Abstract
This single-institute, retrospective cohort study enrolled patients with human epidermal growth factor receptor 2-positive metastatic breast cancer treated with trastuzumab deruxtecan between August 2017 and January 2021 from four previous studies. Of 31 patients, 4 (12.9%) had interstitial lung disease (ILD). The dominant pattern observed on computed tomography was organizing pneumonia (100%), comprising subpleural consolidations in the lung periphery. However, no dominant distribution was observed in radiological lesions of the lungs. Of all the tested patients, lower lobe predominance was noted in 2 (50.0%) patients, upper lobe predominance in 1 (25.0%) patient, and diffused lobe distribution in 1 (25.0%) patient. All events were confined to the Common Terminology Criteria for Adverse Events grade 1 or 2 (100%). None of the patients died. Despite the small number of cases investigated, the incidence of trastuzumab deruxtecan-induced ILD in the Korean population was comparable to that previously reported.
Keywords: Breast Neoplasms; Genes, erbB-2; Lung Diseases, Interstitial; Tomography, X-ray Computed; Trastuzumab
Metastatic human epidermal growth factor receptor 2 (HER2)-positive breast cancer is associated with poor prognosis [1]. Although several anti-HER2 therapies have been developed to improve clinical outcomes, no uniformly accepted third-line therapy has been approved for use after the administration of trastuzumab emtansine, a standard second-line therapy [2,3]. Trastuzumab deruxtecan (DS-8201) is a novel HER2-targeted antibody–drug conjugate comprising a humanized monoclonal antibody that targets HER2, a cleavable tetrapeptide-based linker, and a potent topoisomerase I inhibitor payload [4]. A recent phase 2 study of this drug showed durable antitumor activity in patients with metastatic HER2-positive breast cancer who previously underwent treatment with trastuzumab emtansine [5]. However, interstitial lung disease (ILD) was observed in approximately 14% of the tested patients, including four cases of death (2.2%) attributable to this adverse event. In another phase 2 study of HER2-positive gastric cancer, ILD developed in 10% of patients treated with trastuzumab deruxtecan [6]. Although chest computed tomography (CT) is considered the primary imaging technique for the early diagnosis of ILD, the recommended CT monitoring protocol for trastuzumab deruxtecan-induced ILD has not been established, and its radiological descriptions have not been fully investigated [7]. In particular, no studies have been conducted on Korean populations. Therefore, in this study, we elaborated the pulmonary CT findings of trastuzumab deruxtecan-induced ILD in Korean patients diagnosed with metastatic breast cancer who were treated with trastuzumab deruxtecan.
We retrospectively reviewed the data from four studies (NCT03248492, NCT03523585, NCT03529110, and NCT03734029) in patients treated with trastuzumab deruxtecan at XXX between August 2017 and January 2021. All subjects received a 5.4 mg/kg dose of fam-trastuzumab deruxtecan every 3 weeks, and the disease status was evaluated every 6 weeks. Diagnostic work-up, including CT scan, was also performed if a patient presented with acute onset of pulmonary signs/symptoms. Demographic and radiographic data were obtained by reviewing medical records. This study was approved by the Institutional Review Board of Asan Medical Center in Seoul, Korea (IRB No. 2021-1068). Moreover, as this study was retrospectively conducted using medical records, the need for informed consent was waived.
CT scans were obtained using a SOMATOM definition AS CT scanner (Siemens Healthineers, Erlangen, Germany). The reconstruction intervals were 5 mm, with another 5-mm interval without a gap for the B50 algorithm, and a 1-mm reconstruction of the 5-mm gap for the B60 algorithm [8,9]. Imaging was conducted at sustained full inspiration, and the scanning area ranged from the supraclavicular area to the adrenal gland level. Furthermore, the scans were obtained using 120 kVp and 100 effective mAs with dose modulation. All images were then interpreted and photographed at window settings for the pulmonary parenchyma (width, 1500 HU; level, −700 HU) and mediastinum (width, 450 HU; level, 50 HU).
Three observers (a thoracic radiologist, a pulmonologist, and a medical oncologist) reviewed the CT images and assessed the presence of ground-glass attenuation, consolidation, nodules, and linear opacity, including septal thickening [10]. Additionally, the overall distribution of abnormalities was assessed. Zonal predominance was divided into upper and lower zones. Upper lung zone predominance was considered when most of the abnormalities were above the tracheal carinal level; lower zone predominance was considered when most abnormalities were below that level. Lateral predominance (unilateral or bilateral) was also assessed. The axial distribution was regarded as predominantly central if most abnormalities were in the inner third of the lung; peripheral distribution was marked when abnormalities were seen mostly in the outer third of the lung. The presence of subpleural sparing, defined as relative sparing of the lung immediately adjacent to the pleura, was noted [11]. Pre-existing lung lesions, such as metastatic lung nodules, fibrotic lesions, and pleural effusions, were also recorded.
If the oncologist suspected ILD in patients who received trastuzumab deruxtecan, they consulted an experienced thoracic radiologist and a pulmonologist who examined the CT imaging and suggested further diagnostic work-up, as needed. The same pulmonologist confirmed the ILD diagnosis by considering the patient’s symptoms, physical examination findings, medical history, laboratory findings, microbiological test results, and CT findings based on a drug-induced lung injury guideline [7].
Subsequently, 31 patients were found to have been treated using trastuzumab deruxtecan. The clinical features of the patients are summarized in Table 1. The median age of the patients was 54 years (interquartile range [IQR], 47.0–60.0 years). Furthermore, most of the patients (96.8%) were women. According to the patients’ medical records, two (6.5%) patients had a history of smoking, one (3.2%) had a history of chronic obstructive pulmonary disease, and one (3.2%) had a history of asthma. Most patients (96.8%) had invasive ductal carcinoma, as observed on histopathology. The most common HER2 expression was observed during immunohistochemistry (IHC) 3+ (58.1%), followed by IHC 2+/silver in situ hybridization (+) (35.5%). Additionally, 83.9% (26/31), 71.0% (22/31), and 54.8% (17/31) of the patients had previously undergone trastuzumab, pertuxumab, and trastuzumab emtansine therapies, respectively. The median number of previous cytotoxic chemotherapies was 3.0 (IQR, 2.0–5.0). The most common CT findings at baseline were nodules (58.1%), followed by fibrotic lesions (22.6%). Tumor response was evaluated in 28 of the 31 patients using the Response Evaluation Criteria in Solid Tumors version 1.1. Of the 28 patients, 19 (67.8%) achieved a partial response, 8 (28.6%) experienced stable disease, and 1 (3.6%) experienced progressive disease. Among the 31 patients, 30 (96.8%) experienced at least one adverse event during treatment. The most common adverse events were fatigue (54.8%) and nausea (51.6%). Adverse events, including pneumonitis in four patients, led to six patients (19.4%) being excluded from further analysis. There appear to be no specific characteristics associated with ILD development.
Table 1. Patients’ baseline characteristics.
| Variables | Total | |
|---|---|---|
| Number | 31 | |
| Age (yr) | 54.0 (47.0–60.0) | |
| Sex | ||
| Female | 30 (96.8) | |
| Smoking history | 2 (6.5) | |
| Co-existing pulmonary disease | ||
| Chronic obstructive pulmonary disease | 1 (3.2) | |
| Asthma | 1 (3.2) | |
| Lung metastasis at baseline | 12 (38.7) | |
| Histopathology | ||
| Invasive ductal carcinoma | 30 (96.8) | |
| Invasive lobular carcinoma | 1 (3.2) | |
| HER2 expression | ||
| IHC 3+ | 18 (58.1) | |
| IHC 2+/SISH (+) | 11 (35.5) | |
| IHC 2+/SISIH (−) or IHC 1+ | 3 (9.7) | |
| Hormone receptor (+) | 15 (48.4) | |
| Prior HER2-targeted therapy | ||
| Trastuzumab | 26 (83.9) | |
| Pertuxumab | 22 (71.0) | |
| Trastuzumab emtansine | 17 (54.8) | |
| Number of prior cytotoxic chemotherapies | 3.0 (2.0–5.0) | |
| Chest CT findings at baseline | ||
| Consolidation | 1 (3.2) | |
| Nodule | 18 (58.1) | |
| Linear opacity | 4 (12.9) | |
| Fibrotic lesion | 7 (22.6) | |
| Pleural effusion or mass | 4 (12.9) | |
| Tumor response by the RECIST version 1.1 (n = 28) | ||
| Complete response | 0 (0.0) | |
| Partial response | 19 (67.8) | |
| Stable disease | 8 (28.6) | |
| Progression disease | 1 (3.6) | |
| Adverse events* | ||
| Nausea | 16 (51.6) | |
| Fatigue | 17 (54.8) | |
| Alopecia | 8 (25.8) | |
| Constipation | 8 (25.8) | |
| Anorexia | 13 (41.9) | |
Values are presented as median (interquartile range) or number of patients (%).
HER2 = human epidermal receptor 2; IHC = immunohistochemistry; SISH = silver in situ hybridization; CT = computed tomography; RECIST = Response Evaluation Criteria in Solid Tumors.
*Adverse events reported in more than 25% of patients.
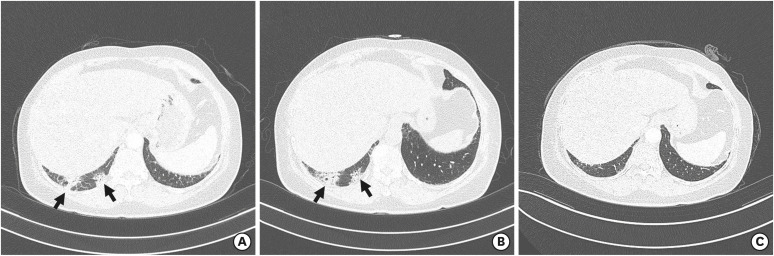
Four patients (12.9%) developed ILD (Table 2). However, only one patient had undergone bronchoalveolar lavage, which identified no organism. The median time until the onset of ILD after the treatment was 307 days (IQR, 221.5–388.0 days). None of these patients presented with symptoms or signs associated with ILD at the time of the initial diagnosis. Three patients (cases 1, 2, and 3) received glucocorticoids as treatment for ILD, and none were hospitalized due to ILD. The most common finding seen in all four patients on CT scans was the presence of consolidation. The consolidation was located in the lung periphery in all patients; lower lobe predominance was observed in two patients, upper lobe predominance in one patient (Figure 1), and diffuse distribution in one patient (Supplementary Video 1). Furthermore, in two of the four patients, the opacities were bilateral, whereas the opacities were unilateral in the other two patients. Two patients showed ground-glass opacities associated with areas of consolidation. Therefore, all CT findings in the four patients were interpreted as organizing pneumonia (OP) patterns [12]. These events were primarily Common Terminology Criteria for Adverse Events grade 1 or 2, and no patient had ILD of grade 3 or higher. Subsequently, follow-up CT in case 3 performed 18 days later showed more extensive areas of consolidation (Figure 2). Radiologic improvements were also noted at follow-up in the two patients (cases 3 and 4), and the duration from onset to recovery was 266 days and 479 days. However, the remaining patients showed no changes in abnormal CT findings until the last examination. The occurrence of ILD led to discontinuation of drugs in all four patients; those patients remained stable from day 183 to day 674 without disease progression.
Table 2. Radiologic findings from the four patients diagnosed with interstitial pneumonitis.
| Patient demographics | CT findings | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Sx | Onset (day) | Steroid use | Resolution | Appearance | Distribution | Pattern | |||||||||
| GGO | Con | N | L | ST | PE | Extent | Axial | SS | Zonal | Lateral | |||||||
| F | 60 | No | 331 | Yes | No | No | Yes | No | No | No | No | M | P | No | Upper | Both | OP |
| F | 47 | No | 201 | Yes | No | Yes | Yes | No | No | No | No | M | P | No | Diffuse | Both | OP |
| F | 61 | No | 283 | Yes | Yes | No | Yes | No | No | No | No | F | P | No | Lower | Right | OP |
| F | 53 | No | 407 | No | Yes | Yes | Yes | No | No | No | No | F | P | No | Lower | Right | OP |
CT = computed tomography; Sx = symptoms; GGO = ground glass opacity; Con = consolidation; N = nodule; L = linear opacity; ST = septal thickening; PE = pleural effusion; P = peripheral; M = multifocal; F = focal; SS = subpleural sparing; OP = organizing pneumonia.
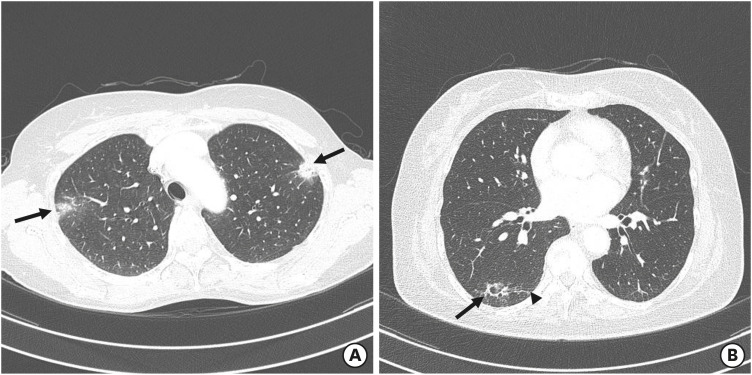
Figure 1. Chest CT scans obtained from patients with trastuzumab deruxtecan-induced lung injury. (A) A CT image obtained from a 60-year-old woman with organizing pneumonia in the upper lobes (case 1 in Table 2) shows peripheral, multifocal, nodular consolidations (arrows). The patient developed drug-induced interstitial lung disease 331 days after beginning treatment with trastuzumab deruxtecan; however, the lung abnormalities remained even after glucocorticoid therapy. (B) A CT image obtained from a 53-year-old woman with organizing pneumonia in the right lower lobe (case 4 in Table 2) who received her first dose of trastuzumab deruxtecan 407 days previously showed focal consolidation and ground-glass opacity (arrow) adjacent to underlying fibrosis (arrowhead). The patient’s condition improved after cessation of trastuzumab deruxtecan without glucocorticoid therapy.
CT = computed tomography.
Figure 2. Chest CT scans of a 61-year-old woman (case 3 in Table 2) with trastuzumab deruxtecan-induced organizing pneumonia. (A) Axial index CT images of the right lower lung shows subpleural consolidations (arrows) in the basal segment. (B) Axial follow-up CT images performed 18 days after index CT show worsening consolidations (arrows). (C) CT examination performed 256 days after index CT shows near-complete resolutions of lung parenchymal abnormality.
CT = computed tomography.
In this study, we reported specific CT findings of trastuzumab deruxtecan-induced ILD in patients with metastatic breast cancer. The OP pattern comprising subpleural consolidations was dominant in all four patients. In addition, the incidence and severity of ILD in patients were similar to those previously reported.
Two recent phase 2 studies reported high incidences of trastuzumab deruxtecan-induced ILD in patients diagnosed with metastatic breast and gastric cancers [5,6]. Earlier studies assessed 184 patients enrolled from North America, Asia, and Europe who received trastuzumab deruxtecan for 10 months [5]. Based on their results, 25 patients (13.6%) experienced drug-induced ILD, most of which were grade 1 or 2 (96%). The median onset duration of ILD in these patients was 193 days (IQR, 42–535). Furthermore, nine patients (36%) showed resolved or resolving (median duration, 34.0 days) symptoms. However, four deaths (2.2%) were attributed to ILD. Another phase 2 study including 187 patients from Japan and South Korea reported an ILD incidence of 10% [6]. According to the study, the median time to onset was 84.5 days. Most events were grade 1 or 2 (75%), and eight patients with ILD (66.7%) showed improved or improving status (median duration, 57.0 days). In our study, we showed an incidence of 12.9%, which is similar to the results of previous studies. In addition, all patients experienced grade 1 or 2 adverse events, whereas half of the patients showed resolved status. Unlike previous studies, both the onset and recovery times for ILD in our cohort were long. However, a pooled analysis of eight single-arm phase 1 and 2 studies examining trastuzumab deruxtecan use showed that 88% of the patients who developed ILD experienced onset within 12 months of treatment [13]. In 48% of the confirmed cases, the onset time identified by the investigators was later than that identified by the ILD adjudication committee. Therefore, no significant difference was observed in terms of the timing of onset compared to previous results. This conclusion was based on the fact that three-quarters of ILD cases in our study developed within the first 12 months.
The CT findings of OP are nonspecific and have been observed in various diseases and neoplastic drugs [14]. A common disease involving interstitial pneumonitis in the form of OP is polymyositis-dermatomyositis (PM-DM) [15]. Approximately one-third of patients with PM-DM develop interstitial pneumonitis [16]. However, no evidence led us to suspect PM-DM in our patients diagnosed with interstitial pneumonitis because there were no symptoms of muscle weakness or autoantibody reactions. In addition, OP is a frequent manifestation of drug reactions, including bleomycin, cyclophosphamide, and methotrexate [17]. Although cyclophosphamide used for chemotherapy to treat breast cancer was proposed to cause CT manifestations of OP, only one of the four patients diagnosed with interstitial pneumonitis was treated with cyclophosphamide approximately 4 years prior [18]. Abnormalities in OP observed using CT resembled those of chronic eosinophilic pneumonia (CEP) [19]. However, approximately 50% of patients diagnosed with CEP have asthma, and a vast majority have peripheral eosinophilia [20]. None of the four patients in this study had asthma and peripheral eosinophilia, suggesting a higher likelihood of trastuzumab deruxtecan-induced OP than CEP. All cases in this study showed OP patterns in their CT scans; this result was similar to that of a phase 1 study including 115 patients who received trastuzumab deruxtecan [21]. That study reported an ILD incidence of 17.4%, including cases of OP (30%). Thus, the clinical diagnosis of trastuzumab deruxtecan-induced OP in the four cases was reliable.
This study is significant as it mainly included the analyses of CT findings of trastuzumab deruxtecan-induced ILD at a single institute. However, this study had several limitations. First, the study was a descriptive, retrospective review of clinical and radiologic records and included only a few patients. Second, bronchoalveolar lavage to exclude the possibility of combined pneumonia caused by infectious organisms was not conducted in most cases. Third, ILD was mainly diagnosed based only on CT findings, and no correlation with pathological findings was found as biopsies were not performed. Finally, CT scan to detect ILD was performed only if the patient presented with ILD-related pulmonary symptoms. Since four patients who were diagnosed with trastuzumab deruxtecan-induced ILD in this study showed no symptoms suggesting ILD, ILD might have been underestimated in our cohort.
To summarize, the incidence of trastuzumab deruxtecan-induced ILD in the Korean population was comparable to that observed in previous trials. Moreover, the most commonly encountered CT finding in trastuzumab-induced ILD is the OP pattern, which comprises mainly consolidations in the subpleural areas. The presence of these findings in patients undergoing treatment with trastuzumab deruxtecan should arouse suspicion for drug-induced ILD.
Acknowledgments
This study was supported by the Ulsan University. The Scientific Publications Team of Asan Medical Center provided editorial support in the form of writing assistance, collation of authors’ comments, and grammatical editing.
Footnotes
Conflict of Interest: Kyung Hae Jung participated as a consultant on the advisory boards of Roche, AstraZeneca, Novartis, and Takeda. Sung-Bae Kim received research funding from Novartis, Sanofi-Aventis, and DongKook Pharm Co. and held stock from Genopeaks, NeogeneTC and participated in advisory boards from Novartis, AstraZeneca, Liily, Dae Hwa Pharmaceutical Co. Ltd, ISU Abxis, and Daiichi-Sankyo.
- Supervision: Jeong JH, Kim JE, Ahn JH, Jung KH.
- Writing - original draft: Jang YJ.
- Writing - review & editing: Kim SB.
SUPPLEMENTARY MATERIAL
Axial CT images of a 47-year-old woman (case 2 in Table 2) with an approximately 200-day history of trastuzumab deruxtecan with diffuse organizing pneumonia. Axial CT images of the upper, right middle, right lower, and left lower lungs show bilateral, multifocal consolidations and ground-glass opacities characterized as organizing pneumonia patterns.
References
- 1.Goddard KA, Weinmann S, Richert-Boe K, Chen C, Bulkley J, Wax C. HER2 evaluation and its impact on breast cancer treatment decisions. Public Health Genomics. 2012;15:1–10. doi: 10.1159/000325746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krop IE, Kim SB, González-Martín A, LoRusso PM, Ferrero JM, Smitt M, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–699. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 5.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 7.Kubo K, Azuma A, Kanazawa M, Kameda H, Kusumoto M, Genma A, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig. 2013;51:260–277. doi: 10.1016/j.resinv.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Schaller S, Wildberger JE, Raupach R, Niethammer M, Klingenbeck-Regn K, Flohr T. Spatial domain filtering for fast modification of the tradeoff between image sharpness and pixel noise in computed tomography. IEEE Trans Med Imaging. 2003;22:846–853. doi: 10.1109/TMI.2003.815073. [DOI] [PubMed] [Google Scholar]
- 9.Gierada DS, Bierhals AJ, Choong CK, Bartel ST, Ritter JH, Das NA, et al. Effects of CT section thickness and reconstruction kernel on emphysema quantification relationship to the magnitude of the CT emphysema index. Acad Radiol. 2010;17:146–156. doi: 10.1016/j.acra.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 11.Silva CI, Müller NL, Lynch DA, Curran-Everett D, Brown KK, Lee KS, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology. 2008;246:288–297. doi: 10.1148/radiol.2453061881. [DOI] [PubMed] [Google Scholar]
- 12.Ujita M, Renzoni EA, Veeraraghavan S, Wells AU, Hansell DM. Organizing pneumonia: perilobular pattern at thin-section CT. Radiology. 2004;232:757–761. doi: 10.1148/radiol.2323031059. [DOI] [PubMed] [Google Scholar]
- 13.Yin O, Iwata H, Lin CC, Tamura K, Watanabe J, Wada R, et al. Exposure-response relationships in patients with HER2-positive metastatic breast cancer and other solid tumors treated with trastuzumab deruxtecan. Clin Pharmacol Ther. 2021;110:986–996. doi: 10.1002/cpt.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch DA, Travis WD, Müller NL, Galvin JR, Hansell DM, Grenier PA, et al. Idiopathic interstitial pneumonias: CT features. Radiology. 2005;236:10–21. doi: 10.1148/radiol.2361031674. [DOI] [PubMed] [Google Scholar]
- 15.Tansey D, Wells AU, Colby TV, Ip S, Nikolakoupolou A, du Bois RM, et al. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004;44:585–596. doi: 10.1111/j.1365-2559.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- 16.Connors GR, Christopher-Stine L, Oddis CV, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest. 2010;138:1464–1474. doi: 10.1378/chest.10-0180. [DOI] [PubMed] [Google Scholar]
- 17.Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018;7:356. doi: 10.3390/jcm7100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva CI, Müller NL. Drug-induced lung diseases: most common reaction patterns and corresponding high-resolution CT manifestations. Semin Ultrasound CT MR. 2006;27:111–116. doi: 10.1053/j.sult.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Arakawa H, Kurihara Y, Niimi H, Nakajima Y, Johkoh T, Nakamura H. Bronchiolitis obliterans with organizing pneumonia versus chronic eosinophilic pneumonia: high-resolution CT findings in 81 patients. AJR Am J Roentgenol. 2001;176:1053–1058. doi: 10.2214/ajr.176.4.1761053. [DOI] [PubMed] [Google Scholar]
- 20.Alam M, Burki NK. Chronic eosinophilic pneumonia: a review. South Med J. 2007;100:49–53. doi: 10.1097/01.smj.0000242863.17778.1d. [DOI] [PubMed] [Google Scholar]
- 21.Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:816–826. doi: 10.1016/S1470-2045(19)30097-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Axial CT images of a 47-year-old woman (case 2 in Table 2) with an approximately 200-day history of trastuzumab deruxtecan with diffuse organizing pneumonia. Axial CT images of the upper, right middle, right lower, and left lower lungs show bilateral, multifocal consolidations and ground-glass opacities characterized as organizing pneumonia patterns.