Abstract
Plant-associated endophytes define an important symbiotic association in nature and are established bio-reservoirs of plant-derived natural products. Endophytes colonize the internal tissues of a plant without causing any disease symptoms or apparent changes. Recently, there has been a growing interest in endophytes because of their beneficial effects on the production of novel metabolites of pharmacological significance. Studies have highlighted the socio-economic implications of endophytic fungi in agriculture, medicine, and the environment, with considerable success. Endophytic fungi-mediated biosynthesis of well-known metabolites includes taxol from Taxomyces andreanae, azadirachtin A and B from Eupenicillium parvum, vincristine from Fusarium oxysporum, and quinine from Phomopsis sp. The discovery of the billion-dollar anticancer drug taxol was a landmark in endophyte biology/research and established new paradigms for the metabolic potential of plant-associated endophytes. In addition, endophytic fungi have emerged as potential prolific producers of antimicrobials, antiseptics, and antibiotics of plant origin. Although extensively studied as a “production platform” of novel pharmacological metabolites, the molecular mechanisms of plant–endophyte dynamics remain less understood/explored for their efficient utilization in drug discovery. The emerging trends in endophytic fungi-mediated biosynthesis of novel bioactive metabolites, success stories of key pharmacological metabolites, strategies to overcome the existing challenges in endophyte biology, and future direction in endophytic fungi-based drug discovery forms the underlying theme of this article.
Keywords: anti-infectives, bioactive metabolites, biosynthetic gene clusters (BCG), drug discovery, endophytic fungi, fungal pharmacology, production platforms
1. Introduction
Endophytes represent biological reservoirs of novel natural products, opening new avenues in the frontiers of drug discovery. Plant-associated microorganisms that colonize the internal tissues of all plant species are gaining momentum as key targets for bio-prospection in the search for novel chemical entities [1,2]. The discovery of the acclaimed anticancer drug paclitaxel accelerated research on endophytic biology and yielded prospective “drug candidates” with antimicrobial, immunosuppressant, antioxidant, and anti-neurodegenerative functions [1,2,3]. Currently, the impact of natural products in clinical applications cannot be underestimated [4], with the pharmaceutical sector adopting high-throughput approaches to screen plant secondary metabolites as potential lead molecules. According to a study by Newman and Cragg [5], among the 1562 drugs approved by the FDA, 141 comprised botanical drugs, 64,320 drugs were derivatives of natural products, while 61 drugs were synthesized based on natural pharmacophore [5].
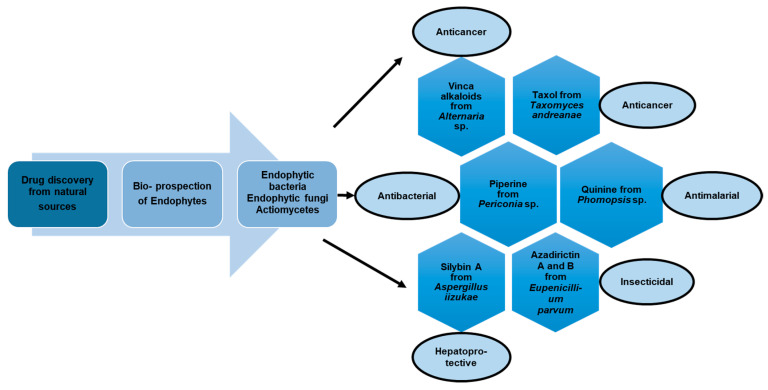
With the emerging threat of drug-resistant microbes and antimicrobial resistance (AMR), it has become essential to discover novel antimicrobials to counter AMR. However, the low pace of discovery and indiscriminate use of the existing antibiotics have further necessitated the exploration of novel antimicrobial entities to compensate for the drying drug pipeline [2,6,7,8]. The rich yet less-explored diversity of endophytic entities and their considerable potential to impact the pharmaceutical industry have facilitated the discovery of secondary metabolites of therapeutic significance (Figure 1. Bio-prospection of endophytes and discovery of novel, high-value metabolites of commercial significance, including hinnuliquinone, a potent inhibitor of the HIV-1 protease [9]; cytonic acids A and B, human cytomegalovirus protease inhibitors [10]; pestacin and isopestacin, antioxidants [11]; paclitaxel, antineoplastic agents [12]; and lariatins A and B, anti-HIV agents [13]. The discovery and commercial production of paclitaxel from different endophytic species revolutionized the pharmaceutical industry (with FDA approval in 1992), and the diterpene natural product garnered commercial sales of over $3 billion in 2004 [14].
Figure 1.
Bio-prospection of endophytes and discovery of novel, high-value metabolites of commercial significance.
With increasing research on the discovery of natural products from biological species, endophytes are increasingly being explored as production platforms for bioactive metabolites with diverse chemical structures. The discovery of taxol from the endophytic fungus Taxomyces andreanae in 1993 [15], and subsequently from other endophytic species, led to renewed research on pharmacological metabolites from endophytic sources. Furthermore, studies have established that endophytes mimic host metabolism and can produce, induce, and modify the metabolic chemical entities within hosts [16,17]. Endophytic fungi are one of the most promising candidates for endophyte-mediated natural product discovery. Diverse bioactive metabolites produced by endophytic fungi have demonstrated socio-economic importance and found applications in agriculture (as biofertilizers/biostimulants) and the environment (bioremediation), as biofuels/biocatalysts, in addition to their pharmacological attributes. Table 1 provides an overview of endophytic fungi in the environment, including multifaceted applications, key examples, and translational outcomes. As useful metabolites with multifaceted environmental implications are increasingly being discovered, different strains of endophytic fungi are being investigated and the associated limitations are being addressed for maximum use/multifaceted applications.
Table 1.
Endophytic fungi in environment: significant multi-faceted applications, key examples, and translational outcomes.
| Biological Application | Endophytic Fungi | Plant Species | Outcome | Reference |
|---|---|---|---|---|
| Plant growth promotion and agriculture | ||||
| Plant growth promotion (PGP) | Penicillium sp. 21 | Camellia sinensis | Mineral-solubilizing function (Ca3(PO4)2 and rock phosphate) | [18,19] |
| Penicillium sp. 2 | ||||
| Aspergillus sp. MNF | ||||
| Trichoderma gamsii (NFCCI 2177) | Lens esculenta | Solubilization of Tricalcium phosphate | [20] | |
| Trichoderma peudokoningi | Solanum lycopersicum | Siderophore production, HCN and ammonia production | [21] | |
| Chaetomium globosum | ||||
| Fusarium oxysporum | ||||
| Ophiosphaerella sp. | Triticum aestivum | PGP activities | [22] | |
| Cochliobolus sp. | ||||
| Cladosporium sphaerospermum | Glycine max | Solubilize calcium phosphate | [23] | |
| Fusarium tricinctum RSF-4L | S. nigrum | Production of phytohormones (gibberellins) | [24] | |
| Alternaria alternata RSF-6L | ||||
| Endophytic fungi | Hordeum murinum subsp. murinum | IAA production, PGP activities Plant yield increase |
[25] | |
| Biofertilizers/biostimulants for crops | Aspergillus sp. | S. officinarum | Phosphorous solubilization | [26] |
| Penicillium sp. 1 | ||||
| Penicillium sp. 2 | ||||
| Cochliobolus sp. | T. aestivum | Phosphorous solubilization | [26] | |
| Curvularia sp. | ||||
| Fusarium equiseti | Pisum sativum | Phosphorous solubilization | [27] | |
| Coniothyrium aleuritis isolate 42 | Lycopersicon esculentum | Plant biomass increase, fruit yield | [28] | |
| Pichia guilliermondii isolate F15 | ||||
| Fusarium oxysporum strain NSF2 | ||||
| Fusarium proliferatum strain AF04 | ||||
| Aspergillus nidulans strain FH5 | ||||
| Trichoderma spirale strain YIMPH30310 | ||||
| Biocontrol function | Fusarium verticillioides | Zea mays | Restrict Ustilago maydis growth | [29] |
| Penicillium sp. | Cucumis sativus | Biocontrol of Fusarium oxysporum f. sp. cucumerinum | [30] | |
| Guignardia mangiferae | ||||
| Hypocrea sp. | ||||
| Neurospora sp. | ||||
| Eupenicillium javanicum | ||||
| Lasiodiplodia theobromae | ||||
| Biotic/abiotic stress tolerance | Piriformospora indica | Hordeum vulgare | Drought stress tolerance | [31] |
| P. indica | Brassica rapa | Drought stress tolerance | [32] | |
| P. indica | H. vulgare | Biotic/abiotic stress tolerance | [25] | |
| Bioremediation | Mucor sp. MHR-7 | Brassica campestris | Metal toxicity reduction | [33] |
| Rhizopus sp. CUC23 | Lactuca sativa | Chromium detoxification | [34] | |
| A. fumigatus ML43 | ||||
| Penicillum radicum PL17 | ||||
| Endophytic fungi | Agrostis stolonifera | Bioremediation of lead | [35] | |
| Neotyphodium coenophialum | Festuca arundinacea, Festuca pratensis | Bioaugmentation, total petroleum hydrocarbons (TPH) and polycyclic aromatic hydrocarbons (PAHs) removal from the soil | [36] | |
| N. uncinatum | ||||
|
Verticillium sp. Xylaria sp. |
Plants from Ecuadorian Amazon | Degradation of Petroleum hydrocarbon | [37] | |
| Endophytic fungi | - | Bioremediation of synthetic plastic polymers | [38] | |
| Curvularia sp. | Mangrove sp. | Heavy metal biosorption | [39] | |
| Neusartorya sp. | ||||
| Bjerkandera adusta SWUSI4 | Sinosenecio oldhamianus | Detoxification of triphenylmethane dyes | [40] | |
| Lasiodiplodia theobromae | Boswellia ovalifoliolata | Heavy metal tolerance | [41] | |
|
Lindgomycetaceae P87 Aspergillus sp. A31 |
Aeschynomene fluminensis | Heavy metal resistance, bioremediation | [42] | |
| Bioactive metabolites for industrial and pharmacological applications | ||||
| Paclitaxel |
Taxomyces andreanae
T. brevifolia |
Pacific yew | Anticancer | [15] |
| Azadirachtin A and B | Eupenicillium parvum | Azadirachta indica | Insecticidal | [43] |
| Subglutinol A | Fusarium subglutinans | Tripterygium wilfordii | Immuno-suppressant | [44] |
| Isopestacin | Pestalotiopsis microspora | Terminalia morobensis | Antifungal, Antioxidant | [11] |
| Podophyllotoxin | Trametes hirsuta | Podophyllum hexandrum | Antiviral, Radio-protective | [45] |
| Forskolin | Rhizoctonia bataticola | Coleus forskohlii | Anti-HIV, Antitumor | [46] |
| Sanguinarine | Fusarium proliferatum | Macleaya cordata | Antihelmintic | [47] |
| Digoxin | Alternaria sp. | Digitalis lanata | Cardiotonic | [48] |
| Quinine | Phomopsis sp. | Cinchona ledgeriana | Antimalarial | [49] |
| Capsaicin | Alternaria alternata | Capsicum annuum | Cardio-protective | [50] |
Although plant-associated endophytes serve as “biosynthetic platforms” of pharmaceutically important secondary metabolites, challenges exist in terms of the limited knowledge of endophyte biology and decreased production of secondary metabolites due to repeated sub-culturing of endophytic strains, projecting a need to adopt a more integrated and systematic approach towards the exploitation of endophytes in drug discovery and research. In this direction, a comprehensive insight into plant–endophyte associations and their dynamics is necessary to understand how and why endophytes biosynthesize secondary metabolites [51]. Another prospective strategy is to develop strain improvement methods using genetic engineering, optimization of culture conditions/media for cultivation, and co-culturing of different endophytic strains. Recent advances in high-throughput technologies and the “genomic revolution” have contributed considerably to natural product research and the discovery of biosynthetic gene clusters (BGCs) [52,53]. Genetic engineering of endophytes is still in its infancy and is often regarded as a progenitor of system biology and functional genomics strategies [54] with the potential for long-term translational success. Endophytes mimic their host plant in independent biosynthesis of secondary metabolites, thus acting as a prospective platform for genetic manipulation [14]. Studies have suggested the inclusion of metabolic pathways and genes in endophytes via genetic recombination between plant hosts and endophytes [55,56]. In addition, horizontal gene transfer, an evolutionary mechanism, has been suggested as an adaptive mechanism for endophytes, and it confers novel traits to the associated microbes [56].
Moreover, approaches in metagenomics, whole-genome sequencing, and omics biology have further enabled access to the less explored natural product reservoirs of plant-associated endophytes. The existing and emerging trends in endophytic fungi-mediated biosynthesis of novel bioactive metabolites, success stories of key pharmacological metabolites, strategies to overcome the existing/forthcoming challenges in endophytic biology, and future directions/outcomes in endophytic fungi-based drug discovery form the underlying theme of this article.
2. Bioactive Metabolites from Endophytic Fungi as Novel Drug Candidates
Plants possessing pharmacological properties and bioactive metabolites are used to treat ailments in modern healthcare [57]. The small molecule drugs approved from 1981–2014 comprise more than 51% of natural products [58]. However, the indiscriminate use of natural products has threatened natural resources, specifically plant species, and led to a shortage of novel bioactive metabolites. Thus, it is imperative to explore other eco-friendly alternatives to produce high-value phytochemicals and plant-associated endophytes, which are emerging as a highly attractive source. The emerging significance of secondary metabolites from endophytes is largely attributed to their multifaceted applications, ranging from pharmaceutical drugs and immune suppressants to agriculture and industrial uses. The majority (38%) of the bioactive metabolites, including antibiotics, were isolated from fungi out of 22,500 microbe-derived compounds [59]. Recently, the slow pace of antibiotic discovery and rising AMR have hampered the drug discovery process.
Plant-associated endophytes comprise bacterial and fungal species that colonize internal plant tissues and complete their partial or entire life cycle inside the host plant. Showing universal occurrence and inhabiting almost all vascular plant species, endophytes demonstrate multifaceted advantages to the host plant by promoting growth, conferring tolerance to biotic/abiotic stresses [29], and biosynthesizing bioactive secondary metabolites [43]. Recent investigations into the ecological perspective of secondary metabolite production include protection against pathogens [60,61], mitigation of abiotic stress in plants [62], and herbivore deterrence [63]. An investigation into the natural product chemistry of endophytes highlighted the remarkable potential of endophytes in the production of phytochemicals and emerging platforms for the fabrication of novel antimicrobial entities [64]. Table 2 presents current state of knowledge on the production of endophytic fungi-mediated secondary metabolites and their pharmacological significance. It is imperative to understand plant–endophyte dynamics and how endophytes are a key source of phytochemicals. Endophytes influence their host and induce favorable responses in different ways, including elicitation of novel phytochemicals, plant growth augmentation, and conferring stress (abiotic/biotic) tolerance to the host plant [64].
Table 2.
Production of endophytic fungi-mediated secondary metabolites and their pharmacological significance.
| Endophytic Fungi | Fungi and Fungus-Like Taxa | Plant Association | Bioactivity | Secondary Metabolite | Class of Compound | Active Concentration | Pathogen(s) | Reference |
|---|---|---|---|---|---|---|---|---|
| Pestalotiopsis foedan | Coelomycetes |
Bruguiera
sexangula |
Antifungal | (3R,4R,6R,7S)-7-hydroxyl-3,7-dimethyl-oxabicyclo [3.3.1] nonan-2-one | Monoterpene lactone | 3.1 µg/mL (MIC) |
Botrytis cinerea
Phytophthora nicotianae |
[65] |
| (3R,4R)-3-(7-methylcyclohexenyl)-propanoic acid | 6.3 µg/mL | [66] | ||||||
| Pestalotiopsis sp. DO14 | Coelomycetes |
Dendrobium
officinale |
Antifungal, Cytotoxic |
(4S,6S)-6-[(1S,2R)-1,2-dihydroxybutyl]-4-hydroxy-4-methoxytetrahydro-2H-pyran-2-one | Monoterpenoid | ≤25 µg/mL (MIC) |
Candida albicans
Cryptococcus neoformans Trichophyton rubrum Aspergillus fumigatus |
[67] |
| (6S,2E)-6-hydroxy-3-methoxy-5-oxodec-2-enoic acid | ||||||||
|
Diaporthe
maritima |
Coelomycetes | Picea sp. | Antifungal | Phomopsolide A | Dihydropyrones | 25 µM (MIC) | Microbotryum violaceum | [68] |
| Phomopsolide B | 250 µM | |||||||
| Phomopsolide C | 250 µM | |||||||
| Scleroderma UFSM Sc1 | Basidiomycetes |
Eucalyptus
grandis |
Antifungal, Insecticidal |
Sclerodol A | Lanostane-type triterpenes |
50 µg/mL (MIC) 50 µg/mL 12.5 µg/mL 25 µg/mL |
C. albicans
C. tropicalis C. crusei C. parapsilosis |
[69] |
| Sclerodol B | 25 µg/mL 25 µg/mL 6.25 µg/mL 12.5 µg/mL |
|||||||
| Fusarium fujikuroi (WF5) | Hyphomycetes |
Eleusine
coracana |
Antifungal | 5-hydroxy 2(3H)-benzofuranone | Furanone | 31.25 µg/mL (MIC) | F. graminearum | [70] |
| Harpagoside | Iridoide glycoside | 31.25 µg/mL | ||||||
|
Trichoderma koningiopsis YIM PH30002 |
Hyphomycetes | Panax notoginseng | Antifungal | Koningiopisin C | Polyketides | 32 µg/mL (MIC) 64 µg/mL 32 µg/mL 16 µg/mL |
F. oxysporum
A. panax F. solani P. cucumerina |
[71] |
| Trichoderma brevicompactum 0248 | Hyphomycetes | Allium sativum | Antifungal | Trichodermin | Sesquiterpene | EC50 of 0.25 µg/mL 2.02 µg/mL 25.60 µg/mL |
R. solani
B. cinerea C. lindemuthianum |
[72] |
| Aspergillus sp. | Hyphomycetes | Gloriosa superba | Antimicrobial, Cytotoxic |
6-methyl-1,2,3-trihydroxy-7,8-cyclohepta-9,12-diene-11-one-5,6,7,8-tetralene-7-acetamide (KL-4) | Tetralene derivative | 25 µg/mL (MIC) 12.5 µg/mL 50 µg/mL |
S. cerevisiae
C. albicans C. gastricus |
[73] |
| Penicillium sp. R22 | Hyphomycetes | Nerium indicum | Antifungal | 3-O-methylviridicatin | Isoquinolone alkaloid | 31.2 µg/mL (MIC) |
A. brassicae
B. cinerea V. mali |
[74] |
| Viridicatol | 31.2 µg/mL |
A. brassicae
A. alternata B. cinerea |
||||||
| 5-hydroxy-8-methoxy-4-phenylisoquinolin-1(2H)-one | 31.2 µg/mL |
A. brassicae
A. alternata V. mali |
||||||
| Trichoderma sp. 09 | Hyphomycetes |
Myoporum
bontioides |
Antifungal | Dichlorodiaportin | Isocoumarin | 6.25–150 µg/mL(MIC) |
C. musae
Rhizoctonia solani |
[75] |
| Dichlorodiaportinolide | ||||||||
|
Fusarium
chlamydosporium |
Hyphomycetes | Anvillea garcinii | Antimicrobial, Cytotoxic |
Fusarithioamide A | Benzamide derivative | 3.1 μg mL−1 (MIC) 4.4 μg mL−1 6.9 μg mL−1 |
B. cereus
S. aureus E. coli |
[76] |
| Curvularia sp., strain M12 | Hyphomycetes | Murraya koenigii | Antifungal | Murranofuran A | Dihydrofurans | 0.5 µg/mL | Phytophthora capsici | [77] |
| Murranolide A | Oxygenated polyketide | IC50 50–100 µg/mL | ||||||
| Murranopyrone | Dihydropyrones | 50–100 µg/mL | ||||||
| Murranoic acid A | Dienoic acid | 50–100 µg/mL | ||||||
| Fusarium sp. | Hyphomycetes |
Mentha
longifolia |
Antimalarial Antifungal |
Fusaripeptide A | Cyclodepsipeptide | IC50 0.24 µM 0.11 µM |
C. glabrata
C. albicans |
[78] |
| Trichothecium sp. | Hyphomycetes |
Phyllanthus
amarus |
Anticancer, Antimetastatic, Antifungal |
Trichothecinol A | Trichothecenes | 20 µg/mL (MIC) |
Cryptococcus albidus HeLa and B16F10 cells MDA-MB-231 cells |
[79] |
| Phoma sp. | Coelomycetes | Fucus serratus | Antimicrobial | Phomafuranol (3R)-5-hydroxymellein Phomalacton Emodin |
Dihydrofuran derivative |
NR | M. violaceum | [80] |
| (3R)-5-hydroxymellein | 5 mm ZOI | |||||||
| Phomalacton | 6 mm | |||||||
| Emodin | 5 mm | |||||||
| Rhizopycnis vagum Nitaf 22 | Coelomycetes |
Nicotiana
tabacum |
Antimicrobial, Cytotoxic |
Rhizopycnin D | Dibenzo-α-pyrone derivatives |
IC50 9.9 µg/mL | M. oryzae | [81] |
| Colletotrichum sp. | Coelomycetes | Gomera | Antibacterial, Antifungal, Antialgal |
Seimatoric acid | Oxobutanoic acid derivative |
NR | Microbotryum violaceum | [82] |
| Colletonoic acid | Benzoic acid derivative | 7 mm ZOI |
B. megaterium
C. fusca |
|||||
| Xylaria sp. XC-16 | Ascomycetes | Toona sinensis | Cytotoxic, Fungicidal |
Cytochalasin Z28 | Cytochalasins | 12.5 µM (MIC) | G. saubinetti | [83] |
|
Penicillium
chrysogenum |
Hyphomycetes |
Cistanche
deserticola |
Neuroprotective | Chrysogenamide A | Macfortine alkaloids | IC50 1 × 10−4 µM | SH-SY5Y cells | [84] |
| Circumdatin G | ||||||||
| Benzamide | ||||||||
| 2′,3′-dihydrosorbicillin(9Z,12Z)-2,3-dihydroxypropyloctadeca-9,12-dienoate | ||||||||
|
Chaetomium globosum CDW7 |
Ascomycetes | Ginkgo biloba | Antifungal | Chaetoglobosin A | Chaetoglobosins | IC50 0.35 µg/mL | S. sclerotiorum | [85] |
| Chaetoglobosin D | 0.62 µg/mL | |||||||
| Coniothyrium sp. | Coelomycetes |
Salsola
oppostifolia |
Antimicrobial | Coniothyrinones A | Hydroxyanthraquinone | 7.5 mm ZOI | Microbotryum violaceum | [86] |
| Coniothyrinones B | 6 mm | |||||||
| Coniothyrinones C | 8 mm | |||||||
| Coniothyrinones D | 7.5 mm | |||||||
| Pestalotiopsis fici | Coelomycetes | Camellia sinensis | Antifungal | Ficipyrone A | α-pyrones | IC50 15.9 µM | Gibberella zeae | [87] |
|
Xylaria sp. strain F0010 |
Ascomycetes |
Abies holophylla Garcinia
hombroniana |
Antioxidant | Griseofulvin | Indanones | IC50 18.0 µg/mL | A. mali | [88,89] |
| 5.0 µg/mL | B. cinerea | |||||||
| 1.7 µg/mL |
Colletotrichum
gloeosporioides |
|||||||
| 11.0 µg/mL | Corticium sasaki | |||||||
| 30.0 µg/mL | F. oxysporum | |||||||
| 1.7 µg/mL | M. grisea | |||||||
| Phaeoacremonium sp. | Hyphomycetes | Senna spectabilis | Antifungal | Isoaigialone B Isoaigialone C |
Lactone derivatives | 5 µg >5 µg |
Cladosporium cladosporioides
C. sphaerospermum |
[90] |
| Aigialone | 5 µg | |||||||
| Aspergillus terreus | Hyphomycetes |
Carthamus
lanatus |
Anti-microbial, Anti-malarial, Anti-leishmanial |
(22E,24R)-stigmasta-5,7,22-trien-3-β-ol | Butyrolactones | IC50 4.38 µg/mL | C. neoformans | [91] |
| Aspernolides F | 5.19 µg/mL | |||||||
|
Penicillium
raciborskii |
Hyphomycetes |
Rhododendron
tomentosum |
Antifungal | Outovirin C | Bridged epipolythiodiket-opiperazines | 0.38 µM (MIC) |
F. oxysporum
B. cinerea V. dahlia |
[92] |
| Mycosphaerella sp. | Ascomycetes |
Eugenia
bimarginata |
Antifungal | 2-amino-3,4-dihydroxy-2-25-(hydroxymethyl)-14-oxo-6,12-eicosenoic acid | Eicosanoic acids | 1.3 to 2.50 µg/mL (MIC) |
C. neoformans
C. gattii |
[93] |
| Myriocin | 0.5 µg/mL | |||||||
| Guignardia sp. | Ascomycetes | Euphorbia sieboldiana | Antifungal | Guignardone N | Meroterpenes and dioxolanone derivatives | FIC 0.23 | C. albicans | [94] |
| Guignardic acid | 0.19 | |||||||
| Hyalodendriella sp. | Ascomycetes | Populus deltoides Marsh × P. nigra L. | Antimicrobial | Hyalodendriol C | Dibenzo-α-pyrones | 19.22–98.47 μg/mL (MIC) |
Bacillus subtilis
Pseudomonas lachrymans Ralstonia solanacearum Xanthomonas vesicatoria Magnaporthe oryzae |
[95,96] |
| Palmariol B | 16.18–92.21 μg/mL | |||||||
| TMC-264 | 16.24–85.46 μg/mL | |||||||
| Penicilliumolide B | 17.81–86.32 μg/mL | |||||||
| Alternariol 9-methyl ether | 107.19–123.19 μg/mL | |||||||
| Fusarium sp. | Hyphomycetes | Ficus carica | Antifungal | Helvolic acid methyl ester | Helvolic acid derivative | 12.5–25 μg/mL (MIC) |
B. cinerea C. gloeosporioides F. oxysporum f. sp. niveum Fusarium graminearum Phytophthora capsici |
[97] |
| Helvolic acid | ||||||||
| Hydrohelvolic acid | ||||||||
| Lopherdermium nitens DAOM 250027 | Ascomycetes | Pinus strobus | Antifungal | Six phenolic bisabolane-type sesquiterpenoids | Phenolic bisabolane-type sesquiterpenoids | 50 μM (MIC) | Microbotryum violaceum | [98] |
| Pyrenophorin | Macrolide | 5 μM | Saccharomyces cerevisiae | |||||
| Epicoccum sp. | Ascomycetes | Theobroma cacao | Antimicrobial, Antifungal | Epicolactone | Polyoxygenated polyketides | 20–80 μg per paper disc (MIC) |
Pythium ultimum
Aphanomyces cochlioides Rhizoctonia solani |
[99] |
| Epicoccolide A | ||||||||
| Epicoccolide B | ||||||||
| Botryosphaeria dothidea KJ-1 | Ascomycetes | Melia azedarach | Antifungal, Antibacterial, Antioxidant Cytotoxic |
Stemphyperylenol | α-pyridone derivative Ceramide derivative |
1.57 μM (MIC) | Alternaria solani | [100] |
| Pycnophorin | 6.25–25 μM | |||||||
| Chaetoglobosin C | ||||||||
| Djalonensone | ||||||||
| Alternariol | ||||||||
| β-sitosterol glucoside | ||||||||
| 5-hydroxymethylfurfural | ||||||||
| Botryosphaeria sp. P483 | Ascomycetes | Huperzia serrata | Antifungal, Nematicidal |
Botryosphaerin H | Tetranorlabdane diterpenoids | ZOI 9, 7, 7, 8, 8 mm |
Gaeumannomyces graminis
Fusarium solani Pyricularia oryzae Fusarium moniliforme F. oxysporum |
[101] |
| 13,14,15,16-tetranorlabd-7-en-19,6β:12,17-diolide | 12, 10, 10, 11, 13 mm | |||||||
| Colletotrichum gloeosporioides | Coelomycetes |
Michelia
champaca |
Antifungal | 2-phenylethyl 1H-indol-3-yl-acetate | ---- | 5 µg 25 µg (MIC) |
Cladosporium cladosporioides
C. sphaerospermum |
[102] |
| Uracil | ||||||||
| Cyclo-(S*-Pro-S*-Tyr) Cyclo-(S*-Pro-S*-Val) 2(2-aminophenyl) acetic acid | ||||||||
| 4-hydroxy-benzamide | ||||||||
| 2(2-hydroxyphenyl) acetic acid | ||||||||
| Phoma sp. WF4 | Coelomycetes |
Eleusine
coracana |
Antifungal | Viridicatol | Viridicatol alkaloid | ZOI 1.8 mm | Fusarium graminearum | [103] |
| Tenuazonic acid | Tenuazonic acid | 2 mm | ||||||
| Alternariol | Alternariol | 1.5 mm | ||||||
| Alternariol monomethyl ether | Ether derivative | 1.5 mm | ||||||
| Chaetomium cupreum ZJWCF079 | Ascomycetes | Macleaya cordata | Antifungal | Ergosta-5,7,22-trien-3beta-ol | NR | EC50 125 µg/mL 190 µg/mL |
Sclerotinia sclerotiorum
B. cinerea |
[104] |
| Microsphaeropsis sp. | Coelomycetes |
Salsola
oppositifolia |
Antifungal | Microsphaerol | Polychlorinated triphenyl diether | ZOI 9 and 5 mm 9 and 7 mm 8 and 3 mm |
Microbotryum violaceum
B. megaterium E. coli |
[105] |
| Seimatosporium sp. | Seimatorone | Naphthalene derivative |
||||||
| Mycosphaerella sp. | Ascomycetes |
Eugenia bimarginata DC. |
Antifungal | 2-amino-3,4-dihydroxy-2-25-(hydroxymethyl)-14-oxo-6,12-eicosenoic acid | Eicosanoic acid | 1.3–2.50 µg/mL (MIC) |
C. neoformans
C. gattii |
[93] |
| Myriocin | 0.5 µg/mL | |||||||
| Pezicula sp. | Ascomycetes |
Forsythia
viridissima |
Antifungal | Mellein | NR | EC50 48.63 µg/mL | B. cinerea | [106] |
| 150.90 µg/mL | Colletotrichum orbiculare | |||||||
| 163.37 µg/mL | Verticillium dahliae | |||||||
| 159.09 µg/mL | Fusarium oxysporium f. sp. | |||||||
| 118.83 µg/mL | Cucumerinum | |||||||
| 161.04 µg/mL | Pyricularia oryzae | |||||||
| 125.36 µg/mL | Pestalotia diospyri | |||||||
| 205.01 µg/mL | Pythium ultimum | |||||||
| 45.98 µg/mL | Sclerotinia sclerotiorum | |||||||
| Nodulisporium sp. A21 | Ascomycetes | Ginkgo biloba | Anti-phytopathogenic, Antifungal |
Sporothriolide | NR | EC50 3.04 µg/mL 200 µg/mL |
Rhizoctonia solani
Magnaporthe oryzae |
[107] |
| Echinacea purpurea | Ascomycetes | Biscogniauxia mediterranea EPU38CA | Antifungal | (−)-5-methylmellein | NR | 300 µM (MIC) | P. obscurans | [108] |
| ---- | P. viticola | |||||||
| (−)-(3R)-8-hydroxy-6-methoxy-3,5-dimethyl-3,4-dihydroisocoumarin | Coumarin | ---- | B. cinerea | |||||
| 300µM | P. viticola | |||||||
| ---- | P. obscurans | |||||||
| Phialophora mustea | Ascomycetes | Crocus sativus | Antimicrobial, Cytotoxic |
Phialomustin C | Azaphilone derivative | IC50 14.3 µM | Candida albicans | [109] |
| Phialomustin D | 73.6 µM | |||||||
|
Plectophomella sp. Physalospora sp. |
Ascomycetes | NR | Antifungal, Antibacterial, Herbicidal |
(−)-Mycorrhizin A | Mycorrhizin | --- |
Ustilago violacea
Eurotium repens |
[110] |
| Cytochalasins E | Cytochalasins | --- |
E. repens
Mycotypha microspore |
|||||
| Cytochalasins K | ||||||||
| Radicinin | Dihydropyranone | --- |
E. repens
M. microspore |
|||||
| Berkleasmium sp. | Ascomycetes |
Dioscorea
zingiberensis |
Antifungal | Diepoxin ζ | Spirobisnaphthalenes | IC50 9.1–124.5 µg/mL | M. oryzae | [111] |
| Palmarumycin C11 | ||||||||
| Palmarumycin C12 | ||||||||
| Cladospirone B | ||||||||
| Palmarumycin C6 | ||||||||
| 1,4,7β-trihydroxy-8-(spirodioxy-1′,8′-naphthyl)-7,8-dihydronaphthalene | ||||||||
| Palmarumycin C8 | ||||||||
| Diaporthe melonis | Ascomycetes |
Annona
squamosa |
Antimicrobial | Diaporthemins A | Dihydroanthracenone atropodiastereomers | NA |
S. aureus 25697 S. aureus ATCC 29213 S. pneumoniae ATCC 49619 |
[112] |
| Diaporthemins B | NA |
S. aureus 25697 S. aureus ATCC 29213 S. pneumoniae ATCC 49619 |
||||||
| Flavomannin-6,6′-di-O-methyl ether | 32 μg/mL (MIC) | Staphylococcus aureus 25697 | ||||||
| 32 μg/mL | S. aureus ATCC 29213 | |||||||
| 2 μg/mL | Streptococcus pneumoniae ATCC 49619 | |||||||
| Cryptosporiopsis quercina | Ascomycetes | Tripterigium wilfordii | Antifungal | Cryptocandin | Lipopeptide | MIC 0.03–0.07 μg/mL |
C. albicans,
Trichophyton mentagrophytes, Trichophyton rubrum |
[113] |
| Colletotrichum gloeosporioides | Ascomycetes |
Artemesia
mongolica |
Antifungal | Colletotric acid | Benzoic acid derivative | MIC 25 μg/mL | Bacillus subtilis | [114] |
| 50 μg/mL | Staphylococcus aureus | |||||||
| 50 μg/mL | Sarcina lutea | |||||||
| 50 μg/mL | Helminthosporium sativum | |||||||
| Alternaria sp. | Ascomycetes | Trixis vauthieri | Anti-trypanosomiasis, Anti-leishmaniasis |
Altenusin | Biphenyl fungal metabolite |
IC50 4.3 μM | Trypanothione reductase (TR) inhibitory activity | [115] |
| Aspergillus sp. strain CY725 | Hyphomycetes | NR | Antibacterial | Helvolic acid | Helvolic acid | 8.0 µg/mL (MIC) |
H. pylori | [116] |
| Monomethylsulochrin | 10.0 µg/mL | |||||||
| Ergosterol | Sterols | 20.0 µg/mL | ||||||
| 3b-hydroxy-5a, 8a-epidioxy-ergosta-6, 22-diene | 30.0 µg/mL | |||||||
| Fusarium sp. IFB-121 | Hyphomycetes |
Quercus
variabilis |
Antibacterial | Fusaruside | Cerebrosides | 3.9 µg/mL (MIC) | B. subtilis | [117] |
| 3.9 µg/mL | E. coli | |||||||
| 1.9 µg/mL | P. fluorescens | |||||||
| (2S,2′R,3R,3′E,4E,8E)-1-O-beta-D-glucopyranosyl-2-N-(2′-hydroxy-3′-octadecenoyl)-3-hydroxy-9-methyl-4,8-sphingadienine | 7.8 µg/mL | B. subtilis | ||||||
| 3.9 µg/mL | E. coli | |||||||
| 7.8 µg/mL | P. fluorescens | |||||||
| Periconia sp. | Ascomycetes | Taxus cuspidata | Antibacterial | Periconicins A | Fusicoccane diterpenes | 3.12 µg/mL (MIC) | Klebsiella pneumoniae | [118] |
| Periconicins B | 25 µg/mL | |||||||
| F. oxysporum | Hyphomycetes |
Lycopersicum
esculentum |
Nematicidal | 3-hydroxypropionic acid | Propionic acid | LD50 12.5–15 µg/mL | M. incognita | [119] |
| Chaetomium sp. | Ascomycetes | Nerium oleander | Antioxidant | NR | NR | IC50 109.8 μg/mL | Inhibited xanthine oxidase activity | [120] |
| Cladosporium cladosporioides | Ascomycetes | Huperzia serrata | Prevent neurodegeneration | Huperzine A | NR | 10 μg/mL | Acetylcholinesterase inhibition activity | [121] |
| Edenia sp. | Ascomycetes | Petrea volubilis | Antiparasitic | Palmarumycin CP17 | NR | IC50 1.34 μM | Leishmania donovani | [122] |
| Palmarumycin CP18 | 0.62 μM | |||||||
| Nodulisporium sp. | Ascomycetes | Erica arborea | Antifungal, Antialgal |
Nodulisporins D | Naphthalene-Chroman Coupling products | ZOI 8 mm | B. megatarium | [123] |
| 7 mm | M. violaceum | |||||||
| 8 mm | C. fusca | |||||||
| Nodulisporins E | 7 mm | B. megatarium | ||||||
| 7 mm | M. violaceum | |||||||
| 5 mm | C. fusca | |||||||
| Nodulisporins F | 8 mm | B. megatarium | ||||||
| 10 mm | M. violaceum | |||||||
| 8 mm | C. fusca | |||||||
| (3S,4S,5R)-2,4,6-trimethyloct-6-ene-3,5-diol | 0 mm | B. megatarium | ||||||
| 8 mm | M. violaceum | |||||||
| 6 mm | C. fusca | |||||||
| 5-hydroxy-2-hydroxymethyl-4H-chromen-4-one | 0 mm | B. megatarium | ||||||
| 6 mm | M. violaceum | |||||||
| 6 mm | C. fusca | |||||||
| 3-(2,3-dihydroxyphenoxy)-butanoic acid | 0 mm | B. megatarium | ||||||
| 6 mm | M. violaceum | |||||||
| 7 mm | C. fusca | |||||||
| Chalara sp. | Ascomycetes |
Artemisia
vulgaris |
Antibacterial | Isofusidienol A | Chromone-3-oxepines | ZOI 23 mm | B. subtilis | [124] |
| Isofusidienol B | 22 mm | |||||||
| Isofusidienol C | 9 mm | |||||||
| Isofusidienol D | 8 mm | |||||||
| Ampelomyces sp. | Ascomycetes | Urospermum picroides | Cytotoxic | 6-O-methylalaternin | NR | 41.7 μM (MIC) |
S. aureus
S. epidermidis Enterococcus faecalis |
[125] |
| Altersolanol A | 37.2–74.4 μM | |||||||
| Alternaria sp. | Ascomycetes | Sonneratia alba | Antibiotic | Xanalteric acid I | Phenolic compounds | 343.40–686.81 μM | S. aureus | [126] |
| Xanalteric acid II | ||||||||
| Pestalotiopsis theae | Coelomycetes | --- | Anti-HIV | Pestalotheol A | NR | NR | HIV-1LAI replication in C8166 cells |
[127] |
| Pestalotheol B | NR | |||||||
| Pestalotheol C | EC50 16.1 μM | |||||||
| Pestalotheol D | NR | |||||||
|
Stemphylium
globuliferum |
Ascomycetes | Mentha pulegium | Cytotoxic | Alterporriol G and H (mixture) | NR | EC50 2.7 μM | L5178Y lymphoma cells |
[128] |
| Altersolanol K | ||||||||
| Altersolanol L | ||||||||
| Stemphypyrone | ||||||||
| Chaetomium sp. | Ascomycetes |
Otanthus
maritimus |
Cytotoxic | Aureonitolic acid | Tetrahydrofuran derivative |
NA | L5178Y mouse lymphoma cells |
[129] |
| Cochliodinol | EC50 7.0 μg/mL | |||||||
| Isocochliodinol | --- | |||||||
| Iindole-3-carboxylic acid | --- | |||||||
| Cyclo(alanyltryptophane) | --- | |||||||
| Orsellinic acid | 2.7 μg/mL | |||||||
| Chaetomium sp. | Ascomycetes |
Eucalyptus
exserta |
Antibacterial | Mollicellin O | Depsidones | IC50 79.44 μg/mL |
S. aureus ATCC29213 |
[130] |
| 76.35 μg/mL | S. aureus N50 | |||||||
| Mollicellin H | 5.14 μg/mL |
S. aureus ATCC29213 |
||||||
| 6.21 μg/mL | S. aureus N50 | |||||||
| Mollicellin I | 70.14 μg/mL |
S. aureus ATCC29213 |
||||||
| 63.15 μg/mL | S. aureus N50 | |||||||
| Cytotoxic | Mollicellin G | 19.64 μg/mL | HepG2 cell line | |||||
| 13.97 μg/mL | Hela cell line | |||||||
| Mollicellin H | 6.83 μg/mL | HepG2 cell line | ||||||
| --- | Hela cell line | |||||||
| Antioxidant | Mollicellin I | --- | HepG2 cell line | |||||
| 21.35 μg/mL | Hela cell line | |||||||
| Mollicellin O | 71.92 μg/mL | DPPH free radical scavenging assay |
||||||
| Alternaria sp. | Ascomycetes |
Polygonum
senegalense |
Cytotoxic | Alternariol | Sulfated derivatives of alternariol and its monomethyl ethers | EC50 1.7 μg/mL | L5178Y lymphoma cells | [131] |
| Alternariol 5-O-sulfate | 4.5 μg/mL | |||||||
| Alternariol 5-O-methyl ether Altenusin Desmethylaltenusin |
7.8 μg/mL | |||||||
| 2,5dimethyl-7-hydroxychromone Tenuazonic acid | 6.8 μg/mL | |||||||
| Altertoxin I | 6.2 μg/mL | |||||||
| 3′-hydroxyalternariol 5-O-methyl ether | --- | |||||||
| Alterlactone | --- | |||||||
| Alternaric acid | --- | |||||||
| Talaroflavone | --- | |||||||
| Altenuene | --- | |||||||
| 4′-epialtenuene | --- | |||||||
|
Phyllosticta
spinarum |
Ascomycetes |
Platycladus
orientalis |
Cytotoxic | Tauranin(+)-(5 S,10 S)-4′-hydroxymethylcyclozonarone | Sesquiterpene quinones | EC50 4.3 μM | NCI-H460 cell lines MCF-7 cell lines SF-268 cell lines PC-3 M cell lines MIA Pa Ca-2 cancer cell lines |
[132] |
| 3-ketotauranin | 1.5 μM | |||||||
| 3alpha-hydroxytauranin | 1.8 μM | |||||||
| 12-hydroxytauranin | 3.5 μM | |||||||
| Phyllospinarone | 2.8 μM | |||||||
| Eupenicillium sp. | Ascomycetes |
Glochidion
ferdinandi |
Cytotoxic | Trichodermamide C | A modified dipeptide | IC50 1.5 μM | Human colorectal carcinoma cell line HCT116 |
[133] |
| 9.3 μM | Human lung carcinoma cell line A549 |
|||||||
| Pestalotiopsis sp. | Coelomycetes |
Rhizophora
mucronata |
Cytotoxic | Pestalotiopsone F | Pestalasins chromones |
EC50 26.89 μM | Murine cancer cell line L5178Y | [134,135] |
Abbreviations: ZOI, Zone of Inhibition; MIC, Minimum Inhibitory Concentration; IC50, Inhibitory Concentration T 50%; EC50, Effective Concentration at 50%; LD50, Lethal Dose Concentration at 50%; FIC, Fractional Inhibitory Concentration; NR, Not Reported; NA, Not Active.
Endophytic fungi produce diverse kinds of bioactive metabolites and are categorized as terpenoids, indole alkaloids, polyketides, and non-ribosomal peptides [136] biosynthesized via different routes. Terpenoids (composed of multiple isoprene units) are biosynthesized via the mevalonate pathway by terpene cyclases, biosynthesis of cyclic terpenes by diterpene cyclase and sesquiterpene cyclases, indole diterpenes by prenyl transferases, and carotenoid formation by phytoene synthases [137]. While indole alkaloids are biosynthesized via the shikimic pathway, polyketides by polyketide synthases from acetyl-CoA and malonyl-CoA units, and nonribosomal are formed independently of ribosomes function by NRPS enzymes, different categories of fungal metabolites originate via different biosynthetic routes [137]. The different categories of metabolites are further classified as flavonoids, coumarins, xanthones, quinones, lignans, and others and demonstrate multifaceted applications. The plethora of bioactive compounds and their analogs have displayed potential pharmacological activities in animal models and as herbal medicines against several human ailments. With scientific breakthroughs in high-throughput technologies and whole-genome sequencing, natural products from endophytes have been explored as alternative sources of novel drug-like candidates, among the 1562 FDA approved drugs, there were 64 original natural products, and 320 drugs were derivatives of natural products [5]. In addition, the development of new technologies has substantially contributed to the re-establishment of drying pipelines for novel candidates in drug discovery programs. The availability of complete genomes of endophytes and the enormous amount of information on different endophyte strains have provided remarkable insights into the molecular mechanisms of endophyte colonization and interaction with the host plant.
The bio-prospection of endophytic fungi suggests that very few fungal strains have been studied in-depth, with others highlighting the potential for the existence of enormous novel candidates. The dominant taxonomic categories for the synthesis of chemical entities are from the orders Ascomycota (~97%), Basidiomycota (~2%), and Mucoromycota (~1%), and the key metabolite-rich strains include Fusarium, Aspergillus, Penicillium, and Alternaria [59,138]. The presence of high-value (anticancer) compounds in endophytic fungi includes novel chemical entities such as terpenoids and steroids (integracides), polyketides (phomones), nitrogen-containing heterocycles (penicisulfuranols), and ester quinones [138]. Other studies have reviewed and reported the presence of metabolites for tropical diseases (including altenusin, viridiol, and cochlioquinone) [139], metabolites mimicking plant secondary metabolites (antioxidant resveratrol), antidiabetics (rohitukine), anticancer (taxol), antihypercholesteromics (lovastatin), and others [140,141]. Several other metabolites of socio-economic importance were identified from endophytic fungi between 2010 and 2017 [141,142]. Among the diverse metabolites, a few significant ones produced from endophytic fungi include saponins as nutraceuticals [143], loline alkaloids as bioinsecticides [144], chitosan as a food additive [145], taxol as a pharmaceutical [146], fatty acids as ingredients in cosmetics [145], and ascotoxin as bioherbicides [147], among others. In a remarkable study, Harpar et al. [148] predicted the relative stereochemistry of ambuic acid, isolated from the endophytic fungi, Pestalotiopsis microspora. The novel solid-state NMR approach led to structural elucidation and a dimeric structure was suggested for ambuic acid, consisting of a hydrogen-bonded adjacent carboxyl group, including the lattice structure details, an excellent study performed for the first time on a natural product.
Furthermore, the quorum-sensing activity of ambuic acid in Gram-positive bacteria suggested its development as a potent antipathogenic drug for targeting the virulence expression of Gram-positive bacteria. The compound, ambuic acid, inhibited quorum sensing in bacteria via the inhibition of gelatinase biosynthesis-activating pheromone (GBAP) biosynthesis [149]. Another interesting area in bioactive metabolites from Muscodor spp. has gained momentum in multi-faceted applications. Diverse types of volatile organic compounds (VOCs) are produced by Muscodor spp. and comprise aldehydes, aromatics, esters, alcohols, terpenoids, and nitrosamides, among others [150]. The endophytic genus highlights multi-faceted attributes in industrial and agricultural applications, followed by food and healthcare, projecting direct and indirect applications. The diverse application of VOCs, isolated from Muscodor spp., includes agricultural applications (antimicrobial agents, for soil fumigation, biofumigation, etc.), food preservation, the perfume industry, and bioactive compounds in healthcare [150]. In addition, non-ribosomal peptides (NRP) and Penicillin (antibiotic) were isolated from a mangrove endophytic fungus, Penicillium chrysogenum MTCC 5108 [151]. Similarly, a polyketide synthase I (PKS I)-based screening method led to the discovery of a new polyketide, penicitriketo from endophytic fungi [152].
Plant-associated endophytes have gained significant momentum in bio-prospection and isolation of bioactive metabolites with multi-faceted attributes. In this direction, literature reviews have provided key insights into the existing and emerging significance of endophytes in drug discovery and research—a few articles worth mentioning include Discussion on biodiversity of endophytes and its exploitation in drug discovery [1], Bioactive metabolites, and their pharmaceutical design in drug development [2], Emerging roles and applications of actinomycetes endophytes [13], Chemical ecology of endophytes and its role in bioactive metabolite production [43], The process of horizontal gene transfer and its implications in the transfer of novel traits in production of bioactive metabolites [56], and comprehensive insight on the diverse metabolites produced by fungal endophytes and their biological functions, among others. The present review on fungal endophytes provides a detailed insight into the existing and emerging prospects of fungal endophytes as “novel candidates” in drug discovery and research and how the different yield enhancement strategies can be adopted to address the associated bottlenecks and enhance bioactive metabolite production.
3. Molecular Mechanisms of Plant–Endophytic Fungi Interactions
Evolutionary studies have suggested an important role of fungal partners in plant adaptation/colonization in terrestrial systems [153]. Several studies have documented the beneficial role of plant-associated endophytic fungi [29,154]; however, biological and chemical barriers need to be addressed to establish plant–fungal associations [43]. The theory of “balanced antagonism” ensures a balanced equilibrium between the adverse effects of endophyte association with plant hosts and the defense response exerted by the plants [155]. To ensure survival within plant hosts, endophytes secrete secondary metabolites that neutralize the toxic effects of plant host defense. Moreover, the invasion of endophytes into plants activates the plant defense system, limiting endophyte development and disease symptoms, if any. Asymptomatic colonization by endophytes is mediated by metabolite production, which counters host defense via multipartite symbiosis [155,156]. The phenomenon of “balanced antagonism” with the competent existing microbes is further adopted by endophytic fungi, balancing plant defense and virulence of endophytic fungi. However, if the plant host defense is overcome by endophytic fungi, it will lead to plant disease via plant–pathogen interactions [157]. A key example of this phenomenon is the production of taxol by Paraconiothyrium SSM001 to tackle host pathogens [61]. Furthermore, the coevolution of endophytes and plant hosts has resulted in the development of sophisticated mechanisms by endophytes to modify plant immune responses [158], such as the suppression of β-glucan-triggered immunity by endophytes in multiple plants [159]. In terms of plant hosts, the evolution of defense mechanisms, including secondary metabolite production to counter pathogens, constitutes an integral host mechanism. In response to this, pathogens have developed resistance mechanisms and undergone structural modifications in some cases to counter plant defense responses [157]. An interesting mechanism displayed by endophytes within plants is promoting plant immunity by monitoring the vascular system; for example, in Pacific yew, known for taxol production by endophytic fungi, the pathogens enter the vascular system through air pockets and cracks in the bark, and the endophytes release taxol as a defense barrier to pathogens [61].
Recent advances in scientific interventions and whole-genome sequencing have contributed substantially to the bio-prospection of endophytic fungi to produce pharmacological metabolites. Endophytic fungi and their metabolic pathways are key targets for research. Although the metabolic pathway has defined a genetic framework to produce a specific metabolite, gene clusters mostly remain silent under laboratory conditions [3]. Moreover, strategies to activate silent gene clusters through co-cultivation of different endophytic fungal strains, mutagenesis, and genetic engineering have been adopted to improve the biosynthetic potential of different endophytic strains, with limited success. Moreover, the bioactive metabolites isolated from endophytic fungi are classified into different categories, including phenolics, flavonoids, saponins, alkaloids, terpenes, and xanthones [160], demonstrating multiple pharmacological properties. However, the decreased production due to repeated sub-culturing of the microbial strains leads to an unstable yield in axenic cultures, which accounts for the few existing challenges in endophyte biology and research. Different researchers have proposed parallel hypotheses on the genetic origin of metabolites (both in endophytes and plants), including the presence of parallel biosynthetic pathways for metabolite production in plants and endophytes. However, examples of taxol gene clusters showed low similarities between plants and microbes [161], suggesting an independent evolution of biosynthetic pathways in different organisms [162]. Another key example is camptothecin (CPT) production by Fusarium solani utilizing the host enzyme strictosidine synthase for CPT biosynthesis, suggesting the horizontal transfer of gene clusters between endophytic fungi and host plants in an evolutionary course [163]. Both endophytic fungi and plants possess resistance mechanisms against CPT and taxol, respectively. Another independent hypothesis claims that host mimicking by endophytic fungi leads to the production of bioactive compounds, such as plant hosts, questioning the actual biosynthesis by endophytic fungi [43]. However, limited knowledge about the molecular mechanisms of plant–associated endophytic fungi is a major challenge in the large-scale commercial production of bioactive metabolites from endophytic fungi.
4. Scientific Approaches for Natural Product-Based Drug Discovery
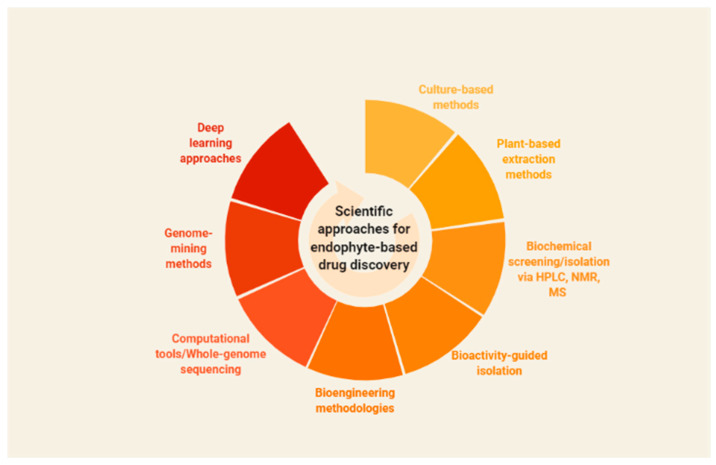
Key strides have been made in the discovery and characterization of valuable bioactive metabolites from natural sources. Substantial efforts to identify novel bioactive metabolites have opened new avenues in natural product-based drug discovery from endophytic fungi. The increasing importance of natural products in pharmacological applications has greatly impacted the large-scale production of high-value metabolites. Plant–microbe associations, particularly endophytic fungi, continue to intrigue researchers worldwide with a considerable potential to impact the pharmaceutical industry [164,165]. With the alarming rise in AMR and declining drug pipelines, novel metabolites from natural sources are increasingly being studied for their therapeutic potential. In this direction, advances in high-throughput technologies and scientific interventions have provided firm grounds as “discovery platforms” in natural product-based drug discovery programs. In addition, the emerging revolution in interdisciplinary strategies comprising computational methods and multi-omics strategies has redefined natural product discovery from plant–endophytic associations [166]. Figure 2 provides a schematic overview of the traditional and emerging scientific approaches for endophyte-based drug discovery.
Figure 2.
Provides a schematic overview of the traditional and emerging scientific approaches for endophyte-based drug discovery (Created with BioRender.com, accessed on 24 January 2022).
5. Traditional Scientific Methods
Recently, there has been an increased exploration of plant–endophytic associations for the discovery of high-value metabolites with potential pharmacological applications. Deep learning methods have defined an interesting platform for the bio-prospection of endophytes, regulatory networks, and the prediction of novel chemical entities [166]. As discussed earlier, plant-associated endophytes have defined an attractive “biosynthetic platform” for the synthesis of novel bioactive metabolites; however, several challenges exist in harnessing these biological sources in drug discovery. For example, most of the secondary metabolite pathways are silenced, and the knowledge of metabolic networks and mechanisms needs to be understood in detail. Furthermore, in silico approaches predict the existence of a wide array of metabolites that remain silent/inactive under native conditions (in planta) [167,168]. In recent decades, natural product discovery from endophytes relied on powerful, low-throughput methods [3], essentially including culture-based methods, plant-based extraction methods, biochemical screening/isolation using HPLC, NMR, and MS, and bioactivity-guided isolation [169], with existing limitations. Toward addressing the existing challenges, sophisticated methods, namely the “one strain-many compounds” (OSMAC) approach, engineering ribosomes, heterologous expression of genes, and promoter studies have been widely explored [170]. The OSMAC method is based on studying a particular strain and its growth under different culture conditions to produce a diverse array of metabolites [168]. These promising tools enhance gene expression in endophytes via biotic/abiotic triggering mechanisms.
Another interesting strategy is the co-cultivation of different endophytic fungal strains to elicit gene expression in silent gene clusters [171]. For example, gene expression for taxol production was reclaimed in Aspergillus terreus when co-cultivated with Podocarpus gracilior leaves [172]. Furthermore, genetic methods have been successful in activating silent BGCs and include inducible/constitutive promoters [173], host ribosome engineering [174], and mutant selection [175]. In addition, for culturable endophytic bacteria, some important high-throughput techniques used include high-throughput elicitor screening with imaging mass spectrometry, with the potential to induce silent gene clusters [176], and most techniques were effective for culturable microbes only.
6. Deep Learning Approaches
One of the key approaches for analyzing large data comprises artificial intelligence, further classified as deep learning and machine learning. These methods predict the distribution pattern of the plant microbiome (environmental niches) and may accurately predict the biosynthesis of bioactive metabolites from endophytes [177], highlighting a potential approach to replace challenging global, comprehensive estimation. In this approach, the initial dataset could be obtained from the increasing multi-omics and genomics combined with plant metabolomics data. The targeted region can then be analyzed through multi-omics methods, further co-integrated with metabolic pathway analysis [178,179,180]. Currently, multiple deep learning and machine learning strategies are employed to improve drug discovery from natural products. The software DeepBGC relies on a word2vec-like word embedding skip-gram neural network and bidirectional long short-term memory (BiLSTM) neural network, subject to a large dataset from microbial communities [181]. Furthermore, bio-prospection of endophytes for bioactive metabolites may be performed by integrating genome metabolic models and deep learning methods. Similarly, for the diversity prediction of chemical entities, deep learning chemi-informatics methods may be employed for efficient prediction. In addition, computational biology methods emphasize the analysis of chemical entities and metabolomics strategies, targeting a predictive model of plant microbiome and associated biochemical changes. In keeping with the target goal, the abovementioned deep learning methods may be suitably altered; for example, the objective may be to find chemical novelty or a target function (antimicrobial) or elucidation of complex structures [166]. The established genome mining tools aim to understand metabolic flux and pathway regulation, regulatory processes, and metabolite interactions. With the enormous amount of data generated from high-throughput experiments, the OSMAC tool, co-integrated with metabolomics information, forms a basis for computational analysis. These novel computational pipelines project revolutionary landmarks in natural product-mediated drug discovery from endophytes.
7. High-Throughput Strategies
Advances in computational biology and whole-genome sequencing have been instrumental in defining new avenues for endophyte-mediated natural product discovery. Comparative genomics aims to understand the diversity of chemical entities from microbes because of the conserved BGCs across species, associated with regulatory genes, uptake, and product transport [166]. Moreover, BGCs, which are specific, are mostly silent/lowly expressed under laboratory conditions, making functional gene prediction difficult. An effective approach toward the discovery of natural products begins with in silico predictions through genome information and proceeds to experimental validation via activation of the biosynthetic pathways [178]. The ongoing efforts in the development and upgradation of computational resources have greatly refined research on natural product-mediated drug discovery. Databases such as the Database of BIoSynthesis clusters CUrated and InTegrated (DoBISCUIT) [182], and ClustScan software [183], and ClusterMine 360 [184] have facilitated the discovery/identification of novel gene clusters. Moreover, the discovery of BGCs via phylogenetic analysis was performed through a useful database, The Minimum Information on Biosynthetic Gene clusters (MIBiG) [185]. Other bioinformatics databases promoting the annotation of BGCs include Reconstruction, Analysis and Visualization of Metabolic Networks’ RAVEN 2.0 software [186], antibiotics and secondary metabolite analysis shell (antiSMASH) [187], and Metaflux [188], and the genome-wide identification and profiling of gene clusters of natural products have become attainable. In addition, bioinformatics software (based on the network algorithm) improves predictions of genome mining methods [189] and may be co-integrated with metabolic modeling approaches [190]. The predictive strategies further extend the understanding of metabolic interactions in microbial cultures [191], bio-kinetic models for the estimation of interspecific interactions among microbes [192], single-cell analysis for endophyte metabolism [192] and transcriptome approaches for comprehensive understanding [193].
8. Pharmacological Metabolites: Case Studies, Mechanism of Action, and Commercial Success
Medicinal plants form the backbone of the traditional system of medicine and are a rich source of pharmacological metabolites for the treatment of diseases and prospects as modern medicines [56]. Since the initial reports of taxol production from the endophyte Taxomyces andreanae, similar to its plant host (Taxus brevifolia) in 1993, endophytes have been increasingly explored for the production of high-value metabolites. Plant-associated endophytes, particularly endophytic fungi, have emerged as production platforms for pharmacological metabolites with diverse therapeutic applications [2]. There has been an upsurge in the production and marketing of pharmacological metabolites worldwide, and a few remarkable examples and their success stories in the pharmaceutical sector have been discussed. Table 3 presents key examples of commercially available drugs from endophytic fungi: key examples, pharmacological functions, bottlenecks, and success stories.
Table 3.
Commercially available drugs from Endophytic fungi: key examples, pharmacological function, bottlenecks, and success stories.
| Marketed Drug | Commerical Market | Endophyte | Pharmacological Functions | Bottlenecks | References | ||
|---|---|---|---|---|---|---|---|
Taxol (Paclitaxel)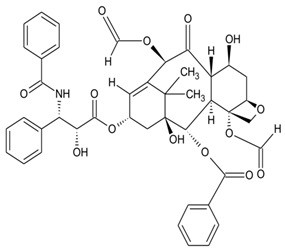
|
$78.77 million (2017) |
Taxomyces andreanae
Taxus brevifolia |
Anticancer-binds to microtubule assembly and delays cell division/growth, Treatment for breast, ovary, and Kaposi’s sarcoma |
Biosynthetic complexities, harvestation of the molecule | [15,194,195] | ||
Camptothecin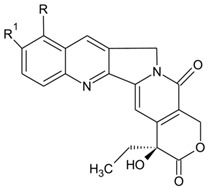
|
$2.2 billion (2008) |
Entrophospora infrequens Alternaria alternata Fomitopsis sp. Phomopsis sp. |
Inhibitor of Topoisomerase enzyme | Yield-loss on repeated sub-culturing |
[196,197] | ||
| R | R1 | ||||||
| Camptothecin (2) | H | H | |||||
| 9 Methoxycamptothecin (3) | OCH3 | H | |||||
| 10-Hydroxycamptothecin (4) | H | OH | |||||
| Vinca alkaloids (Vinblastine and Vincristine) 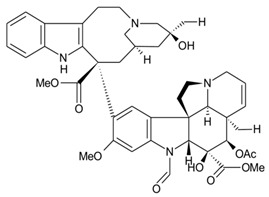
|
- |
Alternaria sp. F. oxysporum |
Anticancer alkaloids, inhibit microtubule assembly leading to destabilization |
Yield-loss on repeated sub-culturing |
[198,199] | ||
Podophyllotoxin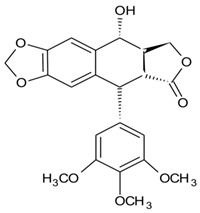
|
$444.1 million (2019) | Phialocephala fortinii | Cytotoxic in U-87 cell line, Antitumor activity in cancer models | Low abundance in plants | [200,45] | ||
Griseofulvin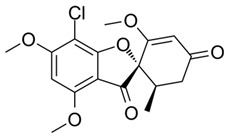
|
$5.8 billion (2019) |
Xylaria sp. strain F0010 |
Antioxidant | Low abundance, production from alternate sources | [89] | ||
Laritin A and B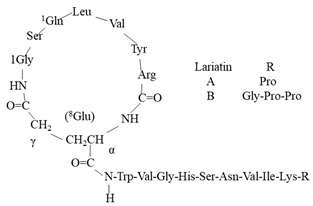
|
- | Rhodococcus jostii | Anti-inflammatory, Anticancer, Immunomodulatory properties | Low abundance, Yield-loss on repeated sub-culturing | [13] | ||
Rohitukine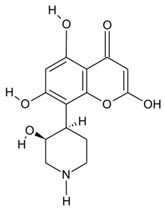
|
- | Fusarium proliferatum | Cytotoxic against the HCT-116 and MCF7 cell lines | Yield-loss on repeated sub-culturing |
[201] | ||
Piperine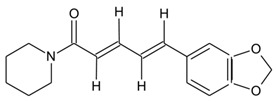
|
$4.87 billion (2020) | Periconia strains | Hepatoprotective Antibacterial |
Yield-loss on repeated sub-culturing |
[202] https://www.expertmarketresearch.com/reports/piperine-market accessed on 12 July 2021 |
||
Altersolanol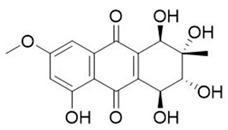
|
- | Alternaria sp. | Antiangiogenic | Yield-loss on repeated sub-culturing |
[203] | ||
Huperzine A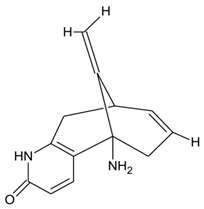
|
- | Shiraia sp. | Cholinesterase inhibitor | Yield-loss on repeated sub-culturing |
[204] | ||
Phomoxanthone A and B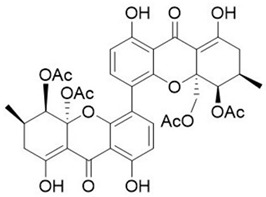
|
- |
Phomopsis sp., BCC 1323 |
Cytotoxic against BC-1 cells, KB, and Vero (non-malignant) cells | Yield-loss on repeated sub-culturing |
[205] | ||
Quinine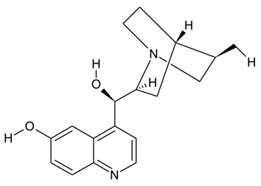
|
$804.98 million | Phomopsis sp. | Antimalarial agent | Yield-loss on repeated sub-culturing |
[206] https://www.prnewswire.com/news-releases/global-quinine-market-review-2015-2018 accessed on 12 July 2021 |
||
Silybin A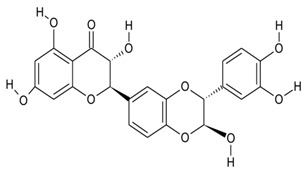
|
- | Aspergillus iizukae | Hepatoprotective | Low abundance, production from alternate sources | [207] | ||
9. Taxol Production
The anticancer drug taxol is one of the most successful drugs marketed globally for its anticancer activity. Taxol was isolated from the medicinal plant Taxus brevifolia, but its concentration was too low (0.001–0.05%) in most species. Therefore, 1 g of taxol production requires 15 kg of tree bark, while the anticancer dose amounts to 2.5 g [208]. To increase taxol production, alternative sources have been investigated to meet the rising therapeutic demands. The bioactive metabolite, taxol was first isolated from the endophytic fungi Taxomyces andreanae [209], and subsequently from other endophyte species. The discovery (in 1960), structural elucidation (1971), and FDA approval (1992) of taxol defined new paradigms in global commercial success, projecting a global market of USD 78.77 million in 2017. In chemotherapies for cancer treatment, paclitaxel is a mitosis inhibitor and is widely used for the treatment of cancers such as cervical, ovarian, and breast cancers. The global consumption trends indicated a market share of 53% (2017), with Europe accounting for 19% of global market trends [210].
Taxol and its derivatives represent a popular class of anticancer drugs produced by different endophytes. The mechanism of action includes inhibition of mitosis and promotion of tubulin depolymerization during cell division [211]. After several research attempts, the taxol-producing endophyte T. andreanae was discovered in Taxus brevifolia, detected via electrospray mass spectroscopy [212] and compared with the standard paclitaxel. The identification of taxol and its anticancer potential was bright, but the existing crisis and the low content in the plant projected major concerns to increase supply. Subsequently, paclitaxel-producing endophytes have been reported (taxol-producing endophytes from Taxus baccata L.), characterized, and validated using HPLC-MS [213]. The five taxol-producing endophytic fungi were Fusarium redolens, Gibberella avenacea, Fusarium tricinctum, Paraconiothyrium brasiliense, and Microdiplodia sp. G16A, with the highest taxol yield of 66.25 μg/L by Fusarium redolens [214]. The diterpenoid metabolite was further isolated from several endophytes belonging to the Ascomycota, Basidiomycota, Deuteromycota, and Zygomycota classes [215,216]. However, successful translational research towards industrial production faces certain challenges currently, including decreased production because of repeated sub-culturing and low concentration in biological species. Comprehensive knowledge of endophyte biology and dynamics is essential for improving the commercial production of important bioactive metabolites [2,217].
Presently, considering the global market for anticancer drugs, research efforts have been made for large-scale production of taxol from endophytic fungi [218,219,220]. Fermentation methods have been optimized for taxol production by supplementing nitrogen/carbon sources, precursors, and elicitors [221] and defined conditions related to temperature, pH of culture, and dissolved oxygen [222]. Moreover, inhibition of the metabolic pathway of sterols (ergosterol) by triadimefon (inhibitor) is effective in enhancing the production of paclitaxel [223]. However, taxol production in endophytic fungal cultures leads to the reprogramming of fungal physiology and the regulatory loss of metabolite production [141]. Co-cultivation of Paraconiothyrium sp. with Alternaria sp., and Phomopsis sp. enhanced taxol production eightfold [224]. Genetic transformation of endophytic fungi is an emerging method for increasing the production of targeted metabolites [220]. Protoplast-mediated transformation [225], Agrobacterium-mediated transformation [226], electroporation [227], and genome editing via CRISPR-Cas [228] are some other scientific approaches employed to enhance taxol production by endophytic fungi.
10. CPT Production
CPT is a monoterpene indole alkaloid that is commercially marketed as an anticancer drug. Initially, it was isolated from the stem and bark of Camptotheca acuminata, a native Chinese tree. CPT has a pentacyclic structure and is a topoisomerase inhibitor that binds to topoisomerase I and DNA complex and stabilizes it, leading to DNA damage and apoptosis (https://en.wikipedia.org/wiki/Camptothecin, accessed on 23 July 2021). In clinical trials, CPT showed anticancer activity against colon, lung, breast, and stomach cancers. The commercial success of CPT is attributed to its applications in cancer chemotherapies, and its derivatives are marketed with the name belotecan, topotecan, irinotecan, and trastuzumab deruxtecan [229,230]. CPT was commercially isolated from Nothapodytes nimmoniana and C. acuminata and has a high global demand for cancer treatment [231]. Although some CPT derivatives are commercially marketed (irinotecan, belotecan, and topotecan), others such as diflomotecan, tenifatecan, genz-644282, chimmitecan, exatecan, lipotecan, silatecan, cositecan, and simmitecan are under clinical trials [232,233]. The increasing demand for CPT has led to indiscriminate use/overharvesting of the two plants, making them an endangered species [234]. To produce 1 ton of CPT, approximately 1000–1500 tons of plant wood chips are required [235], leading to excessive exploitation of the plant species.
The emerging popularity of endophytes as production platforms for high-value metabolites necessitates the potential bio-prospection of endophytes for natural product drug discovery. CPT-producing endophytes have been discovered, including Trichoderma atroviride LY357 [236], Fusarium solani strain ATLOY-8 [237], and Neurospora sp. [238]. However, the variable/inconsistent production, yield loss because of sub-culturing, and low concentrations are the major bottlenecks in the commercial-scale production. Mohinudeen et al. [239] reported the presence of a high-CPT-producing endophyte Alternaria sp. from N. nimmoniana. The endophyte produced up to ~200 μg/g of CPT in axenic cultures and demonstrated cytotoxic activity against cancer cell lines [239]. Regarding the molecular pathways in CPT production, strictosidine synthase, tryptophan decarboxylase, secologanin synthase, and geraniol 10-hydroxylase are some of the key enzymes identified to date. Kusari et al. [43] identified tryptophan decarboxylase, secologanin synthase, and geraniol 10-hydroxylase in endophytic fungi. The absence of the strictosidine synthase gene in endophytes has led to the presumption of the involvement of host genes in CPT biosynthesis. Decreased CPT yield due to sub-culturing was explained by the absence of selection pressure, leading to the degradation of biosynthetic pathways in the endophytic fungi [41], limiting CPT biosynthesis. Recent advances employing endophytes as production platforms for CPT have focused on isolation/fermentation of CPT-producing endophytes, extraction, and detection via different methods, and employing fermentation culture to improve CPT yield [240].
11. Vinca Alkaloids (Vincristine/Vinblastine) Production
Vinca alkaloids are pharmacological metabolites isolated from Madagascar periwinkle (Catharanthus roseus) and are listed on the World Health Organization’s List of Essential Medicines [241]. Vinca alkaloids are used as effective medications for the treatment of different types of cancers, including bladder cancer, melanoma, and lung cancers (https://en.wikipedia.org/wiki/ accessed on 23 July 2021). Vinblastine is an analog of vincristine, and its anticancer mechanism is defined by binding to tubulin, thereby inhibiting microtubule assembly and formation, and is regarded as an effective chemotherapeutic agent. Two of the most successful commercial drugs—vinblastine and vincristine—are present at very low concentrations in plants. Moreover, the estimated global demand for bioactive metabolites (0.3 tons annually) and global market of USD 200 million projects additional requirements from alternative natural resources [242]. According to Balandrin and Klocke [243], 500 g of C. roseus leaves is required to produce 1 g of pure vincristine. Although vinca alkaloids differ slightly in their chemical structure and mechanisms, their clinical activity and toxicity vary. The commercial success and importance of vinca alkaloids have led to studies on endophytes from C. roseus. Several endophytic fungi were isolated and screened to produce vincristine/vinblastine, and a few key examples are Aspergillus, Alternaria, and Cladosporium sp. Moreover, the endophytic fungus Talaromyces radicus (from C. roseus) produced vincristine in substantial amounts (670 μg/L) in modified M2 medium and vinblastine in PDB medium (70 μg/L). Furthermore, vincristine was purified, and it demonstrated cytotoxic activity against cancer cell lines [244].
12. Podophyllotoxin Production
Another prospective pharmacologically important metabolite podophyllotoxin, an aryl tetralin lignin, is widely present in angiosperms and gymnosperms [245]. Podophyllotoxins are emerging as pharmacological metabolites because of their antiviral and cytotoxic activities [246,247]. Some semisynthetic derivatives of podophyllotoxin, teniposide, and etoposide have been approved for the treatment of leukemia and different types of cancers [248]. Because of its low concentration in plants, etoposide was developed and approved by the FDA in 1983. Other analogs were chemically synthesized but had a low yield. Research efforts to enhance the production of podophyllotoxin and its analogs from different species have been conducted. The plant tissue culture method is a reliable and sustainable approach to produce bioactive metabolites from natural sources [249]. The mechanism of action of teniposide and etoposide is marked by interaction with topoisomerase II [250] by two mechanisms: it exerts its action by enhancing topoisomerase II DNA covalent complex levels or the removal of topoisomerase II catalytic function [251]. Moreover, DNA breaks induced by enzymes promote sister chromatid exchange, recombination, translocation, and insertions/deletions [252].
Different strains of endophytic fungi have been studied for their potential to biosynthesize podophyllotoxin, and Phialocephala fortinii, endophytic fungi from Podophyllum peltatum, produced podophyllotoxin in the range of 189 and 0.5 μg/L. Furthermore, the cytotoxic potential of the endophytic fungi was evaluated, and the LD50 values were 2–3 μg/mL [200]. Another species, Fusarium oxysporum, isolated from Juniperus recurva, produced 28 μg/g of dry mass of the metabolite [253]. The bioactive metabolite has been detected/produced by several species of endophytic fungi, a few prominent ones comprise Aspergillus fumigatus from Juniperus communis [248], Trametes hirsuta from Podophyllum hexandrum [45], and Mucor fragilis [254]. The ongoing bio-prospection of different endophytic species for pharmacological metabolites highlights its existing and emerging importance in natural product-mediated drug discovery.
13. Existing/Potential Bottlenecks in Biotechnological Applications
Endophytic fungi have great potential to impact the pharmaceutical industry, subject to the bio-prospection of novel pharmacological metabolites with multifaceted implications. However, little is known about plant–endophyte dynamics, low yield, and loss of yield because sub-culturing and scaling-up cultures constitute some key bottlenecks. An emerging demand and constant supply of novel, high-value metabolites define global trends, considering the drying pipeline of antibiotic arsenals. Although the plant–endophyte interaction remains poorly understood, less information on endophytic interactions with other microbes further hampers endophyte applications. Genetic instability in plant tissue culture, slow fungal growth, and maintenance issues have decreased the use of tissue culture methods [255]. In this regard, fermentation technologies are an attractive platform to produce high-value metabolites from fungal cultures. These methods offer distinct benefits in terms of less expense, rapid growth, and optimization of culture conditions for effective production of the targeted metabolite, and sustainable production is achieved. The factors in scale-up processes, namely, oxygen solubility, viscosity of the medium, temperature, time of cultivation, pH, and others, and their optimization, make the process cumbersome. The culture conditions need to be optimized and regulated properly for the large-scale targeted production of metabolite of interest [168]. Genetic engineering approaches for the activation of BCGs for enhanced production hold potential; however, with very few reported studies on endophyte chassis, the genetic manipulation methods need to be further optimized. The combinational synthesis for complex metabolites highlights associated problems and may be resolved by employing elicitors for increased production of metabolites in a particular metabolic pathway. Other challenges include the complex structure of metabolites and undesired pathway intermediates, resulting in the inhibition of enzyme function. The existing areas of concern include the adverse effects of endophytic fungi in the control of pathogens. For example, endophytes of Crocus sativus may be pathogenic to host plant and result in infection, as endophytes are considered latent pathogens capable of causing diseases [256]. A deep understanding of the associated risk factors with endophytes is necessary to avoid adverse effects and benefit biocontrol applications.
14. Strategies for Yield Enhancement of Pharmacologically Important Metabolites
Substantial efforts to isolate and screen endophytes for high-value metabolites resulted in undesirable/low yields of the target metabolite. Although novel chemical entities such as CPT, paclitaxel, podophyllotoxin, and huperzine A were isolated from endophytic fungi, bottlenecks with large-scale production and commercialization remains a major concern. As the field of endophytes is gaining popularity in multifaceted environmental applications, scientific tools and approaches are aimed at delineating the dynamics of plant–endophyte interactions and enhancing the yield of pharmacologically important metabolites. Co-integration of bioprocess techniques and genetic/metabolomics approaches may be employed to effectively target and produce high yields of the desired metabolites.
15. Metabolic Engineering of Endophytic Fungi
Studies on the engineering of endophytes are preliminary and investigate endophyte chassis to improve the yields of targeted metabolites through genetic strategies. Genetic manipulation of endophytes defines potential future outcomes by introducing key pathway genes via genetic transformation for yield enhancement of high-value metabolites in axenic cultures. Studies on the genetic transformation of taxol-biosynthesizing endophytes include the overexpression of genes and genome rearrangement with mutagenesis for enhanced metabolite production [257,258]. Random mutagenesis approaches for genetic modification of the endophyte genome and induction of metabolites and mutant screening have been adopted [258]. Taxol production was increased (endophyte strain HDF-68) through protoplast fusion of two strains—UL50-6 and UV40-19—leading to a 20–25% increase in taxol yield [259]. Wang et al. [260] showed that the endophytic fungus Phomopsis sp. produces deacetylmycoepoxydiene (DAM) (antitumor metabolite), and genome shuffling of eight parental protoplasts resulted in a high-yielding strain (produced >200-fold DAM) in the transgenic endophytic strain [260]. Similarly, previous studies on Pestalotiopsis microspores (endophytic fungi) aimed to decode the taxol biosynthetic pathway via protoplast transformation [261]. These unexpected results led to the detection of extrachromosomal DNA in the transformants, suggesting a key role in development and adaptation. The translational success and marketing of taxol, led to extensive investigations on taxol-producing endophytes: with the application of genetic engineering defining a key platform in endophyte chassis; some important examples include, restriction enzyme-mediated integration in Ozonium sp. strain BT2 (taxol-producing fungi) [262]; Agrobacterium-mediated genetic transformation in Ozonium sp. EFY21, with enhanced efficiency of transformation [263] and PEG-mediated transformation of Ozonium sp. EFY-21 [264]. In this direction, multiple approaches for genome modification/shuffling of endophytes have been attempted to increase the metabolic flux, either by limiting competitive pathways or by reorientation of precursor supply, strain improvement methods leading to yield enhancement of targeted metabolites. In an interesting study of pathway reconstitution, the taxadiene-biosynthetic cluster was expressed in Escherichia coli and transformed into Alternaria alternata TPF6 for taxadiene production. This study was able to address the challenges associated with the first step of taxadiene production, and a consistent supply of taxadiene in A. alternata TPF6 (61.9 ± 6.3 μg/L) was obtained [265]. The inactivation of the sterol biosynthesis pathway improved the taxol yield dramatically, suggesting the prospects of genetic engineering as a means of yield enhancement of metabolites. Genome editing of filamentous fungi using the CRISPR-Cas system promises to revolutionize the production of natural products. The CRISPR-Cas9 tool aims at Cas9 gene delivery and guides DNA in cassettes and formation of active Cas9/guide RNA complex and requires markers, shuttle vectors, and promoter sequences for efficient expression. The validation of BGCs and targeted metabolite production may be improved by employing the CRISPR-Cas genome editing approach for the transformation of endophytic fungi.
16. Mutagenesis of Endophytic Fungi
To exploit endophytes on a commercial scale, it is imperative to understand and tap into the biosynthetic potential of endophytes to enhance the yield of high-value metabolites. To address the concern toward low-yielding endophyte strains, strain improvement strategies may be employed to enhance yield and other characteristics, including utilization of nitrogen/carbon sources, changes in morphology, and reduction in undesired metabolites [266]. In addition to genetic manipulations, mutagenesis of endophytes by protoplast fusion or random screening offers distinct advantages and prospects in this direction. The genetic framework of microbial strains is altered by employing mutagens and comprises either chemical (nitrosoguanidine, diethyl sulfate, ethyl methyl sulfonate) or physical (X-ray, γ-rays, microwave, etc.) mutagens. Moreover, mutant strains were analyzed using random methods to identify mutants with a particular genotype of interest [267]. Mutations are also induced by using two or more mutagens or one repeatedly [220]. Mutagenesis of endophytic fungi leads to regulatory gene alterations, causing phenotypic changes and enhanced secondary metabolite production, although the process needs to be defined [268]. A key example by Kai et al. [269] discussed the mutagenesis of endophytes and the selection of hygromycin-resistant strains for enhanced taxol production. In endophytic fungi, mutagenesis is attempted by employing mycelia, spores, or protoplasts and applied to taxol-producing endophytes with remarkable success [242]. Although mutagenesis offers a prospective approach, mutation of endophyte mycelium highlights the demerits of genetic divergence in the offspring, and the optimization of conditions for endophyte spore mutagenesis is difficult, suggesting that protoplast fusion is the best way to induce mutagenesis [269]. Studies on mutagenesis of endophytes for strain improvement/yield enhancement focused on optimal conditions for protoplast preparation, optimizing factors such as time, pH, temperature, enzymes, medium, and culture conditions [270,271,272]. A few reports have discussed the yield enhancement of the targeted metabolite via protoplast mutagenesis [269,273] highlighting its beneficial outcomes. Zhao et al. [271] reported the generation of an improved taxol-producing strain (HDF-68) through the fusion of two mutant protoplasts of Nodulisporium sylviforme. The taxol production in the mutant strain increased to 468.62 μg/L compared to the parent endophyte strains, suggesting mutagenesis as a powerful approach for yield enhancement, with some considerations.
17. Co-Culture of Different Endophyte Strains
With the discovery of many endophytes and bio-prospection of their respective strains, the compatibility of endophytes to produce a target metabolite has been explored [153]. The individual endophyte strains are maintained or preserved as monocultures and cultured to produce high-value metabolites; however, the standard culture may not hold the potential to activate the expression of BCGs in endophytic fungi, leading to a decrease in the discovery of novel metabolites. Sequencing of whole genomes showed that multiple genes encoding bacterial/fungal enzymes surpassed the secondary metabolites in these microbes [274]. Moreover, the metabolic processes in microbes are significantly changed with little fluctuation in culture conditions, specifically in endophytes with host-balanced interactions and multipartite interactions [242]. The co-cultivation of different endophytic strains aims to activate some silent BGCs in the presence of compatible microbes and enhances the production of targeted metabolites of interest. This is relevant in the case of antibiotic production when competition governs the biosynthesis of a product in cultures [157]. Corynespora cassiicola SUK2 and Colletotrichum fructicola SUK1 (endophytic fungi), isolated from Nothapodytes nimmoniana (Grah.) Mabb. (Ghanera), demonstrated host-independent biosynthesis of CPT under fermentation conditions, with a significantly higher yield (>1.4-fold) than that of monocultures [275]. Ahamed and Ahring [257] co-cultivated E. coli with Gliocadium roseum (endophytic fungi) and increased hydrocarbon production by 100-fold, compared to pure cultures of G. roseum. Other key examples, namely co-cultivation of Phomopsis and Alternaria, led to an eightfold increase in taxol production [224], and Alternaria and Paraconiothyrium SSM001 co-cultures led to a threefold increase in taxol production, and other examples suggest that co-culturing of compatible endophyte strains highlights an attractive alternative for yield enhancement of high-value metabolites.
18. Optimization of Culture Conditions
Although the production of diverse chemical entities by endophytic fungi is prospective in addressing natural product-mediated drug discovery, the multiple factors that govern production remain poorly understood. The cultural factors that play a key role in metabolite production include temperature, pH, cultivation time, nutrients, and aeration in the medium [276]. The term OSMAC was coined by Bode et al. [277] to show that a single microbial strain may biosynthesize multiple compounds by changing culture conditions, leading to the discovery of high-value metabolites [104]. Optimization can be achieved by considering a one-factor change or multiple-factor variations for fermentation and medium optimization. In addition, the statistical design provides reliable information for experiments by enabling the analysis of experimental variables and making an accurate prediction [278]. Wang et al. [279] discussed the importance of optimization of factors (such as carbon source, pH, and temperature) for Neurospora intermedia DP8-1 (diuron-degrading endophyte) from sugarcane and reported effective biodegradation of up to 99% of the diuron present. In the endophytic fungi F. solani from Ferocactus latispinus, the pH value and nitrogen/carbon ratio were found to affect polyketide production and were optimized, resulting in a higher yield (476 μmol/L) [280]. In addition, a bioreactor was developed to monitor the consistent production of metabolites [281]. The colonization of plant internal tissues by fungal endophytes (dark condition) showed that light treatment decreased taxol production in Paraconiothyrium SSM001 and decreased the level of gene expression [282].
19. Epigenetic Modifiers
The BCGs in fungi are present in the heterochromatin state and are controlled by epigenetic processes, including DNA methylation and histone deacetylation [283]. Gene expression/silencing in fungi is regulated by chromatin modification via DNA methylation/histone deacetylation. Epigenetic modifiers such as histone deacetylase (HDAC) inhibitors have the potential to initiate remodeling of chromatin to activate BGCs [284]. In addition, 5-aza-20-deoxycytidine and 5-azacytidine (DNA methyltransferase) inhibitors are another important class of epigenetic modifiers used for the discovery of natural products. However, an in-depth understanding of secondary metabolite biosynthesis and regulation is essential for the development of new techniques for the isolation of secondary metabolites from fungi [242]. Prospective approaches for discovery/isolation of high-value metabolites include BCG-specific transcription factor overexpression, promoter exchange, or employing inducible promoters for gene activation, and applied to endophytic fungi to alter secondary metabolite networks.
20. Translational Success and Outcome of Endophyte Research
The discovery and commercial production of taxol was a landmark in endophyte biology and research and marked the bio-prospection of endophytes in natural product-mediated drug discovery. The key pharmacological metabolites, including CPT, podophyllotoxin, huperzine A, and vinca alkaloids (vincristine/vinblastine), define a novel paradigm in pharmacology, with a new hope to discover and employ high-value metabolites in therapeutic endeavors. Although endophytes continue to gain popularity as an attractive production platform for pharmaceutical therapeutics at an affordable scale, the existing and emerging bottlenecks regarding the purity of endophyte strains, sub-culturing concerns, low yields, and adverse effects need to be further addressed for maximal utilization. Recent advances in scientific interventions and high-throughput methods have made substantial contributions to endophyte research, aiming to address the projected challenges. Genetic engineering of endophytes comprising gene overexpression and gene constructs, strain mutagenesis, and co-cultivation of compatible strains is targeted toward the improvement of different endophyte strains and high yield of targeted metabolites. With the depletion of natural resources and limited availability of natural products, the bio-prospection of endophytes and their genetic improvement is an important area of research in drug discovery programs.
Abbreviations
antimicrobial resistance AMR; human cytomegalovirus hCMV; food and drug administration FDA; biosynthetic gene clusters BGCs; horizontal gene transfer HGT; strictosidine synthase STR; camptothecin CPT; high performance liquid chromatography HPLC; nuclear magnetic resonance NMR; mass spectrometry MS, one strain many compounds OSMAC; high-throughput elicitor screening with imaging mass spectrometry HiTES-IMS; Database of BIoSynthesis clusters CUrated and InTegrated’ doBISCUIT; Minimum Information on Biosynthetic Geneclusters’ MIBiG; Reconstruction, Analysis and Visualization of Metabolic Networks’ RAVEN 2.0; antibiotics & Secondary Metabolite Analysis Shell antiSMASH; Bidirectional Long Short-Term Memory BiLSTM; neural netword RNN; Protoplast Mediated Transformation PMT; Agrobacterium-mediated transformation AMT; clustered regularly interspaced short palindromic repeats CRISPR; Potato Dextrose Broth PDB; deacetylmycoepoxydiene DAM; restriction enzyme-mediated integration REMI; nitrosoguanidine NTG; diethyl sulfate DES; ethyl methyl sulfonate EMS; histone deacetylase HDAC.
Author Contributions
P.T. and H.B. planned the manuscript. P.T. contributed to literature collection and writing of the manuscript. H.B. made critical suggestions for the improvement. All authors have significantly contributed, revised, and approved the manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out with the support of R&D Program for Forest Science Technology (Project No. 2022450B10-22101) provided by Korea Forest Service (Korea Forestry Promotion Institute).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Staniek A., Woerdenbag J., Kayser O. Endophytes exploiting biodiversity for the improvement of natural product-based drug discovery. J. Plant Interact. 2008;3:75–98 doiorg/101080/17429140801886293. doi: 10.1080/17429140801886293. [DOI] [Google Scholar]
- 2.Tiwari P., Srivastava Y., Bae H. Endophytes: Trend of pharmaceutical design of Endophytes as anti-infective. Curr Top. Med. Chem. 2021;21:1572–1586. doi: 10.2174/1568026621666210524093234. [DOI] [PubMed] [Google Scholar]
- 3.Rashmi M., Venkateswara S.V. Secondary metabolite production by Endophytic fungi: The gene clusters, nature, and expression. In: Jha S., editor. Endophytes and Secondary Metabolites Reference Series in Phytochemistry. 2019. pp. 475–490. [Google Scholar]
- 4.Tiwari P., Katyal A., Khan M.F., Ashraf G.M., Ahmed K. Lead optimization resources in drug discovery studies. Endocr. Metab. Immune Disord.-Drug Targets. 2019;19:754–774. doi: 10.2174/1871530319666190304121826. [DOI] [PubMed] [Google Scholar]
- 5.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari P., Khare T., Shriram V., Bae H., Kumar V. Exploring synthetic biology strategies for producing potent antimicrobial phytochemicals. Biotechnol. Adv. 2021;48:107729. doi: 10.1016/j.biotechadv.2021.107729. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari P., Srivastava Y., Kumar V. Antimicrobial peptides as effective agents against drug-resistant pathogens. In: Kumar V., Shriram V., Paul A., Thakur M., editors. Antimicrobial Resistance. Springer; Singapore: 2022. pp. 289–322. [DOI] [Google Scholar]
- 8.Tiwari P., Srivastava Y., Bajpai M., Sharma A. Bioactive metabolites from natural sources: Prospects and significance in drug discovery and research. Bioingene PSJ. 2021;1:1–14. [Google Scholar]
- 9.Singh S.B., Ondeyka J.G., Tsipouras N., Ruby C., Sardana V., Schulman M., Sanchez M., Pelaez F., Stahlhut M.W., Munshi S., et al. Hinnuliquinone, a C2-symmetric dimeric non-peptide fungal metabolite inhibitor of HIV-1 protease. Biochem. Biophys. Res. Commun. 2004;324:108–113. doi: 10.1016/j.bbrc.2004.08.234. [DOI] [PubMed] [Google Scholar]
- 10.Guo B., Dai J., Ng S., Huang Y., Leong C., Ong W., Carte B.K. Cytonic acids A & B: Novel tridepside inhibitors of hCMV protease from the endophytic fungus Cytonaema species. J. Nat. Prod. 2000;63:602–604. doi: 10.1021/np990467r. [DOI] [PubMed] [Google Scholar]
- 11.Strobel G.A., Ford E., Worapong J., Harper J.K., Arif A.M., Grant D.M., Fung P.C., Chan K. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry. 2002;60:179–183. doi: 10.1016/S0031-9422(02)00062-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P., Zhou P.P., Yu L.J. An endophytic taxol-producing fungus from Taxus media, Cladosporium cladosporioides MD2. Curr. Microbiol. 2009;59:227–232. doi: 10.1007/s00284-008-9270-1. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto A., Takahashi Y. Endophytic actinomycetes: Promising source of novel bioactive compounds. J. Antibiot. 2017;70:514–519. doi: 10.1038/ja.2017.20. [DOI] [PubMed] [Google Scholar]
- 14.Croteau R., Ketchum R.E.B., Long R.M., Kaspera R., Wildung M.R. Taxol biosynthesis and molecular genetics. Phytochem Rev. 2006;5:75–97. doi: 10.1007/s11101-005-3748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stierle A., Strobel G., Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 16.Venieraki A., Dimou M., Katinakis P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hell. Plant Protect. J. 2017;10:51–66. doi: 10.1515/hppj-2017-0006. [DOI] [Google Scholar]
- 17.Ludwig-Müller J. Plants and endophytes: Equal partners in secondary metabolite production? Biotechnol. Lett. 2015;37:1325–1334. doi: 10.1007/s10529-015-1814-4. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N., Sabat J., Parida R., Kerkatta D. Solubilization of tricalcium phosphate and rock phosphate by microbes isolated from chromite, iron and manganese mines. Acta Bot. Croat. 2007;66:197–204. [Google Scholar]
- 19.Nath R., Sharma G., Barooah M. Efficiency of tricalcium phosphate solubilization by two different endophytic Penicillium sp. isolated from tea (Camellia sinensis L.) Eur. J Exp. Biol. 2012;2:1354–1358. [Google Scholar]
- 20.Rinu K., Sati P., Pandey A. Trichoderma gamsii (NFCCI 2177): A newly isolated endophytic, psychrotolerant, plant growth promoting, and antagonistic fungal strain. J. Basic Microbiol. 2014;54:408–417. doi: 10.1002/jobm.201200579. [DOI] [PubMed] [Google Scholar]
- 21.Chadha N., Prasad R., Varma A. Plant promoting activities of fungal endophytes associated with tomato roots from central Himalaya, India and their interaction with Piriformospora indica. Int. J. Pharm. Bio Sci. 2015;6:333–343. [Google Scholar]
- 22.Spagnoletti F., Tobar N., Di Pardo A.F., Chiocchio V., Lavado R. Dark septate endophytes present different potential to solubilize calcium, iron and aluminium phosphates. Appl. Soil Ecol. 2017;111:25–32. doi: 10.1016/j.apsoil.2016.11.010. [DOI] [Google Scholar]
- 23.Hamayun M., Khan S.A., Ahmad N., Tang D.-S., Kang S.-M., Na C.-I., Sohn E.-Y., Hwang Y.-H., Shin D.-H., Lee B.-H. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009;25:627–632. doi: 10.1007/s11274-009-9982-9. [DOI] [Google Scholar]
- 24.Khan A.R., Ullah I., Waqas M., Shahzad R., Hong S.J., Park G.S., Jung B.K., Lee I.J., Shin J.H. Plant growth-promoting potential of endophytic fungi isolated from Solanum nigrum leaves. World J. Microbiol. Biotechnol. 2015;31:1461–1466. doi: 10.1007/s11274-015-1888-0. [DOI] [PubMed] [Google Scholar]
- 25.Johnson L.J., de Bonth A.C., Briggs L.R., Caradus J.R., Finch S.C., Fleetwood D.J., Fletcher L.R., Hume D.E., Johnson R.D., Popay A.J., et al. The exploitation of Epichloae endophytes for agricultural benefit. Fungal Diver. 2013;60:171–188. doi: 10.1007/s13225-013-0239-4. [DOI] [Google Scholar]
- 26.Sane S., Mehta S. Isolation and evaluation of rock phosphate solubilizing fungi as potential biofertilizer. J Fertil Pestic. 2015;6:156–160. doi: 10.4172/2471-2728.1000156. [DOI] [Google Scholar]
- 27.Šišić A., Baćanović J., Finckh M.R. Endophytic Fusarium equiseti stimulates plant growth and reduces root rot disease of pea (Pisum sativum L.) caused by Fusarium avenaceum and Peyronellaea pinodella. Eur. J Plant Pathol. 2017;148:271–282. doi: 10.1007/s10658-016-1086-4. [DOI] [Google Scholar]
- 28.Xia Y., Sahib M.R., Amna A., Opiyo S.O., Zhao Z., Gao Y.G. Culturable endophytic fungal communities associated with plants in organic and conventional farming systems and their effects on plant growth. Sci. Rep. 2019;9:1669. doi: 10.1038/s41598-018-38230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez R.J., White J.F., Jr., Arnold A.E., Redman R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 30.Abro M.A., Sun X., Li X., Jatoi G.H., Guo L.D. Biocontrol potential of Fungal endophytes against Fusarium oxysporum f. sp. cucumerinum causing Wilt in Cucumber. Plant Pathol. J. 2019;35:598–608. doi: 10.5423/PPJ.OA.05.2019.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kord H., Fakheri B., Ghabooli M., Solouki M., Emamjomeh A., Khatabi B., Sepehri M., Salekdeh G.H., Ghaffari M.R. Root endophytic fungus Piriformospora indica improves drought stress adaptation in barley by metabolic and proteomic reprogramming. Environ. Exp. Bot. 2019;157:197–210. doi: 10.1016/j.envexpbot.2018.10.002. [DOI] [Google Scholar]
- 32.Sun C., Johnson J.M., Cai D., Sherameti I., Oelmüller R., Lou B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J. Plant Physiol. 2010;167:1009–1017. doi: 10.1016/j.jplph.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Zahoor M., Irshad M., Rahman H., Qasim M., Afridi S.G., Qadir M., Hussain A. Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol. Environ. Safety. 2017;142:139–149. doi: 10.1016/j.ecoenv.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Bibi S., Hussain A., Hamayun M., Rahman H., Iqbal A., Shah M., Irshad M., Qasim M., Islam B. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere. 2018;211:653–663. doi: 10.1016/j.chemosphere.2018.07.197. [DOI] [PubMed] [Google Scholar]
- 35.Soldi E., Casey C., Murphy B.R., Hodkinson T.R. Fungal endophytes for Grass based bioremediation: An endophytic consortium isolated from Agrostis stolonifera stimulates the growth of Festuca arundinacea in lead contaminated soil. J. Fungi. 2020;6:254. doi: 10.3390/jof6040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soleimani M., Afyuni M., Hajabbasi M.A., Nourbakhsh F., Sabzalian M.R., Christensen J.H. Phytoremediation of an aged petroleum contaminated soil using endophyte infected and non-infected grasses. Chemosphere. 2010;81:1084–1090. doi: 10.1016/j.chemosphere.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 37.Marín F., Navarrete H., Narvaez-Trujillo A. Total petroleum hydrocarbon degradation by endophytic fungi from the Ecuadorian Amazon. Advan. Microbiol. 2018;8:1029–1053. doi: 10.4236/aim.2018.812070. [DOI] [Google Scholar]
- 38. [(accessed on 12 July 2021)]. Available online: https://www.bard.edu.
- 39.Ling O.M., Teen L.P., Mujahid A., Proksch P., Müller M. Initial screening of Mangrove endophytic fungi for antimicrobial compounds and heavy metal biosorption potential. Sains Malaysiana. 2016;45:1063–1071. [Google Scholar]
- 40.Gao T., Qin D., Zuo S., Peng Y., Xu J., Yu B., Song H., Dong J. Decolorization and detoxification of triphenylmethane dyes by isolated endophytic fungus, Bjerkandera adusta SWUSI4 under non-nutritive conditions. Bioresour. Bioprocess. 2020;7:53. doi: 10.1186/s40643-020-00340-8. [DOI] [Google Scholar]
- 41.Aishwarya S.A.N.I., Nagam N., Vijaya T., Netala R.V. Screening and identification of heavy metal-tolerant endophytic fungi Lasiodiplodia theobromae from Boswellia ovalifoliolata an endemic plant of Tirumala hills. Asian J Pharm. Clin. Res. 2017;10:488–491. doi: 10.22159/ajpcr.2017.v10i3.16697. [DOI] [Google Scholar]
- 42.Pietro-Souza W., Pereira F.C., Mello I.S., Stachack F.F.F., Terezo A.J., da Cunha C.N., White J.F., Li H., Soares M.A. Mercury resistance and bioremediation mediated by endophytic fungi. Chemosphere. 2020;240:124874. doi: 10.1016/j.chemosphere.2019.124874. [DOI] [PubMed] [Google Scholar]
- 43.Kusari S., Hertweck C., Spiteller M. Chemical ecology of endophytic funSgi: Origins of secondary metabolites. Chem. Biol. 2012;19:792–798. doi: 10.1016/j.chembiol.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Strobel G.A., Pliam N.B. Immuno suppressants diterpene compounds. Google Patents. 1997
- 45.Puri S.C., Nazir A., Chawla R., Arora R., Riyaz-ul-Hasan S., Amna T., Ahmed B., Verma V., Singh S., Sagar R., et al. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. J. Biotechnol. 2006;122:494–510. doi: 10.1016/j.jbiotec.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Mir R.A., Kaushik P.S., Chowdery R.A., Anuradha M. Elicitation of forskolin in cultures of Rhizactonia bataticola-a phytochemical synthesizing endophytic fungi. Int. Pharm. Pharmaceut. Sci. 2015;7:185–189. [Google Scholar]
- 47.Wang X.J., Min C.L., Ge M., Zuo R.H. An endophytic sanguinarine-producing fungus from Macleaya cordata, Fusarium proliferatum BLH51. Curr. Microbiol. 2014;68:336–341. doi: 10.1007/s00284-013-0482-7. [DOI] [PubMed] [Google Scholar]
- 48.Kaul S., Ahmed M., Zargar K., Sharma P., Dhar M.K. Prospecting endophytic fungal assemblage of Digitalis lanata Ehrh. (foxglove) as a novel source of digoxin: A cardiac glycoside. 3 Biotech. 2013;3:335–340. doi: 10.1007/s13205-012-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maehara S., Simanjuntak P., Ohashi K., Shibuya H. Composition of endophytic fungi living in Cinchona ledgeriana (Rubiaceae) J Nat. Med. 2010;64:227–230. doi: 10.1007/s11418-009-0380-2. [DOI] [PubMed] [Google Scholar]
- 50.Devari S., Jaglan S., Kumar M., Deshidi R., Guru S., Bhushan S., Kushwaha M., Gupta A.P., Gandhi S.G., Sharma J.P., et al. Capsaicin production by Alternaria alternata, an endophytic fungus from Capsicum annum; LC–ESI–MS/MS analysis. Phytochemistry. 2014;98:183–189. doi: 10.1016/j.phytochem.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Meshram V., Gupta M. Endophytic fungi: A quintessential source of potential bioactive compounds. In: Hodkinson T.R., Doohan F.M., Saunders M.J., Murphy B.R., editors. Endophytes for a Growing World. Cambridge University Press; Cambridge, UK: 2019. pp. 277–309. [DOI] [Google Scholar]
- 52.Zazopoulos E., Huang K., Staffa A., Liu W., Bachmann B.O., Nonaka K., Ahlert J., Thorson J.S., Shen B., Farnet C.M. A genomics-guided approach for discovering and expressing cryptic metabolic pathways. Nat Biotech. 2003;21:187–190. doi: 10.1038/nbt784. [DOI] [PubMed] [Google Scholar]
- 53.Lautru S., Deeth R.J., Bailey L.M., Challis G.L. Discovery of a new peptide natural product by Streptomyces coelicolor genome mining. Nat. Chem. Biol. 2005;1:265–269. doi: 10.1038/nchembio731. [DOI] [PubMed] [Google Scholar]
- 54.Tyo K.E., Alper H.S., Stephanopoulos G.N. Expanding the metabolic engineering toolbox: More options to engineer cells. Trends Biotechnol. 2007;25:132–137. doi: 10.1016/j.tibtech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Tan R.X., Zou W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2000;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 56.Tiwari P., Bae H. Horizontal gene transfer and endophytes: An implication for the acquisition of novel traits. Plants. 2020;9:305. doi: 10.3390/plants9030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan S.Y., Zhou S.F., Gao S.H., Yu Z.L., Zhang S.F., Tang M.K., Sun J.N., Ma D.L., Han Y.F., Fong W.F., et al. New perspectives on how to discover drugs from Herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid. Based Complement. Altern. Med. 2013;2:627375. doi: 10.1155/2013/627375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L., Zhang Q.Y., Jia M., Ming Q.L., Yue W., Rahman K., Qin L.-P., Han T. Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Crit. Rev. Microbiol. 2016;42:454–473. doi: 10.3109/1040841X.2014.959892. [DOI] [PubMed] [Google Scholar]
- 59.Berdy J. Bioactive microbial metabolites. J. Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 60.Mousa W.K., Shearer C., Limay-Rios V., Ettinger C.L., Eisen J.A., Raizada M.N. Roothair endophyte stacking in finger millet creates a physicochemical barrier to trap the fungal pathogen Fusarium graminearum. Nat. Microbiol. 2016;1:16167. doi: 10.1038/nmicrobiol.2016.167. [DOI] [PubMed] [Google Scholar]
- 61.Soliman S.S.M., Greenwood J.S., Bombarely A., Mueller L.A., Tsao R., Mosser D.D., Raizada M.N. An endophyte constructs fungicide-containing extracellular barriers for its host plant. Curr. Biol. 2015;25:2570–2576. doi: 10.1016/j.cub.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 62.Hamayun M., Hussain A., Khan S.A., Kim H.Y., Khan A.L., Waqas M., Irshad M., Iqbal A., Rehman G., Jan S., et al. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017;8:686. doi: 10.3389/fmicb.2017.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panaccione D.G., Beaulieu W.T., Cook D. Bioactive alkaloids in vertically transmitted fungal endophytes. Funct. Ecol. 2014;28:299–314. doi: 10.1111/1365-2435.12076. [DOI] [Google Scholar]
- 64.Jamwal V.L., Gandhi S.G. Endophytes as a source of High-value phytochemicals: Present scenario and future outlook. In: Jha S., editor. Endophytes and Secondary Metabolites. Springer Nature; Cham, Switzerland: 2019. Reference Series in Phytochemistry. [DOI] [Google Scholar]
- 65.Xu D., Zhang B.Y., Yang X.L. Antifungal monoterpene derivatives from the plant endophytic fungus Pestalotiopsis foedan. Chem. Biodivers. 2016;13:1422–1425. doi: 10.1002/cbdv.201600114. [DOI] [PubMed] [Google Scholar]
- 66.Deshmukh S.K., Prakash V., Ranjan N. Recent advances in the discovery of bioactive metabolites from Pestalotiopsis. Phytochem. Rev. 2017;16:883–920. doi: 10.1007/s11101-017-9495-3. [DOI] [Google Scholar]
- 67.Wu L.S., Jia M., Chen L., Zhu B., Dong H.X., Si J.P., Peng W., Han T. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules. 2016;21:14. doi: 10.3390/molecules21010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanney J.B., McMullin D.R., Green B.D., Miller J.D., Seifert K.A. Production of antifungal and anti-insect an metabolites by the Picea endophyte Diaporthe maritima sp. nov. Fungal Biol. 2016;120:1448–1457. doi: 10.1016/j.funbio.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Morandini L.M.B., Neto A.T., Pedroso M., Antoniolli Z.I., Burrow R.A., Bortoluzzi A.J., Mostardeiro M.A., da Silva U.F., Dalcol I.I., Morel A.F. Lanostane-type triterpenes from the fungal endophyte Scleroderma UFSMSc1 (Persoon) Fries. Bioorg. Med. Chem. Lett. 2016;26:1173–1176. doi: 10.1016/j.bmcl.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 70.Mousa W.K., Schwan A.L., Raizada M.N. Characterization of antifungal natural products isolated from endophytic fungi of finger millet (Eleusine coracana) Molecules. 2016;21:1171. doi: 10.3390/molecules21091171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu K., Yang Y., Miao C.P., Zheng Y.K., Chen J.L., Chen Y.W., Xu L.H., Guang H.L., Ding Z.T., Zhao L.X. Koningiopisins A-H, polyketides with synergistic antifungal activities from the endophytic fungus Trichoderma koningiopsis. Planta Med. 2016;82:371–376. doi: 10.1055/s-0035-1558228. [DOI] [PubMed] [Google Scholar]
- 72.Shentu X., Zhan X., Ma Z., Yu X., Zhang C. Antifungal activity of metabolites of the endophytic fungus Trichoderma brevicompactum from garlic. Braz. J. Microbiol. 2014;45:248–254. doi: 10.1590/S1517-83822014005000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Budhiraja A., Nepali K., Sapra S., Gupta S., Kumar S., Dhar K.L. Bioactive metabolites from an endophytic fungus of Aspergillus species isolated from seeds of Gloriosa superba Linn. Med. Chem. Res. 2013;22:323–329. doi: 10.1007/s00044-012-0032-z. [DOI] [Google Scholar]
- 74.Ma Y.M., Qiao K., Kong Y., Li M.Y., Guo L.X., Miao Z., Fan C. A new isoquinolone alkaloid from an endophytic fungus R22 of Nerium indicum. Nat. Prod. Res. 2017;31:951–958. doi: 10.1080/14786419.2016.1258556. [DOI] [PubMed] [Google Scholar]
- 75.Li W., Xu J., Li F., Xu L., Li C. A new antifungal isocoumarin from the endophytic fungus Trichoderma sp.09 of Myoporum bontioides A. Gray. Pharmacogn. Mag. 2016;12:259–261. doi: 10.4103/0973-1296.192204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ibrahim S.R.M., Elkhayat E.S., Mohamed G.A.A., Fat’hi S.M., Ross S.A. Fusarithioamide A, a new antimicrobial and cytotoxic benzamide derivative from the endophytic fungus Fusarium chlamydosporium. Biochem. Biophys. Res. Commun. 2016;479:211–216. doi: 10.1016/j.bbrc.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 77.Mondol M.A.M., Farthouse J., Islam M.T., Schueffler A., Laatsch H. Metabolites from the endophytic fungus Curvularia sp. M12 act as motility inhibitors against Phytophthora capsici Zoospores. J. Nat. Prod. 2017;80:347–355. doi: 10.1021/acs.jnatprod.6b00785. [DOI] [PubMed] [Google Scholar]
- 78.Ibrahim S.R.M., Abdallah H.M., Elkhayat E.S., Al Musayeib N.M., Asfour H.Z., Zayed M.F., Mohamed G.A. Fusaripeptide A: New antifungal and anti-malarial cyclodepsipeptide from the endophytic fungus Fusarium sp. J. Asian Nat. Prod. Res. 2018;20:75–85. doi: 10.1080/10286020.2017.1320989. [DOI] [PubMed] [Google Scholar]
- 79.Taware R., Abnave P., Patil D., Rajamohananan P.R., Raja R., Soundararajan G., Kundu G.C., Ahmad A. Isolation, purification and characterization of Trichothecinol-A produced by endophytic fungus Trichothecium sp. and its antifungal, anticancer and antimetastatic activities. Sustain. Chem. Process. 2014;2:8. doi: 10.1186/2043-7129-2-8. [DOI] [Google Scholar]
- 80.Hussain H., Kock I., Al-Harras A., Al-Rawahi A., Abbas G., Green I.R., Shah A., Badshah A., Saleem M., Draeger S., et al. Antimicrobial chemical constituents from endophytic fungus Phoma sp. Asian. Pac. J. Trop. Med. 2014;7:699–702. doi: 10.1016/S1995-7645(14)60119-X. [DOI] [Google Scholar]
- 81.Lai D., Wang A., Cao Y., Zhou K., Mao Z., Dong X., Tian J., Xu D., Dai J., Peng Y., et al. Bioactive dibenzo-α-pyrone derivatives from the endophytic fungus Rhizopycnis vagum Nitaf22. J. Nat. Prod. 2016;79:2022–2031. doi: 10.1021/acs.jnatprod.6b00327. [DOI] [PubMed] [Google Scholar]
- 82.Hussain H., Root N., Jabeen F., Al-Harras A., Al-Rawahi A., Ahmad M., Hassan Z., Abba G., Mabood F., Shah A., et al. Seimatoric acid and colletonoic acid: Two new compounds from the endophytic fungi, Seimatosporium sp. and Colletotrichum sp. Chin. Chem. Lett. 2014;25:1577–1579. doi: 10.1016/j.cclet.2014.06.006. [DOI] [Google Scholar]
- 83.Zhang Q., Xiao J., Sun Q.Q., Qin J.C., Pescitelli G., Gao J.M. Characterization of cytochalasins from the endophytic Xylaria sp. and their biological functions. J. Agric. Food Chem. 2014;62:10962–10969. doi: 10.1021/jf503846z. [DOI] [PubMed] [Google Scholar]
- 84.Lin Z., Wen J., Zhu T., Fang Y., Gu Q., Zhu W. Chrysogenamide A from an endophytic fungus associated with Cistanche deserticola and its neuroprotective effect on SH-SY5Y cells. J. Antibiot. 2008;61:81–85. doi: 10.1038/ja.2008.114. [DOI] [PubMed] [Google Scholar]
- 85.Zhao S.S., Zhang Y.Y., Yan W., Cao L.L., Xiao Y., Ye Y.H., Zhao S.S., Zhang Y.Y., Yan W., Cao L.L., et al. Chaetomium globosum CDW7, a potential biological control strain and its antifungal metabolites. FEMS Microbiol. Lett. 2017;364 doi: 10.1093/femsle/fnw287. [DOI] [PubMed] [Google Scholar]
- 86.Sun P., Huo J., Kurtan T., Mandi A., Antus S., Tang H., Draeger S., Schulz B., Hussain H., Krohn K., et al. Structural and stereochemical studies of hydroxyanthraquinone derivatives from the endophytic fungus Coniothyrium sp. Chirality. 2013;25:141–148. doi: 10.1002/chir.22128. [DOI] [PubMed] [Google Scholar]
- 87.Liu S., Liu X., Guo L., Che Y., Liu L. 2H-Pyran-2-one and2H-Furan-2-one derivatives from the plant endophytic fungus Pestalotiopsis fici. Chem. Biodivers. 2013;10:2007–2013. doi: 10.1002/cbdv.201200361. [DOI] [PubMed] [Google Scholar]
- 88.Rukachaisirikul V., Buadam S., Sukpondma Y., Phongpaichit S., Sakayaroj J., Hutadilok-Towatana N. Indanone and mellein derivatives from the Garcinia-derived fungus Xylaria sp. PSU-G12. Phytochem. Lett. 2013;6:135–138. doi: 10.1016/j.phytol.2012.11.007. [DOI] [Google Scholar]
- 89.Sica V.P., Rees E.R., Tchegnon E., Bardsley R.H., Raja H.A., Oberlies N.H. Spatial and temporal profiling of griseofulvin production in Xylaria cubensis using mass spectrometry mapping. Front. Microbiol. 2016;7:544. doi: 10.3389/fmicb.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silva G.H., Zeraik M.L., de Oliveira C.M., Teles H.L., Trevisan H.C., Pfenning L.H., Nicolli C.P., Young M.C.M., Mascarenhas Y.P., Abreu L.M., et al. Lactone derivatives produced by a Phaeoacremonium sp., an endophytic fungus from Senna spectabilis. J. Nat. Prod. 2017;80:1674–1678. doi: 10.1021/acs.jnatprod.5b00828. [DOI] [PubMed] [Google Scholar]
- 91.Ibrahim S.R.M., Elkhayat E.S., Mohamed G.A., Khedr A.I.M., Fouad M.A., Kotb M.H.R., Ross S.A. Aspernolides F and G, new butyrolactones from the endophytic fungus Aspergillus terreus. Phytochem. Lett. 2015;14:84–90. doi: 10.1016/j.phytol.2015.09.006. [DOI] [Google Scholar]
- 92.Kajula M., Ward J.M., Turpeinen A., Tejesvi M.V., Hokkanen J., Tolonen A., Hakkanen H., Picart P., Ihalainen J., Sahl H.G., et al. Bridged epipolythiodiketopiperazines from Penicillium raciborskii, an endophytic fungus of Rhododendron tomentosum Harmaja. J. Nat. Prod. 2016;79:685–690. doi: 10.1021/np500822k. [DOI] [PubMed] [Google Scholar]
- 93.Pereira C.B., de Oliveira D.M., Hughes A.F.S., Kohlhoff M., Vieira M.L.A., Martins Vaz A.B., Ferreira M.C., Carvalho C.R., Rosa L.H., Rosa C.A., et al. Endophytic fungal compounds active against Cryptococcus neoformans and C. gattii. J. Antibiot. 2015;68:436–444. doi: 10.1038/ja.2015.11. [DOI] [PubMed] [Google Scholar]
- 94.Li T.X., Yang M.H., Wang X.B., Wang Y., Kong L.Y. Synergistic antifungal meroterpenes and dioxolanone derivatives from the endophytic fungus Guignardia sp. J. Nat. Prod. 2015;78:2511–2520. doi: 10.1021/acs.jnatprod.5b00008. [DOI] [PubMed] [Google Scholar]
- 95.Meng X., Mao Z., Lou J., Xu L., Zhong L., Peng Y., Zhou L., Wang M. Benzopyranones from the endophytic fungus Hyalodendriella sp. Ponipodef12 and their bioactivities. Molecules. 2012;17:11303–11314. doi: 10.3390/molecules171011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mao Z., Lai D., Liu X., Fu X., Meng J., Wang A., Wang X., Sun W., Liu Z.L., Zhou L., et al. Dibenzo-α-pyrones: A new class of larvicidal metabolites against Aedes aegypti from the endophytic fungus Hyalodendriella sp. Ponipodef12. Pest Manag. Sci. 2017;73:1478–1485. doi: 10.1002/ps.4481. [DOI] [PubMed] [Google Scholar]
- 97.Liang X.A., Ma Y.M., Zhang H.C., Liu R. A new helvolic acid derivative from an endophytic Fusarium sp. of Ficus carica. Nat. Prod. Res. 2016;30:2407–2412. doi: 10.1080/14786419.2016.1190722. [DOI] [PubMed] [Google Scholar]
- 98.McMullin D.R., Green B.D., Miller J.D. Antifungal sesquiterpenoids and macrolides from an endophytic Lophodermium species of Pinus strobus. Phytochem. Lett. 2015;14:148–152. doi: 10.1016/j.phytol.2015.10.006. [DOI] [Google Scholar]
- 99.Talontsi F.M., Dittrich B., Schueffler A., Sun H., Laatsch H. Epicoccolides: Antimicrobial and antifungal polyketides from an endophytic fungus Epicoccum sp. associated with Theobroma cacao. Eur. J. Org. Chem. 2013;2013:3174–3180. doi: 10.1002/ejoc.201300146. [DOI] [Google Scholar]
- 100.Xiao J., Zhang Q., Gao Y.Q., Tang J.J., Zhang A.L., Gao J.M. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 2014;62:3584–3590. doi: 10.1021/jf500054f. [DOI] [PubMed] [Google Scholar]
- 101.Chen Y.M., Yang Y.H., Li X.N., Zou C., Zhao P.J. Diterpenoids from the endophytic fungus Botryosphaeria sp. P483 of the Chinese herbal medicine Huperzia serrata. Molecules. 2015;20:16924–16932. doi: 10.3390/molecules200916924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chapla V.M., Zeraik M.L., Leptokarydis I.H., Silva G.H., Bolzani V.S., Young M.C.M., Pfenning L.H., Araujo A.R. Antifungal compounds produced by Colletotrichum gloeosporioides, an endophytic fungus from Michelia champaca. Molecules. 2014;19:19243–19252. doi: 10.3390/molecules191119243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mousa W.K., Schwan A., Davidson J., Auzanneau F.I., Strange P., Liu H., Zhou T., Raizada M.N. An endophytic fungus isolated from finger millet (Eleusine coracana) produces anti-fungal natural products. Front. Microbiol. 2015;6:1157. doi: 10.3389/fmicb.2015.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J., Zhang Y.Y., Ding D.D., Yu S.P., Wang L.W. A study on the secondary metabolites of endophytic fungus Chaetomium cupreum ZJWCF079 in Macleaya cordata. Health Res. 2013;33:94–96. [Google Scholar]
- 105.Hussain H., Root N., Jabeen F., Al-Harrasi A., Ahmad M., Mabood F., Hassan Z., Shah A., Green I.R., Schulz B., et al. Microsphaerol and seimatorone: Two new compounds isolated from the endophytic fungi, Microsphaeropsis sp. and Seimatosporium sp. Chem. Biodivers. 2015;12:289–294. doi: 10.1002/cbdv.201400098. [DOI] [PubMed] [Google Scholar]
- 106.Wang J., Wang G., Zhang Y., Zheng B., Zhang C., Wang L. Isolation and identification of an endophytic fungus Pezicula sp. in Forsythia viridissima and its secondary metabolites. World. J. Microbiol. Biotechnol. 2014;30:2639–2644. doi: 10.1007/s11274-014-1686-0. [DOI] [PubMed] [Google Scholar]
- 107.Cao L.L., Zhang Y.Y., Liu Y.J., Yang T.T., Zhang J.L., Zhang Z.G., Shen L., Liu J.Y., Ye Y.H. Anti-phytopathogenic activity of sporothriolide, a metabolite from endophyte Nodulisporium sp. A21 in Ginkgo biloba. Pestic. Biochem. Physiol. 2016;129:7–13. doi: 10.1016/j.pestbp.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 108.Carvalho C.R., Wedge D.E., Cantrell C.L., Silva-Hughes A.F., Pan Z., Moraes R.M., Madoxx V.L., Rosa L.H. Molecular phylogeny, diversity, and bioprospecting of endophytic fungi associated with wild ethnomedicinal North American plant Echinacea purpurea (Asteraceae) Chem. Biodivers. 2016;13:918–930. doi: 10.1002/cbdv.201500299. [DOI] [PubMed] [Google Scholar]
- 109.Nalli Y., Mirza D.N., Wani Z.A., Wadhwa B., Mallik F.A., Raina C., Chaubey A., Riyaz-Ul-Hassan S., Ali A. Phialomustin A-D, new antimicrobial and cytotoxic metabolites from an endophytic fungus, Phialophora mustea. RSC Adv. 2015;5:95307–95312. doi: 10.1039/C5RA18121F. [DOI] [Google Scholar]
- 110.Hussain H., Kliche-Spory C., Al-Harrasi A., Al-Rawahi A., Abbas G., Green I.R., Schulz B., Krohn K., Shah A. Antimicrobial constituents from three endophytic fungi. Asian Pac. J. Trop. Med. 2014;7:S224–S227. doi: 10.1016/S1995-7645(14)60236-4. [DOI] [PubMed] [Google Scholar]
- 111.Shan T., Tian J., Wang X., Mou Y., Mao Z., Lai D., Dai J., Peng Y., Zhou L., Wang M. Bioactive spirobisnaphthalenes from the endophytic fungus Berkleasmium sp. J. Nat. Prod. 2014;77:2151–2160. doi: 10.1021/np400988a. [DOI] [PubMed] [Google Scholar]
- 112.Ola A.R.B., Debb A., Kurtán T., Brötz-Oesterhelt H., Aly A.H., Proksch P. Dihydroanthracenone metabolites from the endophytic fungus Diaporthe melonis isolated from Annona squamosa. Tetrahedron Lett. 2014;55:3147–3150. doi: 10.1016/j.tetlet.2014.03.110. [DOI] [Google Scholar]
- 113.Strobel G.A., Miller R.V., Miller C., Condron M., Teplow D.B., Hess W.M. Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Microbiology. 1999;145:1919–1926. doi: 10.1099/13500872-145-8-1919. [DOI] [PubMed] [Google Scholar]
- 114.Zou W.X., Meng J.C., Lu H., Chen G.X., Shi G.X., Zhang T.Y., Tan R.X. Metabolites of Colletotrichum gloeosporioides, an endophytic fungus in Artemisia mongolica. J. Nat. Prod. 2000;63:1529–1530. doi: 10.1021/np000204t. [DOI] [PubMed] [Google Scholar]
- 115.Cota B.B., Rosa L.H., Caligiorne R.B., Rabello A.L.T., Alves T.M.A., Rosa C.A., Zani C.L. Altenusin, a biphenyl isolated from the endophytic fungus Alternaria sp., inhibits trypanothione reductase from Trypanosoma cruzi. FEMS Microbiol. Lett. 2008;285:177–182. doi: 10.1111/j.1574-6968.2008.01221.x. [DOI] [PubMed] [Google Scholar]
- 116.Li Y., Song Y.C., Liu J.Y., Ma Y.M., Tan R.X. Anti-Helicobacter pylori substances from endophytic fungal cultures. World J. Microbiol. Biotechnol. 2005;21:553–558. doi: 10.1007/s11274-004-3273-2. [DOI] [Google Scholar]
- 117.Shu R.G., Wang F.W., Yang Y.M., Liu Y.X., Tan R.X. Antibacterial and xanthine oxidase inhibitory cerebrosides from Fusarium sp. IFB-121, and endophytic fungus in Quercus variabilis. Lipids. 2004;39:667–673. doi: 10.1007/s11745-004-1280-9. [DOI] [PubMed] [Google Scholar]
- 118.Kim S., Shin D.S., Lee T., Oh K.B. Periconicins, two new fusicoccane diterpenes produced by an endophytic fungus Periconia sp. with antibacterial activity. J. Nat. Prod. 2004;67:448–450. doi: 10.1021/np030384h. [DOI] [PubMed] [Google Scholar]
- 119.Schwarz M., Kopcke B., Weber R.W.S., Sterner O., Anke H. 3-Hydroxypropionic acid as a nematicidal principle in endophytic fungi. Phytochemistry. 2004;65:2239–2245. doi: 10.1016/j.phytochem.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 120.Huang W.-Y., Cai Y.-Z., Hyde K.D., Corke H., Sun M. Endophytic fungi from Nerium oleander L (Apocynaceae): Main constituents and antioxidant activity. World J. Microbiol. Biotechnol. 2007;23:1253–1263 101007/s11274. doi: 10.1007/s11274-007-9357-z. [DOI] [Google Scholar]
- 121.Zhang Z.B., Zeng Q.G., Yan R.M., Wang Y., Zou Z.R., Zhu D. Endophytic fungus Cladosporium cladosporioides LF70 from Huperzia serrata produces Huperzine, A. World J. Microbiol. Biotechnol. 2011;27:479–486. doi: 10.1007/s11274-010-0476-6. [DOI] [Google Scholar]
- 122.Martìnez-Luis S., Della-Togna G., Coley P.D., Kursar T.A., Gerwick W.H., Cubilla-Rios L. Antileishmanial constituents of the Panamanian endophytic fungus Edenia sp. J. Nat. Prod. 2008;71:2011–2014. doi: 10.1021/np800472q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dai J., Krohn K., Draeger S., Schulz B. New Naphthalene-chroman coupling products from the endophytic fungus, Nodulisporium sp. from Erica arborea. Eur. J. Org. Chem. 2009;2009:1564–1569. doi: 10.1002/ejoc.200801106. [DOI] [Google Scholar]
- 124.Lösgen S., Magull J., Schulz B., Draeger S., Zeeck A. Isofusidienols: Novel chromone-3-oxepines produced by the endophytic fungus Chalara sp. Eur. J. Org. Chem. 2008;2008:698–703. doi: 10.1002/ejoc.200700839. [DOI] [Google Scholar]
- 125.Aly A.H., Edrada-Ebel R., Wray V., Müller W.E.G., Kozytska S., Hentschel U., Proksch P., Ebel R. Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides. Phytochemistry. 2008;69:1716–1725. doi: 10.1016/j.phytochem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 126.Kjer J., Wray V., Edrada-Ebel R.A., Ebel R., Pretsch A., Lin W.H., Proksch P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2009;72:2053–2057. doi: 10.1021/np900417g. [DOI] [PubMed] [Google Scholar]
- 127.Li E., Tian R., Liu S., Chen X., Guo L., Che Y. Pestalotheols AD, bioactive metabolites from the plant endophytic fungus Pestalotiopsis theae. J. Nat. Prod. 2008;71:664–668. doi: 10.1021/np700744t. [DOI] [PubMed] [Google Scholar]
- 128.Debbab A., Aly A.H., Edrada-Ebel R.A., Wray V., Müller W.E.G., Totzke F., Zirrgiebel U., Schächtele C., Kubbutat M.H.G., Lin W.H., et al. Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J. Nat. Prod. 2009;72:626–631. doi: 10.1021/np8004997. [DOI] [PubMed] [Google Scholar]
- 129.Aly A.H., Debbab A., Edrada-Ebel R., Wray V., Müller W.E., Lin W., Ebel R., Proksch P. A new tetrahydrofuran derivative from the endophytic fungus Chaetomium sp. isolated from Otanthus maritimus. Z Naturforsch C. 2009;64:350–354. doi: 10.1515/znc-2009-5-608. [DOI] [PubMed] [Google Scholar]
- 130.Ouyang J., Mao Z., Guo H., Xie Y., Cui Z., Sun J., Wu H., Wen X., Wang J., Shan T. Mollicellins O⁻R, four new Depsidones isolated from the endophytic fungus Chaetomium sp. Eef-10. Molecules. 2018;23:3218. doi: 10.3390/molecules23123218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aly A.H., Edrada-Ebel R., Indriani I.D., Wray V., Müller W.E.G., Frank T., Zirrgiebel U., Schächtele C., Kubbutat M.H.G., Lin W.H., et al. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J. Nat. Prod. 2008;71:972–980. doi: 10.1021/np070447m. [DOI] [PubMed] [Google Scholar]
- 132.Wijeratne E.M.K., Paranagama P.A., Marron M.T., Gunatilaka M.K., Arnold A.E., Gunatilaka A.A.L. Sesquiterpene quinones and related metabolites from Phyllosticta spinarum, a fungal strain endophytic in Platycladus orientalis of the Sonoran desert. J. Nat. Prod. 2008;71:218–222. doi: 10.1021/np070600c. [DOI] [PubMed] [Google Scholar]
- 133.Davis R.A., Longden J., Avery V.M., Healy P.C. The isolation, structure determination and cytotoxicity of the new fungal metabolite, trichodermamide C. Bioorg. Med. Chem. Lett. 2008;18:2836–2839. doi: 10.1016/j.bmcl.2008.03.090. [DOI] [PubMed] [Google Scholar]
- 134.Xu J., Kjer J., Sendker J., Wray V., Guan H., Edrada R.A., Müller W.E.G., Bayer M., Lin W.H., Wu J., et al. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorg Med. Chem. 2009;17:7362–7367. doi: 10.1016/j.bmc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 135.Xu J., Kjer J., Sendker J., Wray V., Guan H., Edrada R.A., Lin W.H., Wu J., Proksch P. Chromones from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. J. Nat. Prod. 2009;72:662–665. doi: 10.1021/np800748u. [DOI] [PubMed] [Google Scholar]
- 136.Singh A., Singh D.K., Kharwar R.N., White J.F., Gond S.K. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: Insights, avenues, and challenges. Microorganisms. 2021;9:197. doi: 10.3390/microorganisms9010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zeilinger S., García-Estrada C., Martín J.F. Fungal secondary metabolites in the “OMICS” Era. In: Zeilinger S., Martín J.F., García-Estrada C., editors. Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites. Springer; New York, NY, USA: 2015. pp. 1–12. [Google Scholar]
- 138.Li S.J., Zhang X., Wang X.H., Zhao C.Q. Novel natural compounds from endophytic fungi with anticancer activity. Eur. J. Med. Chem. 2018;156:316–343. doi: 10.1016/j.ejmech.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 139.Rosa L.H., Vieira M.L.A., Cota B.B., Johann S., Alves T.M.A., Zani C.L., Rosa C.A. Endophytic fungi of tropical forests: A promising source of bioactive prototype molecules for the treatment of neglected diseases. In Drug Development-a case study based insight into modern strategies, Chris Rundfeldt L. (eds), Intech Open, London, United Kingdom, 2011; pp. 469–486. ISBN 978-953-307-257-9
- 140.Mohammed S.I., Patil M.P., Patil R.H., Maheshwari V.L. Endophytes: Crop Productivity and Protection. Springer; Berlin/Heidelberg, Germany: 2017. Endophytes: Potential source of therapeutically important secondary metabolites of plant origin; pp. 95–110. [DOI] [Google Scholar]
- 141.Kusari S., Pandey S.P., Spiteller M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry. 2013;91:81–87. doi: 10.1016/j.phytochem.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 142.Porras-Alfaro A., Bayman P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011;49:291–315. doi: 10.1146/annurev-phyto-080508-081831. [DOI] [PubMed] [Google Scholar]
- 143.Nicoletti R., Fiorentino A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture. 2015;5:918–970. doi: 10.3390/agriculture5040918. [DOI] [Google Scholar]
- 144.Aly A.H., Debbab A., Kjer J., Proksch P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010;41:1–16. doi: 10.1007/s13225-010-0034-4. [DOI] [Google Scholar]
- 145.George T.S., Guru K.S.S., Vasanthi N.S., Kannan K.P. Extraction, purification and characterization of chitosan from endophytic fungi isolated from medicinal plants. World J. Sci. Technol. 2011;1:43–48. [Google Scholar]
- 146.Soliman S.S., Trobacher C.P., Tsao R., Greenwood J.S., Raizada M.N. A fungal endophyte induces transcription of genes encoding a redundant fungicide pathway in its host plant. BMC Plant Biol. 2013;13:93. doi: 10.1186/1471-2229-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khan A.L., Hamayun M., Hussain J., Kang S.-M., Lee I.-J. The newly isolated endophytic fungus Paraconiothyrium sp. LK1 produces ascotoxin. Molecules. 2012;17:1103–1112. doi: 10.3390/molecules17011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harper J.K., Barich D.H., Hu J.Z., Strobel G.A., Grant D.M. Stereochemical analysis by solid-state NMR: structural predictions in Ambuic acid. J. Org. Chem. 2003;68:12, 4609–4614. doi: 10.1021/jo020377i. [DOI] [PubMed] [Google Scholar]
- 149.Nakayama J., Uemura Y., Nishiguchi K., Yoshimura N., Igarashi Y., Sonomoto K. Ambuic acid inhibits the biosynthesis of cyclic peptide quormones in gram-positive bacteria. Antimicrob. Agents Chemother. 2009;53:580–586. doi: 10.1128/AAC.00995-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saxena S., Strobel G.A. Marvellous Muscodor spp.: Update on their biology and applications. Microb. Ecol. 2021;82:5–20. doi: 10.1007/s00248-020-01644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Devi P., Rodrigues C., Naik C.G., D’Souza L. Isolation and characterization of antibacterial compound from a Mangrove-endophytic fungus, Penicillium chrysogenum MTCC 5108. Indian J. Microbiol. 2012;52:617–623. doi: 10.1007/s12088-012-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang X., Wang H., Liu T., Xin Z. A PKS I gene-based screening approach for the discovery of a new polyketide from Penicillium citrinum Salicorn 46. Appl. Microbiol. Biotechnol. 2014;98:4875–4885. doi: 10.1007/s00253-014-5572-3. [DOI] [PubMed] [Google Scholar]
- 153.Redecker D., Kodner R., Graham L.E. Glomalean fungi from the Ordovician. Science. 2000;289:1920–1921. doi: 10.1126/science.289.5486.1920. [DOI] [PubMed] [Google Scholar]
- 154.Yu Q.Y., Fang L., Yun M.Q., Ji G.W., Rong S.H., Liang B.L. Endophytic fungi harbored in the root of Sophora tonkinensis Gapnep: Diversity and biocontrol potential against phytopathogens. Microbiol. Open. 2017;6:e437. doi: 10.1002/mbo3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schulz B., Rommert A.K., Dammann U., Aust H.J., Strack D. The endophyte-host interaction: A balanced antagonism? Mycol. Res. 1999;103:1275–1283. doi: 10.1017/S0953756299008540. [DOI] [Google Scholar]
- 156.Schulz B., Haas S., Junker C., Andrée N., Schobert M. Fungal endophytes are involved in multiple balanced antagonisms. Curr. Sci. 2015;109:39–45. [Google Scholar]
- 157.Yan L., Zhao H., Zhao X., Xu X., Di Y., Jiang C., Jin M. Production of bioproducts by endophytic fungi: Chemical ecology, biotechnological applications, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2018;102:6279–6298. doi: 10.1007/s00253-018-9101-7. [DOI] [PubMed] [Google Scholar]
- 158.Zamioudis C., Pieterse C.M. Modulation of host immunity by beneficial microbes. Mol. Plant Microbe Interact. 2012;25:139–150. doi: 10.1094/MPMI-06-11-0179. [DOI] [PubMed] [Google Scholar]
- 159.Wawra S., Fesel P., Widmer H., Timm M., Seibel J., Leson L., Kesseler L., Nostadt R., Hilbert M., Langen Zuccaro A.G. The fungal specific β-glucan-binding lectin FGB1 alters cell-wall composition and suppresses glucan-triggered immunity in plants. Nat. Commun. 2016;7:13188. doi: 10.1038/ncomms13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schulz B., Boyle C., Draeger S., Römmert A.-K., Krohn K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002;106:996–1004. doi: 10.1017/S0953756202006342. [DOI] [Google Scholar]
- 161.Xiong Z.Q., Yang Y.Y., Na Z., Yong W. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus x media. BMC Microbiol. 2013;13:71. doi: 10.1186/1471-2180-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang Y., Zhao H., Barrero R.A., Zhang B., Sun G., Wilson I.W., Xie F., Walker K.D., Parks J.W., Robert B. Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL 62431. BMC Genom. 2014;15:69. doi: 10.1186/1471-2164-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kusari S., Zühlke S., Spiteller M. Effect of artificial reconstitution of the interaction between the plant Camptotheca acuminata and the fungal endophyte Fusarium solani on Camptothecin biosynthesis. J. Nat. Prod. 2011;74:764–775. doi: 10.1021/np1008398. [DOI] [PubMed] [Google Scholar]
- 164.Strobel G., Daisy B., Castillo U., Harper J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004;67:257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 165.Gunatilaka A.A. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006;69:509–526. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aghdam S.A., Brown A.M.V. Deep learning approaches for natural product discovery from plant endophytic microbiomes. Environ. Microbiome. 2021;16:6. doi: 10.1186/s40793-021-00375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kusari P., Kusari S., Spiteller M., Kayser O. Implications of endophyte-plant crosstalk in light of quorum responses for plant biotechnology. Appl. Microbiol. Biotechnol. 2015;99:5383–5390. doi: 10.1007/s00253-015-6660-8. [DOI] [PubMed] [Google Scholar]
- 168.Pan R., Bai X., Chen J., Zhang H., Wang H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: A literature review. Front. Microbiol. 2019;10:294. doi: 10.3389/fmicb.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dias D.A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Baral B., Akhgari A., Metsä-Ketelä M. Activation of microbial secondary metabolic pathways: Avenues and challenges. Synth. Syst. Biotechnol. 2018;3:163–178. doi: 10.1016/j.synbio.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Akone S.H., Pham C.-D., Chen H., Ola A.R.B., Ntie-Kang F., Proksch P. Epigenetic modification, co-culture and genomic methods for natural product discovery. Phys. Sci. Rev. 2018;4:1–13. doi: 10.1515/psr-2018-0118. [DOI] [Google Scholar]
- 172.El-Sayed A.S.A., Mohamed N.Z., Safan S., Yassin M.A., Shaban L., Shindia A.A., Ali G.S., Sitohy M.Z. Restoring the taxol biosynthetic machinery of Aspergillus terreus by Podocarpus gracilior pilger microbiome, with retrieving the ribosome biogenesis proteins of WD40 superfamily. Sci. Rep. 2019;9:11534. doi: 10.1038/s41598-019-47816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Corre C., Song L., O’Rourke S., Chater K.F., Challis G.L. 2-Alkyl-4-hydroxymethylfuran-3-carboxylic acids, antibiotic production inducers discovered by Streptomyces coelicolor genome mining. Proc. Natl. Acad. Sci. USA. 2008;105:17510–17515. doi: 10.1073/pnas.0805530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Anyaogu D.C., Mortensen U.H. Heterologous production of fungal secondary metabolites in Aspergilli. Front. Microbiol. 2015;6:1–6. doi: 10.3389/fmicb.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guo F., Xiang S., Li L., Wang B., Rajasärkkä J., Gröndahl-Yli-Hannuksela K., Ai G., Metsä-Ketelä M., Yang K. Targeted activation of silent natural product biosynthesis pathways by reporter-guided mutant selection. Metab. Eng. 2015;28:134–142. doi: 10.1016/j.ymben.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 176.Xu F., Wu Y., Zhang C., Davis K.M., Moon K., Bushin L.B., Seyedsayamdost M.R. A genetics-free method for high-throughput discovery of cryptic microbial metabolites. Nat. Chem. Biol. 2019;15:161–168. doi: 10.1038/s41589-018-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hoffmeister D., Keller N.P. Natural products of filamentous fungi: Enzymes, genes, and their regulation. Nat. Prod. Rep. 2007;24:393–416. doi: 10.1039/B603084J. [DOI] [PubMed] [Google Scholar]
- 178.Koo T., Lee J., Hwang S. Development of an interspecies interaction model: An experiment on Clostridium cadaveris and Clostridium sporogenes under anaerobic condition. J. Environ. Manag. 2019;237:247–254. doi: 10.1016/j.jenvman.2019.02.084. [DOI] [PubMed] [Google Scholar]
- 179.Ravikrishnan A., Nasre M., Raman K. Enumerating all possible biosynthetic pathways in metabolic networks. Sci. Rep. 2018;8:9932. doi: 10.1038/s41598-018-28007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Toju H., Tanabe A.S., Sato H. Network hubs in root-associated fungal metacommunities. Microbiome. 2018;6:116. doi: 10.1186/s40168-018-0497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hannigan G.D., Prihoda D., Palicka A., Soukup J., Klempir. O., Rampula L., Durcak J., Wurst M., Kotowski J., Chang D., et al. A deep learning genome-mining strategy for biosynthetic gene cluster prediction. Nucleic Acids Res. 2019;47:e110. doi: 10.1093/nar/gkz654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ichikawa N., Sasagawa M., Yamamoto M., Komaki H., Yoshida Y., Yamazaki S., Fujita N. DoBISCUIT: A database of secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 2013;41:408–414. doi: 10.1093/nar/gks1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Starcevic A., Zucko J., Simunkovic J., Long P.F., Cullum J., Hranueli D. ClustScan: An integrated program package for the semi-automatic annotation of modular biosynthetic gene clusters and in silico prediction of novel chemical structures. Nucleic Acids Res. 2008;36:6882–6892. doi: 10.1093/nar/gkn685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Conway K.R., Boddy C.N. ClusterMine360: A database of microbial PKS/NRPS biosynthesis. Nucleic Acids Res. 2013;41:402–407. doi: 10.1093/nar/gks993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Epstein S.C., Charkoudian L.K., Medema M.H. A standardized workflow for submitting data to the minimum information about a biosynthetic gene cluster (MIBiG) repository: Prospects for research-based educational experiences. Stand. Genomic Sci. 2018;13:16. doi: 10.1186/s40793-018-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang H., Marcišauskas S., Sánchez B.J., Domenzain I., Hermansson D., Agren R., Nielsen J., Kerkhoven E.J. RAVEN 2.0: A versatile toolbox for metabolic network reconstruction and a case study on Streptomyces coelicolor. PLoS Comput. Biol. 2018;14:1–17. doi: 10.1371/journal.pcbi.1006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Blin K., Shaw S., Steinke K., Villebro R., Ziemert N., Lee S.Y., Medema M.H., Weber T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47:W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Latendresse M., Krummenacker M., Trupp M., Karp P.D. Construction and completion of flux balance models from pathway databases. Bioinformatics. 2012;28:388–396. doi: 10.1093/bioinformatics/btr681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Faust K., Raes J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012;10:538–550. doi: 10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- 190.Stolyar S., Van Dien S., Hillesland K.L., Pinel N., Lie T.J., Leigh J.A., Stahl D.A. Metabolic modeling of a mutualistic microbial community. Mol. Syst. Biol. 2007;3:92. doi: 10.1038/msb4100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ravikrishnan A., Blank L.M., Srivastava S., Raman K. Investigating metabolic interactions in a microbial co-culture through integrated modelling and experiments. Comput. Struct. Biotechnol J. 2020;18:1249–1258. doi: 10.1016/j.csbj.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mori T., Cahn J.K.B., Wilson M.C., Meoded R.A., Wiebach V., Martinez A.F.C., Helfrich E.J.N., Albersmeier A., Wibberg D., Dätwyler S., et al. Single-bacterial genomics validates rich and varied specialized metabolism of uncultivated Entotheonella sponge symbionts. Proc. Natl. Acad. Sci. USA. 2018;115:1718–1723. doi: 10.1073/pnas.1715496115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kuang X., Sun S., Wei J., Li Y., Sun C. Iso-Seq analysis of the Taxus cuspidata transcriptome reveals the complexity of Taxol biosynthesis. BMC Plant Biol. 2019;19:210. doi: 10.1186/s12870-019-1809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. [(accessed on 12 July 2021)]; Available online: https://dtp.cancer.gov/timeline/flash/success_stories/s2_taxol.
- 195. [(accessed on 12 July 2021)]. Available online: https://en.wikipedia.org/wiki/Paclitaxel.
- 196.Puri S.C., Verma V., Amna T., Qazi G.N., Spiteller M. An endophytic fungus from Nothapodytes foetida that produces camptothecin. J. Nat. Prod. 2005;68:1717–1719. doi: 10.1021/np0502802. [DOI] [PubMed] [Google Scholar]
- 197.Shweta S., Gurumurthy B.R., Ravikanth G., Ramanan U.S., Shivanna M.B. Endophytic fungi from Miquelia dentata Bedd., produce the anti-cancer alkaloid, camptothecine. Phytomedicine. 2013;20:337–342. doi: 10.1016/j.phymed.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 198.Guo B., Li H., Zhang L. Isolation of a fungus producing vinblastine. J. Yunnan Univ. 1998;20:214–215. [Google Scholar]
- 199.Lingqi Z., Bo G., Haiyan L., Songrong Z., Hua S., Su G., Rongcheng W. Preliminary study on the isolation of endophytic fungus of Catharanthus roseus and its fermentation to produce products of therapeutic value. Zhong Cao Yao. 2000;31:805–807. [Google Scholar]
- 200.Eyberger A.L., Dondapati R., Porter J.R. Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin. J. Nat. Prod. 2006;69:1121–1124. doi: 10.1021/np060174f. [DOI] [PubMed] [Google Scholar]
- 201.Kumara P.M., Zuehlke S., Priti V., Ramesha B.T., Shweta S., Ravikanth G., Vasudeva R., Santhoshkumar T.R., Spiteller M., Shaanker R.U. Fusarium proliferatum, an endophytic fungus from Dysoxylum binectariferum Hook. f, produces rohitukine, a chromane alkaloid possessing anti-cancer activity. Anton. Leeuw. 2012;101:323–329. doi: 10.1007/s10482-011-9638-2. [DOI] [PubMed] [Google Scholar]
- 202.Verma V.C., Lobkovsky E., Gange A.C., Singh S.K., Prakash S. Piperine production by endophytic fungus Periconia sp. isolated from Piper longum L. J. Antibiot. 2011;64:427–431. doi: 10.1038/ja.2011.27. [DOI] [PubMed] [Google Scholar]
- 203.Pompeng P., Sommit D., Sriubolmas N., Ngamrojanavanich N., Matsubara K., Pudhom K. Antiangiogenetic effects of anthranoids from Alternaria sp.; an endophytic fungus in a Thai medicinal plant Erythrina variegata. Phytomedicine. 2013;20:918–922. doi: 10.1016/j.phymed.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 204.Su J., Liu H., Guo K., Chen L., Yang M., Chen Q. Research advances and detection methodologies for microbe-derived acetylcholinesterase inhibitors: A systemic review. Molecules. 2017;22:176. doi: 10.3390/molecules22010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Isaka M., Jaturapat A., Rukseree K., Danwisetkanjana K., Tanticharoen M., Thebtaranonth Y. Phomoxanthones A and B, novel xanthone dimers from the endophytic fungus Phomopsis species. J. Nat. Prod. 2001;64:1015–1018. doi: 10.1021/np010006h. [DOI] [PubMed] [Google Scholar]
- 206.Maehara S., Simanjuntak P., Kitamura C., Ohashi K., Shibuya H. Cinchona alkaloids are also produced by an endophytic filamentous fungus living in cinchona plant. Chem. Pharm. Bull. 2011;59:1073–1074. doi: 10.1248/cpb.59.1073. [DOI] [PubMed] [Google Scholar]
- 207.El-Elimat T., Raja H.A., Graf T.N., Faeth S.H., Cech N.B., Oberlies N.H. Flavonolignans from Aspergillus iizukae, a fungal endophyte of milk thistle (Silybum marianum) J. Nat. Prod. 2014;77:193–199. doi: 10.1021/np400955q. [DOI] [PubMed] [Google Scholar]
- 208.Malik S., Cusidó R.M., Mirjalili M.H., Moyano E., Palazón J., Bonfill M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review. Process Biochem. 2011;46:23–34. doi: 10.1016/j.procbio.2010.09.004. [DOI] [Google Scholar]
- 209.Strobel G., Stierle A., Hess W.M. Taxol formation in yew-Taxus. Plant Sci. 1993;92:1–12. doi: 10.1016/0168-9452(93)90060-D. [DOI] [Google Scholar]
- 210. [(accessed on 12 July 2021)]. Available online: https://menafn.com/1100419587/Global-Paclitaxel-Sales-Market-Report-2020.
- 211.Schiff P.B., Horwitz S.B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Strobel G.A., Stierle A., Stierle D., Hess W.M. Taxomyces andreanae a proposed new taxon for a bulbilliferous hyphomycete associated with Pacific yew. Mycotaxon. 1993;47:71–78. [Google Scholar]
- 213.Garyali S., Kumar A., Reddy M.S. Enhancement of taxol production from endophytic fungus Fusarium redolens. Biotechnol. Bioprocess Eng. 2014;19:908–915. doi: 10.1007/s12257-014-0160-z. [DOI] [Google Scholar]
- 214.Garyali S., Kumar A., Reddy M.S. Diversity and antimitotic activity of taxol-producing endophytic fungi isolated from Himalayan yew. Ann. Microbiol. 2014;64:1413–1422. doi: 10.1007/s13213-013-0786-7. [DOI] [Google Scholar]
- 215.Kharwar N.R., Mishra A., Gond K., Stierle S., Stierle A. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011;28:1208–1228. doi: 10.1039/c1np00008j. [DOI] [PubMed] [Google Scholar]
- 216.Hao X., Pan J., Zhu X. Taxol producing fungi. In: Ramawat K.G., Merillon J.M., editors. Natural products. Springer; Berlin, Germany: 2013. pp. 2797–2812. [Google Scholar]
- 217.Shankar Naik B. Functional roles of fungal endophytes in host fitness during stress conditions. Symbiosis. 2019;79:99–115. doi: 10.1007/s13199-019-00635-1. [DOI] [Google Scholar]
- 218.Wang J.F., Li G.L., Lu H.Y., Zheng Z., Huang Y., Su W. Taxol from Tubercularia sp. strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol. Lett. 2000;193:249–253. doi: 10.1111/j.1574-6968.2000.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 219.Sun D.F., Ran X.Q., Wang J.F. Isolation and identification of a taxol-producing endophytic fungus from Podocarpus. Acta Microbiol. Sin. 2008;48:589–595. [PubMed] [Google Scholar]
- 220.Zhou X., Zhu H., Liu L., Lin J., Tang K. A review: Recent advances and future prospects of taxol-producing endophytic fungi. Appl. Microbiol. Biotechnol. 2010;86:1707–1717. doi: 10.1007/s00253-010-2546-y. [DOI] [PubMed] [Google Scholar]
- 221.Zhao K., Sun L., Wang X., Li X., Wang X., Zhou D. Screening of high taxol producing fungi by mutagenesis and construction of subtracted cDNA library by Suppression substracted hybridization for differentially expressed genes. Acta Microbiol. Sin. 2011;51:923–PMID: 22043793. [PubMed] [Google Scholar]
- 222.Zhao K., Lu Y., Jin Y., Ma X., Liu D., Wang X., Wang X. Advances and prospects of taxol biosynthesis by endophytic fungi. Chin. J. Biotech. 2016;2532:1039–1051. doi: 10.13345/j.cjb.150519. [DOI] [PubMed] [Google Scholar]
- 223.Flores-Bustamante Z., Rivera-Orduña F.N., Martinez-Cárdenas A., Flores-Cotera L.B. Microbial paclitaxel: Advances and perspectives. J. Antibiot. 2010;63:460–467. doi: 10.1038/ja.2010.83. [DOI] [PubMed] [Google Scholar]
- 224.Soliman S.S.M., Raizada M.N. Interactions between co-habitating fungi elicit synthesis of taxol from an endophytic fungus in host Taxus plants. Front. Microbiol. 2013;4:1–14. doi: 10.3389/fmicb.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Beltrametti F., Barucco D., Rossi R., Selva E., Marinelli F. Protoplast fusion and gene recombination in the uncommon Actinomycete Planobispora rosea producing GE2270. J. Antibiot. 2007;60:447–454. doi: 10.1038/ja.2007.57. [DOI] [PubMed] [Google Scholar]
- 226.Michielse C.B., Hooykaas P.J.J., van den Hodel C., Ram A. Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori. Nat. Protocol. 2008;3:1671–1678. doi: 10.1038/nprot.2008.154. [DOI] [PubMed] [Google Scholar]
- 227.Meyer V. Genetic engineering of filamentous fungi-progress, obstacles and future trends. Biotechnol. Adv. 2008;26:177–185. doi: 10.1016/j.biotechadv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 228.El-Sayed A.S.A., Abdel Ghany S.E., Ali G.S. Genome editing approaches: Manipulating of lovastatin and taxol synthesis of filamentous fungi by CRISPR/Cas9 system. Appl. Microbiol. Biotechnol. 2017;101:3953–3976. doi: 10.1007/s00253-017-8263-z. [DOI] [PubMed] [Google Scholar]
- 229.Wall M.E., Wani M.C., Cook C.E., Palmer K.H., McPhail A.I., Sim G.A. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966;88:3888–3890. doi: 10.1021/ja00968a057. [DOI] [Google Scholar]
- 230.Samuelsson G. Drugs of Natural Origin: A Textbook of Pharmacognosy, 5 ed. Pharmaceutical Press; Stokkholm, Sweden: 2004. [Google Scholar]
- 231.Kusari S., Zühlke S., Spiteller M. An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J. Nat. Prod. 2009;72:2–7. doi: 10.1021/np800455b. [DOI] [PubMed] [Google Scholar]
- 232.Pizzolato J.F., Saltz L.B. The camptothecins. Lancet. 2003;361:2235–2242. doi: 10.1016/S0140-6736(03)13780-4. [DOI] [PubMed] [Google Scholar]
- 233.Musiol R. An overview of quinoline as a privileged scaffold in cancer drug discovery. Expert Opin. Drug Discov. 2017;12:583–597. doi: 10.1080/17460441.2017.1319357. [DOI] [PubMed] [Google Scholar]
- 234.Isah T., Mujib A. Camptothecin from Nothapodytes nimmoniana: Review on biotechnology applications. Acta Physiol. Plant. 2015;37:106. doi: 10.1007/s11738-015-1854-3. [DOI] [Google Scholar]
- 235.Watase I., Sudo H., Yamazaki M., Saito K. Regeneration of transformed Ophiorrhiza pumila plants producing camptothecin. Plant Biotechnol. 2004;21:337–342. doi: 10.5511/plantbiotechnology.21.337. [DOI] [Google Scholar]
- 236.Pu X., Qu X., Chen F., Bao J., Zhang G., Luo Y. Camptothecin-producing endophytic fungus Trichoderma atroviride LY357: Isolation, identification, and fermentation conditions optimization for camptothecin production. Appl. Microbiol. Biotechnol. 2013;97:9365–9375. doi: 10.1007/s00253-013-5163-8. [DOI] [PubMed] [Google Scholar]
- 237.Clarance P., Khusro A., Lalitha J., Sales J., Paul A. Optimization of camptothecin production and biomass yield from endophytic fungus Fusarium solani strain ATLOY-8. J. Appl. Pharm. Sci. 2019;9:35–46. doi: 10.7324/JAPS.2019.91005. [DOI] [Google Scholar]
- 238.Rehman S., Shawl A.S., Kour A., Andrabi R., Sudan P., Sultan P., Verma V., Qazi G.N. An endophytic Neurospora sp. from Nothapodytes foetida producing camptothecin. Appl. Biochem. Microbiol. 2008;44:203–209. doi: 10.1134/S0003683808020130. [DOI] [PubMed] [Google Scholar]
- 239.Mohinudeen I.A.H.K., Kanumuri R., Soujanya K.N., Soujanya K.N., Shaanker R.U., Srivastava S. Sustainable production of camptothecin from an Alternaria sp. isolated from Nothapodytes nimmoniana. Sci. Rep. 2021;11:1478. doi: 10.1038/s41598-020-79239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ruan Q., Patel G., Wang J., Luo E., Zhou W., Sieniawska E., Hao X., Kai G. Current advances of endophytes as a platform for production of anti-cancer drug camptothecin. Food Chem. Toxicol. 2021;151:112113. doi: 10.1016/j.fct.2021.112113. [DOI] [PubMed] [Google Scholar]
- 241.World Health Organization Model List of Essential Medicines. 2019. [(accessed on 23 July 2021)]. Available online: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists.
- 242.Venugopalan A., Srivastava S. Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol. Adv. 2015;33:873–887. doi: 10.1016/j.biotechadv.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 243.Balandrin M.J., Klocke J.A. Medicinal, aromatic and industrial materials from plants. In: Bajaj Y.P.S., editor. Biotechnology in Agriculture and Forestry: Medicinal and Aromatic Plant. Volume 4. Springer; Berlin, Germany: 1988. pp. 1–36. [Google Scholar]
- 244.Palem P.P., Kuriakose G.C., Jayabaskaran C. An endophytic fungus, Talaromyces radicus, isolated from Catharanthus roseus, produces vincristine and vinblastine, which induce apoptotic cell death. PLoS ONE. 2015;10:e0144476. doi: 10.1371/journal.pone.0144476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Majumder A., Jha S. Biotechnological approaches for the production of potential anticancer leads podophyllotoxin and paclitaxel: An overview. J. Biol. Sci. 2009;1:46–69. [Google Scholar]
- 246.Gordaliza M., Castro M.A., García-Grávalos M.D., Ruiz P., Miguel del Corral J.M., Feliciano A.S. Antineoplastic and Antiviral activities of podophyllotoxin related lignans. Arch. Pharm. 1994;327:175–179. doi: 10.1002/ardp.19943270309. [DOI] [PubMed] [Google Scholar]
- 247.Abd-Elsalam K.A., Hashim A.F. Hidden Fungi as microbial and nano-Factories for anticancer agents. Fungal Genom Biol. 2013;3:e115. doi: 10.4172/2165-8056.1000e115. [DOI] [Google Scholar]
- 248.Kusari S., Lamshoft M., Spiteller M. Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009;107:1019–1030. doi: 10.1111/j.1365-2672.2009.04285.x. [DOI] [PubMed] [Google Scholar]
- 249.Ochoa-Villarreal M., Howat S., Hong S., Jang M.O., Jin Y.W., Lee E.K., Loake G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016;49:149–158. doi: 10.5483/BMBRep.2016.49.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cortés F., Pastor N. Induction of endoreduplication by topoisomerase II catalytic inhibitors. Mutagenesis. 2003;18:105–112. doi: 10.1093/mutage/18.2.105. [DOI] [PubMed] [Google Scholar]
- 251.Nitiss J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer. 2009;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Froelich-Ammon S.J., Osheroff N. Topoisomerase poisons: Harnessing the dark side of enzyme mechanism. J. Biol. Chem. 1995;270:21429–21432. doi: 10.1074/jbc.270.37.21429. [DOI] [PubMed] [Google Scholar]
- 253.Kour A., Shawl A.S., Rehman S., Sultan P., Qazi P.H., Suden P., Khajuria R.K., Verma V. Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J. Microbiol. Biotechnol. 2008;24:1115–1121. doi: 10.1007/s11274-007-9582-5. [DOI] [Google Scholar]
- 254.Huang J.-X., Zhang J., Zhang X.-R., Zhang K., Zhang X., He X.-R. Mucor fragilis as a novel source of the key pharmaceutical agents podophyllotoxin and kaempferol. Pharm. Biol. 2014;52:1237–1243. doi: 10.3109/13880209.2014.885061. [DOI] [PubMed] [Google Scholar]
- 255.Charlwood B.V., Rhodes M.J. Secondary Products from Plant Tissue Culture. Clarendon Press; New York, NY, USA: 1990. [Google Scholar]
- 256.Eaton C.J., Cox M.P., Scott B. What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci. 2011;180:190–195. doi: 10.1016/j.plantsci.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 257.Ahamed A., Ahring B.K. Production of hydrocarbon compounds by endophytic fungi Gliocladium species grown on cellulose. Bioresour. Technol. 2011;102:9718–9722. doi: 10.1016/j.biortech.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 258.Elgendy M.M.A.A., Alzahrani H.A.A., Elbondkly A.M.A. Genome shuffling of Mangrove endophytic Aspergillus luchuensis MERV10 for improving the cholesterol-lowering agent lovastatin under solid state fermentation. Mycobiology. 2016;44:171–179. doi: 10.5941/myco.2016.44.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhao K., Xiao Y., Wang C., Liu D., Zhang Y., Wang X., Li X., Jin T. Screening of taxol biosynthesis-related genes in taxol produced from Nodulisporium sylviforme HDF-68 by mRNA differential display. Ann. Microbiol. 2014;64:1633–1642. doi: 10.1007/s13213-014-0807-1. [DOI] [Google Scholar]
- 260.Wang M., Zhang W., Xu W., Shen Y., Du L. Optimization of genome shuffling for high-yield production of the antitumor deacetylmycoepoxydiene in an endophytic fungus of mangrove plants. Appl. Microbiol. Biotechnol. 2016;100:1–8. doi: 10.1007/s00253-016-7457-0. [DOI] [PubMed] [Google Scholar]
- 261.Long D.M., Smidmansky E.D., Archer A.J., Strobel G.A. In vivo addition of telomeric repeats to foreign DNA generates chromosomal DNAs in the taxol-producing fungus Pestalotiopsis microspora. Fungal Genet. Biol. 1998;24:335–344. doi: 10.1006/fgbi.1998.1065. [DOI] [PubMed] [Google Scholar]
- 262.Wang Y., Guo B., Miao Z., Tang K. Transformation of taxol-producing endophytic fungi by restriction enzyme-mediated integration (REMI) FEMS Microbiol. Lett. 2007;273:253–259. doi: 10.1111/j.1574-6968.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- 263.Liu L., Wei Y.M., Zhou X.W., Lin J., Sun X.F., Tang K.X. Agrobacterium tumefaciens mediated genetic transformation of the Taxol-producing endophytic fungus Ozonium sp. EFY21. Genet. Mol. Res. 2013;12:2913. doi: 10.4238/2013.August.12.7. [DOI] [PubMed] [Google Scholar]
- 264.Wei Y., Zhou X., Liu L., Lu J., Wang Z., Yu G., Hu L., Lin J., Sun X., Tang K. An efficient transformation system of taxol-producing endophytic fungus EFY-21 (Ozonium sp.) Afr. J. Biotechnol. 2010;9:1726–1733. doi: 10.5897/AJB2010.000-3019. [DOI] [Google Scholar]
- 265.Bian G., Yuan Y., Tao H., Shi X., Zhong X., Han Y., Fu S., Fang C., Deng Z., Liu T. Production of taxadiene by engineering of mevalonate pathway in Escherichia coli and endophytic fungus Alternaria alternata TPF6. Biotechnol. J. 2017;12:1600697. doi: 10.1002/biot.201600697. [DOI] [PubMed] [Google Scholar]
- 266.Fidan O., Zhan J. Discovery and engineering of an endophytic Pseudomonas strain from Taxus chinensis for efficient production of zeaxanthin diglucoside. J. Biol. Eng. 2019;13:66. doi: 10.1186/s13036-019-0196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Barrios-Gonzalez J., Fernandez F.J., Tomasini A. Microbial secondary metabolites production and strain improvement. Indian J. Biotechnol. 2003;2:322–333. [Google Scholar]
- 268.Adrio J.L., Demain A.L. Genetic improvement of processes yielding microbial products. FEMS Microbiol. Rev. 2006;30:187–214. doi: 10.1111/j.1574-6976.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- 269.Kai Z., Qingshen S., Yanjun Z., Wenxiang P., Tao J., Dongpo Z. Screening and characterization of a high taxol producing fungus by protoplast mutagenesis. High Technol. Lett. 2009;15:220–226. doi: 10.3772/j.issn.1006-6748.2009.02.020. [DOI] [Google Scholar]
- 270.Xu F., Tao W.Y., Cheng L., Guo L.J. Formation and regeneration of protoplasts of taxol-producing endophytic fungus Fusarium maire. J. Food Sci. Biotechnol. 2006;25:20–24. [Google Scholar]
- 271.Zhao K., Zhou D.P., Ping W.X., Ge J. Study on the preparation and regeneration of protoplast from taxol-producing fungus Nodulisporium sylviforme. Nat. Sci. 2004;2:52–59. [Google Scholar]
- 272.Zhou X., Wei Y., Zhu H., Wang Z., Lin J., Liu L., Tang K. Protoplast formation, regeneration and transformation from the taxol-producing fungus Ozonium sp. Afr. J. Biotechnol. 2008;7:2017–2024. doi: 10.5897/AJB2008.000-5050. [DOI] [Google Scholar]
- 273.Zhou D., Zhao K., Ping W., Ge J., Ma X., Jun L. Study on the mutagenesis of protoplasts from taxol-producing fungus Nodulisporium sylviforme. J. Am. Sci. 2005;1:62. [Google Scholar]
- 274.Scherlach K., Hertweck C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009;7:1753–1760. doi: 10.1039/b821578b. [DOI] [PubMed] [Google Scholar]
- 275.Bhalkar B.N., Patil S.M., Govindwar S.P. Camptothecin production by mixed fermentation of two endophytic fungi from Nothapodytes nimmoniana. Fungal Biol. 2016;120:873–883. doi: 10.1016/j.funbio.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 276.Elmoslamy S.H., Elkady M.F., Rezk A.H., Abdelfattah Y.R. Applying taguchi design and large-scale strategy for mycosynthesis of nanosilver from endophytic Trichoderma harzianum SYA.F4 and its application against phytopathogens. Sci. Rep. 2017;7:45297. doi: 10.1038/srep45297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bode H.B., Bethe B., Höfs R., Zeeck A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chembiochem. 2002;3:619–627. doi: 10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 278.Thiry M., Cingolani D. Optimizing scale-up fermentation processes. Trends Biotechnol. 2002;20:103–105. doi: 10.1016/S0167-7799(02)01913-3. [DOI] [PubMed] [Google Scholar]
- 279.Wang Y., Li H., Feng G., Du L., Zeng D. Biodegradation of diuron by an endophytic fungus Neurospora intermedia DP8-1 isolated from sugarcane and its potential for remediating diuron-contaminated soils. PLoS ONE. 2017;12:e0182556. doi: 10.1371/journal.pone.0182556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gracidarodríguez J., Gómezvaladez A., Tovarjiménez X., Amaroreyes A., Aranacuenca A., Zamudiopérez E. Optimization of the biosynthesis of naphthoquinones by endophytic fungi isolated of Ferocactus latispinus. Biologia. 2017;72:1416–1421. doi: 10.1515/biolog-2017-0177. [DOI] [Google Scholar]
- 281.Bhalkar B.N., Bedekar P.A., Kshirsagar S.D., Govindwar S.P. Solid state fermentation of soybean waste and an up-flow column bioreactor for continuous production of camptothecine by an endophytic fungus Fusarium oxysporum. RSC Adv. 2016;6:56527–56536. doi: 10.1039/C6RA08956A. [DOI] [Google Scholar]
- 282.Soliman S.S.M., Raizada M.N. Darkness: A crucial factor in fungal taxol production. Front. Microbiol. 2018;9:353. doi: 10.3389/fmicb.2018.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pettit R.K. Small-molecule elicitation of microbial secondary metabolites. Microb. Biotechnol. 2011;4:471–478. doi: 10.1111/j.1751-7915.2010.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Brakhage A.A., Schroeckh V. Fungal secondary metabolites-strategies to activate silent gene clusters. Fungal Genet. Biol. 2011;48:15–22. doi: 10.1016/j.fgb.2010.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.