Abstract
Background
The association between visceral adiposity index (VAI) and diabetic complications has been reported in cross-sectional studies, while the effect of VAI on complication development remains unclear. This study aims to evaluate the longitudinal association of VAI and Chinese VAI (CVAI) with the incidence of diabetic nephropathy and retinopathy using a Chinese cohort.
Methods
A total of 8 948 participants with type 2 diabetes from Beijing Health Management Cohort were enrolled during 2013–2014, and followed until December 31, 2019. Nephropathy was confirmed by urine albumin/creatinine ratio and estimated glomerular filtration rate; retinopathy was diagnosed using fundus photograph.
Results
The mean (SD) age was 53.35 (14.66) years, and 6 154 (68.8%) were men. During a median follow-up of 4.82 years, 467 participants developed nephropathy and 90 participants developed retinopathy. One-SD increase in VAI and CVAI levels were significantly associated with an increased risk of nephropathy, and the adjusted hazard ratios (HR) were 1.127 (95% CI 1.050–1.210) and 1.165 (95% CI 1.003–1.353), respectively. On contrary, VAI and CVAI level were not associated with retinopathy after adjusting confounding factors.
Conclusion
VAI and CVAI are independently associated with the development of nephropathy, but not retinopathy in Chinese adults with diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01464-1.
Keywords: Visceral adiposity index, Abdominal obesity, Diabetic nephropathy, Diabetic retinopathy
Background
Type 2 diabetes accounts for around 90% of diabetes, becoming a serious public health issue worldwide. It is estimated that 366 million people are expected to have diabetes by 2030, and the vascular complications have become the leading cause of death in type 2 diabetes [1, 2]. The vascular complications severely impact the patients’ life quality and pose a heavy economic burden on the health care system [3].
Abdominal obesity is recognized as one of the important risk factors of cardiometabolic diseases [4], diabetes [5], and diabetic complications [6]. Interestingly, several studies reported that the distribution of adipose tissue rather than the total amount is more crucial in the development of vascular complications [7]. X-ray computed tomography (CT) and magnetic resonance imaging (MRI) are precise methods to detect abdominal adiposity [8]. However, these methods are expensive, time-consuming and require radiation exposure, and thus they are not practical for frequent clinical use and epidemiological research in general population. Waist circumference (WC) is a major clinical indicator of increased visceral fat, which is unable to distinguish between subcutaneous and visceral fat mass, given the different role of subcutaneous and visceral fat [9]. Thus, some surrogate indexes of visceral adiposity have been established, including visceral adiposity index (VAI) and Chinese visceral adiposity index (CVAI) [10, 11]. Previous studies [11, 12] have found that VAI and CVAI could predict the risk of diabetes in adults. Nevertheless, the current evidence about the association between VAI and diabetic complication is limited. A recent study [13] reported the cross-sectional associations of VAI and CVAI with diabetic vascular complications. However, the effect of VAI and CVAI on the development of diabetic complications remains unclear.
Therefore, this study aims to investigate the longitudinal association of VAI and CVAI with the incidence of diabetic nephropathy and retinopathy using a population-based cohort.
Methods
Data source and study population
The Beijing health management cohort (BHMC) is an open cohort study conducted in Beijing Xiaotangshan Hospital and Beijing Physical Examination Center in Beijing, China. Briefly, the BHMC study is designed to investigate the risk factors and biomarkers for cardiometabolic diseases, such as metabolic syndrome and type 2 diabetes. The details of the study design have been described in previous studies [14, 15].
From 2013 to 2014, a total of 47,543 participants aged 20 years or above underwent a comprehensive health examination, and 10,948 were confirmed to have type 2 diabetes. To minimize the possible influence of reverse causality, 220 participants with a history of retinopathy and 478 participants with nephropathy at baseline were excluded. In addition, we excluded 65 participants with type 1 diabetes and 1027 participants lacking anthropometric measurement data at baseline. Finally, 8948 participants with complete data were annually followed under same identical conditions for the development of nephropathy and retinopathy until December 31, 2019, and the incident cases in each follow-up year were shown in Fig. 1. This study was approved by the Ethics Committee of Capital Medical University (Grant Number: 2020SY031). All participants provided written informed consent.
Fig. 1.
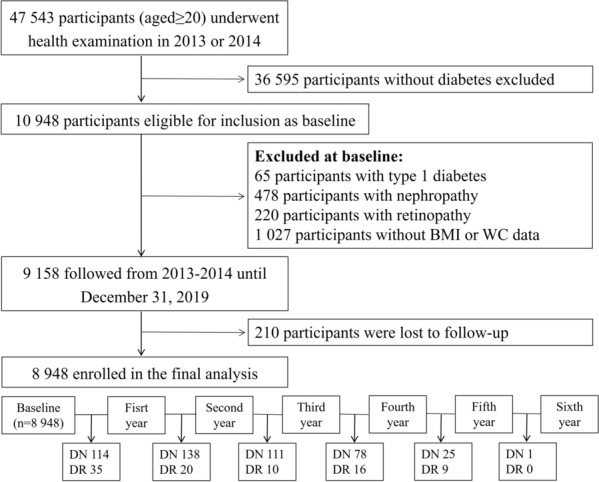
Flow chart of this current study
Data collection
Baseline data on demographic variables and health information, including age, sex, education level, physical activity, smoking status, drinking status, diagnosis history of diseases and medication use were collected via a standard questionnaire by our trained staff. Educational level was categorized as illiteracy or primary school, middle school, high school or above. Smoking status was defined as ‘current smoker’, ‘former smoker’ and ‘never smoked’. Drinking status was defined as ‘current drinking’ and ‘no current drinking’. Physical activity was defined as having moderate or intense exercise ‘ ≥ 20 min per time and ≥ 4 times per week’. Self-reported health conditions included the physician-diagnosed history of diabetes, hypertension and dyslipidemia. The uses of antidiabetic, antihypertensive and lipid lowering medication were defined as a positive response to the following question: ‘Do you take antidiabetic, antihypertensive or lipid lowering medication regularly following the physician's prescription?’.
The clinical characteristics and biochemical examination data were obtained from the electronic medical record system. Anthropometric measurements include height, weight, and WC. Body mass index (BMI) is calculated as weight (in kilograms)/height2 (in metres squared). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were calculated as the average of two measurements on the right arm using a sphygmomanometer after resting for at least 10 min. The overnight fasting venous blood samples were obtained from all participants, and measured in the central laboratory of Beijing Xiaotangshan Examination Center or Beijing Physical Examination Center. The data of fasting blood glucose, glycated haemoglobin (HbA1c), serum total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, serum creatinine, urinary albumin/creatinine ratio (ACR) were collected in this current study. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration serum creatinine equation [16]. Hypertension was defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or use of any antihypertensive medication or self-reported history of hypertension according to the JNC-7 criteria [17]. Diabetes was defined as fasting glucose ≥ 7.0 mmol/l, HbA1c ≥ 6.5% or the use of any antidiabetic medication or self-reported history of diabetes based on the American Diabetes Association [18]. According to the Guidelines on Prevention and Treatment of Dyslipidaemia for Chinese Adults, dyslipidaemia was defined as triglycerides ≥ 2.3 mmol/l, total cholesterol ≥ 6.2 mmol/l, LDL cholesterol ≥ 4.1 mmol/l, HDL cholesterol < 1.0 mmol/l, or any lipid-lowering medication or self-reported history of dyslipidaemia [19].
Definition of exposure and outcome
Baseline VAI level is calculated using WC, BMI, triglycerides and HDL. CVAI level is calculated using age, WC, BMI, triglycerides and HDL cholesterol. The sex specific formula is as follows:
Men:
VAI = WC/(39.68 + 1.88*BMI)*(triglycerides/1.03)*(1.31/HDL cholesterol).
CVAI = − 267.93 + 0.68*age (years) + 0.03*BMI (kg/m2) + 4.00*WC (cm) + 22.00*lg [triglycerides (mmol/l)]−16.32*HDL cholesterol (mmol/l).
Women:
VAI = WC/(36.58 + 1.89*BMI)*(triglycerides/0.813)*(1.52/HDL cholesterol).
CVAI = − 187.32 + 1.71*age (years) + 4.32*BMI (kg/m2) + 1.12*WC (cm) + 39.76*lg(triglycerides (mmol/l))−11.66*HDL cholesterol (mmol/l).
The VAI and CVAI showed significant correlations with visceral adipose tissue volume as described previously [10, 20].
Diabetic retinopathy was confirmed by ophthalmologists using the 45° four-field stereoscopic digital photography (Carl Zeiss Fundus Camera, Germany) centered at the central point of macula and optic disc, including temporal upper, temporal lower, nasal lower, and nasal upper fields. Non-mydriatic digital images of the retina for both eyes were taken, and the retinopathy was diagnosed according to the International Clinical Diabetic Retinopathy Disease Severity Scale [21]. The retinopathy diagnosis could be divided into four severity: mild non-proliferative; moderate non-proliferative; severe non-proliferative and proliferative. The presence of any severity lesion mentioned above was defined as diabetic retinopathy. Diabetic nephropathy was confirmed as urinary ACR ≥ 30 mg/mmol or eGFR < 60 mL/min/1.73m2 according to the organization Kidney Disease Improving Global Outcomes (KDIGO) [22–24].
Statistical analysis
Categorical variables were presented as number (proportions), and continuous variables were presented as mean (standard deviation, SD) or median (interquartile range, IQR). Difference between groups were compared using Chi-square test for categorical variables, and Student's t-test or Mann–Whitney U test for continuous variables, as appropriate.
Baseline VAI and CVAI were centered and standardized before analysis. The follow-up time was calculated from baseline to the year of nephropathy or retinopathy diagnosis, loss to follow-up, or the end date (December 31, 2019), whichever came first. The Cox proportional hazards models were used to investigate the associations of VAI and CVAI with the risk of incident nephropathy and retinopathy. To adjust for potential confounding factors, the following models were established: Model 1, adjusted for age and sex; Model 2, adjusted for age, sex, education level, smoking status, drinking status, physical activity, BMI group, hypertension, dyslipidaemia, fasting glucose and the use of antidiabetic medication. The hazard ratio (HR) and 95% confidence interval (CI) were provided. The restricted cubic spline function was used to analyze the dose–response relationship of VAI and CVAI with outcome. The discriminatory power of VAI and CVAI was shown using time-dependent receiver operating characteristics (ROC) curve and C-statistic. To identify the modification effect of potential variables, the interactive terms were tested in the model. In addition, we calculated the associations of the tertiles of VAI and CVAI with the diabetic complications, and the individuals were divided into lower, middle and upper groups of VAI and CVAI.
We performed multiple sensitivity analyses. First, participants experiencing a event of nephropathy or retinopathy within one year were excluded. Second, we repeated the analysis in people without the use of antidiabetic medication. Third, HbA1c level was further adjusted to explore the stability of our findings.
All the analyses mentioned above were conducted using R software, version 4.1.0 (R Foundation). The difference was considered statistically significant at two-side significance level of P < 0.05.
Results
The final analysis included 8 948 individuals with type 2 diabetes, and 6 154 (68.8%) were men. The mean (SD) age of this population was 53.35 (14.66) years. The median [IQR] of baseline VAI and CVAI were 1.46 [0.90,2.37] and 112.60 [77.19,143.17]. During a median follow-up of 4.82 years, 467 participants developed nephropathy and 90 participants developed retinopathy. Table 1 shows the baseline characteristics of participants according to the complication development or not. The CVAI levels were significantly higher in the nephropathy and retinopathy groups, while the VAI level was only significantly higher in the nephropathy group. The baseline characteristics of participants according to the tertiles of baseline VAI and CVAI index are shown in Additional file 1: Table S1 and Table S2.
Table 1.
Baseline characteristics of the study population
| Overall (n = 8948) | DN- (n = 8481) | DN + (n = 467) | P value | DR- (n = 8858) | DR + (n = 90) | P value | |
|---|---|---|---|---|---|---|---|
| Age, years | 53.35 (14.66) | 52.42 (14.19) | 70.27 (12.70) | < 0.001 | 53.33 (14.70) | 55.88 (10.25) | 0.101 |
| Men, n (%) | 6154 (68.8) | 5825 (68.7) | 329 (70.4) | 0.453 | 6074 (68.6) | 80 (88.9) | < 0.001 |
| Education, n (%) | |||||||
| Primary school or below | 955 (10.7) | 900 (10.6) | 55 (11.8) | 0.320 | 939 (10.6) | 16 (17.8) | 0.003 |
| Middle school | 6054 (67.7) | 5731 (67.6) | 323 (69.2) | 5988 (67.6) | 66 (73.3) | ||
| High school or above | 1939 (21.7) | 1850 (21.8) | 89 (19.1) | 1931 (21.8) | 8 (8.9) | ||
| Physical activity (n, %) | 3755 (42.0) | 3546 (41.8) | 209 (44.8) | 0.228 | 3721 (42.0) | 34 (37.8) | 0.483 |
| Current smoking (n, %) | 2709 (30.3) | 2592 (30.6) | 117 (25.1) | 0.013 | 2668 (30.1) | 41 (45.6) | 0.002 |
| Current drinking (n, %) | 5067 (56.6) | 4832 (57.0) | 235 (50.3) | 0.005 | 5013 (56.6) | 54 (60.0) | 0.588 |
| BMI, kg/m2 | 25.48 (3.36) | 25.46 (3.37) | 25.86 (3.27) | 0.012 | 25.47 (3.37) | 26.23 (3.15) | 0.033 |
| WC, cm | 87.32 (10.18) | 87.17 (10.18) | 90.02 (9.75) | < 0.001 | 87.27 (10.18) | 91.70 (8.33) | < 0.001 |
| Hypertension (n, %) | 3607 (40.3) | 3262 (38.5) | 345 (73.9) | < 0.001 | 3559 (40.2) | 48 (53.3) | 0.015 |
| Dyslipidaemia (n, %) | 3328 (37.2) | 3138 (37.0) | 190 (40.7) | 0.120 | 3282 (37.1) | 46 (51.1) | 0.008 |
| Antidiabetic (n, %) | 1198 (13.4) | 1075 (12.7) | 123 (26.3) | < 0.001 | 1128 (12.7) | 70 (77.8) | < 0.001 |
| Antihypertensive (n, %) | 732 (8.2) | 659 (7.8) | 73 (15.6) | < 0.001 | 728 (8.2) | 4 (4.4) | 0.268 |
| Lipid lowering (n, %) | 492 (5.5) | 438 (5.2) | 54 (11.6) | < 0.001 | 488 (5.5) | 4 (4.4) | 0.835 |
| Fasting glucose, mmol/L | 5.31 [4.94,5.94] | 5.30 [4.93,5.93] | 5.50 [5.04,6.32] | < 0.001 | 5.30 [4.93,5.91] | 8.32 [7.21,12.07] | < 0.001 |
| HbA1c, % | 5.57 [5.31,5.96] | 5.56 [5.30,5.94] | 5.80 [5.54,6.24] | < 0.001 | 5.57 [5.31,5.95] | 7.53 [6.54,9.09] | < 0.001 |
| Triglycerides, mmol/L | 1.27 [0.89,1.87] | 1.27 [0.89,1.88] | 1.21 [0.91,1.77] | 0.479 | 1.27 [0.89,1.87] | 1.38 [0.96,2.19] | 0.143 |
| HDL-C, mmol/L | 1.26 [1.06,1.53] | 1.26 [1.06,1.53] | 1.25 [1.05,1.56] | 0.945 | 1.26 [1.06,1.53] | 1.17 [0.97,1.34] | < 0.001 |
| VAI | 1.46 [0.90,2.37] | 1.45 [0.90,2.36] | 1.76 [1.03,2.80] | 0.043 | 1.46 [0.89,2.37] | 1.45 [0.95,2.32] | 0.984 |
| CVAI | 112.60 [77.19,143.17] | 111.07 [75.68,141.98] | 136.62 [107.49,160.81] | < 0.001 | 112.44 [76.88,143.07] | 125.61 [104.48,154.95] | < 0.001 |
Data are the mean (SD), median [IQR] or number (%)
To convert fasting glucose to mg/dL, multiply by 18; triglycerides to mg/dL multiply by 28.25
DR diabetic retinopathy, DN diabetic nephropathy, BMI body mass index, HbA1c glycated haemoglobin, HDL-C high-density lipoprotein cholesterol, VAI visceral adiposity index, CVAI Chinese visceral adiposity index
In the fully adjusted model, one-SD increase in VAI and CVAI levels were significantly associated with an increased risk of nephropathy, and the HR values were 1.127 (95% CI 1.050–1.210) and 1.165 (95% CI 1.003–1.353), respectively. On contrary, VAI and CVAI levels were not significantly associated with retinopathy after adjusting confounding factors, and the HR values were 1.071 (95% CI 0.950–1.207) and 0.878 (95% CI 0.615–1.252), as shown in Table 2. The full regression results are shown in Additional file 1: Table S3, and fasting glucose level and hypertension had a significant impact on the microvascular complications. The dose–response relationship of VAI and CVAI with the risk of nephropathy development is presented in Fig. 2. The C-statistic values of VAI and CVAI for predicting the development of nephropathy were 0.632 [95% CI 0.585–0.678] and 0.617 [95% CI 0.584–0.651], as shown in Fig. 3. The C-statistic values for predicting retinopathy is presented in Additional file 2: Fig. S1.
Table 2.
Association of visceral adiposity indexes with the development of diabetic nephropathy and retinopathy
| Hazard ratio (95% CI) | ||||
|---|---|---|---|---|
| Model 1 | P value | Model 2 | P value | |
| Nephropathy | ||||
| VAI per-SD increase | 1.154 (1.095–1.217) | < 0.001 | 1.127 (1.050–1.210) | 0.001 |
| CVAI per-SD increase | 1.296 (1.159–1.450) | < 0.001 | 1.165 (1.003–1.353) | 0.045 |
| Retinopathy | ||||
| VAI per-SD increase | 0.739 (0.549–0.995) | 0.047 | 1.071 (0.950–1.207) | 0.264 |
| CVAI per-SD increase | 1.330 (1.038–1.704) | 0.024 | 0.878 (0.615–1.252) | 0.471 |
Model 1: adjusted for age and sex
Model 2: age, sex, BMI group, education level, smoking status, drinking status, physical activity, hypertension, dyslipidaemia, fasting glucose and use of antidiabetic medication
VAI visceral adiposity index, CVAI Chinese visceral adiposity index
Fig. 2.
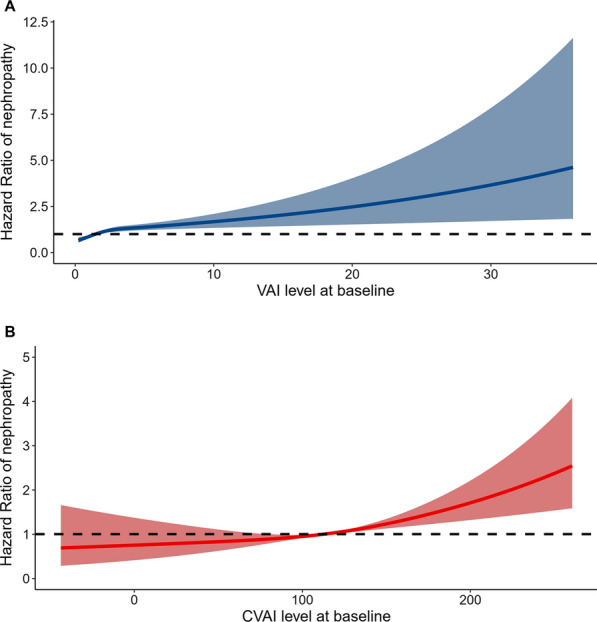
Dose–response relationship of baseline VAI and CVAI with incident nephropathy after adjusting for age and sex
Fig. 3.
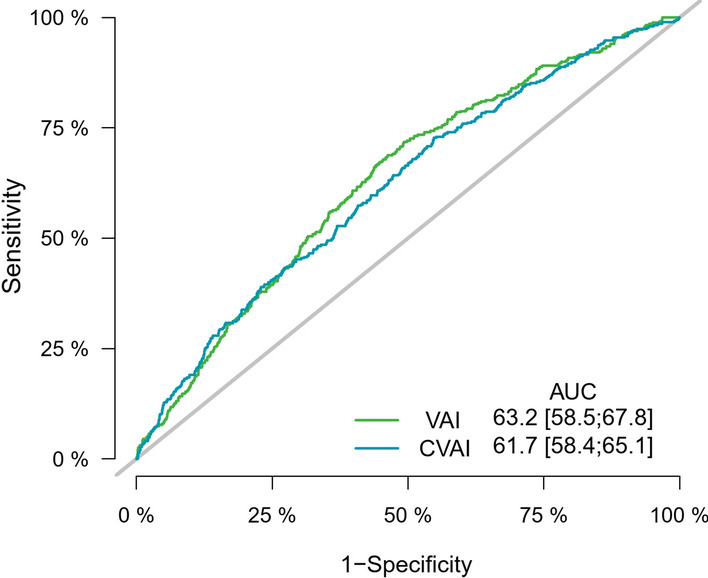
Time-dependent ROC curves of VAI and CVAI for predicting diabetic nephropathy development
In addition, we observed no significant interaction effect of age, sex, obesity, smoking with VAI and CVAI for incident nephropathy (Additional file 1: Table S4). Table 3 summarizes the association of the tertiles of VAI and CVAI with the incidence of nephropathy and retinopathy. Individuals in the highest tertile of VAI were significantly associated with an increased risk of nephropathy, compared with those in the lowest tertile (adjusted HR: 1.338, 95% CI 1.033–1.735, P value: 0.028). Significant association was not observed between CVAI tertiles and incident nephropathy. Tertiles of VAI and CVAI were not associated with the development of retinopathy.
Table 3.
Association of the tertiles of visceral adiposity indexes with the development of diabetic nephropathy and retinopathy
| Hazard ratio (95% CI) | ||||
|---|---|---|---|---|
| Model 1 | P value | Model 2 | P value | |
| Nephropathy | ||||
| VAI (ref: lower) | – | – | ||
| Middle | 1.222 (0.976–1.531) | 0.080 | 1.098 (0.873–1.382) | 0.425 |
| Upper | 1.571 (1.251–1.973) | < 0.001 | 1.338 (1.033–1.735) | 0.028 |
| CVAI (ref: lower) | – | – | ||
| Middle | 1.136 (0.851–1.516) | 0.387 | 0.989 (0.737–1.325) | 0.938 |
| Upper | 1.690 (1.293–2.208) | < 0.001 | 1.317 (0.975–1.780) | 0.073 |
| Retinopathy | ||||
| VAI (ref: lower) | – | – | ||
| Middle | 1.025 (0.587–1.790) | 0.930 | 0.765 (0.431–1.361) | 0.363 |
| Upper | 1.536 (0.915–2.578) | 0.104 | 0.696 (0.354–1.367) | 0.292 |
| CVAI (ref: lower) | – | – | ||
| Middle | 2.198 (1.119–4.317) | 0.022 | 1.369 (0.688–2.722) | 0.371 |
| Upper | 2.335 (1.188–4.589) | 0.014 | 1.134 (0.518–2.484) | 0.754 |
Model 1: adjusted for age and sex
Model 2: age, sex, BMI group, education level, smoking status, drinking status, physical activity, hypertension, dyslipidaemia, fasting glucose and use of antidiabetic medication;
VAI visceral adiposity index, CVAI Chinese visceral adiposity index
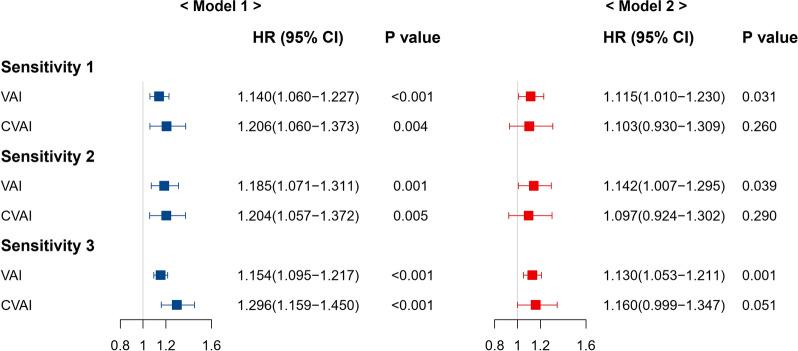
We repeated the analysis after excluding participants who developed nephropathy within one year. Only VAI was significantly associated with incident nephropathy (HR: 1.115, 95% CI 1.010–1.230). After excluding participants using antidiabetic medication, we found that VAI, not CVAI was significantly associated with incident nephropathy (HR: 1.142, 95% CI 1.007–1.295). The results remained almost consistent when HbA1c level was further adjusted, and VAI was independently associated with nephropathy development (HR: 1.130, 95% CI 1.053–1.211), as shown in Fig. 4.
Fig. 4.
Association of VAI and CVAI with incident nephropathy in sensitivity analyses
Discussion
This study evaluated the longitudinal association of visceral adiposity index with the development of nephropathy and retinopathy in a diabetic population. In the current analysis, we found that One-SD increase of VAI and CVAI levels were significantly associated with an increased risk of nephropathy, but not retinopathy in Chinese adults with diabetes after adjusting confounding factors. In the multiple sensitivity analyses, we found that VAI was more independently associated with incident nephropathy than CVAI.
Previous studies found that excess visceral fat, but not general adiposity, is strongly correlated with incident prediabetes and type 2 diabetes in obese adults [25, 26]. Some studies indicated that, as a simple indicator of visceral adipose function, VAI and CVAI could increase the risk of prediabetes and type 2 diabetes. Xia et al. [11, 27] reported that CVAI is a stronger predictor of prediabetes and diabetes than traditional indexes such as BMI and WC. Similarly, TZou et al. [28] found that CVAI and VAI may serve as potential indicators of diabetes. In addition, some studies reported that VAI is correlated with the risk of macrovascular complications in diabetic population [29, 30]. However, data on the associations of VAI and CVAI with diabetic microvascular complications are relatively limited. Several cross-sectional studies reported that VAI is an independent risk factor for excess urinary albumin excretion and diabetic nephropathy [31–33], and CVAI is closely associated with the prevalence of cardiovascular diseases and diabetic nephropathy [13]. In this current study, we found that VAI and CVAI levels at baseline are positively associated with the development of nephropathy in diabetic population using a cohort design.
Although the underlying mechanisms of VAI with diabetic nephropathy have not been fully illustrated, there are several possible explanations accounting for the observed associations. First, VAI is a representative marker to assess visceral adiposity which is strongly related with insulin resistance [34], and insulin resistance may lead to hypo-inflammation [35], endothelium dysfunction [36], and oxidative stress [37], contributing to the development of microangiopathy. Podocytes are insulin sensitive renal cells, and thus the insulin resistance is more likely to cause kidney damage [38]. Second, VAI is a valuable indicator of visceral adipose function [10]. The release of free fatty acids from central and visceral adipose tissue can increase the secretion of pro-inflammatory factors [39], such as TNF-α and interleukin-6, which could cause glomerular endothelial dysfunction and increase urine albumin. Third, obesity may also directly affect renal pathophysiology through the altered renal hemodynamics, as well as the production of adipokines and growth factors [40]. Adipokines are involved in microvascular injury and kidney damage by mediating endothelial dysfunction, inducing oxidative stress, inflammation, activation of the renin–angiotensin–aldosterone system [41] and endoplasmic reticulum stress [42]. All these mechanisms could partly explain the positive association between VAI and the development of nephropathy.
Wan et al. [13] found that VAI and CVAI were associated with a higher prevalence of diabetic nephropathy. While multiple sensitivity analyses in our study did not find a stable association between baseline CVAI and the incidence of diabetic nephropathy. The distinct formulas of calculating VAI and CVAI may account for this difference. In our study, the individuals with diabetic nephropathy were much older. Previous studies have shown that visceral adipose tissue is not associated with incident atherosclerotic cardiovascular events in older men [43]. Therefore, visceral adipose tissue may not have a strong adverse effect on health in relatively older people. CVAI is calculated based on age, BMI, WC, triglycerides, and HDL-C, while VAI is calculated independent of age and thus may be more useful for predicting the development of diabetic nephropathy. More studies in Chinese population are needed to confirm the association of VAI and CVAI with incident diabetic complications.
In this study, we did not find a positive association of VAI and CVAI with incident diabetic retinopathy, which is different from some other studies. Moh et al. [44] reported that visceral adiposity is associated with diabetic retinopathy in an Asian cohort with longstanding type 2 diabetes for 10 years. Of note, the cumulative incidence of diabetic retinopathy was only 1.0% in our study, while the annual incidence of diabetic retinopathy ranged from 2.2% to 12.7% from other Asia, North America, Caribbean, and sub-Saharan Africa studies [45], which indicates the participants in our cohort were at a relatively early stage of diabetes. In addition, the participants were annually physically examined and thus advised by our trained staff about the glucose control. The glucose and HbA1c levels were not severely high in this cohort, which could partially explain the low incidence of diabetic retinopathy. In addition, Moh et al. [44] found that the association between visceral adiposity and diabetic retinopathy became insignificant after controlling for ACR and eGFR, and thus the relationship may be attributable to vascular injury reflected by coexisting renal burden. Therefore, the visceral adiposity could be more strongly associated with renal function and nephropathy, which is also indicated in our study.
Our study is based on a population cohort of people with type 2 diabetes, which could explain the longitudinal associations of VAI and CVAI with the development of diabetic complications. In addition, the data were obtained from the central medical system or using the standardized questionnaire, which allow us to adjust for the potential confounding factors. However, several limitations in our study should be acknowledged. First, the visceral adiposity was not directly measured by MRI method, and we failed to examine the consistence between actual visceral fat mass and VAI in Chinese population. Second, the exact duration of diabetes in this cohort was unavailable, and the duration is an important risk factor of diabetic complication, which could bias the relationship between VAI and diabetic nephropathy. Third, information about the neurological complication is unavailable in this current cohort. We failed to assess the longitudinal association between VAI and the neuropathy development in diabetic population.
Conclusions
Our findings suggest that elevated levels of VAI and CVAI at baseline are significantly associated with increased risk of incident nephropathy, but not retinopathy in diabetic population.
Supplementary Information
Additional file 1: Table S1. Baseline characteristics according to tertiles groups of VAI. Table S2: Baseline characteristics according to tertiles groups of CVAI. Table S3: Full regression results of VAI and CVAI with diabetic nephropathy and retinopathy. Table S4: Interaction effect test of age, sex, obesity and smoking with incident nephropathy.
Additional file 2: Figure S1. Time-dependent ROC curves of VAI and CVAI for predicting diabetic retinopathy development.
Acknowledgements
We thank all the staff and participants of the Beijing Health Management Cohort for their invaluable contributions.
Abbreviations
- VAI
Visceral adiposity index
- CVAI
Chinese visceral adiposity index
- BHMC
Beijing Health Management Cohort
- WC
Waist circumference
- BMI
Body mass index
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- HbA1c
Glycated haemoglobin
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- ACR
Albumin/creatinine ratio
- eGFR
Estimated glomerular filtration rate
- ROC
Receiver operating characteristics
Authors' contributions
Study conception and design: ZYW, XLM, and WW; data collection: JBZ, XPK, YL, JQW, and ZKX; data analysis and interpretation: ZYW, XTL, ZWL, and XL; manuscript writing and reviewing: ZYW, and SQY; study supervision: XHG, and LXT. All authors read and approved the final manuscript.
Funding
Our work was funded by the National Natural Science Foundation of China (Numbers: 81872708 and 82073668).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author (Dr. Xiuhua Guo) on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committees of Capital Medical University. All participants gave informed consent to participate before taking part. The approval number was 2020SY031.
Consent for publication
Not applicable.
Competing interests
The authors have nothing to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhiyuan Wu, Email: wuxiaozhi@ccmu.edu.cn.
Siqi Yu, Email: ysq@mail.ccmu.edu.cn.
Xiaoping Kang, Email: kanspin910@126.com.
Yue Liu, Email: mugglesblue@163.com.
Zongkai Xu, Email: xuzongkai1997@163.com.
Zhiwei Li, Email: 15128472546@163.com.
Jinqi Wang, Email: cmuwangjinqi@163.com.
Xinlei Miao, Email: miaoxinlei1030@163.com.
Xiangtong Liu, Email: lxiangtong@163.com.
Xia Li, Email: x.li2@latrobe.edu.au.
Jingbo Zhang, Email: 13910625118@139.com.
Wei Wang, Email: wei.wang@ecu.edu.au.
Lixin Tao, Email: taolixin@ccmu.edu.cn.
Xiuhua Guo, Email: statguo@ccmu.edu.cn.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 3.Ward A, Alvarez P, Vo L, Martin S. Direct medical costs of complications of diabetes in the United States: estimates for event-year and annual state costs (USD 2012) J Med Econ. 2014;17(3):176–183. doi: 10.3111/13696998.2014.882843. [DOI] [PubMed] [Google Scholar]
- 4.Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin B, Zambon A, Barter P, Fruchart JC, Eckel RH. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 5.Salehinia F, Abdi H, Hadaegh F, Serahati S, Valizadeh M, Azizi F, Hosseinpanah F. Abdominal obesity phenotypes and incident diabetes over 12 years of follow-up: the Tehran Lipid and glucose study. Diabetes Res Clin Pract. 2018;144:17–24. doi: 10.1016/j.diabres.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Zhou R, Li F, Chen G, Fu Q, Gu S, Wu X. Associations between general and abdominal obesity and incident diabetic neuropathy in participants with type 2 diabetes mellitus. J Diabetes. 2021;13(1):33–42. doi: 10.1111/1753-0407.13075. [DOI] [PubMed] [Google Scholar]
- 7.Man RE, Sabanayagam C, Chiang PP, Li LJ, Noonan JE, Wang JJ, Wong TY, Cheung GC, Tan GS, Lamoureux EL. Differential association of generalized and abdominal obesity with diabetic retinopathy in Asian patients with type 2 diabetes. JAMA Ophthalmol. 2016;134(3):251–257. doi: 10.1001/jamaophthalmol.2015.5103. [DOI] [PubMed] [Google Scholar]
- 8.Omura-Ohata Y, Son C, Makino H, Koezuka R, Tochiya M, Tamanaha T, Kishimoto I, Hosoda K. Efficacy of visceral fat estimation by dual bioelectrical impedance analysis in detecting cardiovascular risk factors in patients with type 2 diabetes. Cardiovasc Diabetol. 2019;18(1):137. doi: 10.1186/s12933-019-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taksali SE, Caprio S, Dziura J, Dufour S, Calí AM, Goodman TR, Papademetris X, Burgert TS, Pierpont BM, Savoye M, Shaw M, Seyal AA, Weiss R. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57(2):367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 10.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q, Aleteng Q, Chen Y, Sun YX, Hu Y, Pan BS, Li XY, Gao X. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: A prospective cohort study. Diabetes Metab Res Rev. 2018;34(7):e3048. doi: 10.1002/dmrr.3048. [DOI] [PubMed] [Google Scholar]
- 12.Wei J, Liu X, Xue H, Wang Y, Shi Z. Comparisons of Visceral Adiposity Index, Body Shape Index, Body Mass Index and waist circumference and their associations with diabetes mellitus in adults. Nutrients. 2019 doi: 10.3390/nu11071580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, Zhang W, Zhang H, Xia F, Wang N, Lu Y. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol. 2020;19(1):118. doi: 10.1186/s12933-020-01095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Zhao Z, Mu Y, Zou X, Zou D, Zhang J, Chen S, Tao L, Guo X. Gender differences in the association between serum uric acid and prediabetes: a six-year longitudinal cohort study. Int J Environ Res Public Health. 2018 doi: 10.3390/ijerph15071560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, Miao X, Liu X, Li X, Wang W, Guo X, Tao L. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20(1):134. doi: 10.1186/s12933-021-01330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 18.Johnson EL, Feldman H, Butts A, Billy CD, Dugan J, Leal S, Rhinehart AS, Shubrook JH, Trujillo J, Neumiller JJ. Standards of medical care in diabetes-2020 abridged for primary care providers. Clin Diabetes. 2020;38(1):10–38. doi: 10.2337/cd20-as01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joint Committee for Developing Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults Chinese guidelines on prevention and treatment of dyslipidemia in adults. Chin J Epidemiol. 2007;35:390–419. [PubMed] [Google Scholar]
- 20.Wu J, Gong L, Li Q, Hu J, Zhang S, Wang Y, Zhou H, Yang S, Wang Z. A novel visceral adiposity index for prediction of type 2 diabetes and pre-diabetes in Chinese adults: a 5-year prospective study. Sci Rep. 2017;7(1):13784. doi: 10.1038/s41598-017-14251-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 22.Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013, 3 (1): 19–62. [DOI] [PMC free article] [PubMed]
- 23.Seidu S, Barrat J, Khunti K. Clinical update: The important role of dual kidney function testing (ACR and eGFR) in primary care: Identification of risk and management in type 2 diabetes. Prim Care Diabetes. 2020;14(4):370–375. doi: 10.1016/j.pcd.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto Y, Tanaka M, Okada H, Senmaru T, Hamaguchi M, Asano M, Yamazaki M, Oda Y, Hasegawa G, Toda H, Nakamura N, Fukui M. Metabolically healthy obesity and risk of incident CKD. Clin J Am Soc Nephrol. 2015;10(4):578–583. doi: 10.2215/CJN.08980914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung SH, Ha KH, Kim DJ. Visceral fat mass has stronger associations with diabetes and prediabetes than other anthropometric obesity indicators among Korean adults. Yonsei Med J. 2016;57(3):674–680. doi: 10.3349/ymj.2016.57.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, Li Q, Wang D, Hu Y, Pan BS, Li XJ, Li XY, Gao X. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214. doi: 10.1038/srep38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsou MT, Chang YC, Hsu CP, Kuo YC, Yun CH, Huang WH, Hu KC, Liu CY, Chen YJ, Sung KT, Liu CC, Hung CL, Kuo JY, Chen TY, Hung TC, Yeh HI. Visceral adiposity index outperforms conventional anthropometric assessments as predictor of diabetes mellitus in elderly Chinese: a population-based study. Nutr Metab (Lond) 2021;18(1):87. doi: 10.1186/s12986-021-00608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wung CH, Lee MY, Wu PY, Huang JC, Chen SC. Obesity-related indices are associated with peripheral artery occlusive disease in patients with type 2 diabetes mellitus. J Pers Med. 2021 doi: 10.3390/jpm11060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randrianarisoa E, Lehn-Stefan A, Hieronimus A, Rietig R, Fritsche A, Machann J, Balletshofer B, Häring HU, Stefan N, Rittig K. Visceral adiposity index as an independent marker of subclinical atherosclerosis in individuals prone to diabetes mellitus. J Atheroscler Thromb. 2019;26(9):821–834. doi: 10.5551/jat.47274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun K, Lin D, Li F, Qi Y, Feng W, Ren M, Yan L, Liu D. Visceral adiposity index is associated with increased urinary albumin excretion: a population-based study. Clin Nutr. 2019;38(3):1332–1338. doi: 10.1016/j.clnu.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Yang S, Zhang A, Yang P, Cao X, Li X, Goswami R, Wang Y, Luo T, Liao K, Cheng Q, Xiao X, Li Q. Abdominal obesity is more closely associated with diabetic kidney disease than general obesity. Diabetes Care. 2016;39(10):e179–180. doi: 10.2337/dc16-1025. [DOI] [PubMed] [Google Scholar]
- 33.Ou YL, Lee MY, Lin IT, Wen WL, Hsu WH, Chen SC. Obesity-related indices are associated with albuminuria and advanced kidney disease in type 2 diabetes mellitus. Ren Fail. 2021;43(1):1250–1258. doi: 10.1080/0886022X.2021.1969247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pekgor S, Duran C, Berberoglu U, Eryilmaz MA. The role of visceral adiposity index levels in predicting the presence of metabolic syndrome and insulin resistance in overweight and obese patients. Metab Syndr Relat Disord. 2019;17(5):296–302. doi: 10.1089/met.2019.0005. [DOI] [PubMed] [Google Scholar]
- 35.Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, Ferrannini E. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55(4):1133–1140. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 36.Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. doi: 10.1186/s12933-018-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 38.Bamba R, Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. The visceral adiposity index is a predictor of incident chronic kidney disease: a population-based longitudinal study. Kidney Blood Press Res. 2020;45(3):407–418. doi: 10.1159/000506461. [DOI] [PubMed] [Google Scholar]
- 39.Matulewicz N, Karczewska-Kupczewska M. Insulin resistance and chronic inflammation. Postepy Hig Med Dosw (Online) 2016;70:1245–1258. [PubMed] [Google Scholar]
- 40.Mallamaci F, Tripepi G. Obesity and CKD progression: hard facts on fat CKD patients. Nephrol Dial Transplant. 2013;28(Suppl 4):105–108. doi: 10.1093/ndt/gft391. [DOI] [PubMed] [Google Scholar]
- 41.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 42.Li C, Lin Y, Luo R, Chen S, Wang F, Zheng P, Levi M, Yang T, Wang W. Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney. Am J Physiol Renal Physiol. 2016;310(5):F351–363. doi: 10.1152/ajprenal.00223.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schousboe JT, Kats AM, Langsetmo L, Vo TN, Taylor BC, Schwartz AV, Cawthon PM, Lewis CE, Barrett-Connor E, Hoffman AR, Orwoll ES, Ensrud KE. Central obesity and visceral adipose tissue are not associated with incident atherosclerotic cardiovascular disease events in older men. J Am Heart Assoc. 2018;7(16):e009172. doi: 10.1161/JAHA.118.009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moh A, Neelam K, Zhang X, et al. Excess visceral adiposity is associated with diabetic retinopathy in a multiethnic Asian cohort with longstanding type 2 diabetes. Endocr Res. 2018;43(3):186–194. doi: 10.1080/07435800.2018.1451541. [DOI] [PubMed] [Google Scholar]
- 45.Sabanayagam C, Banu R, Chee ML, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7(2):140–149. doi: 10.1016/S2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Baseline characteristics according to tertiles groups of VAI. Table S2: Baseline characteristics according to tertiles groups of CVAI. Table S3: Full regression results of VAI and CVAI with diabetic nephropathy and retinopathy. Table S4: Interaction effect test of age, sex, obesity and smoking with incident nephropathy.
Additional file 2: Figure S1. Time-dependent ROC curves of VAI and CVAI for predicting diabetic retinopathy development.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author (Dr. Xiuhua Guo) on reasonable request.