Abstract
Objective
To compare pneumonic-type invasive mucinous adenocarcinoma (pIMA) confined to a single lobe with clinical T2, T3, and T4 stage lung cancer without pathological node metastasis regarding survival after curative surgery and to identify prognostic factors for pIMA.
Materials and Methods
From January 2010 to December 2017, 41 patients (15 male; mean age ± standard deviation, 66.0 ± 9.9 years) who had pIMA confined to a single lobe on computed tomography (CT) and underwent curative surgery were identified in two tertiary hospitals. Three hundred and thirteen patients (222 male; 66.3 ± 9.4 years) who had non-small cell lung cancer (NSCLC) without pathological node metastasis and underwent curative surgery in one participating institution formed a reference group. Relapse-free survival (RFS) and overall survival (OS) were calculated using the Kaplan–Meier method. Cox proportional hazard regression analysis was performed to identify factors associated with the survival of patients with pIMA.
Results
The 5-year RFS and OS rates in patients with pIMA were 33.1% and 56.0%, respectively, compared with 74.3% and 91%, 64.3% and 71.8%, and 46.9% and 49.5% for patients with clinical stage T2, T3, and T4 NSCLC in the reference group, respectively. The RFS of patients with pIMA was comparable to that of patients with clinical stage T4 NSCLC and significantly worse than that of patients with clinical stage T3 NSCLC (p = 0.012). The differences in OS between patients with pIMA and those with clinical stage T3 or T4 NSCLC were not significant (p = 0.11 and p = 0.37, respectively). In patients with pIMA, the presence of separate nodules was a significant factor associated with poor RFS and OS {unadjusted hazard ratio (HR), 4.66 (95% confidence interval [CI], 1.95–11.11), p < 0.001 for RFS; adjusted HR, 4.53 (95% CI, 1.59–12.89), p = 0.005 for OS}.
Conclusion
The RFS of patients with pIMA was comparable to that of patients with clinical stage T4 lung cancer. Separate nodules on CT were associated with poor RFS and OS in patients with pIMA.
Keywords: Adenocarcinoma, mucinous; Lung neoplasms; Neoplasm staging; Prognosis
INTRODUCTION
Invasive mucinous adenocarcinoma (IMA), which is histologically diagnosed upon seeing a tall columnar structure with basally located nuclei and abundant intracellular or extracellular cytoplasmic mucin, accounts for approximately 2%–5% of lung adenocarcinomas [1,2]. As a separate category of lung adenocarcinoma, lymph node involvement and distant metastasis are less frequent in IMA than in other subtypes of adenocarcinoma, but aerogenous spread and separate tumors around the main lesion are common [3,4,5].
In the 8th tumor-node-metastasis (TNM) staging system, pneumonic-type lung adenocarcinoma has been introduced as a rare form of lung cancer, most of which are pneumonic-type IMA (pIMA) [6,7]. According to these criteria, there is diffuse distribution of adenocarcinoma throughout one or more regions of the lung on pathological investigation, as well as a regional distribution, similar to a pneumonic infiltrate or consolidation on CT. The tumor stage of pneumonic-type lung cancer is either determined by tumor size or designated as clinical T3 if multiple pulmonary sites of involvement are confined within a single lobe [7]. This exception to tumor size-based staging is made because the exact size measurement of pneumonic-type lung cancer can be quite difficult when the lesion is not clearly demarcated because of consolidation and ground-glass opacity. Currently, no study has validated the prognosis of pIMA according to the T category in the 8th TNM staging system. Furthermore, other than the presence of pneumonic consolidation on CT, there have been no reports regarding detailed CT findings that may predict the prognosis of pIMA.
Therefore, the purpose of our study was to compare pIMA confined to a single lobe with clinical stage T2, T3, and T4 lung cancer without pathological node metastasis regarding survival after curative surgery, and identify prognostic factors for pIMA.
MATERIALS AND METHODS
This retrospective study was approved by the Institutional Review Boards of Asan Medical Center (institution A, IRB No. 2020-0841) and Samsung Medical Center (institution B, IRB No. 2020-04-142), which waived the requirement for informed consent from patients.
Study Population
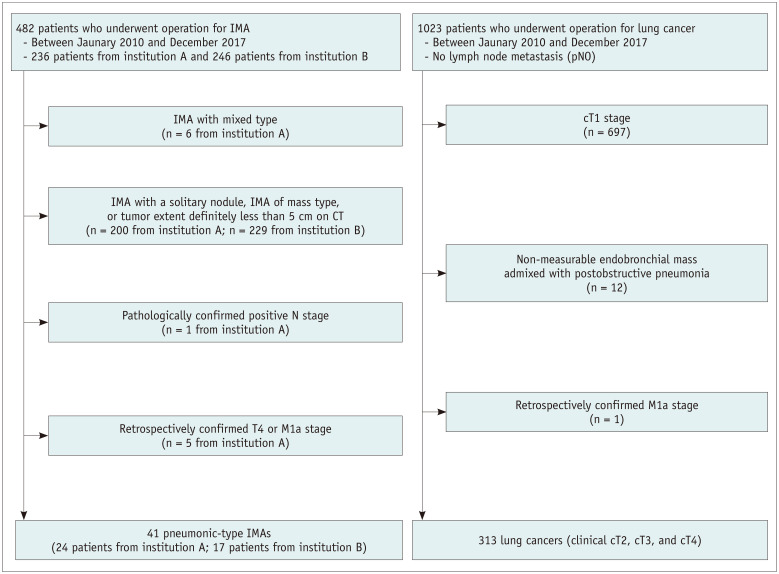
In both institutions A and B, pathologic evaluation based on the renewed lung adenocarcinoma scheme has been applied since 2010 in advance of the official publication of the new classification [1]. Patients who had undergone curative surgery for IMA between January 2010 and December 2017 were searched, and 482 patients with IMA were identified from 11532 consecutive patients with lung adenocarcinoma (236 cases at institution A from 6150 consecutive patients and 246 at institution B from 5382 consecutive patients).
From the 482 cases, 441 patients were excluded to establish a pIMA population with clinical stage T3 disease: 1) mixed invasive mucinous/non-mucinous adenocarcinoma on pathology, 2) IMA manifesting as a nodule or mass, 3) pIMAs with a single disease focus measuring less than 5 cm on CT, 4) pathologically confirmed node metastasis, and 5) pIMAs with preoperatively confirmed T4 or M1a stage based on subsequent follow-up CT scans. Finally, 41 patients were included in the study (Fig. 1).
Fig. 1. Patient selection flow chart.
IMA = invasive mucinous adenocarcinoma
The surgical database was searched for the same period for patients with surgically confirmed non-small cell lung cancer without lymph node metastasis in institution A, and 1023 patients were identified. Among them, 710 patients were excluded for the following reasons: 1) clinical stage T1 lung cancer on preoperative CT, 2) endobronchial mass admixed with postobstructive pneumonia making precise size measurement impossible on CT, and 3) retrospectively confirmed stage M1a disease. Clinical stages were assigned according to the 8th edition of TNM classification. Finally, 313 patients with clinical T2, T3, and T4 (cT2, cT3, and cT4) lung cancer were included in the reference group. None of the patients in the pIMA or reference groups underwent preoperative treatment. All preoperative chest CT scans were performed within 30 days before surgery (median, 7.3 days; range, 0–30 days).
Follow-Up after Surgery for Survival Analysis
Relapse-free survival (RFS) and overall survival (OS) were determined based on the electronic medical records of the two institutions. RFS was calculated from the date of surgery until either the date of recurrence (event, defined as local tumor recurrence, distant metastasis, or death), or until the date that the patient was last known to be free of recurrence (censored). OS was calculated from the date of surgery until either death from any cause (event) or the date the patient was last known to be alive (censored).
CT Acquisition
All CT data were acquired with contrast enhancement in the supine position at full inspiration. The scan coverage was from the lung base to the level of the thoracic inlet. In institution A, chest CTs were obtained using 16- or 64-detector row equipment from two different manufacturers (Somatom Definition, and Sensation-16, Siemens Medical Solutions; and Lightspeed 16, Lightspeed VCT, and Discovery, GE Medical Systems). The acquisition parameters were as follows: 120 kVp, 30–200 mAs; pitch, 0.875–1; and collimation, 1–1.25 mm. Dose modulation using automatic exposure control was applied. Intravenous contrast media (90–120 mL of 300–370 mgI/mL non-ionic contrast) was injected at a rate of 3 mL/s and scanning started after a delay of 50 seconds. Images were reconstructed using a sharp kernel with a slice thickness/interval of 1/1, 1.25/1.25, 2.5/2.5, and 3/3 mm.
In institution B, CT scans were obtained using 16- or 64-detector row CT scanners (LightSpeed16 and LightSpeed VCT, GE Medical Systems) with the following parameters: collimation, 0.625 mm; 120 kVp; 150 to 200 mA; pitch, 1.35 or 1.375; and section thickness, 1.25 mm. After the initial scan without contrast material administration, CT scanning was again performed 90 seconds after the administration of contrast material (100 mL iopamidol [Iomeron 300; Bracco]) delivered at a rate of 1.5 mL/s using a power injector. This was followed by a 20 cm3 saline flush at a rate of 1.5 mL/s. Non-contrast data were reconstructed using a bone algorithm, whereas contrast-enhanced imaging data were reconstructed using soft tissue algorithms.
Image Analysis
Using preoperative CT images, 41 pIMAs were evaluated for the presence of a cavity within the tumor, separate nodules within a lobe, and extent of tumor with a fraction of involved segments in a lobe, which was quantified as the number of involved segments divided by the number of segments of the corresponding lobe. The initial recurrence pattern of pIMA was also evaluated for the presence of nodules and consolidation. All image interpretations were performed based on a consensus between two radiologists (with 9 years and 16 years of experience in chest radiology). For the reference group, clinical staging was performed on CT images according to the 8th TNM guidelines [8], with assessments based on a consensus between two radiologists (with 9 and 16 years of experience in chest radiology).
For positron emission tomography (PET)/CT evaluation, a circular region of interest (ROI) was drawn at the most intense area of fluorodeoxyglucose (FDG) uptake in the pIMA. FDG uptake within the ROIs was then analyzed to determine the maximum standardized uptake value (SUVmax).
Pathological Data Collection
One of several pathologists at either institution evaluated surgically resected lung cancers and performed comprehensive histological subtyping. The predominant histologic subtypes determined according to the 2015 WHO classification of lung cancers were collected from pathologic reports.
Statistical Analysis
The Mann-Whitney U test, Kruskal-Wallis test, and chi-squared test were used for comparisons between the pIMA and reference groups. RFS and OS were estimated using the Kaplan-Meier method, and the log-rank test was used to evaluate differences between subgroups.
Univariable and multivariable analyses using Cox proportional hazards regression were performed to assess the effect of various clinicopathologic and radiologic factors on RFS and OS in patients with pIMA. As some variables were correlated with each other, such as multiple nodules, fraction of involved segments in a lobe, cavity, and age, the Cox models were fitted using only one of the corresponding correlated variables in the multivariable analysis. SUVmax was not included in the multivariable analysis because only 29 patients underwent PET/CT in the pIMA group. For each Cox model, the highest likelihood score (chi-squared) statistic and number of best predictors were determined through minimization of the Akaike information criterion.
Logistic regression analysis was performed to verify the correlations between preoperative CT findings and recurrence patterns. A hazard rate curve for death was drawn to measure the impact of the recurrence pattern on death during follow-up after surgery. Data were analyzed using R version 4.0.1 (the R Foundation for Statistical Computing). For pairwise comparisons of survival outcomes between cT3, cT4, and pIMA patients, p < 0.017 (i.e., 0.05/3) was considered statistically significant. Statistical significance was set at p < 0.05.
RESULTS
Patient Characteristics
The patient characteristics are summarized in Table 1. The pIMA group included 41 patients, and the reference group included 205 cT2, 71 cT3, and 37 cT4 stage patients. The mean follow-up period was 47.2 months (range, 4.5–109.8 months) for the pIMA group and 52.0 months (range, 3.3–116.4 months) for the reference group, with there being no statistically significant difference (p = 0.435). There was no statistically significant difference in the survival rate of patients with pIMA between institutions A and B (Supplementary Fig. 1).
Table 1. Demographic and Clinical Characteristics of the pIMA Group and Reference Group.
| pIMA (n = 41) | Reference Group (n = 313) | P | |||
|---|---|---|---|---|---|
| Age, years | 66.0 ± 9.9 | 66.3 ± 9.4 | 0.718* | ||
| Age according to clinical staging, years | 0.668† | ||||
| cT2 | 65.9 ± 9.2 | ||||
| cT3 | 66.7 ± 9.8 | ||||
| cT4 | 67.3 ± 9.8 | ||||
| Sex | < 0.001‡ | ||||
| Male | 15 (36.6) | 222 (70.9) | |||
| Female | 26 (63.4) | 91 (29.1) | |||
| Smoking history | < 0.001‡ | ||||
| Ever smoker | 12 (29.3) | 206 (65.8) | |||
| Never smoker | 29 (70.7) | 107 (34.2) | |||
| Involved lobe | < 0.001‡ | ||||
| Upper lobe | 3 (7.3) | 164 (52.4) | |||
| Right upper lobe | 2 | 104 | |||
| Left upper lobe | 1 | 60 | |||
| Right middle lobe | 2 (4.9) | 16 (5.1) | |||
| Lower lobe | 36 (87.8) | 133 (42.4) | |||
| Right lower lobe | 18 | 86 | |||
| Left lower lobe | 18 | 47 | |||
| Clinical T-stage | |||||
| cT2 | 205 (65.5) | ||||
| cT3 | 71 (22.7) | ||||
| cT4 | 37 (11.8) | ||||
| Subtype | |||||
| Squamous | 118 (37.7) | ||||
| Adenocarcinoma | 177 (56.5) | ||||
| Others | 18 (5.8) | ||||
| Surgical procedure | 0.818‡ | ||||
| Lobectomy | 39 (95.1) | 294 (93.9) | |||
| Bilobectomy | 2 (4.9) | 16 (5.1) | |||
| Pneumonectomy | 3 (1.0) | ||||
| SUVmax | 6.3 ± 3.5 (n = 29) | 9.8 ± 5.8 (n = 270) | 0.001* | ||
| Follow-up, months | 47.2 ± 24.4 | 52.0 ± 25.41 | 0.435* | ||
| Follow-up period according to clinical staging, months | |||||
| cT2 | 55.2 ± 24.2 | ||||
| cT3 | 48.8 ± 26.5 | ||||
| cT4 | 40.2 ± 25.3 | ||||
Data are presented as mean ± SD or n (%) values. Unless otherwise indicated, data are the number of patients with percentage in parentheses. The other subtypes in the reference group were seven large cell neuroendocrine carcinomas, six adenosquamous carcinomas, and single cases of mucoepidermoid, carcinosarcoma, sarcomatoid carcinoma, signet ring cell carcinoma, and pleomorphic carcinoma.
*Mann-Whitney U test, †Kruskal-Wallis test, ‡Chi-square test. pIMA = pneumonic-type invasive mucinous adenocarcinoma, SD = standard deviation, SUVmax = maximum standardized uptake value
Comparison of Survival Outcomes
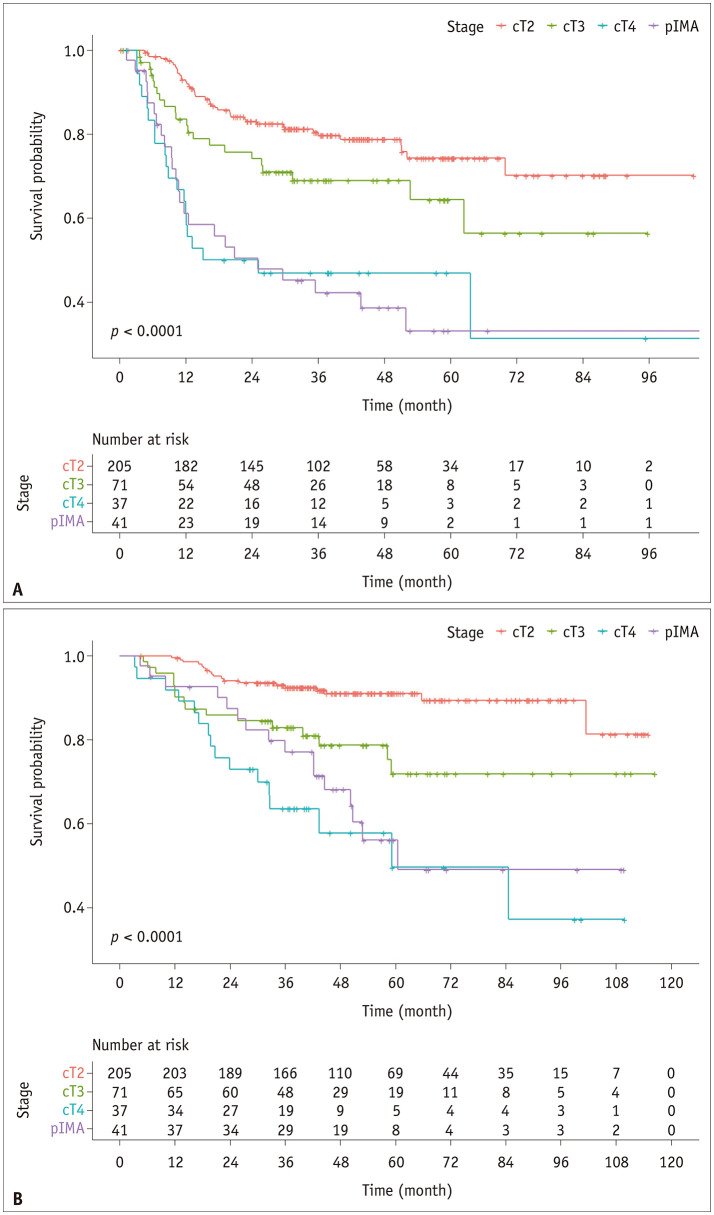
Among the 41 patients with pIMA, 24 cases of recurrence (58.5%) were identified during the follow-up period. The 5-year RFS rate was 33.1%. In the reference group, 85 cases of recurrence (27.1%) were observed during the follow-up period. The 5-year RFS rates were 74.3%, 64.3%, and 46.9% for stage cT2, cT3, and cT4 disease, respectively (Fig. 2). The RFS of the pIMA group was comparable to that of patients with cT4 cancer (p = 0.82; Bonferroni-corrected significance criterion, 0.05/3 = 0.017), and differed significantly from that of patients with cT3 cancer in a pairwise comparison (p = 0.012; Bonferroni-corrected significance criterion, 0.05/3 = 0.017).
Fig. 2. Kaplan-Meier survival curves of relapse-free survival (A) and overall survival (B) for pIMA and reference groups (cT2, cT3, and cT4).
pIMA = pneumonic-type invasive mucinous adenocarcinoma
In patients with pIMA, 16 cases of death (39.0%) were identified during the follow-up period, with a 5-year OS rate of 56.0%. The 5-year OS rates of patients with and without recurrence were 45.8% and 82.4%, respectively (p = 0.09). In the reference group, 49 patients (15.7%) died during the follow-up period. The 5-year OS rates were 91%, 71.8%, and 49.5% for patients with cT2, cT3, and cT4 cancer, respectively (Fig. 2). The OS curve of the pIMA group intersected with that of the cT3 stage at approximately 24 months, and thereafter remained close to that of the cT4 stage (Fig. 2). The differences in the OS curves between cT3 and pIMA and between cT4 and pIMA were not statistically significant (cT3 and pIMA, p = 0.11; cT4 and pIMA, p = 0.37; Bonferroni-corrected significance criterion, 0.05/3 = 0.017).
Prognostic Factors for pIMA
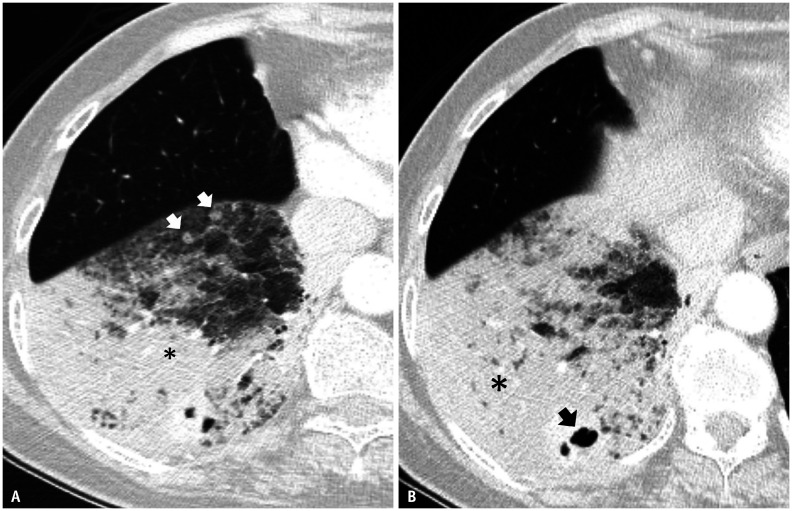
The mean fraction of segments in a lobe with pIMA was 0.55 (range, 0.2–1.0). The presence of a cavity within the tumor and separate nodules within a lobe were identified in 18 (43.9%) and 15 (56.1%) patients, respectively (Fig. 3). pIMAs were significantly more common in a lower lobar location (87.8%) than tumors in the reference group (p < 0.001) (Table 1).
Fig. 3. Pneumonic-type invasive mucinous adenocarcinoma on CT.
A, B. Enhanced CT obtained from a 65-year-old female patient with a lung window setting (3 mm thickness) shows a patchy area of ground-glass opacity and consolidation (asterisks), suggesting pneumonic-type adenocarcinoma with separate nodules (arrows, A) and a cavity (arrow, B) in the right lower lobe.
In the univariable analysis, the fraction of involved segments in a lobe (unadjusted hazard ratio [HR], 1.32; 95% confidence interval [CI], 1.13–1.53; p < 0.001), presence of a cavity (unadjusted HR, 3.51; 95% CI, 1.52–8.13; p = 0.003), presence of separate nodules (unadjusted HR, 4.66; 95% CI, 1.95–11.11; p < 0.001), and SUVmax (unadjusted HR, 1.23; 95% CI, 1.06–1.43; p = 0.006) were significant factors associated with RFS. In the multivariable analysis, the presence of separate nodules within the lobe showed the strongest association with RFS, followed by the fraction of involved segments in a lobe (for an increase of 0.1) and presence of a cavity (Table 2).
Table 2. Univariable and Multivariable Cox Proportional Hazard Regression Analysis of Factors Affecting Relapse-Free Survival in Patients with pIMA (n = 41).
| Variables | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | ||
| Age, years | 0.98 (0.94–1.02) | 0.337 | |||
| Sex | |||||
| Male | Reference | ||||
| Female | 1.19 (0.51–2.78) | 0.697 | |||
| Smoking status | |||||
| Non-smoker | Reference | ||||
| Ex- or current-smoker | 1.17 (0.50–2.74) | 0.716 | |||
| Lesion characteristics | |||||
| Fraction of involved segments in a lobe (unit = 0.1) | 1.32 (1.13–1.53) | < 0.001 | 1.32 (1.13–1.53) | < 0.001 | |
| Cavity | 3.51 (1.52–8.13) | 0.003 | 3.51 (1.52–8.13) | 0.003 | |
| Separate nodule(s) | 4.66 (1.95–11.11) | < 0.001 | 4.66 (1.95–11.11) | < 0.001 | |
| SUVmax (unit = 1) | 1.23 (1.06–1.43) | 0.006 | |||
| Adjuvant treatment | |||||
| None | Reference | ||||
| Adjuvant chemotherapy | 1.75 (0.77–3.97) | 0.180 | |||
CI = confidence interval, HR = hazard ratio, pIMA = pneumonic-type invasive mucinous adenocarcinoma, SUVmax = maximum standardized uptake value
In terms of OS, the presence of separate nodules within a lobe and SUVmax values were statistically significant prognostic factors. In the multivariable analysis, the presence of separate nodules was the only statistically significant predictor (adjusted HR, 4.53; 95% CI, 1.59–12.89; p = 0.005) (Table 3).
Table 3. Univariable and Multivariable Cox Proportional Hazard Regression Analysis of Factors Affecting Overall Survival in Patients with pIMA (n = 41).
| Variables | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | ||
| Age, years | 1.04 (0.99–1.09) | 0.114 | |||
| Sex | |||||
| Male | Reference | ||||
| Female | 0.98 (0.33–2.87) | 0.968 | |||
| Smoking status | |||||
| Non-smoker | Reference | ||||
| Ex- or current-smoker | 1.15 (0.39–3.38) | 0.794 | |||
| Smoking amount (pack-years) | 1.00 (0.97–1.03) | 0.917 | |||
| Lesion characteristics | |||||
| Fraction of involved segments in a lobe (unit = 0.1) | 1.19 (0.98–1.44) | 0.082 | |||
| Cavity | 1.95 (0.69–5.48) | 0.206 | |||
| Separate nodule(s) | 4.53 (1.59–12.89) | 0.005 | 4.53 (1.59–12.89) | 0.005 | |
| SUVmax (unit = 1) | 1.32 (1.13–1.53) | < 0.001 | |||
| Adjuvant treatment | |||||
| None | Reference | ||||
| Adjuvant chemotherapy | 1.11 (0.40–3.08) | 0.995 | |||
CI = confidence interval, HR = hazard ratio, pIMA = pneumonic-type invasive mucinous adenocarcinoma, SUVmax = maximum standardized uptake value
Recurrence Patterns
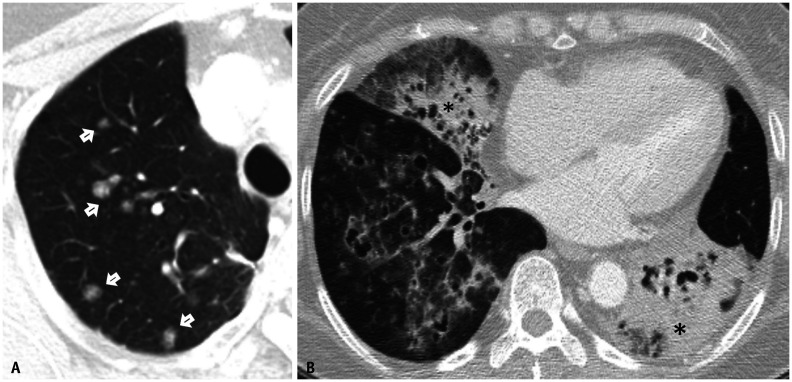
All 24 cases of recurrence were confined within the lung parenchyma; five patients (20.8%) had a single nodule without consolidation, seven patients (29.2%) had multiple nodules without consolidation, and 12 patients (50%) had consolidation with or without nodules (Fig. 4). In 11 of the 12 patients who had recurrence with nodules, ground-glass nodules were the predominant features of recurrence.
Fig. 4. Two different patterns of recurrence in patients with pneumonic-type invasive mucinous adenocarcinoma.
A. Nodular recurrence in a 62-year-old female patient (arrows). The relapse-free survival and overall survival in this patient were 17.17 months and 60.5 months, respectively. B. Recurrence with consolidation in a 76-year-old male patient (asterisks). The relapse-free survival and overall survival in this patient were 5.07 months and 27.47 months, respectively.
We reclassified these recurrence patterns into nodular recurrence (12 patients) and recurrence with consolidation (12 patients). All 12 patients (100%) who had recurrence with consolidation and seven patients (58.3%) with nodular recurrence experienced recurrence within 2 years.
DISCUSSION
The current lung cancer system emphasizes tumor size and tumor stage changes every centimeter up until the T2 stage. However, in pneumonic-type lung cancer, accurate measurement of tumor size may be difficult. Therefore, the current staging system suggests that pneumonic-type lung cancer in a single lobe can be considered a T3 stage. Although pIMA is just one representative subtype of lung cancer manifesting as pneumonic type, there is no available report comparing the survival outcomes of pIMA according to the current lung cancer staging system. In our study, we found that the RFS of pIMA was worse than that of other cancers graded as stages cT3 according to the current staging system, and similar to those graded as stage cT4.
The poor RFS of pIMA relative to cT3 disease may stem from the strong tendency for characteristic microscopic skip lesions in pIMA, lesions that are relevant to the aerogenous spread of tumor cells [9,10]. Cha and Shim [9] hypothesized that tumor cells travel in the background of abundant alveolar mucin and become situated in the alveolar walls away from the primary lesion. Accordingly, as abundant alveolar mucin might be seen as consolidative lung parenchymal opacity on CT, we assume that the consolidative features of pIMA itself may reflect a higher potential for microscopic tumor spread. This is associated with the presence of separate nodules on CT, which was the only predictor for both RFS and OS, and with the finding that all cases of pIMA recurrence were intrapulmonary parenchymal metastases.
In contrast to RFS, the OS curve for pIMA crossed that of cT3 stage disease at approximately 24 months after surgery, and then gradually approximated that of cT4 disease. This may be due to the recurrence patterns of pIMA. We classified parenchymal recurrence into two patterns, nodular recurrence (12 patients, 50%) and recurrence with consolidation (12 patients, 50%), which eventually progressed to consolidation on follow-up CT. Therefore, the OS curve of pIMA did not decrease in the same manner as the RFS curve of pIMA. However, our study population was small and the heterogeneous pattern of the survival curve requires further verification in a larger study.
One CT finding of note in our study on pIMA is that the fraction of involved segments in a lobe was not an independent predictor of poor OS. Considering that pIMA is composed of both a mucinous spread component and an invasive component, and the exact invasive tumor size cannot be inherently differentiated on CT, our results suggest that CT measurement of pIMA may not reliably reflect tumor burden. As evidence, Saito et al. [11] reported that the pathological size of mucin spread and radiological size of IMA were not significantly different, whereas the pathological invasive tumor size was significantly smaller than the radiological size. In contrast, SUVmax was found to be a significant predictor of both poor RFS and OS in the univariable analysis, although SUVmax was not included in the multivariable analysis because of missing cases. As SUVmax reflects the metabolic activity of the tumor, it could provide more accurate prognostic information than the tumor extent on CT.
Our study has several limitations. First, the sample size was small. Although the patients with pIMA who had undergone curative surgery were recruited from two tertiary hospitals, the final number of patients included in this study was still low. Second, the number of cases without lymph node metastasis was limited to the cT3 and cT4 comparison patients, even though surgically resected lung cancer cases were recruited for a period of 8 years. Therefore, the OS curve showed significant differences between clinical stages in the reference group; however, there was only marginal significance (p = 0.06) between cT3 and cT4. Third, the follow-up protocols, which may vary depending on the physician, could skew the survival data. However, as a rule, both institutions followed the surveillance guidelines, with 3 to 6 months of regular follow-up until 2 years after surgery [12]. Fourth, the reference group was not statistically identical to the pIMA group with respect to sex and smoking status. Fortunately, sex and smoking status were not significant factors for survival outcomes in either group. Finally, the reference group included adenocarcinoma and non-adenocarcinoma. Given the subtype of pIMA, it would be better to include only adenocarcinoma. However, the current lung cancer staging system does not differentiate according to subtypes, and the aim of our study was to determine the clinical tumor stage of pIMA. Therefore, we included patients with various subtypes in the reference group.
In conclusion, patients with pIMA on CT showed comparable RFS after curative surgery in patients with cT4 non-small cell lung cancer. The presence of separate nodules on CT was significantly associated with poor OS and RFS in patients with pIMA.
Footnotes
Conflicts of Interest: Sang Min Lee and Joon Beom Seo who is on the editorial board of the Korean Journal of Radiology was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
- Conceptualization: Sang Min Lee, Ho Yun Lee.
- Data curation: Wooil Kim, Sang Min Lee, Ho Yun Lee.
- Formal analysis: Wooil Kim, Sang Min Lee, Jung Bok Lee, Ho Yun Lee.
- Funding acquisition: Ho Yun Lee.
- Investigation: Wooil Kim, Sang Min Lee, Joon Beom Seo, Hong Kwan Kim, Jhingook Kim, Ho Yun Lee.
- Methodology: Wooil Kim, Jung Bok Lee, Sang Min Lee, Ho Yun Lee.
- Project administration: Wooil Kim, Sang Min Lee, Joon Beom Seo, Ho Yun Lee.
- Resources: Sang Min Lee, Ho Yun Lee.
- Software: Wooil Kim, Jung Bok Lee, Sang Min Lee.
- Supervision: Sang Min Lee, Joon Beom Seo, Hong Kwan Kim, Jhingook Kim, Ho Yun Lee.
- Validation: Wooil Kim, Sang Min Lee, Joon Beom Seo, Hong Kwan Kim, Jhingook Kim, Ho Yun Lee.
- Visualization: Wooil Kim, Sang Min Lee, Ho Yun Lee.
- Writing—original draft: Wooil Kim, Sang Min Lee, Ho Yun Lee.
- Writing—review & editing: Wooil Kim, Sang Min Lee, Joon Beom Seo, Hong Kwan Kim, Jhingook Kim, Ho Yun Lee.
Funding Statement: This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP; Ministry of Science, ICT, & Future Planning) (NRF-2020R1F1A1068226).
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available because of institutional policy, but are available from the corresponding author on reasonable request.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2021.0465.
References
- 1.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011;8:381–385. doi: 10.1513/pats.201107-042ST. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Lee HY, Cha MJ, Lee KS, Lee HY, Kwon OJ, Choi JY, et al. Prognosis in resected invasive mucinous adenocarcinomas of the lung: related factors and comparison with resected nonmucinous adenocarcinomas. J Thorac Oncol. 2016;11:1064–1073. doi: 10.1016/j.jtho.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Gaikwad A, Gupta A, Hare S, Gomes M, Sekhon H, Souza C, et al. Primary adenocarcinoma of lung: a pictorial review of recent updates. Eur J Radiol. 2012;81:4146–4155. doi: 10.1016/j.ejrad.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Austin JH, Garg K, Aberle D, Yankelevitz D, Kuriyama K, Lee HJ, et al. Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology. 2013;266:62–71. doi: 10.1148/radiol.12120240. [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Detterbeck FC, Marom EM, Arenberg DA, Franklin WA, Nicholson AG, Travis WD, et al. The IASLC lung cancer staging project: background data and proposals for the application of TNM staging rules to lung cancer presenting as multiple nodules with ground glass or lepidic features or a pneumonic type of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol. 2016;11:666–680. doi: 10.1016/j.jtho.2015.12.113. [DOI] [PubMed] [Google Scholar]
- 8.Detterbeck FC, Nicholson AG, Franklin WA, Marom EM, Travis WD, Girard N, et al. The IASLC lung cancer staging project: summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eighth edition of the TNM classificatio. J Thorac Oncol. 2016;11:639–650. doi: 10.1016/j.jtho.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Cha YJ, Shim HS. Biology of invasive mucinous adenocarcinoma of the lung. Transl Lung Cancer Res. 2017;6:508–512. doi: 10.21037/tlcr.2017.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadota K, Nitadori JI, Sima CS, Ujiie H, Rizk NP, Jones DR, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol. 2015;10:806–814. doi: 10.1097/JTO.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito T, Tsuta K, Honda O, Ishida M, Yamaka R, Tanaka N, et al. Prognostic impact of mucin spread, tumor cell spread, and invasive size in invasive mucinous adenocarcinoma of the lung. Lung Cancer. 2020;146:50–57. doi: 10.1016/j.lungcan.2020.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Schneider BJ, Ismaila N, Altorki N. Lung cancer surveillance after definitive curative-Intent therapy: ASCO guideline summary. JCO Oncol Pract. 2020;16:83–86. doi: 10.1200/JOP.19.00722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed during the study are not publicly available because of institutional policy, but are available from the corresponding author on reasonable request.