosis] or liver biopsy and (6) a diagnosis of hypertension based on office blood pressure (BP) measurement by physicians or International Classification of Diseases (ICD) codes. Exclusion criteria were as follows: (1) cross-sectional studies, editorials, congress abstracts and case reports; (2) studies that did not exclude different causes of liver steatosis; (3) studies with a median follow-up <1 year; (4) studies that did not report a measure of association with 95% CI for the outcome of interest and (5) studies that were performed in the pediatric population.
Data extraction and quality assessment
All titles and abstracts were independently examined by two investigators (S.C. and G.P.) and full-texts of potentially relevant articles were obtained and scrutinized separately by the same authors. We resolved discrepancies by consensus, referring back to the original articles. Information was extracted from all studies on study design, country, follow-up duration, the outcome of interest and covariates included in the multivariable regression models. In case of multiple publications on the same subjects, we included only the most up-to-date and comprehensive one. The risk of bias was assessed independently by two authors (S.C. and G.P.) and discrepancies were resolved by discussion. Studies were evaluated for their quality following the Newcastle-Ottawa Scale (NOS) [19]. This scale allocates a maximum of nine points for three major domains: selection of participants (maximum of four points), comparability of study groups (maximum of two points) and ascertainment of outcomes of interest (maximum of three points).
Data synthesis and statistical analysis
Hazard ratios or ORs and corresponding 95% CI were considered as the measure of association of interest for each eligible study. We extracted the effect size from the statistical model reporting the maximum extent of adjustment for confounders. Adjusted hazard ratios and OR were pooled to calculate an overall estimate of effect size. Because we expected a relatively large heterogeneity in results, as it is a common finding when evaluating observational studies on different cohorts with varying degrees of adjustment, we used the random-effects model using the method of Der Simonian and Laird, with the estimate of heterogeneity being taken from the Mantel–Haenszel model. Statistical heterogeneity was evaluated by visual inspection of the forest plot, as well as by the Cochrane Q test and the I2 statistics, which represents the proportion of the observed variability that cannot be explained by chance alone.
A funnel plot was constructed to evaluate the presence of publication bias by plotting the logarithm of the effect measure against the logarithm of its standard error. We also used both the Egger’s test [20] and the rank correlation Begg’s test [21]. To evaluate the possible sources of heterogeneity and the robustness of our findings, we performed prespecified subgroup-sensitivity analyses by geographical location, methodology used to diagnose NAFLD and degree of covariate adjustment (with special focus on adjustment for baseline BP values and measures of adiposity). Moreover, additional sensitivity analyses were conducted to evaluate whether the pooled effect estimate was strongly influenced by a specific study. This was performed by omitting one study each time and recalculating the pooled effect estimate on the remaining studies. All statistical analyses were performed with Stata 13.0 (Stata Corp, College Station, Texas, USA). A two-tailed P value <0.05 was considered significant.
Results
Search results
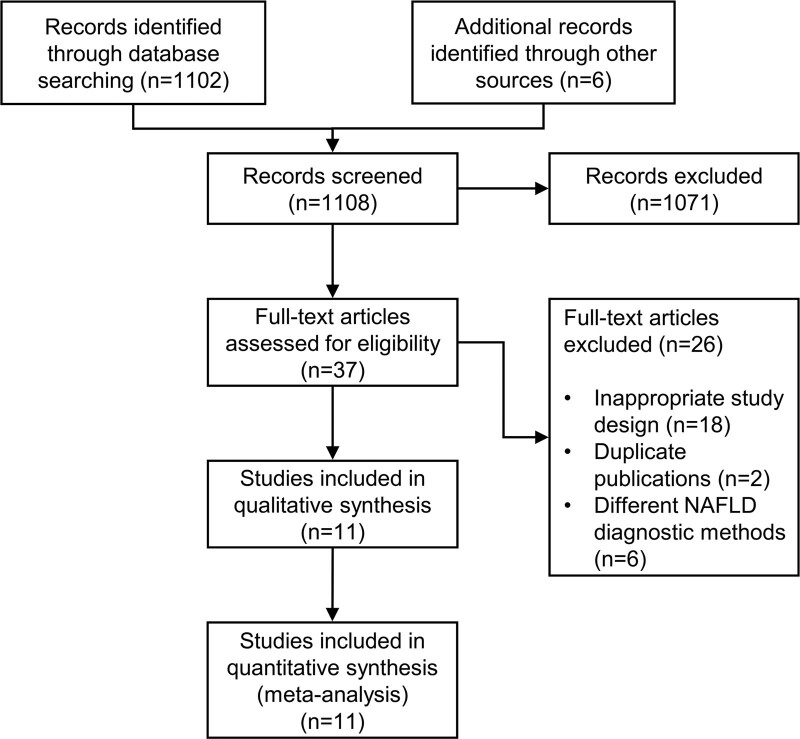
From a total of 1108 articles identified by literature research, 1071 were excluded by title and abstract screening. We examined the full text of the remaining 37 studies. After excluding articles with a cross-sectional design or that did not report the outcome of interest (n = 18), 2 studies were not included because they reported results on the same population of two included studies, and 6 were excluded because they used different diagnostic methods to define NAFLD (mainly γ-GT levels), leading to a final number of 11 included studies that were analyzed and assessed for quality. A PRISMA flow diagram of the study selection is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Features of the included articles
The main characteristics of the included studies are reported in Table 1. All were observational (either prospective or retrospective) cohort studies and most of them were performed on middle-aged individuals sampled from the general population. Overall, they included 390 348 individuals (52% men) with a mean follow-up of 5.7 years (ranging from 2.6 to 9 years). Eight studies were carried out in Asia (South Korea and China), two in Europe (France and Germany) and one in the USA. Excluding one study that did not report the prevalence of NAFLD but segregated the population in FLI quartiles [29], the mean prevalence of NAFLD was 21.5%. One study was performed only in men, while all the rest included a combined sample of men and women.
Table 1.
Overview of the included studies investigating the association between nonalcoholic fatty liver disease and incident hypertension
| Author | Year | Country | Follow-up (years) | Sample | Male (%) | NAFLD diagnostic method | NAFLD at baseline (%) | Diabetes at baseline (%) | Definition of hypertension | Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|
| Bonnet et al., [22] | 2017 | France | 9 | 2886 | 45.2 | Fatty liver index | 7.6 | NA | BP ≥140/90 mmHg or use of BP lowering drugs | Age, sex, smoking, FPG and alcohol intake |
| Fan et al.,[23] | 2007 | China | 6 | 1146 | 90.5 | Ultrasound | 31.2 | 6.5 | BP ≥140/90 mmHg | Age |
| Huh et al.,[24] | 2015 | South Korea | 2.6 | 1521 | 31.8 | Fatty liver index | 8.2 | NA | BP ≥140/90 mmHg or use of BP lowering drugs | Age, sex, SBP, DBP, smoke, exercise, alcohol, diabetes |
| Kim et al.,[25] | 2017 | South Korea | 5.1 | 2119 | 54.1 | Ultrasound | 19.8 | 2.8 | BP ≥140/90 mmHg or use of BP lowering drugs | Age, sex, smoking, waist circumference, triglycerides, HDL, LDL, uric acid |
| Lau et al.,[26] | 2010 | Germany | 5 | 2417 | 63.4 | Ultrasound | 39.4 | 7.2 | BP ≥140/90 mmHg or use of BP lowering drugs | Age, sex, waist circumference |
| Liu et al.,[27] | 2018 | China | 5 | 6704 | 36.3 | Ultrasound | 30 | 11.1 | BP ≥140/90 mmHg or use of BP lowering drugs or self-reported diagnosis | Age, sex, smoking, alcohol, physical activity, education, family history, SBP, waist circumference, change in BMI |
| Ma et al.,[28] | 2016 | USA | 6.2 | 1051 | 54.1 | CT | 17.8 | 2.6 | BP ≥140/90 mmHg or use of BP lowering drugs | Age, sex, smoking, physical activity, alcohol intake, SBP, DBP, BMI, change in BMI |
| Roh et al.,[29] | 2020 | South Korea | 5.2 | 334280 | 48.3 | Fatty liver index | NA | 0.0 | ICD-10 code | Age, sex, alcohol, SBP, DBP, glucose, total cholesterol |
| Ryoo et al.,[30] | 2014 | South Korea | 5 | 22090 | 100 | Ultrasound | 34.2 | 2.8 | BP ≥140/90 mmHg or use of BP lowering drugs | Age, BMI, triglyceride, creatinine, transaminases, smoking, exercise, diabetes |
| Sung et al.,[31] | 2014 | South Korea | 5 | 11448 | 69.4 | Ultrasound | 19.9 | 2.1 | BP ≥140/90 mmHg or use of BP lowering drugs | Age, sex, smoking, alcohol, exercise, SBP, BMI, diabetes, GGT, HOMA-IR, eGFR, change in BMI |
| Zhou and Cen [32] | 2018 | China | 9 | 4686 | 67.8 | Fatty liver index | 6.5 | NA | BP ≥140/90 mmHg or use of BP lowering drugs | Age, sex, waist circumference, SBP, DBP, FPG, HDL-C, TG |
BP, blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GGT, gamma-glutamyl transpeptidase; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostatic model of insulin resistance; ICD, International Classification of Diseases; LDL, low density lipoprotein; NA, not available; NAFLD, nonalcoholic fatty liver disease; TG, triglycerides.
Six studies used ultrasonography to diagnose NAFLD (n = 45 924 individuals), one study used computed tomography (n = 1051 individuals) and the remaining four used the FLI (n = 343 373). Definition of hypertension was consistent in most studies and as BP ≥140/90 mmHg or the initiation of antihypertensive treatment, with one study identifying patients using ICD codes. As reported in Supplementary Table S3, Supplemental digital content 1, http://links.lww.com/EJGH/A725 4, 6 and 1, studies were considered at low (receiving at least 8 stars), medium (7 stars) and high risk of bias (<7 stars) according to NOS, respectively, thus indicating an overall low to medium risk of bias.
Association between nonalcoholic fatty liver disease and incident hypertension
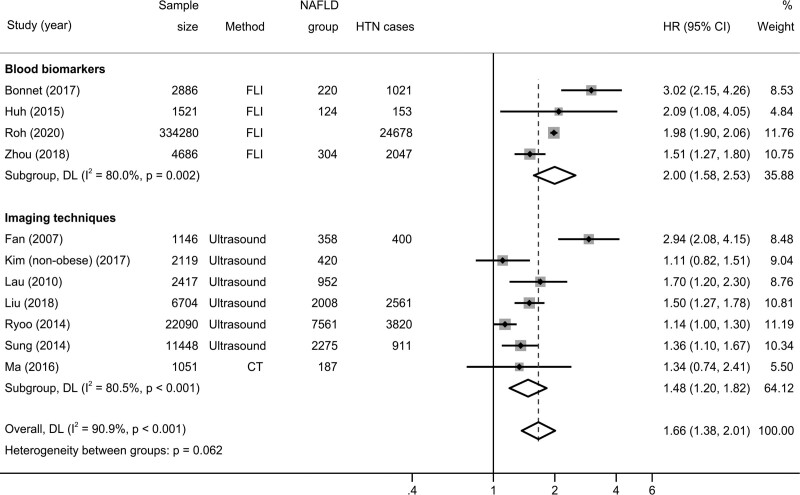
As shown in Fig. 2, the pooled hazard ratios for incident hypertension was 1.66 (95% CI, 1.38–2.01; test for overall effect z = 5.266; P < 0.001) when pooling adjusted effect estimates. The test for heterogeneity was significant (Cochran’s Q = 109.85; degrees of freedom (df) = 10; P < 0.001). No study suggested a decreased risk of incident hypertension in patients with NAFLD.
Fig. 2.
Forest plot and pooled estimates on the effect of NAFLD on the risk of incident hypertension in 11 eligible studies, stratified based on the methodology used for NAFLD diagnosis. CI, confidence interval; HR, hazard ratio; HTN, hypertension; NAFLD, nonalcoholic fatty liver disease.
When the analysis was stratified based on the methodology used to identify patients with NAFLD, the association of interest was consistent in both studies using FLI (n = 4 studies; hazard ratios 2.00; 95% CI, 1.58–2.53; test for overall effect z = 5.766; P < 0.001) and studies using imaging techniques such as ultrasonography or CT (n = 7 studies; hazard ratios 1.48; 95% CI, 1.20–1.82; test for overall effect z = 3.657; P < 0.001), with borderline heterogeneity between the two groups (Cochran’s Q = 3.49; degrees of freedom (df) = 1; P = 0.062).
Sensitivity analyses and risk of publication bias
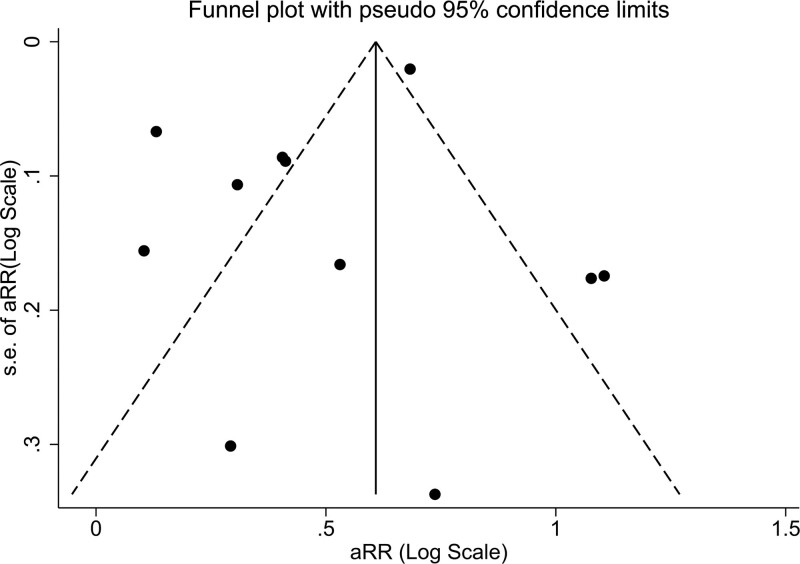
Subgroup analyses based on follow-up duration, degree of adjustment for covariates and geographical region were performed to explore possible sources of heterogeneity and are shown in Table 2. Notably, an increased risk of incident hypertension in patients with NAFLD was evident in all subgroups. No significant impact was found with regards to the duration of follow-up, geographical region and adjustment for baseline BP values. On the other hand, we found that adjustment for adiposity measure at baseline (either BMI or waist circumference or both) attenuated the extent of the association. Indeed, the hazard ratio was 2.44 (95% CI, 1.84–3.22; test for overall effect z = 6.229; P < 0.001) for those not performing the adjustment (n = 4 studies) and 1.36 (95% CI, 1.20–1.54; test for overall effect z = 4.871; P < 0.001) for those performing it (n = 7 studies), with a significant between-group heterogeneity in the outcome measure (Cochrane Q = 14; df = 1; P < 0.001). No evidence of significant publication bias was found by using asymmetry analysis in the funnel plot (Fig. 3). Furthermore, both Egger’s test (P = 0.247) and rank correlation Begg’s test (P = 0.312) did not show statistically significant asymmetry. Finally, sensitivity analysis (Supplementary Figure S1, Supplemental digital content 1, http://links.lww.com/EJGH/A725) showed that there was no significant trend suggesting that the overall result was influenced by any individual study.
Table 2.
Subgroup-sensitivity analyses on studies investigating the association between nonalcoholic fatty liver disease and incident hypertension
| Hazard ratios (95% CI) | Test for overall effect | Study number | Between group heterogeneity | |
|---|---|---|---|---|
| Duration of follow-up | ||||
| <6 years | 1.48 (1.16–1.89) | z = 3.182, P = 0.001 | 7 | P = 0.176 |
| ≥6 years | 2.10 (1.35–3.20) | z = 3.325, P = 0.001 | 4 | |
| Adjustment for baseline BP | ||||
| Absent | 1.78 (1.16–2.73) | z = 2.647, P = 0.008 | 5 | P = 0.655 |
| Present | 1.60 (1.33–1.93) | z = 4.881, P < 0.001 | 6 | |
| Adjustment for adipositya | ||||
| Absent | 2.44 (1.84–3.22) | z = 6.229, P < 0.001 | 4 | P < 0.001 |
| Present | 1.36 (1.20–1.54) | z = 4.871, P < 0.001 | 7 | |
| Geographical region | ||||
| Europe/USA | 1.97 (1.23–3.15) | z = 2.830, P = 0.005 | 3 | P = 0.401 |
| Asia | 1.58 (1.27–1.96) | z = 4.078, P < 0.001 | 8 | |
All studies included in Fig. 2 were analyzed in these subgroup analyses.
Inclusion of either BMI or waist circumference in the multivariable logistic regression model.
BP, blood pressure; CI, confidence interval.
Fig. 3.
Funnel plot of selected studies describing the relationship between effect size and standard error on the log scale. The vertical line represents the pooled effect size and the dashed lines represent the pseudo 95% confidence intervals.
Discussion
In this large meta-analysis including 11 observational cohort studies involving 390 348 adult individuals free from hypertension at baseline from different geographical locations, we show that NAFLD is associated with a hazard ratio of 1.66 (95% CI, 1.38–2.01) for incident hypertension over a mean follow-up of 5.7 years. The extent of the association did not differ when the analysis was stratified based on diagnostic modality (blood-based panels versus imaging techniques), country of origin and adjustment for baseline BP values. On the other hand, the hazard ratio from studies that adjusted their estimates for adiposity measures (waist circumference and BMI) at baseline or at follow-up was significantly lower than that of studies that did not perform this correction, even though the association remained significant. This aspect underlies the important role of obesity as a potential confounder.
The results of the present study expand those of two previous meta-analyses focusing on the role of γ-GT as a predictor of incident hypertension [14,15]. The most recent, by Kunutsor et al., [14] which included 14 studies for a total of 44 582 individuals, found that in a comparison of extreme thirds of baseline γ-GT levels, the relative risk for hypertension was 1.32 (95% CI, 1.23–1.43), with the heterogeneity of estimates from different studies being explained by mean age, duration of follow-up and degree of confounder adjustment. Compared to these results, we have significantly increased the sample size (about nine times) and identified NAFLD with more accurate diagnostic methods, as γ-GT levels might be affected by a series of unrelated conditions such as alcoholic liver disease, cholestatic liver disease and induction by drugs [33]. On the other hand, we cannot provide evidence on whether NAFLD severity in terms of inflammation and fibrosis impacts the magnitude of this association, as was recently suggested in a biopsy-based study involving patients with NAFLD and different degrees of histologic changes [34]. On this aspect, additional cohort studies of well-characterized NAFLD patients are needed.
From a pathophysiological standpoint, several mechanisms might account for the role of NAFLD as a potential driver of hypertension in the general population [6]. It is well known that liver steatosis is strongly associated with insulin resistance and hyperinsulinemia. Apart from increasing the risk of developing type 2 diabetes, insulin resistance is associated with low-grade systemic inflammation and endothelial dysfunction, which might lead to vasoconstriction. Moreover, the action of insulin on sodium handling is frequently preserved in insulin resistance and contributes to sodium retention and arterial hypertension [35]. Other pathways linking the two conditions are represented by oxidative stress, hyperactivity of the sympathetic nervous system and the angiotensin aldosterone systems as well as increased risk of chronic kidney disease [36].
The current meta-analysis has several limitations that deserve to be acknowledged. First, given the observational nature of the included studies, it is not possible to definitely prove a causality link between the exposure and the outcome. Second, while most studies adjusted for several potential confounders including age, cigarette smoke and baseline BP values (as shown in Table 1), the possibility of residual confounding by unmeasured factors cannot be excluded. As an example, some studies did not adjust for baseline BMI and waist circumference. It should be noted, however, that these parameters are included in the FLI equation and adjustment might therefore reduce the diagnostic ability of the score to correctly identify patients with steatosis and therefore bias results towards the null. It was therefore not possible to combine models that accounted for the same variables. While sensitivity analyses showed consistency of the association of interest independently of geographical region, most studies included Asian patients, who tend to develop NAFLD at lower BMI levels compared to patients of Caucasian origin and this aspect may influence the observed effect of adiposity in modulating the relationship between NAFLD and hypertension.
Third, interpretation of our results demands cautiousness given the high degree of heterogeneity found between studies. While no study found a lower risk of hypertension in patients with NAFLD, variability in the magnitude of the association might result from a combination of factors including covariate adjustment, methods for NAFLD diagnosis and other potential unmeasured variables. It should also be noted that thresholds for significant alcohol consumption differed among the included studies, and not all of them systematically screened all participants for different forms of liver disease and use of steatogenic medications. More detailed analysis of heterogeneity would require pooling individual participants’ data from the different studies.
Fourth, none of the included studies used a gold standard technique such as liver biopsy or magnetic resonance spectroscopy to diagnose NAFLD. In fact, while these two techniques are more reliable than both liver ultrasonography and FLI, they are expensive and time-consuming, making them unsuitable for large-scale population studies. Moreover, liver biopsy is an invasive technique with possible (although rare) life-threatening complications, raising ethical concerns related to its use in apparently healthy subjects [37,38].
Our analysis also has some important strengths. It incorporates data from large epidemiological studies from Asia, Europe and the US including a representative pool of patients with NAFLD seen in clinical practice. Moreover, the large number of both exposed individuals and events yields high statistical power to precisely quantify the association between NAFLD and incident hypertension. Finally, there was no sign of significant publication bias affecting the analysis when evaluated by both Egger’s and Begg’s tests.
In conclusion, this large meta-analysis shows that NAFLD (diagnosed by either FLI or imaging techniques) is significantly associated with a ~1.7-fold increased risk of developing hypertension over a mean of 5.7 years. Moreover, obesity is an important confounder responsible for significant heterogeneity between studies and affecting the extent of this association. This underlies the need to carefully screen patients with NAFLD for the development of hypertension and the associated risk of cardiovascular events. Further studies evaluating whether NAFLD severity in terms of inflammation and fibrosis impacts on the risk of developing hypertension are needed.
Acknowledgements
All authors made substantial contributions to the conception and design or acquisition, analysis and interpretation of data. All authors drafted the article or revised it critically for important intellectual content. All authors approved the final version of the manuscript to be published. GP is the guarantor of this work.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.eurojgh.com
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Huang TD, Behary J, Zekry A. Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern Med J 2020; 50:1038–1047. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67:328–357. [DOI] [PubMed] [Google Scholar]
- 4.Ciardullo S, Monti T, Perseghin G. Prevalence of liver steatosis and fibrosis detected by transient elastography in adolescents in the 2017-2018 National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol 2021; 19:384–390.e381. [DOI] [PubMed] [Google Scholar]
- 5.Ciardullo S, Monti T, Perseghin G. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care 2021; 44:519–525. [DOI] [PubMed] [Google Scholar]
- 6.Zhao YC, Zhao GJ, Chen Z, She ZG, Cai J, Li H. Nonalcoholic fatty liver disease: an emerging driver of hypertension. Hypertension 2020; 75:275–284. [DOI] [PubMed] [Google Scholar]
- 7.Ciardullo S, Monti T, Sala I, Grassi G, Mancia G, Perseghin G. Nonalcoholic fatty liver disease and advanced fibrosis in US adults across blood pressure categories. Hypertension 2020; 76:562–568. [DOI] [PubMed] [Google Scholar]
- 8.Ciardullo S, Monti T, Grassi G, Mancia G, Perseghin G. Blood pressure, glycemic status and advanced liver fibrosis assessed by transient elastography in the general United States population. J Hypertens 2021; 39:1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikonomou D, Georgiopoulos G, Katsi V, Kourek C, Tsioufis C, Alexopoulou A, et al. Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol 2018; 30:979–985. [DOI] [PubMed] [Google Scholar]
- 10.Lorbeer R, Bayerl C, Auweter S, Rospleszcz S, Lieb W, Meisinger C, et al. Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. J Hypertens 2017; 35:737–744. [DOI] [PubMed] [Google Scholar]
- 11.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al.; Authors/Task Force Members. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 12.Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, et al. Hypertension. Nat Rev Dis Primers 2018; 4:18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020; 16:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunutsor SK, Apekey TA, Cheung BM. Gamma-glutamyltransferase and risk of hypertension: a systematic review and dose-response meta-analysis of prospective evidence. J Hypertens 2015; 33:2373–2381. [DOI] [PubMed] [Google Scholar]
- 15.Liu CF, Gu YT, Wang HY, Fang NY. Gamma-glutamyltransferase level and risk of hypertension: a systematic review and meta-analysis. PLoS One 2012; 7:e48878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 17.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010; 42:503–508. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 22.Bonnet F, Gastaldelli A, Pihan-Le Bars F, Natali A, Roussel R, Petrie J, et al.; D.E.S.I.R., RISC Study Groups. Gamma-glutamyltransferase, fatty liver index and hepatic insulin resistance are associated with incident hypertension in two longitudinal studies. J Hypertens 2017; 35:493–500. [DOI] [PubMed] [Google Scholar]
- 23.Fan JG, Li F, Cai XB, Peng YD, Ao QH, Gao Y. Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol 2007; 22:1086–1091. [DOI] [PubMed] [Google Scholar]
- 24.Huh JH, Ahn SV, Koh SB, Choi E, Kim JY, Sung KC, et al. A prospective study of fatty liver index and incident hypertension: the KoGES-ARIRANG study. PLoS One 2015; 10:e0143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SS, Cho HJ, Kim HJ, Kang DR, Berry JR, Kim JH, et al. Nonalcoholic fatty liver disease as a sentinel marker for the development of diabetes mellitus in non-obese subjects. Dig Liver Dis 2018; 50:370–377. [DOI] [PubMed] [Google Scholar]
- 26.Lau K, Lorbeer R, Haring R, Schmidt CO, Wallaschofski H, Nauck M, et al. The association between fatty liver disease and blood pressure in a population-based prospective longitudinal study. J Hypertens 2010; 28:1829–1835. [DOI] [PubMed] [Google Scholar]
- 27.Liu P, Tang Y, Guo X, Zhu X, He M, Yuan J, et al. Bidirectional association between nonalcoholic fatty liver disease and hypertension from the Dongfeng-Tongji cohort study. J Am Soc Hypertens 2018; 12:660–670. [DOI] [PubMed] [Google Scholar]
- 28.Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol 2017; 66:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roh JH, Park JH, Lee H, Yoon YH, Kim M, Kim YG, et al. A close relationship between non-alcoholic fatty liver disease marker and new-onset hypertension in healthy korean adults. Korean Circ J 2020; 50:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryoo JH, Suh YJ, Shin HC, Cho YK, Choi JM, Park SK. Clinical association between non-alcoholic fatty liver disease and the development of hypertension. J Gastroenterol Hepatol 2014; 29:1926–1931. [DOI] [PubMed] [Google Scholar]
- 31.Sung KC, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J Hepatol 2014; 60:1040–1045. [DOI] [PubMed] [Google Scholar]
- 32.Zhou K, Cen J. The fatty liver index (FLI) and incident hypertension: a longitudinal study among Chinese population. Lipids Health Dis 2018; 17:214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009; 136:477–85.e11. [DOI] [PubMed] [Google Scholar]
- 34.Ampuero J, Aller R, Gallego-Durán R, Crespo J, Calleja JL, García-Monzón C, et al.; HEPAmet Registry. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J Hepatol 2020; 73:17–25. [DOI] [PubMed] [Google Scholar]
- 35.Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol 2016; 12:721–737. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Schattenberg JM, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut 2020;gutjnl-2020-323082. doi: 10.1136/gutjnl-2020-323082 [DOI] [PubMed] [Google Scholar]
- 37.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009; 49:1017–1044. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.